Abstract
Recycling of the nitrogenous waste uric acid (UA) of wood-feeding termites by their gut bacteria is one of the significant aspects of symbiosis for the conservation of nitrogen sources. Diverse anaerobic UA-degrading bacteria comprising 16 species were isolated from the gut of eight termite species, and were assigned to Clostridia, Enterobacteriaceae, and low G+C Gram-positive cocci. UA-degrading Clostridia had never been isolated from termite guts. UA-degrading ability was sporadically distributed among phylogenetically various culturable anaerobic bacteria from termite guts. A strain of Clostridium sp., which was commonly isolated from three termite species and represented a probable new species in cluster XIVa of clostridia, utilized UA as a nitrogen source but not as a sole carbon and energy source. This feature is in clear contrast to that of well-studied purinolytic clostridia or previously isolated UA degraders from termite guts, which also utilize UA as a sole carbon and energy source. Ammonia is the major nitrogenous product of UA degradation. Various purines stimulated the growth of this strain when added to an otherwise growth-limiting, nitrogen poor medium. The bacterial species involved the recycling of UA nitrogen in the gut microbial community of termites are more diverse in terms of both taxonomy and nutritional physiology than previously recognized.
Keywords: symbiosis, uric acid, nitrogen recycling, anaerobic bacteria, termite
Uric acid (UA) is a major nitrogenous waste excreted from animals such as terrestrial insects, birds, and certain reptiles (57 and references therein). Because of its poor solubility in water (only 60 mg L−1 at 20°C), its excretion as a nontoxic solid minimizes water loss. Not merely regarded as nitrogenous waste, UA is apparently utilized as a nitrogen source or metabolic reserve in some insects, particularly those existing on a nitrogen-poor diet (e.g. 6, 10, 18, 44). Nitrogen acquisition is a primary concern of insects feeding on diets mostly composed of plant materials.
Wood-feeding termites are one of the best-studied insects, and their strategy to recycle UA nitrogen, and therefore to conserve combined nitrogen, is clearly elucidated (41–44). Wood-feeding termites store UA in their fat bodies and transfer it via Malpighian tubules to the hindgut, where gut microbiota recycle UA nitrogen. Termite tissues lack uricase or any UA utilizing activity, whereas gut microbiota degrade UA and consequently the feces of termites contain only small amounts of UA (43, 44). UA degradation in the termite gut is an anaerobic process responsible primarily for gut bacteria (44). It is estimated that an amount of uric acid nitrogen equivalent to 30% of the total nitrogen in an average termite colony may be recycled or redistributed annually through the action of gut uricolytic bacteria (3, 44). Besides the decomposition and utilization of cellulose and acquisition of new nitrogen by fixation of atmospheric N2, the recycling of UA nitrogen is an important and significant aspect of the symbiosis with gut microbiota (3, 4). Termite-gut microbiota form a complex, highly structured, but stable microbial community comprising largely yet-uncultivated, novel and diverse species (9, 32, 39).
Uricolytic bacteria have been isolated from the termite gut, and well studied physiologically (41, 42). The isolates are assigned to any of Streptococcus sp. (according to the original description), Sebaldella (formerly Bacteroides) termitidis, and Citrobacter sp. Despite the repeated extensive isolation, however, only a single species of the subterranean termite Reticulitermes flavipes has been investigated. There are more than 270 termite genera and a marked difference in gut microbiota has been disclosed among genera (11, 56); therefore, studies on the diversity of UA-degrading bacteria in various termites and characterization of their nutritional physiology are of particular significance to understand the nature of the gut microbial community and the symbiosis with host termites. In this study, we isolated and identified anaerobic uricolytic bacteria from eight various termite species. An isolate in the genus Clostridium (designated strain NkU-1) was further investigated for its properties of UA utilization.
Materials and Methods
Isolation and taxonomic characterization
Termite species used in this study are shown in Table 1. Uricolytic bacteria in the gut of termites were isolated according to basically the same methods described by Potrikus and Breznak (41). Worker termites were collected directly from the infested wood, and after washing the surface of the body with distilled water, their gut was removed from the body and squeezed into 0.4% (w/v) NaCl solution under anaerobic conditions. After appropriate dilution with the same solution, the gut homogenate was inoculated onto the top layer of the isolation plate (see below). The composition of culture media is described as a percentage (w/v) in this study unless indicated otherwise. The isolation plate (BHIU) comprised double-layer agar of brain-heart infusion (Difco) 3.7%, cystein-HCl 0.05%, and resazulin 10−4% containing 1.0 and 0.1% of UA and 0.6 and 1.5% agar in the top and bottom layers, respectively. The inoculated plate was anaerobically incubated at 25°C for 14 days. The colonies surrounded by a clear zone in the otherwise opaque medium were counted as presumptive uricolytic bacteria. Anaerobic manipulation and cultivation were performed in an anaerobic chamber (Bactron; Amco) with an O2-free atmosphere of N2/H2/CO2 (80/10/10, v/v/v). In the case of the termite Reticulitermes speratus, we applied a different method to identify UA-degrading bacteria; anaerobic bacteria were first isolated in medium that did not contain UA, as described previously (37), and after identification, representatives were examined for UA degradation by inoculating the isolates onto the BHIU plate.
Table 1.
Anaerobic uricolytic bacteria present in the gut of diverse termites
| Termitea | Body weight (mg termite−1)b | CFU per gut | % urico lytic | No. of isolates identified | Identified bacterial groupc | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Clostridia | Enterobacteriaceae | Low G+C Gram-positive cocci | |||||
| Reticulitermes speratus (Rs) | 2.1 | 4.2×105 | 5.0 | 2d | 0 | 2 | 0 |
| Coptotermes formosanus (Cf) | 3.5 | 8.2×105 | 4.9 | 1e | 1 | 0 | 0 |
| Neotermes koshunensis (Nk) | 15.3 | 2.2×106 | 48.6 | 4e | 4 | 0 | 0 |
| Glyptotermes fuscus (Gf) | 3.6 | 3.5×106 | 0.6 | 3e | 0 | 2 | 1 |
| Cryptotermes domesticus (Cd) | 5.4 | 8.5×105 | 1.2 | 31 | 8 (2) | 5 (3) | 18 (2) |
| Hodotermopsis sjoestedti (Hs) | 63.8 | 8.4×106 | 0.1 | 52 | 52 (2) | 0 | 0 |
| Odontotermes formosanus (Of) | 2.7 | 2.1×105 | 9.5 | 19 | 4 | 15 (3) | 0 |
| Nasutitermes takasagoensis (Nt) | 3.8 | 1.5×106 | 2.0 | 42 | 39 | 0 | 3 |
Initials of termite species used for name tag of the isolate are given in parentheses. Rs and Cf are subterranean termites (Rhinotermitidae). Nk, Gf, and Cd: dry-wood termites (Kalotermitidae). Hs: damp-wood termite (Termopsidae). Of and Nt belong to Termitidae; Of: fungus-grower; Nt: wood-feeder. All the termite species were collected in Japan; Rs in Saitama prefecture, Hs on Yakushima island, and Cf, Nk, Gf, Cd, Of, and Nt on Iriomote island.
