Abstract
The relative importance of overweight after childhood and excess weight gain during adulthood remains unclear. In 39,909 male participants of the Health Professionals Follow-Up Study who were 40–75 years of age in 1986 and were followed until 2008, we documented 8,755 incident cases of obesity-related chronic diseases (type 2 diabetes mellitus, cardiovascular diseases, and colorectal, renal, pancreatic, and esophageal cancers). We calculated composite and cause-specific hazard ratios using a model that included body mass index (BMI; weight (kg)/height (m)2) at 21 years of age, weight change since age 21 years, smoking, alcohol consumption, and family histories of myocardial infarction, colon cancer, and diabetes. Compared with a BMI at 21 years of 18.5–22.9, the composite hazard ratio for a BMI of 23–24.9 was 1.22 (95% confidence interval (CI): 1.16, 1.29), that for a BMI of 25.0–27.4 was 1.57 (95% CI: 1.48, 1.67), that for a BMI of 27.5–29.9 was 2.40 (95% CI: 2.17, 2.65), and that for a BMI ≥30.0 was 3.15 (95% CI: 2.76, 3.60). The composite hazard ratios for adult weight gain compared with a stable weight were 1.12 (95% CI: 1.03, 1.22) for a gain of 2.5–4.9 kg, 1.41 (95% CI: 1.31, 1.52) for a gain of 5–9.9 kg, 1.72 (95% CI: 1.59, 1.86) for a gain of 10–14.9 kg, and 2.45 (95% CI: 2.27, 2.63) for a gain ≥15 kg. Adiposity in early adulthood and adult weight gain were both associated with marked increases in the risk of major chronic diseases in middle-aged and older men, and these associations were already apparent at modest levels of overweight and weight gain.
Keywords: cardiovascular disease, diabetes mellitus, epidemiology, mortality, obesity
The high and increasing prevalence of overweight and obesity in children and adolescents (1) is a major public health concern. Obesity in adolescence and early adulthood has been related to premature morbidity and mortality in adulthood (2–11). The health consequences of moderate overweight in early adulthood are less clear. Furthermore, most studies on obesity in adolescence have focused on all-cause mortality (2, 7–10) or a single disease (5, 12–14) as the outcome; few studies have evaluated the impact of excess adiposity in early adulthood on the incidence of a variety of chronic diseases while taking into account competing events (3, 15).
To interpret the findings about adiposity in early adulthood and the risk of chronic diseases at older ages, it is important to consider how adult weight changes may modify this association. Data on the association between weight gain since adolescence and health outcomes in middle or older age are limited (9, 13, 16–21). Particularly, the relative importance of adiposity in early adulthood and adult weight gain for the onset of chronic diseases in middle and older age remains unclear.
We therefore prospectively evaluated the associations of adiposity in early adulthood and subsequent weight change with the incidence of chronic diseases in middle-aged and older men who were followed for 22 years. We studied major chronic diseases that have been consistently associated with adult obesity so that we could clearly distinguish the associations of moderate overweight and weight gain in early adulthood with those diseases.
METHODS
Study design and population
The Health Professional Follow-up Study is an ongoing prospective cohort study of 51,529 male dentists, optometrists, osteopaths, pharmacists, podiatrists, and veterinarians aged 40–75 years at baseline in 1986. The Human Subjects Committee of the Harvard School of Public Health approved this study, and all participants gave written consent.
At baseline, the men completed a questionnaire that collected information about their medical history, diet, and lifestyle factors. Information on medical history was updated via mailed questionnaires every second year. For the present analysis, we excluded 8,385 men who reported a history of one of the studied end points or any chronic disease at baseline that may be associated with either obesity or weight loss (2,148 with cancer, 1,605 with diabetes, 2,185 with cardiovascular disease (CVD), 2,403 with asthma or chronic obstructive pulmonary diseases, and 44 with renal failure). Furthermore, 5 men for whom we were missing date of death, 15 men for whom we were missing date of birth, and 3 men who were outside the baseline age range of 40–75 years were consecutively excluded. Of the remaining participants, 1,888 men were missing information on the initial questionnaires about recalled weight at 21 years of age and 1,283 men were missing information on weight change during the 5 years before baseline. These participants were excluded, as were 32 men who had a body mass index (BMI, defined as the weight in kilograms divided by the square of the height in meters) below 15 at 21 years of age and 9 men who had a BMI below 15 at the baseline of the study. After these exclusions, 39,909 men were included in the analysis.
Data collection
At study baseline, the participants reported their current height and weight, their recalled weight at 21 years of age, and the weight change that occurred in the 5 years before the study baseline. In a validation study among 123 participants, the Pearson correlation coefficient between self-reported weight and the mean of 2 standardized technician-measured weights was 0.97, and the mean self-reported weight was 1.06 kg (95% confidence interval (CI): 0.44, 1.71) lower than the measured weight (22). BMI at the age of 21 years was calculated as the weight at 21 years in kilograms divided by the square of the height reported in 1986 in meters. We defined adult weight change as the change in weight between the age of 21 years and the baseline of the study in 1986.
The baseline questionnaire also included questions about whether the participants had first-degree family members with a history of diabetes, myocardial infarction, or colon cancer and about lifestyle factors, such as cigarette smoking and alcohol consumption. Participants were asked about their mean weekly time spent engaged in 8 recreational activities during the previous year (23). The total energy expended during physical activity was expressed in hours per week of metabolic equivalents. Participants reported their average consumption of approximately 130 foods and beverages during the previous year on a validated semiquantitative food frequency questionnaire (24).
Obesity-related diseases
As the primary end point of this study, we defined a composite outcome of the first occurrence of fatal or nonfatal chronic obesity-related disease. In this outcome variable, we included major chronic diseases that have been consistently associated with obesity: CVD (defined as myocardial infarction or stroke) (25), type 2 diabetes mellitus (25, 26), and certain cancers (cancers of the rectum, colon, kidney, pancreas, and esophagus) (25, 26), as well as death from these diseases.
Every 2 years after the baseline of the study, participants were asked to report new diseases that had been diagnosed during the past 2 years on their follow-up questionnaires. Response rates for these questionnaires have been consistently over 90%. We asked all men who reported incident diagnoses of CVD or cancer to confirm the report and provide permission to review their medical records. Study investigators who were unaware of the subjects' risk factor status reviewed the medical records to confirm the diagnoses. All men who reported a diagnosis of diabetes on any of the biennial follow-up questionnaires received a supplementary questionnaire about symptoms, diagnostic tests, and medication. Deaths during follow-up were reported by family members, coworkers, or postal authorities or were identified through the National Death Index (27, 28). Physicians reviewed death certificates and hospital or pathology reports to classify individual causes of death and were unaware of participants' reported questionnaire results. A detailed description of definitions and confirmation of the diagnoses can be found in Web Appendix 1, available at http://aje.oxfordjournals.org/.
