Significance
More than 92% of ER− breast cancer patients die with moderate to high NOS2. In this report, we show that tumor cell NOS2, through formation of a specific flux of NO, drives ER− disease to a more aggressive phenotype. Correlation between NOS2-related genes from patient cohorts, in vitro, and animal experiments show an inflammatory loop mediated by NOS2 that makes ER− breast cancer an aggressive disease.
Abstract
Inflammation is widely recognized as an inducer of cancer progression. The inflammation-associated enzyme, inducible nitric oxide synthase (NOS2), has emerged as a candidate oncogene in estrogen receptor (ER)-negative breast cancer, and its increased expression is associated with disease aggressiveness and poor survival. Although these observations implicate NOS2 as an attractive therapeutic target, the mechanisms of both NOS2 induction in tumors and nitric oxide (NO)-driven cancer progression are not fully understood. To enhance our mechanistic understanding of NOS2 induction in tumors and its role in tumor biology, we used stimulants of NOS2 expression in ER− and ER+ breast cancer cells and examined downstream NO-dependent effects. Herein, we show that up-regulation of NOS2 occurs in response to hypoxia, serum withdrawal, IFN-γ, and exogenous NO, consistent with a feed-forward regulation of NO production by the tumor microenvironment in breast cancer biology. Moreover, we found that key indicators of an aggressive cancer phenotype including increased S100 calcium binding protein A8, IL-6, IL-8, and tissue inhibitor matrix metalloproteinase-1 are up-regulated by these NOS2 stimulants, whereas inhibition of NOS2 in MDA-MB-231 breast cancer cells suppressed these markers. Moreover, NO altered cellular migration and chemoresistance of MDA-MB-231 cells to Taxol. Most notably, MDA-MB-231 tumor xenographs and cell metastases from the fat pad to the brain were significantly suppressed by NOS2 inhibition in nude mice. In summary, these results link elevated NOS2 to signals from the tumor microenvironment that arise with cancer progression and show that NO production regulates chemoresistance and metastasis of breast cancer cells.
Inflammation is a major component of the tumor microenvironment and a driving force in cancer initiation, promotion, and progression (1–3). Epithelial cancers express markers of inflammation that promote disease progression and drug resistance through evasion of cell death pathways and increased tumor metastasis. Rapid cancer growth leads to tumor hypoxia and nutrient deprivation, which promotes chronic inflammatory feed-forward signaling and selection of resistant tumors that are clinically challenging and sometimes untreatable.
Several proinflammatory proteins such as COX2, NF-κB, IL-6, IL-8, S100 calcium binding protein A8 (S100A8), and VEGF are markers of chronic inflammation in the tumor microenvironment. In addition, these proinflammatory mediators directly correlate with inducible nitric oxide synthase (NOS2), which is an emerging biomarker of aggressive tumors that predicts poor survival in patients with elevated tumor NOS2 expression (4–8). These and other clinical studies warrant an improved mechanistic understanding of intratumoral NOS2 regulation and endogenous NO production, which may be therapeutically beneficial.
Toward this end, our laboratory and others have used NO donors to study NO signaling in cancer. However, intratumoral NOS2 induction by components of the tumor microenvironment, which produces endogenous NO at levels that promote disease progression and predict poor outcome, has not been examined. Herein, we have used cell culture conditions that simulate chronic inflammation in the tumor microenvironment, which include nutrient deprivation by serum withdrawal (SW), hypoxia, inflammatory cytokines, and NO donors to examine physiologic mechanisms of NOS2 induction and downstream effects of NO target activation in both estrogen receptor positive and negative (ER+ and ER−) breast cancer cells. In addition, we show in vivo effects of NOS2 inhibition on tumor growth and metastases in mice. Using this approach, we provide evidence of NOS2 as a key driver of feed-forward signaling that promotes chronic inflammation and cancer progression.
Results
NOS2 Drives Tumor Growth and Metastasis.
