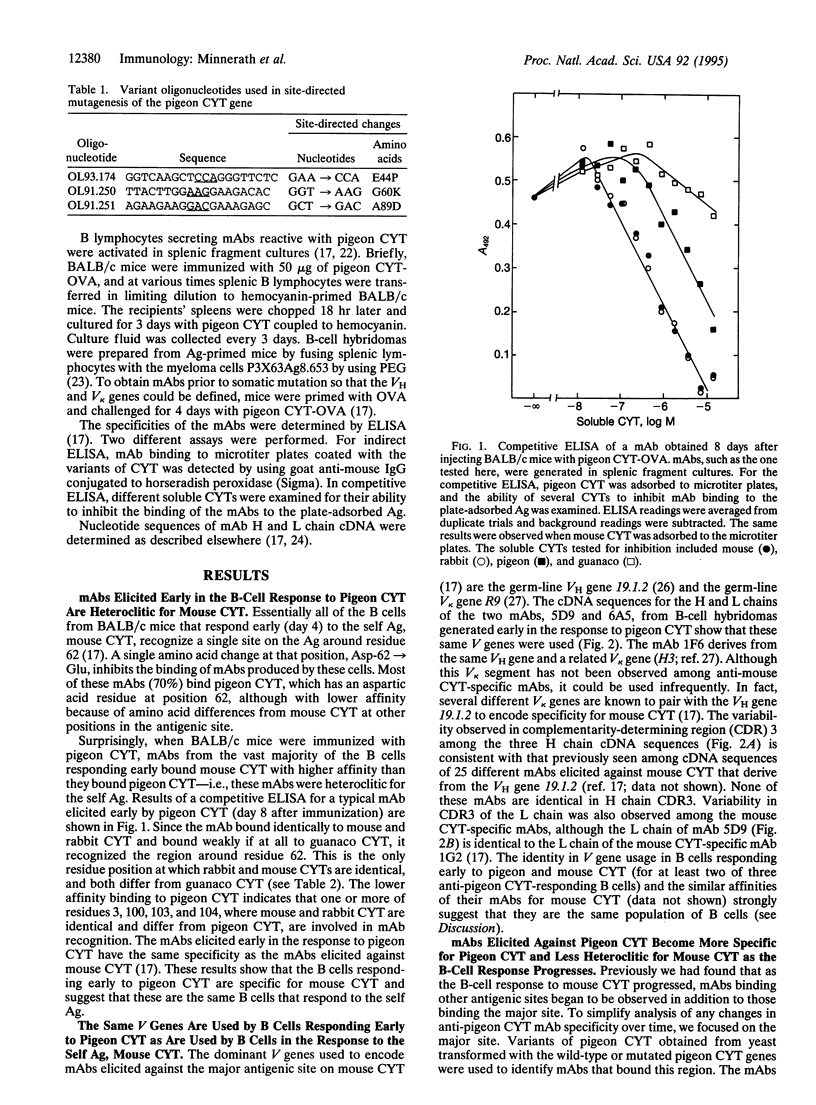
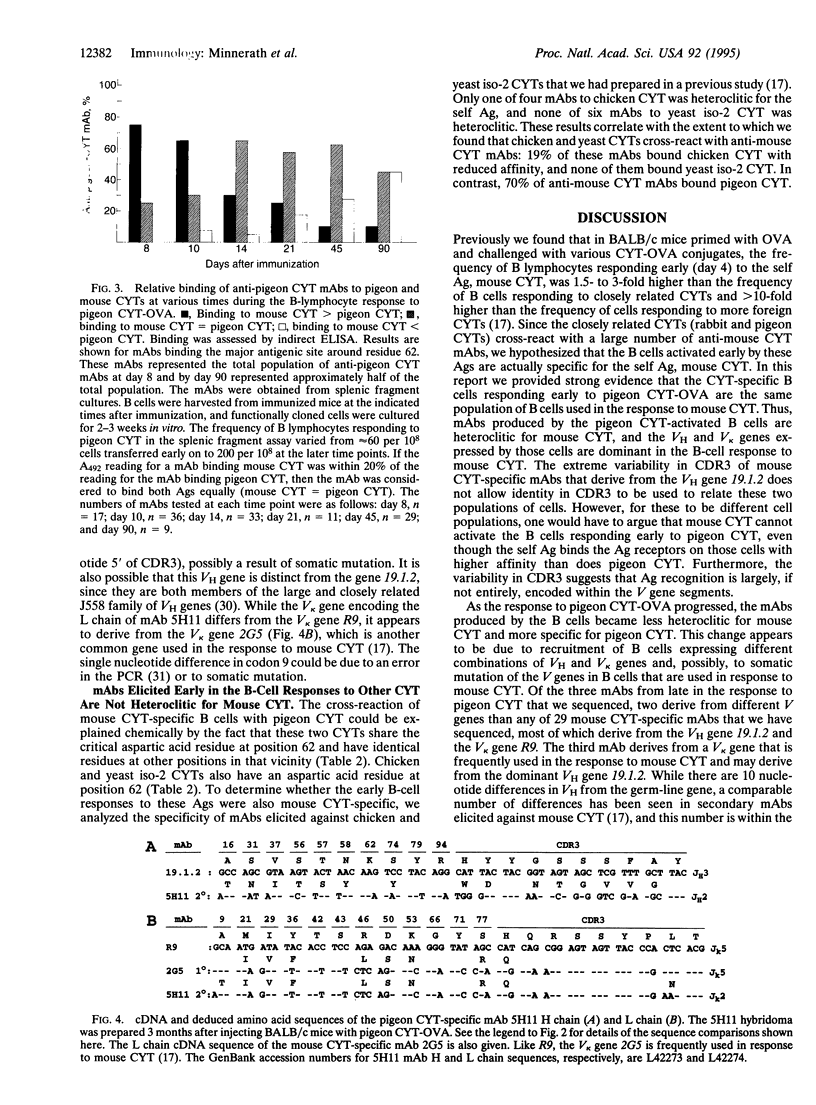
Abstract
Direct evidence is presented in support of the longstanding but unproven hypothesis that B lymphocytes specific for self antigens (Ags) can be used in the immune response to foreign Ags. We show that the B cells in BALB/c mic responding early to pigeon cytochrome c (CYT) produce antibodies that recognize and bind the major antigenic site on mouse CYT with greater affinity than they bind pigeon CYT i.e., they are heteroclitic for the self Ag. Furthermore, these B cells express the same combination of immunoglobulin variable region (V) genes that are known to be used in B-cell recognition of mouse CYT. Over time, the response to pigeon CYT becomes more specific for the foreign Ag through the recruitment of B cells expressing different combinations of V genes and, possibly, somatic mutation of the mouse CYT specific B cells from early in the response. Cross-recognition of pigeon CYT by mouse CYT-specific B cells results from the sharing of critical amino acid residues by the two Ags. Although B-cell recognition of the self Ag, mouse CYT, is very specific, which limits the extent to which foreign Ags can cross-activate the autoreactive B cells, it is possible that polyreactive B cells to other self Ags may be used more frequently in response to foreign Ags.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein S., Pritchard-Briscoe H., Anderson T. A., Crosbie J., Gammon G., Loblay R. H., Basten A., Goodnow C. C. Induction of self-tolerance in T cells but not B cells of transgenic mice expressing little self antigen. Science. 1991 Mar 8;251(4998):1223–1225. doi: 10.1126/science.1900950. [DOI] [PubMed] [Google Scholar]
- Akolkar P. N., Sikder S. K., Bhattacharya S. B., Liao J., Gruezo F., Morrison S. L., Kabat E. A. Different VL and VH germ-line genes are used to produce similar combining sites with specificity for alpha(1----6)dextrans. J Immunol. 1987 Jun 15;138(12):4472–4479. [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The natural autoantibodies system: between hypotheses and facts. Mol Immunol. 1993 Aug;30(12):1133–1142. doi: 10.1016/0161-5890(93)90160-d. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Carlson S. S., Mross G. A., Wilson A. C., Mead R. T., Wolin L. D., Bowers S. F., Foley N. T., Muijsers A. O., Margoliash E. Primary structure of mouse, rat, and guinea pig cytochrome c. Biochemistry. 1977 Apr 5;16(7):1437–1442. doi: 10.1021/bi00626a031. [DOI] [PubMed] [Google Scholar]
