Background: Roles for AGS3/Gpsm1 in the immune system are not defined.
Results: Loss of AGS3 expression results in defective leukocyte chemotaxis, calcium mobilization, and ERK1/2 and Akt activation.
Conclusion: AGS3 is required for efficient chemokine receptor signal integration.
Significance: These studies extend the functional repertoire for AGS3 in the immune system, providing unexpected regulatory mechanisms for immune function.
Keywords: Chemokines, Chemotaxis, Cxcr4, G-protein-coupled Receptors (GPCR), G Proteins, AGS3, Gαi, Gβγ, GPR Motif, Gpsm1
Abstract
Activator of G-protein signaling 3 (AGS3, gene name G-protein signaling modulator-1, Gpsm1), an accessory protein for G-protein signaling, has functional roles in the kidney and CNS. Here we show that AGS3 is expressed in spleen, thymus, and bone marrow-derived dendritic cells, and is up-regulated upon leukocyte activation. We explored the role of AGS3 in immune cell function by characterizing chemokine receptor signaling in leukocytes from mice lacking AGS3. No obvious differences in lymphocyte subsets were observed. Interestingly, however, AGS3-null B and T lymphocytes and bone marrow-derived dendritic cells exhibited significant chemotactic defects as well as reductions in chemokine-stimulated calcium mobilization and altered ERK and Akt activation. These studies indicate a role for AGS3 in the regulation of G-protein signaling in the immune system, providing unexpected venues for the potential development of therapeutic agents that modulate immune function by targeting these regulatory mechanisms.
Introduction
Integration of signals emanating from chemokine receptors represents one of the most commonly used mechanisms for leukocyte distribution and recruitment to lymphoid organs and the periphery. Such signal integration involves not only the regulated expression of individual chemokine receptors and the stoichiometries of the core signaling triad of receptor, heterotrimeric G-protein, and effectors but also cell type-specific accessory proteins that modulate signals across this core signaling system. Such accessory proteins bestow upon leukocytes and other cells the ability to tightly control signaling pathways to maximize signal efficiency, strength, and duration while at the same time providing flexibility to quickly adapt to changes in environmental stimuli (1, 2).
Perturbations of heterotrimeric G-protein signal input or duration result in defective leukocyte development, trafficking, motility, and overall chemokine responsiveness (3–9). In addition, accessory proteins at the GPCR2-G-protein interface also play key roles in regulating leukocyte function by modulating G-protein activity and responsiveness to chemokines. Many regulators of G-protein signaling (RGS) proteins are expressed in leukocytes (1, 10) and play important functions in regulating chemokine responsiveness. For example, RGS1 plays an important role in modulating lymphocyte motility and trafficking (4). RGS1−/− lymphocytes move more rapidly in response to chemokines, suggesting that modulating the duration of Gαi activation in response to chemokines plays an important role in leukocyte activation and trafficking (4). Additional mechanisms for modulation of Gαi activity are also likely important for spatio-temporal regulation of leukocyte responsiveness and for integration of signals from multiple chemokines at any given time.
Another group of accessory proteins, the activators of G-protein signaling (AGS) proteins, were identified in a yeast-based functional screen for receptor-independent activators of heterotrimeric G-proteins (11, 12) and can be broadly categorized into three groups based on their input into the G-protein activation/deactivation cycle (13). Group II AGS proteins are characterized by the presence of up to four G-protein regulatory (GPR) motifs (also referred to as LGN or GoLoco motifs (14, 15)), which bind free Gαi/o/t subunits in the GDP-bound conformation and act as guanine nucleotide dissociation inhibitors (16, 17). GPR motif-containing proteins thus provide a novel mode of signal input to heterotrimeric G-proteins that may operate distinct from the superfamily of GPCRs and may also function as binding partners for Gαi subunits independent of heterotrimer formation. These theories have broad implications for signal processing and provide a mechanism for unexpected functions of G-proteins as signal transducers within the cell.
A member of the Group II AGS proteins, AGS3 (gene name, G-protein signaling modulator-1 (Gpsm1))3 contains seven tetratricopeptide repeats, which are involved in protein-protein interactions and four G-protein regulatory (GPR)/GoLoco motifs, allowing AGS3 to simultaneously bind up to four Gαi-GDP subunits free of Gβγ. Previous data suggest functional roles for AGS3 in such diverse processes as neuronal plasticity and addiction, autophagy, membrane protein trafficking, polycystic kidney disease, cardiovascular regulation, and metabolism (18–28). As part of an expanded approach to more fully understanding the in vivo role of AGS3 in G-protein signal processing, we previously reported the generation of a conditional AGS3-null mouse strain, which is a valuable model to dissect physiological functions of AGS3 (18, 23, 26).
Our goal in this study was to define the functional role of AGS3 in leukocytes, beginning with its role in chemokine receptor signal integration. Our data suggest that AGS3 assists in the integration of signals from the receptor to the chemotactic machinery, including calcium mobilization, ERK1/2 and Akt phosphorylation, and leukocyte motility. These data indicate key roles for AGS3 in the integration of chemokine receptor signaling and expand the functional repertoire of accessory proteins in the immune system.
EXPERIMENTAL PROCEDURES
Materials
Recombinant mouse GM-CSF, CXCL12, and CCL19 were purchased from BioAbChem Inc. (Ladson, SC). AGS3-antisera generated by immunization of rabbits with a GST-AGS3 fusion protein encoding the GPR domain (Ala461–Ser650) of AGS3 was kindly provided by Dr. Dzwokai Ma (University of California, Santa Barbara, CA). Gαi1/2 and Gαi3 antisera were kindly provided by Dr. Thomas Gettys (Pennington Biomedical Research Center, Baton Rouge, LA). Protease inhibitor cocktail tablets (Complete Mini) were obtained from Roche Applied Science. Gallein was obtained from Tocris (Bristol, UK). Other materials were obtained as described elsewhere (29, 30).
Mice
Generation of Gpsm1−/− mice3 used in this study was previously described (18). Wild-type and Gpsm1−/− female littermates at 6–12 weeks of age from Gpsm1+/− intercrosses were used. Gpsm1+/− breeding pairs were generated from backcrosses onto C57BL/6J mice for more than 12 generations. Tissues and lysates were prepared and processed for immunoblotting as described (31).
Flow Cytometry and Cell Sorting
Single-cell suspensions from spleen and thymus were prepared from female mice (Gpsm1+/+ and Gpsm1−/− littermates) at 6 weeks of age by crushing freshly dissected tissues between frosted glass slides in PBS. After lysing red blood cells with ammonium-chloride-potassium (ACK) lysis buffer (170 mm NH4Cl, 170 mm Tris), cells were counted and washed with PBS with 1% BSA and 0.05% NaN3 (PBS-BSA). A total of 106 cells were first incubated with anti-FcγIII (CD16/CD32) for 30 min at 4 °C to block Fc receptors, and then cells were incubated with primary FITC or phycoerythrin-conjugated antibodies in PBS-BSA for 30 min at 4 °C (BD Pharmingen). Cells were washed twice in PBS-BSA, resuspended in 500 μl of PBS-BSA, and analyzed on a flow cytometer (BD Pharmingen). CD4 and CD8 mAbs were used to characterize thymus cell subsets. The following mAbs were used to characterize spleen cell subsets: total B cells (B220+, CD3−); follicular B cells (B220+, CD21intermediate+, CD23+); marginal zone B cells (B220+, CD21high+, CD23−); double positive T cells (CD4+, CD8+); and double negative T cells (CD4−, CD8−).
