Abstract
Two novel ethylene-assimilating bacteria, strains ETY-M and ETY-NAG, were isolated from seawater around Japan. The characteristics of both strains were investigated, and phylogenetic analyses of their 16S rRNA gene sequences showed that they belonged to the genus Haliea. In C1–4 gaseous hydrocarbons, both strains grew only on ethylene, but degraded ethane, propylene, and propane in addition to ethylene. Methane, n-butane, and i-butane were not utilized or degraded by either strain. Soluble methane monooxygenase-type genes, which are ubiquitous in alkene-assimilating bacteria for initial oxidation of alkenes, were not detected in these strains, although genes similar to particulate methane monooxygenases (pMMO)/ammonia monooxygenases (AMO) were observed. The phylogenetic tree of the deduced amino acid sequences formed a new clade near the monooxygenases of ethane-assimilating bacteria similar to other clades of pMMOs in type I, type II, and Verrucomicrobia methanotrophs and AMOs in alpha and beta proteobacteria.
Keywords: short-chain alkene, Haliea, particulate methane monooxygenase (pmo)
Ethylene occurs in the atmosphere at approximately 0.1–10 ppbv (41) and affects atmospheric chemistry and the global climate. It provides a sink for hydroxyl radicals and plays a key role in the production and destruction of ozone in the troposphere (10). Ethylene acts as a hormone in higher plants and is involved in the regulation of plant physiological processes. In addition, it can inhibit methane consumption activity and ammonium oxidation of soil (47). Higher plants generate ethylene from methionine via 1-aminocyclopropane-1-carboxylic acid (ACC). Many species of bacteria, yeasts, and molds produce ethylene from methionine via 2-keto-4-methylthiobutyric acid or from glutamic acid via 2-oxoglutarate (13).
Most isolates assimilating short-chain alkenes, ethylene, and/or propylene are terrestrial Gram-positive bacteria from the CMNR group or strains of Xanthobacter(37), such as Rhodococcus rhodochrous B-276 (formally, Nocardia corallina) (12, 39), Xanthobacter autotrophicus Py2 (17, 49), Nocardioides sp. JS614 (28), and Mycobacterium(15, 22). These strains oxidize alkenes by alkene monooxygenases to epoxyalkanes, which are further metabolized by epoxyalkane: coenzyme M transferase (5, 23). The alkene monooxygenases in these strains are soluble diion monooxygenases similar to soluble methane monooxygenases, phenol hydroxylases, toluene monooxygenases, and short-chain alkane mono-oxygenases (21, 25). The alkene monooxygenases are divided into two groups: those from Xanthobacter autotrophicus Py2 and the remainder. The former has an operon arrangement of an alpha subunit hydroxylase–ferredoxin–gamma subunit hydroxylase-coupling protein–beta subunit hydroxylase–reductase and the latter exhibit beta subunit hydroxylase-coupling protein–alpha subunit hydroxylase–reductase.
Ethylene is also produced in the sea, and ethylene concentrations in rock pools were recorded to range between 47.2 and 856.4 pmol L−1(3). A seasonal cycle was observed with a summer maximum and concentrations varied from 17 to 951 pmol L−1 in coastal waters. Ethylene concentrations in surface water vary in the range of 1.8–39.2 nL L−1 and generally show a vertical maximum at the pycnocline (approximately 100 m depth), where elevated concentrations of chlorophyll-a, dissolved oxygen, and nutrients were also found in the western Atlantic (36). Photochemical transformation of dissolved organic matter in surface water results in the production of ethylene (34). Some micro- and macroalgae, photosynthetic bacteria, and cyanobacteria produce ethylene, probably via ACC or acrylate from methionine (3, 27, 32). Few reports have examined the physiological effects of ethylene on algae, which is surprising given that ethylene may play a multifaceted role in algae, having driven the loss of chlorophyll-a(32) and having contributed to mastoparan-induced cell death in green algae (48). Nevertheless, no reports on ethylene-degrading marine micro-organisms currently exist.
We isolated ethylene-assimilating bacteria from seawater and investigated their characteristics, particularly those related to ethylene assimilation, to clarify the role of bacteria in ethylene circulation in the sea.
Materials and Methods
Growth conditions
A 5VM medium containing 100 mg NH4NO3, 10 mg KH2PO4, 2.5 mg Fe(III)EDTA, 2.75 mg vitamin B12, 2.5 mg biotin, 500 mg thiamine–HCl, 372 mg Na2EDTA, 0.25 mg CuSO4·5H2O, 5.75 mg ZnSO4·7H2O, 4.55 mg MnCl2·4H2O, 0.6 mg of CoCl2·6H2O, 0.27 mg (NH4)6Mo7O24·4H2O, and 5 mg yeast extract (Difco, Detroit, MI, USA) in 1 L filtered seawater (pH 8.1) was used to isolate and culture ethylene-assimilating bacteria. Ethylene was supplied by replacing 50% of the air in a culture vessel. Solid medium used for culturing 5VM media and 1% gellan gum. The culture was incubated at 25°C.
Escherichia coli strains were grown in Luria–Bertani (LB) broth containing 1% polypeptone, 0.5% yeast extract, and 1% NaCl medium supplemented with ampicillin (100 μg/mL) when necessary.
