Background: The mechanism underlying the re-entering of mature miRNAs into the nucleus remains unknown.
Results: IPO8 mediates the transport of mature miRNAs into the nucleus through binding to Ago2.
Conclusion: IPO8 is a candidate carrier for nuclear import of mature miRNAs.
Significance: This finding extends the gene regulatory network of miRNAs.
Keywords: MicroRNA, Nuclear Transport, Polymerase Chain Reaction (PCR), RNA Processing, siRNA
Abstract
Mature microRNAs (miRNAs), ∼22-nucleotide noncoding RNAs regulating target gene expression at the post-transcriptional level, have been recently shown to be transported into the nucleus where they modulate the biogenesis of other miRNAs or their own expression. However, the mechanism that governs the transport of mature miRNAs from cytoplasm to nucleus remains unknown. Here, we report that importin 8 (IPO8), a member of the karyopherin β (also named the protein import receptor importin β) family, plays a critical role in mediating the cytoplasm-to-nucleus transport of mature miRNAs. Specifically knocking down IPO8 but not other karyopherin β family proteins via siRNA significantly decreases the nuclear transport of various known nucleus-enriched miRNAs without affecting their total cellular levels. IPO8-mediated nuclear transport of mature miRNAs is also dependent on the association of IPO8 with the Argonaute 2 (Ago2) complex. Cross-immunoprecipitation and Western blot analysis show that IPO8 is physically associated with Ago2. Knocking down IPO8 via siRNA markedly decreases the nuclear transport of Ago2 but does not affect the total cellular Ago2 level. Furthermore, dissociating the binding of miRNAs with Ago2 by trypaflavine strongly reduces the IPO8-mediated nuclear transport of miRNAs.
Introduction
MicroRNAs (miRNAs),5 a class of 19–24-nucleotide noncoding RNAs (ncRNAs), are widely involved in post-transcriptional gene regulation (1). According to the canonical model, miRNAs recognize target mRNAs and exert their roles predominantly in the cytoplasm. Accumulating evidences, however, have demonstrated that certain mature miRNAs can re-enter the nucleus (2–4). Földes-Papp et al. (5) were the first to report the expression of miR-122 in liver cells and show unequivocal proof for the relocation of cytoplasm-assembled single-stranded miR-122 into the cell nucleus. Hwang et al. (4) demonstrated that miR-29b could be imported into the cell nucleus. Interestingly, they demonstrated that, at least for miR-29b, it is a distinctive hexanucleotide element (AGUGUU) that distinguishes miR-29b from its family member miR-29a and guides its nucleus import. Consistently, studies have also reported the presence of active miRNA effectors, such as Argonaute proteins, in the nucleus (6). These findings suggest that there are mechanisms to guide the cytoplasmic-nuclear transport of miRNAs and Argonaute proteins and that miRNAs, after re-entering into the nucleus, may also have biological functions. Indeed, Meister et al. (2) showed that nucleus miR-21 could guide the cleavage of target RNA, suggesting a role of nucleus miRNA in RNA processing. Further studies by us (7) and others (8) demonstrated that mature miRNAs, such as miR-709 or let-7, were not only transported into the nucleus but also modulated the biogenesis of other miRNAs or its own expression in the nucleus. Identification of the relocation and function of mature miRNAs in the nucleus significantly extended the gene regulatory network of miRNAs. However, the mechanism that governs the transport of mature miRNAs from cytoplasm to nucleus remains completely unknown.
The karyopherin β family receptors are the major transporters that are specific for nucleocytoplasmic cargoes in mammalian cells. A major function of these transporters is to mediate the transport between the cytoplasm and nucleus of macromolecules that contain nuclear import or export signals (9, 10). As they constantly shuttle between the nucleus and cytoplasm, these karyopherin β family receptors can be found in the nucleus and cytoplasm. All members have the ability to recognize specific cargoes, Ran GTP or nucleoporins. The interaction between the karyopherin β family receptors and nucleoporin repeats contributes to the import and export of karyopherin β family receptor/cargoes through the central transporter of the nuclear pore complex (11–13). It remains unknown whether or how the karyopherin β family receptors mediate the transport of mature miRNAs from the cytoplasm into the nucleus.
