Abstract
The creation of stable flow cultures of hepatocytes is highly desirable for the development of platforms for drug toxicity screening, bio-artificial liver support devices, and models for investigating liver physiology and pathophysiology. Given that hepatocytes cultured using the collagen overlay or ‘sandwich’ configuration maintain a wide range of differentiated functions, we describe a simple method for adapting this culture configuration within a microfluidic device. The device design consists of a porous membrane sandwiched between two layers of PDMS resulting in a two-chambered device. In the bottom chamber, hepatocytes are cultured in the collagen sandwich configuration, while the top chamber is accessible for flow. We demonstrate that hepatocytes cultured under flow exhibit higher albumin and urea secretion, and induce cytochrome P450 1A1 activity in comparison to static cultures. Furthermore, over two weeks, hepatocytes cultured under flow show a well-connected cellular network with bile canaliculi formation, whereas static cultures result in the formation of gaps in the cellular network that progressively increase over time. Although enhanced functional response of hepatocytes cultured under flow has been observed in multiple prior studies, the exact mechanism for this flow induced effect remains unknown. In our work, we identified that hepatocytes secrete higher level of collagen in the flow cultures; inhibiting collagen secretion within the flow cultures reduced albumin secretion and restored the appearance of gaps in the cellular network similar to the static cultures. These results demonstrate the importance of the increased collagen secretion by hepatocytes cultured under flow as a mechanism to maintain well-connected cellular network and differentiated function.
1. Introduction
The ability to maintain long-term stable culture of primary hepatocytes is important for creating bio-artificial liver support systems1, drug toxicity screening platforms2, and models for investigating liver physiology and pathophysiology3. Since liver plays a central role in drug metabolism and detoxification, it is one of the principal target organs for studying toxicity of drugs. Drug-induced hepatic injury is the most frequent reason cited for withdrawal of approved drugs4. In vitro systems are often used as models to predict drug toxicity and pharmacokinetics for clinical cases. Typically, these drug toxicity studies are conducted in static cultures. However, in vivo hepatocytes are exposed to drugs under flow conditions. Microfluidic devices can be used for culturing hepatocytes in a well-controlled flow microenvironment. Several groups have taken advantage of the small geometries and diverse material types to culture hepatocytes in microfluidic devices5–7. There is a considerable interest in developing strategies that promote the maintenance of stable hepatocyte phenotype and function in microfluidic devices for up to a few weeks.
After isolation, hepatocytes rapidly lose their differentiated functions and require specialized extracellular matrix (ECM) culture conditions, such as the collagen overlay or sandwich configuration, for long-term culture8, 9. Other approaches that facilitate hepatocytes in maintaining their function include co-cultivation with other cell types such as fibroblasts10, 11, and endothelial cells12; or spheroid formation13. Although initially used in static cultures, these approaches have also been adapted for flow systems. Typically, hepatocyte fibroblast co-cultures are patterned under an open configuration and the introduction of flow requires assembly of the pre-seeded devices14, 15. Similarly, macro scale bioreactors have been developed that enable hepatocyte seeding in sandwich configuration, but perfusion requires a somewhat cumbersome assembly post-seeding16, 17. Another approach relies on seeding hepatocytes with the non-parenchymal cells on a scaffold within the multi-well flow plate where the cells assemble into spheroids18. Additionally, hepatocytes have been cultured in microfluidic devices without collagen overlay, or in co-cultures where monocultures of hepatocytes are either packed at high density6 or induced to form aggregates5, 7. Instead of directly exposing hepatocytes to flow, these approaches rely on media transport through narrow side channels that enclose the cells. Although, these microfluidic approaches provide interesting solutions, hepatocyte functional data is only reported for up to one week of culture5–7.
Almost universally, whenever hepatocytes in perfusion/flow cultures are compared to their corresponding static cultures, under low shear stress conditions, the cells show higher function in flow cultures, presumably due to greater nutrient exchange. However, the exact mechanism through which flow induces higher function remains unknown. In this work, we first demonstrate a simple method for culturing hepatocytes under the collagen sandwich configuration within a fully assembled microfluidic device that is amenable to flow on top of the collagen gel. Furthermore, we identify the importance of increased collagen secretion by hepatocytes during flow condition in comparison to static cultures as a mechanism by which flow enhances and stabilizes hepatocyte differentiated function in microfluidic devices.
