Abstract
We investigated the prevalence of qnr and aac(6′)-Ib-cr genes in water-borne environmental bacteria and in clinical isolates of Enterobacteriaceae, as well as the subtypes of qnr. Environmental bacteria were isolated from surface water samples obtained from 10 different locations in Hangzhou City, and clinical isolates of Citrobacter freundii were isolated from several hospitals in four cities in China. qnrA, qnrB, qnrS, and aac(6′)-Ib-cr genes were screened using PCR, and the genotypes were analyzed by DNA sequencing. Ten of the 78 Gram-negative bacilli isolated from water samples were C. freundii and 80% of these isolates carried the qnrB gene. qnrS1 and aac(6′)-Ib-cr genes were detected in two Escherichia coli isolates and qnrS2 was detected in one species, Aeromonas punctata. The qnr and aac(6′)-Ib-cr genes were present in 75 (72.8%) and 12 (11.6%) of 103 clinical isolates of C. freundii, respectively. Of the clinical C. freundii isolates with the qnr gene, 65 isolates (63.1%) carried qnrB, but only three (2.9%) and one (1.0%) carried qnrA1 and qnrS2, respectively, while five isolates carried both qnrA1 and qnrB, and one isolate carried both qnrS1 and qnrB. The qnrB9 gene was the dominant qnrB subtype, followed by qnrB8 and qnrB6. Southern hybridization studies indicated that the qnr genes are located on different plasmids. Plasmids isolated from both environmental and clinical C. freundii isolates appeared to be homogenous.
Keywords: Enterobacteriaceae, environmental bacteria, quinolone resistance gene
Quinolones, which have a broad spectrum of antibacterial activity, have been widely used for chemotherapy and have led to increased resistance of bacteria. Resistance to quinolones is mainly due to chromosomally mediated mechanisms, including mutations in quinolone targets (DNA gyrase and topoisomerase IV) and decreased accumulation of quinolones (porin alternation or overexpression of efflux pump systems) (21). The first plasmid-mediated quinolone resistance (PMQR) determinant was identified in Klebsiella pneumoniae in 1998 (11). Cloning of the gene identified this determinant as a 657-bp fragment encoding a protein with 218 aminoacid residues, which was named Qnr (more recently termed QnrA) (29); QnrB and QnrS were discovered subsequently (5, 6). Very recently, two novel qnr genes, qnrC and qnrD, were reported (3, 30). In addition to Qnr, two new types of PMQR determinants have been described. The aac (6′)-Ib-cr aminoglycoside acetyltransferase gene, whose product is capable of acetylating ciprofloxacin and norfloxacin, was discovered in qnrA-positive Escherichia coli in 2006 (18). QepA, a plasmid-mediated fluoroquinolone efflux pump, was identified in two clinical isolates of E. coli, one from Belgium and one from Japan, in 2007 (14, 33).
Qnr and aac (6′)-Ib-cr determinants have now been identified worldwide in many different enterobacterial species (17, 19, 26). These determinants can also be detected in E. coli isolates from poultry and swine (35) and in Enterobacteriaceae from pets, livestock and poultry (10). To better understand the transfer and prevalence of drug-resistant pathogens and determinants in the human-environment system, we collected bacteria from both water samples and clinical patients, and analyzed the qnr and aac (6′)-Ib-cr genes. We found that a high percentage of the environmental samples shared the qnrB gene in common with the clinical samples.
Materials and Methods
Bacterial strains
Water samples were collected from 10 distinct aquatic environments (including West Lake, Qiantang River, Jinghang Grand Canal, Xixi Wetland, Jiefang River, Huajiachi Lake, Jiuxi River, Tiesha River, two fountains in Qingchun Square and the 2nd Affiliated Hospital of Zhejiang University [SAHZU]) in Hangzhou, China during October to November 2008. Five to ten representative sites in each locality were selected for sample collection. We selected those sampling location to represent the main water environments of the city, including artificial fountain, rivers with running water, large volume lakes, and small ponds. Bacteria in water samples (1 L samples) were concentrated by centrifugation and inoculated onto blood-, MacConkey-, and thiosulphate citrate bile salts sucrose (TCBS)-agar plates. Clinical isolates of Citrobacter spp. were isolated from SAHZU and collected from several other hospitals in four cities (Beijing, Shanghai, Hangzhou, and Wenzhou) in China during January to December 2008, in a drug resistance surveillance program. All the collected isolates were from sources such as sputum, urine and bodily secretions. All of these isolates were identified using the Vitek System (bioMérieux, Hazelwood, MO, USA).