Statistical analysis
BMI at 21 years of age was categorized according to the World Health Organization criteria, with 2 additional cutoff points at 23 and 27.5 as recommended by a World Health Organization expert consultation (29). This allowed a detailed examination of the associations of BMI with the outcome parameters within the “normal weight” and “overweight” ranges (<18.5, 18.5–22.9 (reference category), 23.0–24.9, 25.0–27.4, 27.5–29.9, and ≥ 30.0). Adult weight change was calculated as the difference between the reported weight at the baseline of the study in 1986 and the recalled weight at the age of 21 years and was grouped into 7 categories, using stable weight (<2.5-kg weight change) as the reference category (other weight-change categories: <−5.0 kg (n = 1,779); −5.0 to −2.5 kg (n = 1,406); −2.4 to 2.4 kg (n = 7,298); 2.5 to 4.9 kg (n = 5,909); 5.0 to 9.9 kg (n = 9,823); 10.0 to 14.9 kg (n = 6,546); and ≥15.0 kg (n = 7,148)). We calculated age-standardized mean values and proportions of characteristics of the participants at the baseline of the study for categories of BMI at age 21 years and tested differences in baseline characteristics between BMI categories with 1-way analysis of variance for continuous variables and with χ2 tests for categorical variables. Because participants could have suffered from multiple diseases, which could possibly result in competing risks, we studied a composite end point of the first occurrence of fatal or nonfatal diabetes, CVD, or obesity-related cancer. In addition, we studied the separate outcomes using cause-specific hazards, censoring on any prior event that was not the event of interest (30). In these analyses of the cause-specific hazards, only persons without any prior event were included in the separate outcome variables. For example, the participants who first experienced a diagnosis of diabetes and later during follow-up experienced a cardiovascular event were counted as cases in the cause-specific hazard of diabetes but were censored in the cause-specific hazard of CVDs.
Time of follow-up was defined as the number of months between the month of returning the baseline questionnaire in 1986 and the month during which the first major chronic obesity-related diseases was reported, the month of death, or the end of the follow-up on January 31, 2008, whichever occurred first. We calculated incidence rates with 95% confidence intervals for both the composite end point and the cause-specific outcomes. We performed Cox proportional hazards regression using age in months as the time scale and the month of baseline questionnaire return as the entry time to estimate the composite and cause-specific hazard ratios and 95% confidence intervals for categories of BMI at age 21 years and adult weight change as compared with the reference categories. We evaluated the proportionality of the hazards during time (i.e., age) by including an interaction term for the exposure variables with age and tested with the log likelihood ratio statistic whether inclusion of these interaction terms improved the fit of the models.
We evaluated potential confounding by cigarette smoking, alcohol consumption, physical activity level, family history of myocardial infarction, colon cancer and diabetes, and dietary factors at baseline (quintiles of energy-adjusted intake of cereal fiber, vegetables, fruit, ω-3 fatty acids, red meat, trans fat, and ratio of polyunsatured fat to saturated fat). In separate analyses, we mutually adjusted for BMI at age 21 years and adult weight change to examine their independent contributions. All analyses were also performed for all-cause mortality as the outcome.
To test for trends, we calculated the median values of BMI at 21 years of age within each category of BMI at 21 years and modeled these median values as a continuous variable in all models with BMI at 21 years. Similarly, we modeled the medians of weight change within each adult weight-change category as a continuous variable in all adult weight-change models. To minimize confounding by cigarette smoking, we also conducted the analyses in never smokers. To reduce the impact of potentially unintentional weight loss, we also conducted the analyses after excluding the weight change that occurred during the 5 years before baseline. In addition, we performed the analyses of weight change in 3 strata of BMI at 21 years of age (<20.0, 20.0–24.9, and ≥25.0). Finally, we examined joint associations of BMI at 21 years of age in 3 categories (18.5–22.9, 23–24.9, and ≥25; participants with a BMI <18.5 were excluded) and subsequent weight change in 4 categories (<−2.5 kg, −2.5 to 2.4 kg, 2.5 to 9.9 kg, and ≥10.0 kg). We used SAS software, version 9 (SAS Institute, Inc., Cary, North Carolina) for all analyses.
RESULTS
Participants had a mean age of 53.8 (standard deviation, 9.6) years, a mean BMI at age 21 years of 23.0 (standard deviation, 2.9), and a mean subsequent weight change of 7.9 (standard deviation, 8.8) kg. The baseline characteristics of the study population (in 1986) according to BMI at age 21 years are shown in Table 1. Men who had a low BMI at 21 years of age were older and less physically active at baseline, whereas men who were overweight or obese at 21 years of age were more likely to be former smokers and to have a family history of myocardial infarction or diabetes. The mean adult weight gain was larger in men who had a lower BMI at 21 years of age. Nevertheless, overweight and obesity tended to track over time, with participants who had higher BMIs at baseline being in the highest categories of BMI at 21 years of age. Compared with participants who were younger at baseline, participants who were 50–65 years of age at baseline more often had a family history of disease, were more likely to be former smokers, and were less likely to be physically active at baseline (Table 1).
Table 1.
Age-Standardized Characteristics of Participants (n = 39,909) by Categories of BMI at 21 Years of Age, Health Professionals Follow-up Study, United States, 1986a
| Characteristic and Age at Baseline, yearsb | BMI at 21 Years of Agec |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 (n = 1,296) |
18.5–22.9 (n = 19,065) |
23.0–24.9 (n = 10,669) |
25.0–27.4 (n = 6,674) |
27.5–29.9 (n = 1,491) |
≥30.0 (n = 714) |
