Background: Low intensity pulsed ultrasound (LIPUS) is a mechanical stimulus clinically used to promote bone fracture healing.
Results: LIPUS suppresses adipogenesis and promotes osteogenesis of mesenchyme stem/progenitor cell lines by inhibiting PPARγ2 through ROCK-Cot/Tpl2-MEK-ERK pathway.
Conclusion: LIPUS influences multilineage differentiation of mesenchymal stem and progenitor cells.
Significance: LIPUS may be a new clinical approach to chronic bone metabolic disorders, including osteoporosis.
Keywords: Adipogenesis, Cell Differentiation, MAP Kinases (MAPKs), Mechanotransduction, Mesenchymal Stem Cells, LIPUS, Osteogenesis
Abstract
Mesenchymal stem cells (MSCs) are pluripotent cells that can differentiate into multilineage cell types, including adipocytes and osteoblasts. Mechanical stimulus is one of the crucial factors in regulating MSC differentiation. However, it remains unknown how mechanical stimulus affects the balance between adipogenesis and osteogenesis. Low intensity pulsed ultrasound (LIPUS) therapy is a clinical application of mechanical stimulus and facilitates bone fracture healing. Here, we applied LIPUS to adipogenic progenitor cell and MSC lines to analyze how multilineage cell differentiation was affected. We found that LIPUS suppressed adipogenic differentiation of both cell types, represented by impaired lipid droplet appearance and decreased gene expression of peroxisome proliferator-activated receptor γ2 (Pparg2) and fatty acid-binding protein 4 (Fabp4). LIPUS also down-regulated the phosphorylation level of peroxisome proliferator-activated receptor γ2 protein, inhibiting its transcriptional activity. In contrast, LIPUS promoted osteogenic differentiation of the MSC line, characterized by increased cell calcification as well as inductions of runt-related transcription factor 2 (Runx2) and Osteocalcin mRNAs. LIPUS induced phosphorylation of cancer Osaka thyroid oncogene/tumor progression locus 2 (Cot/Tpl2) kinase, which was essential for the phosphorylation of mitogen-activated kinase kinase 1 (MEK1) and p44/p42 extracellular signal-regulated kinases (ERKs). Notably, effects of LIPUS on both adipogenesis and osteogenesis were prevented by a Cot/Tpl2-specific inhibitor. Furthermore, effects of LIPUS on MSC differentiation as well as Cot/Tpl2 phosphorylation were attenuated by the inhibition of Rho-associated kinase. Taken together, these results indicate that mechanical stimulus with LIPUS suppresses adipogenesis and promotes osteogenesis of MSCs through Rho-associated kinase-Cot/Tpl2-MEK-ERK signaling pathway.
Introduction
Multipotent mesenchymal stem cells (MSCs)2 in bone marrow give rise to several cell lineages, including adipocytes, osteocytes, and chondrocytes. Several studies have shown that the differentiating directions of MSCs into adipocytes and osteoblasts are controlled in a reciprocal fashion (1). The strict control of MSC differentiation is crucial for maintaining homeostasis of bone marrow between bone formation and fatty marrow, and the failed control of MSC differentiation often causes bone metabolic diseases (2). For example, biased MSC differentiation toward adipogenesis is strongly related to osteoporosis and other chronic bone loss diseases (3). Similarly, an increased number of adipocytes and a decreased number of osteoblasts are often found in age-related physiological bone reduction (4).
It has been well established that both osteogenic and adipogenic differentiations are regulated by critical master transcription factors. PPARγ2 is a master adipogenic transcription factor that is related to age-related osteoporosis (5). Down-regulation of PPARγ2 promotes osteogenic differentiation of MSCs (6). Conversely, up-regulation of Runx2 accelerates osteogenic differentiation of MSCs, inducing increased bone mass (7). These findings suggest that transcriptional activities of these two master regulators may be regulated in inverse manners.
Mechanical stress is one of the effective regulators of MSC differentiation. Several kinds of mechanical stress, such as shear stress, cellular stretch, and centrifugal force, are known to affect osteoblast differentiation in various ways (8). Low intensity pulsed ultrasound (LIPUS) is mechanical stress that is already used as a clinical application to promote the healing of bone fracture (9). Several in vitro studies have shown that LIPUS facilitates osteoblast differentiation, represented by increased Osteocalcin mRNA expression and extracellular calcification (10). We have reported previously that mRNA expression of several chemokines in mature osteoblasts that is mediated by angiotensin 2 type 1 receptor is also induced with LIPUS treatment (11). In the site of bone fracture, MSCs are the cellular source of osteoprogenitor cells (12). Thus, it seems reasonable to presume that the therapeutic advantage of LIPUS in bone fracture may be associated with the direct effects of LIPUS on not only osteoblasts but also MSCs. However, it remains mostly unknown how LIPUS affects the biological functions of MSCs.
Mitogen-activated protein kinase (MAPK) cascades are well known signaling pathways controlling cellular proliferation, differentiation, and death in a variety of cell types (13). It has been reported that MAPK family proteins, including ERK, p38 MAPK, and c-Jun N-terminal kinase (JNK), are activated by various mechanical stimuli (14). We and other groups have reported previously that ERKs are crucial signaling molecules in LIPUS-induced cellular responses (11, 15). However, detailed signaling pathways of MAPK activation by mechanical stress are still poorly understood.
In this study, we explored how LIPUS affects MSC differentiation. We found that LIPUS treatment of MSCs is inhibitory to adipogenic differentiation but promotive of osteogenic differentiation of MSCs. These effects of LIPUS on MSC differentiation were dependent on LIPUS-induced ERK activation. Through analyses with specific chemical inhibitors, Cot/Tpl2 kinase, a serine/threonine kinase known to be involved in LPS signaling, was found to be essential for the LIPUS-mediated ERK activation. Moreover, Cot/Tpl2 activation by LIPUS was found to be mediated by a further upstream kinase, ROCK. Thus, these data indicated that LIPUS effectively modulates MSC differentiation through ROCK-Cot/Tpl2-MEK-ERK signaling pathway.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Tpl2 kinase inhibitor (TKI), a specific Cot/Tp2 kinase inhibitor; Y-27632, a specific ROCK inhibitor; and U0126, a specific MEK inhibitor, were purchased from Merck KGaA, Wako (Osaka, Japan), and Funakoshi (Tokyo, Japan), respectively. Antibodies recognizing phosphorylated forms of ERKs, p38 kinases, and JNKs were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against ERK1/2, p38 kinases, JNK1/2, Cot/Tpl2, IκBα, and PPARγ were also from Cell Signaling Technology. A phosphospecific antibody against Cot/Tpl2 was obtained from Bioss (Woburn, MA). Antibodies against β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the phosphorylated form of PPARγ were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Cell Culture
3T3-L1, a mouse preadipocyte cell line, was obtained from DS Pharma Biomedical Co. Ltd. (Osaka, Japan) and maintained in Dulbecco's modified Eagle's medium (DMEM) (Wako) containing 10% fetal bovine serum (FBS), 50 units/ml penicillin, and 50 mg/ml streptomycin. ST2, a mouse mesenchymal stem cell line, was obtained from RIKEN Cell Bank (Tsukuba, Japan) and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Wako) containing 10% FBS, 50 units/ml penicillin, and 50 mg/ml streptomycin. MC3T3-E1, a mouse osteoblast cell line, was obtained from RIKEN Cell Bank and maintained in Eagle's α-minimal essential medium (Wako) containing 10% FBS, 50 units/ml penicillin, and 50 mg/ml streptomycin. 10T(1/2), a mouse mesenchymal stem cell line, was obtained from RIKEN Cell Bank and maintained in DMEM containing 10% FBS, 50 units/ml penicillin, and 50 mg/ml streptomycin. 10T(1/2) cells were treated with 20 μm 5′-azacytidine for 3 days to induce differentiation. Adipogenic differentiation of 3T3-L1 and ST2 was induced by the addition of 0.5 mm 3-isobutyl-1-methylxanthine (IBMX), 1.7 μm insulin, and 1 μm dexamethasone in the culture medium. Osteogenic differentiation of ST2 was induced by the addition of 280 μm l-ascorbic acid 2-phosphate trisodium and 5 mm β-glycerophosphate in the culture medium. The bilineage differentiation of 10T(1/2) cells was induced by the addition of 20 μm 5′-azacytidine for 3 days.
Ultrasound Application
Cells were stimulated using a LIPUS-generating system (Teijin Pharma Ltd., Tokyo, Japan), which was described previously (16). The LIPUS signal consisted of a series of 1.5-MHz, 200-μs burst sine waves at 1.0 kHz and was delivered at an intensity of 30 milliwatts/cm2. The pattern and intensity of the LIPUS signal used in this study were essentially the same as that used in clinical practice and in animal model experiments.
Oil Red O Staining
Lipid droplet appearance was determined by oil red O staining. The cells were washed with Ca2+-free phosphate-buffered saline (PBS) twice and fixed in 10% formaldehyde in PBS for 1 h at 4 °C. After three washes with distilled water and one wash with 60% isopropanol in distilled water, the cells were stained in 0.5% (w/v) oil red O in isopropanol for 15 min at room temperature. The remaining dye was washed out by three washes with distilled water.
Alizarin Red S Staining
Matrix mineralization was visualized by alizarin red S staining. The cells were rinsed with Ca2+-free PBS twice and fixed in 10% formaldehyde in PBS for 20 min at 4 °C. After three washes with distilled water, the cells were stained in 1% alizarin red S solution for 15 min at room temperature. The remaining dye was washed out by three washes with distilled water.
RNA Interference of Cot/Tpl2 and ROCK1
3T3-L1 cells, MC3T3-E1 cells, and ST2 cells were transfected with small interference RNA (siRNA) duplexes specific for murine Cot/Tpl2 (r(GAGAACAUUGCUGAGUUAU)dTdT and r(AUAACUCAGCAAUGUUCU)dTdT) or ROCK1 (r(GYGGYAAAGGYAAYCGGCAT)dTdT and Ur(GCCGAUUACCUUUACCAC)dTdT) obtained from Sigma-Aldrich or nontargeting control siRNA duplexes (Control siRNA-A, Santa Cruz Biotechnology Inc.) using Hilymax (Dojondo) according to the manufacturer's instructions.