Antimicrobial susceptibility testing
The minimal inhibitory concentration (MIC) of ciprofloxacin, levofloxacin and nalidixic acid against bacteria was determined using the agar dilution method according to Clinical and Laboratory Standards Institute (CLSI) recommendations (4). MIC results were determined after incubation at 35°C for 16–20 hours. Muller-Hinton agar was purchased from Oxoid (Hampshire, UK).
PCR amplification and sequencing
Screening of qnrA, qnrB, qnrS and aac(6′)-Ib genes was carried out by PCR amplification using specific primers (8, 20). Colonies were boiled to prepare DNA templates for PCR. The reaction was conducted in a Tpersonal thermal cycler (Whatman Biometra, Goettingen, Germany) as previously described (20). The PCR products were sequenced using an ABI3730 Sequencer (Applied Biosystems, Carlsbad, CA, USA), and the obtained sequences were compared with the sequences deposited in GenBank.
Transconjugation and transformation studies
For these studies the donors were the qnr-carrying strains isolated in this study, while rifampicin-resistant E. coli J53 was used as the acceptor. The transconjugated strains were screened on a medium including sulfamethoxazole or levofloxacin. The detailed experimental method was as described by Wang et al.(30).
Southern hybridization
The amplified products of qnrB and qnrS of the environmental isolates were labeled using the DIG High Primer DNA Labeling and Detection Starter Kit I (Roche, Diagnostics, Mannheim, Germany), according to the manufacturer’s instructions. Plasmid and chromosomal DNA was extracted from the qnr-carrying water-borne strains using a kit from Axygen (Axygen Scientific, Union City, NJ, USA). The DNA was trans-blotted to a nylon membrane from ethidium bromide (EB)-free 0.8% agar gel after 1.5 h of electrophoresis, and was then hybridized to the probe using the DIG High Primer DNA Labeling and Detection Starter Kit I according to the manufacturer’s instructions.
Results
Bacteria isolated from aquatic environments
Seventy-eight Gram-negative bacilli were isolated from water samples, including 33 Enterobacteriaceae, 21 Aeromonas spp., ten Acinetobacter spp., ten Pseudomonas spp., two Alcaligenes spp., two Plesiomonas spp. and ten Citrobacter freundii (Table 1). Gram-negative cocci or Gram-positive bacteria were not obtained in the current study.
Table 1.
Species distribution of bacteria isolated from aquatic environments and their MICs of ciprofloxacin, levofloxacin and nalidixic acid (μg mL−1)
| Strain | No. | Ciprofloxacin | Levofloxacin | Nalidixic acid | Resourcea | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MIC, or MIC50/MIC90 | MIC Range | MIC, or MIC50/MIC90 | MIC Range | MIC, or MIC50/MIC90 | MIC Range | |||
| C. freundii | 10 | ≤0.125/0.25 | ≤0.125~64 | 0.125/0.25 | ≤0.125~1 | 4/8 | 2~16 | WL, QTR, JHGC, XXW, JFR, HJCL |
| E. coli | 9 | 0.25/64 | ≤0.125~64 | 0.03/2 | 0.03~2 | 4/>256 | 1~>256 | WL, JHGC, JFR, HJCL, JXR |
| K. pneumoniae | 6 | ≤0.125 | ≤0.125 | 0.03/0.06 | 0.03~0.06 | 2/4 | 0.5~4 | QTR, XXW, JFR, HJCL, JXR |
| E. cloacae | 2 | ≤0.125 | ≤0.125 | 0.03 | 0.03 | 2 | 2 | XXW, JXR |
| E. aerogenes | 1 | ≤0.125 | —b | 0.06 | — | 2 | — | HJCL |
| E. intermedius | 1 | 0.25 | — | 0.06 | — | 1 | — | HJCL |
| Proteus penneri | 1 | ≤0.125 | — | 0.05 | — | 1 | — | HJCL |
| Pantoea sp. | 1 | ≤0.125 | — | 0.06 | — | 128 | — | JFR |
| Kluyvera spp. | 2 | ≤0.125 | ≤0.125 | ≤0.015/0.03 | ≤0.015~0.03 | 1/4 | 1~4 | QTR, JFR |
| Aeromonas spp. | 21 | 0.25/16 | 0.125~32 | 0.25/16 | 0.125~16 | 128/>256 | 64~>256 | WL, JHGC, XXW, JFR, HJCL, JXR, TSR, FQCS, FH |
| Acinetobacter spp. | 10 | 0.5/16 | ≤0.125~32 | 0.06/0.5 | 0.06~1 | 2/64 | 0.5~128 | QTR, XXW, JXR, TSR, FQCS |
| Pseudomonas spp. | 10 | ≤0.125/1 | ≤0.125~32 | 0.06/0.5 | 0.06~1 | 1 | 0.5~1 | WL, QTR, JHGH, JFR, HJCL, JXR, TSR |
| Alcaligenes spp. | 2 | ≤0.125 | ≤0.125~0.25 | ≤0.015 | ≤0.015 | 1 | 1 | WL, TSR |
| Plesiomonas shigelloides | 2 | ≤0.125 | ≤0.125 | ≤0.015 | ≤0.015 | 1 | 1 | HJCL |
WL, West Lake; QTR, Qiantang River; JHGC, Jinghang Grand Canal; XXW, Xixi Wetland; JFR, Jiefang River; HJCL, Huajiachi Lake; JXR, Jiuxi River; TSR, Tiesha River; FQCS, fountain in Qingchun Square; FH, fountain at 2nd Affiliated Hospital of Zhejiang University
only one isolate.