Sampling and isolation of bacteria
Surface seawater was collected in 120-mL glass vials from several Japanese coasts during July 1998 and May 1999, and cultured with 5VM medium and ethylene. After growth was observed in the liquid medium, a portion of the broth was streaked onto solid medium and cultured with ethylene, and any visible colonies were transferred to liquid medium. This isolation procedure was repeated at least five times, at which point the purity of the isolated strain was confirmed by microscopic observation and by the lack of growth of other bacteria on Marine Agar 2216 (Difco) plates.
Growth characteristics
The temperature range for growth was estimated by growing the isolates in liquid 5VM medium with ethylene at 4°C, 10°C, 20°C, 30°C, 37°C, and 45°C. To test the salt-dependence of growth, 0, 0.26, 2.6, 13, 26, 52, 79, or 132 g of NaCl was added to 1 L modified 5VM medium, in which seawater was replaced by 1.54 g CaCl2·2H2O, 100 mg KBr, 3 mg KF, 700 mg KCl, 30 mg H3BO3, 4.09 g K2SO4, 200 mg KHCO3, 17 mg SrCl2·6H2O, and 11.1 g MgCl2·6H2O in 1 L distilled water. The isolates were cultured in these media with ethylene.
To test their utilization of carbon sources with liquid 5VM medium, gaseous hydrocarbon was added by replacing 50% of the gas phase; alcohol was added at 1% and other carbon sources were added at 0.1%. We tested the nitrogen sources for the growth of isolates by adding them at 0.1% to liquid 5VM medium without NH4NO3 containing 0.1% sodium acetate as a carbon source. The growth of isolates was assessed by the turbidity of the media as well as protein concentrations, which were determined using a modified Lowry method (2).
Oxidation of short-chain alkanes and alkenes by resting cells
The isolated ethylene-assimilating strains were cultured with 5VM medium and ethylene. The cultured cells were harvested by centrifugation at 7,000 × g and suspended in sterilized seawater at about one-tenth of the volume of their broth. The suspended cells were transferred to 13.5-mL glass vials, which were sealed with Teflon-lined rubber septa. Five or 10 μL nitrogen gas containing alkene and alkane gases was added to the vials. The vials were maintained at room temperature for 5 h, after which 0.1 mL of 5 N NaOH solution was added and the total hydrocarbon gases in the vials were extracted and analyzed using a gas chromatographic system (42) (GC-17A; Shimadzu, Tokyo, Japan) equipped with FID as a detector.
Transmission electron microscopy (TEM)
Transmission electron microscopy analysis of the purified strains, which were cultured at 22°C for 20 days, was performed by negative staining using a JEM-2000EX (JEOL, Tokyo, Japan).
Determination of DNA G+C content, cellular fatty acid profile, and ubiquinones
DNA G+C content, cellular fatty acid profile, and ubiquinones was determined by TechnoSuruga (Shizuoka, Japan).
Isolation of total DNA and sequencing
Total DNA of ETY-M and ETY-NAG and DNA manipulations for E. coli was isolated according to Sambrook using the standard protocols (35). The 16S rRNA coding sequence was amplified by total DNA as a template and primers 9F (5′-GAGTTTGATCCTG GCTCAG-3′) (4) and 1510R (5′-GGTTACCTTGTTACGACTT-3′) (46). The mmoX-like gene was amplified by using mmoX206F (5′-ATCGCBAARGAATAYGCSCG-3′) (1) or mmoX1 (5′-CGGT CCGCTGTGGAAGGGCATGAAGCGCGT-3′) (29) as a forward primer, and mmoX886R (5′-ACCCANGGCTCGACYTTGAA-3′) (1) ormmoXr901(5′-TGGGTSAARACSTGGAACCGCTGGGT-3′)(38) as a reverse primer. The pmoA-like gene was obtained by PCR amplification using the forward primer A189f (5′-GGNGACTGG GACTTCTGG-3′) and reverse primer A682r (5′-GAASGCN GAGAAGAASGC-3′) (19). PCR amplification was performed using an S1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA) using Ex Taq DNA polymerase (Takara Bio, Otsu, Japan) under the following conditions: for the 16S rRNA gene, a pre-denaturing step at 96°C for 2 min followed by 30 cycles at 96°C for 30 s, 55°C for 1 min, and 72°C for 2 min, and final elongation at 72°C for 7 min. PCR amplification of the pmoA-like gene and mmoX-like gene are described elsewhere (30). The amplified fragments of the 16S rRNA gene (1.5 kb) and the pmoA-like gene (0.5 kb) were cloned into pMD20, T-vector (Takara Bio), then transformed in E. coli DH5α as a host strain. DNA sequencing was carried out by cycle sequencing using the BigDye terminator v3.1 cycle sequencing kit (Applied Biosystems/Life Technologies, Carlsbad, CA, USA) with the ABI PRISM 310NT Genetic Analyzer (Applied Biosystems/Life Technologies). Accumulated sequencing data were analyzed with GENETYX-MAC software ver.15 (Genetyx, Tokyo, Japan).