In the present study, we explored the role of karyopherin β family receptors in regulating the nuclear transport of mature miRNAs. Our results showed that importin 8 (IPO8), a member of the protein import receptor importin β (also named karyopherin β) family, plays a critical role in mediating the cytoplasm-to-nucleus transport of mature miRNAs, and IPO8-mediated transportation requires the help of Argonaute 2 (Ago2) complex.
EXPERIMENTAL PROCEDURES
Reagents, Cells, and Antibodies
The mouse L929 cell line was purchased from the China Cell Culture Center (Shanghai, China). Cells were maintained at 37 °C in a humidified 5% CO2 incubator in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) that contained 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. The anti-Ago2 (ab57113 for immunoprecipitation, ab32381 for immunoblotting), anti-importin-β (ab2811), anti-importin-4 (ab99271), anti-importin-7 (ab99273), and anti-importin-11 (ab118415) antibodies were purchased from Abcam (Hangzhou, China). Trypaflavine (TPF) (14) was purchased from Sigma-Aldrich. The monoclonal anti-GAPDH and anti-histone H2A antibodies and protein G-Agarose (sc-2003) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-importin-8 (LS-C47292) antibody was purchased from LifeSpan BioSciences (Seattle, WA). Alexa Fluor 488-conjugated anti-mouse and Alexa Fluor 594-conjugated anti-rabbit antibodies were purchased from Molecular Probes. The miR-709 mimics and IPO8 siRNA were purchased from RIBOBIO (Guangzhou, China). After testing the effect of all four IPO8 siRNA oligonucleotides, we selected oligonucleotide (5′-GUAUACAGCUGCAAAGAAAdTdT-3′) and used this single siRNA in an IPO8 knockdown experiment.
Preparation of Nuclear Extracts
The nuclear fraction of cells was extracted using a PARISTM kit (Ambion). Cells were washed three times with PBS on ice followed by centrifugation at 300 × g for 5 min. Cell pellets were resuspended in cell fraction buffer from the PARISTM kit, incubated on ice for 10 min, and then centrifuged at 500 × g for 5 min at 4 °C. Nuclear pellets were homogenized with the cell disruption buffer from the PARISTM kit.
RNA Isolation and qRT-PCR of mRNA and Mature miRNAs
Total cellular RNA was extracted using a miRNeasy mini kit (Qiagen, Shanghai, China). The qRT-PCR was performed using TaqMan probes (Applied Biosystems) for mature miRNAs or SYBR Green (Takara, Mountain View, CA) for mRNA. Briefly, total RNA was reverse-transcribed to cDNA using AMV reverse transcriptase (Takara) and a stem-loop RT primer or reverse primer (Applied Biosystems). Real-time PCR was performed on an Applied Biosystems 7900 sequence detection system (Applied Biosystems). All of the reactions, including the no-template controls, were run in triplicate. After the reactions, the cycle threshold (CT) values were determined using fixed threshold settings. The miRNA expression in the cells or nuclei was normalized to U6 snRNA, and mRNA expression in the cells was normalized to GAPDH.
Transfection of Cells with miRNA Mimic and siRNA
Cells were seeded in 6-well plates or 10-mm dishes, and they were transfected the following day using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For the transfection, 20 pmol of RNA per 105 cells was used. Cells were harvested 48 h after transfection for real-time PCR analysis and Western blotting.
Immunoprecipitation and immunoblotting
Cells were lysed with lysis buffer (20 mm Tris-HCl, 150 mm NaCl, 0.5% Nonidet P-40, 2 mm EDTA, 0.5 mm DTT, 1 mm NaF, 1 mm PMSF, and 1% protease inhibitor cocktail from Sigma, pH 7.5) for 30 min on ice. The lysates were cleared by centrifugation (16,000 × g) for 10 min at 4 °C and then immunoprecipitated with anti-Ago2 antibody, anti-importin-8 antibody, or normal IgG followed by protein G-Agarose beads. After the elution, the RNA was extracted using a miRNeasy mini kit. The anti-Ago2 or anti-importin-8 antibody was used for the Western blot analysis.