Quinolone susceptibility of environmental and clinical isolates
The MIC50 and MIC90 of ciprofloxacin against 78 water-borne environmental isolates were ≤0.125 μg mL−1 and 16 μg mL−1, respectively. The MICs of ciprofloxacin, levofloxacin and nalidixic acid, as well as the MIC50/MIC90 ratios and the MIC range against each genus are shown in Table 1. The MIC50 and MIC90 of ciprofloxacin and levofloxacin against the water-borne environmental C. freundii were ≤0.125 μg mL−1 and 0.25 μg mL−1; 0.125 μg mL−1 and 0.25 μg mL−1, respectively, while those of nalidixic acid were 4 μg mL−1 and 8 μg mL−1, respectively.
The overall MIC50 and MIC90 of ciprofloxacin against 103 clinical isolates of C. freundii were 2 μg mL−1 and 32 μg mL−1, respectively. There was little difference in the MIC50 and MIC90 for samples collected from hospitals in different cities. The MIC50 and MIC90 against clinical samples from the four cities were 4 μg mL−1 and 16 μg mL−1, ≤0.125 μg mL−1 and 32 μg mL−1, 0.5 μg mL−1 and 16 μg mL−1, and 1 μg mL−1 and 32 μg mL−1, for samples from Hangzhou, Wenzhou, Shanghai, and Beijing respectively. The MIC50 and MIC90 of ciprofloxacin against Citrobacter braakii were 4 μg/ml and >128 μg mL−1, respectively. The MIC of ciprofloxacin against all isolates of Citrobacter koseri and Citrobacter amalonaticus was ≤0.125 μg mL−1.
Prevalence of the qnr and aac(6′)-Ib-cr genes in environmental and clinical isolates
Ten of the water-borne environmental isolates were C. freundii and 80% of these carried the qnrB gene (Table 2). The qnrS gene was detected in one E. coli and one Aeromonas sp. The aac(6′)-Ib gene was detected in other E. coli strain and another Aeromonas sp. The two Aeromonas spp. were identified as Aeromonas punctata based on the results of sequencing of the gyrB gene (32). The qnrS1 gene in E. coli and the qnrS2 gene in A. punctata were identified by sequencing. One of the three isolates that carried the aac(6′)-Ib gene carried the -cr variant. E. coli and A. punctata were all isolated from Huajiachi Lake. Three of the eight C. freundii isolates that carried the qnrB gene were isolated from Huajiachi Lake, two were from West Lake and one each was isolated from the Qiantang River, Jinghang Grand Canal and the Xixi Wetland.
Table 2.
