Background: Serotonin (5-HT) contributes to the pathogenesis of chronic inflammatory diseases known to have defects in apoptotic cell removal (i.e. efferocytosis).
Results: 5-HT impairs macrophage efferocytosis via 5-HT transporter-dependent activation of the RhoA/Rho kinase pathway.
Conclusion: Results show a novel mechanism by which 5-HT might disrupt resolution of inflammation.
Significance: Targeting the 5-HT transporter may have a therapeutic role in chronic inflammatory disorders.
Keywords: Autoimmune Diseases, Inflammation, Lung, Phagocytosis, RhoA, Efferocytosis, Rho Kinase, Serotonin Receptors
Abstract
Serotonin (5-hydroxytryptamine; 5-HT) is a CNS neurotransmitter increasingly recognized to exert immunomodulatory effects outside the CNS that contribute to the pathogenesis of autoimmune and chronic inflammatory diseases. 5-HT signals to activate the RhoA/Rho kinase (ROCK) pathway, a pathway known for its ability to regulate phagocytosis. The clearance of apoptotic cells (i.e. efferocytosis) is a key modulator of the immune response that is inhibited by the RhoA/ROCK pathway. Because efferocytosis is defective in many of the same illnesses where 5-HT has been implicated in disease pathogenesis, we hypothesized that 5-HT would suppress efferocytosis via activation of RhoA/ROCK. The effect of 5-HT on efferocytosis was examined in murine peritoneal and human alveolar macrophages, and its mechanisms were investigated using pharmacologic blockade and genetic deletion. 5-HT impaired efferocytosis by murine peritoneal macrophages and human alveolar macrophages. 5-HT increased phosphorylation of myosin phosphatase subunit 1 (Mypt-1), a known ROCK target, and inhibitors of RhoA and ROCK reversed the suppressive effect of 5-HT on efferocytosis. Peritoneal macrophages expressed the 5-HT transporter and 5-HT receptors (R) 2a, 2b, but not 2c. Inhibition of 5-HTR2a and 5-HTR2b had no effect on efferocytosis, but blockade of the 5-HT transporter prevented 5-HT-impaired efferocytosis. Genetic deletion of the 5-HT transporter inhibited 5-HT uptake into peritoneal macrophages, prevented 5-HT-induced phosphorylation of Mypt-1, reversed the inhibitory effect of 5-HT on efferocytosis, and decreased cellular peritoneal inflammation. These results suggest a novel mechanism by which 5-HT might disrupt efferocytosis and contribute to the pathogenesis of autoimmune and chronic inflammatory diseases.
Introduction
Serotonin (5-hydroxytryptamine; 5-HT)2 is a CNS neurotransmitter best known for its role regulating mood, appetite, sleep, and cognitive functions such as memory and learning (1). 5-HT also has a variety of important endocrine-like functions outside of the CNS, which include its ability to regulate vasoconstriction, proliferation, osteoclastogenesis, insulin secretion, and platelet function (1–7). The literature also suggests that 5-HT is an important regulator of the immune system with a role in the pathogenesis of autoimmunity (8, 9), inflammatory bowel disease (10–12), pulmonary hypertension (13, 14), acute lung injury (15, 16), asthma (17), and chronic obstructive pulmonary disease (18–21).
5-HT is produced by two isoforms of tryptophan hydroxylase (tph), tph-2 in the CNS and tph-1 in the periphery(2). Despite its critical role in the CNS, over 95% of the 5-HT in the body is produced by enterochromaffin cells in the gut, where it regulates intestinal motility (6, 7). To a large extent, enterochromaffin cells also supply 5-HT to the rest of the body by secreting it into the blood where it is incorporated into platelets (via the 5-HT transporter) and delivered to distant organs and cells. Both structural and immune cells, such as macrophages, respond to 5-HT through one of 14 G protein-coupled receptors or via a 5-HT transporter-dependent process called serotonylation (1, 2, 22, 23). Both mechanisms result in activation of the RhoA/Rho kinase (ROCK) pathway, which is a well known regulator of the cytoskeleton and phagocytosis, including phagocytosis of apoptotic cells (efferocytosis).
Apoptotic cells are recognized, eaten, and digested through efferocytosis, a distinctive process that maintains physiologic homeostasis and resolves inflammation following an infectious or non-infectious provocation (24–26). Efferocytosis utilizes a unique but redundant repertoire of bridging proteins and receptors that recognize externalized ligands on the surface of apoptotic cells. Interactions between efferocytosis receptors and apoptotic cell ligands have at least three significant effects: 1) phagocytosis of dying cells through activation of Rac-1; 2) induction of mediators that suppress the innate immune response; and 3) suppression of adaptive immunity. Failed or dysfunctional efferocytosis has been implicated in the pathogenesis of chronic inflammatory diseases including, systemic lupus erythematosus, rheumatoid arthritis, obesity, cardiovascular disease, neurodegenerative disease, and lung diseases such as cystic fibrosis, asthma, chronic granulomatous disease, and chronic obstructive pulmonary disease (24–31). In each disease, the cause(s) for impaired efferocytosis is complex, but in several cases the final common pathway involves activation of the RhoA/ROCK pathway (32–35), a well described negative regulator of efferocytosis (36). Because of the growing body of evidence linking 5-HT signaling with chronic inflammation and activation of the RhoA/ROCK pathway, this study was designed to determine the impact of 5-HT on efferocytosis and to define the mechanisms involved.
MATERIALS AND METHODS
Reagents and Antibodies
X-vivo10 media was obtained from Lonza (Walkersville, MD). Reagents purchased from Mediatech Inc. (Manassas, VA) included DMEM, RPMI 1640, PBS, and Hanks' balanced salt solution. FBS was purchased from ATLANTA Biologicals (Lawrenceville, GA). Serotonin (5-HT) and Y-27632 were obtained from Sigma and C3 transferase was purchased from Cytoskeleton Inc. (Denver, CO). Thioglycollate (TG) was purchased from Fluka Analytical (St. Louis, MO). 5-HT receptor 2a (HTR2a) antibody was purchased from Novus Biologicals (Littleton, CO), antibody against 5-HT receptor 2b (HTR2b) was purchased from Acris Antibodies, Inc. (San Diego, CA), antibody against the 5-HT transporter (5-HTT) was purchased from Abcam (Cambridge, MA), and antibody against β-actin was purchased from Sigma. RS-96544 hydrochloride and RS-127445 hydrochloride were from Tocris Biosciences (Bristol, UK). Fluoxetine HCl was from Santa Cruz (Santa Cruz, CA). Carboxylated beads and latex beads were purchased from Bangs Laboratories, Inc. (Fishers, IN).
Experimental Animals
Mice were housed and studied under institutional animal care and use committee-approved protocols at the animal facility of the National Jewish Health and University of Colorado Anschutz Medical Campus and National Jewish Health. Experiments were performed on 8–12-week-old 5-HTT-deficient (KO) mice (B6.129(Cg)-Slc6a4tm1Kpl/J) and age/sex-matched, C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) (37–39).
Human Subjects
The study was approved by and performed in accordance with the ethical standards of the institutional review board on human experimentation at the University of Colorado Anschutz Medical Campus and National Jewish Health. Written informed consent was obtained from each subject.
Primary Cell Preparation and Culture
TG-elicited peritoneal macrophages were obtained according to previously established methods. Briefly, mice were injected intraperitoneally with 1.5 ml of a 4% sterile and aged (1 month) solution of TG medium. At 3 days post-injection, mice were euthanized with CO2, and the peritoneal cavity was lavaged with 10 ml of sterile Hanks' balanced salt solution. Peritoneal cells were collected, centrifuged at 1,000 × g for 10 min at 4 °C, and resuspended in Xvivo10 media and cultured with humidification in 10% CO2 at 37 °C. After 1 h of culture, non-adherent cells were aspirated and pre-warmed fresh media was added to each well. For resident peritoneal macrophages isolations, naive mice were used and the harvested cells handled as above. Human alveolar macrophages were isolated by bronchoalveolar lavage from healthy volunteers. Cells were resuspended in X-vivo10 media with 10% human serum and plated on 96-well tissue culture plates. Cells were incubated for 24 h in 10% CO2 at 37 °C. Then medium was replaced with serum-free X-vivo10 media for experimentation.
Induction of Apoptosis
Murine thymocytes were isolated from the thymi of 3–4-week-old C57BL/6J mice by first passing thymi through a 40-μm cell strainer to separate individual cells. Thymocytes and Jurkat T cells were washed with PBS, resuspended in RPMI media containing 10% FBS at 2 × 106 cells/ml, exposed to UV irradiation at 254 nm for 10 min, and cultured for 3 h in 5% CO2 at 37 °C before use.
IgG Opsonization
Human erythrocytes were opsonized, as described (34), by adding anti-human erythrocyte rabbit IgG fraction (ICN Pharmaceuticals, Inc., Aurora, OH) and incubated for 1 h at room temperature before the experiments.