Quantitative Polymerase Chain Reaction Analysis
Total RNA was isolated with TRI reagent (Molecular Research Center Inc, Cincinnati, OH) and reverse transcribed with reverse transcriptase (Toyobo, Osaka, Japan) in the presence of an oligo(dT) primer and RNase inhibitor (Takara Bio Inc., Otsu, Japan) at 37 °C for 1 h. Real time PCR was conducted using Step-One Plus (Invitrogen). The cDNA synthesized from 0.5 μg of total RNA was amplified in a 10-μl volume with 0.11× SYBR Green I (Cambrex, Rockland, ME), 0.1 mm dNTPs, 0.2 μm each primer, 0.1 μm ROX reference dye (Invitrogen), and 1 unit of Blend Taq DNA polymerase (Toyobo) under the following conditions: 95 °C for 2 min and then 55 PCR cycles at 95 °C for 30 s, 60 °C for 20 s, and 72 °C for 20 s. Fluorescent signals were measured in real time, and then each sample was quantified according to the manufacturer's instructions. To determine the absolute number of copies of the target transcript, PCR product dilutions ranging from 103 to 108 copies were used to generate standard curves. The primer sequences used in PCR are shown in Table 1.
TABLE 1.
Primers used in this study
nt, nucleotides.
| Gene symbol | Primers (5′–3′) | GenBankTM accession number | Amplification length |
|---|---|---|---|
| bp (nt) | |||
| C/ebpa | ACAGAAGGTGCTGGAGTTGA | NM_007678 | 125 (1062–1186) |
| CCTTGACCAAGGAGCTCTCA | |||
| C/ebpb | CATGCACCGCCTGCTG | NM_001287738 | 98 (107–205) |
| CAGTCGGGCTCGTAGTAGAA | |||
| C/ebpd | AGGCAGGGTGGACAAGC | NM_007679 | 112 (120–231) |
| GTAGGCGCTGAAGTCGATG | |||
| Fabp4 | CGACAGGAAGGTGAAGAGCA | NM_024406 | 122 (296–417) |
| ATTCCACCACCAGCTTGTCA | |||
| Gapdh | TCAAGAAGGTGGTGAAGCAG | NM_008084 | 110 (839–948) |
| GGTGGAAGAGTGGGAGTTGC | |||
| Myod1 | CTGCTCTGATGGCATGATGG | NM_010866 | 112 (811–922) |
| CTTCCCTGGCCTGGACTC | |||
| Myogenin | GTCCCAACCCAGGAGATCA | NM_031189 | 128 (591–718) |
| CATGGTTTCGTCTGGGAAGG | |||
| Osteocalcin | CTCACAGATGCCAAGCCCA | NM_007541 | 98 (107–204) |
| CCAAGGTAGCGCCGGAGTCT | |||
| Pparg2 | TGAGCACTTCACAAGAAATTACCA | NM_011146 | 117 (113–229) |
| TGTCAAAGGAATGCGAGTGG | |||
| Rpl13a | GCTTACCTGGGGCGTCTG | NM_009438 | 149 (427–575) |
| ACATTCTTTTCTGCCTGTTTCC | |||
| Runx2 | CCGTGGCCTTCAAGGTTGT | NM_009820 | 118 (635–752) |
| TTCATAACAGCGGAGGCATTT |
Western Blot Analysis
Cells were lysed in RIPA lysis buffer (150 mm NaCl, 1.0% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mm Tris (pH 8.0), 0.1% Na3VO4, and protease inhibitor mixture). Cell lysates were separated by SDS-PAGE and electrotransferred to Immobilon polyvinylidene difluoride membranes (Merck Millipore). The membranes were blocked for 1 h in 5% skim milk in TBST (20 mm Tris-HCl (pH 7.6), 0.15 m sodium chloride, and 0.1%Tween 20), washed three times with TBST, incubated for 2 h with primary antibodies in TBST, washed three times with TBST, and incubated for 1 h with horseradish peroxidase-conjugated anti-mouse or -rabbit immunoglobulin (Merck KGaA) diluted 1:5000 in 5% skim milk in TBST. After three washes in TBST, the blots were developed with the enhanced chemiluminescence substrate (PerkinElmer Life Sciences) according to the manufacturer's instructions.
RESULTS
LIPUS Induced ERK Phosphorylation in Preadipocytes, Osteoblasts, and MSCs
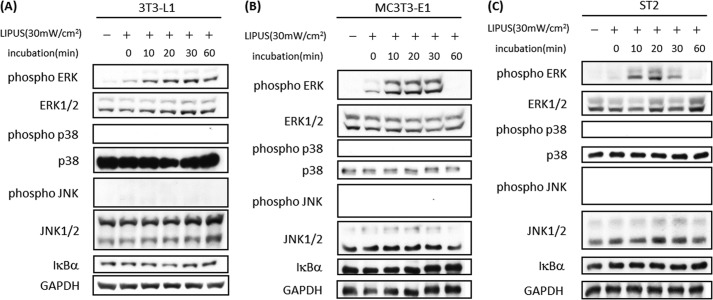
MSCs differentiate into precursors of osteoblasts and adipocytes in bone marrow (1). For the purpose of exploring the effects of LIPUS on these cell types, we stimulated a mouse preadipocyte line, 3T3-L1; a mouse osteoblast cell line, MC3T3-E1; and a mouse MSC line, ST2, with LIPUS and analyzed the induced intracellular signaling pathways (Fig. 1). LIPUS rapidly induced significant phosphorylation of ERK in 3T3-L1 (Fig. 1A), MC3T3-E1 (Fig. 1B), and ST2 cells (Fig. 1C). However, phosphorylation of p38 and JNK as well as the degradation of IκBα was not induced by LIPUS treatment in any of the three cell lines (Fig. 1).
FIGURE 1.
LIPUS induces ERK phosphorylation in adipocytes and mesenchymal stem cells. A, preadipocyte 3T3-L1 cells were stimulated by LIPUS (30 milliwatts (mW)/cm2) for 20 min. Cells were lysed in RIPA lysis buffer at the indicated time after the LIPUS stimulation. Cell lysates were separated by SDS-PAGE, and Western blotting was performed with the indicated antibodies. B, osteoblast MC3T3-E1 cells were stimulated by LIPUS and analyzed as in A. C, ST2, a mouse mesenchymal stem cell line, was stimulated by LIPUS and analyzed as in A.
LIPUS Stimulation Suppressed Adipogenic Differentiation and Promoted Osteogenic Differentiation
Previous studies have reported that osteogenic differentiation of osteoblasts is significantly promoted by daily LIPUS stimulation (10). However, the influence of LIPUS on adipogenic differentiation has never been elucidated. Thus, we induced adipogenic differentiation of 3T3-L1 cells with or without daily LIPUS stimulation. Adipogenic differentiation was evaluated by the appearance of lipid droplets in the cytoplasm visualized by oil red O staining. As a result, lipid droplet appearance was significantly reduced by daily LIPUS treatment (Fig. 2A). We also examined the effect of LIPUS on the gene expression of adipogenic marker proteins. Consistent with the results of lipid staining, mRNA expression levels of the examined adipogenic marker genes Fabp4, Pparg2, C/ebpa, C/ebpb, and C/ebpd were significantly suppressed by LIPUS (Fig. 2B).
FIGURE 2.
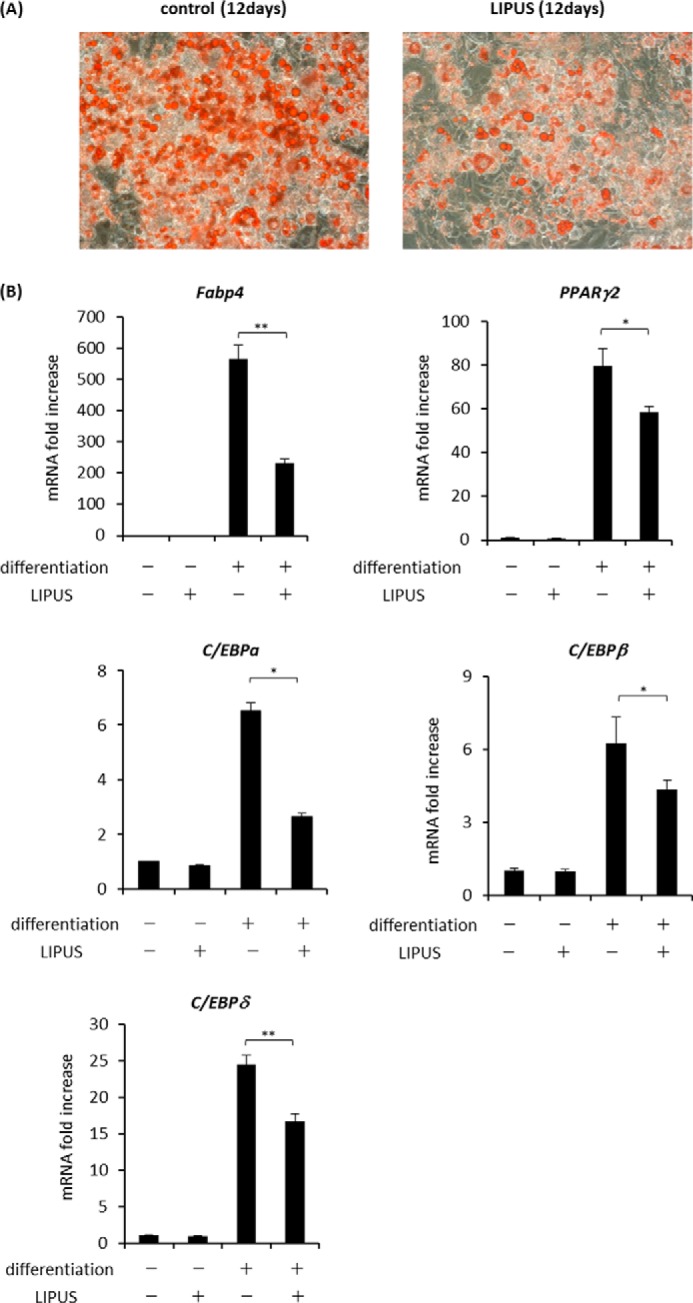
LIPUS suppresses adipogenic differentiation of 3T3-L1 cells. A, 3T3-L1 cells were induced to differentiate by a combination of dexamethasone, insulin, and IBMX for 12 days with or without daily stimulation by LIPUS for 20 min. Cells were stained with oil red O to determine lipid droplet appearance. B, 3T3-L1 cells were induced to differentiate by a combination of dexamethasone, insulin, and IBMX for 12 days with or without daily stimulation by LIPUS for 20 min. Total RNAs were isolated and reverse transcribed. The gene expressions of adipogenic markers were analyzed by real time PCR. The same experiments were performed at least three times. Relative mRNA expression levels are compared with Rpl13a. Error bars represent S.D. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01).