2. Materials and Methods
2.1. Microfluidic Device Fabrication
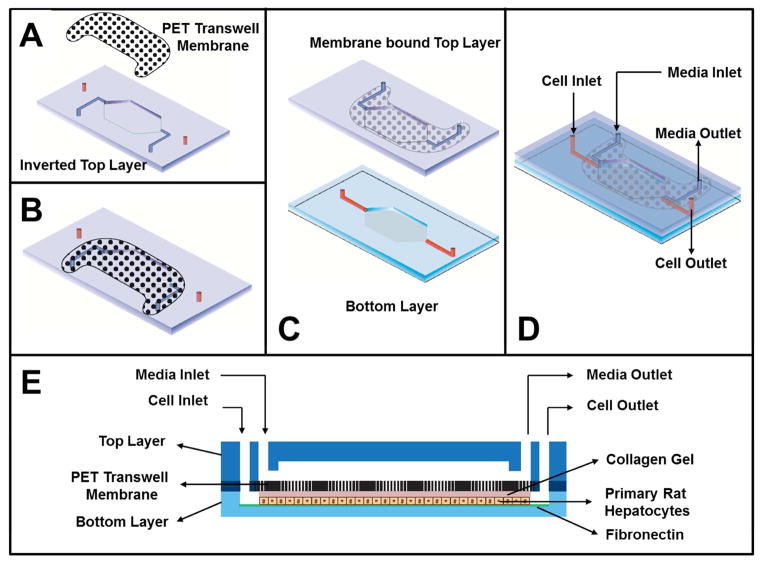
A dual-layered, membrane-based microfluidic device (Figure 1) was fabricated at the BioMEMS Resource Center at the Massachusetts General Hospital and assembled in-house. Silicon-wafer templates served as negative molds to generate the top and bottom layers of the device in poly(dimethyl)siloxane (PDMS, Sylgard 184, Dow Corning) using standard soft-lithography protocols19. Two sets of inlet and outlet ports were punched into the top PDMS layer using a 0.75 mm dermal punch. A 3.0 μm pore-sized PET membrane (8 x 105 pores/cm2 and ~ 6 mm2 exposed surface area/cm2) obtained from transwell membrane insert (BD Biosciences) was cut to desired shape using a laser cutter. The inverted top layer was first bonded to the membrane. Briefly, a thin layer of PDMS pre-polymer was spin-coated onto a clean glass coverslip and a clean top layer was placed onto it for the PDMS to spread on the surface around the channel20. A clean laser-cut membrane was applied to the PDMS pre-polymer coated surface of the top layer and bonded carefully while ensuring the fluidic channels remained free of PDMS pre-polymer (Figure 1A). The membrane bonded firmly to the top layer after baking at 120°C for 1–2 hours (Figure 1B). The membrane-bound top layer and the bottom layer were treated with plasma for 30 seconds using a vacuum plasma cleaner. Next, the two layers were aligned and brought in conformal contact prior to baking at 70°C for 20 min (Figure 1C and 1D). All devices were UV-sterilized for 15 min and the bottom cell culture chamber was coated with 50 μg/mL fibronectin obtained from bovine plasma (Sigma-Aldrich) in PBS and maintained at 37°C for 1 hour before introducing primary hepatocytes. The dimensions of the bottom chamber was 10 mm2 x 0.1 mm (Surface area x Height) and the overall volume of the top and bottom chambers were ~ one μL each.
Figure 1. Schematic of various steps involved in fabrication of microdevice.
A) A thin layer of PDMS pre-polymer is stamped on the feature facing side of the top layer and a PET-based membrane is carefully placed on the surface to cover the entire fluidic channel. B) The membrane sticks firmly to the top layer after baking at 120°C for 1–2 h. C) Membrane-bound top layer is bonded to the bottom layer using plasma cleaner. D) Schematic of the assembled device. E) Cross-sectional view of the assembled device shows that hepatocytes in collagen gel is introduced and cultured in the bottom layer and growth medium is introduced through the top layer.