In Vitro Phagocytosis Assay
In vitro phagocytosis assays were performed as previously described (32–34, 40). Briefly, macrophages were plated in 24-well plates at a concentration of 3 × 105 cells/well on baked glass coverslips. Cells were cultured in serum-free Xvivo10 media. 5-HT was not detectable by ELISA in fresh Xvivo10 media or treatment-naive peritoneal macrophage cultures. Cells were treated with the indicated concentrations of 5-HT for 24 h prior to performing the phagocytosis assay. In some experiments, cells underwent additional treatments with the RhoA inhibitor, C3 transferase at 1 μg/ml, or the ROCK inhibitor, Y-27632 at 10 μm for 3 h. Co-culture experiments were then performed by adding apoptotic cells at a 10:1 ratio (apoptotic cells to macrophages). Cells were co-cultured for 60 min at 37 °C in 10% CO2. Each well was washed 5 times with ice-cold PBS to remove uningested apoptotic cells and stained with modified Wright-Giemsa (Fisher Scientific, Kalamazoo, MI). Phagocytosis was determined by visual inspection of samples by light microscopy and was expressed as the phagocytic index (PI) as described. The PI was calculated by counting total apoptotic cell ingestions divided by 400 macrophages multiplied by 100. Each condition was tested in duplicate. In all cases, during analysis, the reader was blinded to the sample identification. Experiments using human alveolar macrophages were performed in a similar manner, except that 100,000 cells were plated in a 96-well tissue culture plate and co-culture experiments were performed over 3 h.
Western Blotting
Immunoblot analysis was carried out as described previously with some modifications (32). Briefly, macrophages (1.0 × 106 cells/well) were plated in each well of a 6-well tissue culture plate. Following stimulation, the cells were lysed in RIPA buffer (20 mm HEPES, pH 7.4, 150 mm NaCl, 1 mm dithiothreitol, 0.5% Triton X-100, and 1× protease inhibitor mixture set I), resolved on 7.5% SDS-PAGE, and blotted onto nitrocellulose membranes. The membranes were probed with primary antibodies at 4 °C overnight and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Proteins were visualized by enhanced chemiluminescence (Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. Equal loading of proteins in each lane was confirmed by reprobing with the corresponding antibodies against the native proteins or β-actin. The results shown are representative of at least three separate experiments.
Neurotransmitter Transporter Uptake Assay
Neurotransmitter uptake was assessed using the Neurotransmitter Transporter Uptake Assay Kit (Molecular Devices, Sunnyvale, CA). Experiments were performed according to the manual. Briefly, TG-elicited peritoneal macrophages from 5-HTT KO mice and wild-type mice were cultured in a 96-well plate at 1 × 105 cells per well in Hanks' balanced salt solution. The neurotransmitter tracer was added to each well and the plate was incubated for 60 min in 5% CO2 at 37 °C. The tracer becomes fluorescent when internalized through the 5-HT, dopamine, and norepinephrine transporters. The fluorescent signal was read using a Synergy H1 Hybrid Reader (BioTek, Winooski, VT). Specificity for uptake through the 5-HT transporter was determined using genetic deletion of the 5-HT transporter.
RNA Isolation and Real-time PCR
RNA was extracted using RNeasy Mini Kit (Qiagen, Valecia, CA). The protocol utilized the on-column DNase digestion with RNase-free DNase set (Qiagen). The on-column DNase digestion was also performed per the manufacturer's instructions. The RNA quantity and quality was determined using the NanoDrop Spectrophotometer (Thermo Scientific). 0.5 μg of total RNA was reverse transcribed utilizing the High Capacity RNA to cDNA kit (Applied Biosystems, Foster City, CA). For quantitative real-time PCR, 3 μl of cDNA was brought up to 9 μl with nuclease-free water and combined with 1 μl of TaqMan Gene Expression Assay and 10 μl of TaqMan Gene Expression Master Mix (Applied Biosystems). Assays were performed in triplicate under standard real-time PCR conditions (95 °C for 10 min and 40 cycles of 95 °C for 15 s followed by 60 °C for 1 min) using a 7300 Real-time PCR system (Applied Biosystems) with sequence detection software. Inventoried TaqMan Gene Expression Assays (Applied Biosystems) were used to measure mRNA expression levels for glyceraldehyde-3-phosphate dehydrogenase (GAPDH, assay ID Mm03302249_g1), HTR2a (htr2a, assay ID Mm00555764_m1), HTR2b (htr2b, assay ID Mm00434123_m1), and 5-HT transporter (SLC6A4, assay ID Mm00439391_m1). GAPDH was used as the endogenous control.
Statistical Analysis
Data are presented as mean ± S.E., in bar graphs for repeated observations from two or more separate experiments, or in scatter plots for independent observations from two groups (5-HTT KO and wild-type controls). For neurotransmitter uptake, paring was indicated in scatter plots for paired observations from 5-HTT KO and wild-type controls. The means for repeated observations were conservatively compared using ANOVA. When ANOVA indicated significance, the Dunnett's test was used to compare groups versus a reference group adjusting for multiple comparisons. For two-way ANOVA, interaction, genotype, and treatment effects were tested and Tukey-Kramer post hoc analysis was conducted. Significant tests of percent differences from media control were conducted for Rho kinase activity in Fig. 3, C and D, using F-tests and adjusting for multiple testing using Bonferroni t-statistics. (Data are presented as mean ± S.E. in percent of control where media control at 100% is shown as a reference.) The means for independent observations between 5-HTT KO and wild-type controls were robustly compared using the Mann-Whitney test. Paired t test was used for the paired data for neurotransmitter uptake. All data were analyzed using Prism statistical software for the Macintosh (GraphPad Software, Inc., La Jolla, CA) and SAS vs9.2 (SAS Institute, Cary, NC).
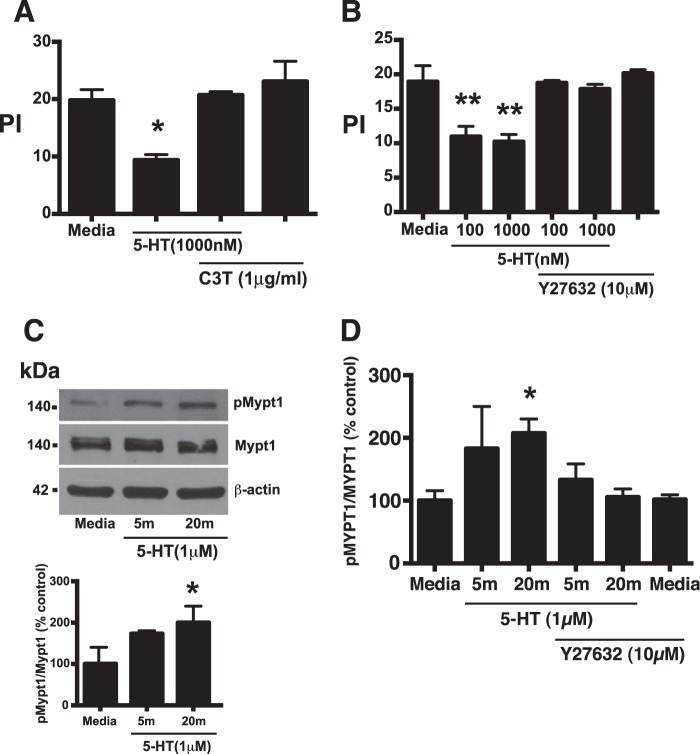
FIGURE 3.
5-HT impairs efferocytosis through activation of the RhoA/Rho kinase pathway. A, murine TG-elicited macrophages were treated with 5-HT at 1,000 nm for 24 h. The RhoA inhibitor, C3 transferase (1 μg/ml), was added either alone or in the presence of 5-HT for 3 h prior to the assay. Apoptotic thymocytes were co-cultured with macrophages at a 10:1 ratio for 1 h in 10% CO2 at 37 °C. Uningested apoptotic thymocytes were washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 3 per group. *, one-way ANOVA, p < 0.05 versus media control (Dunnett's test). B, murine TG-elicited macrophages were treated with 5-HT at 100 or 1,000 nm for 24 h. The ROCK inhibitor, Y-27632 (10 μm), was added either alone or in the presence of 5-HT for 3 h prior to the assay. Apoptotic thymocytes were co-cultured with macrophages at a 10:1 ratio for 1 h in 10% CO2 at 37 °C. Uningested apoptotic thymocytes were washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 3 per group. **, one-way ANOVA, p < 0.05, p < 0.01 versus media control (Dunnett's test). C, murine TG-elicited peritoneal macrophages were stimulated with 5-HT (1 μm) for the indicated times. Phosphorylation of myosin phosphatase 1 (Mypt1) at Thr-696 increased upon 5-HT stimulation. Representative immunoblots are shown in the upper panel. In the lower panel, densitometry was used to determine the ratio of phospho-Mypt1 to total Mypt1. Data represent the mean ± S.E. as a percent of media control for n = 3 per group. Media control = 0.2545 ± 0.1182. *, F-test, p < 0.05 for significance of percent difference from media control (Bonferroni t statistic). D, murine TG-elicited peritoneal macrophage were stimulated with 5-HT (1 μm) for the indicated times in the presence and absence of the Rho kinase inhibitor, Y-27632 (10 μm), and examined for the ratio of phospho-Mypt-1 to total Mypt1 by Western blot. Data represent the mean ± S.E. as percent of media control for n = 5 per group. Media control = 0.1807 ± 0.0286. *, F-test, p < 0.05 for significance of percent control from media control (Bonferroni t statistic).