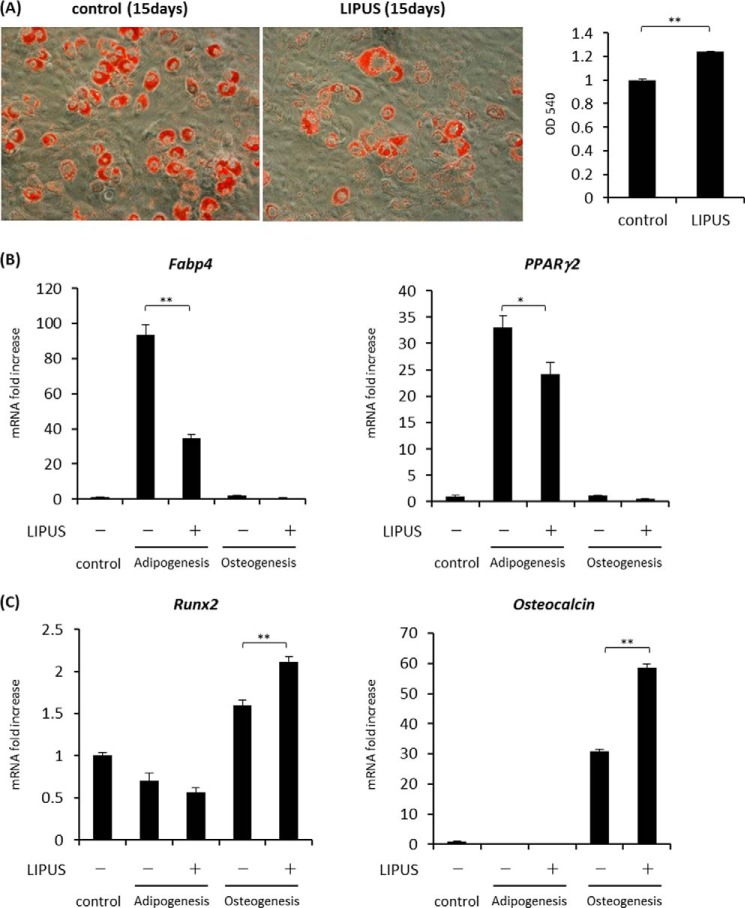
We also analyzed the effects of LIPUS treatment on adipogenic and osteogenic differentiation of a mouse MSC line, ST2 cells. ST2 cells were cultured in either adipogenic or osteogenic differentiation medium with or without LIPUS stimulation. In the inducing condition of adipogenesis, lipid droplet appearances and expression of Fabp4 and Pparg2 were significantly inhibited by LIPUS treatment (Fig. 3, A and B). Conversely, in the induction medium of osteogenesis, the mRNA levels of two osteogenic marker genes, Runx2 and Osteocalcin, were significantly increased by LIPUS (Fig. 3C). These results indicate that LIPUS may be inhibitory to adipogenic differentiation but promotive of osteogenic differentiation of MSCs. Notably, in contrast to the above results with ST2 cells, LIPUS treatment had little effect on the induction of Runx2 or Osteocalcin in osteogenic differentiation experiments using MC3T3-E1, a mouse osteoblast cell line, and mouse calvaria-derived primary osteoblasts (data not shown), indicating that the promotive effect of LIPUS on osteogenic differentiation may be dependent on the cell differentiation stage.
FIGURE 3.
LIPUS-induced effects on adipogenic and osteogenic differentiation of ST2 cells. A, ST2 cells were cultured in adipogenic differentiation media (dexamethasone, insulin, and IBMX) with or without daily 20-min stimulation by LIPUS. After 15 days, cells were stained with oil red O solution to determine lipid droplet appearance. B, after the treatments as in A, total RNAs were isolated and reverse transcribed. The gene expressions were analyzed by real time PCR. Each experiment was repeated at least three times. Relative mRNA expression levels compared with Rpl13a are shown. Error bars represent S.D. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01). C, ST2 cells were cultured in osteogenic differentiation media (280 μm l-ascorbic acid 2-phosphate trisodium and 5 mm β-glycerophosphate) with or without daily 20-min stimulation by LIPUS. After 15 days, the expression levels of osteogenic marker genes were compared with Rpl13a. Error bars represent S.D. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01).
LIPUS-induced Signals Are Mediated by Cot/Tpl2 Kinase
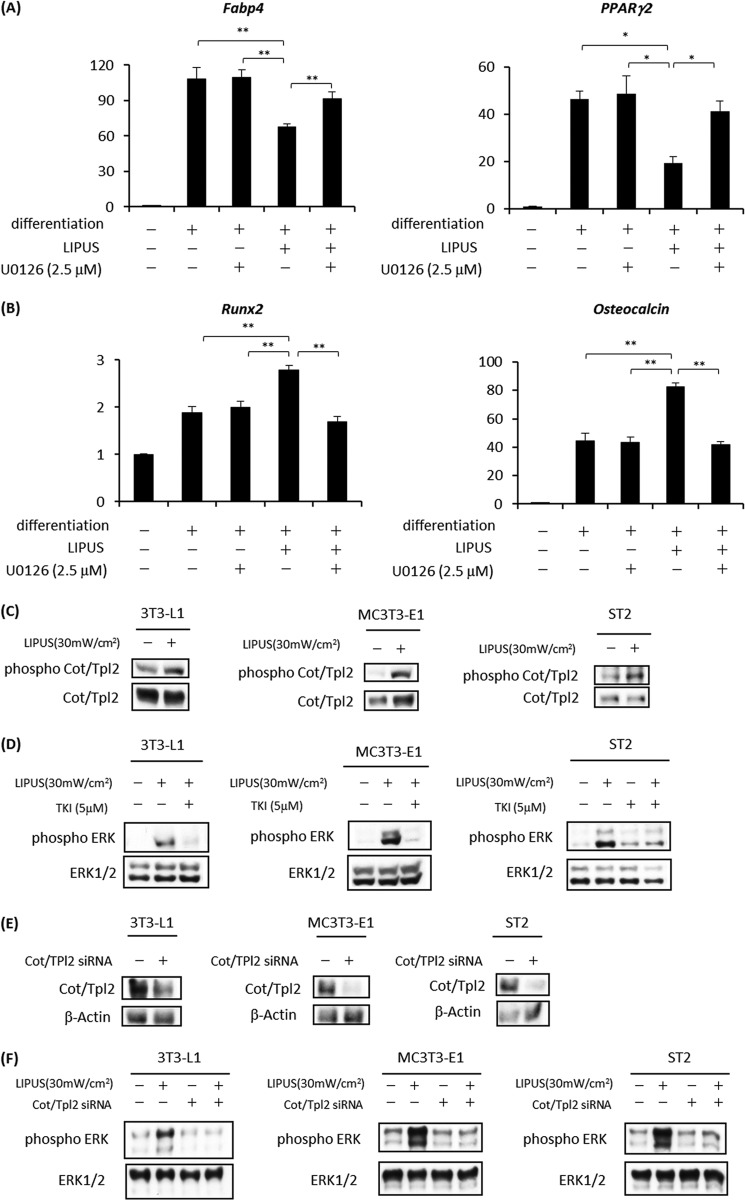
As LIPUS efficiently phosphorylated ERKs among the three kinds of MAPKs (Fig. 1), we next explored the involvement of ERK in the LIPUS effects on ST2 cells. Cells were cultured in either adipogenic or osteogenic differentiation medium with or without LIPUS treatment. Before the LIPUS stimulation, cells were treated with U0126, a specific MEK inhibitor, for 60 min. Cell culture medium was changed to remove U0126 after each LIPUS stimulation. We found that LIPUS-induced inhibition of adipogenic marker mRNA expression was efficiently blocked by U0126 treatment in ST2 cells (Fig. 4A). On the other hand, LIPUS-induced promotion of Runx2 and Osteocalcin mRNA expression during osteogenic differentiation was significantly inhibited by U0126 (Fig. 4B).
FIGURE 4.
LIPUS-induced ERK phosphorylation is mediated by Cot/Tpl2 activation. A, ST2 cells were cultured in adipogenic differentiation medium (dexamethasone, insulin, and IBMX). Cells were treated with or without 2.5 mm U0126 for 60 min and stimulated by LIPUS for 20 min every day. After each LIPUS treatment, cell culture media were changed to remove U0126. After 15 days, total RNAs were isolated, reverse transcribed, and analyzed by real time PCR. Each experiment was repeated at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a mRNA are shown. Error bars represent S.D. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01). B, ST2 cells were cultured in osteogenic differentiation media (l-ascorbic acid 2-phosphate trisodium and β-glycerophosphate) with or without 2.5 mm U0126 for 60 min and stimulated by LIPUS for 20 min every day. After each LIPUS treatment, cell culture media were changed to remove U0126. The expressions of osteogenic marker genes were analyzed as in A. C, 3T3-L1, MC3T3-E1, and ST2 cells were stimulated by LIPUS for 20 min. Cells were lysed in RIPA lysis buffer immediately after the stimulation. Cell lysates were separated by SDS-PAGE, and levels of phosphorylated and total Cot/Tpl2 proteins were determined by Western blotting. D, 3T3-L1, MC3T3-E1, and ST2 cells were pretreated with 5 μm TKI (a Cot/Tpl2-specific inhibitor) for 30 min followed by LIPUS stimulation for 20 min. Cells lysates were prepared in RIPA lysis buffer and separated by SDS-PAGE. The levels of phosphorylated and total ERK proteins were determined by Western blotting. E and F, 3T3-L1, MC3T3-E1, and ST2 cells were transiently transfected with either Cot/Tpl2 siRNA or control siRNA. The effects of Cot/Tpl2 siRNA on Cot/Tpl2 protein expression levels (E) and LIPUS-induced ERK phosphorylation (F) were confirmed by Western blotting. mW, milliwatts.
We next explored the upstream signaling mechanisms controlling ERK phosphorylation. We examined the possible involvement of Cot/Tpl2, an essential upstream kinase for the LPS-induced ERK phosphorylation in a variety of cell types (17–21). We found that Cot/Tpl2 was rapidly phosphorylated in LIPUS-stimulated 3T3-L1, MC3T3-E1, and ST2 cells (Fig. 4C). Notably, pretreatment with TKI, a specific Cot/Tpl2 inhibitor, significantly decreased the level of LIPUS-induced ERK phosphorylation in each of these three cell lines (Fig. 4D). Furthermore, transient transfection with Cot/Tpl2-specific siRNA significantly suppressed the protein expression level of Cot/Tpl2 (Fig. 4E) and LIPUS-induced phosphorylation of ERK in all three cell lines (Fig. 4F).