2.2. Primary Rat Hepatocyte Isolation and Cell Seeding into the Microfluidic Device
Rat hepatocytes were freshly isolated before each experiment from adult female Lewis rats (Charles River Laboratories, Massachusetts) weighing 180–200 g, by a modified procedure as described previously21. All animals were treated in accordance with National Research Council guidelines, and the studies were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital. Typically, 200–300 million hepatocytes with 90–95% viability, as determined by trypan blue exclusion, were isolated and immediately used for seeding devices. A suspension of primary rat hepatocytes, at a final concentration of 10 million cells (M)/mL, was primarily prepared by mixing one part of 20 M/mL cells suspended in media and one part ice-cold collagen pre-gel solution (9 parts 1.25 mg/mL type I rat tail collagen mixed with 1 part 10X DMEM). The cell suspension was introduced into the bottom cell culture chamber using a pipette tip. The device was placed in a 37°C cell culture incubator for one hour to complete the collagen gel formation, which resulted in embedding of hepatocytes in the collagen gel within the bottom chamber of the device. These cells embedded in collagen gel were cultured in serum-free Williams E medium supplemented with, 0.05 U/L insulin, 0.01428 mg/L glucagon, 20 ng/mL epidermal growth factor (EGF), 7.5 μg/mL hydrocortisone, 200 U/mL penicillin, and 200 μg/mL streptomycin at 37 °C in humidified 10% CO2 environment. Media in the top chamber was replaced every 24 hours in devices maintained in static condition. Flow (5 or 20 μL/hour) of fresh media was introduced in the top chamber of perfusion devices after 48 hours of seeding and continued thereafter. Tygon tubing (0.01″ ID×0.03″OD, Cole Parmer) was used for all fluidic connections and media perfusion. In some experiments, cis-hydroxyproline (cHP, 40μg/mL) was added to the culture medium for cells under perfusion for inhibiting collagen secretion22.
2.3. Hepatocyte Morphology and Polarization
Hepatocyte morphology and monolayer integrity were assessed by phase contrast microscopy. Hepatic polarization and bile canalicular network were observed using a solution of 2μM CellTracker™ Green CMFDA (Life Technologies) in a Live Cell Imaging Solution (Life Technologies). CMFDA remains in the cytosol in non-polarized hepatocytes that lack any canaliculi network, and is excreted by polarized hepatocytes into canaliculi when they are present (Figure 2). Reagents were introduced through the top chamber using a pipette tip for both these assays.
Figure 2. Continuous perfusion of media maintained cell morphology and monolayer integrity for two weeks.
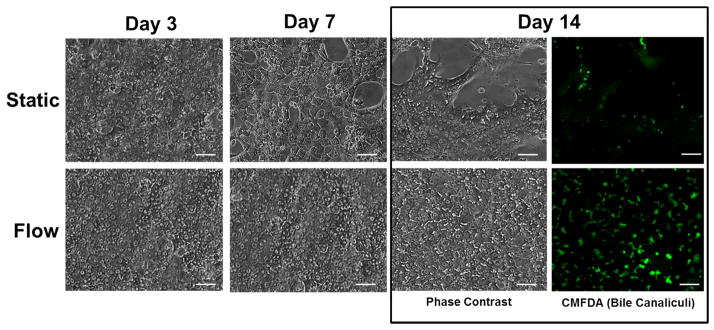
Representative phase contrast images from day 3, 7, and 14 of hepatocyte cultures maintained under static and flow conditions. Static cultures show a gradual change in morphology with low nuclear clarity, appearance of gaps in cellular network unlike flow/perfusion cultures that show stable morphology with clear cell nuclei and well-connected cellular network. Perfusion also induces polarization of hepatocytes and formation of extensive bile canalicular networks by 14 days compared to static cultures. CMFDA is a fluorophore that remains in the cytoplasm of non-polarized hepatocytes and is excreted into bile canalicular spaces in polarized hepatocytes. Image scale bar: 20 μm
2.4. Hepatocyte Function: Albumin, Urea and CYP450 assay
Albumin (Figure 3A) and urea (Figure 3B) contents in the cell culture supernatant samples collected from the microfluidic devices were measured using in vitro rat albumin ELISA kit (Abcam) and Urea BUN assay kits (Stanbio) following the manufacturer’s protocols.
