Abstract
Traditionally, sensory signaling in the urinary bladder has been largely attributed to direct activation of bladder afferents. There is substantive evidence that sensory systems can be influenced by non-neuronal cells, such as the urothelium, which are able to respond to various types of stimuli that can include physiological, psychological and disease-related factors. The corresponding release of chemical mediators (through activation of a number of receptors/ion channels) can initiate signaling mechanisms between and within urothelial cells, as well as other cell types within the bladder wall including bladder nerves. However, the mechanisms underlying how various cell types in the bladder wall respond to normal filling and emptying, and are challenged by a variety of stressors (physical and chemical) are still not well understood. Alterations or defects in signaling mechanisms are likely to contribute to the pathophysiology of bladder disease with symptoms including urinary urgency, increased voiding frequency and pain. This review will discuss some of the components involved in control of lower urinary tract function, with an emphasis on the sensor and transducer roles of the urothelium.
Keywords: afferents, barrier function, mucosa, sensory function, uroepithelium
Introduction
The bladder wall contains the mucosal layer, the muscularis propria and the adventitia/serosa. The urinary bladder mucosa consists of the urothelium, a basement membrane and the lamina propria. The urothelium lines the renal pelvis, ureters, bladder, upper urethra and glandular ducts of the prostate, and forms the interface between the urinary space and the underlying vasculature, connective, nervous, and muscular tissues.1 The urothelium is considered a stratified epithelium, and is comprised of multiple epithelial layers, including a superficial or apical layer composed of large hexagonal cells (diameters of 25–250 μm) called “umbrella” (also termed superficial or facet) cells.1,2 Adjacent to the umbrella cell layer is an intermediate layer (10–25 μm in diameter) of cells and beneath this layer are basal cells (10 μm in diameter), which is in contact with underlying connective tissue and capillary bed. The thickness of the urothelium can significantly vary depending on the degree of bladder distension, and the number of epithelial layers seems to be species dependent.
At the trigone region, the epithelium is thought to show a dilated or stretched appearance due in part to the basement membrane that might limit the degree of contraction in this region of the bladder. There seems to be no apparent difference between the urothelium of the trigone compared with the detrusor, in contrast to cells from the proximal urethra. Although the urethral epithelium has been less systematically studied, this epithelium becomes columnar in nature and is accompanied by a lack of urothelial-specific differentiation markers.3,4 These mucus-secreting cells are often exposed to the lumen and can share similarities to that of gastrointestinal enterochromaffin cells. In addition, these epithelial cells are also covered with microvilli, which might function in increasing cell surface area, affecting bacterial adherence and fluid transport, and might also serve a sensor role for chemical, as well as mechanical, signals. Within the urethral epithelium in a number of species are peptide/amine-containing neuroendocrine cells termed paraneurons.5–7 It has been speculated that these neuroendocrine cells (which are also found in epithelia of the respiratory and gastrointestinal tracts) might exhibit both a secretory and a sensory function; however, the functional significance of these cells in the lower urinary tract has yet to be determined.
Interest has grown in understanding the basis for interactions between the urothelium and the underlying LP, which lies between the basement membrane of the mucosa and the detrusor muscle. An extensive vascular network (including lymphatic vessels) is also located beneath the urothelium, and likely to be a source of mediators that can affect cell survival and signaling.8 The LP is composed of several types of cells, including fibroblasts, sensory nerve endings and a layer of spindle shaped cells likely to play an important role in signaling mechanisms in the bladder wall.9–11 Although the appropriate nomenclature used to describe these cells is still under debate, they have been termed IC, myofibroblasts or ICC. In addition, there is evidence that these ICC-LP cells (which have been typically identified using cellular markers such as c-kit or vimentin) are likely to have a stellate-shaped morphology, and have close contacts with both afferent and efferent bladder nerves.12–15 There is evidence that these ICC-LP cells are able to form a “network” or functional syncytium connected by gap junctions, as shown by immunohistochemistry and also by functional recordings. In addition, these cells might show a differential response to a variety of mediators based on their location in different regions of the urinary bladder. Although the functions of ICC cells in bladder physiology and pathology have not yet been established, it is likely that these ICC cells could functionally link signals from the urothelium with underlying cells in the bladder wall.
Afferent nerves
The bladder and lower urinary tract serves to store and evacuate urine, and is controlled by neural pathways organized at both spinal and supraspinal levels.16–20 These afferent pathways provide input to the reflex circuits that control bladder filling and emptying, and are also the source of non-painful sensations of fullness as well as pain. It has been shown that the plexus of afferent nerves is most dense in the regions of the bladder neck and proximal urethra. The lower urinary tract is regulated by three sets of peripheral nerves that include sacral parasympathetic (pelvic nerves), thoracolumbar sympathetic (hypogastric nerves and sympathetic chain) and somatic nerves (pudendal nerves). For example, afferents traveling in the pelvic nerve are thought to be involved in monitoring bladder volume (during bladder storage) and bladder contraction amplitude (during voiding). These nerves also contain the efferent parasympathetic, sympathetic, and motor fibres supplying the bladder, urethra and sphincters.