Primary Cells
Bone marrow cells from WT or Gpsm1−/− mouse femurs and tibiae were filtered through a 40-μm nylon cell strainer, red blood cells were lysed with ammonium-chloride-potassium lysis buffer, and cells were resuspended in DC media (RPMI 1640 supplemented with 10% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 20 ng/ml recombinant mouse GM-CSF) and plated 4–5 × 105 cells/ml in a 100-mm tissue culture dish. On day 4, 10 ml of fresh DC media was added to each dish. On day 8, nonadherent and loosely adherent cells were harvested, washed, and replated in fresh DC medium containing 10 ng/ml recombinant mouse GM-CSF to generate immature dendritic cells (iDC). On day 9, cells were treated with or without 200 ng/ml LPS for the indicated times or for 24 h to generate mature dendritic cells (mDC). Spleens of WT or Gpsm1−/− mice were gently crushed between frosted glass slides in serum-free RPMI. Red blood cells were lysed with ammonium-chloride-potassium lysis buffer, and splenocytes were washed in PBS (Ca2+-, Mg2+-free) supplemented with 0.1% BSA and 2 mm EDTA and then resuspended at 5 × 107 cells/ml or 1 × 108 cells/ml for subsequent B cell or T cell isolation, respectively. Cell isolation was performed according to Invitrogen Dynabeads protocol for untouched B cell isolation or negative T cell isolation.
Chemotaxis
Corning Transwell 24-well inserts (6.5-mm diameter, 5.0-μm pore size) or 96-well inserts (5.0-μm pore size) were used for all chemotaxis assays. For dendritic cell chemotaxis, serum-free RPMI with or without 250 ng/ml CXCL12 or CCL19 was added to the lower chamber, and ∼3 × 106 cells/ml were loaded into the upper chamber. Where indicated, dendritic cells were preincubated with 10 μm gallein (final concentration) or vehicle (0.02% DMSO final concentration) in serum-free RPMI for 30 min at 37 °C prior to measuring chemotaxis. In these cases, 10 μm gallein (final concentration) and vehicle (0.02% DMSO final concentration) were included in both upper and lower chambers as described previously (32). For lymphocytes, serum-free RPMI supplemented with 0.05 μm β-mercaptoethanol with or without 300 ng/ml CXCL12 or CCL19 was added to the lower chambers, and ∼1 × 107 lymphocytes/ml were added to the upper chamber. Chemotaxis chambers were incubated at 37 °C, 5% CO2 for 20 h for dendritic cells and 5 h for lymphocytes. The upper chamber was then removed, and cells migrating to the lower chamber were counted. The percentage of cells migrating to chemokine was determined by subtracting the average cells migrating to vehicle alone and then comparing with the actual number of input cells.
Determination of Intracellular Calcium Levels
Freshly harvested splenocytes of WT and Gpsm1−/− mice were seeded into clear bottom, black-walled 96-well plates at 1 × 106 cells/well in 100 μl of serum-free, phenol red-free RPMI, and cells were serum-starved for at least 1 h. Cells were incubated in the Calcium 5 assay kit dye for 1 h at 37 °C, and then the plate was allowed to come to room temperature for 15 min prior to analysis. CXCL12 or CCL19 was added at a final concentration of 200 ng/ml by the FLIPRtetra instrument, and measurements were taken every second for at least 300 s. Bone marrow cells were harvested from WT and Gpsm1−/− female mice between 6 and 10 weeks of age and cultured to dendritic cells as described above. DCs were seeded at 2.5 × 105 cells/well and serum-starved for ∼1 h followed by incubation in the calcium dye for 1 h. CXCL12 was added at a final concentration of 200 ng/ml by the FLIPRtetra, and measurements, reported as relative light units, were taken every second for at least 300 s. Negative control and response over baseline corrections were applied using the ScreenWorks 3.1 software.
Immunoblotting
Single-cell suspensions from spleen and thymus were prepared by crushing freshly dissected tissues between frosted glass slides in PBS. After lysing red blood cells with ice-cold 0.17 m NH4Cl, 0.17 m Tris for 5 min at room temperature followed by centrifugation at 500 × g for 5 min at room temperature, cells were pelleted and resuspended in 1% Nonidet P-40 lysis buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 5 mm EDTA, 1% Nonidet P-40) on ice for 20 min followed by centrifugation at 10,000 × g for 30 min at 4 °C. Protein concentration was determined by a Pierce BCA protein assay. Protein samples were loaded on denaturing 10% polyacrylamide gels and then were transferred to polyvinylidene difluoride membranes for immunoblotting as described (31). Cell pellets processed for phosphorylated proteins were lysed in 1% Nonidet P-40 buffer with protease and phosphatase inhibitors (50 mm NaF, 5 mm sodium pyrophosphate, 40 mm β-glycerophosphate, and 200 μm Na3VO4) on ice for 20 min followed by centrifugation at 10,000 × g for 30 min at 4 °C. Samples were subjected to SDS-PAGE, and proteins were transferred to PVDF membranes and immunoblotted for anti-phospho-Akt (Ser483) (Life Technologies), anti-phospho-ERK (Tyr402) (Santa Cruz Biotechnology, Dallas, TX), or total ERK (Abcam, Cambridge, MA) and total Akt (Cell Signaling Technology, Boston, MA) antibodies. Densitometric quantification of the immunoblotted bands was performed using ImageJ densitometry software (Version 1.46r, National Institutes of Health, Bethesda, MD). Selected bands were quantified based on their relative intensities and normalized to total ERK or total Akt.
RESULTS
Increasing evidence indicates a growing number of cellular and physiological roles for accessory proteins such as AGS3 and other proteins containing the GPR motif in dynamic signaling systems such as the central nervous system (CNS) where signal modulation and adaptation of G-protein signaling systems are key to the responsiveness of the system (19–21, 28, 33). The dynamic processing of signals in the immune system also involves highly specialized, spatially integrated, G-protein signaling mechanisms (1, 34). As an initial approach to define the role of GPR proteins in such modes of signal integration, we studied the role of the GPR protein AGS3 in chemotactic signaling in immune cells.
Analysis of Protein Expression and Leukocyte Populations from AGS3/Gpsm1−/− Mice
To explore potential functional roles for AGS3 in leukocytes, we took advantage of a recently developed AGS3/Gpsm1-null mouse model (18).3 AGS3 (Mr ∼74,000) was abundant in cells isolated from the thymus and spleen of wild-type mice, but as expected, was completely absent from the same tissues in Gpsm1−/− mice (Fig. 1). Immunoblot analysis revealed no obvious compensatory alterations in expression of the AGS3/Gpsm1-related protein LGN/Gpsm2. Expression analysis of Gαi2 and Gαi3 subunits, which are the predominantly expressed Gαi subunits in hematopoietic cells (35–37), indicated that Gαi2 and Gαi3 expression was largely unaffected with a modest increase in expression of Gαi2 in Gpsm1−/− splenocytes and thymocytes (Fig. 1).
FIGURE 1.
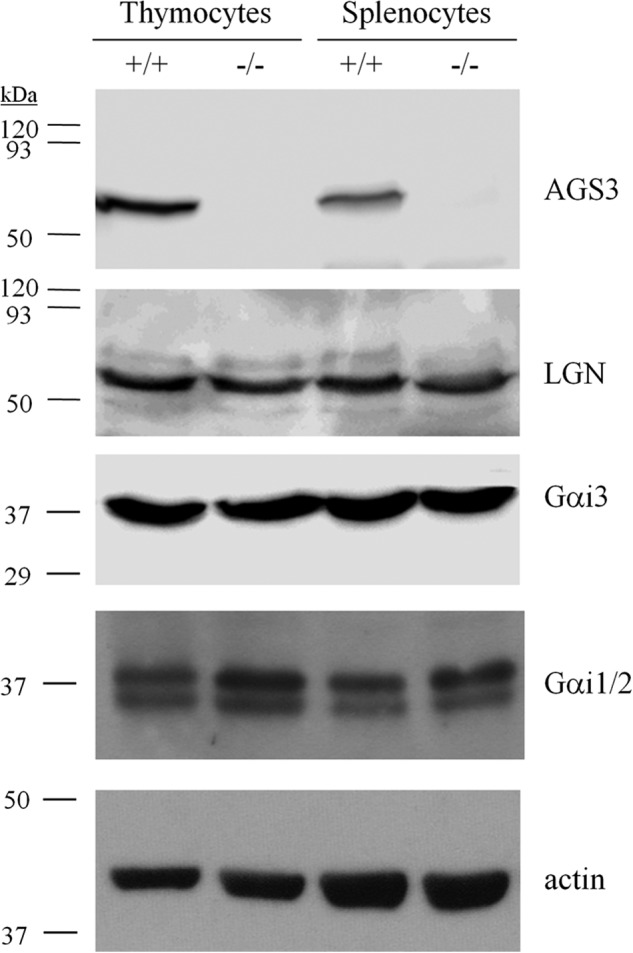
Immunoblot of WT and Gpsm1−/− mouse thymocyte and splenocyte lysates. Thymocytes and splenocytes were isolated following red blood cell lysis and filtering to remove cell and tissue aggregates as described under “Experimental Procedures.” Lysates were prepared with 1% Nonidet P-40 lysis buffer and subjected to SDS-PAGE (100 μg/lane) and immunoblotting with AGS3, LGN, Gαi2, Gαi3, and actin-specific antisera as described under “Experimental Procedures.”
