Background: Desmoglein-2 mediates intestinal epithelial intercellular adhesion.
Results: Galectin-3 was identified to associate with the ectodomain of desmoglein-2 and inhibit the degradation of desmoglein-2 and enhance intercellular adhesion.
Conclusion: Galectin-3 lattices stabilize desmoglein-2 at the cell surface to regulate intercellular adhesion in intestinal epithelial cells.
Significance: This study identifies a novel role of galectin-3 in controlling desmosome structure and function.
Keywords: Cell-Cell Interaction, Desmosome, Galectin, Intestinal Epithelium, Protein Stability, Desmoglein-2, Galectin-3f
Abstract
The desmosomal cadherins, desmogleins, and desmocollins mediate strong intercellular adhesion. Human intestinal epithelial cells express the desmoglein-2 isoform. A proteomic screen for Dsg2-associated proteins in intestinal epithelial cells identified a lectin referred to as galectin-3 (Gal3). Gal3 bound to N-linked β-galactosides in Dsg2 extracellular domain and co-sedimented with caveolin-1 in lipid rafts. Down-regulation of Gal3 protein or incubation with lactose, a galactose-containing disaccharide that competitively inhibits galectin binding to Dsg2, decreased intercellular adhesion in intestinal epithelial cells. In the absence of functional Gal3, Dsg2 protein was internalized from the plasma membrane and degraded in the proteasome. These results report a novel role of Gal3 in stabilizing a desmosomal cadherin and intercellular adhesion in intestinal epithelial cells.
Introduction
The epithelial lining of the gastrointestinal tract serves as an important barrier that separates luminal contents from underlying tissue compartments, thereby playing an important role in host defense and mucosal homeostasis. Intestinal epithelial (IEC)3 intercellular junctions include adherens junctions and desmosomes (DM) that mediate cell-cell adhesion, thereby providing mechanical strength to the epithelium. Intercellular junctions are actively remodeled during epithelial proliferation, migration, and differentiation. Intercellular junction proteins regulate signaling events to control these biological processes (1–6). Thus, altered function of intercellular junction proteins contributes not only to the compromised epithelial barrier but also to changes in epithelial homeostasis observed in disease states associated with mucosal inflammation and neoplasia (1, 7–11).
Transmembrane cadherin glycoproteins in desmosomes include desmoglein (Dsg) and desmocollin (Dsc) that mediate calcium-dependent intercellular adhesion in epithelial cells (4, 12, 13). N-terminal DM cadherin ectodomains bind in trans between neighboring cells to mediate intercellular adhesion, whereas their C-terminal tails associate with plaque proteins and ultimately with intermediate filaments to stabilize the protein complexes and provide mechanical strength to the epithelium (14).
Four human DSG genes are expressed in a tissue- and differentiation-specific manner (15–18) The human simple columnar epithelium only express the Dsg2 isoform (19). Dsg2 has highly conserved extracellular repeat domains that contain N-linked glycans (20, 21). The Dsg2 distal cadherin repeat domain self-associates and also interacts with Dsc2 to mediate intercellular adhesion (4, 22).
Although a number of studies have described mechanisms by which Dsg(s) are stabilized by protein associations with the cadherin cytoplasmic domains, our understanding of proteins that bind to their extracellular domains to mediate adhesion is not well understood. Thus, to identify proteins that regulate Dsg2-mediated intercellular adhesion, we performed mass spectrometry of proteins that co-immunoprecipitated with Dsg2 in intestinal epithelial cells. This study identified a lectin referred to as galectin-3 (Gal3) in a complex with Dsg2. Galectins are β-galactoside-binding proteins that are not only localized in the nucleus and cytoplasm, but are also secreted and bind cell surface glycans (23–27). Gal3 has a C terminus carbohydrate recognition domain (28). A unique feature of Gal3 is the N terminus self-association domain that enables Gal3 oligomerization after glycoprotein binding, thereby facilitating lattice formation at the cell surface (29–31). This ability of Gal3 to oligomerize at the cell surface provides a unique property by which it can influence the stability of proteins at the cell surface. Gal3 has been reported to regulate cell-cell adhesion and other key homeostatic properties such as cell-matrix adhesion, proliferation, and differentiation by associating with N-cadherin, β1 integrin, epithelial growth factor receptor, and transforming growth factor β receptor (32–39). We observed that Gal3 association with Dsg2 is mediated by N-linked glycans on Dsg2 and that this lactose-sensitive interaction promotes Dsg2 stability at the cell surface and epithelial intercellular adhesion.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
SKCO-15 and T84 human model intestinal epithelial cell lines were cultured and maintained as described previously (9). MG262 (Enzo) or chloroquine (MP Biomedical) was used at concentrations of 10 ng/ml and 200 μg/ml, respectively. The Gal3 N-terminal antibody M3/38.1.2.8 HL.2 was harvested from a hybridoma TIB166 (American Type Culture Collection) and used at a concentration of 20 μg/ml. Lipofectamine 2000 was used for siRNA transfections according to the manufacturer's protocol (Invitrogen). The following siRNA (Sigma-Aldrich) sequences were used: siCtrl (CCUAAGGUUAAGUCGCCCUCG), siGal3_1 (GAGUCAUUGUUUGCAAUAC), and siGal3_2 (CAGAAUUGCUUUAGAUUUC). Sucrose (Sigma-Aldrich) and α-lactose (Sigma-Aldrich) were both used at a concentration of 20 mm and incubated for 18 h unless stated otherwise. Two days before antibody, lactose, or siRNA treatment, cells were cultured in 1% FBS containing media and then treated/incubated overnight in serum-free DMEM (Cellgro).
Immunoblots
Lysates were prepared as described previously (40). After SDS-PAGE, resulting gels were transferred to PVDF membranes (Millipore) overnight. For human intestinal epithelial cell lines, the following primary antibodies were used: Dsg2 clone AH12.2 (2, 41), E-cadherin clone HECD-1 (42), GAPDH (Sigma-Aldrich, G9545), Gal3 clone EPR2774 (Novus Biologicals), and Flotillin-1 clone 18 (BD Biosciences). HRP-labeled secondary antibodies were from Jackson ImmunoResearch Laboratories, and infrared dye-labeled secondary antibodies were from Kerry Perry Laboratories. For mouse intestinal epithelial cells, the primary antibody against Dsg2 was used: clone EPR6767 (Novus). Additionally, immunoblots from mouse lysates were imaged using an Odyssey scanner (LI-COR).
Co-immunoprecipitations
SK-CO15 monolayers (∼106 cells) were lysed in 100 mm KCl, 2 mm NaCl, 1 mm Na2ATP, 3.5 mm MgCl2, 10 mm HEPES, 1% Triton X-100, with protease inhibitor cocktails (Sigma-Aldrich). Postnuclear fractions were precleared for 2 h with Sepharose beads conjugated with FLAG antibody clone M2 (Sigma-Aldrich). Precleared lysates were then used to immunoprecipitate Dsg2 or Gal3. The following antibodies were used for immunoprecipitation: Dsg2 clone AH12.2 and Gal3 clone M3/38.1.2.8 HL.2 (American Type Culture Collection).