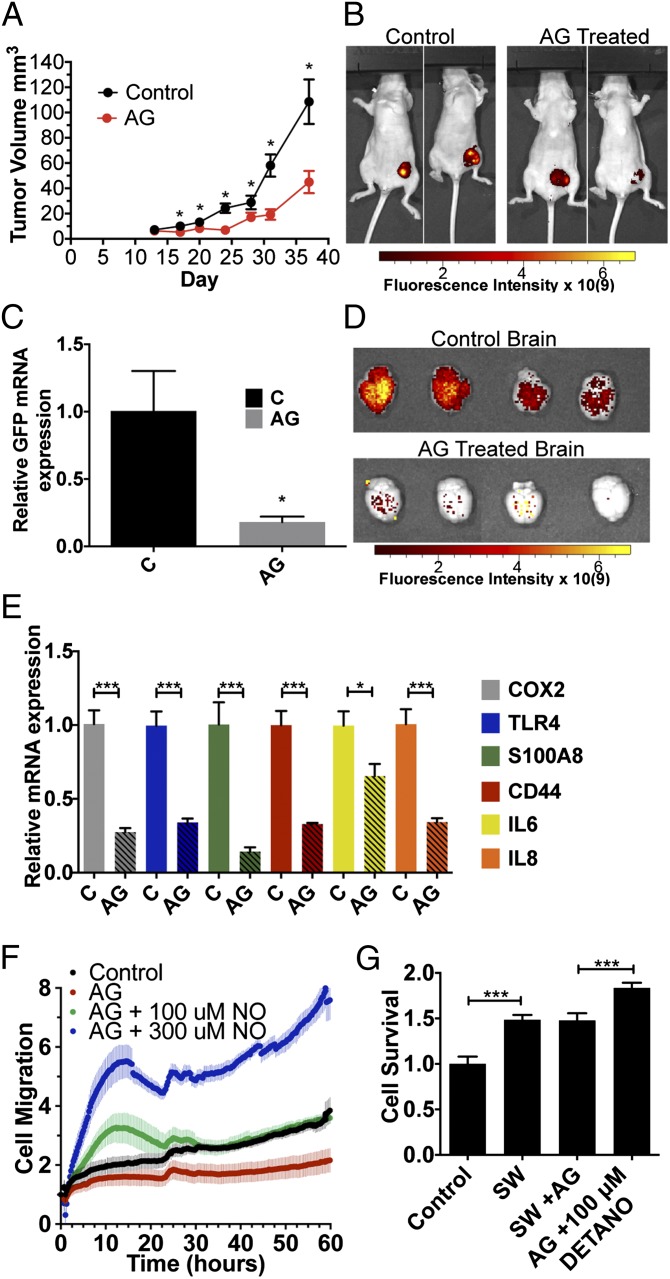
The effects of NOS2 inhibiton via amminoguanidine (AG) on tumor progression was examined in a xenograph model of green fluorescent protein-tagged MDA-MB-231 (MDA-MB-231-GFP) breast cancer cells implanted in the mammary fat pad of female nude mice. Fig. 1 demonstrates significantly reduced tumor growth in AG-treated mice (Fig. 1A); after 37 d of treatment, AG suppressed tumor growth by 59% compared with control (44.9 ± 8.8 mm3 vs. 109 ± 17 mm3, respectively). These results are supported by GFP fluorescence imaging shown in Fig. 1B, which provides a visual index of reduced tumor cell proliferation in AG-treated mice compared with control animals.
Fig. 1.
NO effects on MDA-MB-231 cells in vivo and in vitro. (A) Tumor volume of control and AG-treated GFP-tagged MDA-MB-231 tumor-bearing mice (n = 10 and 11 at day 37). (B) GFP fluorescence intensity at tumor site in control and AG-treated mice at day 45. (C and D) Representative brain GFP mRNA expression and GFP protein fluorescence intensity, respectively, from MDA-MB-231-GFP cell brain metastasis at day 45 after fat pad injection in control and AG-treated mice. (E) mRNA levels of COX2, TLR4, S100A8, CD44, IL-6, and IL-8 in tumors of control and AG-treated mice (day 45). (F) DETA/NO addition mediates cell migration. (G) Taxol drug resistance (at 10 nM) in MDA-MB-231 cells treated with SW with or without AG and DETA/NO (±SEM, *P ≤ 0.05, ***P ≤ 0.001).
The effect of NOS2 inhibition on metastatic potential of MDA-MB-231-GFP tumors was also examined. MDA-MB-231-GFP cell brain metastasis at day 45 after fat pad injection was quantified by real-time PCR and fluorescence imaging of the GFP tag. Fig. 1 C and D shows a dramatic reduction in the quantified mRNA and fluorescent protein levels, respectively of GFP from the metastasized MDA-MB-231-GFP cells in the brains of AG-treated mice compared with control animals. Together, these results demonstrate that NOS2 inhibition dramatically reduces tumor growth and metastasis and provides evidence that NOS2 is a key driver of breast cancer disease progression in this model.
ER− patients with high NOS2 tumor levels were found to have a gene signature that was predictive of outcome (4). Genes included in in this signature were IL-8, IL-6, S100A8, CD44, and Toll-like receptor 4 (TLR4), all of which are activated by NO donors in ER− cell lines. To investigate the effect of AG on this gene signature, we analyzed their expression in dissected tumors from this model by RT-PCR. As seen in Fig. 1E, the mRNA expression of COX2, TLR4, S100A8, CD44, IL-6, and IL-8 all significantly decrease in AG-treated mice compared with control. Therefore, this model mimics the patient data and shows NOS2 inhibition reduces markers that are associated with poor outcome.
Cell Migration.
Earlier reports have demonstrated NO-induced migration of ER− breast cancer cells (4). To examine a role of NO in MDA-MB-231 cell migration induced by SW, we used the xCelligence RTCA instrument that measures cell movement in real time. The cells were plated in serum-free RPMI 1640 in the presence of l-Arg and allowed to adhere overnight. AG was added with or without varying concentrations of the NO donor 1-[N-(2-Aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA/NO, 100 μM or 300 μM), and real-time migration of the cells to serum containing media was monitored for 60 h. Fig. 1F shows abolished cell mobility by AG compared with control cells. Titration of 100 μM DETA/NO reversed the effects of AG and increased cell migration of AG-inhibited cells over 24 h, which returned to baseline levels and is consistent with the temporal release of 100 nM steady-state NO flux released by 100 μM DETA/NO under the same conditions (Fig. S1). In contrast, 300 μM DETA/NO markedly increased cell migration that was sustained through 60 h. These results suggest NO flux-dependent regulation of cell migration. Interestingly, SW also induced NOS2 expression, nitrite production (Fig. S2 A and B), and secretion of prometastatic cytokines such as IL-6 and IL-8, which was partially inhibited by AG (Fig. S3).
