Abstract
Rationale
Cannabinoid antagonists purportedly have greater effects in reducing the intake of highly palatable food compared to less palatable food. However, this assertion is based on free-feeding studies in which the amount of palatable food eaten under baseline conditions is often confounded with other variables, such as unequal access to both food options and differences in qualitative features of the foods.
Objective
We attempted to reduce these confounds by using a model of choice that programmed the delivery rates of sucrose and carrot-flavored pellets.
Methods
Lever-pressing of ten lean (Fa/Fa or Fa/fa) and ten obese (fa/fa) Zucker rats was placed under three conditions in which programmed ratios for food pellets on two levers were 5:1, 1:1, and 1:5. In Phase 1, responses on the two levers produced one type of pellet (sucrose or carrot); in Phase 2, responses on one lever produced sucrose pellets and on the other lever produced carrot pellets. After responses stabilized under each food ratio, acute doses of rimonabant (0, 3, and 10 mg/kg) were administered before experimental sessions. The number of reinforcers and responses earned per session under each ratio and from each lever was compared.
Results and Conclusions
Rimonabant reduced reinforcers in 1:5 and 5:1 food ratios in Phase 1, and across all ratios in Phase 2. Rimonabant reduced sucrose and carrot-flavored pellet consumption similarly; rimonabant did not affect bias toward sucrose, but increased sensitivity to amount differences in lean rats. This suggests that relative amount of food, not palatability, may be an important behavioral mechanism in the effects of rimonabant.
Keywords: cannabinoids, concurrent schedules of reinforcement, obesity, rimonabant, Zucker rats (fa/fa)
Introduction
Endocannabinoid drugs that block the CB1 receptor are well characterized in their ability to reduce free-food intake (Arnone et al. 1997; Carai et al. 2006; Colombo et al. 1998; Gessa et al. 2006; Mathes et al. 2008; Simiand et al. 1998; Thornton-Jones et al. 2005). Rimonabant is perhaps the most researched of the CB1 antagonists in terms of this effect. One research trend specifically suggests that rimonabant is more effective at reducing intake of foods that are more palatable, that is, foods with higher fat or sugar concentration compared to those with lower fat or sugar concentration (Arnone et al. 1997; Carai et al. 2006; Mathes et al. 2008; Simiand et al. 1998).
Despite the consistency of rimonabant’s effects on palatability, there are a number of methodological limitations in these studies (Arnone et al. 1997; Carai et al. 2006; Mathes et al. 2008; Simiand et al. 1998) that prevent this trend in the literature from being conclusive. First, in each of these studies, standard laboratory pellets (considered the less palatable alternative, based on macronutrient content and lower intake levels) were typically available outside of experimental sessions; the more palatable alternative was available only during experimental sessions. Therefore, the duration of availability of both food alternatives was not held constant, which could reduce motivation for the food type that was available outside of the session (e.g., Houston & McNamara, 1989; Hursh 1980). Second, the differences in experience with each food type during baseline and vehicle conditions were not held constant. Higher intake during baseline or vehicle conditions was observed with the more palatable alternative; this could confound the interpretation of the drug effect as well, because more contact with a food reinforcer can alter its reinforcing efficacy (Nevin et al. 1983). Another limit to free-feeding studies includes asymmetry in the qualitative features of the food options. For example, there were differences in textures, density, and viscosity (e.g., hard chow as the less palatable alternative vs. a liquid or a fluffy whip as the more palatable alternative). These features may also affect consumption and possibly interact with drug effects.
One well-established technique that could control for these limitations is an operant choice procedure in which food reinforcers that are identical, except for macronutrient content, are arranged on two levers. On each lever, a food pellet is delivered after the first response after a variable interval (VI) of time passes. A VI 20-s schedule, for example, would arrange delivery of a reinforcer when the first response is made after an average of 20 seconds elapses. This type of schedule can limit baseline rates of food reinforcement by programming an average number of reinforcers per minute (for example, with the VI 20-s schedule, 3 reinforcers per minute are programmed), even for a highly palatable food that may draw more behavior toward it.
In this procedure, allocation of behavior to the two levers is a measure of choice, and many studies support that allocation of behavior generally matches the relative ratio of reinforcement gleaned from each lever. In other words, if three times as many food reinforcers are earned on one lever compared to another, then three times as many responses will occur on that lever compared to the other (e.g., Alsop and Elliffe 1988; Baum 1979; Herrnstein 1961). In some cases, a consistent bias may develop. This may happen, for example, when one food alternative is more palatable than another. Allocation of responses toward the more palatable food alternative can be observed and quantified across different ratios of reinforcer delivery; this allocation of responses toward a more palatable food exceeds allocation of what relative frequency predicts (Baum 1979; Baum 1974; Pierce and Epling 1983). For example, if there is a 1:1 arrangement of two different food reinforcers, and more than half of the behavior, say 75%, is allocated toward one of the pellet types, then bias or preference for that pellet type is inferred. Bias has been quantified in this manner across a number of studies, e.g., as preference for different types of food (Buckley and Rasmussen 2012; Matthews and Temple 1979; Miller 1976), as well as other qualitative features of reinforcement (Rasmussen and Newland 2009; Rasmussen and Newland 2008; Sumpter et al. 1995).
In the present study, the effects of rimonabant on palatable food choice were evaluated using this choice procedure. Concurrently available VI schedules were used to examine allocation of behavior, first, to two identical types of pellets, and second, to different types of identically sized and calorically similar food pellets that differed only in sucrose content. Because this procedure allows baseline relative amounts consumed to be limited, rimonabant-related effects on palatability can be asserted with greater confidence.
In addition, these effects were examined in lean and obese Zucker rats to determine differential sensitivity to rimonabant. Obese Zucker rats, which carry two recessive fa alleles, weigh more than lean Zucker rats (Fa/fa or Fa/Fa), and have higher free-food intake than lean Zucker rats (Yen, Shaw, & Yu, 1977; Zucker & Zucker, 1961). Obese Zucker rats have also demonstrated higher sensitivity to differences in food amounts compared to lean rats (Buckley and Rasmussen 2012). One of the mechanisms that contributes to these differences is defective leptin signaling (Haynes et al. 1997), which is also linked to higher endocannabinoid levels in the hypothalamus (Di Marzo et al. 2001) and more CB1 receptor binding sites in the limbic regions of the brain (Thanos et al. 2008). These differences likely contribute to altered sensitivity to cannabinoid compounds (Smith and Rasmussen 2010), including rimonabant (Rasmussen and Huskinson 2008; Thanos et al. 2008; Vickers et al. 2003). We hypothesized that obese Zucker rats may be more sensitive to the effects of rimonabant, as they have been shown to be more sensitive to cannabinoid drugs.