Figure 3. Cultures under flow maintain higher functions.
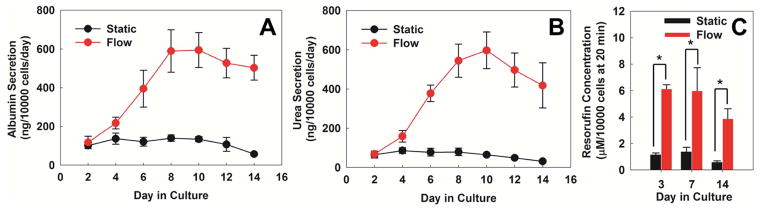
Hepatocytes cultured under continuous perfusion of media have higher and stable A) Albumin secretion, B) urea secretion, and C) induced CYP 1A1 activity (as measured by resorufin concentration) over a two week period compared to static cultures. *p<0.01
CYP1A1 activities (Figure 3C) were assessed by induction of culture with 3-methylcholanthrene (3-MC) at a final concentration of 2μM in media. Static devices were exposed to 3-MC 24 hours before measuring CYP activity. In flow devices, induction was either done by continuous perfusion of 3-MC (16.7 nM) for 24 hours such that the amount of 3-MC exposed to the cells were similar to the static devices, or by static induction with 2μM 3-MC for 24 hours (Figure S2). CYP1A1 conversion of ethoxyresorufin to resorufin after 20 minutes of cell exposure to the substrate solution (10 μM ethoxyresorufin + 80μM dicumarol in phenol red free Earle’s Balanced Salt Solution (EBSS)) added to the top chamber of the device and incubated at 37°C was determined by measuring the fluorescence at 520–530 nm excitation and 580–590nm emission. CYP1A1 activity was reported in terms of resorufin concentration.
2.5. Collagen Gene Expression and Protein Analysis
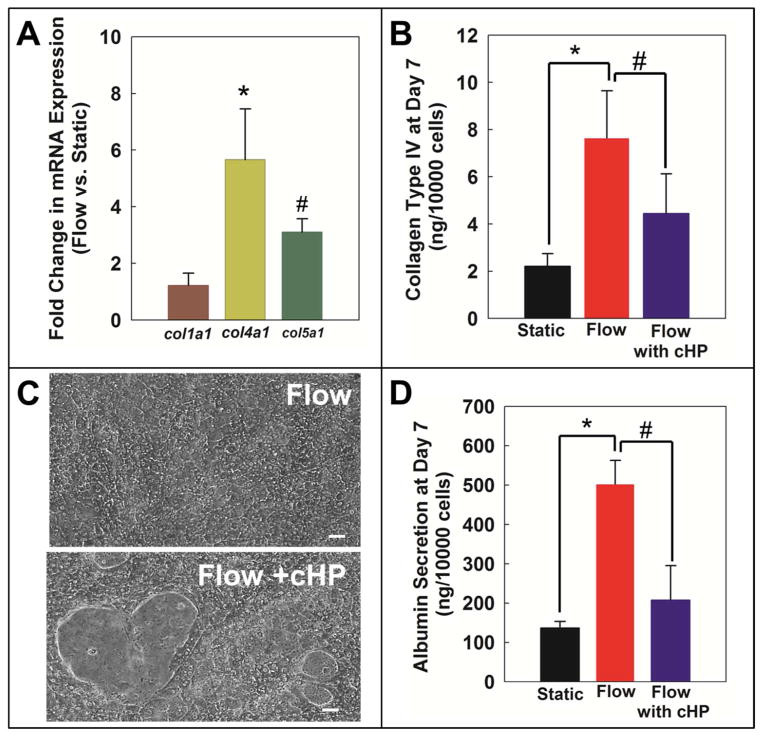
For gene expression studies, hepatocyte cell pellets were collected from the microfluidic devices by perfusion of collagenase through the bottom and top layer, washed with 1X ice-cold PBS and centrifuged, and stored at −80°C. Total RNA from cell pellets was isolated using RNeasy Mini Kit (Qiagen) as per the manufacturer’s instructions. RT-PCR was performed on day 7 hepatocytes to assess relative differences in Collagen Type I (col1a1), Type IV (col4a1), and Type V (col5a1) mRNA expression (Figure 4A) in flow and static devices using OneStep RT-PCR kit (Qiagen) following the manufacturer’s protocol.