RESULTS
Effect of 5-HT on Efferocytosis
To investigate the effect of 5-HT on efferocytosis, murine resident and TG-elicited peritoneal macrophages were isolated and co-cultured with apoptotic murine thymocytes or carboxylated beads in the presence or absence of 5-HT. Carboxylated beads are frequently used as an apoptotic cell surrogate because they are negatively charged and are ingested via an efferocytosis-like mechanism (36). Carboxylated beads are also indigestible, so they can distinguish between effects on ingestion versus digestion (41). TG-elicited peritoneal macrophages were isolated and cultured for 24 h in serum-free Xvivo10 containing 0.1% low endotoxin BSA and various concentrations of 5-HT (i.e. 0–1,000 nm). Peritoneal macrophages were then co-cultured with apoptotic thymocytes at a 10:1 ratio (apoptotic cell to macrophage) for 60 min or carboxylated beads at a 2:1 ratio (beads to macrophages) for 15 min, washed, stained, and assessed for ingestions by blinded, visual assessment. Examples of ingestion and binding of apoptotic thymocytes by peritoneal macrophages are shown in Fig. 1, A and B. 5-HT inhibited ingestion of both apoptotic cells and carboxylated beads by both resident and TG-elicited peritoneal macrophages (Fig. 1).
FIGURE 1.
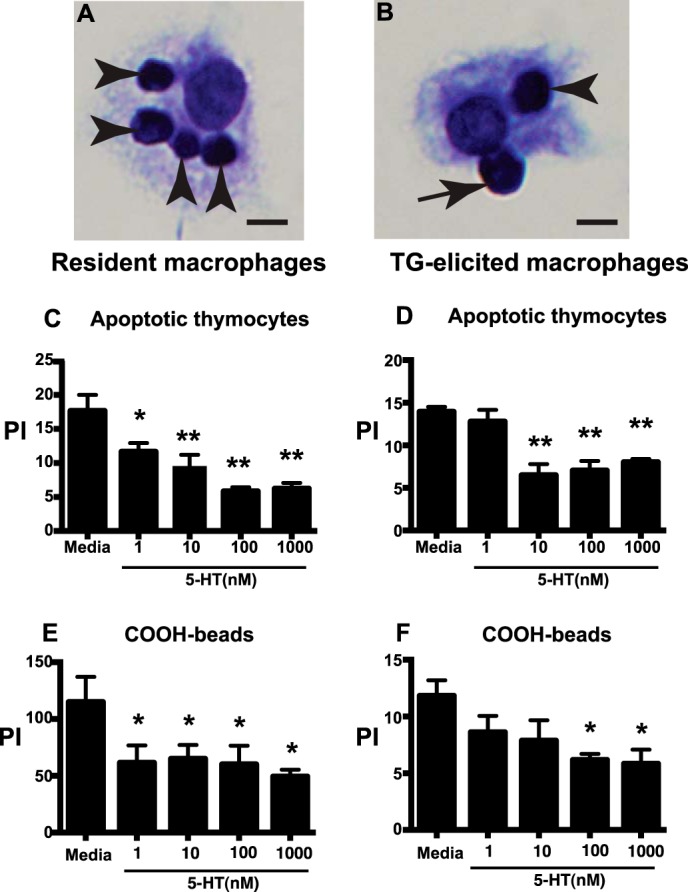
5-HT impairs ingestion of apoptotic cells and carboxylated beads by murine peritoneal macrophages. A and B, photomicrographs (40x original magnification, bar = 5 μm) show binding (arrows) and ingestion (arrowheads) of apoptotic thymocytes by murine peritoneal macrophages. C and D, murine resident (C) and TG-elicited (D) peritoneal macrophages were treated with 5-HT at 1, 10, 100, and 1,000 nm for 24 h then apoptotic thymocytes were co-cultured with macrophages at a 10:1 ratio for 1 h in 10% CO2 at 37 °C. Uningested apoptotic thymocytes were then washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 3 per group. *, one-way ANOVA, p < 0.05 versus media control (Dunnett's Test); **, p < 0.01 versus media control (Dunnett's test). E and F, murine resident (E) and TG-elicited (F) peritoneal macrophages were treated with 5-HT at 1, 10, 100, and 1,000 nm for 24 h, then carboxylated beads were co-cultured with macrophages at a 2:1 ratio for 20 min in 10% CO2 at 37 °C. Uningested beads were washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 3 per group. *, one-way ANOVA, p < 0.05, p < 0.05 versus media control (Dunnett's test).
Several groups have studied the effect of 5-HT on phagocytosis of targets that are unrelated to efferocytosis by murine peritoneal macrophages, such as bone marrow-derived macrophages and microglia, but the effects have been variable (42–44). We performed similar experiments using resident and TG-elicited peritoneal macrophages and found that 5-HT inhibited ingestion of latex beads, but had no effect on ingestion of IgG-opsonized erythrocytes through the Fcγ receptor (Fig. 2). These results indicate that the suppressive effect of 5-HT on efferocytosis is not uniform across all mechanisms of phagocytosis.
FIGURE 2.
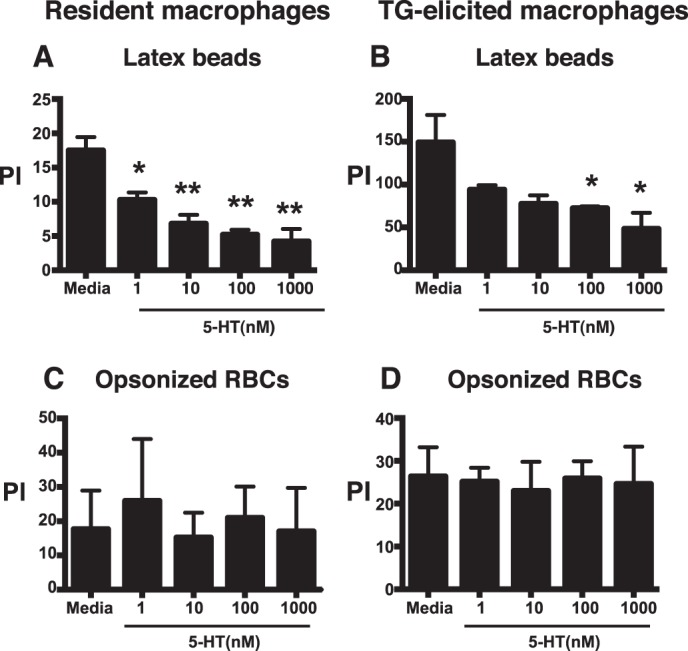
5-HT impairs phagocytosis of latex beads by murine peritoneal macrophages, but has no effect on uptake of IgG-opsonized erythrocytes through the Fcγ receptor. A and B, mouse resident (A) and TG-elicited (B) peritoneal macrophages were treated with 5-HT at 1, 10, 100, and 1,000 nm for 24 h, then latex beads were co-cultured at a 1:1 ratio for 10 min in 10% CO2 at 37 °C. Uningested beads were washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 3 per group, *, one-way ANOVA, p < 0.05 versus media control (Dunnett's Test). **, p < 0.01 versus media control (Dunnett's test). C and D, mouse resident (C) and TG-elicited (D) peritoneal macrophages were treated with 5-HT at 1, 10, 100, and 1,000 nm for 24 h then co-cultured with IgG-opsonized erythrocytes (RBCs) at a 10:1 ratio in 10% CO2 at 37 °C. Uningested RBCs were then washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 3 per group.
5-HT Inhibits Efferocytosis through Activation of the RhoA/ROCK Pathway
The RhoA/ROCK pathway is a potent negative regulator of efferocytosis. 5-HT activates the RhoA/ROCK pathway, leading us to hypothesize that 5-HT suppresses efferocytosis via a RhoA/ROCK-dependent mechanism. To test this hypothesis, TG-elicited peritoneal macrophages were treated with 5-HT in the presence or absence of the specific RhoA inhibitor, C3 transferase, or the specific ROCK inhibitor, Y-27632. C3 transferase and Y-27632 both reversed the ability of 5-HT to inhibit efferocytosis (Fig. 3, A and B). ROCK is a serine/threonine kinase that phosphorylates myosin phosphatase-1 (Mypt-1) at Thr-696 (25). Our results demonstrated that 5-HT increases phosphorylation of Mypt-1 at Thr-696 (Fig. 3C) in a Y-27632-sensitive manner (Fig. 3D), supporting that 5-HT increases ROCK activity.
Expression of 5-HT Receptors and Transporter in Murine Peritoneal Macrophages
5-HT has been shown to activate Rho GTPases by interacting with one of 14 known G protein-coupled receptors (23) or via a 5-HT transporter-dependent mechanism (5). We queried the open public gene array database of the Immunologic Gene Project to determine which of the 5-HT receptors or the 5-HTT are expressed in mouse peritoneal macrophages. The Immgen database showed that the major 5-HT receptors expressed on resident or TG-elicited murine peritoneal macrophages were 5-HT receptor 2a (5-HTR2a), 5-HT 2b (5-HTR2b), and the 5-HTT. We confirmed the expression of 5-HTR2a, 5-HTR2b, and 5-HTT by real-time PCR (Fig. 4A) and Western blotting (Fig. 4, B–D). In contrast, 5-HTR2c was not expressed (Fig. 4A).