Drug Resistance.
Chemoresistance is a recurring clinical problem in cancer treatment. Because NOS2 predicts poor cancer survival, its potential contribution to chemoresistance to Taxol was examined. MDA-MB-231 cells were pretreated by SW (24 h) with or without AG or DETA/NO, and then exposed to Taxol (10 nM) for 18 h. The cells were then trypsinized and plated for survival. Compared with the untreated control, SW abated Taxol cell killing, which was augmented by 100 μM DETA/NO (Fig. 1G). These results indicate NO-mediated resistance to breast cancer cell killing by Taxol under conditions of nutrient deprivation. Collectively, these results show promotion of tumor growth, metastasis, and drug resistance by NOS2-derived NO.
Cytokine Stimulation of NOS2.
Cytokines that induce NOS2 are present in the tumor microenvironment. Thus, cytokine-induced NOS2 expression was examined in breast cancer cell lines. Fig. S4A demonstrates a twofold increase in NOS2 protein expression in MCF-7 and MDA-MB-231 but not MDA-MB-468 breast cancer cells exposed for 24 h to a cytokine mix (CM). NOS2 enzymatic activity was assessed by nitrite levels in the media by using the Griess assay. Neither MCF-7 nor MDA-MB-231 nitrite levels changed after 24-h exposure of IFN-γ, IL-1β, TNF-α, or lipopolysaccharide (LPS) alone. However, nitrite production significantly increased in MDA-MB-468 cells after 24 h of IFN-γ or TNF-α (Fig. S4B). CM increased nitrite levels 2.5-, 2-, and 2.8-fold in MCF-7, MDA-MB-231, and MDA-MB-468 cells, respectively, (Fig. S4C), which was inhibited by AG.
The timing of NOS2 mRNA induction was examined for each CM-stimulated cell line at 4, 24, and 48 h (Fig. S4D). NOS2 mRNA induction in the least aggressive MCF-7 cells peaked at 24 h and was increased 50-fold relative to control. The more aggressive MDA-MB-231 cells exhibited peak NOS2 expression at 4 h with a 170-fold increase. Interestingly, NOS2 expression in the intermediately aggressive MDA-MB-468 cells was similar to MCF-7 cells with maximal induction at 24 h; however, the relative increase in NOS2 levels was much greater and peaked at >4,000-fold above control levels. The human NOS2 gene contains cytokine responsive elements. Accordingly, CM stimulated NOS2 promoter activity in MCF-7, MDA-MB-231, and MDA-MB-468 cells increased 2.2-, 4.2-, and 1.7-fold, respectively, as measured by a luciferase reporter assay (Fig. S4E). It should be noted that the message level was orders of magnitude higher than protein expression and nitrite levels and has been documented in other cell lines, indicating that there is a tight regulation of NOS2 that has not been completely elucidated (9). However, it has been shown in human DLD-1 cells that cytokines can up-regulate miRNA-939 (10), which is able to block protein translation and may be more active in this cell line. The temporal profile of NOS2 mRNA expression was also different in each cell line, further emphasizing the importance of understanding how and when NOS2 is up-regulated, and its impact on the aggressive phenotype of these different cancer cells.
Nutrient Deprivation by Serum Withdrawal.
Nutrient deprivation is a common feature of rapidly growing tumors. Cancer cells adapt to nutrient deprivation by altering metabolism, nutrient uptake, angiogenesis, and autophagy (11, 12). SW of cells grown in culture mimics nutrient deprivation, and we investigated the effects of SW on NOS2 expression. Fig. S2B shows significant nitrite production in MCF-7, MDA-MB-231, and MDA-MB-468 cells after 24–48 h of SW. NOS2 mRNA levels increased for each cell line at each time point, with maximal increases of 5.0, 12, and 2.5 in MCF-7, MDA-MB-231, and MDA-MB-468, respectively (Fig. S2C). NOS2 luciferase promoter activity increased by 1.8-, 4.0- and 2.1-fold, respectively, in the same cell lines (Fig. S2D). In addition, NOS2 protein levels increased after 48 h SW for each cell line (Fig. S2A).
IFN-γ Stimulation.
IFN-γ is a well-known mediator of NOS2 gene expression. IFN-γ alone induced NOS2 mRNA in the MDA-MB-231 cells (Fig. S5A). Moreover, 4-h stimulation with IFN-γ followed by SW increased nitrite accumulation at 48 h (Fig. S5B). NOS2 protein levels increased in response to 4-h IFN-γ treatment followed by SW compared with control (Fig. S5C). In contrast, type 1 IFN-α or IFN-β failed to up-regulate NOS2 mRNA or protein in these cells, indicating that NOS2 induction in breast cancer cells is specific to type 2 IFN-γ (Fig. S5 B and C).
Hypoxia.