Figure 4. Flow stabilizes cellular network and albumin synthesis in hepatocytes by enhancing collagen secretion.
A) Cultures maintained under flow express higher level of collagen Type IVa1 and Type Va1 genes compared to static cultures. B) Even at a functional level, Collagen Type IV protein is produced in higher amounts in cultures under flow compared to static. C) In flow cultures, addition of 40 μg/mL cHP from Day 2 to Day 7 reduces collagen secretion by hepatocytes and disrupts cellular network as seen in static cultures. D) cHP also reduces albumin synthesis in flow cultures. Image scale bar: 20 μm.*p<0.01, #p<0.05
The total Collagen Type IV produced by hepatocytes on day 7 under static and flow conditions (Figure 4B) were quantified using Collagen Type IV (CoL-4) Elisa kit (Mybiosource) as per manufacturer’s protocol.
2.6. Statistical Analysis
Each experiment was performed in duplicates from at least three different rat isolations. Quantitative data was plotted as the mean±standard error of the mean wherever indicated. Statistical analysis was performed using Student’s unpaired two-way t-test. Differences were considered statistically significant when p<0.05.
3. Results and discussion
3.1. Microfluidic Device Design
It is well established that culturing hepatocytes under sandwich configuration results in adoption of an in vivo-like morphology and polarity and maintenance of wide range of differentiated functions8, 9, 21, 23. Our goal was to design a microfluidic device that permits fluid flow in conjunction with sandwich culture of hepatocytes. In a single layered device, introduction of collagen gel on top of hepatocytes can clog the channel without leaving any space for fluid flow. Thus, our device design includes (Figure 1) a porous membrane sandwiched between two layers of PDMS that results in a two-chambered device. In recent reports, porous membranes have been utilized for fabricating two chamber microfluidic devices24, 25. For our device, the hepatocytes that were suspended in the collagen pre gel solution are introduced into the bottom chamber and allowed to gel. These results in hepatocytes embedded in collagen gel in the bottom chamber, while the top chamber remains open for introducing flow on top of the membrane. The intervening membrane acts as a barrier preventing collagen gel from entering the top chamber, while at the same time permitting soluble factor exchange between the top and the bottom chambers through the pores of the membrane.
3.2. Effect of Flow on Hepatocytes Morphology, and Function
In vivo, endothelial cells and the extracellular matrix of the space of Disse separate the sinusoidal flow from hepatocytes. Hepatocytes continuously exchange nutrients and other soluble factors via flow occurring within the sinusoids. In order to simulate this effect and test the effect of flow, the top chamber in the device was continuously perfused with cell culture medium starting from day 2 of culture. As shown in Figure 2, hepatocytes in flow cultures display flattened cuboidal morphology with sustained and well-connected cellular network over a period of two weeks. Furthermore, they also exhibit an extended network of bile canaliculi as seen in the fluorescence micrograph obtained from the CMFDA assay. CMFDA, by itself, is not fluorescent and it passively enters in the cell cytoplasm where it is cleaved by an esterase resulting in formation of the fluorescent product. This fluorescent product is a substrate of the transporter protein MRP-2 expressed on the apical domain, which leads to its accumulation in bile canaliculi26. In contrast to flow condition, hepatocytes in static cultures within the microfluidic device started to show gaps in the cellular network that progressively increased with time (Figure 2). This led to lack of bile canaliculi network formation.