Materials and method
Subjects
Twenty experimentally-naïve male Zucker rats (lean, n=10; obese, n=10) were acquired from Harlan (Livermore, CA) when they were approximately 21 days of age. They were housed in individual cages in a climate-controlled room, with a 12-h light/dark cycle (light beginning at 6:00 a.m.). Rats were given ad libitum access to standard rat chow and water until they were 2 months old. After this point, access to food was restricted to a daily 2-hr free-feeding session immediately following experimental sessions; this established food as a reinforcer. This restriction procedure results in both lean and obese Zucker rats consuming 2.3–2.6% of their body weight in food (Rasmussen et al. 2010; Rasmussen and Huskinson 2008; Smith and Rasmussen 2010). This also allows deprivation levels to be held constant between lean and obese rats and prevents rapid weight gain, which is linked to health problems in the obese Zucker rat. During the course of the present study, body mass for lean Zucker rats ranged from 274–444 g; obese Zucker rats ranged from 430–698 g.
Drug design
Doses (0, 3, and10 mg/kg) of rimonabant (National Institute of Mental Health Chemical Synthesis and Drug Supply Program) were dissolved in a 1:1:18 ethanol (Sigma), Cremaphor (Sigma), and saline solution (1 ml/kg volume) and was administered via i.p. injection 1 hr prior to the start of experimental sessions.
Apparatus
Seven Coulbourn ® Habitest standard rat operant conditioning chambers were used for training and experimental sessions. Each chamber was placed inside a sound-attenuating cubicle, with a fan for air circulation and a speaker providing white noise to reduce extra-chamber noise. Each chamber contained two levers, one on either side of a feeding trough, with a cue light above each lever. When a reinforcer contingency was in place, the cue lights were illuminated. The depression of each lever controlled a pellet feeder on the outside of the chamber, which delivered single 45-mg sucrose (3.4 kcal/g; 94.5 % sucrose) or carrot-flavored (3.4 kcal/g; 3.1 % sucrose) pellets obtained from TestDiet®. A standard computer with Graphic State® software controlled reinforcer contingencies and collected data. The computer was situated in a room adjacent to and separate from the room containing operant conditioning chambers. All sessions were conducted in the morning at the same time (±15 min) from Monday to Friday.
To first verify quantify palatability, six Sprague-Dawley rats, using procedures similar to those that will be described, were trained to press two levers for access to sucrose vs. standard grain pellets, sucrose vs. carrot-flavored (grain) pellets, or standard grain vs. carrot-flavored pellets. Allocation of behavior was quantified using the generalized matching equation (Baum 1974), which we have used previously to mathematically determine bias toward different food types (see Buckley and Rasmussen 2012). Sucrose was found to have the highest bias values, followed by standard grain pellets, and then carrot-flavored pellets. The heightened bias toward sucrose, in addition to the differences in sucrose concentration (95% vs. 3%), support what other studies have found in terms of sucrose being a more palatable reinforcer compared to rat chow (Koch et al. 2000; Mathes et al. 2008; Simiand et al. 1998) Based on these results, sucrose pellets were used as the more palatable food pellet and carrot-flavored grain pellets as the least palatable. In addition, both carrot-flavored pellets and sucrose pellets were distinguishable (as measured by bias values) from grain-based pellets, so pellet novelty (in terms of similarity to food given outside the session) also can be controlled.
Choice Procedure
Training
Rats were trained to press left and right levers by randomly assigning them to one of two conditions: For half of the rats in each group, sucrose was available from both levers (SUC/SUC); for the other half of rats in each group, carrot-flavored pellets were available from both levers (CAR/CAR). Rats were trained in a similar procedure to that described in Buckley and Rasmussen (2012). Each rat was placed into a chamber and a continuous schedule of reinforcement was programmed on each lever, concurrently, for 1 hr, such that each lever-press on either lever would result in delivery of a pellet. After a rat had earned more than 70 reinforcers on one lever, lever-press training was considered acquired for that lever, and the lever was placed on extinction (signaled by no cue light illumination; no pellets were delivered) for subsequent sessions, until the lever-press training (70 responses) occurred for the second lever. If neither lever had been trained after 5 sessions, hand shaping was used to train responding to both levers. Experimental sessions began after a rat was trained to press both levers.
Phase 1: Same Reinforcers (CAR/CAR or SUC/SUC)
The purpose of this phase was to examine behavioral allocation to different reinforcement ratios when the pellet types were the same. This allowed us to determine any bias toward a lever and to examine rimonabant effects that could be explained by relative amount before introducing a second pellet type (in Phase 2).
Concurrent variable-interval variable-interval (conc VI VI) schedules were arranged using the same pellets on which each rat was trained, with one VI schedule programmed on each lever. We used the procedure described in Buckley and Rasmussen (2012), such that reinforcers were programmed on two levers (i.e., left vs. right) using three different conc VI VI schedules of reinforcement: conc VI 12-s VI 60-s, conc VI 20-s VI 20-s, and conc VI 60-s VI 12-s. These schedules yielded programmed reinforcer ratios that were 5:1, 1:1, and 1:5, respectively (for sake of simplicity, we will refer to each schedule by the programmed ratio of reinforcement). Each VI timer for each reinforcer ran independently of the other. A 3-sec changeover delay was inserted when a rat switched from one lever to the other; this ensured switching from one schedule to the other was not adventitiously reinforced. Sessions lasted for 30 min. Each schedule was in effect until stable responding was established. Stability was defined as the last three consecutive sessions that did not show an increasing or decreasing trend in ratios of responses to each lever, and the ratios did not vary by more than 10% from the mean of the last three sessions. In addition, there could be no increasing or decreasing trend in overall response rate.
Following stability of responding under each schedule, rats were injected i.p. with either 0, 3, or 10 mg/kg of rimonabant one hour prior to the experimental session. Each rat received each dose on each schedule in increasing order. After the highest dose was administered, doses were repeated in a non-systematic order such that each dose was administered three times for each schedule in a counterbalanced fashion. At least three days separated administration of doses of rimonabant to ensure there were no drug effects from previous sessions and to minimize tolerance.
Phase 2: Different Reinforcers (SUC/CAR or CAR/SUC)
After the rimonabant dose-response determination was complete for Phase 1, the novel type of food pellet (e.g., sucrose if the rat had CAR/CAR in Phase 1 and vice versa) replaced food from one of the feeders, such that one type of food pellet was consistently available from each lever (e.g., sucrose on the left lever and carrot on the right). Location of the pellet (left vs. right lever) was counterbalanced within groups. Then concurrent schedule sessions and drug injections were conducted in a manner identical to Phase 1, except the type of pellets available on each lever was different.
Data Analysis
Data from the last three stable sessions of each concurrent schedule and for each dose of rimonabant were used in the analysis. The mean reinforcers and responses obtained on each lever for Phases 1 and 2 were compared.
Phase 1: Same Reinforcers
Total reinforcers and responses obtained per session were recorded for analysis. Mean reinforcers and responses earned per session following vehicle administration were compared using a three-way mixed ANOVA, with phenotype (lean vs. obese) and pellet type (SUC/SUC vs. CAR/CAR) as between-subjects factors, and pellet ratio (5:1 vs. 1:1 vs. 1:5) as a within-subjects factor. In addition, mean total reinforcers and responses earned on each lever within sessions were compared using a mixed ANOVA, with phenotype and pellet type as between-subjects factors, and VI component (VI 12-s vs. VI 60-s vs. VI 20-s) and position (left vs. right) as within-subjects factors.