Phylogenetic analysis
Genetic analyses were conducted using 16S rRNA gene fragments of strains ETY-M and ETY-NAG, which were deposited in GenBank. Homology searches were conducted using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/). The sequences were aligned using the CLUSTALW ver. 1.83 program. Phylogenetic trees were constructed using TreeViewX software with the neighbor-joining method. Bootstrap analysis with 100 trial replications was performed to determine the reliability of the clustering patterns.
GenBank accession numbers
The GenBank accession numbers of the 16S rRNA gene sequences are ETY-M (AB646259) and ETY-NAG (AB646260).
Results and Discussion
Isolation, phenotypic characterization, and phylogenetic analyses of two ethylene-assimilating bacteria, strains ETY-M and ETY-NAG
Two strains of ethylene-assimilating bacteria, strain ETY-M and ETY-NAG, were isolated from seawater from Yakushima and Tokyo Bay, Japan, respectively. The two isolated strains exhibited attachment of growing cells to the inner wall of the culture vessel in the liquid medium but did not grow well on Marine Agar 2216 plates.
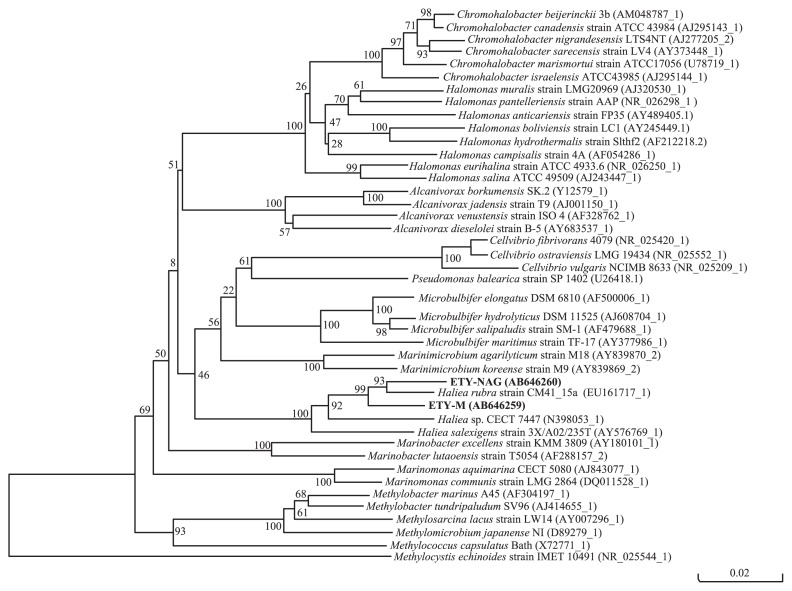
The 16S rRNA gene sequences were analyzed to examine the phylogeny of the strains. The analyses showed that the most closely related bacteria belonged to genus Haliea (Gammaproteobacteria). A BLAST search of strains ETY-M and ETY-NAG revealed similarities to the bacteria Haliea sp. MOLA 104 and Haliea rubra strain CM41_15a (43, 44), which were 97% and 96% identical, respectively. The phylogenetic tree showed that the two isolated strains were in the clade comprising Haliea spp. (Fig. 1).
Fig. 1.
Phylogenetic tree of 16S rRNA genes constructed using a neighbor-joining dendrogram. Methylocystis echinoides served as an outgroup. Numbers to the right are accession numbers in the database. Scale bar indicates 0.02 substitutions per 100 base positions. Numbers at tree nodes are bootstrap values from 100 trials.
Strains ETY-M and ETY-NAG were Gram-negative. Characteristics of both strains are shown in Table 1 with three type strains of Haliea spp. Strains ETY-M and ETY-NAG and Haliea spp. were isolated from marine samples and all strains needed NaCl for growth. All strains produced pigments although the colors were different.
Table 1.
Characterization of ETY-M, ET-NAG, and other Haliea species
| Characteristic | Strains | ||||
|---|---|---|---|---|---|
|
| |||||
| ETY-M | ETY-NAG | 3X/A02/235 | CM41_15a | 7SM29T | |
| Cell morphology | Short Rods | Short Rods | Straight Rods | Straight Rods | Short Rods |
| Cell dimensions (μm) | 0.4–0.45 × 1.2–1.3 | 0.75–1.0 × 0.5–0.6 | 0.3–0.7 × 1.3–1.9 | 0.5 × 2.7 | 0.4–0.5 × 1.1–1.3 |
| Colony color (agar medium) | Purple (5VM) | Yellow (5VM) | Cream (MA) | Red (MA) | Yellow (MA) |
| Growth of nutrient agar (MA) | − | − | + | + | + |
| Flagella | − | + | + | − | + |
| DNA G+C content (mol%) | 65.2 | 58.8 | 61.4 | 64.8 | 62.1 |
| Growth temperature range (°C) | 20–37 | 20–30 | 10–37 | 15–44 | 15–40 |
| Optimum | 30 | 30 | 25–30 | 30 | 28 |
| Salinity range (g L−1) | 13.2–52.7 | 26.4–52.7 | 7–70 | 7–42 | 3.5–150 |
| Optimum | 13.2 | 26.4 | 40 | 35 | unknown |
| Growth substrate | |||||
| Glucose | − | − | − | + | − |
| Maltose | − | + | − | (+) | − |
| Sucrose | − | + | − | − | − |
| Arabinose | − | − | − | − | − |
| Xylose | − | − | N.A. | N.A. | − |
| Fructose | − | − | − | (+) | − |
| Mannose | − | − | (+) | (+) | − |
| Cellobiose | − | − | − | − | (+) |
| Mannitol | − | − | − | N.A. | − |
| Citrate | − | − | − | + | − |
| Succinate | − | − | + | − | − |
| Gluconate | − | − | N.A. | N.A. | − |
| Pyruvate | + | + | + | − | + |
| Acetate | + | + | − | − | + |
| Glycerol | − | (+) | + | − | − |
| Alanine | + | − | − | − | + |
| Glutamate | − | + | (+) | − | + |
| Aspartate | − | + | + | − | + |
| Serine | + | − | − | − | − |
| Gas hydrocarbon utilization of | |||||
| Methane | − | − | N.A. | N.A. | N.A. |
| Ethane | − | − | N.A. | N.A. | N.A. |
| Propane | − | − | N.A. | N.A. | N.A. |
| Ethylene | + | + | N.A. | N.A. | N.A. |
| Propylene | − | − | N.A. | N.A. | N.A. |
| Methanol | − | − | N.A. | N.A. | N.A. |
| Ethanol | + | + | N.A. | N.A. | N.A. |
| 1-Propanol | (+) | − | N.A. | N.A. | N.A. |
| 2-propanol | − | − | N.A | N.A | N.A |
MA, marine agar 2216; +, positive; −, negative; (+), weakly positive; N.A., data not available.