Prevalence of qnr and aac(6′)-Ib-cr genes in water-borne environmental and clinical bacteria
| Resource | Strain | No. | Number of positive isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| qnrA | qnrB | qnrS | qnrA+qnrB | qnrS+qnrB | aac(6′)-Ib | aac(6′)-Ib-cr | ||||
| Aquatic environment | C. freundii | 10 | 0 | 8 (80.0) | 0 | 0 | 0 | 0 | 0 | |
| E. coli | 9 | 0 | 0 | 1 (11.1) | 0 | 0 | 1 (11.1) | 1 (11.1) | ||
| Aeromonas spp. | 21 | 0 | 0 | 1 (4.8) | 0 | 0 | 2 (9.5) | 0 | ||
| Others | 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
|
| ||||||||||
| Hospital | C. freundii | Hangzhou | 51 | 3 (5.9) | 38 (74.5) | 0 | 4 (7.8) | 1 (2.0) | 25 (49.0) | 10 (19.6) |
| Wenzhou | 14 | 0 | 7 (50.0) | 0 | 0 | 0 | 2 (14.3) | 1 (7.1) | ||
| Shanghai | 7 | 0 | 4 (57.1) | 1 (14.3) | 1 (14.3) | 0 | 1 (14.3) | 1 (14.3) | ||
| Beijing | 31 | 0 | 16 (51.6) | 0 | 0 | 0 | 5 (16.1) | 0 | ||
| Total | 103 | 3 (2.9) | 65 (63.1) | 1 (1.0) | 5 (4.8) | 1 (1.0) | 33 (32.0) | 12 (11.6) | ||
| C. braakii | 7 | 2 (28.6) | 1 (14.3) | 0 | 0 | 0 | 4 (57.1) | 3 (42.9) | ||
| C. koseri | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| C. amalonaticus | 3 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | ||
Of the 103 clinical isolates of C. freundii, 75 (72.8%) and 12 (11.6%) carried the qnr and aac(6′)-Ib-cr genes, respectively. qnrA-, qnrB- and qnrS-type alleles were detected in eight (7.8%), 71 (68.9%), and two (1.9%) isolates. Some of these isolates carried two types of qnr alleles (Table 2). The rate of qnr carriage among C. freundii isolates from Beijing, Shanghai, and Wenzhou was a little higher than 50%, while that from Hangzhou was 74.5%. The sequences of the qnrA genes of the isolates all matched that of qnrA1(12), and the sequences of the qnrS genes of the isolates from Hangzhou and Shanghai matched those of qnrS1 and qnrS2(12), respectively. We used three different restriction endonucleases (ApaI, HindIII and XbaI) to digest the two plasmids. The restriction fingerprinting of the plasmids was different, indicating that although the two plasmids are of a similar size, they are different. The common subtypes of qnrB detected in 79 water-borne environmental and clinical isolates of C. freundii were qnrB9 (27 isolates), qnrB8 (16 isolates) (12), qnrB6 (11 isolates), and qnrB4 (6 isolates), and other subtypes, including qnrB10, qnrB11, qnrB12, qnrB13, qnrB16, qnrB17, and qnrB18, were also detected (7). We compared the qnrB sequences of the clinical and environmental strains, but no mutations in the qnrB gene were found in genes that originated from either the environmental or clinical strains.
Interestingly, 11 of 12 isolates that carried aac(6′)-Ib-cr also carried one or two types of qnr. Four isolates carried qnrA1, qnrB8, and aac(6′)-Ib-cr, three isolates carried qnrA1 and aac(6′)-Ib-cr, two isolates carried qnrB6 and aac(6′)-Ib-cr, two isolates carried qnrB9 and aac(6′)-Ib-cr, and one isolate carried only aac(6′)-Ib-cr. Of the clinical isolates of other Citrobacter spp., two of the seven C. braakii isolates carried both qnrA1 and aac(6′)-Ib-cr; one C. braakii isolate carried qnrB11; one C. braakii isolate carried aac(6′)-Ib-cr; one of three C. amalonaticus isolates carried qnrB9. No qnr or aac(6′)-Ib-cr gene was detected in C. koseri.
Distribution of the MIC of ciprofloxacin against isolates carrying qnr- or aac(6′)-Ib-cr-genes
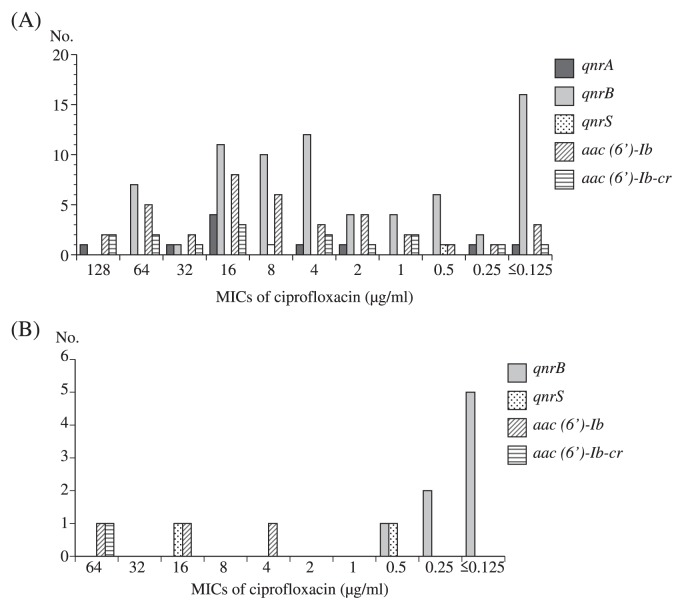
A wide range of MICs of ciprofloxacin against isolates that carried the qnr- or aac(6′)-Ib-cr-genes was observed, which varied from ≤0.125 μg mL−1 to 128 μg mL−1 (Fig. 1). The MIC of ciprofloxacin against most qnrA1- or aac(6′)-Ib-cr-carrying isolates was ≥0.25 μg mL−1, except for one clinical isolate of C. freundii that carried qnrA1, qnrB8, and aac(6′)-Ib-cr (MIC ≤0.125 μg mL−1). Similarly, the MIC of ciprofloxacin against four qnrS-carrying isolates (4/200, 2.0%) was ≥0.5 μg mL−1; however, for qnrB-carrying isolates, the MIC of ciprofloxacin against 16 of 71 clinical isolates (21.9%), and five of eight water-borne environmental isolates of C. freundii (62.5%) was ≤0.125 μg mL−1.