FIGURE 4.
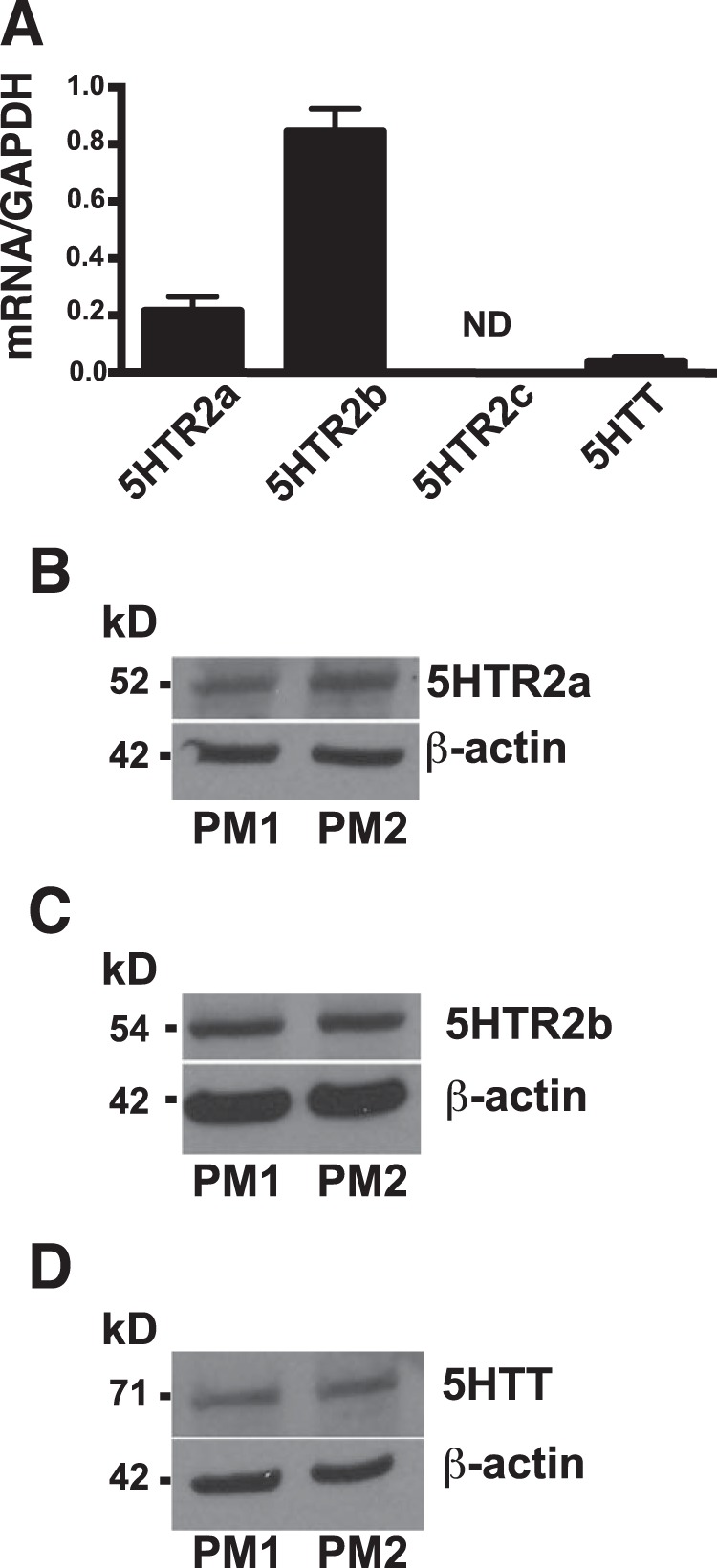
5-HT receptor 2a, 2b, and the 5-HT transporter are expressed in mouse peritoneal macrophages. A, real-time PCR was performed to measure the expression by mRNA. 5-HT receptor 2a (5HTR2a), 5-HT receptor 2b (5HTR2b), and the 5-HTT were expressed in TG-elicited peritoneal macrophages, but 5-HT receptor 2c (5HTR2c) was not detected. Data represent the mean ± S.E. for n = 3 per group. B–D, Western blotting is shown for two representative peritoneal macrophage lysates (PM1 and PM2) evaluating expression of 5-HT receptors and transporter. B, HTR2a (n = 4); C, HTR2b (n = 10); and D, 5-HTT (n = 8) were all detected in mouse peritoneal macrophages.
Effect of 5-HT Receptor and 5-HTT Blockade on 5-HT-impaired Efferocytosis
To determine the mechanism for 5-HT impaired efferocytosis, apoptotic cell ingestion by peritoneal macrophages was examined following 5-HT receptor blockade using the 5-HTR2a antagonist, R-96544, the 5-HTR2b antagonist, RS-127445, and the 5-HTT antagonist, fluoxetine. 5-HT impaired efferocytosis, but was unaffected by either R-96544 or RS-127445 (Fig. 5, A and B). In contrast, fluoxetine prevented the ability of 5-HT to suppress efferocytosis by TG-elicited peritoneal macrophages (Fig. 5C). These data suggest that 5-HT impairs efferocytosis via a 5-HTT-dependent mechanism, and not through a 5-HTR2a or -2b-dependent mechanism.
FIGURE 5.
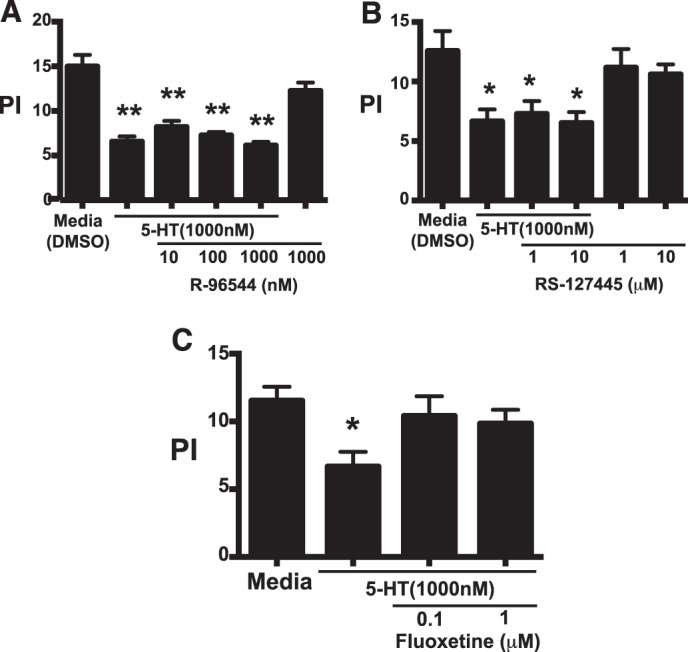
5-HT utilizes the 5-HT transporter, but not 5-HT receptors 2a or 2b, to regulate efferocytosis by murine peritoneal macrophages. A and B, TG-elicited macrophages were treated with 5-HT at 1,000 nm in the presence and absence of: A, the 5-HT receptor 2b inhibitor, R-96544; B, the 5-HT receptor 2b inhibitor, RS-127445; or C, the 5-HT transporter inhibitor, fluoxetine, at the indicated concentrations for 24 h. Apoptotic thymocytes were co-cultured with macrophages at a 10:1 ratio for 1 h in 10% CO2 at 37 °C. Uningested apoptotic thymocytes were washed off and a PI was determined by blinded visual inspection. 5-HT does not inhibit efferocytosis via 5HTR2a- or 5HTR2b-dependent pathways. Data represent the mean ± S.E. for n = 4–5 replicates per group, *, one-way ANOVA, p < 0.05 versus DMSO control (Dunnett's test); **, p < 0.01 versus DMSO control (Dunnett's test). C, TG-elicited macrophages were treated with 5-HT at 1.0 μm for 24 h in the presence and absence of the 5-HT transporter inhibitor, fluoxetine, at 0.1 and 1.0 μm. Apoptotic thymocytes were then co-cultured with macrophages at a 10:1 ratio for 1 h in 10% CO2 at 37 °C. Uningested apoptotic thymocytes were washed off and a PI was determined by blinded visual inspection. Data represent the mean ± S.E. for n = 5 per group, *, one-way ANOVA, p < 0.05 versus media control (Dunnett's test).