The effect of flow on hepatocytes was further evaluated by measuring albumin and urea secretions and cytochrome P450 1A1 (CYP1A1) activity. Hepatocyte cultures subjected to flow showed higher albumin and urea secretion in comparison to static cultures (Figures 3a and 3b), confirming previous reports14, 15, 17. The differences in albumin and urea secretions for flow vs static conditions became greater with time during the first week of culture and then it leveled off. The above results from the flow experiments were obtained at the media flow rate of 5 μl/hour. We evaluated if the hepatocyte function can be enhanced by increasing the media flow rate. We observed that at 20 μl/hour, albumin and urea secretion at the end of the first week of culture was much lower (Figure S1) as compared to 5 μl/hour. Thus, additional flow experiments and their comparison to the static cultures were conducted at the flow rate of 5 μl/hour.
Higher function of hepatocytes in flow cultures was further supported by the CYP1A1 activity. The CYP activity was measured after treating hepatocytes with 3-MC, which is a known inducer of CYP1A1, for 24 hours. In contrast to albumin and urea secretions, the difference in CYP activity between the flow and static cultures was starkly pronounced as early as day 3 of culture (Figure 3C, p<0.01). This indicates that even relatively short duration of flow (24 hours) can greatly enhance the CYP activity of hepatocytes in comparison to static induction. It is important to note that in the flow devices, the inducer concentration was reduced to 16.7 nM (vs 2μM in static) in order to maintain the same total amount of inducer in flow and static conditions over the 24 hour period. Similar results were obtained on days 7 and 14, where the flow cultures showed higher CYP activity than the static cultures. In order to decouple the contribution of continuous 3-MC exposure in flow devices, control experiments were conducted where hepatocytes maintained in perfusion cultures were also treated with 3-MC under static condition for 24 hours and compared to static devices. Figure S2 indicates that the CYP activity of hepatocytes on day 7 was higher in flow than in static cultures (p<0.05) even though the 3-MC treatment was conducted under static condition. The CYP activity was highest for the condition where hepatocytes were maintained in flow cultures and the 3-MC treatment was also conducted in flow condition. These results underscore the dynamic interplay of multiple flow elicited factors that can influence hepatocyte function in microdevices.
3.3. Effect of Flow on Collagen Secretion by Hepatocytes
In the sandwich configuration, it is believed that the layer on top of hepatocytes acts as a barrier for retention of endogenous collagen secreted by hepatocytes and this freshly secreted collagen is responsible for the maintenance of hepatocytes differentiated function. In light of this understanding, we hypothesized that in the flow cultures higher function could be due to greater level of collagen secreted by hepatocytes. In order to test this hypothesis, we first evaluated expression of collagen Ia1, IVa1, and Va1 genes in flow and static conditions, as previous reports indicate that hepatocytes secrete different types of collagens including type I, IV, and V27. In our experiments, we observed that perfused hepatocytes expressed more type IVa1 (p<0.01) and Va1 collagens in comparison to static cultures, while the level was similar for collagen type Ia1 (Figure 4a). Since the greatest flow-induced increase in gene expression was observed for collagen type IVa1, we further evaluated collagen type IV secretion by hepatocytes. Figure 4b indicates that indeed flow enhanced collagen type IV protein secretion by hepatocytes (~4 fold, p<0.01) in comparison to static condition.
In the past reports, collagen synthesis and secretion by hepatocytes has been directly linked to the level of proline both in monoculture23 and co-culture28. One plausible reason for higher collagen synthesis in flow cultures could be due to hepatocytes being exposed to greater amounts of proline compared to static cultures. Since the same media composition was used for both static and flow cultures, hepatocytes in the flow cultures inherently were exposed to a much larger amount of various factors, including proline, present in the cell culture medium. One way of mitigating the effect of proline on collagen secretion by hepatocytes is by introducing cHP in the culture medium. Past reports indicate that cHP gets incorporated in freshly synthesized collagen and disrupts the triple helix structure of collagen molecules, which interferes with their secretion22. In our experiments, we observed that addition of cHP to flow cultures resulted in a decrease of Type IV collagen secretion. Strikingly, addition of cHP to the flow cultures also resulted in formation of gaps in the cellular network of hepatocytes that were not observed in the regular flow cultures (Figure 4c). Furthermore, addition of cHP resulted in reduction of albumin secretion by hepatocytes in the flow cultures (Figure 4d, p<0.05) thereby further supporting the role of higher collagen secretion in the flow cultures as a mechanism for more functional hepatocytes.