Immunofluorescence
Immunofluorescence microscopy was used to identify the subcellular localization of importin-8 and Ago2 in L929 cells. Cells were cultured on 4-well chamber slides. At the time of harvest, cells were fixed with 4% paraformaldehyde and then permeabilized with 0.01% Triton X-100 for 10 min and subsequently probed with antibodies against importin-8 and Ago2 followed by incubation with fluorescent-tagged secondary antibodies (488 and 594 nm). All samples were treated with DAPI dye for nuclear staining (358 nm). For confocal microscopy, the Olympus FV1000 confocal microscope was used.
Statistical Analysis
All of the images of the Western blots and qRT-PCR assays were representative of at least three independent experiments. The qRT-PCR was performed in triplicate. The data were presented as the mean ± S.D. for at least three independent experiments. The differences were considered to be statistically significant at p < 0.05, assessed using Student's t test.
RESULTS
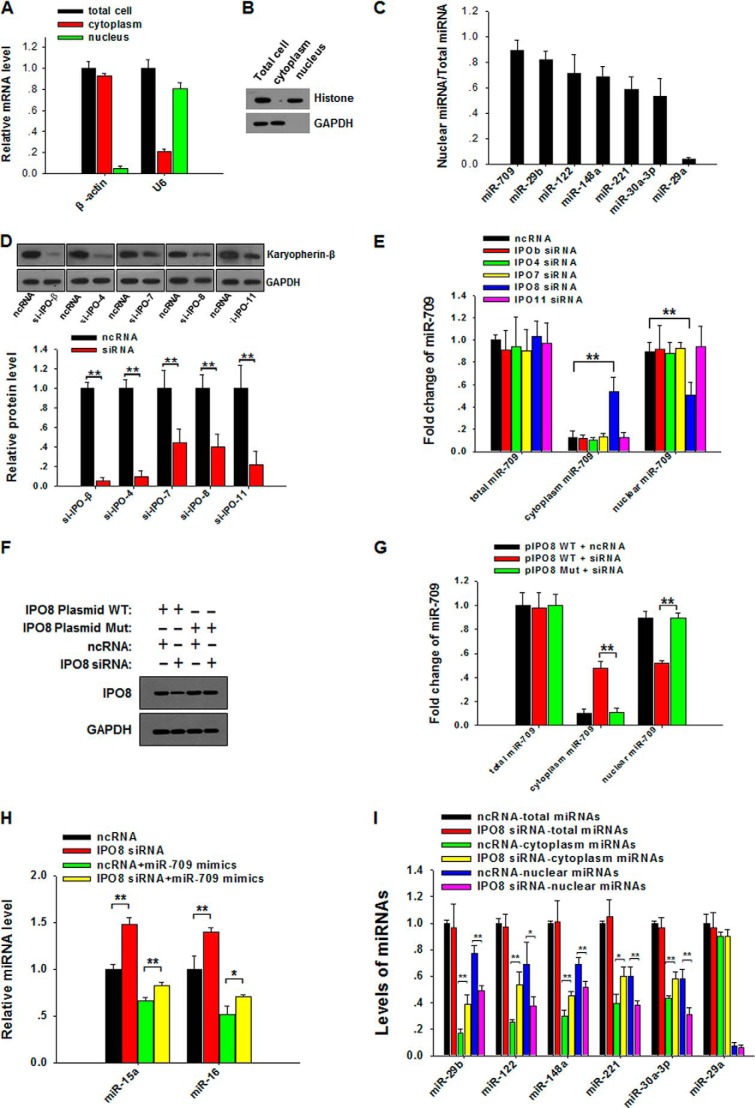
To determine which karyopherin β family member is involved in transporting mature miRNAs into the nucleus, we screened the proteins in the karyopherin β family for their effect on the nuclear distribution of miR-709, a mouse miRNA that is specifically localized to the cell nucleus (7). In this experiment, a nuclear fraction with high purity was isolated from mouse cell line L929 cells. As shown in Fig. 1, A and B, the isolated nuclear fraction displayed a high level of nuclear markers (U6 snRNA and histones) but a low level of cytoplasmic markers (β-actin and GAPDH). Additional evidence for the purity of the isolated nuclear fraction came from qRT-PCR identification of six miRNAs that had previously been detected in the nucleus, including miR-29b (4, 7), miR-122 (5), miR-148a (15), miR-221 (7), miR-30a-3p (7), and miR-709 (7) (Fig. 1C). As a cytoplasmic miRNA (4, 7), miR-29a was not detected in the isolated nuclear fraction (Fig. 1C). By knocking down the expression of each karyopherin β family member in L929 cells via specific siRNAs (Fig. 1D), we found that the nuclear distribution of miR-709 in L929 cells was strongly blocked when cells were transfected with IPO8 siRNA but not with the siRNAs against other karyopherin β proteins (Fig. 1E). Interestingly, IPO8 knockdown did not affect the total cellular level of miR-709, and down-regulation of nuclear miR-709 led to the accumulation of miR-709 in the cytoplasm. To exclude the possibility that the reduction of the nuclear level of miR-709 is due to the off-target effect of IPO8 siRNA, we performed a rescue experiment using an siRNA-resistant IPO8-expressing plasmid. In this experiment, five bases within the siRNA-binding sequence (ttg tat(c) aca(g) gct(c) gca(g) aag(a) aaa) of wild-type IPO8-expressing plasmid (pIPO8 WT) were mutated to generate an IPO8 mutant-expressing plasmid (pIPO8 Mut). With the mutation, pIPO8 Mut expresses normal IPO8 but is not targeted by IPO8 siRNA. We next transfected L929 cells with pIPO8 WT or pIPO8 Mut and then challenged the cells with IPO8-siRNA or control oligonucleotide. After 48 h, cellular levels of IPO8 and nuclear levels of miR-709 were assayed by Western blot and qRT-PCR, respectively. As can be seen, the reduction of both IPO8 (Fig. 1F) and the nuclear transport of miR-709 (Fig. 1G) by IPO8 siRNA was completely rescued by transfection with pIPO8 Mut.