AGS3 and other proteins with the GPR motif together with Gαi and Gβγ play key roles in asymmetric cell division of many types of stem and progenitor cells, thus influencing cell fate decisions (38, 39). In addition, defects in Gαi signaling result in aberrant lymphocyte differentiation (7, 35, 40). We therefore hypothesized that loss of AGS3 may result in defective lymphocyte differentiation. However, analysis of lymphocyte subsets from spleens and thymi of naive Gpsm1−/− mice indicated no statistically significant differences in the relative distribution of B and T cell and other leukocyte subsets as compared with wild-type mice (Table 1).
TABLE 1.
Lymphocyte populations in the spleen and thymus of Gpsm1+/+ and Gpsm1−/− mice
The percentage of cell populations found in the spleen and thymus of Gpsm1+/+ or Gpsm1−/− mice is shown. The results are the mean ± S.E. from four mice for each genotype. Statistical significance was calculated using a Student's t test.
| Genotype | Gpsm1+/+ | Gpsm1−/− |
|---|---|---|
| Total splenic cell populations | ||
| B220+ | 49 ± 1 | 50 ± 2 |
| Non B, Non T | 10 ± 1 | 12 ± 1 |
| CD3+ | 38 ± 1 | 35 ± 2 |
| Percentage of cells from splenic B220+ population | ||
| Follicular | 50 ± 3 | 50 ± 3 |
| Marginal Zone | 14 ± 3 | 13 ± 5 |
| Percentage of cells from splenic CD3+ population | ||
| CD4+ | 55 ± 2 | 53 ± 1 |
| CD4+ CD8+ | 1.2 ± 0.1 | 1.2 ± 0.2 |
| CD4− CD8− | 6.3 ± 1.1 | 7.8 ± 0.4 |
| CD8+ | 38 ± 2 | 37 ± 1 |
| Percentage of cells from thymic CD3+ population | ||
| CD4+ | 8 ± 2 | 7 ± 2 |
| CD4+ CD8+ | 85 ± 4 | 86 ± 3 |
| CD4− CD8− | 3.4 ± 0.5 | 3.3 ± 0.3 |
| CD8+ | 3.3 ± 0.7 | 3.2 ± 0.7 |
Leukocyte Stimulation Enhances AGS3 Protein Levels
As we observed no obvious defects in lymphocyte subset differentiation, we next explored whether AGS3 levels were affected by stimulation of leukocytes. Indeed, dramatic up-regulation of AGS3 expression was observed in primary B cells stimulated with either LPS or IgM and primary T cells stimulated with anti-CD3 and interleukin (IL)-2 (Fig. 2A). In addition, AGS3 levels were also markedly increased over time in primary bone marrow-derived dendritic cells (BMDCs) after treatment with LPS (Fig. 2B). Up-regulation of AGS3 upon immune cell activation suggests a role for AGS3 in regulating immune responses; furthermore, these results indicate that AGS3 levels are responsive to changes in the cellular environment, which is consistent with previous reports of regulation of AGS3 expression by cell stress and maladaptation (19–21, 23, 24, 26).
FIGURE 2.
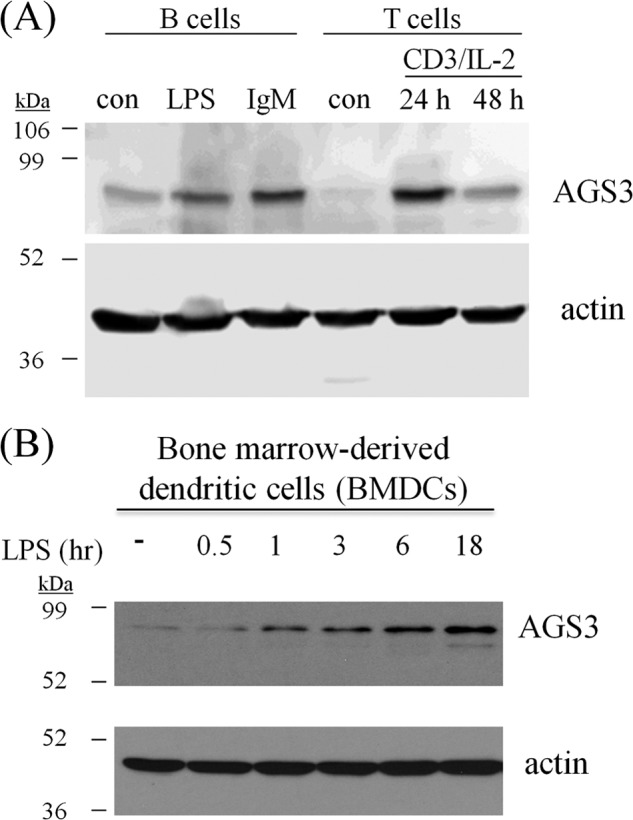
AGS3 is up-regulated in lymphocytes and BMDCs upon stimulation. A, purified B and T lymphocytes from C57BL/6J mice were isolated as described under “Experimental Procedures.” Purified B cells were stimulated with 20 μg/ml anti-IgM F(ab′)2 fragment or 1 mg/ml LPS for 12 h. Purified T cells were stimulated with 0.1 μg/ml anti-CD3 and 20% IL-2 for 24–48 h. After treatment, cells were washed and lysed in SDS sample buffer and subjected to SDS-PAGE and immunoblotting with AGS3 and actin-specific antisera. con, control. B, BMDCs were prepared as described under “Experimental Procedures.” After 8 days, immature dendritic cells were cultured in the absence (−) or presence of 200 ng/ml LPS for the times indicated in the figure. After treatment, cells were washed and lysed with 1% Nonidet P-40 buffer and subjected to SDS-PAGE and immunoblotting with AGS3 and actin-specific antisera as described under “Experimental Procedures.”
Defective Chemotaxis in AGS3/Gpsm1−/− Leukocytes
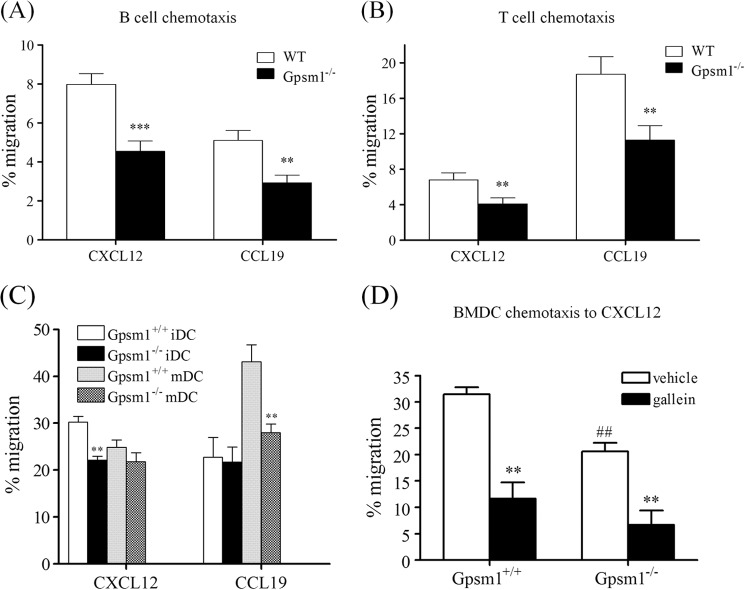
Gαi signal integration is a critical component of chemokine signaling (e.g. (41). We therefore sought to determine the effect of the loss of AGS3 on chemokine-directed signaling events. As an initial approach to address this question, we analyzed the chemotactic responses of leukocytes isolated from WT and Gpsm1−/− mice. B and T lymphocytes isolated from Gpsm1−/− mice showed nearly a 50% reduction in chemotaxis toward either CXCL12/SDF-1α or CCL19 as compared with WT lymphocytes (Fig. 3, A and B). Similarly, BMDCs from AGS3-null mice showed ∼30% reduction in migration toward the chemokine CXCL12 (Fig. 3C, left panel). There was no significant difference in random migration between WT and Gpsm1−/− lymphocytes or BMDCs,4 suggesting that the chemotactic defect in Gpsm1−/− cells was not due to an overall decrease in cell motility. Chemokine receptor expression levels were not altered in Gpsm1−/− BMDCs or lymphocytes as compared with WT cells as determined by flow cytometry, indicating that the chemotactic defect in Gpsm1−/− leukocytes was not due to loss of chemokine receptor expression.4
FIGURE 3.