|||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Age at baseline (1986), years | 57.1 (10.1) | 54.8 (9.9) | 52.7 (9.2) | 52.3 (9.1) | 52.1 (9.0) | 52.1 (9.1) | ||||||
| BMI at baseline (1986) | 22.8 (2.7) | 24.2 (2.4) | 25.8 (2.3) | 27.5 (3.0) | 29.3 (3.5) | 33.6 (8.9) | ||||||
| <50.0 | 22.0 (2.6) | 23.7 (2.1) | 25.6 (2.2) | 27.3 (2.9) | 29.4 (3.6) | 32.8 (7.6) | ||||||
| 50.0–64.9 | 23.3 (2.7) | 24.5 (2.4) | 26.0 (2.4) | 27.8 (3.0) | 29.3 (3.5) | 33.5 (8.4) | ||||||
| ≥65.0 | 23.4 (2.8) | 24.6 (2.5) | 26.0 (2.5) | 27.1 (2.9) | 28.9 (3.3) | 36.2 (12.9) | ||||||
| BMI at 21 years of age | 17.6 (0.7) | 21.1 (1.2) | 23.8 (0.5) | 25.9 (0.7) | 28.5 (0.7) | 33.6 (6.2) | ||||||
| <50.0 | 17.6 (0.7) | 21.2 (1.1) | 23.8 (0.5) | 25.9 (0.7) | 28.5 (0.7) | 32.9 (5.0) | ||||||
| 50.0–64.9 | 17.6 (0.7) | 21.1 (1.2) | 23.8 (0.5) | 25.9 (0.7) | 28.5 (0.7) | 33.3 (5.9) | ||||||
| ≥65.0 | 17.5 (0.7) | 20.9 (1.2) | 23.8 (0.5) | 25.9 (0.7) | 28.6 (0.6) | 36.4 (8.8) | ||||||
| Weight change since 21 years of age, kg | 16.9 (8.9) | 9.6 (7.7) | 6.5 (7.4) | 5.2 (9.2) | 2.5 (11.1) | −0.9 (16.2) | ||||||
| <50.0 | 14.4 (8.1) | 7.8 (6.8) | 5.8 (7.0) | 4.7 (9.0) | 2.8 (11.3) | −1.0 (15.1) | ||||||
| 50.0–64.9 | 18.4 (9.2) | 10.7 (7.9) | 7.0 (7.6) | 6.2 (9.3) | 2.6 (11.1) | −0.1 (17.5) | ||||||
| ≥65.0 | 18.6 (8.7) | 11.3 (8.2) | 6.9 (7.7) | 3.9 (9.0) | 1.1 (10.4) | −3.4 (15.1) | ||||||
| Family history of MI | 31 | 32 | 33 | 33 | 35 | 39 | ||||||
| <50.0 | 28 | 29 | 29 | 31 | 32 | 35 | ||||||
| 50.0–64.9 | 33 | 36 | 36 | 35 | 36 | 43 | ||||||
| ≥65.0 | 33 | 31 | 33 | 35 | 38 | 35 | ||||||
| Family history of colon cancer | 9 | 8 | 8 | 9 | 9 | 8 | ||||||
| <50.0 | 8 | 6 | 7 | 8 | 6 | 8 | ||||||
| 50.0–64.9 | 10 | 9 | 9 | 10 | 11 | 9 | ||||||
| ≥65.0 | 10 | 8 | 10 | 11 | 10 | 10 | ||||||
| Family history of diabetes | 13 | 14 | 14 | 14 | 16 | 16 | ||||||
| <50.0 | 12 | 12 | 12 | 12 | 13 | 16 | ||||||
| 50.0–64.9 | 14 | 14 | 15 | 15 | 18 | 19 | ||||||
| ≥65.0 | 15 | 16 | 15 | 18 | 20 | 10 | ||||||
| Current smoking | 12 | 10 | 10 | 10 | 11 | 11 | ||||||
| <50.0 | 11 | 9 | 9 | 11 | 13 | 14 | ||||||
| 50.0–64.9 | 13 | 11 | 11 | 10 | 10 | 8 | ||||||
| ≥65.0 | 10 | 8 | 8 | 9 | 8 | 14 | ||||||
| Past smoking | 41 | 42 | 41 | 42 | 46 | 47 | ||||||
| <50.0 | 31 | 33 | 36 | 37 | 38 | 40 | ||||||
| 50.0–64.9 | 47 | 47 | 44 | 45 | 51 | 54 | ||||||
| ≥65.0 | 51 | 51 | 49 | 49 | 51 | 48 | ||||||
| Physical activity, MET hours/week | 15.2 (21.3) | 19.1 (24.1) | 21.1 (25.8) | 20.9 (27.5) | 19.3 (28.1) | 17.8 (22.7) | ||||||
| <50.0 | 16.1 (24.2) | 20.8 (25.9) | 22.8 (28.3) | 23.1 (30.1) | 20.5 (28.5) | 21.2 (26.0) | ||||||
| 50.0–64.9 | 14.7 (19.3) | 18.1 (22.8) | 19.9 (23.2) | 19.7 (24.0) | 18.3 (25.9) | 16.1 (21.1) | ||||||
| ≥65.0 | 14.4 (18.9) | 17.8 (22.7) | 19.9 (26.1) | 18.7 (29.3) | 18.8 (33.0) | 13.9 (15.2) | ||||||
| Alcohol consumption, g/day | 11.7 (17.0) | 11.7 (15.4) | 11.5 (15.4) | 11.2 (15.3) | 10.1 (14.2) | 9.9 (14.8) | ||||||
| <50.0 | 9.8 (15.0) | 10.4 (14.1) | 10.6 (14.0) | 10.4 (14.6) | 9.4 (13.1) | 8.4 (12.3) | ||||||
| 50.0–64.9 | 13.0 (17.7) | 12.7 (16.2) | 12.2 (16.3) | 11.8 (15.9) | 11.1 (15.0) | 11.3 (16.9) | ||||||
| ≥65.0 | 13.5 (19.7) | 11.9 (16.2) | 12.1 (16.6) | 11.3 (15.4) | 9.0 (14.7) | 10.1 (14.3) | ||||||
Abbreviations: BMI, body mass index; MET h/wk, metabolic equivalent hours per week; MI, myocardial infarction; SD, standard deviation.
a P < 0.001 for all continuous variables across categories of BMI (weight (kg)/height (m)2) at 21 years of age, except for age at baseline in participants younger than 50 years of age (P = 0.514) and for MET h/wk in participants 65 years of age or older (P = 0.002) using analysis of variance F test. P < 0.001 for all categorical variables across categories of BMI at age 21 years, except for a family history of colon cancer (overall P = 0.086; for participants 50.0–64.9 years of age, P = 0.023), family history of diabetes (overall P = 0.140; for participants <50.0 years of age, P = 0.042), current smoking (overall P = 0.168; for participants 50.0–64.9 years of age, P = 0.002), and past smoking (overall P = 0.002; for participants ≥65.0 years of age, P = 0.005), using χ2 test.
b Characteristics were reported as current characteristics on the enrollment questionnaire in 1986 when participants were 40–75 years of age except for BMI at age 21 years, which was calculated with the height reported in 1986 and the recalled weight at 21 years of age.
c Data (except for age) have been standardized to the age distribution of the study population. The age categories are <50.0, 50.0–64.9, and ≥65 years.
During a maximum of 22 years (726,084 person-years) of follow-up, we documented 2,970 incident cases of type 2 diabetes mellitus, 4,861 of CVD, and 1,741 of obesity-related cancers. In total, 793 men suffered from multiple events, 24 of whom experienced all 3 events. As a result, the composite end point included 8,755 first events. In the cause-specific analyses, we additionally censored 20 cases who reported 2 events in the same month of follow-up (i.e., it was unknown which of the events occurred first).
Tables 2 and 3 show the incidence rates and hazard ratios of the composite end point and the cause-specific analyses according to BMI at 21 years of age and subsequent weight change. As compared with having a BMI of 18.5–22.9 at 21 years of age, having a BMI of 23 or higher was consistently associated with higher risks of all 3 chronic diseases: type 2 diabetes mellitus, CVD, and cancer (Table 2). A weight gain of 2.5 kg or more during adulthood was associated with a higher risk of both type 2 diabetes mellitus and CVD, but the association between weight gain and obesity-related cancers was weaker (Table 3). For both BMI at age 21 years and adult weight gain, associations for type 2 diabetes were stronger than those for CVD or cancer. With regard to all-cause mortality, having a BMI at age 21 years of 25 or higher was associated with a higher risk than was having a BMI between 18.5 and 22.9. A weight gain of 10 kg or more was associated with a higher risk of all-cause mortality during follow-up as compared with having a stable weight throughout adulthood (Tables 2 and 3). Weight loss during adulthood was associated with lower risks of type 2 diabetes mellitus and cancer but not with lower risks of CVD and all-cause mortality (Table 3). Results after stratification for BMI at 21 years of age are shown in Web Table 1 and described in Web Appendix 2.
Table 2.