Body weights of these termite species were measured in the previous report (36). Based on these values, CFU per mg individual termite was estimated as 0.78–9.72×105.
Number of species identified within the group is shown in parentheses when multiple species were identified.
In Rs, anaerobic bacteria were first isolated and, after identification, representatives were examined for the degradation of UA.
Uricolytic bacteria were first classified by colony morphology, and only a few representatives were identified. In the other termite species (Cd, Hs, Of, and Nt), a colony of the uricolytic bacteria was randomly picked up and classified based on the sequence similarity of 16S rRNA gene.
Clear zone-forming uricolytic bacteria were isolated and purified after successive passages on streak plates; their purity was confirmed by microscopic observation including Gram staining of cells and direct sequencing of PCR product of the 16S rRNA gene. The 16S rRNA gene was amplified, purified, and sequenced partially with 750R primer as described previously (35, 38). The entire sequence of the amplified DNA was determined in some strains using primers described previously (33, 35, 38). Taxonomic assignment of the strains was then performed based on the 16S rRNA sequence using the Naïve Bayesian classifier (ver. 2.2) (58) provided by the Ribosomal Database Project (http://rdp.cme.msu.edu/index.jsp), with an 80% confidence threshold. For detailed comparisons, the quality-checked sequences of type strains in “The All-Species Living Tree” project (61) were obtained from the SILVA rRNA database project (http://www.arb-silva.de/) and used. Phylogenetic analysis with the maximum likelihood method was performed with RAxML 7.2.6 (53) using the GTRGAMMAI model and default settings. The classification was confirmed by Gram staining, cell shape, aerobic growth, catalase and oxidase activity, and oxidative-fermentative (O-F) test when the strain grew aerobically. Representative strains of the uricolytic isolates were deposited in the Japan Collection of Microorganisms (JCM) and their accession numbers are shown in Table 2. The DNA sequences for representative strains of the identified species were deposited in the DDBJ/EMBL/GenBank database under accession numbers AB673451 to AB673466.
Table 2.
Strains representing uricolytic isolates from termite guts and their closest relatives
| Representative strain | Termite host (abundance)a | Closest relativeb | Sequence identityc |
|---|---|---|---|
| Clostridia | |||
| NkU-1 (JCM 10519) | Nk, Of (4/19), Hs (47/52), | Clostridium saccharolyticum (cluster XIVa) (Y18185) | 98.9% |
| Cd6 (JCM 10514)d | Cd (7/31), Nt (39/43) | Clostridium sphenoides (cluster XIVa) (AB075772) | 99.9% |
| Cd13 (JCM 10515) | Cd (1/31) | Clostridium bifermentans (cluster XI) (AB075769) | 100% |
| Hs50 (JCM 10522) | Hs (5/52) | Clostridium sporogenes (cluster I) (X68189) | 99.3% |
| CfU-1 (JCM 10513) | Cf | Clostridium subterminale (cluster I) (AF241844) | 100% |
| Enterobacteriaceae (Gammaproteobacteria) | |||
| RsN-1 (JCM 17987)d | Rs | Enterobacter amnigenus (AB004749) | 99.3% |
| GfU-1 (JCM 17988) | Gf | Enterobacter aerogenes (AB004750) | 98.8% |
| Cd20b (JCM 17989) | Cd (2/31) | Enterobacter asburiae (AB004744) | 98.7% |
| Cd15a (JCM 17990) | Cd (1/31) | Enterobacter cowanii (AJ508303) | 98.0% |
| Cd22 (JCM 17991) | Cd (2/31) | Serratia nematodiphila (EU036987) | 99.9% |
| Of17 (JCM 17992)d | Of (9/19) | Trabulsiella odontotermitis (DQ453129) | 99.5% |
| Of6 (JCM 17993) | Of (4/19) | Citrobacter farmeri (AF025371) | 99.6% |
| Of24 (JCM 17994) | Of (2/19) | Cedecea neteri (AB086230) | 99.7% |
| Low GC Gram positive cocci (Bacilli) | |||
| Cd31 (JCM 10523)d | Gf, Cd (2/31) | Lactococcus lactis subsp. lactis (AB100803) | 100% |
| Cd23 (JCM 10521) | Cd (16/31) | Enterococcus caccae (AY943820) | 99.4% |
| Nt1 (JCM 10537) | Nt (3/43) | Staphylococcus epidermidis (D83363) | 99.3% |
Abbreviations of termite hosts are shown in Table 1. Abundance data shown in parentheses for the four termites Hs, Cd, Nt, and Of correspond to the number of allied isolates divided by the number of examined isolates with a slash.
Accession number of the 16S rRNA gene sequence of type strain used for comparison is shown in parentheses. In Clostridium spp., the clostridia cluster defined by Collins et al.(5) is also shown. To our knowledge, there is no report of obvious anaerobic UA degradation in these close relatives except for C. sphenoides (this study).
Almost the entire sequence of the amplified fragment was determined and compared in strains NkU-1 and Cd23, and strains in Enterobacteriaceae, Only a partial sequence (less than 690 bp) was compared in the other strains.
Strain Cd6 showed 99.4% sequence identity to clone HsH-8 from the gut of Hs (30). Strain RsN-1 showed 99.6% sequence identity to clone Rs-M74 from Rs (11). Strain Of17 showed 99.2% sequence identity to clone BOf3-07 from Of (52) and 99.6% identity to clone Nt2-100 from Nt (13). Strain Cd31 showed 100% sequence identity to clone RsaP87 from R. santonensis(60).