Chemotaxis of immune cells from WT and Gpsm1−/− mice to chemokines CXCL12 and CCL19. A and B, B and T cells were separately isolated from freshly harvested splenocytes of WT and Gpsm1−/− mice. Cells were loaded in Transwell migration chambers with the bottom chamber containing serum-free RPMI supplemented with 0.05 μm β-mercaptoethanol in the absence and presence of 300 ng/ml CXCL12 or CCL19 as indicated. After 5 h at 37 °C, cells in the bottom chamber were counted, and the percentage of cells migrating as compared with input was determined by subtracting the number of cells migrating to the bottom chamber in the absence of chemokine. Data are represented as the mean ± S.E. of three independent experiments with at least triplicate determinations. **, p < 0.01; ***, p < 0.001. C, bone marrow cells were harvested from WT and Gpsm1−/− mice and cultured to iDCs or mDCs as described under “Experimental Procedures.” Dendritic cells were loaded in Transwell migration chambers with the bottom chamber containing serum-free RPMI in the absence and presence of 250 ng/ml CXCL12 or CCL19 as indicated. After 20 h at 37 °C, cells in the bottom chamber were counted, and the percentage of cells migrating as compared with input was determined by subtracting the number of cells migrating to the bottom chamber in the absence of chemokine. Data are represented as the mean ± S.E. of three independent experiments with at least triplicate determinations. **, p < 0.01. D, BMDCs were harvested and cultured as described above and pretreated with 10 μm gallein or vehicle for 30 min prior to measuring chemotaxis to CXCL12 as described under “Experimental Procedures.” **, p < 0.01 for gallein-treated as compared with vehicle control for each genotype. ##, p < 0.01 for Gpsm1−/− vehicle control as compared with Gpsm1+/+ vehicle control. Differences for gallein treatment groups between genotypes were not statistically significant.
Treatment of BMDCs with inflammatory signals such as LPS induces differentiation from iDCs into mDCs. Chemokine receptor expression levels and subsequent chemokine responsiveness in DCs are very sensitive to this maturation process, and DCs respond by rapidly changing their chemokine receptor expression profiles and chemokine sensitivity (42, 43). Although CXCR4 expression increases during maturation, chemotactic responses to its agonist CXCL12 are apparently reduced (44–46). In addition, CCR7 levels increase dramatically upon maturation, and DC responsiveness to the CCR7 agonist CCL19 is significantly enhanced (42–47). We measured WT and Gpsm1−/− BMDC chemotaxis to CXCL12 and CCL19 before and after treatment with LPS for 24 h. As shown in Fig. 3C, our results for WT BMDC chemotaxis before and after maturation (iDC and mDC, respectively) are consistent with previously published observations (44–46) in that iDCs preferentially responded to CXCL12 (Fig. 3C, left panel) and mDCs preferentially responded to CCL19 (Fig. 3C, right panel). One would predict that if AGS3 is up-regulated upon LPS treatment (Fig. 2) and if AGS3 plays a positive role in integrating chemokine signals, then the chemotactic defect in DCs from Gpsm1−/− mice would be greater for mDCs as compared with iDCs. As expected, Gpsm1−/− iDCs exhibited an ∼25% decrease in chemotaxis to CXCL12 (Fig. 3C, left panel); however, Gpsm1−/− mDCs showed an ∼40% decrease in chemotaxis to CCL19 as compared with WT mDCs (Fig. 3C, right panel). These data are consistent with the hypothesis that chemokine signal integration is sensitive to AGS3 levels and that AGS3 plays a positive role in chemokine signal processing.
A possible explanation for AGS3 function in chemokine signal integration is that by binding Gαi subunits prior to reformation of Gαiβγ heterotrimer, AGS3 could enhance or prolong Gβγ signaling. As an initial approach to address this question, we treated WT and Gpsm1−/− dendritic cells with gallein, a Gβγ inhibitor. Although the chemotactic response to chemokine was reduced in Gpsm1−/− dendritic cells, the magnitude of inhibition of chemokine-induced chemotaxis by the Gβγ antagonist gallein was similar in both WT and Gpsm1−/− dendritic cells (Fig. 3D). These data suggest that the reduced chemotaxis observed in Gpsm1−/− cells may result from the loss of a positive modulatory role of AGS3 on Gβγ signaling.
Impaired Chemokine-mediated Signal Processing in AGS3/Gpsm1−/− Leukocytes
To further define the effects of loss of AGS3 on chemokine signaling, we investigated chemokine-induced mobilization of intracellular calcium in WT and Gpsm1−/− leukocytes (Fig. 4). An increase in intracellular calcium plays a critical role in cell motility and chemotaxis (48, 49). Indeed, Gpsm1−/− splenocytes and BMDCs showed diminished chemokine-induced calcium responses (Fig. 4), consistent with the chemotactic defect in Gpsm1−/− cells as compared with WT cells.
FIGURE 4.

Gpsm1−/− splenocytes and dendritic cells have impaired chemokine-stimulated calcium responses. A and B, freshly harvested splenocytes of WT and Gpsm1−/− mice were seeded into clear bottom, black-walled 96-well plates at 1 × 106 cells/well in 100 μl of serum-free, phenol red-free RPMI in the absence of serum for at least 1 h. Cells were incubated in Calcium 5 assay dye for 1 h at 37 °C and incubated at room temperature for 15 min prior to analysis. CXCL12 (A) or CCL19 (B) was added at a final concentration of 200 ng/ml by the FLIPRtetra, and measurements (relative light units, RLU) were taken every second for at least 300 s. C, bone marrow cells were harvested from WT and Gpsm1−/− mice and cultured to BMDCs as described under “Experimental Procedures.” BMDCs were seeded at 250,000 cells/well in the absence of serum for ∼1 h. BMDCs were incubated in Calcium 5 dye for 1 h at 37 °C and incubated at room temperature for 15 min prior to analysis. CXCL12 was added at a final concentration of 200 ng/ml by the FLIPRtetra, and measurements (relative light units RLU) were taken every second for at least 300 s. Data are representative of three independent experiments with triplicate determinations.
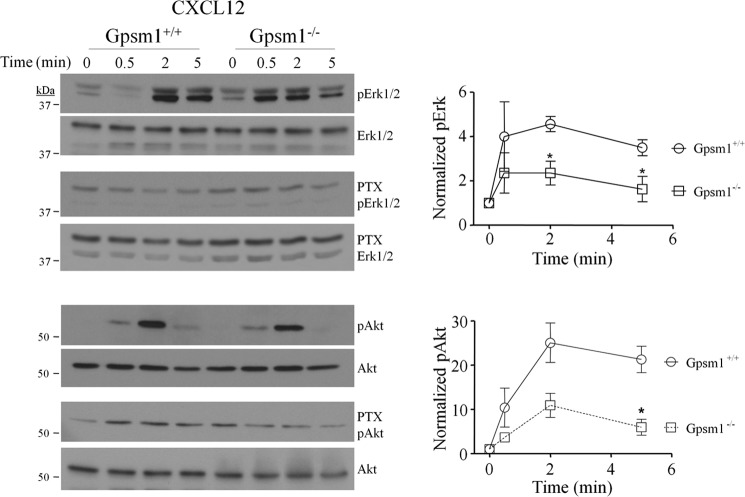
In addition to calcium mobilization, phosphorylation of ERK1/2 and Akt is also attributed to chemokine signaling. Stimulation of chemokine receptors such as CXCR4 activates the kinase ERK1/2 by stimulating its phosphorylation (50), which in turn is an important regulator of cell motility in many cell types including lymphocytes and dendritic cells (41, 51). Interestingly, Gpsm1−/− BMDCs showed significant impairment of CXCL12-stimulated phosphorylation of ERK1/2 as well as Akt (Fig. 5). In addition, pretreatment with pertussis toxin blocked CXCL12-induced ERK1/2 and Akt phosphorylation in BMDCs from both WT and Gpsm1−/− mice to the same extent (Fig. 5). GM-CSF-stimulated ERK1/2 phosphorylation, acting through a tyrosine kinase receptor pathway, was not affected by the loss of AGS3 expression in Gpsm1−/− BMDCs.4 Splenocytes from Gpsm1−/− mice also showed significant defects in both CXCL12-stimulated and CCL19-stimulated ERK1/2 and Akt phosphorylation (Fig. 6).