Mass Spectrometry
T84 monolayers (∼109 cells) were washed in Hanks' balanced salt saline with calcium and magnesium and harvested in PBS with 0.05% Triton X-100. Postnuclear fractions were precleared for 2 h with Sepharose beads conjugated with an isotype-matched control mouse IgG (Sigma, I5381), and Dsg2 was immunoprecipitated using AH12.2 antibody. Immunoprecipitates were then electrophoresed in 7.5% polyacrylamide gels and silver-stained. The protein bands were excised, and mass spectrometry was performed by the Emory Microchemical Core Facility by matrix-assisted laser desorption ionization/time of flight (MALDI-TOF)-mass spectrometry (MS) and nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Immunofluorescence Labeling and Confocal Microscopy
For immunofluorescence labeling, cells were fixed in 100% ethanol at −20 °C for 20 min. 10-μm sections were made from intestinal tissue frozen in OCT (Tissue-Tek). The following primary antibodies were used for immunofluorescence labeling of SKCO15 cells: Dsg2 clone AH12.2 and Gal3 clone M3/38. To immunostain murine frozen sections, Dsg2 clone EPR6767 and Gal3 (clone AF1197; R&D Systems) were used. Goat anti-mouse Alexa Fluor 555 IgG and a donkey anti-rat Alexa Fluor 488 IgG conjugated secondary antibodies were purchased from Invitrogen, and images of labeled proteins were captured and analyzed using a Zeiss LSM 510 confocal microscope.
Recombinant Proteins
The extracellular domain Dsg2 was cloned into pcDNA3.0 with a His tag and transfected into CHO or HEKT29T cells with 25 μg/ml polyethylenimine. Supernates were collected, and Dsg2 was purified using nickel beads (Pierce). Recombinant human Gal3 was purchased from Abcam (ab89487). The extracellular domain of Dsg2 was deglycosylated with peptide-N-glycosidase F according to the manufacturer's instructions.
ELISA
Immulon 2 HB plates (Thermo Scientific) were coated with 5 μg of Dsg2 ectodomain (ecDsg2) and incubated with casein (Roche Applied Science) and 0.5 μg/ml Gal3 in PBS for 1 h. 20 mm sucrose or lactose (Sigma-Aldrich) was added to the wells during Gal3 incubation. Gal3 binding was detected using 0.5 μg/ml M3/38.1.2.8 HL.2 (American Type Culture Collection) and a goat anti-rat HRP-conjugated antibody (Jackson ImmunoResearch Laboratories).
Dispase Assay
Monolayers were treated with 2 mg/ml dispase (Roche Applied Science) in Hanks' balanced salt saline with calcium and magnesium for 30 min to degrade the extracellular matrix. Monolayers were then subjected to orbital shaking, and pictures were taken to document the extent of monolayer fragmentation.
In Vivo Loop Model
Mice were anesthetized, and the small intestine was exteriorized. After securing the ends of a 2-cm section with surgical thread, antibodies or vehicle (PBS) were injected into the lumen of the loop (43, 44). After 2 h of treatment, mice were euthanized, and small intestinal loops were resected. Intestinal epithelial crypts were isolated from the mucosa by incubating with a cell recovery solution (BD Biosciences) for 15 min. Harvested crypt epithelial cells were lysed in radioimmunoprecipitation assay precipitation buffer supplemented with protease inhibitor cocktail (Sigma-Aldrich). For antibody treatment, 0.5 μg/μl of a Gal3 antibody (clone M3/38.1.2.8 HL.2; American Type Culture Collection) or Gal-1 antibody (clone 201066; R&D Systems) was used. All animal experiments were performed in accordance with protocols approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Lipid Raft Isolation
Cells were harvested in Hanks' balanced salt saline with calcium and magnesium with 1.5% Triton X-100 supplemented containing a protease inhibitor cocktail (Sigma) and homogenized with a Dounce tissue homogenizer (Wheaton). A 5–30% (w/w) sucrose gradient was constructed over the lysate. The gradients were then centrifuged for 19 h (4 °C, 39,000 rpm) in a Beckman SW41 rotor. Fractions (0.5 ml) were collected and analyzed.
Statistics
Immunoblots were quantified using ImageJ (National Institutes of Health) or ImageStudioLite (LI-COR). A z test was performed for control-normalized experiments. An unpaired Student's t test was performed for all other experiments. Results were considered significant when p < 0.05.
RESULTS
Gal3 Associates with Dsg2 in a Glycosylation-dependent Manner
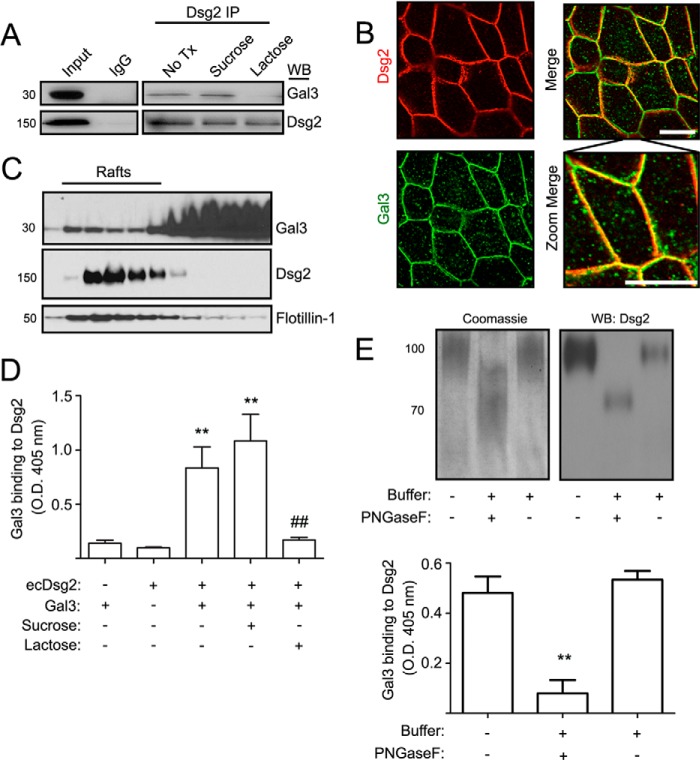