PYG medium (8) was used for the culture of strain NkU-1 for standard taxonomic characterization. Utilization of sugars and other characteristics were examined using a commercially available identification kit, API20A (BioMerieux), according to the manufacturer’s instructions. The G+C content of DNA was determined by a previously reported method (54). Fatty acid analysis was conducted as described previously (14). Type strains of Clostridium spp., C. sphenoides JCM 1415T, C. symbiosum JCM 1297T, C. celerecrescens DSM 5628T, C. aerotolerans DSM 5434T, and C. xylanolyticum DSM 6555T, which were obtained from JCM and German Collection of Microorganisms and Cell Cultures, were used as references mainly for taxonomic studies. The UA degradation ability of these type strains was examined as described above using the BHIU plate. Previously isolated strains from R. flavipes(41) were also used as references for UA degradation.
Nutritional studies
Most nutritional experiments of strain NkU-1 used growth-limiting Y basal medium, which comprised 0.01% yeast extract (Difco), 2.5% (v/v) 40× salt solution, 5% (v/v) 20× KP buffer, 0.4% (v/v) vitamin solution, and 10−4% resazurin-Na; 40× salt solution comprised 2% MgSO4·7H2O, 0.04% NaCl, 0.06% FeSO4·7H2O, 0.26% CaCl2·2H2O, and Na-thioglycolate; 20x KP buffer comprised 5.24% KH2PO4 and 10.71% K2HPO4; and vitamin solution contained 0.05% thiamine, 0.05% riboflavin, 0.05% pyridoxine-HCl, 0.05% calcium pantothenate, 0.01% nicotinic acid, 0.01% biotin, 0.01% folic acid, and 0.01% p-aminobenzoic acid. GY medium was supplemented by 1% glucose in Y medium. TYU medium comprising 1% tryptone (Difco), 0.1% yeast extract, 0.1% UA, 2.5% (v/v) 40× salt solution, and 0.4% (v/v) vitamin solution was also used for the culture of several strains to examine UA degradation. In the case of strain Cd23, GY instead of TYU medium was used for this purpose. UA was solubilized as 2% solution in 0.5 N NaOH, filter sterilized and added at an appropriate final concentraion, and before inoculation, a predetermined amount of sterile 0.5 N HCl was added to neutralize the medium. Purines to be added to the medium were solubilized as described above for UA and in the previous report (41). UA, purine-related compounds, and other chemicals were obtained from Nacalai Tesque (Kyoto, Japan) or various commercial sources. The culture in a glass bottle was sealed with a butyl rubber stopper in the anaerobic chamber and incubated at 37°C for three days with gentle shaking unless indicated otherwise.
In liquid culture, cell growth was measured by optical density (OD) at 660 nm or by the protein amount using Bio-Rad Protein Assay Reagent after adding NaOH (final 1N) and boiling the culture. UA was measured spectrophotometrically by the difference in its specific absorbance at 292 nm before and after treatment with pig liver uricase (Toyobo). NH3 was determined enzymatically by NH3-dependent oxidation of NADPH (measured by absorbance at 340 nm) with beef liver glutamate dehydrogenase (Roche Applied Science). UA and NH3 in growth media were assayed after removal of cells by centrifugation. All measurements were performed in duplicate and mean values are shown.
Results and Discussion
Diversity of UA-degrading bacteria
Anaerobic uricolytic bacteria were successfully detected on the BHIU plate from eight termite species at rates ranging 0.1 to 48.6% CFU (Table 1). They corresponded to the population level of 2.1×103 to 4.0×104 cells per gut except in the case of Neotermes koshunensis (1.1×106 cells per gut). Because of the low culturability of gut bacteria (0.6% to 13% of total gut bacteria (37, 50)), isolated uricolytic bacteria seem to represent mere minor populations in the guts. Nevertheless, diverse uricolytic bacteria were identified in this study (see below). A soil-feeding termite Pericapritermes nitobei (Termitidae) was also examined, but uricolytic bacteria were not obtained, probably because its food (soil organic matter) is rich in nitrogenous compounds and recycling of UA is less significant in this termite.
The uricolytic isolates were taxonomically classified, and 16 different species were identified (Table 2). They were classified into any of the three bacterial groups Clostridia, Enterobacteriaceae (Gammaproteobacteria), and low G+C Gram-positive cocci (Bacilli). To our knowledge, this is the first report for the isolation of uricolytic bacteria in Clostridia from termite guts, although a variety of Clostridia strains have been isolated (for reviews, see references 3, 4). Bacteria related to S. termitidis (belonging to Fusobacteria) could not be isolated in this study.
Strains belonging to Clostridia occurred in six of the eight examined termite species (Table 1). On the basis of the 16S rRNA gene sequence, they were classified into five species in any of the clusters XIVa, XI, and I of clostridia, as defined by Collines et al.(5) (Table 2). Species represented by strain NkU-1 (cluster XIVa) were common to three termite species and occurred frequently among uricolytic isolates from Hodotermopsis sjoestedti. Species represented by strain Cd6 (cluster XIVa), which occurred commonly in two termites and frequently among uricolytic isolates from Nasutitermes takasagoensis, were closely related to C. sphenoides and C. celerecrescens (each 99.9% identity). Indeed, type strains of these two Clostridium species degraded UA in the BHIU plate medium as much as strains NkU-1 and Cd6 (determined by clear zone formation), whereas those of C. xylanolyticum and C. aerotorelance did not (data not shown). All of the isolates in Clostridia were distantly related to well-studied UA-degrading Clostridium species, C. acidiurici, C. purinolyticum (both belonging to cluster XII), and C. cylindrosporum (forms a unique lineage near cluster I or II) (45, 49, 57). From avian ceca, phenotypically diverse uricolytic bacteria are isolated and many apparently belong to Clostridia(1, 48), although most were distinct from the isolates in this study and classified into the genus Eubacterium or Peptostreptococcus.
From R. speratus, various anaerobic bacteria affiliated to the genera Enterobacter, Clostridium, Lactococcus, Bacillus, and Bacteroides were isolated in addition to previously reported Disgonomonas sp. (37). Nevertheless, only Enterobacter sp. (strain RsN-1) could degrade UA on the BHIU plate. The isolates in Clostridium and Lactococcus from R. speratus corresponded to completely different species from the uricolytic strains from other termites. Furthermore, Lactococcus spp. are those of major culturable bacteria in termite guts, but only a few among numerous isolates have demonstrated UA-degrading ability (2, 41, 50); therefore, UA degraders were sporadically distributed in phylogenetically diverse culturable anaerobic bacteria even in the same genus (i.e. Lactococcus or Clostridium).