The two major subtypes of afferents are Aδ (myelinated) and C (unmyelinated) fibers.16,18 Ultrastructure and immunohistochemical studies (used to identify the terminations of sensory afferents in the bladder wall) have shown that Aδ fibers are distributed mainly within the detrusor smooth muscle and are responsive to detrusor stretch, which occurs during bladder filling. In contrast, C-type fibers seem to be more widespread and are distributed in the detrusor muscle, within the lamina propria and in close proximity to the urothelium. There are at least two subclasses of C-type bladder afferent fibers including peptidergic-containing afferents (project to spinal cord lamina I) and those that do not contain peptides (project to spinal cord inner lamina II). Peptidergic-axons seem to be localized throughout the bladder wall, but most species show a dense distribution within the lamina propria next to the urothelium.21
Mechanosensitive afferents are able to respond to bladder filling with a range of thresholds from volumes that would be encountered under normal bladder filling to levels of distension that would be considered noxious and give rise to pain.16,18,22 Those afferents with lower thresholds have small myelinated axons, whereas unmyelinated fibres have generally higher thresholds for activation. High-threshold afferents are also likely to terminate in the deeper muscle layers or in the serosa and respond to high levels of stretch that distort the bladder wall, but might also become sensitized in response to inflammation. This is in contrast to a subtype of afferent that is non-responsive or silent under physiological conditions, but can be sensitized during inflammation.23 There are also recent studies that identified and characterized sacral afferents responding to changes in fluid flow through the urethra.24 These observations show that the properties of these flow-responsive afferents seem to parallel that of cutaneous afferents. This could be important in terms of restoration of bladder emptying after spinal cord injury.
There is considerable interest in mechanisms underlying sensitization of C-fiber afferents, as these nerves are thought to play a key role in symptoms of BPS/IC, as well as patients with urgency sensations at lower than normal bladder volumes.25,26 The pathophysiological mechanisms underlying visceral pain hypersensitivity are not well known. For example, afferents (in particular C-type fibers) can synthesize a number of putative neurotransmitters and can express a wide-range of receptors, such as TRPV1, TRPA1, tropomyosin-related kinase A (responds to nerve growth factor), muscarinic and purinergic subtypes.27–29 In turn, afferents can respond to mediators (such as prostaglandin, serotonin, ATP, histamine, bradykinin, and neurotrophic factors such as NGF) released during inflammation, injury and ischemia from a number of cell types, as well as chemicals present in the urine. For example, NGF, which is highly expressed within the urothelium,30 has attracted a great deal of interest as playing a key role in regulating neural plasticity in response to bladder injury or inflammation. Increased levels of NGF (and other trophic factors) have been detected both in BPS/IC patient biopsies, as well as in urine.31 In addition, the generation of transgenic mice with NGF over-expression restricted to the urothelium has shown a number of changes in bladder function (urinary frequency; pelvic pain) similar to that shown in BPS/IC patients.32 These findings highlight the importance of the bladder urothelium in pathology-induced changes in bladder function and sensation.
Chronic conditions that involve a continuous tissue inflammation or injury can induce changes in sensory pathways resulting in a heightened response to both noxious and non-noxious stimuli. Much of our understanding underlying these plastic changes in the pain pathway have centered on neuronal mechanisms. However, there is increasing evidence that spinal cord glial cells (referred to as astrocytes and microglia) drive the creation and maintenance of allodynia and hyperalgesia, thus playing a significant role in pain processing.33 Though glial cells (like another non-neuronal cell, the urothelial cell) have been cast in a supporting role to neurons, activation of these spinal cord glial cells has been implicated in both the initiation and amplification of persistent pain. For example, remote activation of spinal cord glial cells in response to a peripheral nerve injury can enhance and prolong the response of afferent nerves, thus playing a prominent role in pain amplification.
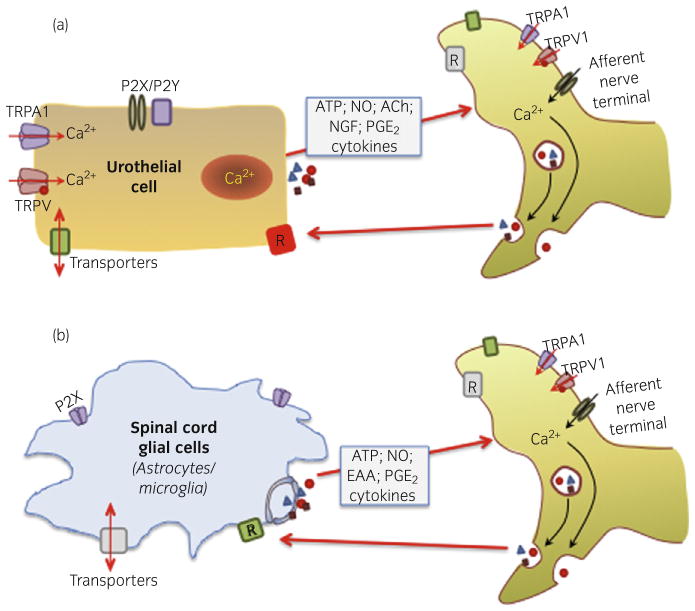
Although most studies have focused on glial cell involvement in peripheral nerve injury, evidence has shown that spinal cord activation of glial cells might be involved in the development and maintenance of central sensitization in various chronic pain conditions. When activated, glial cells can show changes in morphology, as well as function (expression of receptors/channels and release of mediators). This in turn, can lead to changes in neuronal function and ultimately influence pain transmission (Fig. 1).34 Alterations in glial cell morphology and function have been reported in models of colonic irritation, as well as in a naturally-occurring chronic model of idiopathic bladder cystitis diagnosed in cats (termed feline IC).35,36 In this model, there is a pronounced upregulation of immuno-intensity of astrocytic marker glial fibrillary acid protein in dorsal horn regions that receive pelvic afferent input. It is clear that afferent endings respond to a variety of stimuli that reduce the threshold for activation and alter the responsiveness (or excitability), thereby enhancing the gain of the transducer.