Effect of 5-HTT Deficiency on 5-HT-impaired Efferocytosis by Murine Peritoneal Macrophages
The ability of fluoxetine to prevent 5-HT from suppressing efferocytosis suggests involvement of the 5-HTT. However, inhibitor experiments can never be definitive due to recognized and unrecognized off-target effects. To address this critical issue, experiments were performed using peritoneal macrophages from 5-HTT KO and control mice. Rudd and colleagues (45) previously used real-time PCR, Western blot analysis, and [3H]5-HT uptake to demonstrate the presence of a functional 5-HT transporter in peritoneal macrophages. We confirmed the presence of a functional 5-HT transporter in peritoneal macrophages using a neuroendocrine uptake assay, where a masked tracer becomes fluorescent upon internalization through the 5-HT, dopamine, or the norepinephrine transporters. Specificity of the assay depends on the use of specific inhibitors or preferably by genetic deletion of a given transporter. Results showed that internalization of the tracer by 5-HTT KO peritoneal macrophages was decreased compared with wild-type controls (Fig. 6A). To confirm the involvement of 5-HTT in the regulation of efferocytosis, TG-elicited peritoneal macrophages from wild-type and 5-HTT KO mice were treated with 5-HT for 24 h and then assessed for their ability to ingest apoptotic thymocytes. As was seen with fluoxetine (Fig. 5C), 5-HT inhibited efferocytosis by wild-type macrophages, but did not suppress efferocytosis by macrophages from 5-HTT KO mice (Fig. 6B). Similarly, 5-HT increased phosphorylation of Mypt-1 at Thr-696 in macrophages from wild-type mice, but not from 5-HTT KO mice (Fig. 6C). Together these results indicate that 5-HT acts through its transporter to activate the Rho kinase pathway and inhibit efferocytosis in murine peritoneal macrophages.
FIGURE 6.
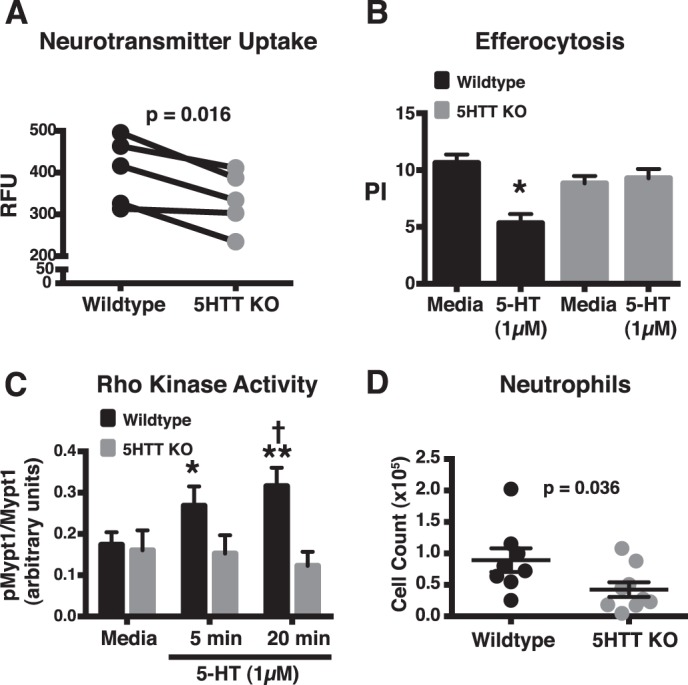
Effect of 5-HTT deficiency on 5-HT uptake, efferocytosis, Rho kinase activity, and peritoneal inflammation. A, function of the 5-HTT in TG-elicited peritoneal macrophages was investigated using a neuroendocrine uptake assay, where a tracer becomes fluorescent upon internalization. The tracer can be internalized by the 5-HT, dopamine, or the norepinephrine transporters, so specificity depends on genetic deletion of a given transporter. TG-elicited peritoneal macrophages were isolated from 5-HTT KO (gray circles) and wild-type control mice (black circles), and uptake of the fluorescent tracer was assessed and expressed as relative fluorescent units (RFU). Lines indicate paired experiments. p = 0.016, wild-type versus 5-HTT KO mice (paired t test). B, TG-elicited peritoneal macrophages from wild-type mice (black columns) and 5-HTT KO mice (gray columns) were treated with media alone or media containing 5-HT (1 μm) for 24 h. Apoptotic thymocytes were co-cultured with macrophages at a 10:1 ratio for 1 h in 10% CO2 at 37 °C. Uningested apoptotic thymocytes were washed off and a PI was determined by blinded visual inspection. 5-HT decreased efferocytosis in wild-type macrophages. Data represent the mean ± S.E. for n = 8 per group. Two-way ANOVA, p < 0.001 for interaction, and p < 0.01 for treatment effect (media versus 5-HT treatment). *, Tukey-Kramer post hoc analysis, p < 0.001 for wild-type 5-HT treatment versus wild-type media control; p < 0.01 for wild-type 5-HT treatment versus 5-HTT KO 5-HT treatment; p < 0.05 for wild-type 5-HT treatment versus 5-HTT KO media control. C, murine TG-elicited peritoneal macrophages from wild-type (black columns) and 5-HTT KO mice (gray columns) were stimulated with 5-HT (1 μm) for the indicated times. Densitometry was used to determine the ratio of phosphomyosin phosphatase 1 (pMypt1) to total Mypt1 by arbitrary units. 5-HT increased phosphorylation of Mypt1 at Thr-696 in wild-type but not in 5-HTT KO macrophages. Data represent the mean ± S.E. for n ≤ 13 per group. Two-way ANOVA, p = 0.03 for interaction, and p < 0.01 for genotype effect (wild-type versus 5-HTT KO). *, Tukey-Kramer post hoc analysis p < 0.03 for wild-type media control versus wild-type 5-HT treatment for 5 min; **, p < 0.01 for wild-type media control versus wild-type 5-HT treatment for 20 min; †, p < 0.03 for wild-type 5-HT treatment for 20 min versus 5-HTT KO 5-HT treatment for 20 min. D, 5-HTT KO (gray circles) and wild-type (black circles) mice were injected with TG. Four days later peritoneal lavage was performed and total cell counts and differentials were taken. Neutrophils were decreased in 5-HTT KO mice compared with wild-type mice. Data represent the mean ± S.E. for n = 8–10 mice per group. p = 0.036, wild-type versus 5-HTT KO mice (Mann-Whitney test).
Effect of 5-HTT Deficiency on Cellular Peritoneal Inflammation
Efferocytosis promotes the resolution of inflammation, leading to the hypothesis that 5-HTT deficiency may accelerate the resolution phase of the inflammatory response by preserving efferocytosis. To examine this hypothesis, cell counts were performed on peritoneal exudates 4 days post-intraperitoneal injection with TG in wild-type and 5-HTT KO mice. These experiments showed a decrease in neutrophils (Fig. 6D) in 5-HTT KO mice versus wild-type controls. In contrast, 5-HTT deficiency had no effect on macrophages (wild-type: 2.1 × 107 ± 3.9 × 106 versus 5-HTT KO: 1.5 × 107 ± 1.8 × 106, p = 0.23, Mann Whitney test). These results suggest that suppression of 5-HTT activity promotes the resolution phase of inflammation.
5-HT Inhibits Efferocytosis by Human Alveolar Macrophages through a 5-HTT and ROCK-dependent Process
Because the repertoire of expressed 5-HT receptors and transporter may vary significantly between species and macrophage type, we sought to determine whether 5-HT has similar effects on efferocytosis by human alveolar macrophages. Like TG-elicited peritoneal macrophages, normal human alveolar macrophages expressed 5-HTT protein by Western blot (Fig. 7A). In addition, 5-HT suppressed efferocytosis of apoptotic Jurkat T-cells, an effect that was reversed by the 5-HTT inhibitor, fluoxetine (Fig. 7B), and the ROCK inhibitor, Y-27632 (Fig. 7C). Together, these results suggest that 5-HT inhibits efferocytosis by both human and murine macrophages via a 5-HTT/ROCK-dependent mechanism.
FIGURE 7.