UA utilization and taxonomy of Clostridium sp. strain NkU-1
The degradation of UA was initially examined in liquid culture in several strains. Clostridium sp. strain NkU-1, its closely related three strains from the same termite, and Lactococcus sp. strain Cd31 degraded UA almost completely, whereas Enterococcus sp. strain Cd23, Enterobacter sp. strains GfU-1, and its close relative isolated from the same termite degraded UA only partially (56%, 31%, and 12% of added UA after 4 days culture, respectively). Incomplete UA degradation is quite a similar phenomenon to those reported previously for isolates from the termite gut (41, 42). Indeed, in the case of strain UAD-1 (originally described as group N Streptococcus sp. (41) but our preliminary analysis indicated that it belonged to the genus Enterococcus), incomplete degradation of UA has been shown unless formate or a formicogenic substrate as a reductant is present in the medium (42). The previously isolated strain of Citrobacter sp. degrades only a small amount of UA in liquid culture, although it forms a prominent clear zone in plate medium and the degradation of UA on the plate is verified (41, 42). Strain NkU-1 represents one of the most common uricolytic bacteria, it degraded UA well in liquid culture, and the physiology of related species from the termite gut has not been characterized so far. Thus, the following taxonomic and nutritional investigations focused on this strain.
The cells of strain NkU-1 were motile, peritrichous, straight rods, 2.0–4.0×0.8–1.0 μm, and often occurred in short chains in the log growth phase. Spores were oval and terminal or subterminal. A major fermentation product from glucose (PYG medium) was acetate (73%), but formate, propionate, and butylate were also detected (10.1–4.8%). The temperature for optimum growth was 35°C and optimum pH for growth was 7.2; the specific growth rate was 0.71 μ per hour under the optimal condition. Catalase, oxidase, and urease were negative. Indol was produced. Strain NkU-1 utilized a variety of carbohydrates and produced acids from glucose, mannitol, lactose, saccharose, maltose, salicin, xylose, arabinose, gelatin, esculin, glycerol, cellobiose, mannose, melezitose, raffinose, rhamnose, and trehalose, but not from sorbitol.
Based on 16S rRNA gene analysis, the closest relatives of strain NkU-1 were Clostridium saccharolyticum (98.9% identity) and Clostridium amygdalium (98.8%), although their relationships were not well resolved in the phylogenetic tree (Fig. 1). C. saccharolyticum and C. amygdalium showed 98.9% identity. The sequence of strain NkU-1 showed less than 98.6% identity to the other species. C. saccharolyticum shows similar characteristics, utilizing a variety of carbohydrates to strain NkU-1, but differs in that it is negative in Gram staining, is non-motile, and has no flagella (26). C. amygdalium is moderately thermophilic (optimum temperature 45°C) and shows a narrower spectrum of carbohydrate utilization (45). In addition, G+C content of C. saccharolyticum and C. amygdalium (28 and 32%, respectively) is lower than that of strain NkU-1 (41.1±1.2%; n=4); therefore, strain NkU-1 likely corresponds to a novel species in the clostridia cluster XIVa, although further study, such as measuring DNA relatedness, is necessary.
Fig. 1.
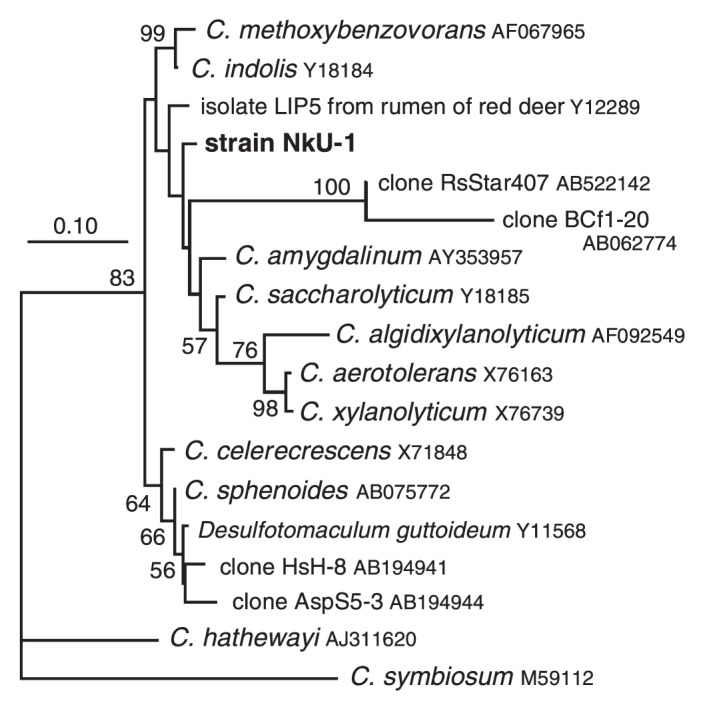
A maximum likelihood tree showing the phylogenetic position of strain NkU-1 in the clostridia cluster XIVa. The tree was reconstructed with 1,345 unambiguously aligned nucleotide sequences of 16S rRNA gene using C. symbiosum as an outgroup. In addition to type strains and isolate LIP5 (17), the analysis included sequences of four clones from termite guts: RsStar407 from R. santonensis(16), BCf1–20 from C. formosanus(51), HsH-8 from H. sjoestedti(30), and AspS5-3 from Archotermopsis sp. (30). The accession number for each sequence is shown. Percent bootstrap value in 1,000 replicates when above 50% is shown at each node. Scale bar represents 0.10 substitutions per position.
In the phylogenetic tree (Fig. 1), two clone sequences, RsStar407 and BCf1–20, from the gut of the termites Reticulitermes santonensis(16) and C. fromosanus(51), respectively, nested very closely to strain NkU-1, although the branch leading to these sequences was long and the sequence identities to strain NkU-1 were relatively low (95.7% and 94.2%, respectively). They are probably phylogenetically closely related to strain NkU-1, but rapidly evolving species inhabit these termites. An isolate from the rumen of red deer (17) is also closely related to strain NkU-1 with 98.7% sequence identity.