FIGURE 5.
Gpsm1−/− dendritic cells exhibit defects in CXCL12-stimulated phosphorylation of ERK1/2 and Akt. Single cell suspensions of Gpsm1+/+ and Gpsm1−/− cultured dendritic cells were pretreated in the absence or presence of 100 ng/ml pertussis toxin (PTX) for 18 h prior to the addition of 200 ng/ml CXCL12 as described under “Experimental Procedures.” At the indicated times, cells were lysed in 1% Nonidet P-40 lysis buffer containing protease and phosphatase inhibitors, and lysates (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF, and immunoblotted with anti-phospho-Akt (Ser483) (pAkt), anti-phospho-ERK (Tyr204) (pErk1/2), or total Akt- or total ERK-specific antibodies. Representative immunoblots are shown in the left panels, and densitometric analysis of at least three independent experiments (represented as means ± S.E.) are shown in the right panels. *, p < 0.05.
FIGURE 6.
Gpsm1−/− splenocytes exhibit defects in CXCL12- and CCL19-stimulated phosphorylation of ERK1/2 and Akt. A and B, single cell suspensions of Gpsm1+/+ and Gpsm1−/− freshly isolated splenocytes were treated with 200 ng/ml CXCL12 (A) or CCL19 (B) as described under “Experimental Procedures.” At the indicated times, cells were lysed in 1% Nonidet P-40 lysis buffer containing protease and phosphatase inhibitors, and lysates (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF, and immunoblotted with anti-phospho-Akt (Ser483) (pAkt), anti-phospho-ERK (Tyr204) (pErk1/2), or total Akt- or total ERK-specific antibodies. Representative immunoblots are shown in the left panels, and densitometric analysis of at least three independent experiments (represented as mean ± S.E.) are shown in the right panels. *, p < 0.05; **, p < 0.01.
DISCUSSION
Accessory proteins for G-protein signaling systems have revealed surprising diversity in modes of heterotrimeric G-protein signal integration, including but not limited to the modulation of signal strength, duration, location, termination, and the formation of signal transduction complexes (for review, see Refs. 2, 13, 16, and 17). Although functional roles for the accessory protein AGS3 have been described in asymmetric cell division, neuronal plasticity and addiction, autophagy, polycystic kidney disease and renal injury, cardiovascular regulation, and metabolism (18–28, 33), the data described in this study are the first to demonstrate a role for AGS3 in the regulation of chemokine responses in hematopoietic cells. Indeed, there are relatively few reports of GPCR signal modulation by GPR motif-containing proteins (21, 28, 52–56), underscoring the significance of the current study, which, in contrast to the previous studies, makes use of primary cells obtained from genetic null (Gpsm1−/−)3 mice.
In this study we demonstrate that the loss of AGS3 expression results in aberrant chemokine-directed cell motility, Ca2+ flux, and ERK and Akt activation. These data suggest expanding functional roles for accessory proteins that, like AGS3, contain the GPR motif in the integration of chemokine receptor signals. As compared with the dramatic defects in chemokine responsiveness in Gαi2-null mice (3–9), the ∼30–40% reduction in agonist-induced chemotaxis rather than a complete loss of chemotaxis in Gpsm1−/− leukocytes is consistent with its effect as a modulatory protein. With respect to the role of AGS3 in chemokine receptor signal integration, three distinct hypotheses present themselves: 1) the chemokine receptor couples to the AGS3-Gαi complex in a manner analogous to Gαiβγ heterotrimer and releases Gαi-GTP to modulate the chemotactic response; 2) the Gαi-AGS3 module itself initiates the formation of an additional, noncanonical signaling complex distinct from Gβγ that modulates chemokine-directed signaling events; and/or 3) by virtue of its GPR motifs, AGS3 influences Gαi and Gβγ subunit interactions to prolong or enhance Gβγ signaling to facilitate directed migration.
In the first hypothesis, the chemokine receptor couples to the Gαi-AGS3 complex (29) to integrate downstream signaling events involving both Gαi and Gβγ. In heterologous systems, the Gαi-AGS3 module appears to directly engage with and is regulated by Gαi-coupled GPCRs (29) including CXCR4.5 It is not immediately clear how such a signaling system may directly impact GPCR-initiated G-protein signaling events; however, studies of Gαi subunit selectivity for chemokine-induced responses (5, 35, 57, 58) may suggest that Gαi subunits (particularly Gαi2) do have a direct impact on chemokine signaling in addition to regulating Gβγ, and thus the AGS3-Gαi module may be operative within this context.
The second hypothesis is that the AGS3-Gαi-GDP complex generated subsequent to receptor activation by AGS3 binding to Gαi-GDP prior to reassociation with Gβγ acts in the context of a novel, noncanonical signaling complex that integrates with Gβγ-regulated events to influence chemotaxis. Interestingly, a recent study suggests that the Gαi-AGS3 module acting through the AGS3 binding protein mammalian inscuteable (mInsc) and the Par3-atypical PKC (aPKC) complex is involved in regulating directed migration of neutrophils (57). These observations would suggest that Gαi-GDP bound to GPR motif(s) would either function as an active signaling entity (38) or serve as a platform for additional signal input as proposed previously (16).
However, cellular responses to chemokine receptor activation are generally thought to be predominantly due to the actions of Gβγ subunits, stemming from original observations by Neptune and Bourne (59), Charo and co-workers (60), and in Dictyostelium (61). Gβγ-mediated stimulation of PI3Kγ (62, 63), phospholipase Cβ (PLCβ) (64), ERK1/2 (65, 66), and exchange factors for small GTPases Rac and Cdc42 (67, 68) (reviewed in Ref. 69) as well as other scaffolding proteins (70) appears to underlie the requirement of Gβγ for regulating chemoattractant-directed cell motility. More recently, small molecule inhibitors of Gβγ further implicate a role for Gβγ in mediating chemokine responses in multiple cell types (32, 71) (Fig. 3D).
Within this context, the third hypothesis is that AGS3 may influence interactions between Gαi and Gβγ subunits and thus impart a positive modulatory effect on cellular responses to chemokines. As AGS3 GPR motifs compete with Gβγ for Gαi binding (12, 29, 72, 73), one possibility is that AGS3 may “grab” free Gαi-GDP prior to reassociation with Gβγ and thus enhance or prolong Gβγ-regulated effector activation (13, 16), and this hypothesis does have some support in the broader context of GPR proteins influencing Gαiβγ subunit interactions (12, 23, 24, 26, 33, 53–56). The loss of AGS3 may thus lead to reduced chemokine-mediated Gβγ signaling, which is the most likely explanation for the observed defects in Gpsm1−/− leukocyte chemotaxis (Fig. 3), calcium mobilization (Fig. 4), and activation of ERK1/2 and Akt (Figs. 5 and 6), all of which are known Gβγ effector pathways as discussed above. Taken together, the data strongly suggest that AGS3 plays a modulatory role on chemokine-mediated Gβγ signaling. As other GPR proteins are also expressed in immune tissues and cells, including LGN/Gpsm2 (Fig. 1) (31, 74), AGS4/Gpsm3 (75),4 and RGS14 (76), it is possible that these proteins may be partially masking the effects of the loss of AGS3 in this process. Defining the roles of these GPR proteins in chemokine signal integration may reveal additional functional capacity of the GPR motif in this context and is a focus of current efforts.