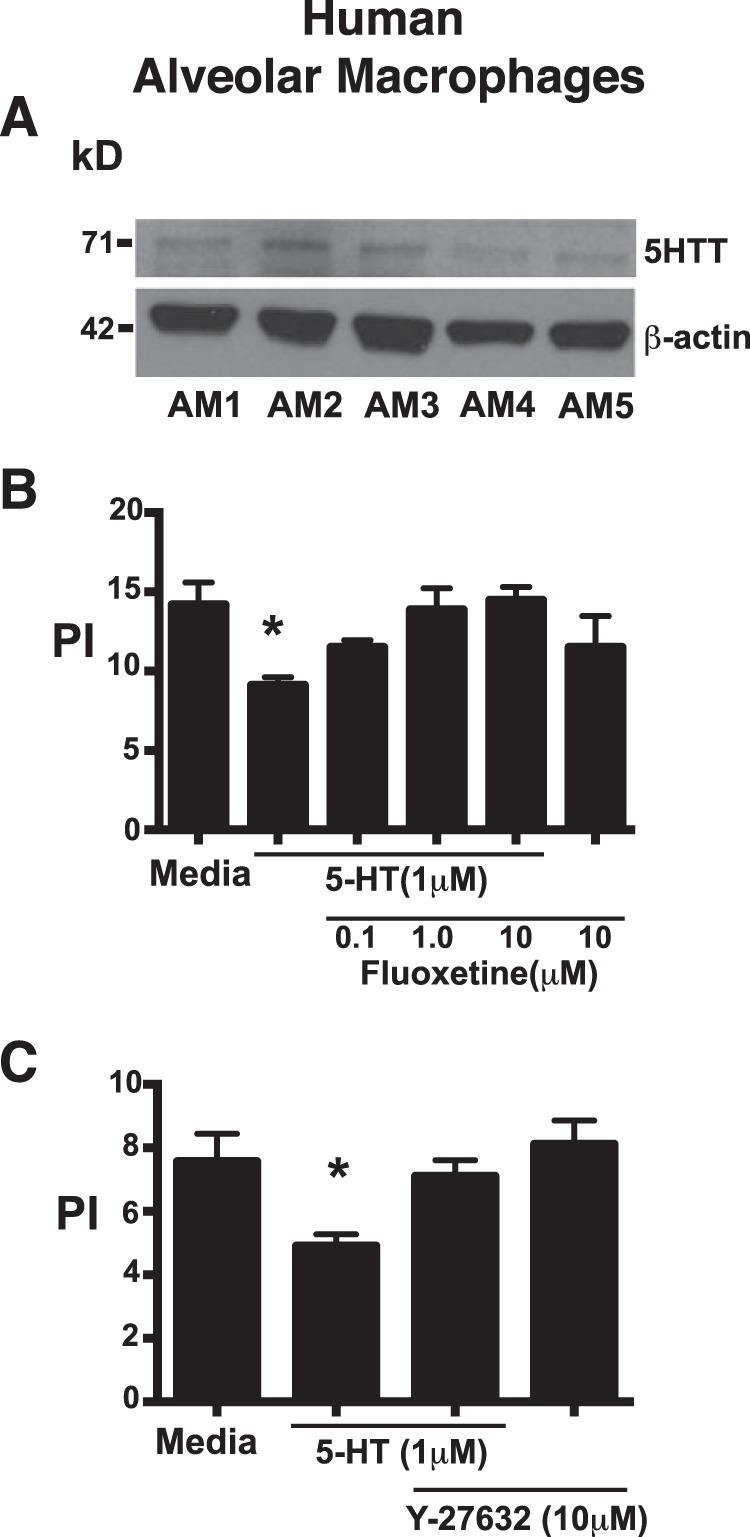
5-HT inhibits efferocytosis by human alveolar macrophages via a mechanism that involves the 5-HT transporter and Rho kinase. A, Western blots were performed on lysates from human alveolar macrophages from n = 5 normal subjects and probed for the presence of the 5-HT transporter. B, human alveolar macrophages were treated with 5-HT at 1.0 μm for 24 h in the presence and absence of the 5-HT transporter inhibitor, fluoxetine, at 0.1, 1.0, and 10 μm. Apoptotic human Jurkat T-cells were then co-cultured with macrophages at a 10:1 ratio for 3 h in 10% CO2 at 37 °C. Uningested apoptotic cells were washed off and PI was determined by visual inspection. Data represent the mean ± S.E. for n = 3 per group, *, one-way ANOVA, p < 0.05 versus media control (Dunnett's test). C, human alveolar macrophages were treated with 5-HT at 1.0 μm for 24 h in the presence and absence of the ROCK inhibitor, Y-27632, at 10 μm. Apoptotic human Jurkat T-cells were then co-cultured with macrophages at a 10:1 ratio for 3 h in 10% CO2 at 37 °C. Uningested apoptotic cells were washed off and a PI was determined by visual inspection. Data represent the mean ± S.E. for n = 4 per group, *, one-way ANOVA, p < 0.05 versus media control (Dunnett's test).
DISCUSSION
Our study demonstrates for the first time the potential of the neuroendocrine system to regulate efferocytosis and thereby influence the maintenance of homeostasis as well as the resolution of inflammation. Our data confirm the hypothesis that 5-HT inhibits efferocytosis by activating the RhoA/ROCK pathway, a well known, potent, negative regulator of efferocytosis (32–36). Unexpectedly, 5-HT did not inhibit efferocytosis via one of its many G protein-linked receptors, but rather suppressed efferocytosis through a 5-HT transporter-dependent mechanism, a protein that is better known as a regulator of neurotransmission than of macrophage biology. Because over 95% of 5-HT is produced outside of the CNS (2, 7), these results may have wide-ranging implications for health and disease.
Results show that 5-HT effectively inhibits phagocytosis of apoptotic cells, carboxylated beads, and latex beads by peritoneal macrophages, but has no effect on ingestion of IgG-opsonized erythrocytes via the Fcγ receptor. Few prior studies have investigated the ability of 5-HT to regulate phagocytosis and none have examined efferocytosis or ingestion through the Fcγ receptor. Existing studies are conflicting. For example, Sternberg et al. (44) explored the ability of 5-HT to regulate phagocytosis of latex beads by bone marrow-derived macrophages isolated from SJL mice. They found that 5-HT enhanced latex bead ingestion when cells were simultaneously exposed to small concentrations of IFNγ but inhibited ingestion when exposed to larger concentrations. In contrast, Freire-Garabal et al. (42) found that a short exposure to 5-HT increased latex bead phagocytosis by resident peritoneal macrophages from BALB/c mice. More recently Krabbe et al. (43) demonstrated that 5-HT inhibits ingestion of microspheres by microglia, resident macrophages of the CNS. These studies differ substantially from ours in regard to mouse strain, macrophage type, and 5-HT exposure (e.g. time and concentration). Together, these differences make a direct comparison difficult. More recently, an intriguing study demonstrated the importance of 5-HT in effective erythropoiesis, where the absence of 5-HT decreased differentiation of erythroid precursors and increased phagocytosis of erythrocytes (46). Like apoptotic cells, aging erythrocytes externalize phosphatidylserine on their outer membrane allowing them to be recognized and removed by phagocytes (47–50). These findings suggest that physiologic levels of 5-HT regulate erythropoiesis, in part by inhibiting their recognition and removal from circulation by phagocytes.
5-HT may exert its effects through any of the 14 known G protein-linked 5-HT receptors or through the 5-HTT (7, 22), suggesting a sizable dimension of potential effects. Specific macrophage populations contain their own unique repertoire of 5-HT receptors. For example, our data demonstrate that peritoneal macrophages express 5-HTR2a and 5-HTR2b, but do not express 5-HTR2c. In contrast, murine alveolar macrophages express much higher levels of 5-HTR2c and lower levels of 5-HTR2a and 5-HTR2b (51). Murine alveolar macrophages also express higher amounts of 5-HTT than do peritoneal macrophages (www.immgen.org). Despite the presence of 5-HTR2a and 5-HT2b, 5-HT appears to inhibit efferocytosis through a 5-HTT-dependent mechanism.
The results support the conclusion that 5-HT inhibits efferocytosis via a RhoA/ROCK-dependent mechanism. 5-HT is known to activate the RhoA/ROCK pathway through any of its G protein-linked receptors or through a recently described 5-HTT-dependent mechanism, called serotonylation (5, 23, 52). Our data indicate that 5-HT impairs efferocytosis via a 5-HTT-dependent mechanism, not by activating 5-HTR2a or 5-HTR2b, suggesting that serotonylation may be involved. During serotonylation, transglutaminase-2 transamidates proteins by covently linking 5-HT to glutamine residues.
There are multiple potential mechanisms by which serotonylation may regulate RhoA activity. First, like other Rho GTPases, RhoA may be directly serotonylated (5, 52). Serotonylated GTPases are resistant to GTP hydrolysis, rendering them constitutively active. Serotonylated RhoA has been demonstrated to be a critical mechanism for normal hemostasis by regulating platelet attachment (5). Increased serotonylated RhoA has also been implicated in the pathogenesis of pulmonary hypertension via enhanced activation of ROCK and increased vasoconstriction (14). Second, 5-HT receptor-associated G proteins are suggested to be targets for activation through serotonylation (2). This would indicate that 5-HT receptors may activate RhoA through mechanisms that are separate from traditional receptor/ligand interactions. Finally, two recent studies demonstrated that the extracellular matrix glycoprotein, fibronectin, is a target for serotonylation (53, 54). Although no direct link for RhoA/ROCK activation has been made, fibronectin is known to bind both α5β1 and α5β3 integrins, which results in RhoA activation (55).
The studies presented here represent the beginning of our understanding of the mechanisms by which 5-HT regulates efferocytosis. Further studies will be necessary to determine the involvement of serotonylation and identify whether the target of serotonylation is RhoA or one of the other possible targets that regulate RhoA activity. Likewise, the ability of 5-HT to impair efferocytosis will need to be studied in the context of human diseases with impaired efferocytosis (and their animal models) as outlined above, in particular those diseases where 5-HT has already been implicated in disease pathogenesis. It is this connection that will suggest the therapeutic potential of pharmacologically regulating the 5-HT pathway.
In summary, we have shown the ability of neuroendocrine signaling via 5-HT to inhibit efferocytosis via a pathway that depends on the serotonin transporter and activation of the RhoA/ROCK pathway (Fig. 8). Future studies will focus on elucidating the multiple potential mechanisms by which 5-HTT regulates RhoA/ROCK activation and demonstrating the involvement of this pathway in the pathogenesis of diseases associated with increased peripheral 5-HT, such as rheumatoid arthritis (8, 9), inflammatory bowel disease (10–12), pulmonary hypertension (13, 14), asthma (17), and chronic obstructive pulmonary disease (18–21).
FIGURE 8.