Fig. 1.
Distribution of MIC of ciprofloxacin among isolates with qnr or aac(6′)-Ib-cr gene. (A) Clinical isolates. (B) Water-borne environmental isolates.
Southern hybridization
Southern hybridization using a qnrS probe identified a specific band (Table 3 and Fig. 2) corresponding to the plasmid DNA of environmental E. coli or Aeromonas sp. isolates, whereas no band that corresponded to chromosomal DNA was observed, suggesting that the qnrS gene is located on the plasmid with an approximate size of 53 kb. Using Southern hybridization we also confirmed that a qnrB probe hybridized to plasmid DNA but not to chromosomal DNA of three of the qnrB-carrying environmental C. freundii isolates. No hybridized band was identified from either the plasmid or the chromosomal DNA of five other qnrB-carrying isolates. Since no hybridization was observed for chromosomal DNA, this indicates that qnrB genes are not found on the chromosomes. It is possible that lower copy numbers of the qnrB gene are located on the plasmids of these strains.
Table 3.
Summary of Southern hybridization of environmental isolates carrying qnrB or qnrS genes
| Strain | No. | Number of positive isolates | |||
|---|---|---|---|---|---|
|
| |||||
| qnrB on chromosome | qnrB on plasmid | qnrS on chromosome | qnrS on plasmid | ||
| qnrB-positive | |||||
| C. freundii | 8 | 0 | 3 | ||
| qnrS-positive | |||||
| E. coli | 1 | 0 | 1 | ||
| Aeromonas sp. | 1 | 0 | 1 | ||
Fig. 2.
Southern hybridization analysis for determination of the location of gnr gene. (A) Southern hybridization analysis of plasmid or chromosomal DNAs targeting gnrS gene. Lane 1, Positive control for qnrS gene; Lane 2 or 5, plasmid DNAs from environmentally isolated E. coli and A. punctata, respectively; Lane 3 or 4, chromosomal DNAs from environmentally isolated E. coli and A. punctata, respectively. (B) Southern hybridization analysis of plasmid DNAs from eight environmentally isolated C. fruendii targeting gnrB gene. (C) Southern hybridization analysis of chromosomal DNAs from eight environmentally isolated C. fruendii targeting gnrB gene.
Discussion
C. freundii is one of the normal flora in human and animal intestines; however, it is also an opportunistic pathogen, which can cause diarrhea, septicaemia, meningitis, and brain abscess. It was ranked as No. 13 of the most frequently isolated pathogenic Gram-negative bacteria in 2009 in Zhejiang Province. In this study, the presence of qnr and aac(6′)-Ib-cr genes in water-borne environmental bacterial isolates was screened by PCR and it was found that the prevalence of qnrB in C. freundii was as high as 80.0%. The prevalence of such genes in clinical isolates of C. freundii was therefore investigated. As expected, the rate of qnr and qnrB carriage in these isolates was high (72.8% and 63.1%, respectively). Moreover, 11.6% of the clinical isolates of C. freundii carried the aac(6′)-Ib-cr gene, which was not detected in environmental C. freundii.