This study extends our previous work in defining functional roles of GPR proteins in the intact animal using the AGS3/Gpsm1−/− mouse model (18, 23, 24, 26). This model should continue to provide a valuable platform for further exploration into the functional impacts of GPR-Gαi module regulation on leukocyte function by examining other GPR proteins expressed in the immune system.
Acknowledgments
We thank Ellen Maher, Christine Webster, and Hunter Matthews for technical support. The authors thank Dr. Thomas Gettys (Pennington Biomedical Research Center, Baton Rouge, LA) for Gαi1/2 and Gαi3 antisera and Dr. Dzwokai Ma (University of California, Santa Barbara) for AGS3 antisera.
The research presented in this article was supported in part by the Flow Cytometry and Cell Sorting Shared Resource, funded by a Cancer Center Support grant P30 CA138313 to the Hollings Cancer Center at the Medical University of South Carolina and in part by the National Center for Research Resources and the Office of the Director of the National Institutes of Health through grant number C06 RR015455 and S10 RR027777. This work was supported by Medical University of South Carolina (MUSC) institutional funds (to J. B. B.); National Institutes of Health Grants GM086510 (to J. B. B.), DA025896 (to S. M. L.), AR056670 (to X. Z.), and T32CA119945 (to M. B. O.); the Merit Review Award from the Department of Veterans Affairs (to X. Z.); National Institutes of Health NIGMS SC-INBRE Grant P20GM103499 (to M. B. O.); and the intramural research program of the National Institute of Allergy and Infectious Diseases (to H. C. and J. H. K.).
In 2004, the Human Genome Organization (HUGO) committee classified the gene names for multiple GPR motif-containing proteins with the designation “G-protein signaling modulator (Gpsm) 1–4.” Therefore, by convention, the gene name for AGS3 is Gpsm1, and thus it is more appropriate to refer to the genotype of AGS3-null mice as Gpsm1−/−. We refer to the protein encoded by the Gpsm1 gene as AGS3.
M. Branham-O'Connor, W. G. Robichaux, III, and J. B. Blumer, unpublished observations.
W. G. Robichaux III and J. B. Blumer, unpublished observations. Using a recently developed bioluminescence resonance energy transfer (BRET) platform in HEK293 cells, we observed that when coexpressed with CXCR4, AGS3-Rluc–Gαi2-YFP BRET signals were reduced by ∼40% by the CXCR4 agonist CXCL12. We also observed a Gαi2-dependent, agonist-regulated complex between AGS3-Rluc and CXCR4-Venus. Both effects were blocked by either the CXCR4 antagonist AMD3100 or by pertussis toxin pretreatment.
- GPCR
- G-protein-coupled receptor
- GPR
- G-protein regulatory
- RGS
- regulators of G-protein signaling
- AGS
- activators of G-protein signaling
- BMDC
- bone marrow-derived dendritic cell
- DC
- dendritic cell
- iDC
- immature dendritic cell
- mDC
- mature dendritic cell
- DMSO
- dimethyl sulfoxide
- LGN
- Leu-Gly-Asn-enriched protein.
REFERENCES
- 1. Cho H., Kehrl J. H. (2009) Regulation of immune function by G protein-coupled receptors, trimeric G proteins, and RGS proteins. Prog. Mol. Biol. Transl. Sci. 86, 249–298 [DOI] [PubMed] [Google Scholar]
- 2. Sato M., Blumer J. B., Simon V., Lanier S. M. (2006) Accessory proteins for G proteins: Partners in signaling. Annu. Rev. Pharmacol. Toxicol. 46, 151–187 [DOI] [PubMed] [Google Scholar]
- 3. Cho H., Kamenyeva O., Yung S., Gao J. L., Hwang I. Y., Park C., Murphy P. M., Neubig R. R., Kehrl J. H. (2012) The loss of RGS protein-Gαi2 interactions results in markedly impaired mouse neutrophil trafficking to inflammatory sites. Mol. Cell. Biol. 32, 4561–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han S. B., Moratz C., Huang N. N., Kelsall B., Cho H., Shi C. S., Schwartz O., Kehrl J. H. (2005) Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity 22, 343–354 [DOI] [PubMed] [Google Scholar]
- 5. Hwang I. Y., Park C., Kehrl J. H. (2007) Impaired trafficking of Gnai2+/− and Gnai2−/− T lymphocytes: implications for T cell movement within lymph nodes. J. Immunol. 179, 439–448 [DOI] [PubMed] [Google Scholar]
- 6. Pero R. S., Borchers M. T., Spicher K., Ochkur S. I., Sikora L., Rao S. P., Abdala-Valencia H., O'Neill K. R., Shen H., McGarry M. P., Lee N. A., Cook-Mills J. M., Sriramarao P., Simon M. I., Birnbaumer L., Lee J. J. (2007) Gαi2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc. Natl. Acad. Sci. U.S.A. 104, 4371–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudolph U., Finegold M. J., Rich S. S., Harriman G. R., Srinivasan Y., Brabet P., Boulay G., Bradley A., Birnbaumer L. (1995) Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nat. Genet. 10, 143–150 [DOI] [PubMed] [Google Scholar]
- 8. Skokowa J., Ali S. R., Felda O., Kumar V., Konrad S., Shushakova N., Schmidt R. E., Piekorz R. P., Nürnberg B., Spicher K., Birnbaumer L., Zwirner J., Claassens J. W., Verbeek J. S., van Rooijen N., Köhl J., Gessner J. E. (2005) Macrophages induce the inflammatory response in the pulmonary Arthus reaction through Gαi2 activation that controls C5aR and Fc receptor cooperation. J. Immunol. 174, 3041–3050 [DOI] [PubMed] [Google Scholar]
- 9. Zarbock A., Deem T. L., Burcin T. L., Ley K. (2007) Gαi2 is required for chemokine-induced neutrophil arrest. Blood 110, 3773–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moratz C., Harrison K., Kehrl J. H. (2004) Regulation of chemokine-induced lymphocyte migration by RGS proteins. Methods Enzymol. 389, 15–32 [DOI] [PubMed] [Google Scholar]
- 11. Cismowski M. J., Takesono A., Ma C., Lizano J. S., Xie X., Fuernkranz H., Lanier S. M., Duzic E. (1999) Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat. Biotechnol. 17, 878–883 [DOI] [PubMed] [Google Scholar]
- 12. Takesono A., Cismowski M. J., Ribas C., Bernard M., Chung P., Hazard S., 3rd, Duzic E., Lanier S. M. (1999) Receptor-independent activators of heterotrimeric G-protein signaling pathways. J. Biol. Chem. 274, 33202–33205 [DOI] [PubMed] [Google Scholar]
- 13. Blumer J. B., Smrcka A. V., Lanier S. M. (2007) Mechanistic pathways and biological roles for receptor-independent activators of G-protein signaling. Pharmacol. Ther. 113, 488–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponting C. P. (1999) Raf-like Ras/Rap-binding domains in RGS12− and still-life-like signalling proteins. J. Mol. Med. (Berl.) 77, 695–698 [DOI] [PubMed] [Google Scholar]
- 15. Siderovski D. P., Diversé-Pierluissi M. a., De Vries L. (1999) The GoLoco motif: a Gαi/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem. Sci. 24, 340–341 [DOI] [PubMed] [Google Scholar]
- 16. Blumer J. B., Oner S. S., Lanier S. M. (2012) Group II activators of G-protein signalling and proteins containing a G-protein regulatory motif. Acta Physiol. (Oxf.) 204, 202–218 [DOI] [PubMed] [Google Scholar]