The DNA G+C contents of strains ETY-M and ETY-NAG were 65.2 and 58.8 mol%, respectively, which is in accordance with the ranges reported for other Haliea species (Table 1). In addition, ETY-M and ETY-NAG exhibit ubiquinone Q-8, as do other Haliea species. The cellular fatty acid profile of strain ETY-M was determined, and five components of fatty acids were detected at concentrations greater than 1%: C18:1ω7c, 37.5%; (C16:1ω7c or C15:0 iso 2OH), 28.0%; C16:0, 21.0%; C14:0, 6.5%; C10:0 3OH, 3.2%. This profile is most similar to H. rubra in Haliea spp. Additional characteristics of both strains are compared with Haliea strains in Table 1.
With respect to carbon sources except for hydrocarbons, both strains showed good growth with the addition of acetate and pyruvate, as did the H. mediterranea strain 7SM29 (26), whereas strain ETY-NAG also assimilated maltose, sucrose, aspartate, and glutamate, and strain ETY-M also assimilated serine and alanine. As a nitrogen source, both strains utilized ammonium sulfate but not potassium nitrate. Furthermore, strain ETY-M utilized aspartate and arginine and strain ETY-NAG utilized arginine as nitrogen sources in addition to the amino acids they used for carbon sources. Therefore, on the basis of 16S rRNA gene sequence analyses as well as their physiological and biochemical characteristics, both strains were identified as Haliea spp.
Strains ETY-M and ETY-NAG specifically assimilate ethylene
No strains of the genus Haliea have been reported to assimilate gaseous hydrocarbons, including ethylene. The assimilation of various gas hydrocarbons by strains ETY-M and ETY-NAG was investigated, and both strains were found to assimilate only ethylene, but not methane, ethane, propane, or propylene (Table 1). Only two strains, R. rhodochrous B-276 and Mycobacterium E20, have been reported to grow well on both alkanes and alkenes (37). Mycobacterium E20 utilized ethylene, but grew poorly on propylene and butene as well as ethane and higher alkanes (7), although ethylene was oxidized by the monooxygenases differently than for alkane oxidation (8). R. rhodochrous B-276 grew well on ethylene, propylene, propane, 1-butene, butane, and butadiene, but not on ethane or methane (14). X. autotrophicus Py2, as well as some other isolates, were able to use ethylene and propylene but not ethane, propane, or butane (45). Of the seven ethylene- or propylene-utilizing Mycobacterium strains tested, only one strain used both ethylene and propylene (9).
To test the conversion of gaseous hydrocarbons in both strains, the degradation of gaseous hydrocarbons by resting cells was performed with methane, ethane, propane, i-butane, n-butane, ethylene, and propylene (Table 2). Methane, i-butane, and n-butane were hardly degraded by both strains, while ethane, ethylene, and propylene were markedly degraded by both strains. Ethane, ethylene, and propylene were degraded to 0.48%, 0.02%, and 1.0%, respectively, by strain ETY-M, and to 0.06%, 0.15%, and 4.6%, respectively, by strain ETY-NAG. Propane was degraded a little more slowly than ethane, ethylene, and propylene by both strains, and was degraded to 9.3–58% by strain ETY-M and to 26% by strain ETY-NAG. Thus, C1 or C4 gases, such as methane, i-butane, and n-butane, were hardly degraded, while C2 or C3 gases, such as ethane, ethylene propane, and propylene, were degraded well by both strains. This indicated that ETY-M and ETY-NAG are able to specifically degrade C2 and C3 gas hydrocarbons, but not to assimilate them, except for ethylene.
Table 2.