Fig. 1.
Hypothetical model showing possible bi-directional interactions between non-neuronal cells (A-urothelium; B-spinal cord glial cells) and nerves. Both urothelial cells and glial cells can be targets for transmitters and mediators released from nerves. In turn, these non-neuronal cells can release a number of mediators that can alter the responsiveness (or excitability) of sensory nerves. This suggests that non-neuronal cells might have the ability to change the gain of the system, thereby altering sensory functions.
Urothelial integrity and repair
The apical surface of the urothelium is also covered with a sulfated polysaccharide glycosaminoglycan or mucin layer that is thought to act as a non-specific anti-adherence factor and as a defense mechanism against infection.37 In addition, as the bladder fills the umbrella cells appear flat and stretched, and this change in morphology and cell size is accompanied by vesicular traffic (i.e. exocytosis/endocytosis), which adds membrane to the apical surface thereby increasing the overall urinary bladder surface area. This process of ongoing replacement of apical membrane by newly fused discoid vesicles also serves to maintain the urothelial barrier.38–40 These processes allow the bladder to accommodate varying changes in bladder volume and also to restore leaky urothelial membrane areas during bladder filling and emptying. There is evidence that factors, such as ATP (released from the urothelium), can bind to urothelial-purinergic receptors in an autocrine manner and thereby modulate membrane traffic.38 Exocytosis/endocytosis (vesicular recycling) might also play an important role in modulating the release of a number of neurotransmitters/mediators, as well as regulation of many receptors and ion channels in urothelial cells.
Epithelial integrity is maintained through a complex process of migration and proliferation (to restore cell numbers), and differentiation (to restore function).41 Urothelial cells typically show a slow rate of proliferation or turnover.42,43 There is significant interest in identifying urothelial stem cells with the goal of using these cells for bladder repair and regeneration. Although some investigators have suggested that a population of progenitor cells might reside in the basal cell layer, at present a definitive identification of urothelial progenitor and stem cells remains elusive. Although some studies suggest that neither urine-derived factors nor cyclic mechanical changes are likely to contribute to urothelial proliferation and differentiation, an accelerated proliferation can occur in various bladder pathologies.1,44,45 For example, after damage to the umbrella cell layer, the urothelium rapidly undergoes both functional and structural changes in order to restore the barrier in response to infection, inflammation or injury. After disruption of the barrier, in the early stages of regeneration the superficial cells might appear smaller in size and are often covered with microvilli.46 In some pathologies, a defect in maturation of superficial umbrella cells have been reported, though the factors that might be involved in these processes have not been identified. The processes underlying urothelial repair are complex, involving several structural elements, signaling pathways, trophic factors and a cellular environment that is constantly undergoing cyclic mechanical deformation.
Conditions, such as BPS/IC, senescence or spinal cord injury, are also associated with changes in the urothelial barrier (Fig. 2).47,48 For example, studies in aged animals have shown significant alterations to the bladder urothelium including areas of mucosal denudation. There is evidence that instillation of liposomes composed of phospholipids results in a repair or restoration of the urothelial barrier in animals after injury or inflammation.49,50 Although the mechanism underlying liposome interaction in epithelium is not known, studies have shown that different lipid species can improve the barrier function and might even have an anti-inflammatory role. In this regard, a study using intravesical liposomes on a single subject with ulcerative BPS/IC has shown promise to repair the dysfunctional urothelium and decrease bladder symptoms.51
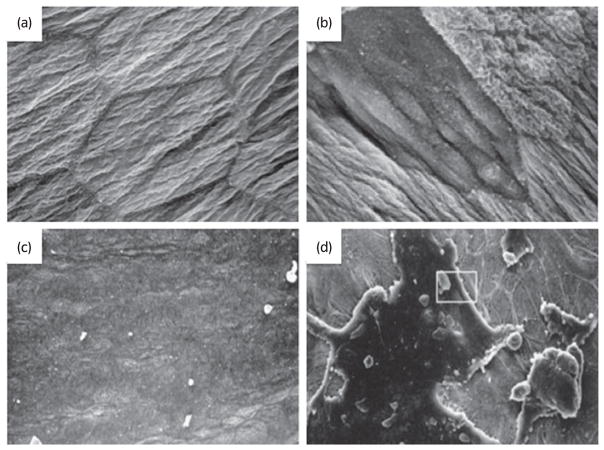
Fig. 2.
Urothelial alterations with bladder pathology. Scanning electron micrograph of apical surface of umbrella cell layer from (a) normal rat and (b) 2 h after spinal cord injury (reproduced from Apodaca et al.47 with permission). Scanning electron micrograph of hydro-distended bladders from (c) C-control (normal, unaffected) cats and in (d) cats diagnosed with feline interstitial cystitis (depicting regions where the umbrella cells layer is disrupted; reproduced from Lavelle et al.48 with permission).
Studies have shown that various types of physiological and psychological stress lead to a failure of urothelial and suburothelial “defensive” systems, thereby promoting changes in both urothelial barrier and signaling functions. For example, stress-mediated activation of the HPA axis can result in increased production of corticotrophin-releasing factor, which can regulate neuroendocrine and autonomic responses to stress.52,53 Psychological stress and HPA axis alterations can exert deleterious effects on mucosal immune function in a number of structures, such as the gut and the skin.54,55 Stress-related dysregulation of signaling between the brain and target structures (including the urinary bladder) can lead to a disruption of the epithelial barrier and alteration in sensory function, as well as an increased prevalence of infection. For example, psychological stress through HPA axis activation has been shown to inhibit microbial peptides and increase the severity of cutaneous infection in mice.56 Thus, it is likely that epithelia in many target organs can express the equivalent of the HPA-stress axis that might act as a defense system and exhibit similar roles in coordinating localized responses to stress in addition to other (sensory/barrier) functions.