Hazard Ratios of Fatal and Nonfatal Obesity-Related Diseases by Categories of BMIa at 21 Years of Age (n = 39,909), Health Professionals Follow-up Study, United States, 1986–2008
| Disease and BMI Category | No. of Events | Age-Adjusted HR | 95% CI | Multivariate HRb | 95% CI | HR Adjusted for Subsequent Weight Changec | 95% CI |
|---|---|---|---|---|---|---|---|
| Obesity-related diseasesd,e | |||||||
| <18.5 | 353 | 1.29 | 1.16, 1.44 | 1.28 | 1.15, 1.43 | 0.99 | 0.88, 1.10 |
| 18.5–22.9 | 3,969 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 23.0–24.9 | 2, 145 | 1.05 | 0.99, 1.10 | 1.05 | 1.00, 1.11 | 1.22 | 1.16, 1.29 |
| 25.0–27.4 | 1,571 | 1.29 | 1.22, 1.37 | 1.29 | 1.21, 1.37 | 1.57 | 1.48, 1.67 |
| 27.5–29.9 | 460 | 1.86 | 1.69, 2.05 | 1.81 | 1.64, 1.99 | 2.40 | 2.17, 2.65 |
| ≥30.0 | 257 | 2.34 | 2.06, 2.65 | 2.27 | 2.00, 2.58 | 3.15 | 2.76, 3.60 |
| Type 2 diabetes mellitus | |||||||
| <18.5 | 113 | 1.58 | 1.30, 1.92 | 1.57 | 1.30, 1.91 | 0.99 | 0.82, 1.21 |
| 18.5–22.9 | 1,080 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 23.0–24.9 | 613 | 1.03 | 0.93, 1.14 | 1.03 | 0.93, 1.14 | 1.36 | 1.23, 1.50 |
| 25.0–27.4 | 603 | 1.67 | 1.51, 1.85 | 1.66 | 1.50, 1.84 | 2.33 | 2.11, 2.58 |
| 27.5–29.9 | 207 | 2.76 | 2.38, 3.20 | 2.64 | 2.27, 3.06 | 4.18 | 3.59, 4.87 |
| ≥30.0 | 119 | 3.53 | 2.92, 4.27 | 3.34 | 2.76, 4.04 | 5.96 | 4.91, 7.24 |
| Cardiovascular disease | |||||||
| <18.5 | 182 | 1.20 | 1.03, 1.40 | 1.19 | 1.02, 1.38 | 1.01 | 0.87, 1.18 |
| 18.5–22.9 | 2,163 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 23.0–24.9 | 1,108 | 1.03 | 0.96, 1.11 | 1.04 | 0.96, 1.12 | 1.14 | 1.06, 1.23 |
| 25.0–27.4 | 697 | 1.11 | 1.02, 1.21 | 1.10 | 1.01, 1.20 | 1.24 | 1.14, 1.36 |
| 27.5–29.9 | 192 | 1.52 | 1.31, 1.76 | 1.49 | 1.28, 1.72 | 1.73 | 1.48, 2.02 |
| ≥30.0 | 99 | 1.77 | 1.45, 2.16 | 1.72 | 1.40, 2.10 | 2.01 | 1.63, 2.48 |
| Obesity-related cancerf | |||||||
| <18.5 | 58 | 1.16 | 0.89, 1.52 | 1.14 | 0.87, 1.49 | 1.07 | 0.81, 1.40 |
| 18.5–22.9 | 715 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 23.0–24.9 | 421 | 1.14 | 1.01, 1.29 | 1.15 | 1.02, 1.30 | 1.20 | 1.06, 1.36 |
| 25.0–27.4 | 268 | 1.23 | 1.07, 1.42 | 1.24 | 1.08, 1.43 | 1.33 | 1.15, 1.54 |
| 27.5–29.9 | 60 | 1.36 | 1.04, 1.77 | 1.36 | 1.04, 1.77 | 1.53 | 1.17, 2.02 |
| ≥30.0 | 37 | 1.88 | 1.35, 2.62 | 1.90 | 1.37, 2.65 | 2.21 | 1.56, 3.12 |
| All-cause mortality | |||||||
| <18.5 | 421 | 1.21 | 1.09, 1.33 | 1.18 | 1.07, 1.31 | 1.07 | 0.97, 1.18 |
| 18.5–22.9 | 4,602 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 23.0–24.9 | 2,115 | 1.02 | 0.97, 1.07 | 1.02 | 0.97, 1.08 | 1.07 | 1.01, 1.13 |
| 25.0–27.4 | 1,385 | 1.15 | 1.08, 1.22 | 1.15 | 1.08, 1.22 | 1.20 | 1.13, 1.29 |
| 27.5–29.9 | 347 | 1.40 | 1.26, 1.56 | 1.38 | 1.24, 1.54 | 1.44 | 1.28, 1.61 |
| ≥30.0 | 191 | 1.73 | 1.50, 2.00 | 1.73 | 1.50, 2.01 | 1.79 | 1.54, 2.09 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
a Weight (kg)/height (m)2. Trend of linearity over BMI categories was tested by modeling the median values of BMI of each category as a continuous variable in all presented models (P < 0.001 for all models).
b Additionally adjusted for cigarette smoking (categories of never smoker, past smoker, and current smoker of 1–4, 5–14, 15–24, 25–34, 35–44, or ≥45 or more cigarettes/day), alcohol consumption (0, 0–5, 5–10, 10–20, 20–30, 30–45, or ≥45 g/day), and family history of myocardial infarction, colon cancer, and diabetes.
c Additionally adjusted for weight change between the age of 21 years and the study baseline (<−5.0, −5.0 to −2.5, −2.4 to 2.4, 2.5 to 4.9, 5.0 to 9.9, 10.0 to 14.9, and ≥15.0 kg).
d Type 2 diabetes mellitus, cardiovascular disease, or obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers).
e Person-time values of obesity-related diseases by category of BMI were 22,168 person-years, 346,976 person-years, 198,378 person-years, 121,347 person-years, 25,612 person-years, and 11,604 person-years, respectively. Incidence rates were 1,592 per 100,000 person-years, 1,144 per 100,000 person-years, 1,081 per 100,000 person-years, 1,295 per 100,000 person-years, 1,796 per 100,000 person-years, and 2,215 per 100,000 person-years, respectively.
f Colorectal, renal, pancreatic, and esophageal cancers.
Table 3.
Hazard Ratios of Fatal and Nonfatal Obesity-Related Diseases by Weight Change Between 21 Years of Age and 1986a (n = 39,909), Health Professionals Follow-up Study, United States, 1986–2008
| Disease and Weight Change, kg | No. of Events | Age-Adjusted HR | 95% CI | Multivariate HRb | 95% CI | HRc Adjusted for BMId at Age 21 Years | 95% CI |
|---|---|---|---|---|---|---|---|
| Obesity-related diseasese,f | |||||||
| <−5.0 | 313 | 1.18 | 1.04, 1.34 | 1.15 | 1.01, 1.30 | 0.77 | 0.68, 0.88 |
| −5.0 to −2.5 | 207 | 0.96 | 0.83, 1.11 | 0.95 | 0.82, 1.10 | 0.81 | 0.70, 0.94 |
| −2.4 to 2.4 | 1,089 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2.5 to 4.9 | 969 | 1.07 | 0.98, 1.17 | 1.08 | 0.99, 1.17 | 1.12 | 1.03, 1.22 |
| 5.0 to 9.9 | 2,051 | 1.34 | 1.25, 1.44 | 1.34 | 1.24, 1.44 | 1.41 | 1.31, 1.52 |
| 10.0 to 14.9 | 1,644 | 1.58 | 1.46, 1.70 | 1.57 | 1.45, 1.70 | 1.72 | 1.59, 1.86 |
| ≥15.0 | 2,482 | 2.24 | 2.09, 2.41 | 2.21 | 2.06, 2.37 | 2.45 | 2.27, 2.63 |
| Type 2 diabetes mellitus | |||||||
| <−5.0 | 54 | 1.01 | 0.75, 1.36 | 0.98 | 0.73, 1.32 | 0.50 | 0.37, 0.68 |
| −5.0 to −2.5 | 40 | 0.93 | 0.66, 1.30 | 0.92 | 0.66, 1.29 | 0.69 | 0.49, 0.97 |
| −2.4 to 2.4 | 223 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2.5 to 4.9 | 211 | 1.17 | 0.97, 1.41 | 1.17 | 0.971.42 | 1.26 | 1.04, 1.52 |
| 5.0 to 9.9 | 605 | 2.02 | 1.74, 2.36 | 2.02 | 1.73, 2.35 | 2.21 | 1.89, 2.58 |
| 10.0 to 14.9 | 525 | 2.67 | 2.28, 3.12 | 2.67 | 2.28, 3.13 | 3.07 | 2.63, 3.60 |