- 17. McCudden C. R., Hains M. D., Kimple R. J., Siderovski D. P., Willard F. S. (2005) G-protein signaling: back to the future. Cell. Mol. Life Sci. 62, 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blumer J. B., Lord K., Saunders T. L., Pacchioni A., Black C., Lazartigues E., Varner K. J., Gettys T. W., Lanier S. M. (2008) Activator of G protein signaling 3 null mice: I. Unexpected alterations in metabolic and cardiovascular function. Endocrinology 149, 3842–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowers M. S., Hopf F. W., Chou J. K., Guillory A. M., Chang S. J., Janak P. H., Bonci A., Diamond I. (2008) Nucleus accumbens AGS3 expression drives ethanol seeking through Gβγ. Proc. Natl. Acad. Sci. U.S.A. 105, 12533–12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bowers M. S., McFarland K., Lake R. W., Peterson Y. K., Lapish C. C., Gregory M. L., Lanier S. M., Kalivas P. W. (2004) Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron 42, 269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fan P., Jiang Z., Diamond I., Yao L. (2009) Up-regulation of AGS3 during morphine withdrawal promotes cAMP superactivation via adenylyl cyclase 5 and 7 in rat nucleus accumbens/striatal neurons. Mol. Pharmacol. 76, 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groves B., Gong Q., Xu Z., Huntsman C., Nguyen C., Li D., Ma D. (2007) A specific role of AGS3 in the surface expression of plasma membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 104, 18103–18108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwon M., Pavlov T. S., Nozu K., Rasmussen S. A., Ilatovskaya D. V., Lerch-Gaggl A., North L. M., Kim H., Qian F., Sweeney W. E., Jr., Avner E. D., Blumer J. B., Staruschenko A., Park F. (2012) G-protein signaling modulator 1 deficiency accelerates cystic disease in an orthologous mouse model of autosomal dominant polycystic kidney disease. Proc. Natl. Acad. Sci. U.S.A. 109, 21462–21467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nadella R., Blumer J. B., Jia G., Kwon M., Akbulut T., Qian F., Sedlic F., Wakatsuki T., Sweeney W. E., Jr., Wilson P. D., Lanier S. M., Park F. (2010) Activator of G protein signaling 3 promotes epithelial cell proliferation in PKD. J. Am. Soc. Nephrol. 21, 1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pattingre S., De Vries L., Bauvy C., Chantret I., Cluzeaud F., Ogier-Denis E., Vandewalle A., Codogno P. (2003) The G-protein regulator AGS3 controls an early event during macroautophagy in human intestinal HT-29 cells. J. Biol. Chem. 278, 20995–21002 [DOI] [PubMed] [Google Scholar]
- 26. Regner K. R., Nozu K., Lanier S. M., Blumer J. B., Avner E. D., Sweeney W. E., Jr., Park F. (2011) Loss of activator of G-protein signaling 3 impairs renal tubular regeneration following acute kidney injury in rodents. FASEB J. 25, 1844–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vural A., Oner S., An N., Simon V., Ma D., Blumer J. B., Lanier S. M. (2010) Distribution of activator of G-protein signaling 3 within the aggresomal pathway: role of specific residues in the tetratricopeptide repeat domain and differential regulation by the AGS3 binding partners Giα and mammalian inscuteable. Mol. Cell. Biol. 30, 1528–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao L., McFarland K., Fan P., Jiang Z., Inoue Y., Diamond I. (2005) Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc. Natl. Acad. Sci. U.S.A. 102, 8746–8751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oner S. S., An N., Vural A., Breton B., Bouvier M., Blumer J. B., Lanier S. M. (2010) Regulation of the AGS3·Gαi signaling complex by a seven-transmembrane span receptor. J. Biol. Chem. 285, 33949–33958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oner S. S., Maher E. M., Breton B., Bouvier M., Blumer J. B. (2010) Receptor-regulated interaction of activator of G-protein signaling-4 and Gαi. J. Biol. Chem. 285, 20588–20594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blumer J. B., Chandler L. J., Lanier S. M. (2002) Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J. Biol. Chem. 277, 15897–15903 [DOI] [PubMed] [Google Scholar]
- 32. Lehmann D. M., Seneviratne A. M., Smrcka A. V. (2008) Small molecule disruption of G protein βγ subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol. Pharmacol. 73, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanada K., Tsai L. H. (2005) G protein βγ subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122, 119–131 [DOI] [PubMed] [Google Scholar]
- 34. Kehrl J. H., Hwang I. Y., Park C. (2009) Chemoattract receptor signaling and its role in lymphocyte motility and trafficking. Curr. Top. Microbiol. Immunol. 334, 107–127 [DOI] [PubMed] [Google Scholar]
- 35. Huang T. T., Zong Y., Dalwadi H., Chung C., Miceli M. C., Spicher K., Birnbaumer L., Braun J., Aranda R. (2003) TCR-mediated hyper-responsiveness of autoimmune Gαi2−/− mice is an intrinsic naive CD4+ T cell disorder selective for the Gαi2 subunit. Int. Immunol. 15, 1359–1367 [DOI] [PubMed] [Google Scholar]
- 36. Su A. I., Cooke M. P., Ching K. A., Hakak Y., Walker J. R., Wiltshire T., Orth A. P., Vega R. G., Sapinoso L. M., Moqrich A., Patapoutian A., Hampton G. M., Schultz P. G., Hogenesch J. B. (2002) Large-scale analysis of the human and mouse transcriptomes. Proc. Natl. Acad. Sci. U.S.A. 99, 4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Su A. I., Wiltshire T., Batalov S., Lapp H., Ching K. A., Block D., Zhang J., Soden R., Hayakawa M., Kreiman G., Cooke M. P., Walker J. R., Hogenesch J. B. (2004) A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. U.S.A. 101, 6062–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gönczy P. (2008) Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 [DOI] [PubMed] [Google Scholar]
- 39. Knoblich J. A. (2010) Asymmetric cell division: recent developments and their implications for tumour biology. Nat. Rev. Mol. Cell Biol. 11, 849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dalwadi H., Wei B., Schrage M., Spicher K., Su T. T., Birnbaumer L., Rawlings D. J., Braun J. (2003) B cell developmental requirement for the Gαi2 gene. J. Immunol. 170, 1707–1715 [DOI] [PubMed] [Google Scholar]
- 41. Delgado-Martín C., Escribano C., Pablos J. L., Riol-Blanco L., Rodríguez-Fernández J. L. (2011) Chemokine CXCL12 uses CXCR4 and a signaling core formed by bifunctional Akt, extracellular signal-regulated kinase (ERK)1/2, and mammalian target of rapamycin complex 1 (mTORC1) proteins to control chemotaxis and survival simultaneously in mature dendritic cells. J. Biol. Chem. 286, 37222–37236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C. R., Qin S., Lanzavecchia A. (1998) Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28, 2760–2769 [DOI] [PubMed] [Google Scholar]
- 43. Sozzani S., Allavena P., D'Amico G., Luini W., Bianchi G., Kataura M., Imai T., Yoshie O., Bonecchi R., Mantovani A. (1998) Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161, 1083–1086 [PubMed] [Google Scholar]
- 44. Dehring D. A., Clarke F., Ricart B. G., Huang Y., Gomez T. S., Williamson E. K., Hammer D. A., Billadeau D. D., Argon Y., Burkhardt J. K. (2011) Hematopoietic lineage cell-specific protein 1 functions in concert with the Wiskott-Aldrich syndrome protein to promote podosome array organization and chemotaxis in dendritic cells. J. Immunol. 186, 4805–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Humrich J. Y., Humrich J. H., Averbeck M., Thumann P., Termeer C., Kämpgen E., Schuler G., Jenne L. (2006) Mature monocyte-derived dendritic cells respond more strongly to CCL19 than to CXCL12: consequences for directional migration. Immunology 117, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ricart B. G., John B., Lee D., Hunter C. A., Hammer D. A. (2011) Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J. Immunol. 186, 53–61 [DOI] [PubMed] [Google Scholar]
- 47. Vecchi A., Massimiliano L., Ramponi S., Luini W., Bernasconi S., Bonecchi R., Allavena P., Parmentier M., Mantovani A., Sozzani S. (1999) Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. J. Leukoc. Biol. 66, 489–494 [DOI] [PubMed] [Google Scholar]