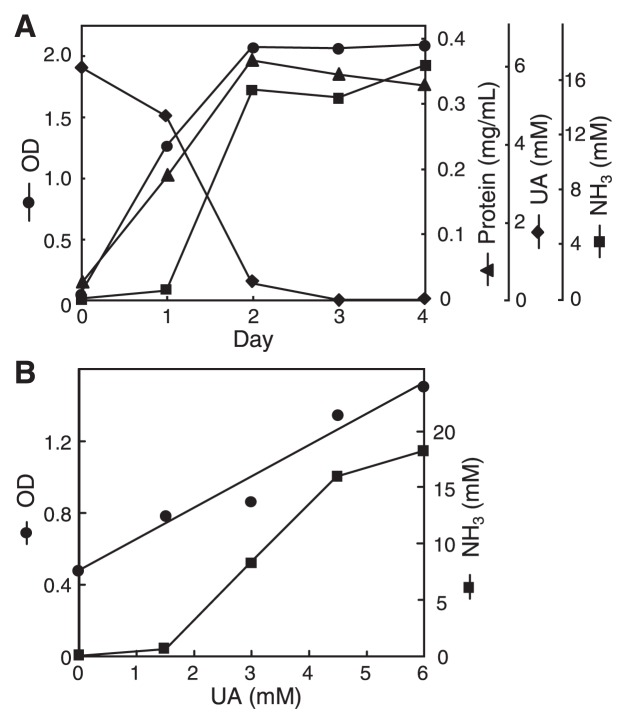
Addition of glucose, cellobiose, fructose, lactose, or xylose stimulated the growth of strain NkU-1 in otherwise growth-limiting medium (Y medium), but UA did not, although UA was partially degraded and NH3 was produced (Table 3). The result indicated that strain NkU-1 did not utilize UA as a sole carbon and energy source. Strain NkU-1 efficiently utilized UA as a nitrogen source when these carbon sources were supplied (Table 3), and UA served as a good nitrogen source as ammonium chloride and ammonium sulfate (data not shown). UA was completely or largely degraded in the presence of these carbon sources and a large amount of NH3 was produced in the medium. Time course and dose-effect analyses (Fig. 2) confirmed the dependence of UA degradation on cell growth. Only a low level of NH3 was excreted at the vigorously growing stage of the cells (Day 1) or at a low level of UA supply (1.5 or 3.0 mM) because nitrogen of degraded UA could be first assimilated into growing cells. Taking this amount of nitrogen into consideration (calculated based on the ratio between the increase of protein from Days 0 to 1 and the amount of nitrogen of degraded UA minus excreted NH3 at Day 1), entire recovery of UA nitrogen was obtained as excreted NH3 at the later stage of cell growth (98% and 106% at Days 3 and 4, respectively); therefore, strain NkU-1 likely produced a stoichiometric amount of NH3 by UA degradation (4 mol NH3 from 1 mol UA).
Table 3.
Utilization of uric acid by strain NkU-1 as a nitrogen source with various carbon sources
| Carbon source | Addition of UA | OD | Protein (mg mL−1) | NH3 produced (mM) | UA remained (mA) |
|---|---|---|---|---|---|
| None | − | 0.18 | 0.050 | 0.11 | NT |
| None | + | 0.19 | 0.078 | 6.41 | 4.66 |
| Glucose | − | 0.79 | 0.086 | 0.59 | NT |
| Glucose | + | 1.21 | 0.170 | 18.23 | 0 |
| Cellobiose | − | 0.78 | 0.082 | 0 | NT |
| Cellobiose | + | 1.14 | 0.147 | 20.58 | 0 |
| Fructose | − | 0.47 | 0.064 | 0 | NT |
| Fructose | + | 1.21 | 0.142 | 15.29 | 0 |
| Lactose | − | 0.44 | NT | 0 | NT |
| Lactose | + | 0.94 | NT | 20.29 | 0.41 |
| Xylose | − | 0.39 | NT | 0.53 | NT |
| Xylose | + | 0.70 | NT | 18.15 | 1.42 |
UA was added to 5.95 mM. NT: not tested.
Fig. 2.
Time course (A) and dose effect (B) of utilization of uric acid as a nitrogen source by strain NkU-1. (A) GY medium supplemented with 0.1% UA was used, and cell growth (OD and protein), UA in the culture, and produced NH3 were measured at 24-hour intervals. (B) GY medium supplemented with the defined concentration of UA was used and OD, protein content (data not shown), and produced NH3 were measured after three days culture. Linear correlations were obtained for OD (R2=0.96) and protein (R2=0.90).
In addition to UA, purine, hypoxanthine, guanine, and xanthine, and the corresponding ribonucleotides to the latter three (inosine, guanosine, and xantosine) stimulated the cell growth of strain NkU-1 (OD and protein concentration (mg mL−1) ranged from 0.90 to 0.95 and 0.11 to 0.15, respectively, after three days culture) in otherwise growth-limiting, carbon-rich but nitrogen-poor GY medium (OD and protein reached only 0.36 and 0.04, respectively). Allantoin, allantoic acid, and ribose (ribose does not contain any nitrogen) did not increase cell growth (OD and protein (mg mL−1) reached less than 0.39 and 0.06, respectively). When ammonium chloride as a positive control was added to GY medium, OD and protein (mg mL−1) reached 0.93 and 0.12, respectively. The results suggest that strain NkU-1 utilizes various purines as a nitrogen source.
The cell suspension of strain NkU-1 degraded UA anaerobically but not aerobically, and UA degradation was detected only in the cell suspension prepared with the culture grown in the presence of UA (data not shown). Aerobic UA degradation generally involves uricase (urate oxidase), which needs molecular oxygen for the catalyzed reaction, and produces allantoin and allantoic acid as intermediate compounds (57). The anaerobic UA degradation pathway and the involved enzymes are considered completely different from those of aerobic, as reported in purinolytic clostridia (48).
Significance of the isolates and symbiotic UA recycling
A unique feature of strain NkU-1 with respect to UA utilization is that it apparently does not use UA as a carbon and energy source, which is in clear contrast to that of previous isolates from the gut of R. flavipes and three purinolytic clostridia species; they utilize UA as a carbon and energy as well as nitrogen source and UA increases the cell yield when incorporated into growth-limiting media (41, 42, 49). Because carbohydrates derived from plant matter are rich in the gut environment and strain NkU-1 can utilize many of them, there is less demand for UA as a carbon and energy source. As opposed to the purinolytic clostridia, none of the isolates from termite guts show an absolute requirement of UA for growth. Strain NkU-1 likely utilizes various purines as a nitrogen source as in the cases of purinolytic clostridia species. The previously isolated strain UAD-1 utilizes only UA effectively, whereas the other examined strains display rather broader versatilities of purines (41). Although the limited versatility reasonably relates to the UA-forming ability of the host termites, as discussed previously (41), the ecological significance of the difference of the versatility of purines is unclear.