Hypoxia activates various oncogenic and metabolic pathways (13) and is a hallmark of aggressive tumors (14). Hypoxia increases HIF-1α stabilization and accumulation and regulates important inflammatory genes, including NOS2, which increased in MCF-7 (15) and MDA-MB-231 cells (16) under hypoxic conditions. We investigated the effect of hypoxia on NOS2 protein expression by incubating breast cancer cell lines under 1% O2 with or without SW for 24 h. As seen in Fig. S5D, protein levels increased in each cell line after 24-h hypoxia compared with cells maintained in room air and this increase occurred only in the absence of serum, indicating that cellular stress in conjunction with hypoxia is required for a prolonged NOS2 up-regulation.
Together, these results show that hypoxia and nutrient deprivation, conditions that arise in the tumor microenvironment with disease progression and further promote disease progression and metastatic spread, induce increased NOS2 expression and activity in human breast cancer cells.
Regulation of NOS2 by AG.
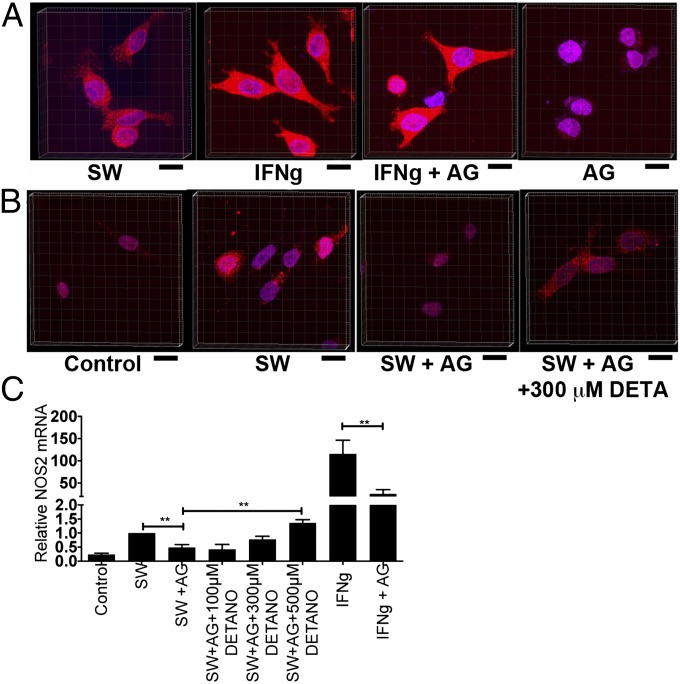
Nitric oxide participates in positive and negative feedback regulation of NOS2 through diverse mechanisms involving translation (17, 18), posttranslational modification, and enzymatic activity (19). The affect of AG on NOS2 induction was examined by confocal microscopy of IFN-γ/SW-treated MDA-MB-231 cells. Fig. 2A demonstrates basal NOS2 protein fluorescence in the SW control, which significantly increased in both cytoplasmic and nuclear compartments after IFN-γ treatment. Whereas the addition of AG minimally effected NOS2 protein expression in IFN-γ treated cells, AG treatment dramatically reduced baseline NOS2 protein expression compared with SW-treated MDA-MB-231 cells (Fig. 2 A and B), which suggests that basal levels of NOS2-derived NO mediate feed-forward NOS2 protein regulation. To examine a potential role of NO in feed-forward NOS2 regulation, DETA/NO was titrated back into AG-inhibited MDA-MB-231 cells. Titration with 300–500 μM DETA/NO increased NOS2 protein (Fig. 2B) and mRNA (Fig. 2C) expression to control levels in AG-inhibited MDA-MB-231 cells. Furthermore, IFN-γ stimulated cells exhibited a 50% reduction of NOS2 mRNA after incubation with AG (Fig. 2C). Together, these results show a requirement of low NO flux (200–500 nM steady-state NO) for feed-forward NOS2 regulation in aggressive MDA-MB-231 breast cancer cells.
Fig. 2.
Confocal microscopy of NOS2 protein expression in MDA-MB-231–treated cells. (A) SW and IFN-γ with or without AG-treated cells. (B) SW with or without AG or 300 μM DETA/NO. (C) AG decreases NOS2 mRNA expression in SW and IFN-γ–treated cells and titration of DETA/NO reestablishes NOS2 mRNA expression to control levels in SW+AG treated cells (±SEM, **P ≤ 0.01). (Scale bars: 20 μm.)
Downstream Targets of NOS.
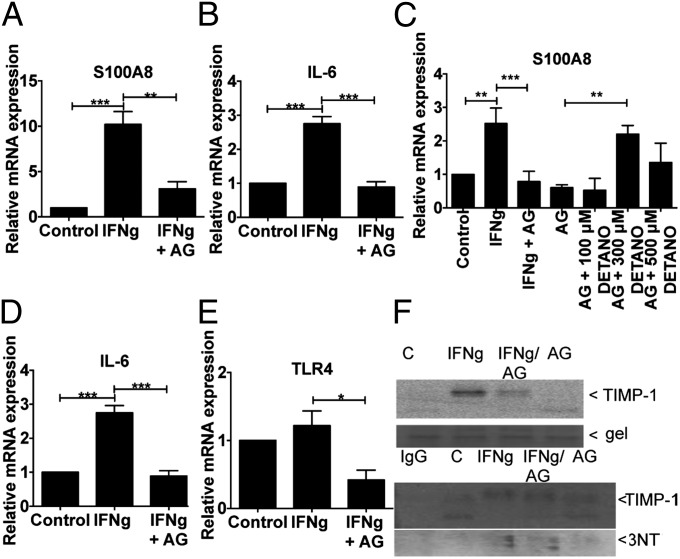
High-NOS2 ER− breast tumors exhibit increased expression of biomarkers, which predict poor breast cancer survival including IL-8, IL-6, TLR4, and S100A8 (4). To further examine NO regulatory mechanisms of these biomarkers under conditions simulating a tumor microenvironment, MDA-MB-231 cells were treated with IFN-γ for 4 h, followed by 48 h of SW with or without AG. Fig. 3 A and B shows induction of S100A8 and IL-6 by INF-γ and SW that is abated by AG. The effect of exogenous NO on AG inhibited S100A8 expression was also examined. Addition of DETA/NO (Fig. 3C) increased S100A8 mRNA levels in AG-inhibited MDA-MB-231 cells in a biphasic manner with maximal expression occurring at 300 μM DETA/NO (∼400 nM steady-state flux). The endogenous receptor for S100A8 is TLR4. AG abated TLR4 basal expression and that of IL-8 (Fig. 3 D and E). These results suggest a role of NO in the regulation and maintenance of these key tumor biomarkers.