- 48. Evans J. H., Falke J. J. (2007) Ca2+ influx is an essential component of the positive-feedback loop that maintains leading-edge structure and activity in macrophages. Proc. Natl. Acad. Sci. U.S.A. 104, 16176–16181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marks P. W., Maxfield F. R. (1990) Transient increases in cytosolic free calcium appear to be required for the migration of adherent human neutrophils. J. Cell Biol. 110, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tilton B., Ho L., Oberlin E., Loetscher P., Baleux F., Clark-Lewis I., Thelen M. (2000) Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J. Exp. Med. 192, 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sagar D., Lamontagne A., Foss C. A., Khan Z. K., Pomper M. G., Jain P. (2012) Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J. Neuroinflammation 9, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Conley J. M., Watts V. J. (2013) Differential effects of AGS3 expression on D(2L) dopamine receptor-mediated adenylyl cyclase signaling. Cell. Mol. Neurobiol. 33, 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kinoshita-Kawada M., Oberdick J., Xi Zhu M. (2004) A Purkinje cell specific GoLoco domain protein, L7/Pcp-2, modulates receptor-mediated inhibition of Cav2.1 Ca2+ channels in a dose-dependent manner. Brain Res. Mol. Brain Res. 132, 73–86 [DOI] [PubMed] [Google Scholar]
- 54. Sato M., Gettys T. W., Lanier S. M. (2004) AGS3 and signal integration by Gαs- and Gαi-coupled receptors: AGS3 blocks the sensitization of adenylyl cyclase following prolonged stimulation of a Gαi-coupled receptor by influencing processing of Gαi. J. Biol. Chem. 279, 13375–13382 [DOI] [PubMed] [Google Scholar]
- 55. Webb C. K., McCudden C. R., Willard F. S., Kimple R. J., Siderovski D. P., Oxford G. S. (2005) D2 dopamine receptor activation of potassium channels is selectively decoupled by Gα-specific GoLoco motif peptides. J. Neurochem. 92, 1408–1418 [DOI] [PubMed] [Google Scholar]
- 56. Wiser O., Qian X., Ehlers M., Ja W. W., Roberts R. W., Reuveny E., Jan Y. N., Jan L. Y. (2006) Modulation of basal and receptor-induced GIRK potassium channel activity and neuronal excitability by the mammalian PINS homolog LGN. Neuron 50, 561–573 [DOI] [PubMed] [Google Scholar]
- 57. Kamakura S., Nomura M., Hayase J., Iwakiri Y., Nishikimi A., Takayanagi R., Fukui Y., Sumimoto H. (2013) The cell polarity protein mInsc regulates neutrophil chemotaxis via a noncanonical G protein signaling pathway. Dev. Cell 26, 292–302 [DOI] [PubMed] [Google Scholar]
- 58. Wiege K., Le D. D., Syed S. N., Ali S. R., Novakovic A., Beer-Hammer S., Piekorz R. P., Schmidt R. E., Nürnberg B., Gessner J. E. (2012) Defective macrophage migration in Gαi2- but not Gαi3-deficient mice. J. Immunol. 189, 980–987 [DOI] [PubMed] [Google Scholar]
- 59. Neptune E. R., Bourne H. R. (1997) Receptors induce chemotaxis by releasing the βγ subunit of Gi, not by activating Gq or Gs. Proc. Natl. Acad. Sci. U.S.A. 94, 14489–14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arai H., Tsou C. L., Charo I. F. (1997) Chemotaxis in a lymphocyte cell line transfected with C-C chemokine receptor 2B: evidence that directed migration is mediated by βγ dimers released by activation of Gαi-coupled receptors. Proc. Natl. Acad. Sci. U.S.A. 94, 14495–14499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peracino B., Borleis J., Jin T., Westphal M., Schwartz J. M., Wu L., Bracco E., Gerisch G., Devreotes P., Bozzaro S. (1998) G protein β subunit-null mutants are impaired in phagocytosis and chemotaxis due to inappropriate regulation of the actin cytoskeleton. J. Cell Biol. 141, 1529–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. (1994) A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits. Cell 77, 83–93 [DOI] [PubMed] [Google Scholar]
- 63. Stephens L. R., Eguinoa A., Erdjument-Bromage H., Lui M., Cooke F., Coadwell J., Smrcka A. S., Thelen M., Cadwallader K., Tempst P., Hawkins P. T. (1997) The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89, 105–114 [DOI] [PubMed] [Google Scholar]
- 64. Wang T., Dowal L., El-Maghrabi M. R., Rebecchi M., Scarlata S. (2000) The pleckstrin homology domain of phospholipase C-β2 links the binding of Gβγ to activation of the catalytic core. J. Biol. Chem. 275, 7466–7469 [DOI] [PubMed] [Google Scholar]
- 65. Crespo P., Xu N., Simonds W. F., Gutkind J. S. (1994) Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature 369, 418–420 [DOI] [PubMed] [Google Scholar]
- 66. Koch W. J., Hawes B. E., Allen L. F., Lefkowitz R. J. (1994) Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by Gβγ activation of p21ras. Proc. Natl. Acad. Sci. U.S.A. 91, 12706–12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ueda H., Nagae R., Kozawa M., Morishita R., Kimura S., Nagase T., Ohara O., Yoshida S., Asano T. (2008) Heterotrimeric G protein βγ subunits stimulate FLJ00018, a guanine nucleotide exchange factor for Rac1 and Cdc42. J. Biol. Chem. 283, 1946–1953 [DOI] [PubMed] [Google Scholar]
- 68. Welch H. C., Coadwell W. J., Ellson C. D., Ferguson G. J., Andrews S. R., Erdjument-Bromage H., Tempst P., Hawkins P. T., Stephens L. R. (2002) P-Rex1, a PtdIns(3,4,5)P3- and Gβγ-regulated guanine-nucleotide exchange factor for Rac. Cell 108, 809–821 [DOI] [PubMed] [Google Scholar]
- 69. Khan S. M., Sleno R., Gora S., Zylbergold P., Laverdure J. P., Labbé J. C., Miller G. J., Hébert T. E. (2013) The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharmacol. Rev. 65, 545–577 [DOI] [PubMed] [Google Scholar]
- 70. Sun Z., Tang X., Lin F., Chen S. (2011) The WD40 repeat protein WDR26 binds Gβγ and promotes Gβγ-dependent signal transduction and leukocyte migration. J. Biol. Chem. 286, 43902–43912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kirui J. K., Xie Y., Wolff D. W., Jiang H., Abel P. W., Tu Y. (2010) Gβγ signaling promotes breast cancer cell migration and invasion. J. Pharmacol. Exp. Ther. 333, 393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bernard M. L., Peterson Y. K., Chung P., Jourdan J., Lanier S. M. (2001) Selective interaction of AGS3 with G-proteins and the influence of AGS3 on the activation state of G-proteins. J. Biol. Chem. 276, 1585–1593 [DOI] [PubMed] [Google Scholar]
- 73. Ghosh M., Peterson Y. K., Lanier S. M., Smrcka A. V. (2003) Receptor- and nucleotide exchange-independent mechanisms for promoting G protein subunit dissociation. J. Biol. Chem. 278, 34747–34750 [DOI] [PubMed] [Google Scholar]
- 74. Oliaro J., Van Ham V., Sacirbegovic F., Pasam A., Bomzon Z., Pham K., Ludford-Menting M. J., Waterhouse N. J., Bots M., Hawkins E. D., Watt S. V., Cluse L. A., Clarke C. J., Izon D. J., Chang J. T., Thompson N., Gu M., Johnstone R. W., Smyth M. J., Humbert P. O., Reiner S. L., Russell S. M. (2010) Asymmetric cell division of T cells upon antigen presentation uses multiple conserved mechanisms. J. Immunol. 185, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giguère P. M., Billard M. J., Laroche G., Buckley B. K., Timoshchenko R. G., McGinnis M. W., Esserman D., Foreman O., Liu P., Siderovski D. P., Tarrant T. K. (2013) G-protein signaling modulator-3, a gene linked to autoimmune diseases, regulates monocyte function and its deficiency protects from inflammatory arthritis. Mol. Immunol. 54, 193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cho H., Kozasa T., Takekoshi K., De Gunzburg J., Kehrl J. H. (2000) RGS14, a GTPase-activating protein for Giα, attenuates Giα- and G13α-mediated signaling pathways. Mol. Pharmacol. 58, 569–576 [DOI] [PubMed] [Google Scholar]