FIGURE 1.
IPO8 transports mature miRNAs into the nucleus. A, quality controls of the preparation of cytoplasmic and nuclear fractions. Levels of β-actin mRNA and U6 snRNA in purified nuclear fractions were detected by qRT-PCR. B, levels of GAPDH and histone detected by Western blot (WB) in purified nuclear fractions. C, levels of six known nuclear miRNAs in purified nuclear fractions from L929 cells. D, L929 cells were transfected with the siRNAs against IPOβ, IPO4, IPO7, IPO8, or IPO11, respectively. Protein levels were detected by WB (upper panel). Quantitative analysis of WB results is shown in the lower panel. E, nuclear and cytoplasmic expression levels of miR-709 in L929 cells transfected with control oligonucleotide (ncRNA), IPOβ siRNA, IPO4 siRNA, IPO7 siRNA, IPO8 siRNA, or IPO11 siRNA. F, L929 cells transfected with wild-type IPO8-expressing plasmid (pIPO8 WT) or mutant IPO8-expressing plasmid (pIPO8 Mut) were treated with IPO8 siRNA or control oligonucleotide. At 48 h after transfection, cell lysates were blotted for IPO8 or GAPDH. G, the reduction of nuclear miR-709 level induced by IPO8 siRNA was rescued when cells were transfected with pIPO8 Mut. H, cellular level of miR-15a and miR-16 in L929 cells after transfection with control miRNA mimics, miR-709 mimics, or IPO8 siRNA 48 h after ncRNA or IPO8 siRNA transfection. I, nuclear and cytoplasmic levels of other five nuclear miRNAs in L929 cells transfected with IPO8 siRNA. *, p < 0.05; **, p < 0.01.
Because our previous study showed that nuclear miR-709 could suppress the biogenesis of miR-15a and miR-16 (7), we speculated that blockade of nuclear transport of miR-709 would elevate the level of miR-15a and miR-16. Indeed, the IPO8 siRNA treatment significantly increased the cellular levels of miR-15a and miR-16 (Fig. 1H). These results suggest that IPO8 may be the receptor that mediates mature miR-709 transport from cytoplasm to nucleus. We next determined whether IPO8 modulated the nuclear transport of the other five nuclear miRNAs. As shown in Fig. 1I, IPO8 knockdown also significantly reduced the nuclear levels of all five miRNAs, whereas the total cellular levels of the five miRNAs were not affected. As a cytoplasmic miRNA, miR-29a was tested as a control in this experiment. As can be seen, IPO8 knockdown affected neither the nuclear level nor the total cellular level of miR-29a. The role of IPO8 in mediating the transport of mature miRNAs into the nucleus was also observed in human cell line 293T cells (data not shown).