Fig. 3.
NO effects on biomarker expression in MDA-MB-231 cells treated with IFN-γ (4 h) and SW (48 h). (A–E) mRNA expression of S100A8, IL-6, IL-8, and TLR4 induced by IFN-γ. (F) TIMP1 protein expression (Upper) and nitration (3NT blot) of TIMP1 immuno-precipitates (Lower) in IFN-γ–stimulated cells treated with or without AG (±SEM, *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001).
Tissue inhibitor matrix metalloproteinase-1 (TIMP1) is an additional biomarker that predicts poor breast cancer patient survival and correlates with increased PI3k/Akt activation in patients with high NOS2 tumor expression (20). TIMP1 expression and tyrosine nitration (3NT) was examined under conditions of IFN-γ/SW with or without AG. Fig. 3F shows IFN-γ/SW-induced TIMP1 protein that was abated by AG. Also, immunoprecipitated TIMP1 protein exhibited increased 3NT levels. Toward this end, we have reported maximal TIMP1 nitration at two tyrosine residues under conditions of ∼400 nM steady-state NO flux, which maximally activated PI3k/Akt signaling through interaction with CD63 (20).
Discussion
Elevated tumor NOS2 predicts poor survival in breast and other types of cancer (4, 5, 21, 22). We used murine tumor xenographs and cell culture conditions that mimic an aggressive tumor microenvironment including inflammation (cytokines), nutrient deprivation (SW), and hypoxia to examine pathways leading to tumor NOS2 expression and downstream targets of NOS2-derived NO that predict aggressive tumor phenotypes (4). Using this approach coupled with pharmacological NOS2 inhibition, we provide mechanistic evidence strongly supporting a role of NOS2-derived NO in breast cancer disease progression as defined by enhanced tumor biomarker expression, tumor growth, and metastatic burden, which are abated by NOS2 inhibition. The mRNA of predictive biomarkers found in high NOS2 ER− breast tumors (COX2, TLR4, S100A8, CD44, IL-6, and IL-8) were decreased by NOS2 inhibition in our mouse model. Collectively, our results implicate NOS2 as a key driver of cancer progression toward metastatic disease.
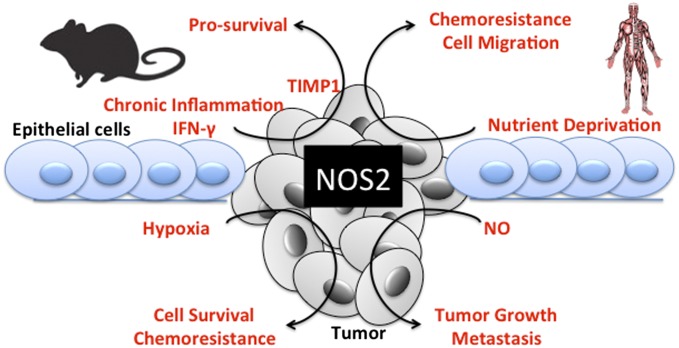
In this report, components of the tumor microenvironment promote NOS2 expression as confirmed by the assessment of increased NOS2 mRNA, protein, and nitrite production. Under these conditions, we show up-regulation of NO-targeted biomarkers (IL-6, IL-8, S100A8/TLR4, and TIMP1) of cancer progression (4, 20) that promote tumor cell survival, proliferation, and migration, which are suppressed by NOS2 inhibition. We also show intracellular mechanisms of NO-mediated feed-forward NOS2 regulation. Together, these results demonstrate that the tumor microenvironment comprises an atmosphere well suited for NOS2 expression and NOS2-derived NO (Fig. 4), which are important for maintenance and progression of aggressive tumor phenotypes.
Fig. 4.
Summary of NOS2 induction and its downstream signaling in ER− breast cancer.