Drug effects also were analyzed separately for lean and obese rats, as behavioral differences between the phenotypes following vehicle administration (and baseline) were found. A mixed ANOVA was used, with pellet type as a between-subjects factor, and dose of rimonabant and pellet ratio (5:1 vs. 1:1 vs. 1:5) as within-subjects factors. In addition, because of differences between reinforcers and responses under each concurrent schedule, two-way mixed ANOVAs were completed for each concurrent schedule separately, with pellet type as a between-subjects factor, and dose of rimonabant as a repeated-measured factor, to measure the effects of rimonabant on reinforcers earned under each concurrent schedule. Effects of rimonabant on reinforcers and responses under each of the different VI components (VI 12-s vs. VI 60-s vs. VI 20-s) were also evaluated separately for lean and obese rats using a mixed ANOVA with pellet type as a between-subjects factor, and dose of rimonabant, VI component, and position as within-subjects factors, and followed up with two-way mixed ANOVAs for each component with pellet group and dose of rimonabant as factors.
For all ANOVAs, Mauchly’s W was computed to assess violations of the assumption of sphericity. Where Mauchly’s W was significant, the Greenhouse-Geisser correction was applied to the degrees of freedom if epsilon was less than 0.75, and the Huynh-Feldt correction was applied if epsilon was greater than or equal to 0.75.
Phase 2: Different reinforcers
Mean total number of reinforcers and reinforcers obtained were analyzed similarly to Phase 1, with the following changes: in analyses of each pellet ratio, pellet type was no longer a between-subjects factor, as all rats had access to both SUC and CAR. In analyses of reinforcers from individual VI components (VI 12-s vs. VI 60-s vs. VI 20-s), pellet type was included as a within-subjects factor. Analyses of drug effects on reinforcers obtained were similar to Phase 1, except that pellet type was excluded as a between-subjects factor in pellet ratio analyses, and pellet type was added as a within-subjects factor to analyses of reinforcers from individual lever components.
Matching analysis
To determine rimonabant’s effects on bias that comes from food palatability, the generalized matching equation (Baum 1974; Buckley and Rasmussen 2012; Rasmussen and Newland 2008) was fit to the data from responses to the left and right levers (B1 and B2) and reinforcers earned on the left and right levers (r1 and r2):
The equation illustrates that the ratio of responses of the left to right levers is a function of the ratio of left to right reinforcers earned, with free parameters a and log k representing the slope (sensitivity to different densities of reinforcement) and the y-intercept of the line (bias), respectively. In perfect matching, in which response allocation depends completely on the ratio of reinforcement, a is equal to 1 and log k is equal to 0. Deviations from matching can be characterized with these free parameters. A value of a < 1 means that allocation of responses is less sensitive to the more extreme differences in VI schedules, i.e., fewer responses are allocated to the richer side and/or more responses to the leaner side, than would be predicted by perfect matching; a > 1 represents suggests hypersensitivity to these differences. Deviations from log k represent bias. In this study, we subtracted any bias from Phase 1 (in which the same food pellets were programmed), which may come from bias toward the left or right lever, from bias in Phase 2 to obtain a bias measure based solely on differences in the two food pellets, i.e., palatability.
Results
Phase 1. SUC/SUC and CAR/CAR
Pellet ratios
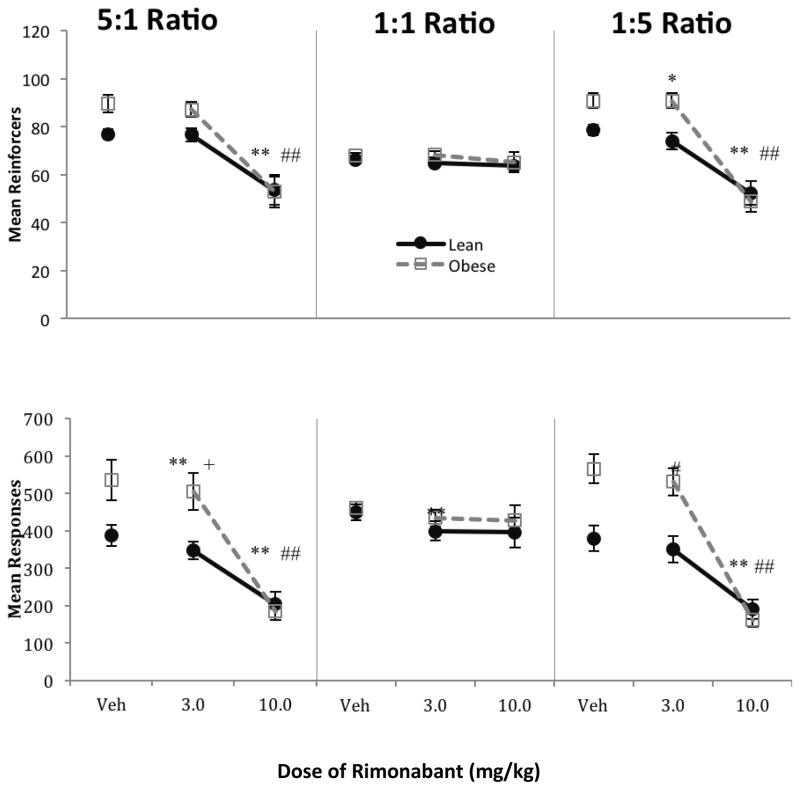
The top of Figure 1 shows that following vehicle administration, obese rats earned significantly more reinforcers than lean rats overall [F(1,16) = 10.83, p = 0.005, ηp2 = 0.40]. These patterns were similar for responses, represented in the bottom panels. Because response-related statistical information throughout the results section is highly redundant with the reinforcer data, they will not be presented in detail. Pellet type (carrot vs. sucrose) was not found to be significant in any of the reinforcer or response analyses in Phase 1, so it will not be discussed further.
Fig. 1.
Mean total (left + right) reinforcers (top) and responses (bottom) per session as a function of dose of rimonabant under three food reinforcement ratios 5:1 (left), 1:1 (center), and 1:5 (right) in Phase 1. Lean rats are shown in closed circles, obese rats in open squares. Error bars = 1 SEM. Post hoc contrasts shown in Table 1a. Symbols represent post hoc contrasts comparing dose to vehicle.
* p < 0.05, ** p < 0.01
Separate analyses of drug effects on reinforcers conducted for each group confirmed a main effect of rimonabant for lean rats [F(1.06,8.51) = 15.36, p = 0.004, ηp2 = 0.66] and obese rats [F(1.14,9.15) = 74.56, p < 0.001, ηp2 = 0.90] Two-way ANOVAS conducted for both groups for each schedule revealed a main effect of drug for the 5:1 schedule (lean: [F(1.03,8.21) = 11.85, p = 0.008, ηp2 = 0.60]; obese: [F(1.05,8.37) = 19.73, p = 0.002, ηp2 = 0.71]) and 1:5 schedules (lean: [F(1.07,8.56) = 10.82, p = 0.009, ηp2 = 0.57]; obese: [F(2,16) = 73.70, p < 0.001, ηp2 = 0.90]). Table 1A shows post hoc contrasts of specific effects of dose of rimonabant compared to vehicle for the 5:1 and 1:5 ratio schedules. There was no effect of drug under the 1:1 schedule with either group (p > 0.4). These statistical patterns were similar for responses (lower panel) for both groups.