Urinary tract infections produced by UPEC are initiated by bacterial adherence to uroplakin proteins on the apical surface of umbrella cells.57,58 Studies have shown that indwelling catheters have been cited as a prime site for bacterial colonization and a common cause of infection.59 The bacteria can form attachments to the irregularities of the catheter surface and develop into biofilms that can play a role in the persistence of infection. It has also been suggested that urothelial differentiation (and increased uroplakin III expression) plays a pivotal role in sensitizing urothelial cells to UPEC-induced infection and possible cell death.60 In addition, there is also a role for endotoxin (lipopolysaccharide, LPS) on the bacterial cell wall in mediating the pain associated with UPEC infection.61
In summary, modification of the urothelium and/or loss of epithelial integrity in a number of pathological conditions can result in the passage of toxic/irritating urinary constituents through the urothelium or release of neuroactive substances from the urothelium. This might result in chronic or persistent changes in the properties of neuronal and non-neuronal cells that can lead to sensory symptoms, such as urinary frequency, urgency, and pain during bladder filling and emptying.
Urothelial-cell interactions
Although urothelial cells are often viewed as bystanders in the process of visceral sensation, recent evidence has supported the view that these cells function as primary transducers of some physical and chemical stimuli, and are able to communicate with underlying cells including bladder nerves, smooth muscle and inflammatory cells. The urothelium is able to respond to a wide variety of mechanical stresses during bladder filling and emptying by activating a number of possible transducer proteins. Possibilities of mechanical signals include bladder pressure, tension in the urothelium or bladder wall, torsion, geometrical tension, movement of visceral organs and even urine tonicity. Alterations in the composition of urine are a type of stress whose contents can vary in both their rate of delivery, as well as the particular constituents.
Additional lines of evidence suggest that urothelial cells participate in the detection of both physical and chemical stimuli. Studies have shown that both afferent and autonomic efferent nerves are located in close proximity (with some penetrating) to the urothelium, and can be activated by a variety of transmitters and mediators released in part by the urothelium.21,62–64 Peptidergic, purinergic and TRPV1-immunoreactive nerve fibers presumed to arise from afferent neurons in the lumbosacral dorsal root ganglia are distributed throughout the urinary bladder musculature, as well as in a plexus beneath and extending into the urothelium. In addition, immunohistochemical studies have also shown both adrenergic (tyrosine hydroxylase) positive nerves, as well as cholinergic (choline acetyltransferase) positive nerves, are in close proximity to the urothelium.
A number of neuronal “sensor molecules” (receptors/ion channels) have been identified in urothelium using both in vivo and in vitro (cell culture and isolated tissue) preparations. Expression of these various receptors enable the urothelium to respond to a number of sensory inputs (physical and chemical) from a variety of sources (for review see1,65–67). These inputs include increased stretch during bladder filling; soluble factors (many found in the urine), such as epidermal growth factor; or chemical mediators/peptides/transmitters, such as substance P, calcitonin gene-related peptide, corticotrophin releasing factor, acetylcholine, adenosine or norepinephrine released from nerves, inflammatory cells and even blood vessels. Various stimuli can lead to a number of outputs from the urothelium including the secretion of numerous chemical substances, such as neurotrophins, peptides, ATP, acetylcholine, prostaglandins, prostacyclin, NO and cytokines. In this regard, the diffusion of such signaling molecules (referred to as volume transmission or paracrine signaling) between the uroepithelium and underlying cell layers has been examined by measuring the propagation of calcium and membrane potential events. Calcium wave propagation might be a common way of translating extracellular stimuli into functional processes that can spread as a “wave” to nearby cells, ultimately leading to release of neuroactive mediators. A range of stimuli are able to enhance this propagation including mechanical stretch and in a number of pathological conditions.68–70 Though a number of studies have shown a directionality of this type of signal transfer, it remains to be determined as to how signaling occurs between the apical-basal epithelium as well as within the superficial (apical) layers. Examples of some urothelial-signaling pathways and potential therapeutic targets in bladder pathophysiology are considered below.
Cholinergic signaling
Urothelial cells express the receptor proteins and mRNA for all the muscarinic subtypes (M1–M5), and also exhibit the machinery necessary for the synthesis and release of acetylcholine.71–75 Although the significance of cholinergic signaling is still being investigated, acetylcholine is likely to act in a paracrine manner to stimulate underlying nerves and smooth muscle, as well as in an autocrine manner to stimulate urothelial (nicotinic; muscarinic) receptors. For example, there is evidence that muscarinic receptors influence urothelial-signaling by enhancing intrinsic detrusor contractions, as well as afferent signaling.69 Some studies have suggested that cholinergic mechanisms might be involved in the release of (yet unidentified) inhibitory factors from the urothelium that depress muscle contractility.76,77 Overall, the release of urothelial-derived acetylcholine and corresponding actions on bladder function is likely to be multifactorial and subject to autoregulation (inhibition/facilitation), which can also be modulated by changes in receptor expression and receptor blockade. Thus, examining the complexities of the non-neuronal acetylcholine synthesis and release machinery (and associated changes in receptor localization/function) could be beneficial to understanding the lack of efficacy/increased adverse effects in some patients, as well as for the development of future therapies.