The less aggressive MCF-7 breast cancer cell model generated significant nitrite levels in response to a CM, but not by cytokines administered as single agents (23). Herein, we show IFN-γ–induced NOS2 and nitrite accumulation in metastatic MDA-MB-231 breast cancer cells. Despite its role in tumor surveillance, the diverse functions of IFN-γ in cancer are beginning to emerge because of unbalanced IFN/JAK/STAT signaling, and it is postulated to suppress tumor immune surveillance leading to the selection of more aggressive, clinically resistant tumors (24–26). In support of these findings, distinct aggressive tumor phenotypes expressing high IFN-γ predict poor patient survival (27). Also, elevated serum IFN-γ predicted cancer recurrence and reduced disease-free survival in melanoma patients (28). Because INF-γ up-regulates NOS2 in aggressive breast cancer cells, we explored potential mechanistic effects linking IFN-γ signaling with elevated NOS2 tumor expression that predicted poor breast cancer specific survival in ER− patients (4). To accomplish this mechanistic investigation, we examined the promoters of 44 genes up-regulated in high NOS2 expressing tumors (4). Among these genes, our analysis identified IFN regulatory factor-1 (IRF1) binding sites in the promoters of the stem cell biomarker CD44 and the basal-like breast cancer biomarker S100A8. IRF1 is also necessary for transcriptional activation of the NOS2 gene (29), and this finding implicates an IFN signature as a key mediator of NOS2 expression and downstream effects in these aggressive breast tumors (4).
IL-8 and IL-6 are two additional biomarkers of disease progression identified in high NOS2 expressing ER− breast tumors (4) and triple negative breast cancer patients (30). Herein, we show IFN-γ induced the mRNA expression of IL-6 that was abated by NOS2 inhibition via AG. Circulating IL-6 is elevated in cancer patients and known to increase STAT3 and initiate downstream binding of STAT and NF-κB in the NOS2 promoter. This scenario provides an additional mechanism for feed-forward NOS2 regulation within the tumor microenvironment. IFN-γ also induced S100A8 in MDA-MB-231 cells, which was also abolished by AG. Interestingly, AG-inhibited S100A8 mRNA was restored to IFN-γ–induced levels by the addition of 300 μM DETA/NO. Baseline expression of the S100A8 receptor TLR4 and IL-8 were suppressed by AG, indicating a tight regulation of these proteins by basal levels of NOS2-derived NO in MDA-MB-231 cells. Collectively, these results support a role of IFN-γ within the breast cancer tumor microenvironment that promotes prosurvival mechanisms in specific breast cancer disease states and implicates the IFN-γ/JAK/STAT pathway as a potential therapeutic target in ER− breast cancer patients with high NOS2 tumor expression. Further elucidation of these and other signatures should improve personalized therapeutic efficacy (26).
Nutrient deprivation (SW) and hypoxia are also common features of tumors and are important when considering the regulation of inflammatory proteins associated with poor survival (31, 32). Hypoxia increases NOS2 and COX2 mRNA in MDA-MB-231 (16) and NOS2 mRNA in MCF-7 cells (15). Our studies show NOS2 protein up-regulation in breast cancer cells after 24-h incubation in 1% O2. Moreover, SW increased cell migration of MDA-MB-231 cells at 1% O2 (33), and our results (Fig. 2F) implicate NOS2 regulation as a key driver leading to tumor cell migration under these conditions. Herein, SW alone significantly increased NOS2 mRNA and protein in all cell lines. In tumors, nutrient deprivation results from limited vascularization and forces tumor adaptation via activation of angiogenic, invasion, and survival pathways (12). NO augments tumor cell survival, implicating NOS2 as a mediator of tumor survival under these conditions (4, 20, 34, 35). Importantly, the current study shows positive feed-forward regulation of NOS2 protein and mRNA expression by exogenous NO under SW conditions, because AG suppressed NOS2 levels, which then rebounded in the presence of exogenous NO donor. We have shown that the TIMP1 protein predicted poor breast cancer survival and activated Akt/BAD signaling in patients with high tumor NOS2 expression (20). Also, TIMP1 nitration correlated with NO-induced PI3k/Akt/BAD prosurvival signaling in MDA-MB-231 cells (20). Herein, we show IFN-γ followed by SW-induced TIMP1 protein expression and nitration in MDA-MB-231 cells, which was suppressed by AG. These results suggest that IFN-γ promotes tumor survival.
The regulation of NOS2 in breast tumors depends on various conditions that are associated with the tumor microenvironment including cytokines, hypoxia, nutrient deprivation, and various metabolic factors. This report presents evidence that NOS2 induction may be sustained by feed-forward regulatory loops involving components of the tumor microenvironment including NO, IL-6, and COX2.
Conclusion
Herein, we show that pharmacological NOS2 inhibition abated tumor growth and metastatic burden of aggressive MDA-MB-231 xenographs in mice. Moreover, low baseline NOS2 expression was observed in metastatic MDA-MB-231 cells grown in culture, which diminished by NOS2 inhibition, then reaccumulated in the presence of exogenous NO. Similarly, NOS2 inhibition blocked MDA-MB-231 cell migration, which was restored to basal levels by the addition of 100 μM DETA/NO. Importantly, NOS2 inhibited cell migration was significantly enhanced above basal levels by the addition of 300 μM DETA/NO, which suggests NO flux-dependent regulation of tumor cell migration. Furthermore, NO donor pretreatment or SW suppressed Taxol cell killing of MDA-MB-231 cells, indicating the promotion of tumor survival and chemoresistance by NOS2-derived NO. These results suggest a requirement of feed-forward NOS2 regulation for the maintenance of a basal NO flux that perpetuates protumorigenic signaling leading to cancer progression and metastases.