Dsg2 is a key structural component of desmosomes in IECs (4, 5). To identify proteins that regulate Dsg2-mediated intercellular adhesion, we performed mass spectrometry of proteins that co-immunoprecipitated with Dsg2 using a model intestinal epithelial cell line (T84). These studies identified Gal3 in a complex with Dsg2. The presence of Gal3 in a Dsg2 protein complex was further confirmed by immunoblotting for Gal3 after Dsg2 immunoprecipitation (Fig. 1A). To determine whether Gal3 association with Dsg2 is mediated through glycan binding, IECs were incubated with lactose that competitively inhibits galectin-glycan interaction prior to Dsg2 immunoprecipitation. The disaccharide lactose that is composed of glucose-galactose has been used as a competitor of galectin binding (45). In contrast, the disaccharide sucrose composed of glucose-fructose does not inhibit galectin recognition of glycans and can therefore be used as a negative control for lactose-mediated inhibition of galectin glycan binding. Indeed, Gal3 co-immunoprecipitation with Dsg2 was inhibited by lactose, but not sucrose. These findings support a galactoside-mediated association of Dsg2 with Gal3. Immunofluorescence labeling and confocal microscopy demonstrated localization of Dsg2 and Gal3 in the lateral membrane of cell-cell junctions (Fig. 1B). Because Dsg2 is enriched in lipid rafts (2), experiments were performed to determine whether Gal3 co-sediments with Dsg2 in lipid rafts. As shown in Fig. 1C, Dsg2 was enriched in IEC lipid raft fractions that also contained Gal3. Additionally, ELISA demonstrated direct binding of recombinant ecDsg2 and Gal3 (Fig. 1D). The binding of ecDsg2 and Gal3 was inhibited by lactose but not sucrose, further confirming galactoside-mediated interaction of these proteins (Fig. 1D). Dsg2 contains multiple extracellular N-linked glycans (20, 21). Thus, we next determined whether these N-linked glycans mediate Gal3 association with Dsg2. Incubation of recombinant ecDsg2 with peptide-N-glycosidase F resulted in a molecular mass shift from 100 to 70 kDa, supporting the presence of N-linked glycans on the ectodomain of Dsg2. Lastly, peptide-N-glycosidase F inhibited binding of ecDsg2 and Gal3 (Fig. 1E). Taken together, these findings support Gal3 association with N-linked glycans in ecDsg2.
FIGURE 1.
Gal3 associates with Dsg2 in a glycosylation-dependent manner. A, Dsg2 was immunoprecipitated (IP) from SKCO15 cells in the absence and presence of 20 mm sucrose or lactose. Representative immunoblots showing Gal3 and Dsg2 are in the Dsg2 protein complex. Immunoblots (WB) are representative of three independent experiments. No Tx, no treatment. B, immunofluorescence labeling and confocal microscopy to localize Dsg2 and Gal3 in SKCO-15 cells. Scale bar = 20 μm. C, isolation of membrane rafts from SKCO15 cells by floatation in continuous sucrose gradients (5–30%). Gradient fractions were immunoblotted for Gal3, Dsg2, and Flotillin-1 protein. D, ELISA to measure binding of recombinant Gal3 to immobilized Dsg2 ectodomain in the presence and absence of 20 mm sucrose and lactose. The results represent the mean ± S.D. from three independent experiments. Gal3 and Dsg2 or Gal3 and Dsg2 with sucrose versus Gal3 or Dsg2 only, **, p < 0.01. Lactose versus sucrose treatment, ##, p < 0.01. E, the ectodomain of Dsg2 was treated with either a deglycosylation buffer alone (buffer) or buffer with peptide-N-glycosidase F to remove N-linked glycans. Samples subjected to SDS-PAGE were either stained with Coomassie Brilliant Blue G-250 or immunoblotted to detect Dsg2 protein (upper panel). ELISA was performed to determine binding of Dsg2 ectodomain with Gal3 in the presence and absence of peptide-N-glycosidase F (lower panel). The results represent the mean ± S.D. from three independent experiments. **, p < 0.01.
Intercellular Adhesion Is Controlled by Gal3
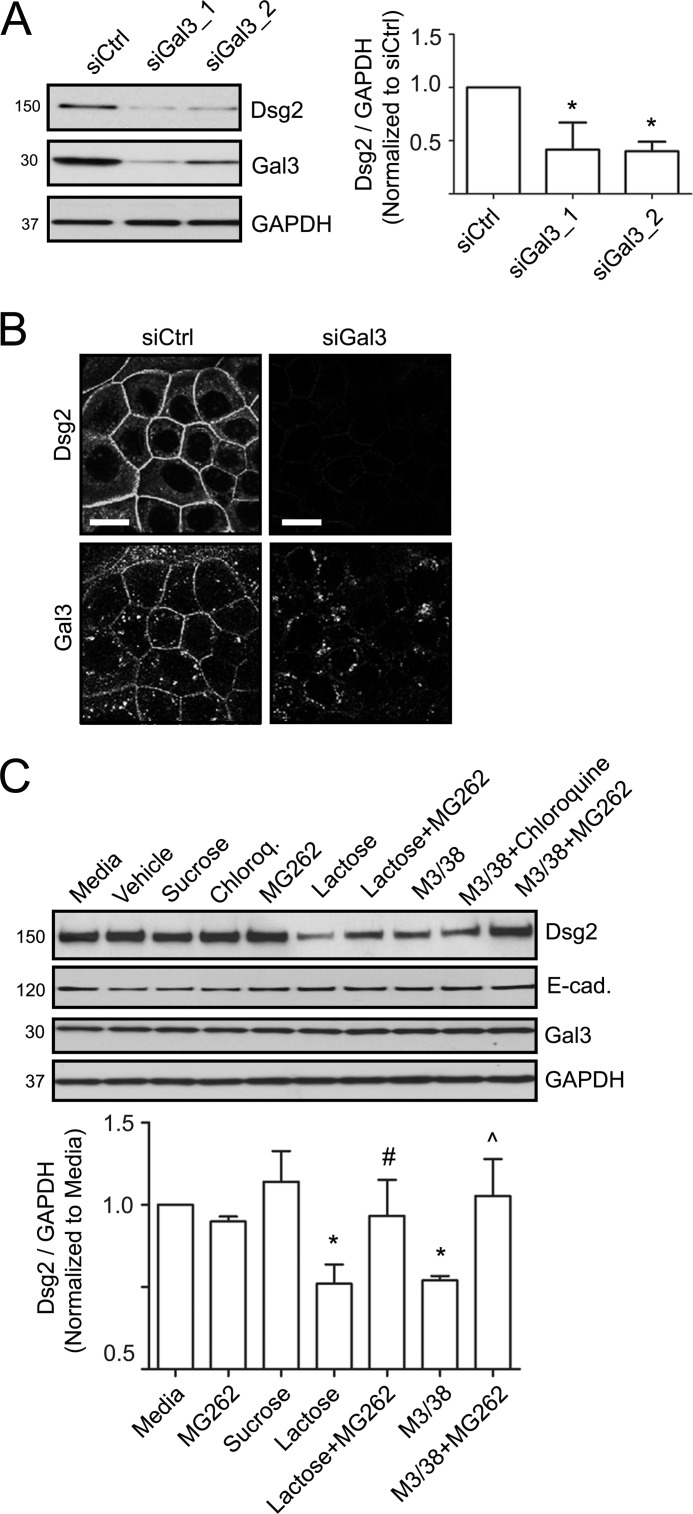
The contribution of Gal3 in controlling epithelial cell-cell adhesion was explored because it associates with Dsg2 that regulates intercellular adhesion. Gal3 was down-regulated with siRNA, and intercellular adhesion was measured using a previously published dispase assay (4, 5). The immunoblot in Fig. 2A confirms siRNA-mediated down-regulation of Gal3. A nonsilencing siRNA was used as control. Additionally, a Gal3 antibody M3/38 that binds to its N terminus self-association domain (46), lactose, and sucrose were used independently to analyze the contributions of Gal3 to epithelial intercellular adhesion. As shown in Fig. 2B, down-regulation of Gal3 (>10-fold, p < 0.05), M3/38 antibody (>10-fold, p < 0.05), or lactose (>10-fold, p < 0.001) resulted in increased monolayer fragmentation, but neither sucrose nor control siRNA increased monolayer fragmentation consistent with decreased intercellular adhesion. These findings support a role of Gal3 in regulating IEC intercellular adhesion.