Nitric oxide
Investigators have also shown that the urothelium expresses both inducible nitric oxide synthase and endothelial NOS.78,79 Urothelial-derived NO can be released in response to mechanical, as well as chemical, stimulation and might either facilitate or inhibit the activity of bladder afferent nerves conveying bladder sensation. For example, reduced levels of NO (through experimental manipulation or pathology) have been shown to result in a bladder hyperactivity that is suggestive of an inhibitory role of NO in bladder function.80 In this regard, activation of urothelial-receptors and the release of inhibitory mediators might explain, in part, the mechanism of action for therapies (e.g. β3-adrenergic receptor agonists) in treatment of bladder disorders such as overactive bladder.
Purinergic signaling
The mechanism underlying the release of chemical mediators from the urothelium, including whether all sensory “inputs” stimulate membrane turnover (i.e. vesicular exocytosis), is not well understood. What little is known about the roles and dynamics of membrane-bound cytoplasmic vesicles in urothelial cell physiology is derived from measurements of membrane capacitance and microscopy of fixed tissues and cells. For example, there is evidence that once released, ATP can act as an important autocrine mediator, which can induce membrane turnover as well as enhance both stretch-induced exocytosis and endocytosis.38 ATP was the first neurotransmitter shown to be released directly from the urothelium by several mechanisms and can interact with P2X (2/3) receptors expressed on nearby afferent neurons. In addition, it has been shown that pathology results in augmented release of ATP from the urothelium, which can enhance excitability of nearby bladder nerves.29,81–84 Urothelial-release of ATP (and autocrine stimulation of urothelial-nucleotide receptors) is likely to contribute to the release of a variety of mediators with the resulting signal likely to be dependent on a number of factors. These include the subtype of purinergic (or adenosine) receptor expressed, as well as the expression of ATPases and other ectonucleotidases. These ecto-enzymes can be secreted or membrane-bound and act to degrade (ATP and UTP) to respective nucleotides, including conversion into adenosine, which activates its own class of P1 receptors also expressed within the urothelium.83,84 Although evidence supports a role for ATP modulating symptoms in several bladder disorders, the mechanisms underlying activation of the micturition pathway at lower bladder volumes (during urgency) are not well understood.
Clinical significance of urothelial signaling
Defects in urothelial sensor molecules and urothelial-cell signaling are likely to contribute to the pathophysiology of bladder diseases. For example, a number of bladder conditions (BPS/IC, spinal cord injury, chemically-induced cystitis) are associated with augmented release of urothelial-derived ATP, which is likely to result in altered sensations or changes in bladder reflexes induced by excitation of purinergic receptors on nearby sensory fibers. ATP can also act in an autocrine manner that could facilitate its own release (and contribute to basal levels of other mediators) from urothelial cells. Augmented expression/release of urothelial-derived chemical mediators is likely to reduce the threshold for activation of nearby bladder afferents. In addition, intercellular communication mediated by gap junctions in myofibroblasts could provide a mechanism for the long-distance spread of signals from the urothelium to the detrusor muscle. Thus, the urothelium has the potential for amplifying signals, both within the urothelium and the bladder wall, and contributing to a gain of function in sensory processing. Factors that can impact on this “gain of function” include alterations in levels of trophic factors, as well as stress and steroid hormones. For example, altered levels of circulating estrogens might play a role in urinary bladder dysfunction, including urgency and frequency. The resulting structural and functional abnormalities might lead to enhanced signaling between the urothelium and underlying cells.
There is evidence that epithelial cells in different organ systems might express similar receptor subtypes.85–87 Accordingly, epithelial cells could use multiple signaling pathways, whose intracellular mechanisms differ according to location and environmental stimuli. This would permit a greater flexibility for the cell to regulate function and respond to complex changes in their surrounding microenvironment. Whether urothelial-sensor molecules all feed into a diverse array of signaling pathways or share similarities with systems such as olfaction, whereby hundreds of receptors share identical transduction cascades,88 is yet to be uncovered.
Changes in epithelial signaling/barrier function would not be unique to the urinary bladder. For example, airway epithelia in asthmatic patients, as well as keratinocytes in certain types of skin diseases, also show a number of similar abnormalities and compromised repair processes.89 This is particularly relevant given the high incidence of associated diseases that can include both visceral and somatic conditions, many of which show a shared loss of epithelial barrier function. For example, recent studies suggest that BPS/IC might share a number of commonalities with other functional pain syndromes.90,91 Patients with BPS/IC also suffer a variety of comorbid disorders that can include irritable bowel syndrome, fibromyalgia, rheumatoid arthritis and even asthma. Although the causes(s) remain unknown, changes in epithelial sensor/barrier function, neurogenic inflammation and even autoimmune involvement might play an important role in the pathogenesis of BPS/IC and comorbid disorders. In addition, multiple organ system involvement might be due in part to organ cross talk, which can exacerbate pelvic pain. Although mechanistic differences are certain to exist, a number of these visceral (and somatic) disorders might also share common features, including reports of stress as a contributing factor in exacerbating the symptoms. In animals, stress-sensitization has been associated with the induction of hyperalgesic states similar to many functional pain syndromes.
Taken together, epithelial cells can respond to a number of challenges (including environmental pollutants and mediators released from nerves or nearby inflammatory cells), resulting in altered expression and/or sensitivity of various receptor/channels as well as changes in release of mediators, all of which could impact sensory function.
Acknowledgments
LAB received NIH/NIDDK grants R37 DK54824 and R01 DK57284.