A common feature of strain NkU-1 with previous isolates from the termite gut is the excretion of NH3 as the major product of UA degradation. As discussed previously (41, 44), NH3 produced by UA degradation can be cycled back to the host insect tissue directly or indirectly after assimilation by gut microbiota. For the latter scheme, some behaviors of host termites such as proctodeal trophallaxis (the exchange of hindgut content among nestmates), necrophagy, and cannibalism likely play important roles (7, 21, 55).
Cockroaches also stored UA in their fat bodies and utilization of uric acid nitrogen by intracellular symbionts (Blattabacterium sp.) has been hypothesized (6). In the genome sequence of the Blattabacterium symbiont, however, there is no candidate gene for uricolytic enzymes (47), suggesting that the symbiont does not play a role, at least in the initial step of UA utilization. One of the plausible alternative explanations is the recycling of UA by gut bacteria as in the case of termites. In a shield bug, bacteria localized extracellularly in the lumen of a specialized structure (so-called swollen crypt) in the midgut (15) have been demonstrated to be responsible for uricolysis during a prereproductive non-feeding period of the host, although associated uricase activity is emphasized (18). Therefore, UA recycling by gut bacteria seems to be a general mechanism for the conservation of nitrogen in terrestrial insects, particularly whose natural diets are low in combined nitrogen. Many insects depend on plant materials and they usually suffer from poverty of nitrogen sources. Meanwhile, yeast-like endosymbionts of planthoppers have been demonstrated to be involved in uric acid recycling, although the related yeast-like symbionts of aphids are not (10). More extensive investigations of the nature of symbiotic recycling of UA in insects are ecologically very important.
Because most studies on anaerobic microbial degradation of UA were conducted more than three decades ago, modern molecular studies are anticipated. Approaches using functional key genes such as nifH, nirK, and amoA are clearly powerful tools for studies on nitrogen cycles in certain environments (e.g. 19, 22, 23, 25, 40, 46, 62, 63) as well as in the termite-gut microbial community (20, 28, 29, 34, 36, 59), but anaerobic UA degradation has not been well-defined genetically; no key gene for this process has been identified. Although experiments with stable isotope measuring or labeling (e.g. 24, 55) may be applicable, the isolation and characterization of anaerobic UA-degrading bacteria are of special significance for further physiological, biochemical, and genome studies, as generally discussed for as-yet-uncultured but ecologically important microorganisms (27, 31). The strains isolated in this study will be very useful for these future studies.
Acknowledgements
We thank J.A. Breznak for kindly providing the uricolyic strains, and Y. Kosako for advice on identification of strains. This work was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science No. 20658024 to M.O. and by Biomass Engineering Program from RIKEN. A.T. was an International Program Associate of RIKEN Joint Graduate School Program with Tokyo Institute of Technology.
References
- 1.Barnes EM, Impey CS. The occurrence and properties of uric acid decomposing anaerobic bacteria in the avian caecum. J Appl Bact. 1974;37:393–409. doi: 10.1111/j.1365-2672.1974.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Tholen A, Overmann J, Brune A. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood- and soil-feeding termites by molecular and culture-dependent techniques. Arch Microbiol. 2000;173:126–137. doi: 10.1007/s002039900120. [DOI] [PubMed] [Google Scholar]
- 3.Breznak JA. Ecology of prokaryotic microbes in the guts of wood- and litter-feeding termites. In: Abe T, Bignell DE, Higashi M, editors. Termites: Evolution, Sociality, Symbioses, Ecology. Kluwer Academic Publishers; Dordrecht: 2000. pp. 209–231. [Google Scholar]
- 4.Brune A, Ohkuma M. Role of the termite gut microbiota in symbiotic digestion. In: Bignell DE, Roisin Y, Lo N, editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht: 2011. pp. 439–475. [Google Scholar]
- 5.Collins MD, Lawson PA, Willems A, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 6.Donellan JF, Kilby BA. Uric acid metabolism by symbiotic bacteria from the fat body of Periplaneta americana. Comp Biochem Physiol. 1967;22:235–252. doi: 10.1016/0010-406x(67)90184-3. [DOI] [PubMed] [Google Scholar]
- 7.Fujita A, Shimizu I, Abe T. Distribution of lysozyme and protease, and amino acid concentration in the guts of a wood-feeding termite, Reticulitermes speratus(Kolbe): possible digestion of symbiont bacteria transferred by trophallaxis. Physiol Entomol. 2001;26:116–123. [Google Scholar]
- 8.Holdeman LV, Cato EP, Moore WEC. Anaerobe Laboratory Manual. 4th ed. Virginia Polytechnic Institute and State University; Blacksburg, VA: 1977. [Google Scholar]
- 9.Hongoh Y. Diversity and genomes of uncultured microbial symbionts in termite gut. Biosci Biotech Biochem. 2010;74:1145–1151. doi: 10.1271/bbb.100094. [DOI] [PubMed] [Google Scholar]
- 10.Hongoh Y, Ishikawa H. Evolutionary studies on uricase of fungal endosymbionts of aphids and planthoppers. J Mol Evol. 2000;51:265–277. doi: 10.1007/s002390010088. [DOI] [PubMed] [Google Scholar]
- 11.Hongoh Y, Ohkuma M, Kudo T. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus(Isoptera; Rhinotermitidae) FEMS Microbiol Ecol. 2003;44:231–242. doi: 10.1016/S0168-6496(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 12.Hongoh Y, Deevong P, Inoue T, et al. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl Environ Microbiol. 2005;71:6590–6599. doi: 10.1128/AEM.71.11.6590-6599.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hongoh Y, Deevong P, Hattori S, Inoue T, Noda S, Noparatnaraporn N, Kudo T, Ohkuma M. Phylogenetic diversity, localization, and cell morphologies of members of the candidate phylum TG3 and a subphylum in the phylum Fibrobacteres, recently discovered bacterial groups dominant in termite guts. Appl Environ Microbiol. 2006;72:6780–6788. doi: 10.1128/AEM.00891-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshi S, Sakata T, Mikuni K, Hashimoto H, Kimura S. Galactosylsucrose and xylosylfructoside alter digestive tract size and concentrations of cecal organic acids in rats fed diets containing cholesterol and cholic acid. J Nutr. 1994;124:52–60. doi: 10.1093/jn/124.1.52. [DOI] [PubMed] [Google Scholar]