Degradation of various alkanes and alkenes by ethylene-grown ETY-M and ETY-NAG
| Substrates | Residual gas hydrocarbons (%) | ||
|---|---|---|---|
|
| |||
| ETY-M (A) | ETY-M (B) | ETY-NAG | |
| Methane | 121 (0.11) | ND | 98 (0.01) |
| Ethane | 0.38 (0.05) | 0.48 (0.07) | 0.06 (1 × 10−4) |
| Ethylene | ND | 0.02 (0.03) | 0.15 (1.3 × 10−4) |
| Propane | 58 (0.1) | 9.3 (0.03) | 26 (0.02) |
| Propylene | ND | 1.0 (0.008) | 4.6 (0.04) |
| i-Butane | 122 (0.06) | 91 (0.05) | 102 (0.01) |
| n-Butane | 118 (0.09) | 90 (0.05) | 102 (0.02) |
For ETY-M, experiments ETY-M (A) and ETY-M (B) were done simultaneously but separately; 5 μL of mixed gas (A) containing 1% each of methane, ethane, propane, i-butane, and n-butane was injected into a vial for ETY-M (A), and 5 μL of mixed gas (B) containing 1% each of ethane, ethylene, propane, propylene, i-butane, and n-butane was injected into a vial for ETY-M (B). For ETY-NAG, 5 μL of mixed gas (A) and (B) was injected into a vial. Total protein concentrations of ETY-M and ETY-NAG, were 0.52 mg L−1 and 0.07 mg L−1, respectively. ND, not determined. Values given in parentheses are standard deviations for three replicates in ETY-M (A) and ETY-M (B) and are the half differences of two replicates in ETY-NAG.
Both strains grew on ethanol but not on 2-propanol. Strain ETY-M grew weakly on 1-propanol whereas strain ETY-NAG did not (Table 1). X. autotrophicus Py2 and Mycobacterium E20 were also able to assimilate ethanol. To clarify the ethylene degradation pathway in strains ETY-M and ETY-NAG, their metabolites and utilization should be investigated, along with other alkene-assimilating bacteria that epoxidize alkene for their growth.
Strains ETY-M and ETY-NAG were isolated from seawater using enrichment culturing with 50% ethylene. Both strains specifically assimilated ethylene in C1–4 gaseous hydrocarbons even though the ethylene concentration in seawater is very low. Ethylene appears to be produced by seaweed as well as some marine microorganisms. These ethylene-assimilating bacteria may coexist with ethylene producers such as members of the methylotrophic genus Methylobacterium, which are ubiquitous on plant surfaces and potentially dominate the phyllosphere population (6). The genus Haliea genus reportedly comprises several percent of the bacterial abundance in mangrove sediments and these species are sensitive to oil contamination (11). Haliea bacteria may interact with mangrove plants via ethylene, which is produced in abundance when plants are wounded; however, no information exists on the utilization of short-chain hydrocarbons by other Haliea spp.
A particulate methane monooxygenase (pmoA)-like gene exists in ETY-M and ETY-NAG
Almost all alkene-assimilating bacteria, including ethylene-assimilating bacteria, carry soluble methane monooxygenase (sMMO)-like genes. To examine the existence of sMMO in both strains, we attempted to amplify the putative mmoX as a target gene, which encodes an α-subunit of the hydroxylase of the sMMO-like gene. The gene was amplified by all combinations of the four primers, i.e., mmoX206F or mmoX1 as a forward primer and mmoX886R or mmoXr901 as a reverse primer; however, no mmoX-like genes were detected in either strain (data not shown). These results suggest that the strains do not carry sMMO-like genes, but rather possess particulate methane monooxygenase (pMMO)-like genes because some pMMO-like enzymes related to short-chain alkane degradation were detected (16, 33). pMMO is a membrane-bound enzyme that requires copper for its activity. The pMMO gene cluster is composed of pmoC, pmoA, and pmoB, and pMMO is analogous to ammonia monooxygenase (AMO), whose gene cluster is composed of amoC, amoA, and amoB(19).