FIGURE 2.
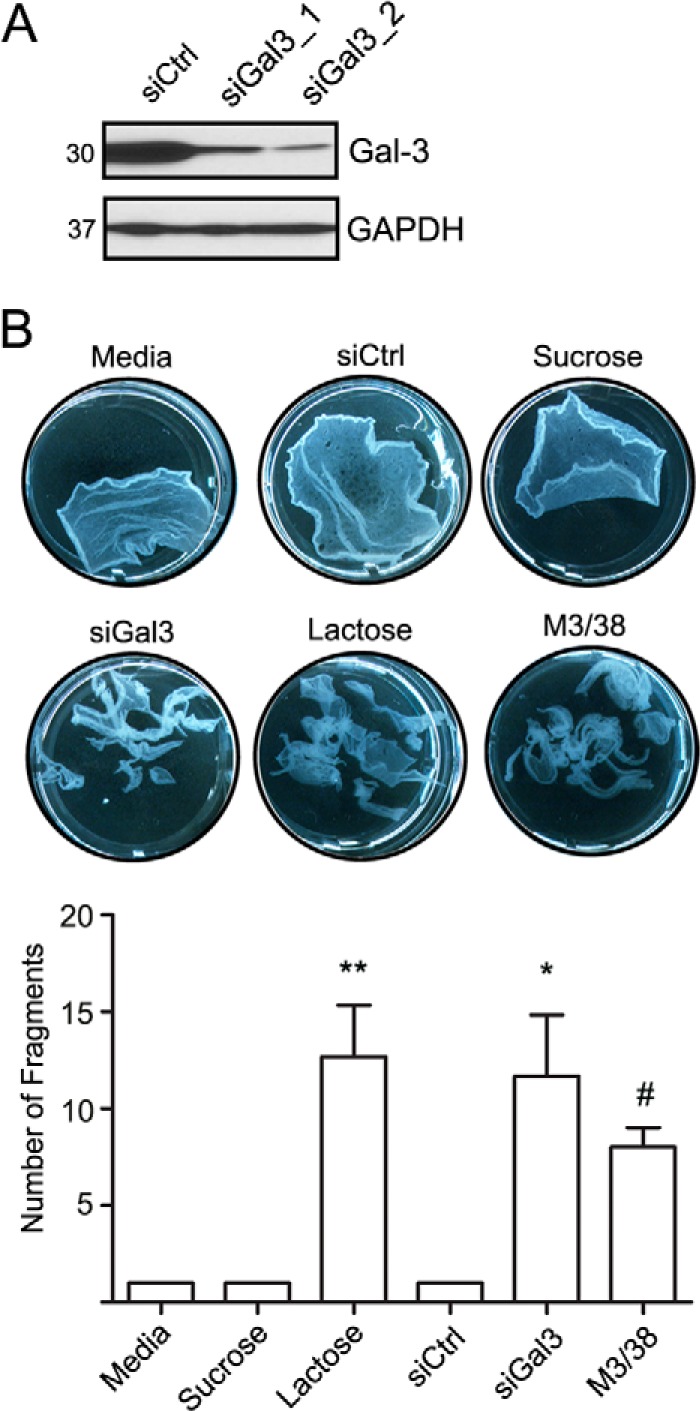
Intercellular adhesion is controlled by Gal3. A, immunoblots to verify down-regulation of Gal3 using two different siRNA sequences (siGal3_1,siGal3_2 in SKCO15 cells. A nonsilencing siRNA control (siCtrl) was included. GAPDH was used as a loading control. B, dispase assay was performed to determine the strength of intercellular adhesion. Gal3 was down-regulated with siRNA (siGal3), or its functional association was inhibited with either lactose or a Gal3 mAb that recognizes its homodimerization domain (M3/38). Monolayers were subjected to the dispase assay, and epithelial fragments were quantified as a measure of cell-cell adhesion. Mean ± S.E., lactose versus sucrose or media, **, p < 0.001. siCtrl versus siGal3, *, p < 0.05. M3/38 versus media, #, p < 0.05.
Dsg2 Protein Stability Is Influenced by Gal3
The above findings independently suggested that Gal3 associates with Dsg2 and regulates cell-cell adhesion. Previous studies have shown that DM cadherins are recruited to the plasma membrane, after which they are incorporated into the junction and stabilized by associating with underlying plaque and cytoskeletal proteins (47). Additionally, remodeling of desmosomes in response to environmental stimuli is associated with destabilization of junction-associated cadherins and their internalization from the cell surface. Internalized cadherins can be degraded or recycled back to the plasma membrane. Additionally, the Gal3 cell surface lattice has been reported to inhibit internalization and degradation of cell surface receptors such as epidermal growth factor receptor (33, 39). Thus, to further explore the relationship between Gal3 and Dsg2, we determined whether Gal3 down-regulation influences Dsg2 protein levels. Indeed, as shown in the Western blot in Fig. 3A, down-regulation of Gal3 resulted in a 2-fold decrease in Dsg2 protein. Consistent with these results, immunofluorescence labeling and confocal microscopy revealed decreased Dsg2 staining in cells treated with Gal3 siRNA (Fig. 3B). Analogous to Gal3 siRNA treatment, incubation of IECs with lactose or Gal3 mAb (M3/38) also decreased Dsg2 steady state protein levels when compared with cells that were treated with media alone, control siRNA, or sucrose (Fig. 3C). We next determined whether Gal3 antibody (M3/38) or lactose treatment resulted in proteasomal or lysosomal degradation of Dsg2. Although treatment with lactose and Gal3 antibody (M3/38) decreased Dsg2 protein, co-incubation with MG262 to inhibit proteasomal degradation but not chloroquine, an inhibitor of lysosomal degradation, restored Dsg2 protein (Fig. 3C).