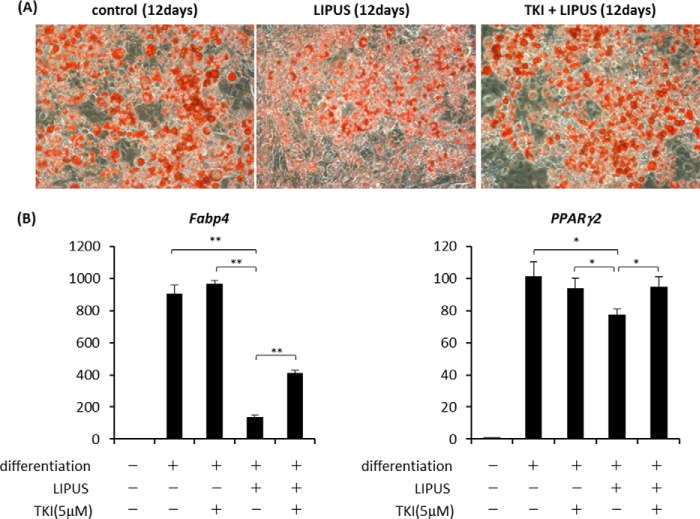
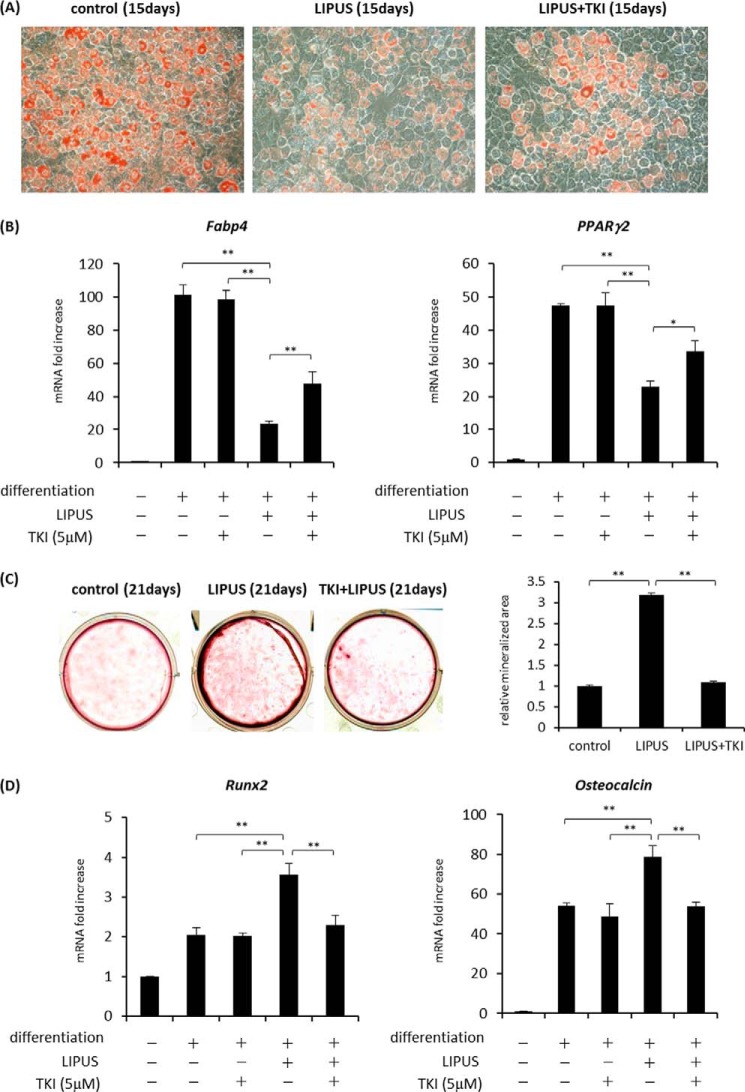
We then explored the involvement of Cot/Tpl2 in LIPUS-induced suppression of adipogenic differentiation. 3T3-L1 cells were induced to differentiate into adipocytes with daily treatment by LIPUS in the presence or absence of TKI. As a result, the LIPUS-induced decrease of lipid droplet appearance was significantly inhibited by the addition of TKI (Fig. 5A). Consistently, the induction of Fabp4 and Pparg2 mRNA, which was inhibited by LIPUS, was recovered by TKI treatment (Fig. 5B). We further examined the role of Cot/Tpl2 in the LIPUS effects on an MSC line, ST2. ST2 cells were cultured in either adipogenic or osteogenic differentiation medium with or without the addition of TKI and LIPUS. Similarly to 3T3-L1 cells, LIPUS-induced inhibition of lipid droplet appearance and adipogenic marker mRNA expression were partially blocked by TKI treatment in ST2 cells when adipogenic differentiation was induced (Fig. 6, A and B). We next examined the involvement of Cot/Tpl2 in the LIPUS-promoted osteogenic differentiation of ST2 cells. LIPUS-induced promotion of matrix mineralization was found to be abrogated by the addition of TKI (Fig. 6C). Consistently, the facilitation of Runx2 and Osteocalcin mRNA expression by LIPUS was completely inhibited by TKI (Fig. 6D). These findings indicated that Cot/Tpl2 activation is essential for the LIPUS-induced promotion of osteogenic differentiation of ST2 cells.
FIGURE 5.
Cot/Tpl2 is involved in the LIPUS-induced suppression of adipogenic differentiation of 3T3-L1 cells. A, 3T3-L1 cells were cultured in adipogenic differentiation media (dexamethasone, insulin, and IBMX) with or without 5 μm TKI and stimulated by daily LIPUS for 20 min. After 12 days, cells were stained with oil red O to determine lipid droplet appearance. B, 3T3-L1 cells were induced to differentiate as in A for 12 days with or without 5 μm TKI and stimulated by daily LIPUS for 20 min. Total RNAs were isolated and reverse transcribed. The gene expressions of adipogenic markers were analyzed by real time PCR. Each experiment was repeated at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a mRNA are shown. Error bars represent S.D. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01).
FIGURE 6.
Cot/Tpl2 is an essential signaling molecule of LIPUS-induced suppression of adipogenesis and promotion of osteogenesis. A, ST2 cells were cultured in adipogenic differentiation media (dexamethasone, insulin, and IBMX) with or without 5 μm TKI and stimulated by daily LIPUS for 20 min. After 15 days, cells were stained with oil red O to determine lipid droplet appearance. B, ST2 cells were induced to differentiate as in A. After 15 days, total RNAs were isolated, reverse transcribed, and analyzed by real time PCR. Each experiment was repeated at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a mRNA are shown. Error bars represent S.D. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01). C, ST2 cells were cultured in osteogenic differentiation media (280 μm l-ascorbic acid 2-phosphate trisodium and 5 mm β-glycerophosphate) with or without 5 μm TKI and stimulated by daily LIPUS for 20 min. After 21 days, cells were stained with alizarin red S for the detection of calcification. The calcified area was photographically measured, and the mineralization ratio relative to control was expressed as mean ± S.D. Statistical significance was determined by Student's t test (**, p < 0.01). D, ST2 cells were induced to differentiate as in C. Total RNAs were isolated and reverse transcribed. The gene expressions of osteogenic markers were analyzed by real time PCR. Each experiment was repeated at least three times with consistent results. Relative mRNA expression levels in comparison with Rpl13a mRNA are shown. Error bars represent S.D. Statistical significance was determined by Student's t test (**p < 0.01).
ROCK Is an Essential Upstream Molecule Regulating Cot/Tpl2-ERK Activation by LIPUS
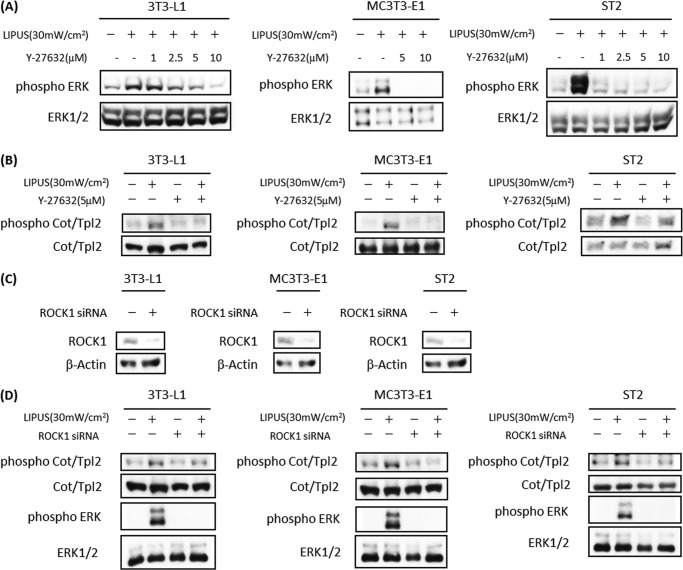
It has been reported that cytoskeletal organization is a key regulator of cell differentiation (22). ROCK is a serine/threonine kinase that plays an important role in mediating cytoskeletal rearrangements (23). Therefore, we examined the involvement of ROCK in LIPUS stimulation. LIPUS-induced ERK phosphorylation was found to be efficiently blocked by Y-27632, a specific ROCK inhibitor, in 3T3-L1, MC3T3-E1, and ST2 cells (Fig. 7A). We further examined the effects of this ROCK inhibitor on LIPUS-induced Cot/Tpl2 phosphorylation and found that it was significantly suppressed by Y-27632 treatment in each of the examined three cell lines (Fig. 7B). We also used siRNA against ROCK to confirm its role in LIPUS-induced signal transduction. Transient transfection with ROCK-specific siRNA efficiently inhibited ROCK protein expression (Fig. 7C) and LIPUS-induced phosphorylation of Cot/Tpl2 and ERK in all three cell lines (Fig. 7D). Therefore, ROCK appears to be an essential upstream molecule in the activation of Cot/Tpl2 and ERK by LIPUS stimulation.
FIGURE 7.
ROCK is an essential upstream molecule in LIPUS-induced Cot/Tpl2 and ERK activation. A, 3T3-L1, MC3T3-E1, and ST2 cells were pretreated with 1, 2.5, 5, or 10 μm Y-27632 (a ROCK-specific inhibitor) for 1 h followed by LIPUS stimulation for 20 min. Cell lysates were separated by SDS-PAGE, and levels of phosphorylated and total ERK proteins were determined by Western blotting. B, 3T3-L1, MC3T3-E1, and ST2 cells were pretreated with 5 μm Y-27632 for 1 h. Levels of phosphorylated and total Cot/Tpl2 proteins were determined as in A. C and D, 3T3-L1, MC3T3-E1, and ST2 cells were transiently transfected with either ROCK1 siRNA or control siRNA. The inhibitory effects of ROCK1 siRNA on ROCK1 protein expression were confirmed by Western blotting (C). Phosphorylation of Cot/Tpl2 and ERK was analyzed by Western blotting (D). mW, milliwatts.