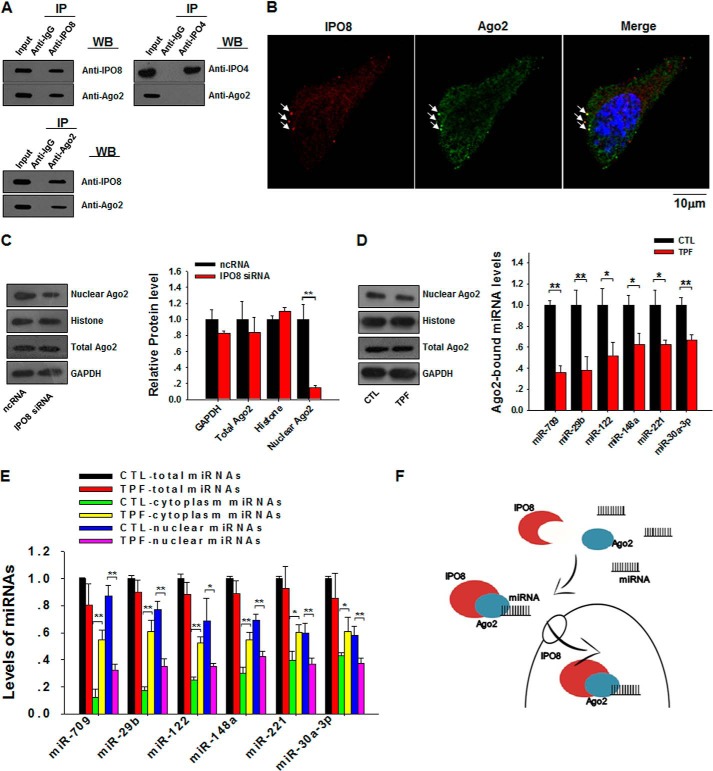
To serve as a carrier for nuclear transport of mature miRNAs, IPO8 must be able to selectively associate with miRNAs that have nuclear import signals. However, such import signals are largely unknown, and so far, there has been no evidence showing that IPO8 can directly bind to mature miRNAs. Given that IPO8 can interact with Ago proteins (16), we postulated that IPO8 might associate with miRNAs through the Ago2 complex. Immunofluorescence labeling and co-immunoprecipitation experiments were then performed to test this hypothesis. As observed in Fig. 2A, co-immunoprecipitation and cross-blotting also clearly demonstrated a binding between IPO8 and Ago2. Serving as a control, immunoprecipitation of IPO4 via anti-IPO4 antibody did not result in co-immunoprecipitation of Ago2. Double-stained L929 cells showed an apparent co-localization of Ago2 (green) and IPO8 (red) (Fig. 2B, arrows). Furthermore, IPO8 knockdown dramatically reduced the nuclear level of Ago2 but not the total cellular level of Ago2 (Fig. 2C), implying that the import of Ago2 into the nucleus is IPO8-dependent. Reduction of the nuclear Ago2 pool by IPO8 knockdown has also been demonstrated recently by Weinmann et al. (16). The involvement of the Ago2 complex in IPO8-mediated cytoplasm-to-nucleus transport of miRNAs is further supported by the observation that TPF, a small molecule that specifically disrupts the association of Ago2 with miRNAs (14, 17), blocked the cytoplasm-to-nuclear transport of all six nuclear miRNAs. As shown in Fig. 2D, TPF treatment did not affect Ago2 level in the nuclear fraction or in the whole cell (left panel) but significantly disrupted the binding of Ago2 with miRNAs (right panel). As expected, although TPF treatment did not change the nuclear transport of Ago2, it strongly reduced the nuclear transport of miR-709 and other nuclear miRNAs (Fig. 2E). Taken together, our results demonstrate for the first time that IPO8, with the help of the Ago2 complex, plays an essential role in mediating miRNA nuclear transport.
FIGURE 2.