| ≥15.0 | 1,077 | 5.28 | 4.57, 6.11 | 5.19 | 4.49, 6.00 | 6.02 | 5.20, 7.00 |
| Cardiovascular disease | |||||||
| <−5.0 | 193 | 1.31 | 1.12, 1.54 | 1.28 | 1.08, 1.50 | 1.02 | 0.86, 1.21 |
| −5.0 to −2.5 | 125 | 1.03 | 0.85, 1.25 | 1.02 | 0.84, 1.24 | 0.94 | 0.77, 1.14 |
| −2.4 to 2.4 | 599 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2.5 to 4.9 | 543 | 1.07 | 0.96, 1.21 | 1.08 | 0.96, 1.22 | 1.11 | 0.98, 1.24 |
| 5.0 to 9.9 | 1,059 | 1.23 | 1.11, 1.36 | 1.23 | 1.11, 1.36 | 1.27 | 1.15, 1.40 |
| 10.0 to 14.9 | 847 | 1.41 | 1.27, 1.57 | 1.41 | 1.27, 1.57 | 1.48 | 1.33, 1.65 |
| ≥15.0 | 1,075 | 1.68 | 1.51, 1.85 | 1.66 | 1.50, 1.84 | 1.76 | 1.59, 1.95 |
| Obesity-related cancerg | |||||||
| <−5.0 | 64 | 0.99 | 0.75, 1.30 | 0.97 | 0.74, 1.27 | 0.78 | 0.58, 1.03 |
| −5.0 to −2.5 | 42 | 0.79 | 0.57, 1.10 | 0.80 | 0.58, 1.10 | 0.73 | 0.53, 1.01 |
| −2.4 to 2.4 | 266 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2.5 to 4.9 | 214 | 0.96 | 0.81, 1.16 | 0.96 | 0.80, 1.15 | 0.98 | 0.82, 1.18 |
| 5.0 to 9.9 | 382 | 1.01 | 0.86, 1.18 | 0.99 | 0.85, 1.16 | 1.03 | 0.88, 1.21 |
| 10.0 to 14.9 | 269 | 1.03 | 0.87, 1.22 | 1.01 | 0.85, 1.20 | 1.07 | 0.90, 1.27 |
| ≥15.0 | 322 | 1.15 | 0.97, 1.35 | 1.11 | 0.94, 1.31 | 1.19 | 1.00, 1.41 |
| All-cause mortality | |||||||
| <−5.0 | 422 | 1.29 | 1.16, 1.44 | 1.27 | 1.14, 1.42 | 1.07 | 0.96, 1.21 |
| −5.0 to −2.5 | 283 | 1.04 | 0.91, 1.18 | 1.04 | 0.91, 1.18 | 0.98 | 0.86, 1.11 |
| −2.4 to 2.4 | 1,311 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 2.5 to 4.9 | 1,097 | 0.96 | 0.88, 1.04 | 0.96 | 0.89, 1.04 | 0.98 | 0.90, 1.06 |
| 5.0 to 9.9 | 2,095 | 1.02 | 0.95, 1.09 | 1.02 | 0.95, 1.09 | 1.05 | 0.98, 1.12 |
| 10.0 to 14.9 | 1,609 | 1.06 | 0.99, 1.15 | 1.05 | 0.98, 1.13 | 1.09 | 1.02, 1.18 |
| ≥15.0 | 2,244 | 1.28 | 1.19, 1.37 | 1.26 | 1.18, 1.35 | 1.31 | 1.22, 1.41 |
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
a Trend of linearity over weight-change categories was tested by modeling the median values of BMI of each category as continuous variable in all presented models (P < 0.001 for all models, except for age-adjusted model of obesity-related cancer, for which P = 0.009).
b Additionally adjusted for cigarette smoking (categories of never smoker, past smoker, and current smoker of 1–4, 5–14, 15–24, 25–34, 35–44, or ≥45 or more cigarettes/day), alcohol consumption (0, 0–5, 5–10, 10–20, 20–30, 30–45, or ≥45 g/day), and family history of myocardial infarction, colon cancer, and diabetes.
c Additionally adjusted for BMI at 21 years of age (<18.5, 18.5–22.9, 23–24.9, 25.0–27.4, 27.5–29.9, and ≥30).
d Weight (kg)/height (m)2.
e Type 2 diabetes mellitus, cardiovascular disease, or obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers).
f Person-time values of obesity-related diseases by category of weight change were 32,878 person-years, 26,736 person-years, 139,634 person-years, 111,889 person-years, 180,498 person-years, 116,565 person-years, and 117,884 person-years, respectively. Incidence rates were 952, 774 per 100,000 person-years, 780, 866 per 100,000 person-years, 1,136 per 100,000 person-years, 1,410 per 100,000 person-years, and 2,105 per 100,000 person-years, respectively.
g Colorectal, renal, pancreatic, and esophageal cancers.
After excluding the weight change that occurred during the 5 years before baseline, adult weight gain became more strongly associated with a higher risk of obesity-related cancer; hazard ratios were 1.16 (95% CI: 0.98, 1.37) for a weight gain of 10.0–14.9 kg and 1.46 (95% CI: 1.23, 1.73) for a weight gain of 15 kg or more as compared with having a stable weight. Associations between adult weight change and the other disease outcomes remained similar in this sensitivity analysis (data not shown). Restriction of the study population to never smokers (n = 18,319), additional adjustment for physical activity level and dietary factors at the baseline of the study, or exclusion of esophageal cancer from the composite outcome did not materially alter the results (data not shown).
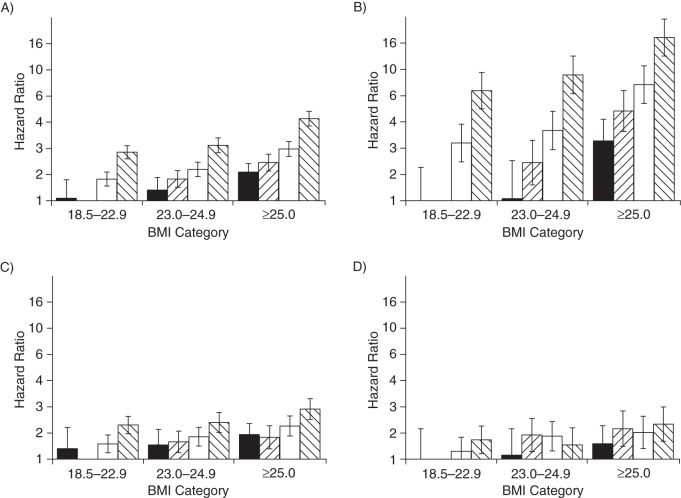
We evaluated the joint associations of BMI at the age of 21 years and adult weight change with the risk of chronic diseases (Figure 1A). First, we compared men with different BMIs at age 21 years who kept a stable weight throughout adulthood. As compared with a stable BMI between 18.5 and 22.9 throughout adulthood, a stable BMI of 23–24.9 was associated with a hazard ratio of 1.47 (95% CI: 1.27, 1.71) and a stable BMI of 25 or more was associated with a hazard ratio of 1.96 (95% CI: 1.69, 2.28). Second, we evaluated whether adult weight gain was associated with a higher risk of chronic disease even in men who had a low BMI at age 21 years. As compared with a stable low BMI (18.5–22.9) throughout adulthood, a weight gain of 2.5–9.9 kg was associated with a hazard ratio of 1.47 (95% CI: 1.30, 1.66), and a weight gain of more than 10 kg with a hazard ratio of 2.35 (95% CI: 2.09, 2.64) in men with a BMI of 18.5–22.9 at age 21 years. Third, we evaluated the combination of being overweight at 21 years of age and having a large weight gain. As compared with men who had a stable low BMI (18.5–22.9), men who had a BMI of 25 or more at 21 years of age and who gained more than 10 kg had a hazard ratio of 4.24 (95% CI: 3.73, 4.83). Overall, the relative importance of early weight and weight change differed by disease, with weight gain being more strongly associated with risk of type 2 diabetes mellitus and BMI in early adulthood more strongly associated with risk of cancers (Figure 1B–D). A stable low BMI was consistently associated with lowest risk of chronic diseases.