FIGURE 3.
Dsg2 protein stability is influenced by Gal3. A, Gal3 protein was down-regulated using two different siRNAs in SKCO15 cells (siGal3_1 and siGal3_2). Immunoblots show decreased Dsg2 protein in cells with down-regulated Gal3. Nonsilencing siRNA (siCtrl) was used as a control, and GAPDH was used to demonstrate equal loading per lane. Mean ± S.E., siCtrl versus siGal3_1, *, p < 0.05. siCtrl versus siGal3_2, *, p < 0.05. B, immunofluorescence labeling and confocal microscopy to assess Dsg2 and Gal3 localization in SKCO15 cells treated with nonsilencing control (siCtrl) and or Gal3 siRNA (siGal3) in SKCO15 cells. Scale bar = 25 μm. C, immunoblots to determine the influence Gal3 mAb (M3/38), lactose, or sucrose on Dsg2 steady state protein levels. MG262 or chloroquine was used to evaluate the influence of Gal3 on proteasomal versus lysosomal degradation of Dsg2. Mean ± S.E., treatment versus media only, *, p < 0.05. Lactose versus Lactose+MG262, #, p < 0.05. M3/38 versus M3/38+MG262, ∧, p < 0.05.
Inhibition of Gal3 in Vivo Decreases Intestinal Epithelial Dsg2 Protein
The above in vitro results using model intestinal epithelial cell lines support a role for Gal3 in regulating Dsg2 protein stability and intercellular adhesion. We next verified the influence of Gal3 on Dsg2 protein in vivo using a murine intestinal loop model (Fig. 4A) (43, 44). The lumens of isolated small intestinal loops from anesthetized mice were perfused with Gal3 mAb (M3/38), Gal-1 mAb (201066), or vehicle (PBS) for 2 h prior to harvesting intestinal epithelial cells for immunoblotting. Analogous to the in vitro results, a 2-fold decrease in Dsg2 protein was observed after infusion of the intestinal lumen with Gal3 antibody (M3/38), but not Gal-1 antibody (201066) or PBS (Fig. 4B). Additionally, immunofluorescence labeling and confocal microscopy of the intestinal mucosa revealed Dsg2 distribution in the lateral membrane of epithelial cell-cell contacts. However, perfusion with the Gal3 antibody (M3/38) induced redistribution of Dsg2 into intracellular compartments (Fig. 4C). Similarly, Gal3 was relocalized from the lateral membrane of intestinal epithelial cells into intracellular vesicle-like structures after perfusion with the Gal3 antibody (M3/38) (Fig. 4C).
FIGURE 4.
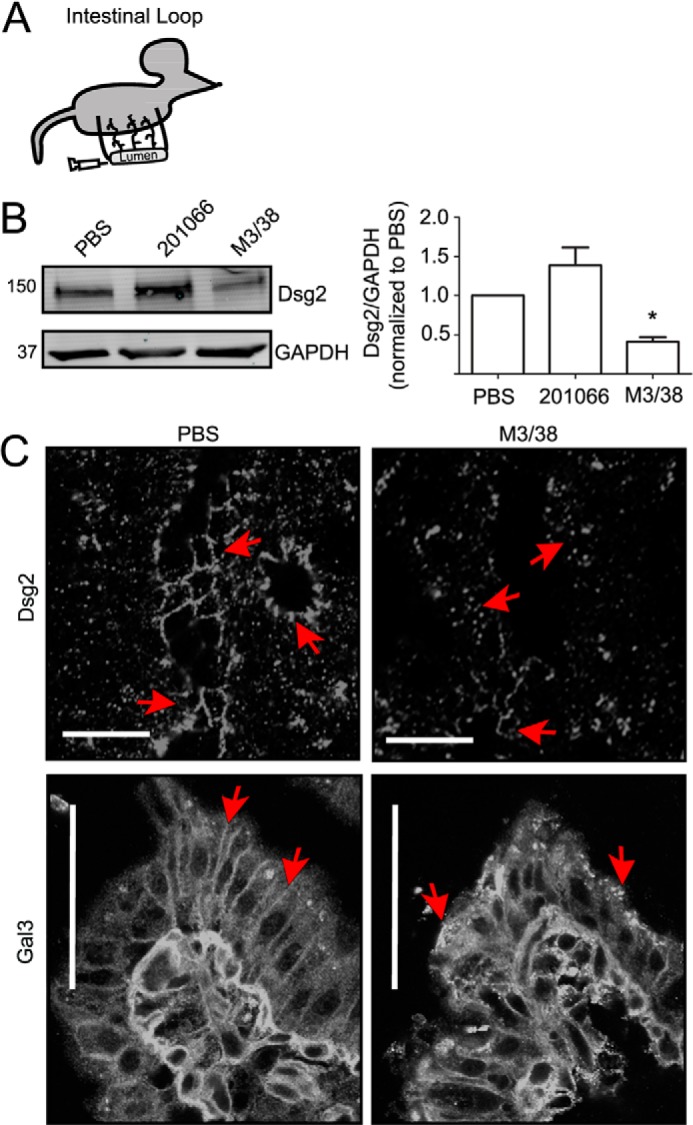
Inhibition of Gal3 in vivo decreases intestinal epithelial Dsg2 protein. A, a graphic representation of the intestinal loop method. B, vehicle alone (PBS), Gal-1 mAb (201066, rat anti-mouse), or a Gal3 mAb (M3/38, rat anti-mouse) was introduced into the lumen of murine small intestinal loops as described under “Experimental Procedures.” 2 h after treatment, intestinal epithelial cells were isolated and immunoblotted for Dsg2 and GAPDH protein. Mean ± S.E., PBS versus Gal3, *, p < 0.05. C, immunofluorescence labeling and confocal microscopy to detect distribution of Dsg2 or Gal3 in frozen sections of the small intestinal loop treated with PBS or M3/38 antibody. Scale bar = 50 μm.