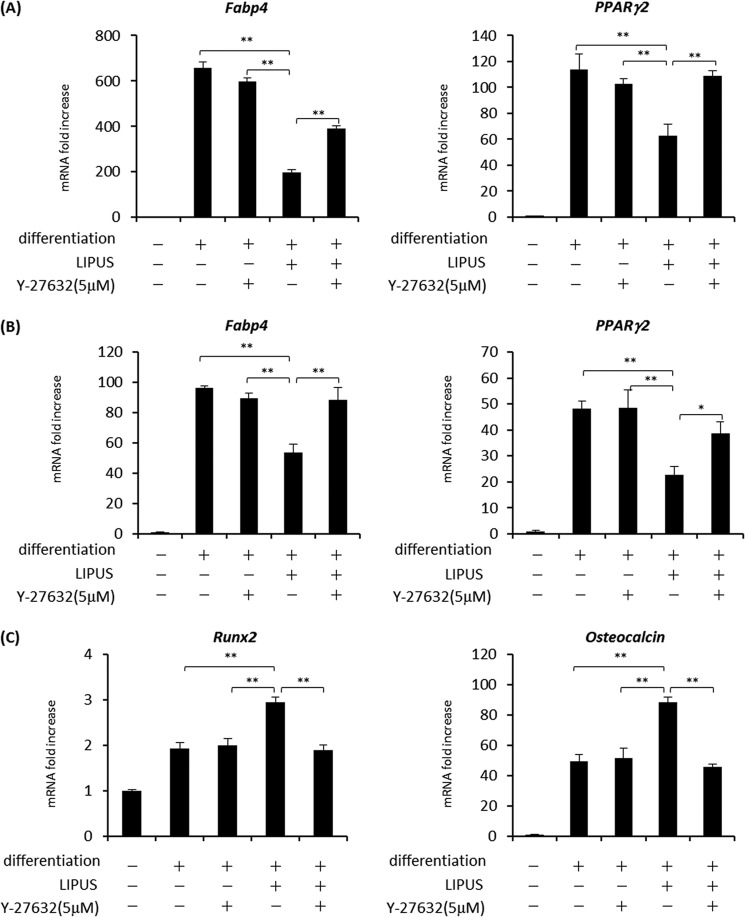
We subsequently examined the role of ROCK in LIPUS-induced regulation of cell differentiation. In adipogenic differentiation of 3T3-L1 cells, the LIPUS-induced inhibition of Fabp4 and Pparg2 mRNA induction was blocked by Y-27632 (Fig. 8A), a result similar to the result of TKI (Fig. 5B). Similar effects of ROCK inhibitor were also observed in ST2 cells (Fig. 8B). On the other hand, the promotion of Runx2 and Osteocalcin mRNA expression by LIPUS was inhibited by Y-27632 during osteogenic differentiation of ST2 cells (Fig. 8C). Taken together, these results indicated that LIPUS suppresses adipogenic and promotes osteogenic differentiation through ROCK-Cot/Tpl2-ERK signaling pathway.
FIGURE 8.
LIPUS suppresses adipogenesis and promotes osteogenesis through ROCK-Cot/Tpl2-ERK pathway. A, preadipocyte 3T3-L1 cells were induced to differentiate into adipocytes with a combination of dexamethasone, insulin, and IBMX for 12 days with or without the addition of 5 μm Y-27632 and stimulated with daily LIPUS for 20 min. Total RNAs were isolated and reverse transcribed, and the gene expressions of adipogenic markers were analyzed by real time PCR. Relative mRNA expression levels were calculated in comparison with the housekeeping Rpl13a mRNA. Error bars represent the S.D. of triplicate values. Statistical significance was determined by Student's t test (**, p < 0.01). Each experiment was performed at least three times with consistent results. A typical result is shown. B, adipogenic differentiation of ST2 cells was induced with a combination of dexamethasone, insulin, and IBMX for 15 days with or without the addition of 5 μm Y-27632 and/or daily 20-min LIPUS stimulation. Analyses of adipogenic gene marker mRNAs were performed as in A. C, osteogenic differentiation of ST2 cells was induced with a combination of l-ascorbic acid 2-phosphate trisodium and β-glycerophosphate for 23 days with or without 5 μm Y-27632 and/or daily LIPUS stimulation for 20 min. The analysis of osteogenic gene marker mRNAs was performed by real time PCR as in A. *, p <0.05.
LIPUS Induces PPARγ Phosphorylation
A previous study has shown that PPARγ2 transcriptional activity is attenuated by the phosphorylation of Ser-112 (24). Thus, we analyzed the possible effects of LIPUS on PPARγ2 phosphorylation and found that PPARγ2 was rapidly phosphorylated by LIPUS, and the phosphorylation lasted for at least 60 min (Fig. 9A). Notably, LIPUS-induced PPARγ2 phosphorylation was significantly inhibited by Y-27632, TKI, and U0126 (Fig. 9B). Additionally, transient transfection with Cot/Tpl2-specific siRNA or ROCK-specific siRNA efficiently inhibited LIPUS-induced phosphorylation of PPARγ2 (Fig. 9C). These results indicated that LIPUS-induced ROCK-Cot/Tpl2-MEK-ERK signaling pathway negatively regulates adipogenesis by regulating not only the expression but also the phosphorylation of PPARγ2.
FIGURE 9.
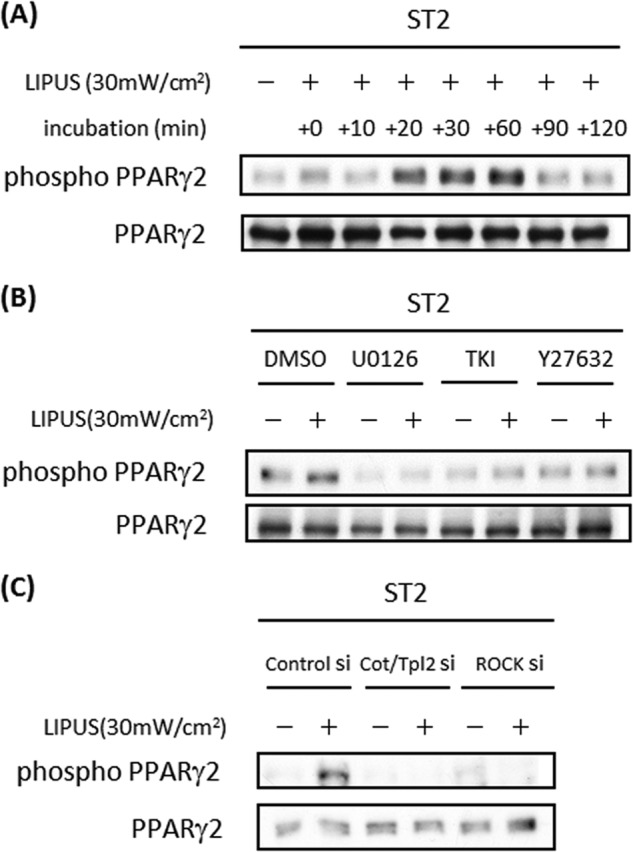
LIPUS induces PPARγ2 phosphorylation in ST2 cells. A, ST2 cells were cultured in adipogenic differentiation medium for 5 days. Cells were stimulated by LIPUS for 20 min and lysed in RIPA lysis buffer at the indicated time after the stimulation. Cell lysates were separated by SDS-PAGE, and levels of phosphorylated and total PPARγ2 proteins were determined by Western blotting. B, ST2 cells were pretreated with 5 μm Y-27632, 5 μm TKI, or 2.5 μm U0126 for 1 h followed by stimulation with LIPUS for 20 min. Western blotting analyses were performed as in A. C, ST2 cells were transiently transfected with Cot/Tpl2 siRNA, ROCK1 siRNA, or control siRNA (si). The analysis of PPARγ2 phosphorylation was performed by Western blotting as in B. mW, milliwatts.
LIPUS Affects the Cell Fate Determination for Differentiation of a Multipotent MSC Cell Line
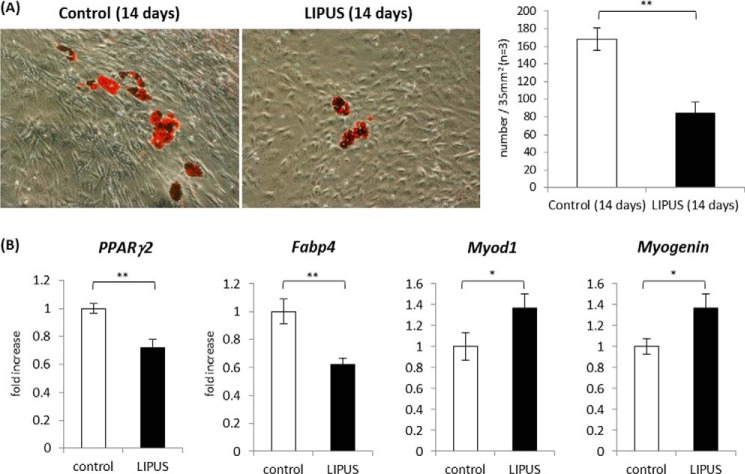
Our data demonstrated that LIPUS exerted inhibitory and promotive effects on adipogenic and osteogenic differentiations, respectively. However, it remained ambiguous whether LIPUS also influenced the cell fate determination of the multipotent stem cell differentiation program. 10T(1/2), a mouse mesenchymal stem cell line, has a multipotency to differentiate into either adipocytes or myoblasts. Pretreatment with 5′-azacytidine for 3 days triggers the differentiation of 10T(1/2) cells, and some cells form lipid droplets, and others show increased expression of myoblast-specific proteins at constant rates in regular DMEM culture medium after a period of days (25). Thus, we used 10T(1/2) cells as our experimental model to evaluate the LIPUS effects on the cell fate determination of MSC differentiation. Following 3-day treatment with 5′-azacytidine, 10T(1/2) cells were cultured for 14 days with or without daily LIPUS treatment. As a result, the number of lipid droplets was significantly decreased by daily LIPUS stimulation (Fig. 10A). Consistent with this observation, the induction of Fabp4 and Pparg2 mRNA was also significantly suppressed (Fig. 10B). Notably, the expression levels of myogenic marker genes myogenic differentiation 1 (Myod1) and Myogenin were significantly promoted by LIPUS treatment (Fig. 10B). These findings suggested that LIPUS influences the fate determination of MSC differentiation toward myoblasts rather than adipocytes.