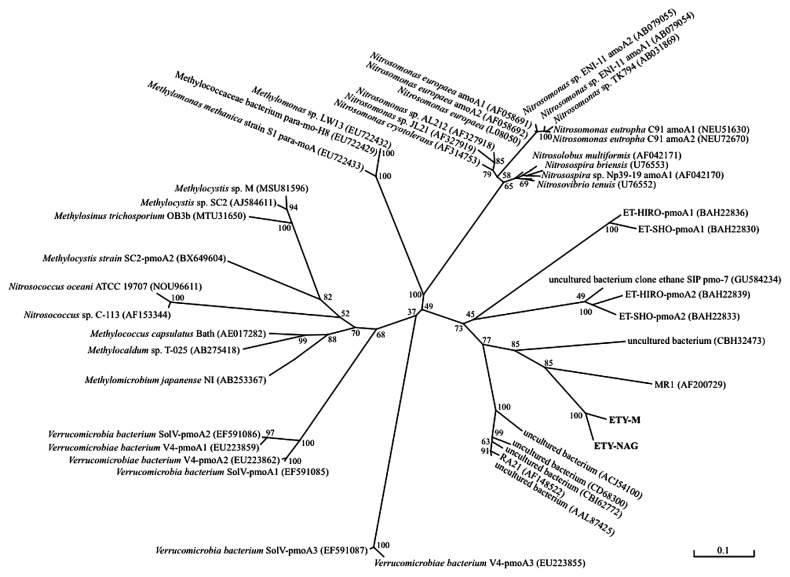
PCR amplification of the pmoA-like gene revealed the presence of the pmoA-like gene in both strains ETY-M and ETY-NAG. The BLAST search results for amplified pmoA-like gene sequences revealed similarities to pmoA (<54% similarity) and ammonia monooxygenase gene (amoA) (<63% similarity). Phylogenetic analysis of these genes indicated that pmoA-like genes of strain ETY-M and ETY-NAG were distant from other pmo-like genes, including methane-, ethane-, and ammonia monooxygenase; however, the genes of strains ETY-M and ETY-NAG clustered nearest to the genes of putative ethane oxidizers that were retrieved from marine sediment by the SIP technique (33) as well as the genes of ethane-assimilating strains ET-HIRO and ET-SHO in GenBank (Fig. 2). The pmoA-like genes of strains ETY-M and ETY-NAG were particularly analogous to those of Nitrosospina and Nitrosomonas, which are beta-proteobacteria (31), compared to those of methanotrophs. In the phylogenetic tree, the pmoA-like genes of both strains formed a new branch of pmoA-like genes of ethylene-assimilating bacteria. Some environmental pmoA/amoA-like clones, which were reported as the RA21 cluster/group (24, 40), were placed near this clade (Fig. 2). Clone RA21 was retrieved from a beech forest soil sample in Denmark and was described as not being placed in any known group of pmo or amo sequences (20). Clone MR1 was also retrieved from forest soil near Marburg, Germany, and was described as a putative ammonium oxidizer (18). These genes could be specific to bacteria that assimilate short-chain hydrocarbons, particularly ethylene or ethane.
Fig. 2.
Phylogenetic tree of pmoA-like genes constructed using a neighbor-joining dendrogram. Numbers to the right are accession numbers in the database. Scale bar indicates 0.1 substitutions per amino acid position. Numbers at tree nodes are bootstrap values from 100 trials.
This is the first report to demonstrate that some strains of Haliea have the ability to degrade gaseous hydrocarbons and that pmoA-like genes are found in isolated bacteria besides methanotrophs and ammonia oxidizers. The genes encoding pMMO or AMO clusters are in the order ‘CAB’ with the exception of archaean AMO and pXMO, which are putatively involved in ammonium oxidation in some methanotrophs, although their specific functions are not clear (40). The gene clusters of pmo-like genes in strains ETY-M and ETY-NAG and their relation to ethylene degradation and these genes will be clarified in future studies.
Acknowledgements
We thank Aya Akiba for her assistance with growth experiments. This research was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan, under a project supporting the formation of research centers in private universities.
References
- 1.Ali H, Scanlan J, Dumont MG, Murrell JC. Duplication of the mmoX gene in Methylosinus sporium: Cloning, sequencing and mutational analysis. Microbiology. 2006;152:2931–2942. doi: 10.1099/mic.0.29031-0. [DOI] [PubMed] [Google Scholar]
- 2.Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 3.Broadgate WJ, Malin G, Küpper FC, Thompson A, Liss PS. Isoprene and other non-methane hydrocarbons from seaweeds: A source of reactive hydrocarbons to the atmosphere. Mar Chem. 2004;88:61–73. [Google Scholar]
- 4.Brosius J, Dult TJ, Sleeter DD, Noller HF. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 5.Coleman NV, Spain JC. Epoxyalkane: Coenzyme M transferase in the ethene and vinyl chloride biodegradation pathways of Mycobacterium strain JS60. J Bacteriol. 2003;185:5536–5545. doi: 10.1128/JB.185.18.5536-5545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corpe WA, Rheem S. Ecology of the methylotrophic bacteria on living leaf surfaces. FEMS Microbiol Ecol. 1989;6:243–250. [Google Scholar]
- 7.De Bont JA, Albers RA. Microbial metabolism of ethylene. Antonie van Leeuwenhoek. 1976;42:73–80. doi: 10.1007/BF00399450. [DOI] [PubMed] [Google Scholar]
- 8.De Bont JA, Attwood MM, Primrose SB, Harder W. Epoxidation of short chain alkenes in Mycobacterium E20: The involvement of a specific mono-oxygenase. FEMS Microbiol Lett. 1979;6:183–188. [Google Scholar]
- 9.De Bont JA, Primrose SB, Collins MD, Jones D. Chemical studies on some bacteria which utilize gaseous unsaturated hydrocarbons. J Gen Microbiol. 1980;117:97–102. [Google Scholar]
- 10.Donahue NM, Prinn RG. Nonmethane hydrocarbon chemistry in the remote marine boundary layer. J Geophys Res. 1990;95:18387–18411. [Google Scholar]