The prevalence of qnr and aac(6′)-Ib-cr genes appears to vary considerably in different studies depending on the criteria used to select the bacterial strains. The overall prevalence of qnr in Enterobacteriaceae has been reported to range from 0.2% to 50%, and aac(6′)-Ib-cr may be more prevalent than qnr(17, 19). The distribution of qnr genes in enterobacterial isolates has been investigated in the UK and Spain (9, 12). The prevalence of qnr genes, especially qnrB, has also been reported for clinically isolated K. pneumoniae and other Enterobacteriaceae species in Asian countries (24, 28). In China, qnr and aac(6′)-Ib-cr genes were detected in 8.0% and 9.9% of extended-spectrum β-lactamase (ESBL)-producing E. coli and K. pneumoniae isolates, respectively, that were collected from six provinces or districts (8). The prevalence of qnrA, qnrB, qnrS, and aac(6′)-Ib-cr genes in Enterobacter cloacae isolates from Anhui Province in China was below 10% for all of the genes (31); however, only a few studies have investigated the prevalence of qnr and aac(6′)-Ib-cr genes in C. freundii, possibly due to the relatively low rate of Citrobacter sp. isolation in a clinical setting compared with that of E. coli, K. pneumonia, and E. cloacae. A Korean study showed that 53 (38.4%) of 138 AmpC-producing C. freundii isolates harbored Qnr determinants (13). Another Korean study detected QnrB determinants in 67.9% of C. freundii, 62.5% of K. pneumoniae, 15.8% of E. cloacae, and 9.4% of E. coli isolates that were resistant to nalidixic acid and to at least one extended-spectrum β-lactam (27). In another study, Enterobacteriaceae isolates from nine teaching hospitals in China were investigated, and the MIC of ciprofloxacin against these isolates was >0.25 μg mL−1. Of the isolates for which the MIC of cefotaxime was >2.0 μg mL−1, qnr was present in 63.3% of C. freundii, 65.5% of K. pneumoniae, 65.7% of E. cloacae, and 6.5% of E. coli isolates. The prevalence of the aac(6′)-Ib-cr gene in these four bacterial species was 26.7%, 21.8%, 8.6%, and 16.9%, respectively (34). In our study, the 103 clinical isolates of C. freundii investigated were collected without any selection criteria; however, the prevalence of qnr and qnrB was as high as 72.8% and 68.9%, respectively, which is similar to that of its prevalence in water-borne environmental C. freundii (80.0%) (Table 2). Clinical isolates of C. braakii displayed a high prevalence of qnr and aac(6′)-Ib-cr (42.9% for both) (Table 2), which may be because C. braakii is a member of the C. freundii complex or may be due to the high MIC of ciprofloxacin against these isolates. It was noted that qnrA1 was always combined with aac(6′)-Ib-cr; nine of ten isolates with qnrA1 also carried aac(6′)-Ib-cr. Of the 15 isolates with aac(6′)-Ib-cr, 13 isolates carried qnr, nine of which were qnrA1 (Table 2). Six variants of qnrA, 19 variants of qnrB, and three variants of qnrS have been identified worldwide (7). All of the qnrA detected in this study were identified as qnrA1. Of the four qnrS-carrying isolates, two isolates carried qnrS1 and two isolates carried qnrS2. The most common subtypes of the qnrB subtype detected in this study were qnrB9, qnrB8 and qnrB6, which is quite different from the results of previous studies (13, 27, 34).
The qnrA and qnrS genes have been shown to originate from water-borne environmental bacteria, Shewanella algae and Vibrio splendidus, respectively (1, 16). A qnrS2 gene was recently identified in a water-borne bacterial species, Aeromonas, isolated from the River Seine in Paris (2) and from a Swiss lake (15). The qnrS2 gene has also been detected in a clinical Aeromonas veronii isolate (22). In the present study, the same qnrS2 gene was detected in the same bacterial species (Table 2). This is the first report of an isolate of the Aeromonas sp. harboring qnrS2 outside Europe (Table 2). As shown in Table 2, eight out of ten water-borne C. fruendii had the qnrB gene (2, 6, 17, 26). These results support the hypothesis that qnr genes originated from water-borne bacteria; however, for the majority of qnrB-carrying environmental C. freundii, the MIC for quinolone is low (Fig. 1B), while for the clinical qnrB-carrying environmental C. freundii, the MIC is widely distributed, indicating that qnrB itself does not contribute to the high level of quinolone resistance, but with the involvement of other mechanisms, such as other qnr genes, gyrA and parC, resistance will be elevated. On the other hand, environmental C. freundii carrying qnrS and aac(6′)-Ib-cr have a higher MIC for quinolone. Such a phenomenon was also observed for the clinical isolates, indicating that those genes contribute to the higher level of quinolone resistance.
Most qnr genes have been reported to be located on plasmid DNA (25), while a few are located on chromosomal DNA (23); however, in our effort to determine the localization of these genes, we were unable to produce transconjugants and transformants that carry qnr genes. The plasmid carrying the qnrB gene may be a non-conjugable plasmid. Our Southern hybridization and endonuclease digestion experiments results clearly indicated that qnrS1-encoding plasmid and qnrS2-encoding plasmid are heterogenous, and that in at least three out of the eight environmental C. freundii strains, qnrB is located on plasmid(s) (data not shown); however, no bands corresponding to qnrB were detected in either plasmid or chromosomal DNA by Southern hybridization of the other qnrB-positive environmental C. freundii. We suspect that the gene might be located on the plasmids in these strains with very low abundance.