Table 1.
Post hoc contrasts for Phase 1 ANOVAs conducted separately for each concurrent schedule (a) or component (b) for lean and obese rats, with pellet type (CAR/CAR vs. SUC/SUC) as a between-subjects factor, and dose of rimonabant as a within-subjects factor. Significant contrasts are shown, which correspond to Figure 1 (a) and Figure 2 (b). Pellet type is omitted, as it was not found to be a significant factor in any analysis.
| (a) Fig 1. Reinforcers by schedule | ||||||
|---|---|---|---|---|---|---|
| Programmed ratio | Dose compared to vehicle | F | df | p | ηp2 | |
|
|
||||||
| Lean | 5:1 schedule | 10.0 mg/kg | 12.7 | (1,8) | 0.008 | 0.61 |
|
| ||||||
| 1:5 schedule | 3.0 mg/kg | 5.88 | (1,8) | 0.042 | 0.42 | |
| 10.0 mg/kg | 16.34 | (1,8) | 0.004 | 0.67 | ||
| Obese | 5:1 schedule | 10.0 mg/kg | 19.23 | (1,8) | 0.002 | 0.71 |
|
| ||||||
| 1:5 schedule | 10.0 mg/kg | 78.53 | (1,8) | < 0.001 | 0.91 | |
| (b) Fig 2. Reinforcers by component | ||||||
|---|---|---|---|---|---|---|
| Programmed component | Dose compared to vehicle | F | df | p | ηp2 | |
|
|
||||||
| Lean | VI 12-s | 10.0 mg/kg | 13.84 | (1,8) | 0.006 | 0.63 |
|
| ||||||
| VI 60-s | 10.0 mg/kg | 17.56 | (1,8) | 0.003 | 0.69 | |
| Obese | VI 12-s | 10.0 mg/kg | 75.2 | (1,8) | < 0.001 | 0.9 |
|
| ||||||
| VI 60-s | 10.0 mg/kg | 71.27 | (1,8) | < 0.001 | 0.9 | |
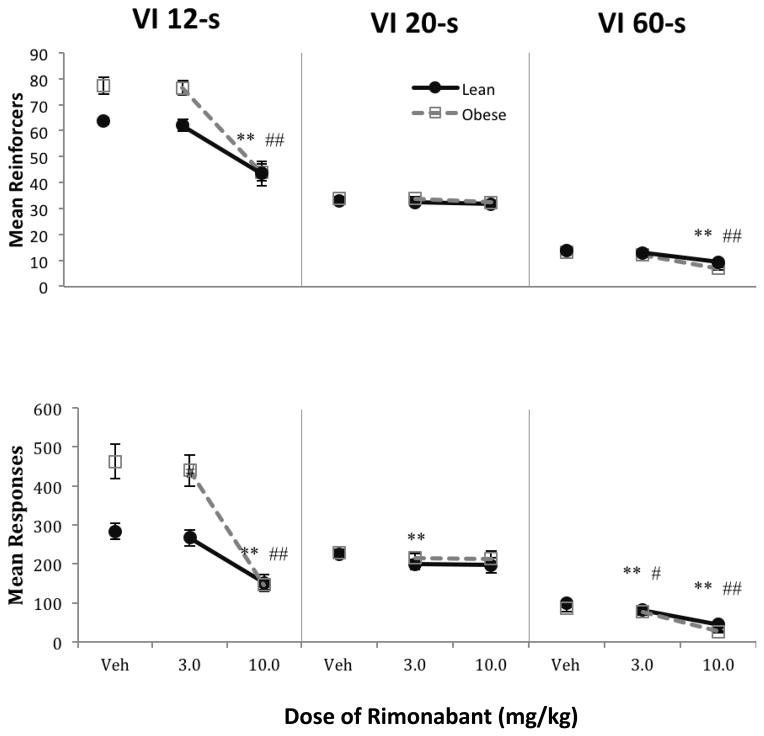
VI components
The top of Figure 2 shows mean reinforcers under the three VI components. Following vehicle administration, obese rats earned more reinforcers than lean rats [F(1,16) = 10.83, p = 0.005, ηp2 = 0.40]. There was also a main effect of VI component [F(1.13,18.08) = 673.02, p < 0.001, ηp2 = 0.99], such that more reinforcers were earned on the VI 12-s components compared to the VI 20-s components [F(1,16) = 459.04, p < 0.001, ηp2 = 0.97], and more on the VI 20-s components compared to VI 60-s components [F(1,16) = 1004.77, p < 0.001, ηp2 = 0.98]. There was a component X phenotype interaction [F(1.13,18.08) = 12.58, p = 0.002, ηp2 = 0.44], in that obese rats earned more reinforcers than lean rats under the VI 12-s components compared to both the VI 20-s components [F(1,16) = 13.85, p = 0.002, ηp2 = 0.46] and the VI 60-s components [F(1,16) = 12.74, p = 0.003, ηp2 = 0.44]. Separate analyses conducted on each group independently confirmed these results.
Fig. 2.
Mean number of reinforcers (top) and responses (bottom) per session in each VI component as a function of dose of rimonabant during Phase 1. Lean rats are shown in black circles, obese rats in gray squares. Error bars = 1 SEM. Post hoc contrasts shown in Table 1b. Symbols represent post hoc contrasts comparing dose to vehicle.
Lean: ** p < 0.01
Obese: ## p < 0.01
Two-way ANOVAs conducted for lean rats revealed that rimonabant reduced reinforcers compared to vehicle for both VI 12-s [F(1.06,8.46) = 10.82, p = 0.010, ηp2 = 0.57] and VI 60-s components [F(1.18,9.45) = 14.78, p = 0.003, ηp2 = 0.65]; the same was found for obese rats (VI 12-s [F(1.14,9.12) = 79.80, p < 0.001, ηp2 = 0.91] and VI 60-s [F(2,16) = 61.62, p < 0.001, ηp2 = 0.89]). Post hoc contrasts comparing each dose of rimonabant to vehicle under VI 12-s and VI 60-s components are shown in Table 1b. The response data in the lower panels resulted in statistical outcomes that were similar to the analyses from the reinforcer data. There were no effects of drug on reinforcers obtained under VI 20-s components for lean or obese rats (ps > 0.41). Position effects (left vs. right) were not found to be significant in any analysis.
Phase 2: SUC/CAR and CAR/SUC
Between Phase 1 and Phase 2, three obese rats were euthanized for various health-related reasons. Of the seven remaining, one rat (F3) only completed two of the three ratios of Phase 2, so its data were excluded from analyses due to assumptions of repeated measures statistical analyses (lean n=10, obese n=6).