DISCUSSION
DMs in intestinal epithelial cells are visualized as multiple spot-welds in the lateral membrane that reside below the adherens junction. Cadherins in these junctions mediate strong intercellular adhesion in addition to controlling epithelial homeostasis (2, 4, 9). Although mass spectroscopy has shown that Dsg2 contains N-linked glycans (20, 21), their functional significance has not been established. However, N-glycans on N-cadherin have been shown to influence cadherin-mediated intercellular binding (48). This study identified Gal3 association with N-linked glycans in Dsg2 ectodomains that stabilizes Dsg2 at the cell surface and inhibits its proteasomal degradation. Furthermore, Gal3 regulates intercellular adhesion in IECs. Previous studies have shown that lactose, peptides specific to Gal3, or the Gal3 mAb (M3/38) weaken intercellular association (32, 49, 50). Interestingly, lactose did not decrease Gal3 levels in whole cell lysates. Additionally, the antibody that binds epitopes in the self-association domain of Gal-3 (M3/38) resulted in the redistribution of Gal3 into intracellular compartments. Importantly, these findings suggest that changes in localization and/or association of Gal3 with its target glycoprotein are important for its function rather than changes in its steady state levels. We therefore believe that disruption of the Gal3 lattice with M3/38 influences Dsg2 stability and intercellular adhesion.
Our study demonstrates a role of Gal3 in regulating the steady state level of a desmosomal cadherin protein. Gal3 has been reported to stabilize cell surface receptors including epithelial growth factor receptor and transforming growth factor β receptor (33, 39). However, not all Gal3-associated receptors are stabilized by Gal3. Gal3 increases the mobility of another cadherin, N-cadherin (35). Additionally, Gal3 promotes internalization of β1 integrin from the cell surface (37, 38).
Gal3 has been reported to self-associate into oligomers of up to five Gal3 molecules. The extracellular Gal3 lattice can be viewed as cell surface microdomains that recruit glycoproteins based on the N-glycan number and branching (39). Gal3 oligomers therefore represent higher order structures that retain cell surface Dsg2 at points of intercellular adhesion in a way that is reminiscent of Gal3 lattice stabilization of epidermal growth factor receptor and transforming growth factor β receptor at the cell surface. Desmosomes are dynamic structures that undergo remodeling in response to a number of physiologic and pathologic stimuli (47). The Gal3 higher order structure presumably keeps Dsg2 localized within cell-cell contacts, thereby inhibiting its internalization and subsequent proteasomal degradation (Fig. 5). However, the precise mechanism by which Gal3 stabilizes Dsg2 remains to be identified. Dsg proteins have an extended C-terminal unique region (DUR) that can mediate Dsg2 self-interaction to influence stabilization of Dsg2 at the cell surface by inhibiting its internalization (5). Similarly, in the extracellular space, Gal3 could influence Dsg2 clustering and associations at the cell surface that could serve as a complementary mechanism to inhibit its internalization from the plasma membrane. Additionally, because E-cadherin undergoes proteasomal degradation (51) in a ubiquitin-dependent manner, Gal3-dependent clustering of Dsg2 within the plasma membrane might inhibit its ubiquitination and proteasomal degradation. Because Dsg2 and Gal3 co-fractionate in lipid rafts, the Gal3 lattice could regulate partitioning of Dsg2 in membrane rafts (52, 53). Thus, a multimeric Gal3/Dsg2 lattice might hinder the ability of Dsg2 to undergo internalization from the plasma membrane and subsequent degradation. In future studies, it will be important to elucidate the relationship between Gal3-mediated stabilization of plasma membrane-associated Dsg2, cytoplasmic domain phosphorylation, and signaling events that serve to strengthen cell-cell adhesion in desmosomes.
FIGURE 5.
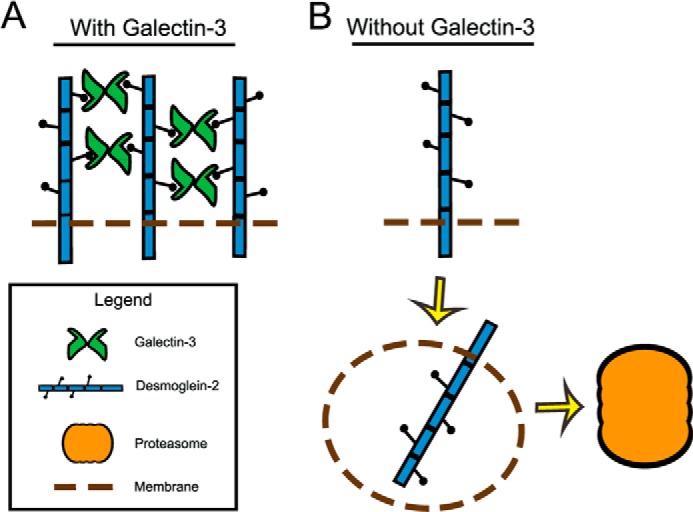
A proposed model for Gal3 lattice regulation of Dsg2 protein. A, Gal3 is secreted into the extracellular space oligomerizes and binds N-linked glycans in Dsg2 ectodomain to stabilize Dsg2 in the plasma membrane. B, in the absence of Gal3, Dsg2 is internalized from the plasma membrane and undergoes proteasomal degradation.
This study highlights an important role of Gal3 and glycosylated Dsg2 in intercellular adhesion, contributing to epithelial barrier integrity. Interestingly, loss of Gal3 protein (54, 55) and Gal3 autoantibodies (56) have been observed in patients with ulcerative colitis, a pathologic state associated with chronic active mucosal inflammation (57, 58). Furthermore, changes in glycosylation of proteins have been reported in patients with chronic inflammatory disorders such as ulcerative colitis and Crohn disease (58, 59). Such altered glycosylation states have been linked to compromised mucin production and function that in turn results in barrier dysfunction with subsequent increase in mucosal inflammation (57). In summary, these studies highlight an important role of Gal3 in controlling Dsg2 protein stability and intercellular adhesion that is intimately linked to barrier function of the intestinal epithelium.
Acknowledgments
We thank Ana Monteiro and Nancy Louis for reviewing this manuscript and Oskar Laur for construct cloning.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK072564, R01 DK061379, and R01 DK079392 (to C. A. P.); R01 DK055679 and DK059888 (to A. N.); and DK064399 (National Institutes of Health Digestive Diseases Research Development Center (DDRDC) tissue culture and morphology grant).
- Dsg2
- Desmoglein-2
- Gal3
- galectin-3
- IEC
- intestinal epithelial cell
- DM
- desmosome
- siCtrl
- nonsilencing control siRNA
- siGal3_1
- galectin-3 siRNA sequence 1
- siGal3_2
- galectin-3 siRNA sequence 2
- ecDsg2
- Dsg2 ectodomain.