Abbreviations & Acronyms
- ATP
adenosine triphosphate
- BPS/IC
bladder pain syndrome/interstitial cystitis
- HPA
hypothalamic–pituitary–adrenal
- IC
interstitial cystitis
- ICC
interstitial cells of Cajal
- IC-LP
interstitial cells-lamina propria
- LP
lamina propria
- NGF
nerve growth factor
- NO
nitric oxide
- PGE2
prostaglandin E2
- UPEC
uropathogenic Escherichia coli
Footnotes
Conflict of interest
None declared.
References
- 1.Khanderwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol. 2009;297:F1477–501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004;5:117–28. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Sun TT. Altered phenotype of cultured urothelial and other stratified epithelial cells: implications for wound healing. Am J Physiol. 2006;291:F9–21. doi: 10.1152/ajprenal.00035.2006. [DOI] [PubMed] [Google Scholar]
- 4.Thomas JC, DeMarco RT, Pope JC. Molecular biology of ureteral bud and trigonal development. Curr Urol Rep. 2005;6:146–51. doi: 10.1007/s11934-005-0084-4. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto Y, Ushiki T, Uchida T, Yamada J, Iwanaga T. Scanning electron microscopic observation of apical sites of open-type paraneurons in the stomach, intestine and urethra. Arch Histol Cytol. 1999;62:181–9. doi: 10.1679/aohc.62.181. [DOI] [PubMed] [Google Scholar]
- 6.Czaja K, Sienkiewica W, Vittoria A, Costagliola A, Cecio A. Neuroendocrine cells in the female urogenital tract of the pig, and their immunohistochemical characterization. Acta Anat (Basel) 1996;157:11–19. doi: 10.1159/000147862. [DOI] [PubMed] [Google Scholar]
- 7.Iwanaga T, Han H, Hoshi O, Kanazawa H, Adachi I, Fujita T. Topographical relation between serotonin-containing paraneurons and peptidergic neurons in the intestine and urethra. Biol Signals. 1994;3:259–70. doi: 10.1159/000109553. [DOI] [PubMed] [Google Scholar]
- 8.Saban MR, Memet S, Jackson DG, et al. Visualization of lymphatic vessels through NF-kappaB activity. Blood. 2004;104:3228–30. doi: 10.1182/blood-2004-04-1428. [DOI] [PubMed] [Google Scholar]
- 9.Aitken KJ, Bagli DJ. The bladder extracellular matrix. Part I: architecture, development and disease. Nat Rev Urol. 2009;6:596–611. doi: 10.1038/nrurol.2009.201. [DOI] [PubMed] [Google Scholar]
- 10.Dixon JS, Gosling JA. Histology and fine structure of the muscularis mucosae of the human urinary bladder. J Anat. 1983;136:265–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Gevaert T, De Vos R, Everaerts W, et al. Characterization of upper lamina propria interstitial cells in bladders from patients with neurogenic detrusor overactivity and bladder pain syndrome. J Cell Mol Med. 2011b;15:2586–93. doi: 10.1111/j.1582-4934.2011.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCloskey KD. Interstitial cells in the urinary bladder –localization and function. Neurourol Urodyn. 2010;29:82–7. doi: 10.1002/nau.20739. [DOI] [PubMed] [Google Scholar]
- 13.McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011;202:233–54. doi: 10.1007/978-3-642-16499-6_11. [DOI] [PubMed] [Google Scholar]
- 14.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol. 2004;171:938–43. doi: 10.1097/01.ju.0000108120.28291.eb. [DOI] [PubMed] [Google Scholar]
- 15.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 16.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009;194:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson KE. Detrusor myocyte activity and afferent signaling. Neurourol Urodyn. 2010;29:97–106. doi: 10.1002/nau.20784. [DOI] [PubMed] [Google Scholar]
- 18.Kanai AJ, Andersson KE. Bladder afferent signaling: recent findings. J Urol. 2010;183:1288–95. doi: 10.1016/j.juro.2009.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 20.de Groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res. 1986;67:167–87. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- 21.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141–55. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Gebhart GA. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol. 2007;99:244–53. doi: 10.1152/jn.01049.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–62. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snellings AE, Yoo PB, Grill WM. Urethral flow-responsive afferents in the cat sacral dorsal root ganglia. Neurosci Lett. 2012;516:34–8. doi: 10.1016/j.neulet.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanno P, Andersson KE, Birder L, Elneil S, Kanai A, Pontari M. Chronic pelvic pain syndrome/bladder pain syndrome: taking stock, looking ahead: ICI-RS 2011. Neurourol Urodyn. 2012;31:375–83. doi: 10.1002/nau.22202. [DOI] [PubMed] [Google Scholar]
- 26.Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology. 2007;69:24–33. doi: 10.1016/j.urology.2006.08.1108. [DOI] [PubMed] [Google Scholar]
- 27.Cruz F, Dinis P. Resiniferatoxin and botulinum toxin type A for treatment of lower urinary tract symptoms. Neurourol Urodyn. 2007;26:920–7. doi: 10.1002/nau.20479. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Freire V, Blanchard MG, Burkhard FC, Kessler TM, Kellenberger S, Monastyrskaya K. Acid-sensing channels in human bladder: expression, function and alterations during bladder pain syndrome. J Urol. 2011;186:1509–16. doi: 10.1016/j.juro.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 29.Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8:S3–S26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochodnicky P, Cruz CD, Yoshimura N, Michel MC. Nerve growth factor in bladder dysfunction: contributing factor, biomarker, and therapeutic target. Neurourol Urodyn. 2011;30:1227–41. doi: 10.1002/nau.21022. [DOI] [PubMed] [Google Scholar]