- Clarke S. H., Huppi K., Ruezinsky D., Staudt L., Gerhard W., Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson J., Radic M. Z., Camper S. A., Hardy R. R., Carmack C., Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991 Jan 24;349(6307):331–334. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- Even J., Griffiths G. M., Berek C., Milstein C. Light chain germ-line genes and the immune response to 2-phenyloxazolone. EMBO J. 1985 Dec 16;4(13A):3439–3445. doi: 10.1002/j.1460-2075.1985.tb04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Goshorn S. C., Retzel E., Jemmerson R. Common structural features among monoclonal antibodies binding the same antigenic region of cytochrome c. J Biol Chem. 1991 Feb 5;266(4):2134–2142. [PubMed] [Google Scholar]
- Grabar P. Hypothesis. Auto-antibodies and immunological theories: an analytical review. Clin Immunol Immunopathol. 1975 Nov;4(4):453–466. doi: 10.1016/0090-1229(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hartley S. B., Crosbie J., Brink R., Kantor A. B., Basten A., Goodnow C. C. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991 Oct 24;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hickey D. R., Jayaraman K., Goodhue C. T., Shah J., Fingar S. A., Clements J. M., Hosokawa Y., Tsunasawa S., Sherman F. Synthesis and expression of genes encoding tuna, pigeon, and horse cytochromes c in the yeast Saccharomyces cerevisiae. Gene. 1991 Aug 30;105(1):73–81. doi: 10.1016/0378-1119(91)90515-d. [DOI] [PubMed] [Google Scholar]
- Jacob J., Przylepa J., Miller C., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993 Oct 1;178(4):1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Klinman N. R. Antibody with homogeneous antigen binding produced by splenic foci in organ culture. Immunochemistry. 1969 Sep;6(5):757–759. doi: 10.1016/0019-2791(67)90140-1. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Minnerath J. M., Crump B. L., Margoliash E., Jemmerson R. Major and minor epitopes on the self antigen mouse cytochrome c mapped by site-directed mutagenesis. Mol Immunol. 1995 Aug;32(11):795–803. doi: 10.1016/0161-5890(95)00050-o. [DOI] [PubMed] [Google Scholar]