Taken together, the above results are consistent with the following mechanism. In the flow cultures, due to greater availability of proline, hepatocytes secrete higher levels of collagen and thus gradually remodel the ECM microenvironment. This endogenously secreted collagen leads to a progressive increase in albumin by various mechanisms, which may include the presence of “ECM responsive elements” in the promoter region of albumin, enhanced sequestration of growth factors, and/or mechanical effects8. Furthermore, the freshly secreted collagen accumulating around hepatocytes perhaps provides “anchors” that prevent the appearance of gaps in the cellular network as the overall ECM environment is remodeled with time. By contrast, in static cultures, less proline is available for collagen synthesis and secretion. This leads to less albumin synthesis and as the ECM environment is remodeled, the amount of collagen produced over time is perhaps not sufficient to provide enough attachment sites, which leads to appearance of gaps in the cellular network. However, we cannot rule out the contribution of other factors such as feedback inhibition on the overall difference in albumin secretion observed between the static and flow cultures. For instance, in the static cultures, there is accumulation of albumin that can potentially inhibit further albumin secretion over a 24 hour period. By contrast, in the flow cultures, albumin is continuously removed and thus any potential feedback inhibition is minimized.
In the flow cultures, perfusion has been shown to be detrimental depending on the level of shear stress experienced by hepatocytes29. Various design schemes, such as culturing hepatocytes in microgrooves15 or separating perfusion channels from cell seeding channel5–7, reduce hepatocyte exposure to flow and thereby facilitate culture in low shear stress conditions. In our design, a 10μm thick membrane and the collagen gel on top of hepatocytes separates them from flow. Thus, detrimental effect of shear stress in our device at the flow rate of 5 μl/hour was likely minimal. Although at the higher flow rate of 20 μl/hour, where we observed greatly reduced albumin and urea secretion, we cannot exclude the detrimental contribution of shear stress on the collagen gel and/or hepatocytes. Another design consideration in the flow devices is the delivery of oxygen to hepatocytes as they are highly metabolically active cells. In the absence of gas exchange, oxygen carried by flowing medium can be the only means of delivering oxygen. However, in our case, since the devices were made up of PDMS, which is highly permeable to oxygen, it is unlikely that in the static and flow cultures there was any consequential difference in delivery of oxygen.
4. Conclusion
In this report, we have developed a simple method for culturing hepatocytes in sandwich configuration within a pre-assembled microfluidic device that is compatible with flow on top of the gel. Hepatocytes showed higher function in the flow cultures as compared to static cultures over a period of two weeks. We observed that the flow can elicit both a quick response as measured by the induced CYP 1A1 activity and a more gradual and sustained effect as indicated by the albumin and urea secretion. We identified the role of greater collagen synthesis as a mechanism for higher function of hepatocytes in the flow cultures.
In the past, the flow cultures for hepatocytes have largely been used for bioartificial liver devices due to an inherent requirement for eventual coupling of devices within a flow circuit. However, there is a growing realization that including flow in drug toxicity platforms and models for investigating liver physiology and pathophysiology will be of great benefit as in vivo hepatocytes experience dynamic flow environment. Development of simple methods for culturing hepatocytes in flow condition will further facilitate achieving this goal.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH)(5UH2TR000503), (K99DK095984 for AB and F32DK098905 for WJM), and Shriner’s Hospital for Children (BU). We thank Dr. Keuye Shen for sharing his expertise in using laser cutter. We acknowledge the support of MGH BioMEMS Resource Center in fabricating microdevices.
Footnotes
The authors have no professional or financial conflicts of interest to disclose.