- 11.Dos Santos HF, Cury JC, do Carmo FL, Dos Santos AL, Tiedje J, van Elsas JD, Rosado AS, Peixoto RS. Mangrove bacterial diversity and the impact of oil contamination revealed by pyrosequencing: Bacterial proxies for oil pollution. PLoS One. 2011;6:e16943. doi: 10.1371/journal.pone.0016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosdike WL, Smith TJ, Dalton H. Adventitious reactions of alkene monooxygenase reveal common reaction pathways and component interactions among bacterial hydrocarbon oxygenases. FEBS J. 2005;272:2661–2669. doi: 10.1111/j.1742-4658.2005.04675.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda H, Ogawa T, Tanase S. Ethylene production by micro-organisms. Adv Microbiol Physiol. 1993;35:275–306. doi: 10.1016/s0065-2911(08)60101-0. [DOI] [PubMed] [Google Scholar]
- 14.Furuhashi K, Taoka A, Uchida S, Karube L, Suzuki S. Production of 1,2-epoxyalkanes from 1-alkenes by Nocardia corallina B-276. Eur J Appl Microbiol Biotechnol. 1981;12:39–45. [Google Scholar]
- 15.Habets-Crützen AQH, de Bont JA. Inactivation of alkane oxidation by epoxides in alkene- and alkane-grown bacteria. Appl Microbiol Biotechnol. 1985;22:428–433. [Google Scholar]
- 16.Hamamura N, Yeager CM, Arp DJ. Two distinct monooxygenases for alkane oxidation in Nocardioides sp. strain CF8. Appl Environ Microbiol. 2001;67:4992–4998. doi: 10.1128/AEM.67.11.4992-4998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamstra RS, Murris MR, Tramper J. The influence of immobilization and reduced water activity on gaseous-alkene oxidation by Mycobacterium PY1 and Xanthobacter PY2 in a gas-solid bioreactor. Biotechnol Bioeng. 1987;29:884–891. doi: 10.1002/bit.260290710. [DOI] [PubMed] [Google Scholar]
- 18.Henckel T, Jäckel U, Schnell S, Conrad R. Molecular analyses of novel methanotrophic communities in forest soil that oxidize atmospheric methane. Appl Environ Microbiol. 2000;66:1801–1808. doi: 10.1128/aem.66.5.1801-1808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmes AJ, Costello AM, Lidstrom ME, Murrell JC. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett. 1995;132:203–208. doi: 10.1016/0378-1097(95)00311-r. [DOI] [PubMed] [Google Scholar]
- 20.Holmes AJ, Roslev P, McDonald IR, Iversen N, Henriksen K, Murrell JC. Characterization of methanotrophic bacterial populations in soils showing atmospheric methane uptake. Appl Environ Microbiol. 1999;65:3312–3318. doi: 10.1128/aem.65.8.3312-3318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes AJ, Coleman NV. Evolutionary ecology and multidisciplinary approaches to prospecting for monooxygenases as biocatalysts. Antonie van Leeuwenhoek. 2008;94:75–84. doi: 10.1007/s10482-008-9227-1. [DOI] [PubMed] [Google Scholar]
- 22.Jin YO, Mattes TE. Adaptation of aerobic, ethene-assimilating Mycobacterium strains to vinyl chloride as a growth substrate. Environ Sci Technol. 2008;42:4784–4789. doi: 10.1021/es8000536. [DOI] [PubMed] [Google Scholar]
- 23.Krum JG, Ensign SA. Heterologous expression of bacterial Epoxyalkane: Coenzyme M transferase and inducible coenzyme M biosynthesis in Xanthobacter strain Py2 and Rhodococcus rhodochrous B276. J Bacteriol. 2000;182:2629–2634. doi: 10.1128/jb.182.9.2629-2634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau E, Ahmad A, Steudler PA, Cavanaugh CM. Molecular characterization of methanotrophic communities in forest soils that consume atmospheric methane. FEMS Microbiol Ecol. 2007;60:490–500. doi: 10.1111/j.1574-6941.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 25.Leahy JG, Batchelor PJ, Morcomb SM. Evolution of the soluble diiron monooxygenases. FEMS Microbiol Rev. 2003;27:449–479. doi: 10.1016/S0168-6445(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 26.Lucena T, Pascual J, Garay E, Arahal DR, Macián MC, Pujalte MJ. Haliea mediterranea sp. nov., a marine gammaproteobacterium. Int J Syst Evol Microbiol. 2010;60:1844–1848. doi: 10.1099/ijs.0.017061-0. [DOI] [PubMed] [Google Scholar]
- 27.Maillard P, Thepenier C, Gudin C. Ethylene production by photosynthetic bacteria, cyanobacteria, and algae. J Mar Biotechnol. 1993;1:97–100. [Google Scholar]
- 28.Mattes TE, Coleman NV, Spain JC, Gossett JM. Physiological and molecular genetic analyses of vinyl chloride and ethene biodegradation in Nocardioides sp. strain JS614. Arch Microbiol. 2005;183:95–106. doi: 10.1007/s00203-004-0749-2. [DOI] [PubMed] [Google Scholar]