- 15.Hosokawa T, Kikuchi Y, Nikoh N, Meng X-Y, Hironaka M, Fukatsu T. Phylogenetic position and peculiar genetic traits of a midgut bacterial symbiont of the stinkbug Parastrachia japonensis. Appl Environ Microbiol. 2010;76:4130–4135. doi: 10.1128/AEM.00616-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda-Ohtsubo W, Faivre N, Brune A. Putatively free-living ‘Endomicrobia’—ancestors of the intracellular symbionts of termite gut flagellates? Environ Microbiol Rep. 2010;2:554–559. doi: 10.1111/j.1758-2229.2009.00124.x. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis GN, Strömpl C, Moore ER, Thiele JH. Isolation and characterization of obligately anaerobic, lipolytic bacteria from the rumen of red deer. Syst Appl Microbiol. 1998;21:135–143. doi: 10.1016/S0723-2020(98)80017-9. [DOI] [PubMed] [Google Scholar]
- 18.Kashima T, Nakamura T, Tojo S. Uric acid recycling in the shield bug, Parastrachia japonensis(Hemiptera: Parastrachiidae), during diapause. J Insect Physiol. 2006;52:816–825. doi: 10.1016/j.jinsphys.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Cao H, Hong Y-G, Gu J-D. Seasonal dynamics of anammox bacteria in estuarial sediment of the Mai Po nature reserve revealed by analyzing the 16S rRNA and hydrazine oxidoreductase (hzo) genes. Microbes Environ. 2011;26:15–22. doi: 10.1264/jsme2.me10131. [DOI] [PubMed] [Google Scholar]
- 20.Lilburn T, Byzek GK, Kim RK, Breznak JA. Nitrogen fixation by symbiotic and free-living spirochetes. Science. 2001;292:2495–2498. doi: 10.1126/science.1060281. [DOI] [PubMed] [Google Scholar]
- 21.Machida M, Kitade O, Miura T, Matsumoto T. Nitrogen recycling through proctodeal trophallaxis in the Japanese damp-wood termite Hodotermopsis japonica(Isoptera, Termopsidae) Insectes Soc. 2001;48:52–56. [Google Scholar]
- 22.Matsumoto S, Ishikawa D, Saeki G, Aoi Y, Tsuneda S. Microbial population dynamics and community structure during the formation of nitrifying granules to treat ammonia-rich inorganic wastewater. Microbes Environ. 2010;25:164–170. doi: 10.1264/jsme2.me10107. [DOI] [PubMed] [Google Scholar]
- 23.Matsutani N, Nakagawa T, Nakamura K, Takahashi R, Yoshihara K, Tokuyama T. Enrichment of a novel marine ammonia-oxidizing archaeon obtained from sand of an eelgrass zone. Microbes Environ. 2011;26:23–29. doi: 10.1264/jsme2.me10156. [DOI] [PubMed] [Google Scholar]
- 24.Mayumi D, Yoshimoto T, Uchiyama H, Nomura N, Nakajima-Kambe T. Seasonal change in methanotrophic diversity and populations in a rice field soil assessed by DNA-stable isotope probing and quantitative real-time PCR. Microbes Environ. 2010;25:156–163. doi: 10.1264/jsme2.me10120. [DOI] [PubMed] [Google Scholar]
- 25.Morimoto S, Hayatsu M, Takada Hoshino Y, Nagaoka K, Yamazaki M, Karasawa T, Takenaka M, Akiyama H. Quantitative analyses of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in fields with different soil types. Microbes Environ. 2011;26:248–253. doi: 10.1264/jsme2.me11127. [DOI] [PubMed] [Google Scholar]
- 26.Murray WD, Khan AW, van den Berg L. Clostridium saccharolyticum sp. nov., a saccharolytic species from sewage sludge. Int J Syst Bacteriol. 1982;32:132–135. [Google Scholar]
- 27.Nakamura K, Tamaki H, Kang MS, Mochimaru H, Lee S-T, Nakamura K, Kamagata Y. A six-well plate method: less laborious and effective method for cultivation of obligate anaerobic microorganisms. Microbes Environ. 2011;26:301–306. doi: 10.1264/jsme2.me11120. [DOI] [PubMed] [Google Scholar]
- 28.Noda S, Ohkuma M, Usami R, Horikoshi K, Kudo T. Culture-independent characterization of a gene responsible for nitrogen fixation in the symbiotic microbial community in the gut of the termite Neotermes koshunensis. Appl Environ Microbiol. 1999;65:4935–4942. doi: 10.1128/aem.65.11.4935-4942.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noda S, Ohkuma M, Kudo T. Nitrogen fixation genes expressed in the symbiotic microbial community in the gut of the termite Coptotermes formosanus. Microbes Environ. 2002;17:139–143. [Google Scholar]
- 30.Noda S, Inoue T, Hongoh Y, Kawai M, Nalepa CA, Vongkaluang C, Kudo T, Ohkuma M. Identification and characterization of ectosymbionts of distinct lineages in Bacteroidales attached to flagellate protists in the gut of termites and a wood-feeding cockroach. Environ Microbiol. 2006;8:11–20. doi: 10.1111/j.1462-2920.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 31.Okabe S, Oshiki M, Kamagata Y, et al. A great leap forward in microbial ecology. Microbes Environ. 2010;25:230–240. doi: 10.1264/jsme2.me10178. [DOI] [PubMed] [Google Scholar]
- 32.Ohkuma M. Symbioses of flagellates and prokaryotes in the gut of lower termites. Trends Microbiol. 2008;16:345–352. doi: 10.1016/j.tim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Ohkuma M, Kudo T. Phylogenetic diversity of the intestinal bacterial community in the termite Reticulitermes speratus. Appl Environ Microbiol. 1996;62:461–468. doi: 10.1128/aem.62.2.461-468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohkuma M, Noda S, Usami R, Horikoshi K, Kudo T. Diversity of nitrogen fixation genes in the symbiotic intestinal microflora of the termite Reticulitermes speratus. Appl Environ Microbiol. 1996;62:2747–2752. doi: 10.1128/aem.62.8.2747-2752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohkuma M, Kudo T. Phylogenetic analysis of the symbiotic intestinal microflora of the termite Cryptotermes domesticus. FEMS Microbiol Lett. 1998;164:389–395. [Google Scholar]
- 36.Ohkuma M, Noda S, Kudo T. Phylogenetic diversity of nitrogen fixation genes in the symbiotic microbial community in the gut of diverse termites. Appl Environ Microbiol. 1999;65:4926–4934. doi: 10.1128/aem.65.11.4926-4934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkuma M, Noda S, Hongoh Y, Kudo T. Diverse bacteria related to the bacteroides subgroup of the CFB phylum within the gut symbiotic community of various termites. Biosci Biotech Biochem. 2002;66:78–84. doi: 10.1271/bbb.66.78. [DOI] [PubMed] [Google Scholar]