Pellet Ratios
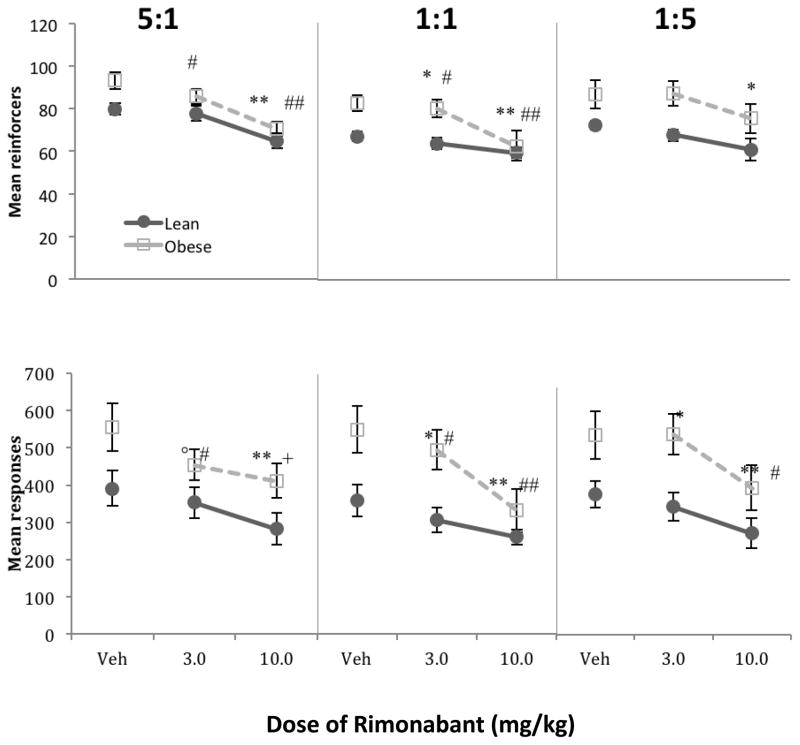
For ease of interpretation, sucrose will be represented as the left lever (or numerator) in the ratios. The top of Figure 3 shows that, following vehicle administration, obese rats earned significantly more reinforcers than lean rats across all ratios [F(1,14) = 13.90, p = 0.002, ηp2 = 0.50]. Analysis of responses, represented in the lower panels, resulted in similar statistical outcomes.
Fig. 3.
Mean total reinforcers (top) and responses (bottom) per session in Phase 2 for lean and obese rats, for each ratio (SUC:CAR). Lean rats are shown in black circles, obese rats are shown in gray squares. Error bars = 1 SEM. Post hoc contrasts shown in Table 2a. Symbols represent post hoc contrasts comparing dose to vehicle.
Lean: * p < 0.05, ** p < 0.01
Obese: # p < 0.05, ## p < 0.01
Separate analyses for each group show that for lean rats, there was a main effect of drug [F(1.28,11.52) = 17.52, p < 0.001, ηp2 = 0.66] and reinforcer ratio [F(2,18) = 6.37, p = 0.008, ηp2 = 0.41] on reinforcers. More reinforcers were earned under the 5:1 (SUC-rich) schedule compared to the 1:1 schedule [F(1,9) = 25.61, p < 0.001, ηp2 = 0.74]. Separate repeated-measures ANOVAs for each schedule revealed main effects of rimonabant under all three ratio schedules for lean rats [F(1.07,9.61) = 12.98, p = 0.005, ηp2 = 0.59; F(2,18) = 9.70, p < 0.001, ηp2 = 0.52; and F(2,18) = 5.24, p = 0.016, ηp2 = 0.37, respectively]. For obese rats, there was a main effect of drug overall [F(2,10) = 26.96, p < 0.001, ηp2 = 0.84]. Separate repeated-measures ANOVAs for each concurrent schedule revealed main effects of drug under the 5:1 (SUC-rich) schedule [F(2,10) = 8.44, p = 0.007, ηp2 = 0.63] and the 1:1 schedule [F(2,10) = 19.93, p < 0.001, ηp2 = 0.80], but not the 1:5 (SUC-lean) schedule (p = 0.13). Post hoc contrasts comparing each dose of rimonabant to vehicle for both lean and obese rats under each ratio schedule are shown in Table 2a. Analysis of response data (lower panels) resulted in similar statistical outcomes.
Table 2.
Post hoc contrasts for Phase 2 ANOVAs conducted separately for each concurrent schedule (a) or component (b) for lean and obese rats with pellet type (CAR vs. SUC), and dose of rimonabant as within-subjects factors. Significant contrasts are shown, which correspond to Figure 3 (a) and Figure 4 (b). Pellet type is omitted for clarity.
| (a) Fig 3. Reinforcers by schedule | ||||||
|---|---|---|---|---|---|---|
| Programmed ratio | Dose compared to vehicle | F | df | p | ηp2 | |
|
|
||||||
| Lean | 5:1 schedule | 10.0 mg/kg | 30.77 | (1,9) | < 0.001 | 0.77 |
|
| ||||||
| 1:1 schedule | 3.0 mg/kg | 5.3 | (1,9) | 0.047 | 0.37 | |
| 10.0 mg/kg | 15.93 | (1,9) | 0.003 | 0.64 | ||
|
| ||||||
| 1:5 schedule | 3.0 mg/kg | 4.52 | (1,9) | 0.062 | 0.33 | |
| 10.0 mg/kg | 6.49 | (1,9) | 0.031 | 0.42 | ||
| Obese | 5:1 schedule | 3.0 mg/kg | 10.05 | (1,5) | 0.025 | 0.67 |
| 10.0 mg/kg | 8.91 | (1,5) | 0.031 | 0.64 | ||
|
| ||||||
| 1:1 schedule | 3.0 mg/kg | 7.99 | (1,5) | 0.037 | 0.62 | |
| 10.0 mg/kg | 22.5 | (1,5) | 0.005 | 0.82 | ||
| (b) Fig 4. Reinforcers by component | ||||||
|---|---|---|---|---|---|---|
| Programmed component | Dose compared to vehicle | F | df | p | ηp2 | |
|
|
||||||
| Lean | VI 12-s | 10.0 mg/kg | 23.59 | (1,9) | < 0.001 | 0.72 |
|
| ||||||
| VI 20-s | 3.0 mg/kg | 5.3 | (1,9) | 0.047 | 0.37 | |
| 10.0 mg/kg | 15.93 | (1,9) | 0.003 | 0.64 | ||
|
| ||||||
| VI 60-s | 3.0 mg/kg | 6.58 | (1,9) | 0.03 | 0.42 | |
| 10.0 mg/kg | 11.53 | (1,9) | 0.008 | 0.56 | ||
| Obese | VI 12-s | 10.0 mg/kg | 6.37 | (1,5) | 0.053 | 0.56 |
|
| ||||||
| VI 20-s | 3.0 mg/kg | 7.99 | (1,5) | 0.037 | 0.62 | |
| 10.0 mg/kg | 22.5 | (1,5) | 0.005 | 0.82 | ||
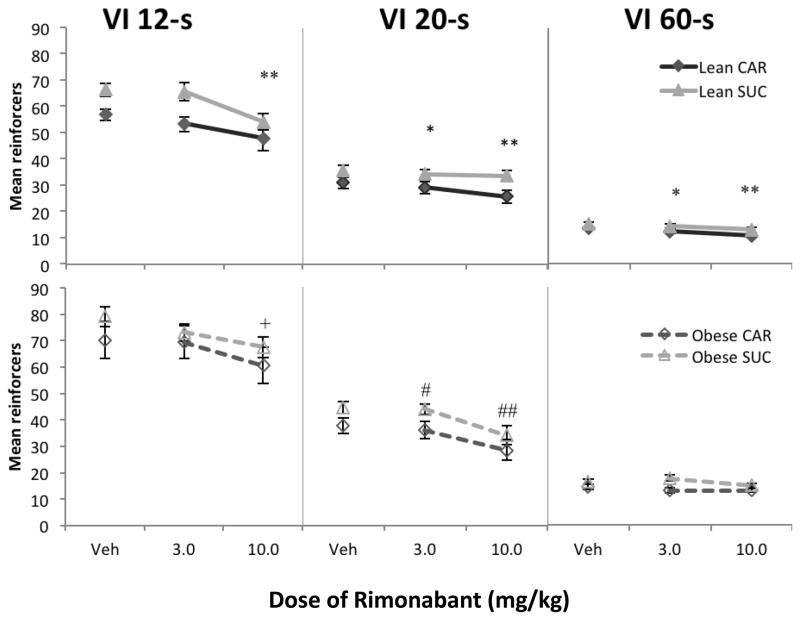
VI components
Figure 4 shows mean reinforcers under each VI component on the sucrose vs. carrot levers as a function of dose of rimonabant. Following vehicle administration, obese rats (bottom) earned more reinforcers on each component compared to lean rats (top) [F(1,14) = 13.90, p = 0.002, ηp2 = 0.50]. There was a main effect of VI component [F(1.28,17.9) = 526.52, p < 0.001, ηp2 = 0.97]; more reinforcers were earned under the VI 12-s components compared to VI 20-s [F(1,14) = 303.57, p < 0.001, ηp2 = 0.96] and more reinforcers were earned on the VI 20-s components compared to VI 60-s [F(1,14) = 646.24, p < 0.001, ηp2 = 0.98]. There was a phenotype X schedule component