IPO8 mediates nuclear transport of mature miRNAs in an Ago2-dependent manner. A, IPO8 and Ago2 were separately immunoprecipitated (IP) from total cell lysates and then cross-blotted by anti-IPO8 and anti-Ago2 antibodies, respectively. B, partial co-localization of Ago2 (green, arrows) with IPO8 (red, arrows) in L929 cells. Nucleus was counterstained with DAPI (blue). C, significant reduction of Ago2 level in the nucleus but not total cellular Ago2 level by IPO8 knockdown. Quantitative analysis of WB results is shown on the right. D, TPF treatment reduces the levels of miRNAs bound to Ago2 complex but not total or nuclear Ago2. E, effect of TPF on nuclear transport of miRNAs. L929 cells were treated with or without 8 μm TPF for 2 days. F, a model for IPO8-mediated cytoplasmic-nuclear shuttling of mature miRNAs. *, p < 0.05; **, p < 0.01.
DISCUSSION
Mammalian miRNAs are matured in the cytoplasm where Dicer, a required miRNA processing enzyme, is located. It is generally believed that mature miRNAs in the nucleus are imported from the cytoplasm. Because the concept of nucleus miRNA itself was only proposed in recent years, very little information about the machinery of importing miRNA, as well as siRNA/RNAi, from cytoplasm into nucleus is available.
Karyopherins are a group of proteins involved in transporting molecules through the pores of the nuclear envelope (18, 19). For Drosha-processed miRNAs to be exported from the nucleus to the cytoplasm in mammalian cells, Exportin-5, a member of the karyopherin family, serves as a major carrier (20). It has been shown that Exportin-5, as well as Exportin-1, can be co-immunoprecipitated with the Argonaute family proteins including Ago1 and Ago2. Weinmann et al. (16) showed evidences that IPO8 interacts with human Argonaute proteins in the cytoplasm and may have additional functions in importation of nuclear miRNA-RISC. Indeed, localization of RISC components, especially Ago2, in the nucleus suggests that RISC itself may play a role in mediating miRNA shuttling between cytoplasm and nucleus. For instance, human Ago2 (21) and Caenorhabditis elegans NRDE-3 (22), both members of the Argonaute family, have been found to be responsible for siRNA nuclear localization. In a model proposed by Ohrt et al. (21), cytoplasmic co-factors for RISC are dissociated with human Ago2 (hAgo2) protein and then form nucleus RISC through which they translocate into the nucleus, bringing along miRNA strands. In the present study, we showed that only IPO8 but not other karyopherin β family members guided the nuclear transport of the complex of nuclear miRNA-RISC. Thus IPO8 is likely a candidate as a carrier for nucleus import of mature miRNAs, as well as their effector protein Ago2. Although the detailed molecular basis for this complex process is yet to be elucidated, we postulate that the process starts with the association of nuclear miRNAs with Ago2, which is then recognized by IPO8, and is finally followed by the IPO8-mediated nuclear transport of miRNAs (Fig. 2F). However, given that the association of Ago2 with miRNAs is generally nonspecific, our data suggest that association of nuclear miRNA with Ago2 is only a necessary condition for their nuclear transport. The selective process of nuclear transport of miRNAs must be guided by additional unknown factors or signals.
The present study presents the first evidence that IPO8 plays a critical role in mediating the transport of mature miRNAs from the cytoplasm to the nucleus, and the nuclear transport of mature miRNAs by IPO8 is dependent on its association with Ago2 complex.
This work was supported by National Basic Research Program of China Grants 2012CB517603 and 2011CB504803863, National Natural Science Foundation of China Grants 31301061, 30890044, 30800946, the Natural Science Foundation of Jiangsu Province Grants BK2011013 and BK20130564), and Specialized Research Fund for the Doctoral Program of Higher Education Grant 20130091120037.
- miRNA
- microRNA
- ncRNA
- noncoding RNA
- miR
- microRNA
- IPO8
- importin 8
- Ago2
- Argonaute 2
- TPF
- trypaflavine
- qRT-PCR
- quantitative RT-PCR
- RISC
- RNA-induced silencing complex
- Mut
- mutant
- WB
- Western blot.