References
- 1.Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, Yarmush ML. Liver Transpl. 2004;10:1331–1342. doi: 10.1002/lt.20229. [DOI] [PubMed] [Google Scholar]
- 2.Khetani SR, Bhatia SN. Nat Biotechnol. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 3.Jindal R, Patel SJ, Yarmush ML. Tissue Eng Part C Methods. 2010 doi: 10.1089/ten.tec.2009.0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee WM. N Engl J Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- 5.Goral VN, Hsieh YC, Petzold ON, Clark JS, Yuen PK, Faris RA. Lab Chip. 2010;10:3380–3386. doi: 10.1039/c0lc00135j. [DOI] [PubMed] [Google Scholar]
- 6.Lee PJ, Hung PJ, Lee LP. Biotechnol Bioeng. 2007;97:1340–1346. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 7.Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H. Lab Chip. 2007;7:302–309. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- 8.Berthiaume F, Moghe PV, Toner M, Yarmush ML. FASEB J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 9.Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. FASEB J. 1989;3:174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia SN, Balis UJ, Yarmush ML, Toner M. J Biomater Sci Polym Ed. 1998;9:1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- 11.Khetani SR, Szulgit G, Del Rio JA, Barlow C, Bhatia SN. Hepatology. 2004;40:545–554. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia SN, Balis UJ, Yarmush ML, Toner M. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 13.Tong JZ, Sarrazin S, Cassio D, Gauthier F, Alvarez F. Biol Cell. 1994;81:77–81. doi: 10.1016/0248-4900(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 14.Kane BJ, Zinner MJ, Yarmush ML, Toner M. Anal Chem. 2006;78:4291–4298. doi: 10.1021/ac051856v. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Berthiaume F, Toner M, Yarmush ML, Tilles AW. Biotechnol Bioeng. 2005;90:632–644. doi: 10.1002/bit.20463. [DOI] [PubMed] [Google Scholar]
- 16.De Bartolo L, Jarosch-Von Schweder G, Haverich A, Bader A. Biotechnol Prog. 2000;16:102–108. doi: 10.1021/bp990128o. [DOI] [PubMed] [Google Scholar]
- 17.Xia L, Ng S, Han R, Tuo X, Xiao G, Leo HL, Cheng T, Yu H. Biomaterials. 2009;30:5927–5936. doi: 10.1016/j.biomaterials.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG. Lab Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald JC, Chabinyc ML, Metallo SJ, Anderson JR, Stroock AD, Whitesides GM. Anal Chem. 2002;74:1537–1545. doi: 10.1021/ac010938q. [DOI] [PubMed] [Google Scholar]
- 20.Chueh BH, Huh D, Kyrtsos CR, Houssin T, Futai N, Takayama S. Anal Chem. 2007;79:3504–3508. doi: 10.1021/ac062118p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn JC, Tompkins RG, Yarmush ML. Biotechnology progress. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 22.Uitto J, Hoffman H, Prockop DJ. Science. 1975;190:1202–1204. doi: 10.1126/science.1198105. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Morgan JR, Tompkins RG, Yarmush ML. FASEB J. 1993;7:586–591. doi: 10.1096/fasebj.7.6.8472895. [DOI] [PubMed] [Google Scholar]
- 24.Huh D, Fujioka H, Tung YC, Futai N, Paine R, 3rd, Grotberg JB, Takayama S. Proc Natl Acad Sci U S A. 2007;104:18886–18891. doi: 10.1073/pnas.0610868104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suda J, Zhu L, Karvar S. Am J Physiol Cell Physiol. 2011;300:C416–424. doi: 10.1152/ajpcell.00467.2010. [DOI] [PubMed] [Google Scholar]
- 27.Tseng SC, Smuckler EA, Stern R. Hepatology. 1983;3:955–963. doi: 10.1002/hep.1840030613. [DOI] [PubMed] [Google Scholar]
- 28.Jindal R, Nahmias Y, Tilles AW, Berthiaume F, Yarmush ML. FASEB J. 2009;23:2288–2298. doi: 10.1096/fj.08-114934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M. Biotechnol Bioeng. 2001;73:379–389. doi: 10.1002/bit.1071. [DOI] [PubMed] [Google Scholar]