Various stimulants present within the tumor microenvironment were found to induce NOS2 expression in vitro. Toward this end, NOS2 was induced in MCF-7, MDA-MB-468, and MDA-MB-231; however, each cell line required different stimulants with varied temporal profiles. Furthermore, NOS2-induced regulation of tumor biomarkers supports NOS2 as a biomarker of disease progression in breast cancer patients (4). NOS2 inhibition in MDA-MB-231 cells decreased basal IL-8 and TLR4 expression. Also, TIMP1, S100A8, and IL-6 were enhanced by IFN-γ in an NO-dependent manner in these cells. The levels of NO that activate these pathways are consistent with ∼200–400 nM steady-state NO flux. These results suggest that NOS2 can be endogenously activated and that NO donors are a viable resource for understanding pathologic NO effects. The heterogeneity of stimulation paradigms is reminiscent of that in tumors and supports a role of flux-dependent NOS2-derived NO in disease progression. The elucidation of factors within the tumor microenvironment that regulate NOS2 and NO flux-driven tumor progression could lead to more personalized therapeutic options for women whose breast tumors express high NOS2. The use of NOS2 inhibitors combined with inhibition of upstream and downstream NO targets (i.e., IFN-γ/JAK/STAT, PI3K/AKT, NF-κB, HIF-1α) or the inhibition of microRNAs that regulate human NOS2, such as miRNA-939, could improve clinical outcome of breast cancer patients. Moreover, NOS2 inhibition suppressed growth and metastatic potential of KRAS mutant MDA-MB-231 (34, 36) cells, which suggests that NOS2 inhibition may provide a viable option for patients with currently untreatable RAS mutant tumors.
Materials and Methods
Cell Culture.
MCF-7, MDA-MB-231, and MDA-MB-468 human breast cancer cells (American Type Culture Collection) were maintained in RPMI 1640 media supplemented with 10% (vol/vol) heat inactivated FBS and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 and room air. Cells were plated in 60-mm dishes at densities of 2 × 106 cells in 4 mL of medium and grown overnight (80–90% confluent). For nitrite analyses, cells were seeded at 2 × 105 in 24-well plates and grown overnight. The medium was replaced with 2 mL (60-mm plates) or 0.5 mL (24-well plates) of serum and phenol-free RPMI 1640 containing penicillin/streptomycin and inflammatory stimulants. Cytokines were added alone or as a CM of 500 U/mL IFN-γ, 20 ng/mL TNF-α, 20 ng/mL IL-1β (R&D Systems), and 20 ng/mL LPS (Sigma). IFN-α and -β (R&D Systems) were added at 500 U/mL hypoxia was achieved by incubating the cells under 1% O2 and 5% CO2 in a Bactron hypoxic chamber (Shel Lab). NOS2 activity was inhibited by the addition of 1 mM AG (Sigma), and cellular effects of NO were independently evaluated by adding the NO donor DETA/NO (T 1/2 = 20 h at 37 °C) at various concentrations (Larry Keefer, National Cancer Institute-Frederick). We selected AG as the primary tool in our studies because of its previous use in clinical trials, its well established oral administration, and because the other common NOS2 inhibitor, 1400W, has shown toxic effects at higher doses (37).
In Vivo Studies.
Female athymic nude mice were supplied from the Frederick Cancer Research and Development Center Animal Production Area (Frederick, MD). The animals were received at 8 wk of age, housed five per cage, and given autoclaved food and water ad libitum. The NOS2 inhibitor AG was administered in filter-sterilized drinking water at 0.5 g/L. Animals were injected with 750,000 GFP-tagged MDA-MB-231 human breast cancer cells in the mammary fat pad at 9–10 wk of age and grown for 1 wk before administration of the NOS2 inhibitor AG. Tumor volume was measured by caliper and calculated as mm3 = [width2 × length]/2, where width is the smaller dimension and presented as mean ± SEM and GFP fluorescence and quantitation are described in SI Materials and Methods. Animal protocols were approved and performed in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council).
Statistical Analyses.
Results are presented as mean ± SEM and determined from at least three independent experiments. Statistical significance was evaluated with Student’s t tests as part of PRISM Graphpad Software and reported as *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
All other methods are summarized in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
The work was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401799111/-/DCSupplemental.