REFERENCES
- 1. Bartel D. P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 2. Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T. (2004) Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 15, 185–197 [DOI] [PubMed] [Google Scholar]
- 3. Politz J. C., Zhang F., Pederson T. (2006) MicroRNA-206 colocalizes with ribosome-rich regions in both the nucleolus and cytoplasm of rat myogenic cells. Proc. Natl. Acad. Sci. U.S.A. 103, 18957–18962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hwang H. W., Wentzel E. A., Mendell J. T. (2007) A hexanucleotide element directs microRNA nuclear import. Science 315, 97–100 [DOI] [PubMed] [Google Scholar]
- 5. Földes-Papp Z., König K., Studier H., Bückle R., Breunig H. G., Uchugonova A., Kostner G. M. (2009) Trafficking of mature miRNA-122 into the nucleus of live liver cells. Curr. Pharm. Biotechnol. 10, 569–578 [DOI] [PubMed] [Google Scholar]
- 6. Robb G. B., Brown K. M., Khurana J., Rana T. M. (2005) Specific and potent RNAi in the nucleus of human cells. Nat. Struct. Mol. Biol. 12, 133–137 [DOI] [PubMed] [Google Scholar]
- 7. Tang R., Li L., Zhu D., Hou D., Cao T., Gu H., Zhang J., Chen J., Zhang C. Y., Zen K. (2012) Mouse miRNA-709 directly regulates miRNA-15a/16–1 biogenesis at the posttranscriptional level in the nucleus: evidence for a microRNA hierarchy system. Cell Res. 22, 504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zisoulis D. G., Kai Z. S., Chang R. K., Pasquinelli A. E. (2012) Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature 486, 541–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Görlich D., Kutay U. (1999) Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15, 607–660 [DOI] [PubMed] [Google Scholar]
- 10. Nakielny S., Dreyfuss G. (1999) Transport of proteins and RNAs in and out of the nucleus. Cell 99, 677–690 [DOI] [PubMed] [Google Scholar]
- 11. Kutay U., Izaurralde E., Bischoff F. R., Mattaj I. W., Görlich D. (1997) Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO J. 16, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radu A., Moore M. S., Blobel G. (1995) The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell 81, 215–222 [DOI] [PubMed] [Google Scholar]
- 13. Kehlenbach R. H., Dickmanns A., Kehlenbach A., Guan T., Gerace L. (1999) A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export. J. Cell Biol. 145, 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Watashi K., Yeung M. L., Starost M. F., Hosmane R. S., Jeang K. T. (2010) Identification of small molecules that suppress microRNA function and reverse tumorigenesis. J. Biol. Chem. 285, 24707–24716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao J. Y., Ma L. M., Guo Y. H., Zhang Y. C., Zhou H., Shao P., Chen Y. Q., Qu L. H. (2010) Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS One 5, e10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinmann L., Höck J., Ivacevic T., Ohrt T., Mütze J., Schwille P., Kremmer E., Benes V., Urlaub H., Meister G. (2009) Importin 8 is a gene silencing factor that targets Argonaute proteins to distinct mRNAs. Cell 136, 496–507 [DOI] [PubMed] [Google Scholar]
- 17. Li L., Zhu D., Huang L., Zhang J., Bian Z., Chen X., Liu Y., Zhang C. Y., Zen K. (2012) Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One 7, e46957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lund E., Güttinger S., Calado A., Dahlberg J. E., Kutay U. (2004) Nuclear export of microRNA precursors. Science 303, 95–98 [DOI] [PubMed] [Google Scholar]
- 19. Shibata S., Sasaki M., Miki T., Shimamoto A., Furuichi Y., Katahira J., Yoneda Y. (2006) Exportin-5 orthologues are functionally divergent among species. Nucleic Acids Res. 34, 4711–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okada C., Yamashita E., Lee S. J., Shibata S., Katahira J., Nakagawa A., Yoneda Y., Tsukihara T. (2009) A high-resolution structure of the pre-microRNA nuclear export machinery. Science 326, 1275–1279 [DOI] [PubMed] [Google Scholar]
- 21. Ohrt T., Mütze J., Staroske W., Weinmann L., Höck J., Crell K., Meister G., Schwille P. (2008) Fluorescence correlation spectroscopy and fluorescence cross-correlation spectroscopy reveal the cytoplasmic origination of loaded nuclear RISC in vivo in human cells. Nucleic Acids Res. 36, 6439–6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guang S., Bochner A. F., Pavelec D. M., Burkhart K. B., Harding S., Lachowiec J., Kennedy S. (2008) An Argonaute transports siRNAs from the cytoplasm to the nucleus. Science 321, 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]