- 31.Lowe EM, Anand P, Terenghi G. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–7. doi: 10.1046/j.1464-410x.1997.00097.x. [DOI] [PubMed] [Google Scholar]
- 32.Schnegelsberg B, Sun TT, Cain G, et al. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol. 2010;298:R534–47. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren K, Dubnew R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthiol. 2008;21:570–9. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birder LA, Wolf-Johnston AS, Chib MK. Beyond neurons: involvement of urothelial and glial cells in bladder function. Neurourol Urodyn. 2010;29:88–96. doi: 10.1002/nau.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradesi S, Golovatschka V, Ennes HS, et al. Role of astrocytes and altered regulation of spinal glutamatergic neurotransmission in stress-induced visceral hyperalgesia in rats. Am J Physiol Gastrointest Liver. 2011;301:G580–9. doi: 10.1152/ajpgi.00182.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parson CL, Boychuk D, Jones S, Hurst R, Callahan H. Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol. 1990;143:139–42. doi: 10.1016/s0022-5347(17)39897-x. [DOI] [PubMed] [Google Scholar]
- 38.Wang EC, Lee JM, Ruiz WG, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–22. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hicks M. The mammalian urinary bladder: an accomodating organ. Biol Rev. 1975;50:215–46. doi: 10.1111/j.1469-185x.1975.tb01057.x. [DOI] [PubMed] [Google Scholar]
- 40.Balestreire EM, Apodaca G. Apical EGF receptor signaling: regulation of stretch-dependent exocytosis in bladder umbrella cells. Mol Biol Cell. 2007;13:830–43. doi: 10.1091/mbc.E06-09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romih R, Korosec P, de Mello W, Jezernik K. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005;320:259–68. doi: 10.1007/s00441-004-1005-4. [DOI] [PubMed] [Google Scholar]
- 42.Martin BF. Cell replacement and differentiation in transitional epithelium: a histological and autoradiographic study of the guinea-pig bladder and ureter. J Anat. 1972;112:433–55. [PMC free article] [PubMed] [Google Scholar]
- 43.Hicks M, Ketterer B, Warren R. The ultrastructure and chemistry of the luminal plasma membrane of the mammalian urinary bladder: a structure with low permeability to water and ions. Phil Trans R Soc Lond. 1974;268:23–38. doi: 10.1098/rstb.1974.0013. [DOI] [PubMed] [Google Scholar]
- 44.Kreft ME, Sterle M, Veranic P, Jezernik K. Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol. 2005;123:529–39. doi: 10.1007/s00418-005-0770-9. [DOI] [PubMed] [Google Scholar]
- 45.Kanai AJ, Zeidel M, Lavelle JP, et al. Manganese superoxide dismutase gene therapy protects against irradiation-induced cystitis. Am J Physiol. 2002;283:F1304–12. doi: 10.1152/ajprenal.00228.2002. [DOI] [PubMed] [Google Scholar]
- 46.Kreft ME, Jezernik K, Kreft M, Romih R. Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann NY Acad Sci. 2009;1152:18–29. doi: 10.1111/j.1749-6632.2008.04004.x. [DOI] [PubMed] [Google Scholar]
- 47.Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol. 2003;284:F966–F76. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- 48.Lavelle JP, Meyers SA, Ruiz WG, Buffington CAT, Zeidel M, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. 2000;278:F540–F53. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman J, Tyagi P, Chancellor MB. Intravesical liposomal (LP09) instillation protects bladder urothelium from chemical irritation. Am Urol Assoc. 2009;182:1506. [Google Scholar]
- 50.Nirmal J, Tyagi P, Dang L, et al. Endocytosis uptake of liposomes in urothelium cells detected by transmission electron microscopy. Am Urol Assoc. 2012;187:1202359. [Google Scholar]
- 51.Peters KM, Hasenau DL, Anthony M, Kaufman J, Killinger KA, Chancellor MB. Novel therapy with intravesical liposomes for ulcerative interstitial cystitis/painful bladder syndrome. LUTS. 2012;4:51–3. doi: 10.1111/j.1757-5672.2011.00108.x. [DOI] [PubMed] [Google Scholar]
- 52.Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology. 2011;94:12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- 53.Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendrix S. Neuroimmune communication in skin: far from peripheral. J Invest Dermatol. 2008;128:260–1. doi: 10.1038/sj.jid.5701171. [DOI] [PubMed] [Google Scholar]
- 56.Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–49. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson G, Palermo J, Schilling J, Roth R, Heuser J, Hultgren S. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–7. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 58.Schilling J, Hultgren S. Recent advances into the pathogenesis of recurrent urinary tract infections: the bladder as a reservoir for uropathogenic Escherichia coli. Int J Antimicrob Agents. 2002;19:457–560. doi: 10.1016/s0924-8579(02)00098-5. [DOI] [PubMed] [Google Scholar]
- 59.Feneley RSL, Kunin CM, Stickler DJ. An indwelling urinary catheter for the 21st century. BJU Int. 2011;109:1746–9. doi: 10.1111/j.1464-410X.2011.10753.x. [DOI] [PubMed] [Google Scholar]