- 38.Ohkuma M, Shimizu H, Thongaram T, Kosono S, Moriya K, Trakulnaleamsai S, Noparatnaraporn N, Kudo T. An alkaliphilic and xylanolytic Paenibacillus species isolated from the gut of a soil-feeding termite. Microbes Environ. 2003;18:145–151. [Google Scholar]
- 39.Ohkuma M, Brune A. Diversity, structure, and evolution of the termite gut microbial community. In: Bignell DE, Roisin Y, Lo N, editors. Biology of Termites: A Modern Synthesis. Springer; Dordrecht: 2011. pp. 413–438. [Google Scholar]
- 40.Onodera Y, Nakagawa T, Takahashi R, Tokuyama T. Seasonal change in vertical distribution of ammonia-oxidizing archaea and bacteria and their nitrification in temperate forest soil. Microbes Environ. 2010;25:28–35. doi: 10.1264/jsme2.me09179. [DOI] [PubMed] [Google Scholar]
- 41.Potrikus CJ, Breznak JA. Uric acid-degrading bacteria in guts of termites [Reticulitermes flavipes(Kollar)] Appl Environ Microbiol. 1980;40:117–124. doi: 10.1128/aem.40.1.117-124.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potrikus CJ, Breznak JA. Anaerobic degradation of uric acid by gut bacteria of termites. Appl Environ Microbiol. 1980;40:125–132. doi: 10.1128/aem.40.1.125-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potrikus CJ, Breznak JA. Uric acid in wood-eating termites. Insect Biochem. 1980;10:19–27. [Google Scholar]
- 44.Potrikus CJ, Breznak JA. Gut bacteria recycle uric acid nitrogen in termites: a strategy for nutrient conservation. Proc Natl Acad Sci USA. 1981;78:4601–4605. doi: 10.1073/pnas.78.7.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rainey FA, Hollen BJ, Small A. Genus I. Clostridium Prazmowski 1880, 23AL. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB, editors. Bergey’s Mannual of Systematic Bacteriology. 3rd ed. Vol. 3. Springer; New York: 2009. pp. 738–834. [Google Scholar]
- 46.Ryuda N, Hashimoto T, Ueno D, Inoue K, Someya T. Visualization and direct counting of individual denitrifying bacterial cells in soil by nirK-targeted direct in situ PCR. Microbes Environ. 2011;26:74–80. doi: 10.1264/jsme2.me10180. [DOI] [PubMed] [Google Scholar]
- 47.Sabree ZL, Kambhampati S, Moran NA. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc Natl Acad Sci USA. 2009;106:19521–19526. doi: 10.1073/pnas.0907504106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiefer-Ullrich H, Andreesen JR. Peptostreptococcus barnesae sp. nov., a gram-positive, anaerobic, obligately purine utilizing coccus from chicken feces. Arch Microbiol. 1985;143:26–31. [Google Scholar]
- 49.Schiefer-Ullrich H, Wagner R, Dürre P, Andreesen JR. Comparative studies on physiology and taxonomy of obligately purinolytic clostridia. Arch Microbiol. 1984;138:345–353. doi: 10.1007/BF00410902. [DOI] [PubMed] [Google Scholar]
- 50.Schultz JE, Breznak JA. Heterotrophic bacteria present in hindguts of wood-eating termites [Reticulitermes flavipes(Kollar)] Appl Environ Microbiol. 1978;35:930–936. doi: 10.1128/aem.35.5.930-936.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shinzato N, Muramatsu M, Matsui T, Watanabe Y. Molecular phylogenetic diversity of the bacterial community in the gut of the termite Coptotermes formosanus. Biosci Biotechnol Biochem. 2005;69:1145–1155. doi: 10.1271/bbb.69.1145. [DOI] [PubMed] [Google Scholar]
- 52.Shinzato N, Muramatsu M, Matsui T, Watanabe Y. Phylogenetic analysis of the gut bacterial microflora of the fungus-growing termite Odontotermes formosanus. Biosci Biotechnol Biochem. 2007;71:906–915. doi: 10.1271/bbb.60540. [DOI] [PubMed] [Google Scholar]
- 53.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinfomatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 54.Tamaoka J, Komagata K. Determination of DNA base composition by reverse-phase high-performance liquid chromatography. FEMS Microbiol Lett. 1984;25:125–128. [Google Scholar]
- 55.Tayasu I, Hyodo F, Abe T. Caste-specific N and C isotope ratios in fungus-growing termites with special reference to uric acid preservation and their nutritional interpretation. Ecol Entomol. 2002;27:355–361. [Google Scholar]
- 56.Thongaram T, Hongoh Y, Kosono S, Ohkuma M, Trakulnaleamsai S, Noparatnaraporn N, Kudo T. Comparison of bacterial communities in the alkaline gut segment among various species of higher termites. Extremophiles. 2005;9:229–238. doi: 10.1007/s00792-005-0440-9. [DOI] [PubMed] [Google Scholar]
- 57.Vogels GD, Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976;40:403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada A, Inoue T, Noda S, Hongoh Y, Ohkuma M. Evolutionary trend of phylogenetic diversity of nitrogen fixation genes in the gut community of wood-feeding termites. Mol Ecol. 2007;16:3768–3777. doi: 10.1111/j.1365-294X.2007.03326.x. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Schmitt-Wagner D, Stingle U, Brune A. Niche heterogeneity determines bacterial community structure in the termite gut (Reticulitermes santonensis) Environ Microbiol. 2005;7:916–932. doi: 10.1111/j.1462-2920.2005.00760.x. [DOI] [PubMed] [Google Scholar]
- 61.Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer KH, Ludwig W, Glöckner FO, Rossello-Mora R. The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol. 2008;31:241–250. doi: 10.1016/j.syapm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Yasuda T, Kuroda K, Hanajima D, Fukumoto Y, Waki M, Suzuki K. Characteristics of the microbial community associated with ammonia oxidation in a full-scale rockwool biofilter treating malodors from livestock manure composting. Microbes Environ. 2010;25:111–119. doi: 10.1264/jsme2.me09175. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida M, Ishii S, Otsuka S, Senoo K. nirK-harboring denitrifiers are more responsive to denitrification-inducing conditions in rice paddy soil than nirS-harboring bacteria. Microbes Environ. 2010;25:45–48. doi: 10.1264/jsme2.me09160. [DOI] [PubMed] [Google Scholar]