References
- 1.Ridnour LA, et al. Molecular pathways: Toll-like receptors in the tumor microenvironment—poor prognosis or new therapeutic opportunity. Clin Cancer Res. 2013;19(6):1340–1346. doi: 10.1158/1078-0432.CCR-12-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain SP, et al. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 2000;60(13):3333–3337. [PubMed] [Google Scholar]
- 3.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91(4):411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn SA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120(11):3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimm EA, Ellerhorst J, Tang CH, Ekmekcioglu S. Constitutive intracellular production of iNOS and NO in human melanoma: Possible role in regulation of growth and resistance to apoptosis. Nitric Oxide. 2008;19(2):133–137. doi: 10.1016/j.niox.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagares-Garcia JA, et al. Nitric oxide synthase as a marker in colorectal carcinoma. Am Surg. 2001;67(7):709–713. [PubMed] [Google Scholar]
- 7.Wang L, Xie K. Nitric oxide and pancreatic cancer pathogenesis, prevention, and treatment. Curr Pharm Des. 2010;16(4):421–427. doi: 10.2174/138161210790232194. [DOI] [PubMed] [Google Scholar]
- 8.Okayama H, et al. NOS2 enhances KRAS-induced lung carcinogenesis, inflammation and microRNA-21 expression. Int J Cancer. 2013;132(1):9–18. doi: 10.1002/ijc.27644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan GC, et al. Epigenetic basis for the transcriptional hyporesponsiveness of the human inducible nitric oxide synthase gene in vascular endothelial cells. J Immunol. 2005;175(6):3846–3861. doi: 10.4049/jimmunol.175.6.3846. [DOI] [PubMed] [Google Scholar]
- 10.Guo Z, et al. miRNA-939 regulates human inducible nitric oxide synthase posttranscriptional gene expression in human hepatocytes. Proc Natl Acad Sci USA. 2012;109(15):5826–5831. doi: 10.1073/pnas.1118118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber K. Energy deregulation: Licensing tumors to grow. Science. 2006;312(5777):1158–1159. doi: 10.1126/science.312.5777.1158. [DOI] [PubMed] [Google Scholar]
- 12.Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: Possible new biochemical target for cancer therapy. Cancer Res. 2000;60(21):6201–6207. [PubMed] [Google Scholar]
- 13.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8(9):705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Tafani M, et al. Up-regulation of pro-inflammatory genes as adaptation to hypoxia in MCF-7 cells and in human mammary invasive carcinoma microenvironment. Cancer Sci. 2010;101(4):1014–1023. doi: 10.1111/j.1349-7006.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tafani M, et al. Induction of autophagic cell death by a novel molecule is increased by hypoxia. Autophagy. 2008;4(8):1042–1053. doi: 10.4161/auto.7070. [DOI] [PubMed] [Google Scholar]
- 17.Griscavage JM, Rogers NE, Sherman MP, Ignarro LJ. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J Immunol. 1993;151(11):6329–6337. [PubMed] [Google Scholar]
- 18.Park SK, Lin HL, Murphy S. Nitric oxide limits transcriptional induction of nitric oxide synthase in CNS glial cells. Biochem Biophys Res Commun. 1994;201(2):762–768. doi: 10.1006/bbrc.1994.1766. [DOI] [PubMed] [Google Scholar]
- 19.Albakri QA, Stuehr DJ. Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J Biol Chem. 1996;271(10):5414–5421. doi: 10.1074/jbc.271.10.5414. [DOI] [PubMed] [Google Scholar]
- 20.Ridnour LA, et al. Nitric oxide synthase and breast cancer: Role of TIMP-1 in NO-mediated Akt activation. PLoS ONE. 2012;7(9):e44081. doi: 10.1371/journal.pone.0044081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekmekcioglu S, et al. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res. 2000;6(12):4768–4775. [PubMed] [Google Scholar]
- 22.Ekmekcioglu S, et al. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119(4):861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 23.Loibl S, et al. Investigations on the inducible and endothelial nitric oxide synthases in human breast cancer cell line MCF-7 - estrogen has an influence on e-NOS, but not on i-NOS. Pathol Res Pract. 2006;202(1):1–7. doi: 10.1016/j.prp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Chapat C, et al. hCAF1/CNOT7 regulates interferon signalling by targeting STAT1. EMBO J. 2013;32(5):688–700. doi: 10.1038/emboj.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodarev NN, et al. Signal transducer and activator of transcription 1 regulates both cytotoxic and prosurvival functions in tumor cells. Cancer Res. 2007;67(19):9214–9220. doi: 10.1158/0008-5472.CAN-07-1019. [DOI] [PubMed] [Google Scholar]
- 26.Weichselbaum RR, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci USA. 2008;105(47):18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaidi MR, Merlino G. The two faces of interferon-γ in cancer. Clin Cancer Res. 2011;17(19):6118–6124. doi: 10.1158/1078-0432.CCR-11-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter GA, et al. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann Surg Oncol. 2001;8(2):116–122. doi: 10.1007/s10434-001-0116-3. [DOI] [PubMed] [Google Scholar]
- 29.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J Exp Med. 1994;180(3):977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartman ZC, et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 2013;73(11):3470–3480. doi: 10.1158/0008-5472.CAN-12-4524-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tafani M, et al. Modulators of HIF1α and NFkB in cancer treatment: Is it a rational approach for controlling malignant progression? Front Pharmacol. 2013;4:13. doi: 10.3389/fphar.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer. 2012;132(12):2721–2729. doi: 10.1002/ijc.27901. [DOI] [PubMed] [Google Scholar]
- 33.Nagelkerke A, et al. Hypoxia stimulates migration of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded protein response. Breast Cancer Res. 2013;15(1):R2. doi: 10.1186/bcr3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Switzer CH, et al. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res. 2012;10(9):1203–1215. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Switzer CH, et al. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res. 2012;14(5):R125. doi: 10.1186/bcr3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim RK, et al. A novel 2-pyrone derivative, BHP, impedes oncogenic KRAS-driven malignant progression in breast cancer. Cancer Lett. 2013;337(1):49–57. doi: 10.1016/j.canlet.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Alderton WK, et al. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 2005;145(3):301–312. doi: 10.1038/sj.bjp.0706168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.