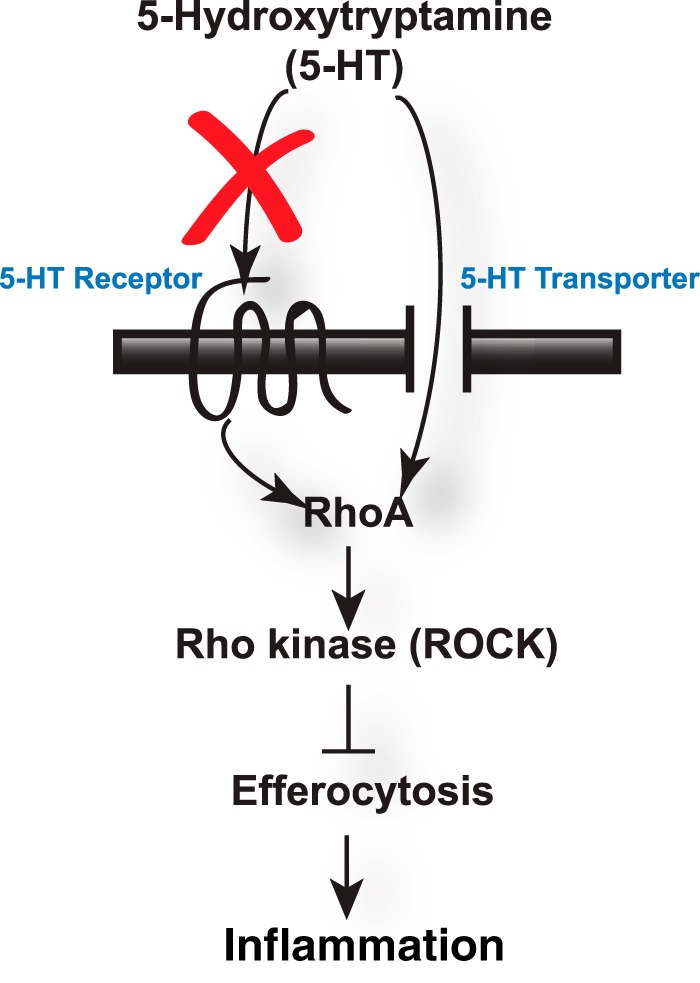
Proposed model for the ability of 5-HT to suppress efferocytosis. 5-HT impairs efferocytosis by activating RhoA and ROCK. 5-HT appears to exert its effect on efferocytosis through the 5-HT transporter and not through 5-HT receptor 2a or 5-HT receptor 2b (red X).
Acknowledgment
We thank Dr. Peter Henson for thoughtful insights during the study and critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-088138 from the NHLBI (to R. W. V.) and Grant R24 AAO19661 from the NIAAA (to E. L. B.) and Clinical Innovator Award CIA092054 from the Flight Attendant Medical Research Institute.
- 5-HT
- 5-hydroxytryptamine
- TG
- thioglycollate
- PI
- phagocytic index
- ANOVA
- analysis of variance
- 5-HTT
- 5-HT transporter.
REFERENCES
- 1. Mössner R., Lesch K. P. (1998) Role of serotonin in the immune system and in neuroimmune interactions. Brain Behav. Immun. 12, 249–271 [DOI] [PubMed] [Google Scholar]
- 2. Walther D. J., Stahlberg S., Vowinckel J. (2011) Novel roles for biogenic monoamines: from monoamines in transglutaminase-mediated post-translational protein modification to monoaminylation deregulation diseases. FEBS J. 278, 4740–4755 [DOI] [PubMed] [Google Scholar]
- 3. Paulmann N., Grohmann M., Voigt J. P., Bert B., Vowinckel J., Bader M., Skelin M., Jevsek M., Fink H., Rupnik M., Walther D. J. (2009) Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 7, e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chabbi-Achengli Y., Coudert A. E., Callebert J., Geoffroy V., Côté F., Collet C., de Vernejoul M. C. (2012) Decreased osteoclastogenesis in serotonin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 109, 2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walther D. J., Peter J. U., Winter S., Höltje M., Paulmann N., Grohmann M., Vowinckel J., Alamo-Bethencourt V., Wilhelm C. S., Ahnert-Hilger G., Bader M. (2003) Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell 115, 851–862 [DOI] [PubMed] [Google Scholar]
- 6. Gershon M. D., Drakontides A. B., Ross L. L. (1965) Serotonin: synthesis and release from the myenteric plexus of the mouse intestine. Science 149, 197–199 [DOI] [PubMed] [Google Scholar]
- 7. Gershon M. D., Tack J. (2007) The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132, 397–414 [DOI] [PubMed] [Google Scholar]
- 8. Seddighzadeh M., Korotkova M., Källberg H., Ding B., Daha N., Kurreeman F. A., Toes R. E., Huizinga T. W., Catrina A. I., Alfredsson L., Klareskog L., Padyukov L. (2010) Evidence for interaction between 5-hydroxytryptamine (serotonin) receptor 2A and MHC type II molecules in the development of rheumatoid arthritis. Eur. J. Hum. Genet. 18, 821–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snir O., Hesselberg E., Amoudruz P., Klareskog L., Zarea-Ganji I., Catrina A. I., Padyukov L., Malmström V., Seddighzadeh M. (2013) Genetic variation in the serotonin receptor gene affects immune responses in rheumatoid arthritis. Genes Immun. 14, 83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghia J. E., Li N., Wang H., Collins M., Deng Y., El-Sharkawy R. T., Côté F., Mallet J., Khan W. I. (2009) Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 137, 1649–1660 [DOI] [PubMed] [Google Scholar]
- 11. Margolis K. G., Pothoulakis C. (2009) Serotonin has a critical role in the pathogenesis of experimental colitis. Gastroenterology 137, 1562–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim J. J., Bridle B. W., Ghia J. E., Wang H., Syed S. N., Manocha M. M., Rengasamy P., Shajib M. S., Wan Y., Hedlund P. B., Khan W. I. (2013) Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J. Immunol. 190, 4795–4804 [DOI] [PubMed] [Google Scholar]
- 13. Eddahibi S., Humbert M., Fadel E., Raffestin B., Darmon M., Capron F., Simonneau G., Dartevelle P., Hamon M., Adnot S. (2001) Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J. Clin. Invest. 108, 1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guilluy C., Eddahibi S., Agard C., Guignabert C., Izikki M., Tu L., Savale L., Humbert M., Fadel E., Adnot S., Loirand G., Pacaud P. (2009) RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am. J. Respir. Crit. Care Med. 179, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 15. Goolaerts A., Roux J., Ganter M. T., Shlyonsky V., Chraibi A., Stéphane R., Mies F., Matthay M. A., Naeije R., Sariban-Sohraby S., Howard M., Pittet J. F. (2010) Serotonin decreases alveolar epithelial fluid transport via a direct inhibition of the epithelial sodium channel. Am. J. Respir. Cell Mol. Biol. 43, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duerschmied D., Suidan G. L., Demers M., Herr N., Carbo C., Brill A., Cifuni S. M., Mauler M., Cicko S., Bader M., Idzko M., Bode C., Wagner D. D. (2013) Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121, 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dürk T., Duerschmied D., Müller T., Grimm M., Reuter S., Vieira R. P., Ayata K., Cicko S., Sorichter S., Walther D. J., Virchow J. C., Taube C., Idzko M. (2013) Production of serotonin by tryptophan hydroxylase 1 and release via platelets contribute to allergic airway inflammation. Am. J. Respir Crit. Care Med. 187, 476–485 [DOI] [PubMed] [Google Scholar]
- 18. Lau W. K., Chan-Yeung M. M., Yip B. H., Cheung A. H., Ip M. S., Mak J. C. (2012) The role of circulating serotonin in the development of chronic obstructive pulmonary disease. PLoS One 7, e31617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soler Artigas M., Wain L. V., Repapi E., Obeidat M., Sayers I., Burton P. R., Johnson T., Zhao J. H., Albrecht E., Dominiczak A. F., Kerr S. M., Smith B. H., Cadby G., Hui J., Palmer L. J., Hingorani A. D., Wannamethee S. G., Whincup P. H., Ebrahim S., Smith G. D., Barroso I., Loos R. J., Wareham N. J., Cooper C., Dennison E., Shaheen S. O., Liu J. Z., Marchini J., Medical Research Council National Survey of Halth and Development (NSHD) Respiratory Study Team, Dahgam S., Naluai A. T., Olin A. C., Karrasch S., Heinrich J., Schulz H., McKeever T. M., Pavord I. D., Heliövaara M., Ripatti S., Surakka I., Blakey J. D., Kähönen M., Britton J. R., Nyberg F., Holloway J. W., Lawlor D. A., Morris R. W., James A. L., Jackson C. M., Hall I. P., Tobin M. D. (2011) Effect of five genetic variants associated with lung function on the risk of chronic obstructive lung disease, and their joint effects on lung function. Am. J. Respir. Crit. Care Med. 184, 786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilk J. B., Shrine N. R., Loehr L. R., Zhao J. H., Manichaikul A., Lopez L. M., Smith A. V., Heckbert S. R., Smolonska J., Tang W., Loth D. W., Curjuric I., Hui J., Cho M. H., Latourelle J. C., Henry A. P., Aldrich M., Bakke P., Beaty T. H., Bentley A. R., Borecki I. B., Brusselle G. G., Burkart K. M., Chen T. H., Couper D., Crapo J. D., Davies G., Dupuis J., Franceschini N., Gulsvik A., Hancock D. B., Harris T. B., Hofman A., Imboden M., James A. L., Khaw K. T., Lahousse L., Launer L. J., Litonjua A., Liu Y., Lohman K. K., Lomas D. A., Lumley T., Marciante K. D., McArdle W. L., Meibohm B., Morrison A. C., Musk A. W., Myers R. H., North K. E., Postma D. S., Psaty B. M., Rich S. S., Rivadeneira F., Rochat T., Rotter J. I., Soler Artigas M., Starr J. M., Uitterlinden A. G., Wareham N. J., Wijmenga C., Zanen P., Province M. A., Silverman E. K., Deary I. J., Palmer L. J., Cassano P. A., Gudnason V., Barr R. G., Loos R. J., Strachan D. P., London S. J., Boezen H. M., Probst-Hensch N., Gharib S. A., Hall I. P., O'Connor G. T., Tobin M. D., Stricker B. H. (2012) Genome Wide Association Studies Identify CHRNA5/3 and HTR4 in the Development of Airflow Obstruction. Am. J. Respir Crit. Care Med. 186, 622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishii T., Wakabayashi R., Kurosaki H., Gemma A., Kida K. (2011) Association of serotonin transporter gene variation with smoking, chronic obstructive pulmonary disease, and its depressive symptoms. J. Hum. Genet. 56, 41–46 [DOI] [PubMed] [Google Scholar]