In summary, there is a high prevalence of plasmid-coded qnr and aac(6′)-Ib-cr genes. The plasmids isolated from both environmental and clinical C. freundii isolates appeared to be homogenous. Further investigations are required to confirm the hypothesis that C. freundii play a role as a reservoir of the qnrB gene and that the aquatic environment is an important vehicle for the spread of PMQR.
Acknowledgements
This work was partially supported by the JSPS Grant-in-Aid for Scientific Research (A) (21256002).
References
- 1.Cattoir V, Poirel L, Mazel D, Soussy CJ, Nordmann P. Vibrio splendidus as the source of plasmid-mediated QnrS-like quinolone resistance determinants. Antimicrob Agents Chemother. 2007;51:2650–2651. doi: 10.1128/AAC.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cattoir V, Poirel L, Aubert C, Soussy CJ, Nordmann P. Unexpected occurrence of plasmid-mediated quinolone resistance determinants in environmental Aeromonas spp. Emerg Infect Dis. 2008;14:231–237. doi: 10.3201/eid1402.070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavaco LM, Hasman H, Xia S, Aarestrup FM. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob Agents Chemother. 2009;53:603–608. doi: 10.1128/AAC.00997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Laboratory and Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 7th ed. CLSI; Wayne, PA, USA: 2006. Approved standard M7-A7 (M100-S16) [Google Scholar]
- 5.Hata M, Suzuki M, Matsumoto M, Takahashi M, Sato K, Ibe S, Sakae K. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother. 2005;49:801–803. doi: 10.1128/AAC.49.2.801-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacoby GA, Walsh KE, Mills DM, Walker VJ, Oh H, Robicsek A, Hooper DC. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob Agents Chemother. 2006;50:1178–1182. doi: 10.1128/AAC.50.4.1178-1182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby G, Cattoir V, Hooper D, Martínez-Martínez L, Nordmann P, Pascual A, Poirel L, Wang M. qnr gene nomenclature. Antimicrob Agents Chemother. 2008;52:2297–2299. doi: 10.1128/AAC.00147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Zhou Z, Qian Y, Wei Z, Yu Y, Hu S, Li L. Plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J Antimicrob Chemother. 2008;61:1003–1006. doi: 10.1093/jac/dkn063. [DOI] [PubMed] [Google Scholar]
- 9.Lavilla S, González-López JJ, Sabaté M, García-Fernández A, Larrosa MN, Bartolomé RM, Carattoli A, Prats G. Prevalence of qnr genes among extended-spectrum β-lactamase-producing enterobacterial isolates in Barcelona, Spain. J Antimicrob Chemother. 2008;61:291–295. doi: 10.1093/jac/dkm448. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Zeng Z, Chen Z, et al. High prevalence of plasmid-mediated quinolone resistance determinants qnr, aac(6′)-Ib-cr, and qepA among ceftiofur-resistant Enterobacteriaceae isolates from companion and food-producing animals. Antimicrob Agents Chemother. 2009;53:519–524. doi: 10.1128/AAC.00886-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Martinez L, Pascual A, Jacoby GA. Quinolone resistance from a transferable plasmid. Lancet. 1998;351:797–799. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 12.Naqvi SM, Jenkins C, McHugh TD, Balakrishnan I. Identification of the qnr family in Enterobacteriaceae in clinical practice. J Antimicrob Chemother. 2009;63:830–832. doi: 10.1093/jac/dkp011. [DOI] [PubMed] [Google Scholar]
- 13.Park YJ, Yu JK, Lee S, Oh EJ, Woo GJ. Prevalence and diversity of qnr alleles in AmpC-producing Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii and Serratia marcescens: a multicentre study from Korea. J Antimicrob Chemother. 2007;60:868–871. doi: 10.1093/jac/dkm266. [DOI] [PubMed] [Google Scholar]
- 14.Perichon B, Courvalin P, Galimand M. Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob Agents Chemother. 2007;51:2464–2469. doi: 10.1128/AAC.00143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picão RC, Poirel L, Demarta A, Silva CS, Corvaglia AR, Petrini O, Nordmann P. Plasmid-mediated quinolone resistance in Aeromonas allosaccharophila recovered from a Swiss lake. J Antimicrob Chemother. 2008;62:948–950. doi: 10.1093/jac/dkn341. [DOI] [PubMed] [Google Scholar]