- Minnerath J. M., Mueller C. M., Buron S., Jemmerson R. B lymphocyte recognition of cytochrome c: higher frequency of cells specific for self versus foreign antigen early in the immune response and V gene usage in the response to self antigen. Eur J Immunol. 1995 Mar;25(3):784–791. doi: 10.1002/eji.1830250324. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Negative selection of lymphocytes. Cell. 1994 Jan 28;76(2):229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Portnoï D., Freitas A., Holmberg D., Bandeira A., Coutinho A. Immunocompetent autoreactive B lymphocytes are activated cycling cells in normal mice. J Exp Med. 1986 Jul 1;164(1):25–35. doi: 10.1084/jem.164.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar B. S., Saegusa J., Onodera T., Notkins A. L. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B cell repertoire. J Immunol. 1984 Dec;133(6):2815–2817. [PubMed] [Google Scholar]
- Roth M. S., Weiner G. J., Allen E. A., Terry V. H., Harnden C. E., Boehnke M., Kaminski M. S., Ginsburg D. Molecular characterization of anti-idiotype antibody-resistant variants of a murine B cell lymphoma. J Immunol. 1990 Jul 15;145(2):768–777. [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Heavy-chain directed B-cell maturation: continuous clonal selection beginning at the pre-B cell stage. Immunol Today. 1994 Jan;15(1):27–32. doi: 10.1016/0167-5699(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Souroujon M., White-Scharf M. E., Andreschwartz J., Gefter M. L., Schwartz R. S. Preferential autoantibody reactivity of the preimmune B cell repertoire in normal mice. J Immunol. 1988 Jun 15;140(12):4173–4179. [PubMed] [Google Scholar]
- Stollar B. D. Autoantibodies and autoantigens: a conserved system that may shape a primary immunoglobulin gene pool. Mol Immunol. 1991 Dec;28(12):1399–1412. doi: 10.1016/0161-5890(91)90042-i. [DOI] [PubMed] [Google Scholar]