interaction [F(1.28,17.9) = 6.59, p = 0.014, ηp2 = 0.32], such that obese rats earned more reinforcers in the VI 12-s [F(1,14) = 8.40, p = 0.012, ηp2 = 0.38] and VI 20-s components [F(1,14) = 14.39, p = 0.002, ηp2 = 0.51] compared to VI 60-s. Across all rats, more sucrose reinforcers were earned compared to carrot reinforcers [F(1,14) = 9.42, p = 0.008, ηp2 = 0.40]. In addition, there was a pellet X schedule component interaction [F(2,28) = 3.49, p = 0.045, ηp2 = 0.20], such that more sucrose reinforcers were earned on the VI 12-s component than carrot-flavored reinforcers delivered on the VI 12-s component; the difference in the amount of sucrose and carrot reinforcers earned was larger in the VI 12-s compared to the VI 60-s component [F(1,14) = 6.49, p = 0.023, ηp2 = 0.32].
Fig. 4.
Mean reinforcers for lean (a) and obese (b) rats in Phase 2 as a function of dose of rimonabant, for each VI component. Data from the CAR lever are shown in black diamonds, and data from the SUC lever are shown in gray triangles. Lean rats = closed symbols; obese rats = open symbols. Error bars = 1 SEM. Post hoc contrasts shown in Table 2b. Symbols represent post hoc contrasts comparing dose to vehicle.
Lean: * p < 0.05, ** p < 0.01
Obese: # p < 0.05, ## p < 0.01, + p = 0.053
Separate analyses of drug effects for both types of rats confirmed a main effect of rimonabant and schedule component for both groups. For lean rats only, more sucrose reinforcers were earned compared to carrot reinforcers overall [F(1,9) = 6.86, p = 0.028, ηp2 = 0.43]. Separate ANOVAs for each concurrent schedule component revealed main effects of rimonabant under the VI 12-s, VI 20-s, and VI 60-s components for lean rats [F(2,18) = 14.56, p < 0.001, ηp2 = 0.62; F(2,18) = 9.70, p < 0.001, ηp2 = 0.52; F(2,18) = 7.14, p = 0.005, ηp2 = 0.44, respectively] and under two of the three components for obese rats (VI 12-s [F(2,10) = 4.59, p = 0.038, ηp2 = 0.48] and VI 20-s [F(1.06,5.3) = 19.93, p = 0.006, ηp2 = 0.80]). Post hoc contrasts for these analyses are shown in Table 2b.
Generalized matching analysis
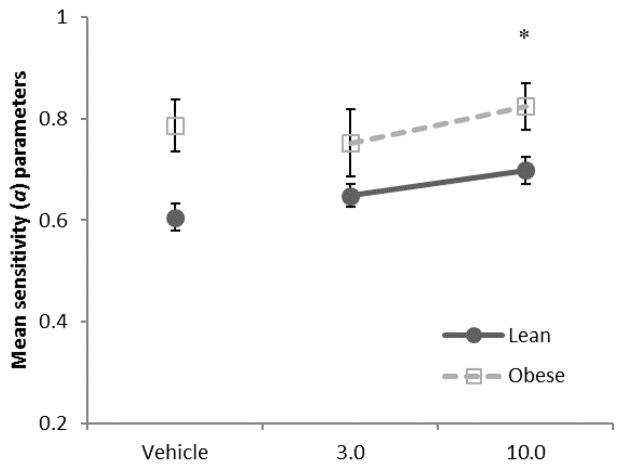
Figure 5 shows mean sensitivity (a) values from the generalized matching equation as a function of dose of rimonabant. Sensitivity values were significantly higher for obese rats than lean rats following vehicle administration [F(1,14) = 12.0, p = 0.004, ηp2 = 0.46]. Drug effects were analyzed separately for lean and obese rats, because there were vehicle differences between the two rat types. For lean rats, there was a main effect of dose of rimonabant [F(2,18) = 3.64, p = 0.047, ηp2 = 0.29]. For obese rats, however, dose of rimonabant was not significant (p = 0.15).
Fig. 5.
Mean sensitivity parameters in Phase 2 (SUC/CAR) as a function of dose of rimonabant. Lean rats are depicted by black circles, and obese rats are depicted by gray squares.
*p = 0.054, ηp2 = 0.35, post hoc contrasts compared to vehicle, for lean rats only
The mean bias (log k) values following vehicle administration were 0.14 (SEM = 0.021) for lean rats and 0.08 (SEM = 0.041) for obese rats. The intercept from the ANOVA regression was significant, suggesting an overall bias toward sucrose for both groups [F(1,14) = 1039.58, p < 0.001, ηp2 = 0.987], though the means were not significantly different between groups. There were no main effects of dose of rimonabant for lean (p = 0.17) or obese rats (p = 0.32) on bias values.
Across all generalized matching analyses, r-squared values ranged from 0.83 to 0.97 for lean rats, and from 0.92 to 0.99 for obese rats, which suggests the generalized matching equation fit the data well. No effects of phenotype, drug, or an interaction were found with r-squared values.
Discussion
Vehicle
The vehicle data from Figures 1 and 3 suggest that the three concurrent schedules that were used produced overall ranges of reinforcers and responses that were similar across all three schedules. When examining vehicle data from the individual schedule components (Figs 2 and 4), rats earned roughly five times more reinforcers and made roughly 5 times more responses on the VI-12-s component compared to the VI-60-s component; this is consistent with what matching would predict, given the programmed ratios of reinforcement. These data, then, suggest that experience with the pellets was similar across schedules and limited, in terms of programmed access to food, which was one aim of using the concurrent schedule procedure.