- 22. Murphy D. L., Fox M. A., Timpano K. R., Moya P. R., Ren-Patterson R., Andrews A. M., Holmes A., Lesch K. P., Wendland J. R. (2008) How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology 55, 932–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Millan M. J., Marin P., Bockaert J., Mannoury la Cour C. (2008) Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 29, 454–464 [DOI] [PubMed] [Google Scholar]
- 24. Vandivier R. W., Henson P. M., Douglas I. S. (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129, 1673–1682 [DOI] [PubMed] [Google Scholar]
- 25. Bratton D. L., Henson P. M. (2011) Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 32, 350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elliott M. R., Ravichandran K. S. (2010) Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 189, 1059–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Vré E. A., Ait-Oufella H., Tedgui A., Mallat Z. (2012) Apoptotic cell death and efferocytosis in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 32, 887–893 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez-Boyanapalli R., Goleva E., Kolakowski C., Min E., Day B., Leung D. Y., Riches D. W., Bratton D. L., Sutherland E. R. (2013) Obesity impairs apoptotic cell clearance in asthma. J. Allergy Clin. Immunol. 131, 1041–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roszer T., Menéndez-Gutiérrez M. P., Lefterova M. I., Alameda D., Núñez V., Lazar M. A., Fischer T., Ricote M. (2011) Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor γ or retinoid X receptor α deficiency. J. Immunol. 186, 621–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huynh M. L., Malcolm K. C., Kotaru C., Tilstra J. A., Westcott J. Y., Fadok V. A., Wenzel S. E. (2005) Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am. J. Respir. Crit. Care Med. 172, 972–979 [DOI] [PubMed] [Google Scholar]
- 31. Fernandez-Boyanapalli R. F., Frasch S. C., McPhillips K., Vandivier R. W., Harry B. L., Riches D. W., Henson P. M., Bratton D. L. (2009) Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 113, 2047–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morimoto K., Janssen W. J., Fessler M. B., McPhillips K. A., Borges V. M., Bowler R. P., Xiao Y. Q., Kench J. A., Henson P. M., Vandivier R. W. (2006) Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 176, 7657–7665 [DOI] [PubMed] [Google Scholar]
- 33. Richens T. R., Linderman D. J., Horstmann S. A., Lambert C., Xiao Y. Q., Keith R. L., Boé D. M., Morimoto K., Bowler R. P., Day B. J., Janssen W. J., Henson P. M., Vandivier R. W. (2009) Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 179, 1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vandivier R. W., Richens T. R., Horstmann S. A., deCathelineau A. M., Ghosh M., Reynolds S. D., Xiao Y. Q., Riches D. W., Plumb J., Vachon E., Downey G. P., Henson P. M. (2009) Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am. J. Physiol. Lung Cell Mol. Physiol. 297, L677–L686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boé D. M., Richens T. R., Horstmann S. A., Burnham E. L., Janssen W. J., Henson P. M., Moss M., Vandivier R. W. (2010) Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol Clin. Exp. Res. 34, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tosello-Trampont A. C., Nakada-Tsukui K., Ravichandran K. S. (2003) Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 278, 49911–49919 [DOI] [PubMed] [Google Scholar]
- 37. Bengel D., Murphy D. L., Andrews A. M., Wichems C. H., Feltner D., Heils A., Mössner R., Westphal H., Lesch K. P. (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol. Pharmacol. 53, 649–655 [DOI] [PubMed] [Google Scholar]
- 38. Murphy D. L., Lesch K. P. (2008) Targeting the murine serotonin transporter: insights into human neurobiology. Nat. Rev. Neurosci. 9, 85–96 [DOI] [PubMed] [Google Scholar]
- 39. Holmes A., Lit Q., Murphy D. L., Gold E., Crawley J. N. (2003) Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2, 365–380 [DOI] [PubMed] [Google Scholar]
- 40. Vandivier R. W., Fadok V. A., Hoffmann P. R., Bratton D. L., Penvari C., Brown K. K., Brain J. D., Accurso F. J., Henson P. M. (2002) Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Invest. 109, 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Erwig L. P., McPhilips K. A., Wynes M. W., Ivetic A., Ridley A. J., Henson P. M. (2006) Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc. Natl. Acad. Sci. U.S.A. 103, 12825–12830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freire-Garabal M., Núñez M. J., Balboa J., López-Delgado P., Gallego R., García-Caballero T., Fernández-Roel M. D., Brenlla J., Rey-Méndez M. (2003) Serotonin up-regulates the activity of phagocytosis through 5-HT1A receptors. Br. J. Pharmacol. 139, 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krabbe G., Matyash V., Pannasch U., Mamer L., Boddeke H. W., Kettenmann H. (2012) Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav. Immun. 26, 419–428 [DOI] [PubMed] [Google Scholar]
- 44. Sternberg E. M., Wedner H. J., Leung M. K., Parker C. W. (1987) Effect of serotonin (5-HT) and other monoamines on murine macrophages: modulation of interferon-gamma induced phagocytosis. J. Immunol. 138, 4360–4365 [PubMed] [Google Scholar]
- 45. Rudd M. L., Nicolas A. N., Brown B. L., Fischer-Stenger K., Stewart J. K. (2005) Peritoneal macrophages express the serotonin transporter. J. Neuroimmunol. 159, 113–118 [DOI] [PubMed] [Google Scholar]
- 46. Amireault P., Hatia S., Bayard E., Bernex F., Collet C., Callebert J., Launay J. M., Hermine O., Schneider E., Mallet J., Dy M., Côté F. (2011) Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 108, 13141–13146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lang K. S., Duranton C., Poehlmann H., Myssina S., Bauer C., Lang F., Wieder T., Huber S. M. (2003) Cation channels trigger apoptotic death of erythrocytes. Cell Death Differ. 10, 249–256 [DOI] [PubMed] [Google Scholar]
- 48. Connor J., Pak C. C., Schroit A. J. (1994) Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J. Biol. Chem. 269, 2399–2404 [PubMed] [Google Scholar]
- 49. de Jong K., Emerson R. K., Butler J., Bastacky J., Mohandas N., Kuypers F. A. (2001) Short survival of phosphatidylserine-exposing red blood cells in murine sickle cell anemia. Blood 98, 1577–1584 [DOI] [PubMed] [Google Scholar]
- 50. McEvoy L., Williamson P., Schlegel R. A. (1986) Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. U.S.A. 83, 3311–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mikulski Z., Zaslona Z., Cakarova L., Hartmann P., Wilhelm J., Tecott L. H., Lohmeyer J., Kummer W. (2010) Serotonin activates murine alveolar macrophages through 5-HT2C receptors. Am. J. Physiol. Lung Cell Mol. Physiol. 299, L272–L280 [DOI] [PubMed] [Google Scholar]
- 52. Guilluy C., Rolli-Derkinderen M., Tharaux P. L., Melino G., Pacaud P., Loirand G. (2007) Transglutaminase-dependent RhoA activation and depletion by serotonin in vascular smooth muscle cells. J. Biol. Chem. 282, 2918–2928 [DOI] [PubMed] [Google Scholar]
- 53. Wei L., Warburton R. R., Preston I. R., Roberts K. E., Comhair S. A., Erzurum S. C., Hill N. S., Fanburg B. L. (2012) Serotonylated fibronectin is elevated in pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 302, L1273–L1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hummerich R., Thumfart J. O., Findeisen P., Bartsch D., Schloss P. (2012) Transglutaminase-mediated transamidation of serotonin, dopamine and noradrenaline to fibronectin: evidence for a general mechanism of monoaminylation. FEBS Lett. 586, 3421–3428 [DOI] [PubMed] [Google Scholar]
- 55. Danen E. H., Sonneveld P., Brakebusch C., Fassler R., Sonnenberg A. (2002) The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159, 1071–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]