Figure 1.
Joint associations between body mass index (BMI) at 21 years of age and adult weight change with fatal and nonfatal obesity-related diseases (A), type 2 diabetes mellitus (B), cardiovascular disease (C), and obesity-related cancer (D) among 38,613 participants in the Health Professionals Follow-up Study, United States, 1986–2008. Obesity-related diseases included type 2 diabetes mellitus, cardiovascular disease, and obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers). Analyses excluded participants with a BMI <18.5 (n = 1,296). Values are hazard ratios from Cox proportional hazards model. Models were adjusted for cigarette smoking (categories of never smoker, past smoker, and current smoker of 1–4, 5–14, 15–24, 25–34, 35–44, or ≥45 or more cigarettes/day), alcohol consumption (0, 0–5, 5–10, 10–20, 20–30, 30–45, or ≥45 g/day), and family history of myocardial infarction, colon cancer, and diabetes.  , an adult weight change <−2.5 kg;
, an adult weight change <−2.5 kg;  , adult weight change of −2.4 to 2.4 kg;
, adult weight change of −2.4 to 2.4 kg;  , adult weight change of 2.5 to 9.9 kg; and
, adult weight change of 2.5 to 9.9 kg; and  , adult weight change ≥10.0 kg. Participants with a BMI of 18.5–22.9 at 21 years of age who maintained a stable weight are the reference category. Bars, 95% confidence intervals.
, adult weight change ≥10.0 kg. Participants with a BMI of 18.5–22.9 at 21 years of age who maintained a stable weight are the reference category. Bars, 95% confidence intervals.
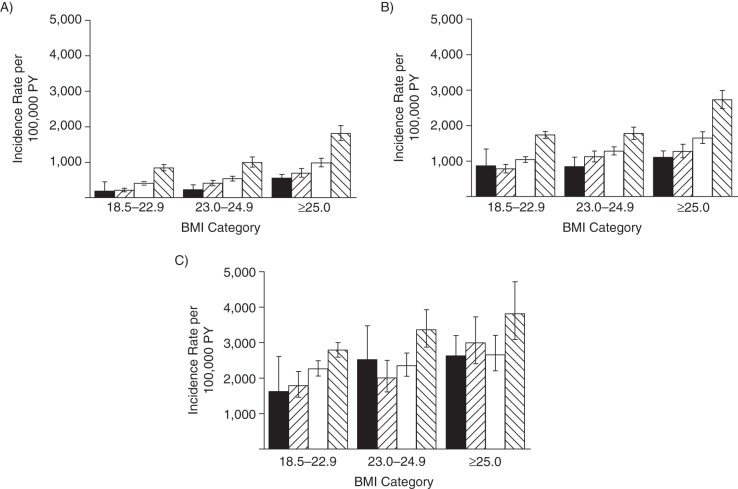
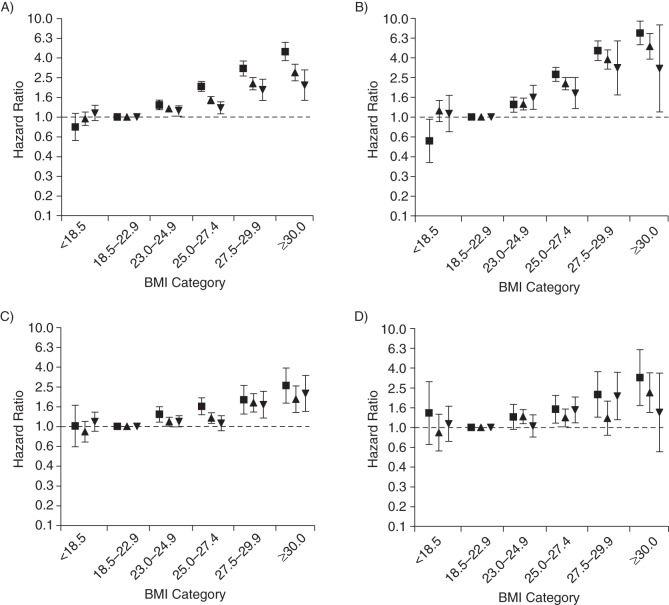
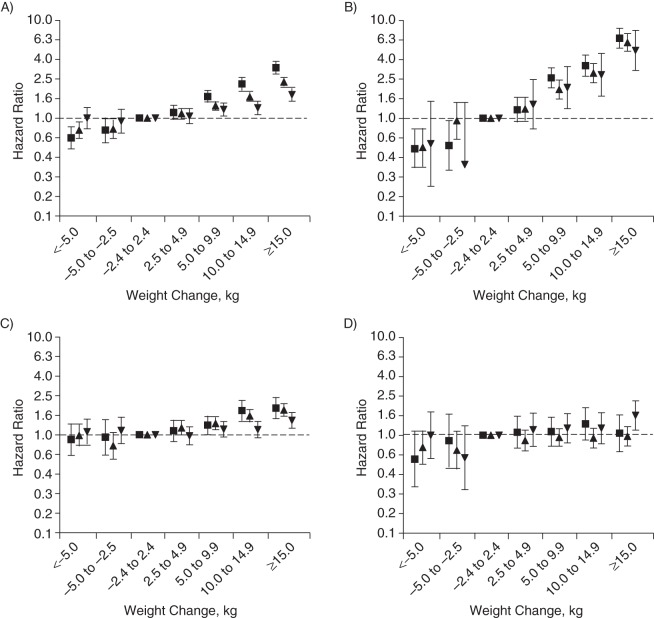
Because the studied associations differed by age (P < 0.001 for interaction), we also present the results stratified by baseline age (Figures 2–4). Although it must be noted that the patterns of associations for BMI at age 21 years and adult weight change in relation to risk of chronic diseases were consistent in the different age groups, baseline age certainly influenced the magnitude of the observed associations. With regard to relative risks, hazard ratios for chronic diseases were generally greater in younger men than in older men (Figures 3 and 4). However, the absolute incidence rates show that the excess incidence of the chronic diseases related to higher BMI at 21 years of age and weight gain during adulthood was greatest in men older than 65 years at baseline (Figure 2).
Figure 2.
Incidence rates per 100,000 person-years (PY) of fatal and nonfatal obesity-related diseases among 38,613 participants in the Health Professionals Follow-up Study, United States, 1986–2008. Rates are displayed by body mass index (BMI) at 21 years of age and adult weight change per age group: A) less than 50 years of age at baseline (n = 15,692), B) 50–64.9 years of age at baseline (n = 17,190) and C) 65 years of age or older at baseline (n = 5,731).  , an adult weight change <−2.5 kg;
, an adult weight change <−2.5 kg;  , adult weight change of −2.4 to 2.4 kg;
, adult weight change of −2.4 to 2.4 kg;  , adult weight change of 2.5 to 9.9 kg; and
, adult weight change of 2.5 to 9.9 kg; and  , adult weight change ≥10.0 kg. Analyses excluded participants with a BMI less than 18.5 (n = 1,296). Obesity-related diseases included fatal or nonfatal type 2 diabetes, cardiovascular diseases, or obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers). Bars, 95% confidence intervals.
, adult weight change ≥10.0 kg. Analyses excluded participants with a BMI less than 18.5 (n = 1,296). Obesity-related diseases included fatal or nonfatal type 2 diabetes, cardiovascular diseases, or obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers). Bars, 95% confidence intervals.
Figure 3.