In Phase 1, when the same pellets were available, the vehicle data also show that obese rats earned more reinforcers and made more responses than lean rats; this was only the case, though, with schedules that had 5:1 and 1:5 ratios of reinforcement. Examination of behavior under the VI components shows that, compared to lean rats, obese rats obtained significantly more reinforcers from the VI 12-s component, which was the richest source of reinforcement. This increase may account for both the higher intake of reinforcers seen in the 5:1 and 1:5 ratio schedules, as well as the heightened sensitivity to different densities of reinforcement (compared to lean rats) that were demonstrated in the matching analysis. In Phase 2, when two types of pellets (SUC vs. CAR) were arranged on two levers, obese rats again earned more reinforcers and made more responses than lean rats overall. In addition, there were more reinforcers and responses earned on SUC levers overall, especially on the VI 12-s component.
Rimonabant
In Phase 1, rimonabant effects consistently appeared with both groups of rats in schedules with 1:5 or 5:1 programmed reinforcer ratios, however, no rimonabant effects were observed in the 1:1 reinforcer ratio (Fig. 1 and 2). Therefore, when two identical pellet types are available, the drug effects appeared to be related to relative quantity. When examining effects within the differing schedule components (Fig. 2), drug effects appeared to manifest similarly in lean and obese Zucker rats — the 10 mg/kg dose of rimonabant reduced reinforcers in the richer (VI 12-s) and leaner (VI 60-s) components similarly. However, partial-eta squared values (i.e., effect sizes), which represent the percent of variance accounted for by each dose compared to control, were larger for obese rats under the 10 mg/kg dose (see Table 1), suggesting that rimonabant reduced the number of reinforcers earned more for obese rats compared to lean rats.
In Phase 2, rimonabant dose dependently reduced he number of reinforcers earned across all ratios for all rats, except under the 1:5 ratio schedule (SUC-lean) for the obese rats, which may have been due to a floor effect. When examining VI components, rimonabant reduced sucrose and carrot-flavored pellets similarly. Reinforcers (whether sucrose or carrot) were reduced across all schedule components for lean rats; for obese rats, reinforcers were reduced for the VI 12-s and VI 20-s components. This suggests that rimonabant may interact with behavior maintained by different ratios of reinforcement when there are food alternatives.
The matching analysis provided several useful pieces of information. First, a bias toward sucrose was quantified; any bias that was found from Phase 1 was subtracted from the bias from Phase 2, thereby allowing a quantification of preference for sucrose (or what some may call palatability). Rimonabant, however, did not affect this bias. In addition, sensitivity values, which quantify behavioral sensitivity to relative amounts of food, did not change at any dose for obese rats. For lean rats, though, rimonabant dose-dependently increased sensitivity to relative food densities to a point at which they were within the range of the sensitivity values of the obese rats. This is the first demonstration of rimonabant-related effects on sensitivity to relative amount, and also the first demonstration of differential effects of rimonabant on matching in lean and obese Zucker rats. It is possible that obese rats’ behavior was insensitive to the effects of rimonabant because of their genotype, though this is unlikely as rimonabant effects were observed in the reinforcer and response data. Moreover, a number of studies show that obese Zucker rats are especially sensitive to CB1 compounds (Rasmussen and Huskinson 2008; Smith and Rasmussen 2010; Vickers et al. 2003). It is more likely that while rimonabant reduced behavior and reinforcers earned overall across all schedules of the obese Zucker rats, the allocation of behavior did not change due to rimonabant. This may be because of a ceiling effect of sensitivity. In other words, their baseline behavior was already highly sensitive to reinforcement densities (slopes near 1), and therefore, could not improve. More research in this area could help clarify this effect.
General Discussion
The aim of this study was to evaluate rimonabant’s effects on palatable food choice by controlling for some limitations of previous studies. The present study better controlled baseline access to each type of food by using variable interval schedules to program limited access to the two food types. This study also equated properties of the two food types, such as, texture, solidity, size, calories, etc. In addition, we subtracted any bias (Phase 1) toward one lever, such that the only difference in behavioral allocation toward the two pellets was related to macronutrient content (i.e., sucrose concentration). Results from this study suggest that rimonabant’s effects are highly related to the relative amount of food available. In Phase 1, when identical food (either sucrose or carrot) was presented on both levers, rimonabant’s effects only occurred in conditions in which the relative amount was different (5:1 and 1:5 ratios), and not in conditions in which the relative amount was the same (the 1:1 ratio). The strongest rimonabant-related reduction in reinforcement was observed in the components with the highest (VI 12-s) and lowest (VI 60-s) amounts of reinforcement available. In Phase 2, when sucrose was programmed to be delivered by one lever and carrot by the other, effects of rimonabant were observed again in the 5:1 and 1:5 ratios. Effects were also observed in the 1:1 ratio, though the effect sizes were smaller. However, in Phase 2, the percent of decrease in carrot and sucrose pellets was similar across all ratios. Even when sucrose and carrot were the richer (i.e., larger) sources of reinforcement, the reductions in intake were similar. Moreover, there was no effect of rimonabant on sucrose bias using the generalized matching equation. These data suggest, then, that the effects of rimonabant on palatable food previously reported (Arnone et al. 1997; Carai et al. 2006; Mathes et al. 2008; Simiand et al. 1998) may be more strongly related to the baseline relative amount of food and may be less related to palatability. In addition, these effects may also be related to other features of the food that are not represented in this study, such as texture, viscosity, or perhaps fat concentration.
This study extends the free-feed literature on rimonabant, as well as free-feed literature with obese rodents, by offering specific behavioral mechanisms of action. First, the vehicle data showed that obese Zucker rats did not exhibit stronger biases toward sucrose than lean Zucker rats, at least with the choices that were offered in the present study. Their data from Phase 1 and Phase 2 suggest that obese rats were more sensitive to differing quantities of reinforcement than lean rats, and this resulted in greater food procurement. This finding replicates baseline data reported in a previous paper (Buckley and Rasmussen 2012). Differential sensitivity to reinforcement rates, then, may be a mechanism that underlies the phenotype of the obese Zucker rat. Rimonabant may also affect sensitivity to reinforcement as well, though this did not manifest in the matching analysis. Some evidence comes from obese Zucker rats exhibiting higher reinforcer rates following vehicle administration when reinforcement rates differed, and when rimonabant was administered, responses to these same schedules were reduced to a greater magnitude compared to lean Zucker rats.
One limitation to this study was the use of only two doses of rimonabant. Obese Zucker rats have a short lifespan compared to lean Zucker rats (about 450 and 660 days, respectively; Azain et al. 2006), and to use three doses would have required an amount of time that would not have allowed for completion of the study. Studies reporting the effects of i.p. administered rimonabant on the food intake of Zucker rats show that doses of 3 and 10 mg/kg are required to see significant behavioral changes, and that 1 mg/kg produces only minimal, and often statistically insignificant, behavioral changes (Gessa et al. 2006; Rasmussen et al. 2012; Rasmussen and Huskinson 2008), which is why these doses were selected for this study.