FIGURE 10.
LIPUS-induced effects on adipogenic and myogenic differentiation of 10T(1/2) cells. A, 10T(1/2), a mouse mesenchymal stem cell line, was treated with 20 μm azacytidine for 3 days. After the treatment, cells were incubated in DMEM with 10% FCS for 14 days. Cells were stained with oil red O to determine lipid droplet appearance. After the staining, the number of lipid droplets was counted under the microscope (n = 3). Error bars represent S.D. Statistical significance was determined by Student's t test (**, p <0.01). B, 10T(1/2) cells were treated with azacytidine as in A and cultured in DMEM with 10% FCS in the presence or absence of daily LIPUS for 20 min. At day 14, analysis of adipogenic and myogenic gene marker mRNAs was performed by real time PCR. Relative mRNA expression levels in comparison with Gapdh mRNA are shown. Statistical significance was determined by Student's t test (*, p <0.05; **, p < 0.01).
DISCUSSION
LIPUS is micromechanical stress that is already clinically applied in bone fracture healing (9). Thus, it is important to identify the types of cells in bone marrow that are responsible for the clinical effectiveness of LIPUS. Bone marrow stroma contains MSCs, multilineage progenitor cells that can differentiate into various cell types, including osteoblasts and adipocytes (26). Previous studies have shown that mechanical stress regulates osteogenic differentiation of osteoblasts (27), and osteoblasts are considered to be a major target cell type of LIPUS in bone marrow (28–31). Here, we found that LIPUS induces intracellular signal transduction, including ERK phosphorylation, in preadipocyte (3T3-L1) and MSC (ST2) cell lines in a manner similar to that for an osteoblast line (MC3T3-E1) (Fig. 1, A and C), indicating that not only osteoblasts but also other cell types in bone marrow may mediate the clinical effectiveness of LIPUS.
One of the significant novel findings of our present study is the inhibitory effect of LIPUS on adipogenic differentiation. When adipogenic differentiation was induced, both lipid droplet appearance and the gene expression of adipogenic differentiation markers were clearly inhibited by daily LIPUS treatment in 3T3-L1 (Fig. 2) and ST2 (Fig. 3) cell lines. Differentiation manners of MSCs are known to be regulated by various local and hormonal factors (1). For example, age-related osteoporosis is related to the biased differentiation of MSCs in bone marrow toward adipocytes rather than osteoblasts (3). The increased adipogenesis, often referred as “fatty marrow,” impairs hematopoiesis in bone marrow presumably due to the direct inhibitory effects of adipocytes on hematopoietic stem cells (32). The age-related fatty marrow has been proposed to be caused by numerous factors, including skeletal immobilization (33). Bone loss caused by skeletal immobilization has been demonstrated in human and experimental animal models. For example, hind limb suspension of rats, an established animal model of skeletal unloading, inhibits long bone formation due to impaired recruitment and functions of osteoblasts (34). Conversely, skeletal immobilization is also known to increase adipogenesis in human bone marrow in addition to its negative effects on osteogenesis (26). These previous findings have indicated that mechanical loading of bone presumably affects MSC differentiation manners toward osteogenesis rather than adipogenesis in bone marrow. However, the detailed molecular mechanisms have not been elucidated.
It is known that adipogenic differentiation is regulated by some core regulatory proteins (35). PPARγ2 is master transcriptional factor of adipogenesis. C/EBPα, C/EBPβ, and C/EBPδ are co-regulatory transcription factors that modulate adipogenic differentiation in association with PPARγ2. Fabp4, a representative adipogenic marker, is involved in the process of lipid droplet accumulation. LIPUS stimulation effectively decreased mRNA of Pparg2, C/ebpa, C/ebpb, C/ebpd, and Fabp4 during adipogenic differentiation of both 3T3-L1 and ST2 cell lines (Fig. 2B). Among these four core regulatory proteins, we presumed that the down-regulation of PPARγ2 is the decisive event for the LIPUS-induced inhibition of adipogenic differentiation as PPARγ2 is the only known factor that is necessary and sufficient for the induction of adipocyte differentiation (36). As a matter of fact, it has been reported previously that the expressions of C/EBPα, C/EBPδ, C/EBPβ, and Fabp4 are all induced by forced PPARγ2 expression in fibroblasts (37). PPARγ2 is a member of the nuclear receptor superfamily and forms a heterodimer with retinoid X receptors to bind PPAR-responsive elements. PPARγ2 is encoded by the Pparg gene, which also produces an alternative splicing variant, PPARγ1. PPARγ1 and PPARγ2 differ only in the N-terminal amino acid sequences, and PPARγ2 has a 5–6-fold increased transcriptional activity of the ligand-independent activation function-1 domain compared with PPARγ1 (38). These two variants are driven by distinct promoters. Although the expression of PPARγ2 is restricted to mature adipocytes, PPARγ1 is ubiquitously expressed, indicating distinct regulatory mechanisms for these two promoters (39, 40). In our present study, daily LIPUS treatment inhibited the adipocyte differentiation-induced Pparg2 mRNA increase (Fig. 2). In contrast, the level of Pparg1 mRNA remained constant during adipogenic differentiation and was not affected by LIPUS treatment (data not shown). These results indicated that LIPUS affects the Pparg2 gene promoter in a specific manner.
Previous reports have revealed that the transcriptional activity of PPARγ2 is modulated by phosphorylation, sumoylation, ubiquitylation, and nitration (41). Phosphorylation of PPARγ2 is presumed to be the most significant negative posttranscriptional regulator among them (42). Ser-112 in PPARγ2 in the mouse and Ser-114 in the human have been reported to be phosphorylated by each of the three MAPK groups (43, 44). Consistent with these reports, we found that Ser-112 phosphorylation of PPARγ2 was induced by LIPUS through ERK activation in ST2 cells (Fig. 9A). These findings indicated that LIPUS inhibits PPARγ2 through two distinct mechanisms: transcriptional repression and phosphorylation-mediated inactivation. Interestingly, both mechanisms appear to require ERK activation. The relative contributions by these two mechanisms to the inhibition of adipogenesis remain unknown at present and require further experiments.
In contrast to the negative effects on adipogenic differentiation, the promotive effects were induced by LIPUS on osteogenic differentiation of ST2 cells, represented by facilitated mRNA expression of Runx2 and Osteocalcin (Fig. 3). Similar to the role of PPARγ2 in adipogenesis, RUNX2 is considered to be the master regulator of osteogenesis. RUNX2 directly enhances the promoter activities of various osteogenic differentiation markers, including Osteocalcin (45). Thus, it seems reasonable to presume that LIPUS promotes osteogenic differentiation of ST2 cells through the transcriptional activation of RUNX2. It should be noted, however, that the expression of osteogenic differentiation marker genes was not altered by daily (20 min/day) LIPUS treatment in a long term (4-week) differentiation experiment of MC3T3-E1, an osteoblast cell line, and primary osteoblasts isolated from calvaria of newborn C57BL/6 mice (data not shown). These seemingly incompatible results may have been caused by different differentiation stages of these two cell lines. Pparg2 mRNA expression was detectable and gradually decreased during osteogenic differentiation in ST2 cells (Fig. 9). Conversely, PPARγ2 expression could not be detected in MC3T3-E1 cells by either quantitative PCR or Western blot analysis (data not shown). Reciprocal crossover regulation has been reported between PPARγ2 and RUNX2 (46–48). Because LIPUS effectively inhibited PPARγ2 activity through phosphorylation (Fig. 9A), one possible explanation is that LIPUS promotes osteogenesis through down-regulation of PPARγ2 and thus did not enhance osteogenic differentiation of MC3T3-E1 cells, which did not express PPARγ2.
Our results have also suggested that LIPUS affects the bilineage differentiation of 10T(1/2) cells toward myoblasts rather than adipocytes (Fig. 10). A previous study has reported that murine G8 myoblasts highly expressing PPARγ and C/EBPα showed markedly reduced levels of MyoD and Myogenin proteins under optimal conditions for muscle differentiation (49). In another study, a PPARγ2-overexpressing bovine embryonic fibroblast cell line grown in adipogenic differentiation medium preferentially differentiated into adipogenic cells even in the presence of ectopic Myod expression (50). Thus, it seems reasonable to suppose that the LIPUS-induced reduction of PPARγ2 transcriptional activity enhances myogenic differentiation. LIPUS-induced alteration of PPARγ2 function might be an important regulator of MSC differentiation toward osteogenesis and myogenesis.
ERK phosphorylation is an important signaling event controlling the differentiation of various cell types, including osteoblasts and adipocytes (13). We found that ERK activation is crucial for the LIPUS-induced inhibitory effects on adipogenesis and promotive effects on osteogenesis (Fig. 4, A and B). This finding is consistent with several previous studies that showed inhibitory effects of ERK activation on adipogenic differentiation. For example, stimulation with oncostatin M inhibited C/EBPβ-induced adipogenic differentiation through ERK signaling pathway in 3T3-L1 cells and mouse embryonic fibroblasts (51). Furthermore, apelin suppresses adipogenic differentiation through an ERK-dependent pathway in preadipocytes and mature adipocytes (52). In contrast, however, other previous studies have shown that ERK activation has promotive effects on adipogenic differentiation. Insulin-, IBMX-, and dexamethasone-induced ERK phosphorylation enhanced the expressions of PPARγ2 and C/EBPα in 3T3-L1 cells (53). In another report, treatment with all-trans-retinoic acid induced commitment of mouse embryonic stem cells into the adipocytic lineage by the ERK signaling pathway (54). We presume that the apparent discrepancies among reports, including ours, are due to the different degrees of adipogenic differentiation in the experimental cell systems. Treatment with the combination of insulin, IBMX, and dexamethasone is widely used as the fundamental inducer to initiate adipogenesis. Retinoic acid was also used as an initial factor to start the differentiation of MSCs into adipocytes. Thus, the activation of ERK appears to be essential in the initial step of adipogenic differentiation. We also found that 3T3-L1 and ST2 cells could not be induced to differentiate by treatment with U0126, a specific MEK inhibitor, in our experimental system (data not shown), indicating that the inhibition of ERK alone does not induce adipogenic differentiation. In our study and the previous reports showing suppressive effects of ERK on adipogenesis (51, 52), cells were stimulated by ERK activators after the initial induction of adipogenic differentiation by insulin, IBMX, and dexamethasone. Thus, it seems reasonable to presume that ERK activation exerts inhibitory effects on adipogenesis after the onset of differentiation.