- 29.Miguez CB, Bourque D, Sealy JA, Greer CW, Groleau D. Detection and isolation of methanotrophic bacteria possessing soluble methane monooxygenase (sMMO) genes using the polymerase chain reaction (PCR) Microb Ecol. 1997;33:21–31. doi: 10.1007/s002489900004. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Hoaki T, Hanada S, Maruyama A, Kamagata Y, Fuse H. Soluble and particulate methane monooxygenase gene clusters in the marine methanotroph Methylomicrobium sp. strain NI. 2007. FEMS Microbiol. Lett. 2007;277:157–164. doi: 10.1111/j.1574-6968.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 31.Norton JM, Alzerreca JJ, Suwa Y, Klotz MG. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol. 2002;177:139–149. doi: 10.1007/s00203-001-0369-z. [DOI] [PubMed] [Google Scholar]
- 32.Plettner I, Steinke M, Malin G. Ethene (ethylene) production in the marine macroalga Ulva(Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): Effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environ. 2005;28:1136–1145. [Google Scholar]
- 33.Redmond MC, Valentine DL, Sessions AL. Identification of novel methane-, ethane-, and propane-oxidizing bacteria at marine hydrocarbon seeps by stable isotope probing. Appl Environ Microbiol. 2010;76:6412–6422. doi: 10.1128/AEM.00271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riemer DD, Milne PJ, Zika RG, Pos WH. Photoproduction of nonmethane hydrocarbons (NMHCs) in seawater. Mar Chem. 2000;71:177–198. [Google Scholar]
- 35.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd ed. Cold Spring Harbor Laboratory Press; NY: 2001. [Google Scholar]
- 36.Seifert R, Delling N, Richnow H, Kempe S, Hefter J, Michaelis W. Ethylene and methane in the upper water column of the subtropical Atlantic. Biogeochemistry. 1999;44:73–91. [Google Scholar]
- 37.Shennan JL. Utilisation of C2–C4gaseous hydrocarbons and isoprene by microorganisms. J Chem Technol Biotechnol. 2006;81:237–256. [Google Scholar]
- 38.Shigematsu T, Hanada S, Eguchi M, Kamagata Y, Kanagawa T, Kurane R. Soluble methane monooxygenase gene clusters from trichloroethylene-degrading Methylomonas sp. strains and detection of methanotrophs during in situ bioremediation. Appl Environ Microbiol. 1999;65:5198–5206. doi: 10.1128/aem.65.12.5198-5206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith TJ, Lloyd JS, Gallagher SC, Fosdike WL, Murrell JC, Dalton H. Heterologous expression of alkene mono-oxygenase from Rhodococcus rhodochrous B-276. Eur J Biochem. 1999;260:446–452. doi: 10.1046/j.1432-1327.1999.00179.x. [DOI] [PubMed] [Google Scholar]
- 40.Tavormina PL, Orphan VJ, Kalyuzhnaya MG, Jetten MSM, Klotz MG. A novel family of functional operons encoding methane/ammonia monooxygenase-related proteins in gammaproteobacterial methanotrophs. Environ Microbiol Rep. 2011;3:91–100. doi: 10.1111/j.1758-2229.2010.00192.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsunogai U, Yoshida N, Gamo T. Carbon isotopic compositions of C2–C5hydrocarbons and methyl chloride in urban, coastal, and maritime atmospheres over the western North Pacific. J Geophys Res. 1999;104:16033–16039. [Google Scholar]
- 42.Tsunogai U, Yoshida N, Ishibashi J, Gamo T. Carbon isotopic distribution of methane in deep-sea hydrothermal plume, Myojin Knoll Caldera, Izu-Bonin arc: Implications for microbial methane oxidation in the oceans and applications to heat flux estimation. Geochim. Cosmochim Acta. 2000;64:2439–2452. [Google Scholar]
- 43.Urios L, Intertaglia L, Lesongeur F, Lebaron P. Haliea salexigens gen. nov., sp. nov., a member of the Gammaproteobacteria from the Mediterranean Sea. Int J Syst Evol Microbiol. 2008;58:1233–1237. doi: 10.1099/ijs.0.65470-0. [DOI] [PubMed] [Google Scholar]
- 44.Urios L, Intertaglia L, Lesongeur F, Lebaron P. Haliea rubra sp. nov., a member of the Gammaproteobacteria from the Mediterranean Sea. Int J Syst Evol Microbiol. 2009;59:1188–1192. doi: 10.1099/ijs.0.002220-0. [DOI] [PubMed] [Google Scholar]
- 45.Van Ginkel CG, de Bont JA. Isolation and characterization of alkene-utilizing Xanthobacter spp. Arch Microbiol. 1986;145:403–407. [Google Scholar]
- 46.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Inubushi K. Ethylene oxidation, atmospheric methane consumption, and ammonium oxidation in temperate volcanic forest soils. Biol. Fertil Soils. 2009;45:265–271. [Google Scholar]
- 48.Yordanova ZP, Iakimova ET, Cristescu SM, Harren FJ, Kapchina-Toteva VM, Woltering EJ. Involvement of ethylene and nitric oxide in cell death in mastoparan-treated unicellular alga Chlamydomonas reinhardtii. Cell Biol Int. 2010;34:301–308. doi: 10.1042/CBI20090138. [DOI] [PubMed] [Google Scholar]
- 49.Zhou NY, Jenkins A, Chan CK, Chion Kwo, Leak DJ. The alkene monooxygenase from Xanthobacter strain Py2 is closely related to aromatic monooxygenases and catalyzes aromatic mono-hydroxylation of benzene, toluene, and phenol. Appl Environ Microbiol. 1999;65:1589–1595. doi: 10.1128/aem.65.4.1589-1595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]