REFERENCES
- 1. Kamekura R., Kolegraff K. N., Nava P., Hilgarth R. S., Feng M., Parkos C. A., Nusrat A. (2013) Loss of the desmosomal cadherin desmoglein-2 suppresses colon cancer cell proliferation through EGFR signaling. Oncogene 10.1038/onc.2013.442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nava P., Laukoetter M. G., Hopkins A. M., Laur O., Gerner-Smidt K., Green K. J., Parkos C. A., Nusrat A. (2007) Desmoglein-2: a novel regulator of apoptosis in the intestinal epithelium. Mol. Biol. Cell 18, 4565–4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eshkind L., Tian Q., Schmidt A., Franke W. W., Windoffer R., Leube R. E. (2002) Loss of desmoglein 2 suggests essential functions for early embryonic development and proliferation of embryonal stem cells. Eur. J. Cell Biol. 81, 592–598 [DOI] [PubMed] [Google Scholar]
- 4. Schlegel N., Meir M., Heupel W. M., Holthöfer B., Leube R. E., Waschke J. (2010) Desmoglein 2-mediated adhesion is required for intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G774–G783 [DOI] [PubMed] [Google Scholar]
- 5. Chen J., Nekrasova O. E., Patel D. M., Klessner J. L., Godsel L. M., Koetsier J. L., Amargo E. V., Desai B. V., Green K. J. (2012) The C-terminal unique region of desmoglein 2 inhibits its internalization via tail-tail interactions. J. Cell Biol. 199, 699–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang H., Yumul R., Cao H., Ran L., Fan X., Richter M., Epstein F., Gralow J., Zubieta C., Fender P., Lieber A. (2013) Structural and functional studies on the interaction of adenovirus fiber knobs and desmoglein 2. J. Virol. 87, 11346–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brooke M. A., Nitoiu D., Kelsell D. P. (2012) Cell-cell connectivity: desmosomes and disease. J. Pathol. 226, 158–171 [DOI] [PubMed] [Google Scholar]
- 8. Dusek R. L., Attardi L. D. (2011) Desmosomes: new perpetrators in tumour suppression. Nat. Rev. Cancer 11, 317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kolegraff K., Nava P., Helms M. N., Parkos C. A., Nusrat A. (2011) Loss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/β-catenin signaling. Mol. Biol. Cell 22, 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yashiro M., Nishioka N., Hirakawa K. (2006) Decreased expression of the adhesion molecule desmoglein-2 is associated with diffuse-type gastric carcinoma. Eur. J. Cancer 42, 2397–2403 [DOI] [PubMed] [Google Scholar]
- 11. Fukushima K., Yonezawa H., Fiocchi C. (2003) Inflammatory bowel disease-associated gene expression in intestinal epithelial cells by differential cDNA screening and mRNA display. Inflamm. Bowel Dis. 9, 290–301 [DOI] [PubMed] [Google Scholar]
- 12. Klessner J. L., Desai B. V., Amargo E. V., Getsios S., Green K. J. (2009) EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol. Biol. Cell 20, 328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcozzi C., Burdett I. D., Buxton R. S., Magee A. I. (1998) Coexpression of both types of desmosomal cadherin and plakoglobin confers strong intercellular adhesion. J. Cell Sci. 111, 495–509 [DOI] [PubMed] [Google Scholar]
- 14. Vasioukhin V., Bowers E., Bauer C., Degenstein L., Fuchs E. (2001) Desmoplakin is essential in epidermal sheet formation. Nat. Cell Biol. 3, 1076–1085 [DOI] [PubMed] [Google Scholar]
- 15. Cheng X., Koch P. J. (2004) In vivo function of desmosomes. J. Dermatol. 31, 171–187 [DOI] [PubMed] [Google Scholar]
- 16. Garrod D. R., Merritt A. J., Nie Z. (2002) Desmosomal cadherins. Curr. Opin. Cell Biol. 14, 537–545 [DOI] [PubMed] [Google Scholar]
- 17. Green K. J., Simpson C. L. (2007) Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 127, 2499–2515 [DOI] [PubMed] [Google Scholar]
- 18. Mahoney M. G., Hu Y., Brennan D., Bazzi H., Christiano A. M., Wahl J. K., 3rd (2006) Delineation of diversified desmoglein distribution in stratified squamous epithelia: implications in diseases. Exp. Dermatol. 15, 101–109 [DOI] [PubMed] [Google Scholar]
- 19. Holthöfer B., Windoffer R., Troyanovsky S., Leube R. E. (2007) Structure and function of desmosomes. Int. Rev. Cytol. 264, 65–163 [DOI] [PubMed] [Google Scholar]
- 20. Chen R., Jiang X., Sun D., Han G., Wang F., Ye M., Wang L., Zou H. (2009) Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J. Proteome Res. 8, 651–661 [DOI] [PubMed] [Google Scholar]
- 21. Wollscheid B., Bausch-Fluck D., Henderson C., O'Brien R., Bibel M., Schiess R., Aebersold R., Watts J. D. (2009) Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol. 27, 378–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Syed S. E., Trinnaman B., Martin S., Major S., Hutchinson J., Magee A. I. (2002) Molecular interactions between desmosomal cadherins. Biochem. J. 362, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moutsatsos I. K., Davis J. M., Wang J. L. (1986) Endogenous lectins from cultured cells: subcellular localization of carbohydrate-binding protein 35 in 3T3 fibroblasts. J. Cell Biol. 102, 477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho M., Cummings R. D. (1995) Galectin-1, a β-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J. Biol. Chem. 270, 5207–5212 [DOI] [PubMed] [Google Scholar]
- 25. Danielsen E. M., van Deurs B. (1997) Galectin-4 and small intestinal brush border enzymes form clusters. Mol. Biol. Cell 8, 2241–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomsen M. K., Hansen G. H., Danielsen E. M. (2009) Galectin-2 at the enterocyte brush border of the small intestine. Mol. Membr. Biol. 26, 347–355 [DOI] [PubMed] [Google Scholar]
- 27. Chabot S., Kashio Y., Seki M., Shirato Y., Nakamura K., Nishi N., Nakamura T., Matsumoto R., Hirashima M. (2002) Regulation of galectin-9 expression and release in Jurkat T cell line cells. Glycobiology 12, 111–118 [DOI] [PubMed] [Google Scholar]
- 28. Cherayil B. J., Chaitovitz S., Wong C., Pillai S. (1990) Molecular cloning of a human macrophage lectin specific for galactose. Proc. Natl. Acad. Sci. U.S.A. 87, 7324–7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmad N., Gabius H. J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 [DOI] [PubMed] [Google Scholar]
- 30. Lepur A., Salomonsson E., Nilsson U. J., Leffler H. (2012) Ligand induced galectin-3 protein self-association. J. Biol. Chem. 287, 21751–21756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morris S., Ahmad N., André S., Kaltner H., Gabius H. J., Brenowitz M., Brewer F. (2004) Quaternary solution structures of galectins-1, -3, and -7. Glycobiology 14, 293–300 [DOI] [PubMed] [Google Scholar]
- 32. Inohara H., Raz A. (1995) Functional evidence that cell surface galectin-3 mediates homotypic cell adhesion. Cancer Res. 55, 3267–3271 [PubMed] [Google Scholar]