Phosphorylation of ERK is induced by various extracellular stimuli, including some types of mechanical stresses (14). Previous studies have demonstrated that focal adhesion kinase, Ras, Raf, and MEK are upstream signaling molecules of ERK phosphorylation by fluid shear stress (55). However, it has been reported that upstream activation cascades of ERK are varied depending on both cell type and the type of mechanical stress (56–59). In our present study, we identified Cot/Tpl2 as an important signaling molecule in LIPUS-induced ERK activation (Fig. 4). Cot/Tpl2 is known as an essential molecule in LPS-induced ERK activation in osteoblasts (17), mast cells (20), and macrophages (18, 21), and our present study is the first report showing the involvement of Cot/Tpl2 in mechanotransduction.
Sensing receptors for some types of mechanical stress have been identified in several studies (11, 60–62). However, it has never been clearly shown how cell differentiation is regulated by the signal transduction of a single mechanical stress-specific receptor (14). Conversely, it has been reported that cytoskeletal organization is a key regulator of some types of cell differentiation (22). In particular, previous studies have reported that MSCs actively change their cytoskeleton (63) and cell membrane shape when their differentiation is induced (64). We found that ROCK, a major molecule involved in cytoskeletal rearrangements, is involved in LIPUS-induced signal transduction. ROCKs (ROCK1 and ROCK2) belong to the AGC (protein kinase A/protein kinase G/protein kinase C) family of serine/threonine kinases and regulate a variety of fundamental cellular functions. Both isoforms, ROCK1 and ROCK2, are ubiquitously expressed (65). Despite some functional differences, they share many downstream targets. ROCK is one of the important effectors of Rho, a small GTPase protein family (66). Following activation by Rho, ROCK functions as a regulator of cytoskeletal remodeling. ROCK induces actin filament stabilization, assembly of actin and actomyosin networks, and microtubule dynamics through phosphorylation of its target proteins, directly contributing to a number of cytoskeleton-mediated processes, including adhesion, contraction, polarity, cytokinesis, motility, permeability, and phagocytosis (23).
Our present data have demonstrated that ROCK is an essential signaling component mediating ERK phosphorylation by LIPUS in MSC, osteoblast, and preadipocyte cell lines (Fig. 7). Recent reports have indicated that ROCK functions as an upstream activator of ERK in several cell types. For example, activation of ROCK induces smooth muscle cell proliferation through ERK phosphorylation (67). However, because ROCK is not considered a direct upstream activator of MEK-ERK signaling pathway, it has remained ambiguous how activated ROCK can induce ERK phosphorylation. Our present data have indicated that Cot/Tpl2, which is an essential MEK kinase in LPS signaling, mediates the ROCK-induced signal to ERK phosphorylation.
Activation of ROCK by mechanical stimuli other than LIPUS has been reported in several cell types (68, 69). A quite recent report, which was published when our study was ongoing, has examined the role of ROCK in mechanical stress-induced MSC differentiation (70). In their report, inhibition of ROCK by Y-23672 suppressed stretch-induced tenogenic differentiation of human bone marrow-derived MSCs. Thus, taken together with our present report, some types of mechanical stress seem to affect differentiation of both human and mouse MSCs.
A previous study has shown that the cytoskeletal structure of SAOS-2, a human osteosarcoma cell line, is dramatically changed after LIPUS stimulation especially with an enhancement of stress fiber formation (30). As Rho families and their effectors, such as ROCK, are essential enzymes in the rearrangement of cellular architectures, their report raises the possibility that LIPUS induces dynamic alterations of actin fibers through activation of ROCK and its downstream signaling molecules. The involvement of ROCK-mediated signaling in Cot/Tpl2 activation has not been reported previously. Two groups examined the involvement of ROCK in reagent-induced phosphorylation of MEK, a downstream kinase of Cot/Tpl2. Treatment with transforming growth factor-α induced MEK activation through ROCK in rat chondrocytes (71). Cholinergic agonists induced activation of Rho and ROCK, which in turn activated MEK and ERK, in rat epithelial cells (72). Our present results suggest the possibility that Cot/Tpl2 might be an upstream kinase in the activation of MEK and ERK by ROCK in the previous reports.
In summary, our study has demonstrated that mechanical stimulus with LIPUS suppresses adipogenesis and promotes osteogenesis of mesenchyme stem and progenitor cell lines. These LIPUS-induced effects are mediated by ROCK-Cot/Tpl2-MEK-ERK signaling pathway and the modulation of PPARγ2 activity. These results possibly suggest new clinical approaches to chronic bone metabolic disorders, such as osteoporosis, using mechanical stimuli, including LIPUS. This study also provides new insights into the molecular mechanisms of cellular effects of LIPUS as well as other mechanical stimuli.
Acknowledgments
We thank Mai Nakashima, Etsuko Kamishikiryo, Momoko Uemura, and Yoko Amita for secretarial assistance.
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Iwadare Scholarship Foundation; and Terayama Foundation and by Teijin Pharma, which supplied the LIPUS device.
- MSC
- mesenchymal stem cell
- LIPUS
- low intensity pulsed ultrasound
- ROCK
- Rho-associated kinase
- Cot/Tpl2
- cancer Osaka thyroid oncogene/tumor progression locus 2
- PPARγ2
- peroxisome proliferator-activated receptor γ2
- Fabp4
- fatty acid-binding protein 4
- RUNX2
- runt-related transcription factor 2
- TKI
- Tpl2 kinase inhibitor
- IBMX
- 3-isobutyl-1-methylxanthine
- RIPA
- radioimmune precipitation assay
- C/EBP
- CCAAT/enhancer-binding protein
- MyoD
- myogenic differentiation.
REFERENCES
- 1. Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 2. Verma S., Rajaratnam J. H., Denton J., Hoyland J. A., Byers R. J. (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 55, 693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nuttall M. E., Patton A. J., Olivera D. L., Nadeau D. P., Gowen M. (1998) Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J. Bone Miner. Res. 13, 371–382 [DOI] [PubMed] [Google Scholar]
- 4. Qiu W., Andersen T. E., Bollerslev J., Mandrup S., Abdallah B. M., Kassem M. (2007) Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J. Bone Miner. Res. 22, 1720–1731 [DOI] [PubMed] [Google Scholar]
- 5. Wan Y. (2010) PPARγ in bone homeostasis. Trends Endocrinol. Metab. 21, 722–728 [DOI] [PubMed] [Google Scholar]
- 6. Ji H., Liu Y., Zhao X., Zhang M. (2011) N-Acetyl-L-cysteine enhances the osteogenic differentiation and inhibits the adipogenic differentiation through up regulation of Wnt 5a and down regulation of PPARG in bone marrow stromal cells. Biomed. Pharmacother. 65, 369–374 [DOI] [PubMed] [Google Scholar]
- 7. Komori T. (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 339, 189–195 [DOI] [PubMed] [Google Scholar]
- 8. Hughes-Fulford M. (2004) Signal transduction and mechanical stress. Sci. STKE 2004, RE12. [DOI] [PubMed] [Google Scholar]
- 9. Romano C. L., Romano D., Logoluso N. (2009) Low-intensity pulsed ultrasound for the treatment of bone delayed union or nonunion: a review. Ultrasound Med. Biol. 35, 529–536 [DOI] [PubMed] [Google Scholar]
- 10. Naruse K., Miyauchi A., Itoman M., Mikuni-Takagaki Y. (2003) Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J. Bone Miner. Res. 18, 360–369 [DOI] [PubMed] [Google Scholar]
- 11. Bandow K., Nishikawa Y., Ohnishi T., Kakimoto K., Soejima K., Iwabuchi S., Kuroe K., Matsuguchi T. (2007) Low-intensity pulsed ultrasound (LIPUS) induces RANKL, MCP-1, and MIP-1β expression in osteoblasts through the angiotensin II type 1 receptor. J. Cell. Physiol. 211, 392–398 [DOI] [PubMed] [Google Scholar]
- 12. Bielby R., Jones E., McGonagle D. (2007) The role of mesenchymal stem cells in maintenance and repair of bone. Injury 38, Suppl. 1, S26–S32 [DOI] [PubMed] [Google Scholar]
- 13. Widmann C., Gibson S., Jarpe M. B., Johnson G. L. (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 79, 143–180 [DOI] [PubMed] [Google Scholar]
- 14. Papachristou D. J., Papachroni K. K., Basdra E. K., Papavassiliou A. G. (2009) Signaling networks and transcription factors regulating mechanotransduction in bone. BioEssays 31, 794–804 [DOI] [PubMed] [Google Scholar]
- 15. de Gusmão C. V., Pauli J. R., Saad M. J., Alves J. M., Belangero W. D. (2010) Low-intensity ultrasound increases FAK, ERK-1/2, and IRS-1 expression of intact rat bones in a noncumulative manner. Clin. Orthop. Relat. Res. 468, 1149–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakao J., Fujii Y., Kusuyama J., Bandow K., Kakimoto K., Ohnishi T., Matsuguchi T. (2014) Low-intensity pulsed ultrasound (LIPUS) inhibits LPS-induced inflammatory responses of osteoblasts through TLR4-MyD88 dissociation. Bone 58, 17–25 [DOI] [PubMed] [Google Scholar]
- 17. Bandow K., Maeda A., Kakimoto K., Kusuyama J., Shamoto M., Ohnishi T., Matsuguchi T. (2010) Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 402, 755–761 [DOI] [PubMed] [Google Scholar]
- 18. Sugimoto K., Ohata M., Miyoshi J., Ishizaki H., Tsuboi N., Masuda A., Yoshikai Y., Takamoto M., Sugane K., Matsuo S., Shimada Y., Matsuguchi T. (2004) A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J. Clin. Investig. 114, 857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bandow K., Kusuyama J., Shamoto M., Kakimoto K., Ohnishi T., Matsuguchi T. (2012) LPS-induced chemokine expression in both MyD88-dependent and -independent manners is regulated by Cot/Tpl2-ERK axis in macrophages. FEBS Lett. 586, 1540–1546 [DOI] [PubMed] [Google Scholar]
- 20. Chiba N., Kakimoto K., Masuda A., Matsuguchi T. (2010) Functional roles of Cot/Tpl2 in mast cell responses to lipopolysaccharide and FcϵRI-clustering. Biochem. Biophys. Res. Commun. 402, 1–6 [DOI] [PubMed] [Google Scholar]