Hazard ratios of fatal and nonfatal obesity-related diseases (A), type 2 diabetes mellitus (B), cardiovascular disease (C), and obesity-related cancer (D) by body mass index (BMI) at 21 years of age among 39,909 participants in the Health Professionals Follow-up Study, United States, 1986–2008. ▪, <50.0 years of age at baseline (n = 16,064); ▴, 50.0–64.9 years of age at baseline (n = 17,777); ▾, ≥65.0 years of age at baseline (n = 6,068). Obesity-related diseases included type 2 diabetes mellitus, cardiovascular diseases, and obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers). Models were adjusted for weight change between the age of 21 years and the baseline of the study (<−5.0, −5.0 to −2.5, −2.4 to 2.4, 2.5 to 4.9, 5.0 to 9.9, 10.0 to 14.9, and ≥15.0 kg), cigarette smoking (categories of never smoker, past smoker, and current smoker of 1–4, 5–14, 15–24, 25–34, 35–44, or ≥45 or more cigarettes/day), alcohol consumption (0, 0–5, 5–10, 10–20, 20–30, 30–45, or ≥45 g/day), and family history of myocardial infarction, colon cancer, and diabetes. Bars, 95% confidence intervals.
Figure 4.
Hazard ratios of fatal and nonfatal obesity-related diseases (A), type 2 diabetes mellitus (B), cardiovascular disease (C), and obesity-related cancer (D) by adult weight change in kilograms per age group among 39,909 participants in the Health Professionals Follow-up Study, United States, 1986–2008. ▪, <50 years of age at baseline (n = 16,064); ▴, 50–65 years of age at baseline (n = 17,777); ▾, ≥65 years of age at baseline (n = 6,068). Obesity-related diseases included type 2 diabetes mellitus, cardiovascular diseases, and obesity-related cancer (colorectal, renal, pancreatic, and esophageal cancers). Models were adjusted for BMI at 21 years of age (<18.5, 18.5–22.9, 23–24.9, 25–27.4, 27.5–29.9, ≥30), cigarette smoking (categories of never smoker, past smoker, and current smoker of 1–4, 5–14, 15–24, 25–34, 35–44, or ≥45 or more cigarettes/day), alcohol consumption (0, 0–5, 5–10, 10–20, 20–30, 30–45, or ≥45 g/day), and family history of myocardial infarction, colon cancer, and diabetes. Bars, 95% confidence intervals.
DISCUSSION
In the present large cohort study of men from the United States, moderate overweight in early adulthood and adult weight gain were substantially and independently associated with a higher risk of major chronic diseases, including type 2 diabetes mellitus, CVD (myocardial infarction and stroke), and cancers of the colon, rectum, kidney, pancreas, and esophagus during 22 years of follow-up. The relative importance of BMI in early adulthood and weight change appeared to differ by disease. Cancers were more strongly associated with BMI in early adulthood, whereas type 2 diabetes mellitus was more strongly associated with adult weight gain. Moderate overweight in early adulthood and adult weight gain were also associated with a higher risk of all-cause mortality. We furthermore observed that men who were lean at the age of 21 years and did not gain weight during adulthood consistently had the lowest risk of the composite outcome and the separate chronic diseases.
To evaluate the overall health impact of adiposity in early adulthood and weight change during adulthood, we used a composite outcome that included several major chronic diseases. Most other studies investigating associations of adiposity in adolescence or adult weight change were limited to 1 or a few health outcomes. Obesity in adolescence and early adulthood has been associated with increased risks of type 2 diabetes (13, 15), coronary heart disease (15, 17, 31–35), and stroke (32). The health consequences of moderate overweight in early adulthood are less clear. A study of 3 historical cohorts in the United Kingdom reported no significant association of being overweight early in life with future ischemic heart disease and stroke, but that study included very few overweight participants (14). The Harvard Growth Study investigated a variety of diseases related to being overweight in adolescence and reported higher risks of coronary heart disease and colorectal cancer in men (3). Whereas the health effects of adult overweight have been controversial (36, 37), 3 mega studies of prospective cohorts that each included approximately a million participants consistently showed that mortality risk was generally lowest with an adult BMI between 20 and 25 in white adults (38, 39). Our results indicate that BMI values in early adulthood at the higher end of these ranges are already associated with substantially higher risks of morbidity and mortality as compared with lower BMI values.
In our study, even modest adult weight gain was associated with higher risks of CVD and type 2 diabetes mellitus, with a particular strong association for diabetes. Large adult weight gain (more than 10–15 kg) was also associated with a higher risk of obesity-related cancers and all-cause mortality. These results are in agreement with those from previous studies, some in the same cohort, that showed that weight gain was associated with increased risks of coronary heart disease (11, 40–43), stroke (16, 41), and most strongly type 2 diabetes mellitus (13, 19, 20). Weight gain during adulthood has also been associated with increased risks of all-cause mortality (5, 11, 21) and mortality due to CVD (9, 18, 21) and certain cancers (9, 18, 21). Our results are also consistent with those from a recent study that showed that coronary heart disease was more closely associated with adolescent BMI and that diabetes was mainly associated with having a high BMI close to the time of diagnosis (16). This supports the hypothesis that processes that cause cancer and atherosclerosis are gradual, whereas the effects of adiposity on insulin resistance and type 2 diabetes may occur more rapidly.
We observed a lower risk of obesity-related diseases associated with a weight loss of 2.5 kg or more during adulthood compared with keeping a stable weight. This reduced risk was mainly due to a markedly lower risk of type 2 diabetes. A recent study showed that persons who were overweight or obese as children but who became nonobese as adults had cardiovascular risk profiles that were similar to those of persons who were never obese (44). We, however, observed that weight loss during adulthood could not completely negate the excess risk of type 2 diabetes, CVD, or cancer in persons who were overweight in early adulthood.
The associations of BMI in early adulthood and subsequent weight change with risk of obesity-related diseases appeared to be modified by age, with higher relative risks in younger participants and higher incidence rates in participants who were older at baseline. Strengths of the present study include the large sample size, the prospective study design, the long follow-up period, and the high response rate during follow-up. Our study also has several potential limitations that need to be considered. First, our analyses relied on self-reported and recalled weight instead of measured weight. Validation studies in this cohort and other cohorts, however, have shown high accuracy of self-reported and recalled weight as compared with measured weight (22, 45–47). The tendency to slightly underreport body weight may have resulted in some overestimation of disease risks in the higher BMI categories in our study (48). However, because underreporting of body weight occurs mainly in obese men (48), this may not have affected our results in the nonobese categories. Second, we were not able to adjust for lifestyle variables at 21 years of age. Adjustment for lifestyle at baseline did not appreciably change the results, but we cannot fully exclude the possibility of residual confounding due to effects of lifestyle at age 21 that are independent of lifestyle at baseline. Third, BMI is not a perfect measure of adiposity because it also reflects variation in lean body mass. In particular, adult weight loss until the baseline of our study may have been affected by poor health (49). Not considering the weight change that occurred during the 5 years before baseline did not materially change the associations between weight change and the combined obesity-related diseases, whereas the association for obesity-related cancers even became stronger. Fourth, between participants, there was a substantial difference in the number of years between age 21 years and the baseline of the study. However, our results were essentially the same for younger and older men. Hence, our study provides information of the impact of adiposity on future morbidity and mortality in men who reach middle age. Finally, our study population included men who were predominantly white, and our findings thus require confirmation in women and in other ethnic groups.
Overweight in childhood and adolescence often continues into adulthood (1, 50, 51) and is associated with cardiovascular risk factors (10, 52–54). The full impact of the current increase in prevalence of childhood obesity can be expected to only become evident decades later with a high incidence of chronic disease and reduced life expectancy (55).