- 16.Poirel L, Rodriguez-Martinez JM, Mammeri H, Liard A, Nordmann P. Origin of plasmid-mediated quinolone resistance determinant QnrA. Antimicrob Agents Chemother. 2005;49:3523–3525. doi: 10.1128/AAC.49.8.3523-3525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirel L, Cattoir V, Nordmann P. Is plasmid-mediated quinolone resistance a clinically significant problem? Clin Microbiol Infect. 2008;14:295–297. doi: 10.1111/j.1469-0691.2007.01930.x. [DOI] [PubMed] [Google Scholar]
- 18.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med. 2006;12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 19.Robicsek A, Jacoby GA, Hooper DC. The worldwide emergence of plasmid-mediated quinolone resistance. Lancet Infect Dis. 2006;6:629–640. doi: 10.1016/S1473-3099(06)70599-0. [DOI] [PubMed] [Google Scholar]
- 20.Robicsek A, Strahilevitz J, Sahm DF, Jacoby GA, Hooper DC. qnr prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob Agents Chemother. 2006;50:2872–2874. doi: 10.1128/AAC.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother. 2003;51:1109–1117. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez-Céspedes J, Blasco MD, Marti S, Alba V, Alcalde E, Esteve C, Vila J. Plasmid-mediated QnrS2 determinant from a clinical Aeromonas veronii isolate. Antimicrob Agents Chemother. 2008;52:2990–2991. doi: 10.1128/AAC.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Cespedes J, Marti S, Soto SM, Alba V, Melción C, Almela M, Marco F, Vila J. Two chromosomally located qnrB variants, qnrB6 and the new qnrB16, in Citrobacter spp. isolates causing bacteraemia. Clin Microbiol Infect. 2009;15:1132–1138. doi: 10.1111/j.1469-0691.2009.02744.x. [DOI] [PubMed] [Google Scholar]
- 24.Shin JH, Jung HJ, Lee JY, Kim HR, Lee JN, Chang CL. High rates of plasmid-mediated quinolone resistance QnrB variants among ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae from urinary tract infections in Korea. Microb Drug Resist. 2008;14:221–226. doi: 10.1089/mdr.2008.0834. [DOI] [PubMed] [Google Scholar]
- 25.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin Microbiol Rev. 2009;22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takasu H, Suzuki S, Reungsang A, Viet PH. Fluoroquinolone (FQ) contamination does not correlate with occurrence of FQ-resistant bacteria in aquatic environments of Vietnam and Thailand. Microbes Environ. 2011;26:135–143. doi: 10.1264/jsme2.me10204. [DOI] [PubMed] [Google Scholar]
- 27.Tamang MD, Seol SY, Oh JY, Kang HY, Lee JC, Lee YC, Cho DT, Kim J. Plasmid-mediated quinolone resistance determinants qnrA, qnrB, and qnrS among clinical isolates of Enterobacteriaceae in a Korean hospital. Antimicrob Agents Chemother. 2008;52:4159–4162. doi: 10.1128/AAC.01633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teo JW, Ng KY, Lin RT. Detection and genetic characterisation of qnrB in hospital isolates of Klebsiella pneumoniae in Singapore. Int. J. Antimicrob Agents. 2009;33:177–180. doi: 10.1016/j.ijantimicag.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Tran JH, Jacoby GA. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci USA. 2002;99:5638–5642. doi: 10.1073/pnas.082092899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Guo Q, Xu X, Wang X, Ye X, Wu S, Hooper DC, Wang M. New plasmid-mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob Agents Chemother. 2009;53:1892–1897. doi: 10.1128/AAC.01400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong Z, Wang P, Wei Y, Wang H, Cao H, Huang H, Li J. Investigation of qnr and aac(6′)-Ib-cr in Enterobacter cloacae isolates from Anhui Province, China. Diagn Microbiol Infect Dis. 2008;62:457–459. doi: 10.1016/j.diagmicrobio.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol. 1995;61:1104–1109. doi: 10.1128/aem.61.3.1104-1109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamane K, Wachino J, Suzuki S, et al. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob Agents Chemother. 2007;51:3354–3360. doi: 10.1128/AAC.00339-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Chen H, Yang Q, Chen M, Wang H. High prevalence of plasmid-mediated quinolone resistance genes qnr and aac(6′)-Ib-cr in clinical isolates of Enterobacteriaceae from nine teaching hospitals in China. Antimicrob Agents Chemother. 2008;52:4268–4273. doi: 10.1128/AAC.00830-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue L, Jiang HX, Liao XP, et al. Prevalence of plasmid-mediated quinolone resistance qnr genes in poultry and swine clinical isolates of Escherichia coli. Vet Microbiol. 2008;132:414–420. doi: 10.1016/j.vetmic.2008.05.009. [DOI] [PubMed] [Google Scholar]