- 21. Dumitru C. D., Ceci J. D., Tsatsanis C., Kontoyiannis D., Stamatakis K., Lin J. H., Patriotis C., Jenkins N. A., Copeland N. G., Kollias G., Tsichlis P. N. (2000) TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103, 1071–1083 [DOI] [PubMed] [Google Scholar]
- 22. Mammoto A., Ingber D. E. (2009) Cytoskeletal control of growth and cell fate switching. Curr. Opin. Cell Biol. 21, 864–870 [DOI] [PubMed] [Google Scholar]
- 23. Amano M., Nakayama M., Kaibuchi K. (2010) Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton 67, 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Beekum O., Fleskens V., Kalkhoven E. (2009) Posttranslational modifications of PPAR-γ: fine-tuning the metabolic master regulator. Obesity 17, 213–219 [DOI] [PubMed] [Google Scholar]
- 25. Taylor S. M., Jones P. A. (1979) Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 17, 771–779 [DOI] [PubMed] [Google Scholar]
- 26. Muruganandan S., Roman A. A., Sinal C. J. (2009) Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 66, 236–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Papachroni K. K., Karatzas D. N., Papavassiliou K. A., Basdra E. K., Papavassiliou A. G. (2009) Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 15, 208–216 [DOI] [PubMed] [Google Scholar]
- 28. Saito M., Soshi S., Tanaka T., Fujii K. (2004) Intensity-related differences in collagen post-translational modification in MC3T3-E1 osteoblasts after exposure to low- and high-intensity pulsed ultrasound. Bone 35, 644–655 [DOI] [PubMed] [Google Scholar]
- 29. Sena K., Leven R. M., Mazhar K., Sumner D. R., Virdi A. S. (2005) Early gene response to low-intensity pulsed ultrasound in rat osteoblastic cells. Ultrasound Med. Biol. 31, 703–708 [DOI] [PubMed] [Google Scholar]
- 30. Hauser J., Hauser M., Muhr G., Esenwein S. (2009) Ultrasound-induced modifications of cytoskeletal components in osteoblast-like SAOS-2 cells. J. Orthop. Res. 27, 286–294 [DOI] [PubMed] [Google Scholar]
- 31. Watabe H., Furuhama T., Tani-Ishii N., Mikuni-Takagaki Y. (2011) Mechanotransduction activates α5β1 integrin and PI3K/Akt signaling pathways in mandibular osteoblasts. Exp. Cell Res. 317, 2642–2649 [DOI] [PubMed] [Google Scholar]
- 32. Naveiras O., Nardi V., Wenzel P. L., Hauschka P. V., Fahey F., Daley G. Q. (2009) Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirkland J. L., Tchkonia T., Pirtskhalava T., Han J., Karagiannides I. (2002) Adipogenesis and aging: does aging make fat go MAD? Exp. Gerontol. 37, 757–767 [DOI] [PubMed] [Google Scholar]
- 34. Wronski T. J., Morey E. R. (1982) Skeletal abnormalities in rats induced by simulated weightlessness. Metab. Bone Dis. Relat. Res. 4, 69–75 [DOI] [PubMed] [Google Scholar]
- 35. Christodoulides C., Lagathu C., Sethi J. K., Vidal-Puig A. (2009) Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 20, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tontonoz P., Hu E., Spiegelman B. M. (1994) Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 37. Spiegelman B. M. (1998) PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes 47, 507–514 [DOI] [PubMed] [Google Scholar]
- 38. Tontonoz P., Hu E., Graves R. A., Budavari A. I., Spiegelman B. M. (1994) mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8, 1224–1234 [DOI] [PubMed] [Google Scholar]
- 39. Tontonoz P., Graves R. A., Budavari A. I., Erdjument-Bromage H., Lui M., Hu E., Tempst P., Spiegelman B. M. (1994) Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPARγ and RXRα. Nucleic Acids Res. 22, 5628–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vidal-Puig A. J., Considine R. V., Jimenez-Liñan M., Werman A., Pories W. J., Caro J. F., Flier J. S. (1997) Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J. Clin. Investig. 99, 2416–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luconi M., Cantini G., Serio M. (2010) Peroxisome proliferator-activated receptor γ (PPARγ): is the genomic activity the only answer? Steroids 75, 585–594 [DOI] [PubMed] [Google Scholar]
- 42. Hu E., Kim J. B., Sarraf P., Spiegelman B. M. (1996) Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 274, 2100–2103 [DOI] [PubMed] [Google Scholar]
- 43. Adams M., Reginato M. J., Shao D., Lazar M. A., Chatterjee V. K. (1997) Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J. Biol. Chem. 272, 5128–5132 [DOI] [PubMed] [Google Scholar]
- 44. Diradourian C., Girard J., Pégorier J. P. (2005) Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie 87, 33–38 [DOI] [PubMed] [Google Scholar]
- 45. Komori T. (2006) Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 99, 1233–1239 [DOI] [PubMed] [Google Scholar]
- 46. Hong J. H., Hwang E. S., McManus M. T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B. M., Sharp P. A., Hopkins N., Yaffe M. B. (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309, 1074–1078 [DOI] [PubMed] [Google Scholar]
- 47. Calo E., Quintero-Estades J. A., Danielian P. S., Nedelcu S., Berman S. D., Lees J. A. (2010) Rb regulates fate choice and lineage commitment in vivo. Nature 466, 1110–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nishikawa K., Nakashima T., Takeda S., Isogai M., Hamada M., Kimura A., Kodama T., Yamaguchi A., Owen M. J., Takahashi S., Takayanagi H. (2010) Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J. Clin. Investig. 120, 3455–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu E., Tontonoz P., Spiegelman B. M. (1995) Transdifferentiation of myoblasts by the adipogenic transcription factors PPARγ and C/EBPα. Proc. Natl. Acad. Sci. U.S.A. 92, 9856–9860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin J., Jin X., Beck S., Kang D. H., Hong Z., Li Z., Jin Y., Zhang Q., Choi Y. J., Kim S. C., Kim H. (2010) In vitro myogenic and adipogenic differentiation model of genetically engineered bovine embryonic fibroblast cell lines. Biotechnol. Lett. 32, 195–202 [DOI] [PubMed] [Google Scholar]
- 51. Miyaoka Y., Tanaka M., Naiki T., Miyajima A. (2006) Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J. Biol. Chem. 281, 37913–37920 [DOI] [PubMed] [Google Scholar]
- 52. Than A., Cheng Y., Foh L. C., Leow M. K., Lim S. C., Chuah Y. J., Kang Y., Chen P. (2012) Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell. Endocrinol. 362, 227–241 [DOI] [PubMed] [Google Scholar]
- 53. Prusty D., Park B. H., Davis K. E., Farmer S. R. (2002) Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 277, 46226–46232 [DOI] [PubMed] [Google Scholar]
- 54. Bost F., Caron L., Marchetti I., Dani C., Le Marchand-Brustel Y., Binétruy B. (2002) Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem. J. 361, 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liedert A., Kaspar D., Blakytny R., Claes L., Ignatius A. (2006) Signal transduction pathways involved in mechanotransduction in bone cells. Biochem. Biophys. Res. Commun. 349, 1–5 [DOI] [PubMed] [Google Scholar]
- 56. Wang B., Du T., Wang Y., Yang C., Zhang S., Cao X. (2011) Focal adhesion kinase signaling pathway is involved in mechanotransduction in MG-63 cells. Biochem. Biophys. Res. Commun. 410, 671–676 [DOI] [PubMed] [Google Scholar]
- 57. Hsu H. J., Lee C. F., Locke A., Vanderzyl S. Q., Kaunas R. (2010) Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One 5, e12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Harris I. S., Zhang S., Treskov I., Kovacs A., Weinheimer C., Muslin A. J. (2004) Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation 110, 718–723 [DOI] [PubMed] [Google Scholar]
- 59. Kawamura S., Miyamoto S., Brown J. H. (2003) Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J. Biol. Chem. 278, 31111–31117 [DOI] [PubMed] [Google Scholar]
- 60. Chao J. T., Davis M. J. (2011) The roles of integrins in mediating the effects of mechanical force and growth factors on blood vessels in hypertension. Curr. Hypertens. Rep. 13, 421–429 [DOI] [PubMed] [Google Scholar]
- 61. Storch U., Mederos y Schnitzler M., Gudermann T. (2012) G protein-mediated stretch reception. Am. J. Physiol. Heart Circ. Physiol. 302, H1241–H1249 [DOI] [PubMed] [Google Scholar]
- 62. Puklin-Faucher E., Sheetz M. P. (2009) The mechanical integrin cycle. J. Cell Sci. 122, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 [DOI] [PubMed] [Google Scholar]
- 64. Rodríguez J. P., González M., Ríos S., Cambiazo V. (2004) Cytoskeletal organization of human mesenchymal stem cells (MSC) changes during their osteogenic differentiation. J. Cell. Biochem. 93, 721–731 [DOI] [PubMed] [Google Scholar]
- 65. Liao J. K., Seto M., Noma K. (2007) Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 50, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amano M., Fukata Y., Kaibuchi K. (2000) Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261, 44–51 [DOI] [PubMed] [Google Scholar]
- 67. Zhao Y., Lv M., Lin H., Cui Y., Wei X., Qin Y., Kohama K., Gao Y. (2013) Rho-associated protein kinase isoforms stimulate proliferation of vascular smooth muscle cells through ERK and induction of cyclin D1 and PCNA. Biochem. Biophys. Res. Commun. 432, 488–493 [DOI] [PubMed] [Google Scholar]
- 68. Sarasa-Renedo A., Tunç-Civelek V., Chiquet M. (2006) Role of RhoA/ROCK-dependent actin contractility in the induction of tenascin-C by cyclic tensile strain. Exp. Cell Res. 312, 1361–1370 [DOI] [PubMed] [Google Scholar]
- 69. Chapados R., Abe K., Ihida-Stansbury K., McKean D., Gates A. T., Kern M., Merklinger S., Elliott J., Plant A., Shimokawa H., Jones P. L. (2006) ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ. Res. 99, 837–844 [DOI] [PubMed] [Google Scholar]
- 70. Xu B., Song G., Ju Y., Li X., Song Y., Watanabe S. (2012) RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J. Cell. Physiol. 227, 2722–2729 [DOI] [PubMed] [Google Scholar]
- 71. Appleton C. T., Usmani S. E., Mort J. S., Beier F. (2010) Rho/ROCK and MEK/ERK activation by transforming growth factor-α induces articular cartilage degradation. Lab. Invest. 90, 20–30 [DOI] [PubMed] [Google Scholar]
- 72. Hodges R. R., Guilbert E., Shatos M. A., Natarajan V., Dartt D. A. (2011) Phospholipase D1, but not D2, regulates protein secretion via Rho/ROCK in a Ras/Raf-independent, MEK-dependent manner in rat lacrimal gland. Invest. Ophthalmol. Vis. Sci. 52, 2199–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]