- 33. Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004) Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 34. Boscher C., Nabi I. R. (2013) Galectin-3- and phospho-caveolin-1-dependent outside-in integrin signaling mediates the EGF motogenic response in mammary cancer cells. Mol. Biol. Cell 24, 2134–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boscher C., Zheng Y. Z., Lakshminarayan R., Johannes L., Dennis J. W., Foster L. J., Nabi I. R. (2012) Galectin-3 protein regulates mobility of N-cadherin and GM1 ganglioside at cell-cell junctions of mammary carcinoma cells. J. Biol. Chem. 287, 32940–32952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lajoie P., Partridge E. A., Guay G., Goetz J. G., Pawling J., Lagana A., Joshi B., Dennis J. W., Nabi I. R. (2007) Plasma membrane domain organization regulates EGFR signaling in tumor cells. J. Cell Biol. 179, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Friedrichs J., Manninen A., Muller D. J., Helenius J. (2008) Galectin-3 regulates integrin α2β1-mediated adhesion to collagen-I and -IV. J. Biol. Chem. 283, 32264–32272 [DOI] [PubMed] [Google Scholar]
- 38. Furtak V., Hatcher F., Ochieng J. (2001) Galectin-3 mediates the endocytosis of β-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 289, 845–850 [DOI] [PubMed] [Google Scholar]
- 39. Lau K. S., Partridge E. A., Grigorian A., Silvescu C. I., Reinhold V. N., Demetriou M., Dennis J. W. (2007) Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129, 123–134 [DOI] [PubMed] [Google Scholar]
- 40. Rankin C. R., Hilgarth R. S., Leoni G., Kwon M., Den Beste K. A., Parkos C. A., Nusrat A. (2013) Annexin A2 regulates β1 integrin internalization and intestinal epithelial cell migration. J. Biol. Chem. 288, 15229–15239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kolegraff K., Nava P., Laur O., Parkos C. A., Nusrat A. (2011) Characterization of full-length and proteolytic cleavage fragments of desmoglein-2 in native human colon and colonic epithelial cell lines. Cell Adh. Migr. 5, 306–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shimoyama Y., Hirohashi S., Hirano S., Noguchi M., Shimosato Y., Takeichi M., Abe O. (1989) Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 49, 2128–2133 [PubMed] [Google Scholar]
- 43. Monteiro A. C., Sumagin R., Rankin C. R., Leoni G., Mina M. J., Reiter D. M., Stehle T., Dermody T. S., Schaefer S. A., Hall R. A., Nusrat A., Parkos C. A. (2013) JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol. Biol. Cell 24, 2849–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sumagin R., Robin A. Z., Nusrat A., Parkos C. A. (2013) Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunol. 10.1038/mi.2013.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W. E., Yagi F., Kasai K. (2002) Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572, 232–254 [DOI] [PubMed] [Google Scholar]
- 46. Ho M. K., Springer T. A. (1982) Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J. Immunol. 128, 1221–1228 [PubMed] [Google Scholar]
- 47. Kowalczyk A. P., Bornslaeger E. A., Norvell S. M., Palka H. L., Green K. J. (1999) Desmosomes: intercellular adhesive junctions specialized for attachment of intermediate filaments. Int. Rev. Cytol. 185, 237–302 [DOI] [PubMed] [Google Scholar]
- 48. Langer M. D., Guo H., Shashikanth N., Pierce J. M., Leckband D. E. (2012) N-Glycosylation alters cadherin-mediated intercellular binding kinetics. J. Cell Sci. 125, 2478–2485 [DOI] [PubMed] [Google Scholar]
- 49. Inohara H., Akahani S., Koths K., Raz A. (1996) Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 56, 4530–4534 [PubMed] [Google Scholar]
- 50. Zou J., Glinsky V. V., Landon L. A., Matthews L., Deutscher S. L. (2005) Peptides specific to the galectin-3 carbohydrate recognition domain inhibit metastasis-associated cancer cell adhesion. Carcinogenesis 26, 309–318 [DOI] [PubMed] [Google Scholar]
- 51. Hartsock A., Nelson W. J. (2012) Competitive regulation of E-cadherin juxtamembrane domain degradation by p120-catenin binding and Hakai-mediated ubiquitination. PLoS One 7, e37476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brennan D., Peltonen S., Dowling A., Medhat W., Green K. J., Wahl J. K., 3rd, Del Galdo F., Mahoney M. G. (2012) A role for caveolin-1 in desmoglein binding and desmosome dynamics. Oncogene 31, 1636–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Resnik N., Sepcic K., Plemenitas A., Windoffer R., Leube R., Veranic P. (2011) Desmosome assembly and cell-cell adhesion are membrane raft-dependent processes. J. Biol. Chem. 286, 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jensen-Jarolim E., Gscheidlinger R., Oberhuber G., Neuchrist C., Lucas T., Bises G., Radauer C., Willheim M., Scheiner O., Liu F. T., Boltz-Nitulescu G. (2002) The constitutive expression of galectin-3 is downregulated in the intestinal epithelia of Crohn's disease patients, and tumour necrosis factor α decreases the level of galectin-3-specific mRNA in HCT-8 cells. Eur. J. Gastroenterol. Hepatol. 14, 145–152 [DOI] [PubMed] [Google Scholar]
- 55. Brazowski E., Dotan I., Tulchinsky H., Filip I., Eisenthal A. (2009) Galectin-3 expression in pouchitis in patients with ulcerative colitis who underwent ileal pouch-anal anastomosis (IPAA). Pathol. Res. Pract. 205, 551–558 [DOI] [PubMed] [Google Scholar]
- 56. Jensen-Jarolim E., Neumann C., Oberhuber G., Gscheidlinger R., Neuchrist C., Reinisch W., Zuberi R. I., Penner E., Liu F. T., Boltz-Nitulescu G. (2001) Anti-Galectin-3 IgG autoantibodies in patients with Crohn's disease characterized by means of phage display peptide libraries. J. Clin. Immunol. 21, 348–356 [DOI] [PubMed] [Google Scholar]
- 57. Campbell B. J., Yu L. G., Rhodes J. M. (2001) Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj. J. 18, 851–858 [DOI] [PubMed] [Google Scholar]
- 58. Bodger K., Halfvarson J., Dodson A. R., Campbell F., Wilson S., Lee R., Lindberg E., Järnerot G., Tysk C., Rhodes J. M. (2006) Altered colonic glycoprotein expression in unaffected monozygotic twins of inflammatory bowel disease patients. Gut 55, 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shinzaki S., Kuroki E., Iijima H., Tatsunaka N., Ishii M., Fujii H., Kamada Y., Kobayashi T., Shibukawa N., Inoue T., Tsujii M., Takeishi S., Mizushima T., Ogata A., Naka T., Plevy S. E., Takehara T., Miyoshi E. (2013) Lectin-based immunoassay for aberrant IgG glycosylation as the biomarker for Crohn's disease. Inflamm. Bowel Dis. 19, 321–331 [DOI] [PubMed] [Google Scholar]