- 60.Thumbikat P, Berry RE, Zhou G, et al. Bacteria-induced uroplakin signaling mediates bladder response to infection. PLoS Pathog. 2009;5:1–17. doi: 10.1371/journal.ppat.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudick CN, Billips BK, Pavlov VI, Yaggie RE, Schaeffer AJ, Klumpp DJ. Host-pathogen interactions mediating pain of urinary tract infection. J Infect Dis. 2010;201:1240–9. doi: 10.1086/651275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birder LA, Nakamura Y, Kiss S, et al. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–60. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- 63.Jen PY, Dixon JS, Gosling JA. Immunohistochemical localization of neuromarkers and neuropeptides in human fetal and neonatal urinary bladder. Br J Urol. 1995;75:230–5. doi: 10.1111/j.1464-410x.1995.tb07317.x. [DOI] [PubMed] [Google Scholar]
- 64.Grol S, van Koeveringe GA, de Vente J, van Kerrebroeck PE, Gillespie JI. Regional differences in sensory innervation and suburothelial interstitial cells in the bladder neck and urethra. BJU Int. 2008;102:870–7. doi: 10.1111/j.1464-410X.2008.07752.x. [DOI] [PubMed] [Google Scholar]
- 65.Birder LA, Ruggieri M, Takeda M, et al. How does the urothelium affect bladder function in health and disease? Neurourol Urodyn. 2012;31:293–9. doi: 10.1002/nau.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Birder LA, DeGroat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int. 2007;72:1057–64. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 68.Ikeda Y, Fry C, Hayashi F, Stolz D, Griffiths D, Kanai A. Role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol. 2007;293:F1018–25. doi: 10.1152/ajprenal.00183.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeda Y, Kanai A. Urotheliogenic modulation of intrinsic activity in spinal cord-transected rat bladders: role of mucosal muscarinic receptors. Am J Physiol. 2008;295:F454–61. doi: 10.1152/ajprenal.90315.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fry CH, Young JS, Jabr RI, McCarthy C, Ikeda Y, Kanai AJ. Modulation of spontaneous activity in the overactive bladder: the role of P2Y agonists. Am J Physiol. 2012;302:F1447–54. doi: 10.1152/ajprenal.00436.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arrighi N, Bodei S, Peroni A, et al. Detection of muscarinic receptor subtypes in human urinary bladder mucosa: age and gender-dependent modifications. Neurourol Urodyn. 2008;27:421–8. doi: 10.1002/nau.20521. [DOI] [PubMed] [Google Scholar]
- 72.Bschleipfer T, Schukowski K, Weidner W, et al. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci. 2007;80:2303–7. doi: 10.1016/j.lfs.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 73.Grol S, Essers PB, van Koeveringe GA, Martinez-Martinez P, de Vente J, Gillespie JI. M3 muscarinic receptor expression on suburothelial interstitial cells. BJU Int. 2009;104:398–405. doi: 10.1111/j.1464-410X.2009.08423.x. [DOI] [PubMed] [Google Scholar]
- 74.Mansfield KJ, Liu L, Mitchelson FJ, Moore KH, Millard RJ, Burcher E. Muscarinic receptor subtypes in human bladder detrusor and mucosa, studied by radioligand binding and quantitative competitive RT-PCR: changes in aging. Br J Pharmacol. 2005;144:1089–99. doi: 10.1038/sj.bjp.0706147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukerji G, Yiangou Y, Grogono J, et al. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol. 2006;176:367–73. doi: 10.1016/S0022-5347(06)00563-5. [DOI] [PubMed] [Google Scholar]
- 76.Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–19. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chess-Williams R. Muscarinic receptors of the urinary bladder: detrusor, urothelial and prejunctional. Auton Autacoid Pharmacol. 2002;22:133–45. doi: 10.1046/j.1474-8673.2002.00258.x. [DOI] [PubMed] [Google Scholar]
- 78.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol. 1995;175:43–53. [PubMed] [Google Scholar]
- 79.Birder LA, Nealen ML, Kiss S, et al. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–70. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol. 2000;164:545–50. [PubMed] [Google Scholar]
- 81.Ford AP, Cockayne DA. ATP and P2X purinoceptors in urinary tract disorders. Handb Exp Pharmacol. 2011;202:485–526. doi: 10.1007/978-3-642-16499-6_22. [DOI] [PubMed] [Google Scholar]
- 82.Burnstock G. Targeting the visceral purinergic system for pain control. Curr Opin Pharmacol. 2012;12:80–6. doi: 10.1016/j.coph.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009;42:3–8. doi: 10.1590/s0100-879x2008005000037. [DOI] [PubMed] [Google Scholar]
- 84.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–8. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 85.Folkerts G, Nijkamp FP. Airway epithelium: more than just a barrier. Trends Pharmacol Sci. 1998;19:334–241. doi: 10.1016/s0165-6147(98)01232-2. [DOI] [PubMed] [Google Scholar]
- 86.Kummer W, Wiegand S, Akinci S, et al. Role of acetylcholine and muscarinic receptors in serotonin-induced bronchoconstriction in the mouse. J Mol Neurosci. 2006;30:67–8. doi: 10.1385/JMN:30:1:67. [DOI] [PubMed] [Google Scholar]
- 87.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–65. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 88.Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–3. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- 89.Bosse Y, Pare PD, Seow CY. Airway wall remodeling in asthma: from the epithelial layer to the adventitia. Curr Allergy Asthma Rep. 2008;8:357–66. doi: 10.1007/s11882-008-0056-0. [DOI] [PubMed] [Google Scholar]
- 90.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat Clin Pract Urol. 2007;4:484–91. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 91.Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol. 2004;172:1242–8. doi: 10.1097/01.ju.0000137953.49304.6c. [DOI] [PubMed] [Google Scholar]