The results seen here suggest that rimonabant-related differences on food palatability that have been reported (Carai et al. 2006; Gessa et al. 2006; Mathes et al. 2008) may be heavily influenced by the relative amount ingested. Rimonabant appears to have stronger effects in situations where a relatively greater amount of food is present, compared to when the amount available is smaller. Moreover, the differences in behavior due to cannabinoid activity, whether due to a genetically influenced alteration in endocannabinoid activity in the obese Zucker rat (Di Marzo et al. 2001; Thanos et al. 2008) or due to a drug-influenced decrease in cannabinoid activity by rimonabant, are largely dependent on the environmental arrangement of food. These findings add to previous research identifying other environmental factors in the behavioral pharmacological effects of rimonabant on food ingestion. For example, when more effort is needed to gain access to food, the effects of rimonabant appear to be stronger than free-feeding situations (Rasmussen et al. 2012; Rasmussen and Huskinson 2008). More research is needed to identify other environmental conditions that may interact with drug effects related to food consumption, as well as differences observed between lean and obese rat strains.
Acknowledgments
We thank Steven Boomhower, Megan Brinton, Brent Call, Tiffany Doherty, Kory Farley, Vanessa Hanson, Conrad Hillman, Casey Johnson, Mathew Luras, Zachary Schumacher, Sasha Scott, Jennifer Stoll, and Jennifer White for assistance with data collection.
This manuscript is part of the first author’s Master’s thesis at Idaho State University and was supported by funding from the WeLEAD project (National Science Foundation SBE-0620073), the Idaho INBRE Program, NIH Grant Nos. P20 RR016454 (National Center for Research Resources) and P20 GM103408 (National Institute of General Medical Sciences), and grants from the Humanities and Social Sciences Research and Graduate Research Committees at Idaho State University.
References
- Alsop B, Elliffe D. Concurrent-schedule performance: Effects of relative and overall reinforcer rate. J Exp Anal Behav. 1988;49:21–36. doi: 10.1901/jeab.1988.49-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Azain MJ, Broderson JR, Martin RJ. Effect of long-term somatotropin treatment on body composition and life span in aging obese Zucker rats. Experimental Biology and Medicine. 2006;231:76–83. doi: 10.1177/153537020623100109. [DOI] [PubMed] [Google Scholar]
- Baum WM. Matching, undermatching, and overmatching in studies of choice. J Exp Anal Behav. 1979;32:269–281. doi: 10.1901/jeab.1979.32-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum WM. On two types of deviation from the matching law: bias and undermatching. J Exp Anal Behav. 1974;22:231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JL, Rasmussen EB. Obese and lean Zucker rats demonstrate differential sensitivity to rates of food reinforcement in a choice procedure. Physiology & Behavior. 2012;108:19–27. doi: 10.1016/j.physbeh.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carai MAM, Colombo G, Maccioni P, Gessa GL. Efficacy of rimonabant and other cannabinoid CB1 receptor antagonists in reducing food intake and body weight: preclinical and clinical data. CNS Drug Reviews. 2006;12:91–99. doi: 10.1111/j.1527-3458.2006.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, et al. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Orrù A, Lai P, et al. Lack of tolerance to the suppressing effect of rimonabant on chocolate intake in rats. Psychopharmacology. 2006;185:248–254. doi: 10.1007/s00213-006-0327-1. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Walsh SA, et al. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. Journal of the Experimental Analysis of Behavior. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Houston AI, McNamara J. The value of food: effects of open and closed economies. Animal Behaviour. 1989;37:546–562. [Google Scholar]
- Mathes CM, Ferrara M, Rowland NE. Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;295:R67–R75. doi: 10.1152/ajpregu.00150.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LR, Temple W. Concurrent schedule assessment of food preference in cows. Journal of the Experimental Analysis of Behavior. 1979;32:245–254. doi: 10.1901/jeab.1979.32-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HL. Matching-based hedonic scaling in the pigeon. J Exp Anal Behav. 1976;26:335–347. doi: 10.1901/jeab.1976.26-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Mandell C, Atak JR. The analysis of behavioral momentum. Journal of the Experimental Analysis of Behavior. 1983;39:49–59. doi: 10.1901/jeab.1983.39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce WD, Epling WF. Choice, matching, and human behavior: A review of the literature. Behav Anal. 1983;6:57–76. doi: 10.1007/BF03391874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Huskinson SL. Effects of rimonabant on behavior maintained by progressive ratio schedules of sucrose reinforcement in obese Zucker (fa/fa) rats. Behavioural Pharmacology. 2008;19:735–742. doi: 10.1097/FBP.0b013e3283123cc2. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Asymmetry of reinforcement and punishment in human choice. Journal of the Experimental Analysis of Behavior. 2008;89:157–167. doi: 10.1901/jeab.2008.89-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Newland MC. Quantification of Ethanol’s Antipunishment Effect in Humans Using the Generalized Matching Equation. Journal of the experimental analysis of behavior. 2009;92:161–180. doi: 10.1901/jeab.2009.92-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen EB, Reilly W, Buckley J, Boomhower SR. Rimonabant reduces the essential value of food in the genetically obese Zucker rat: An exponential demand analysis. Physiology & Behavior. 2012;105:734–741. doi: 10.1016/j.physbeh.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Rasmussen EB, Reilly W, Hillman C. Demand for sucrose in the genetically obese Zucker (fa/fa) rat. Behavioural Processes. 2010;85:191–197. doi: 10.1016/j.beproc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Simiand J, Keane M, Keane PE, Soubrié P. SR 141716, a CB1 cannabinoid receptor antagonist, selectively reduces sweet food intake in marmoset. Behav Pharmacol. 1998;9:179–181. [PubMed] [Google Scholar]
- Smith SL, Rasmussen EB. Effects of 2-AG on the reinforcing properties of wheel activity in obese and lean Zucker rats. Behavioural Pharmacology. 2010;21:292–300. doi: 10.1097/FBP.0b013e32833aec4d. [DOI] [PubMed] [Google Scholar]
- Sumpter CE, Foster TM, Temple W. Predicting and scaling hens’ preferences for topographically different responses. J Exp Anal Behav. 1995;63:151–163. doi: 10.1901/jeab.1995.63-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Ramalhete RC, Michaelides M, et al. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse (New York, NY) 2008;62:637–642. doi: 10.1002/syn.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton-Jones ZD, Vickers SP, Clifton PG. The cannabinoid CB1 receptor antagonist SR141716A reduces appetitive and consummatory responses for food. Psychopharmacology. 2005;179:452–460. doi: 10.1007/s00213-004-2047-8. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, et al. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology. 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]
- Yen TT, Shaw WN, Yu P-L. Genetics of obesity in Zucker rats and Koletsky rats. Heredity. 1977;38:373–377. doi: 10.1038/hdy.1977.100. [DOI] [PubMed] [Google Scholar]
- Zucker LM, Zucker TF. Fatty, a new mutation in the rat. Journal of Heredity. 1961;52:275–278. [Google Scholar]