Our results indicate that weight management in adulthood should not be restricted to high-risk groups with obesity but should also target persons who are modestly overweight. Our results furthermore imply that weight management in adulthood may be more effective for reducing the risk of type 2 diabetes mellitus but that for the risk of cancer in men, BMI in early adulthood is more important than subsequent weight change. For the reduction of the overall risk of chronic diseases, our results underscore the pivotal importance of prevention of excess weight gain in both adolescence and adulthood.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Clinical Epidemiology, Leiden University and Medical Center, Leiden, the Netherlands (Renée de Mutsert); Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Renée de Mutsert, Qi Sun, Walter C. Willett, Frank B. Hu, Rob M. van Dam); Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (Qi Sun, Walter C. Willett, Frank B. Hu); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Walter C. Willett, Frank B. Hu); and Saw Swee Hock School of Public Health and Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore (Rob M. van Dam).
This work was supported by the National Institutes of Health (grants PO1-CA055075, DK58845, P30 DK46200, and U54CA155626), the Netherlands Organization for Scientific Research (NWO) (Rubicon fellowship to Renée de Mutsert), and the National Heart, Lung, and Blood Institute (career development award K99HL098459 to Qi Sun).
We thank the staff of the Health Professionals Follow-Up Study for their valuable contributions.
Preliminary results of this study were presented at the 17th European Congress of Obesity, Amsterdam, The Netherlands, May 6–9, 2009, and published in abstract form (Obes Facts. 2009;2(suppl 2):10).
Conflict of interest: none declared.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmans MD, Kromhout D, de Lezenne CC. The impact of body mass index of 78,612 18-year old Dutch men on 32-year mortality from all causes. J Clin Epidemiol. 1988;41(8):749–756. doi: 10.1016/0895-4356(88)90161-8. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Jacques PF, Dallal GE, et al. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327(19):1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 4.Stevens J, Truesdale KP, Wang CH, et al. Body mass index at age 25 and all-cause mortality in whites and African Americans: The Atherosclerosis Risk in Communities Study. J Adolesc Health. 2012;50(3):221–227. doi: 10.1016/j.jadohealth.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosengren A, Wedel H, Wilhelmsen L. Body weight and weight gain during adult life in men in relation to coronary heart disease and mortality. A prospective population study. Eur Heart J. 1999;20(4):269–277. [PubMed] [Google Scholar]
- 6.van Dam RM, Willett WC, Manson JE, et al. The relationship between overweight in adolescence and premature death in women. Ann Intern Med. 2006;145(2):91–97. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 7.Bjorge T, Engeland A, Tverdal A, et al. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168(1):30–37. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 8.Engeland A, Bjørge T, Tverdal A, et al. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology. 2004;15(1):79–85. doi: 10.1097/01.ede.0000100148.40711.59. [DOI] [PubMed] [Google Scholar]
- 9.Shimazu T, Kuriyama S, Ohmori-Matsuda K, et al. Increase in body mass index category since age 20 years and all-cause mortality: a prospective cohort study (the Ohsaki Study) Int J Obes (Lond) 2009;33(4):490–496. doi: 10.1038/ijo.2009.29. [DOI] [PubMed] [Google Scholar]
- 10.Neovius M, Sundstrom J, Rasmussen F. Combined effects of overweight and smoking in late adolescence on subsequent mortality: nationwide cohort study. BMJ. 2009;338:b496. doi: 10.1136/bmj.b496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yarnell JW, Patterson CC, Thomas HF, et al. Comparison of weight in middle age, weight at 18 years, and weight change between, in predicting subsequent 14 year mortality and coronary events: Caerphilly Prospective Study. J Epidemiol Community Health. 2000;54(5):344–348. doi: 10.1136/jech.54.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuemmeler BF, Pendzich MK, Tercyak KP. Weight, dietary behavior, and physical activity in childhood and adolescence: implications for adult cancer risk. Obes Facts. 2009;2(3):179–186. doi: 10.1159/000220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Lawlor DA, Martin RM, Gunnell D, et al. Association of body mass index measured in childhood, adolescence, and young adulthood with risk of ischemic heart disease and stroke: findings from 3 historical cohort studies. Am J Clin Nutr. 2006;83(4):767–773. doi: 10.1093/ajcn/83.4.767. [DOI] [PubMed] [Google Scholar]
- 15.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364(14):1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rexrode KM, Hennekens CH, Willett WC, et al. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277(19):1539–1545. doi: 10.1001/jama.1997.03540430051032. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273(6):461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 18.Jeffreys M, McCarron P, Gunnell D, et al. Body mass index in early and mid-adulthood, and subsequent mortality: a historical cohort study. Int J Obes Relat Metab Disord. 2003;27(11):1391–1397. doi: 10.1038/sj.ijo.0802414. [DOI] [PubMed] [Google Scholar]
- 19.Koh-Banerjee P, Wang Y, Hu FB, et al. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol. 2004;159(12):1150–1159. doi: 10.1093/aje/kwh167. [DOI] [PubMed] [Google Scholar]
- 20.Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17(9):961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Willett WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7(1):81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 25.James WPT, Jackson-Leach R, Mhurchu CN, et al. Overweight and obesity (high body mass index) In: Ezzati M, Lopez AD, Rodgers A, et al., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004. pp. 497–596. [Google Scholar]
- 26.World Cancer Research Fund/American Institute for Cancer Research. Washington, DC: American Institute for Cancer Research; 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. [Google Scholar]
- 27.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 28.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 29.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 30.Koller MT, Raatz H, Steyerberg EW, et al. Competing risks and the clinical community: irrelevance or ignorance? Stat Med. 2012;31(11-12):1089–1097. doi: 10.1002/sim.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bibbins-Domingo K, Coxson P, Pletcher MJ, et al. Adolescent overweight and future adult coronary heart disease. N Engl J Med. 2007;357(23):2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 32.Falkstedt D, Hemmingsson T, Rasmussen F, et al. Body mass index in late adolescence and its association with coronary heart disease and stroke in middle age among Swedish men. Int J Obes (Lond) 2007;31(5):777–783. doi: 10.1038/sj.ijo.0803480. [DOI] [PubMed] [Google Scholar]
- 33.Owen CG, Whincup PH, Orfei L, et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes (Lond) 2009;33(8):866–877. doi: 10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray L, Lee IM, Sesso HD, et al. Body weight in early and mid-adulthood in relation to subsequent coronary heart disease mortality: 80-year follow-up in the Harvard Alumni Study. Arch Intern Med. 2011;171(19):1768–1770. doi: 10.1001/archinternmed.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galanis DJ, Harris T, Sharp DS, et al. Relative weight, weight change, and risk of coronary heart disease in the Honolulu Heart Program. Am J Epidemiol. 1998;147(4):379–386. doi: 10.1093/oxfordjournals.aje.a009460. [DOI] [PubMed] [Google Scholar]
- 36.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Kit BK, Orpana H, et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chei CL, Iso H, Yamagishi K, et al. Body mass index and weight change since 20 years of age and risk of coronary heart disease among Japanese: the Japan Public Health Center-Based Study. Int J Obes (Lond) 2008;32(1):144–151. doi: 10.1038/sj.ijo.0803686. [DOI] [PubMed] [Google Scholar]
- 41.Stevens J, Erber E, Truesdale KP, et al. Long- and short-term weight change and incident coronary heart disease and ischemic stroke: The Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2013;178(2):239–248. doi: 10.1093/aje/kws461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rimm EB, Stampfer MJ, Giovannucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117–1127. doi: 10.1093/oxfordjournals.aje.a117385. [DOI] [PubMed] [Google Scholar]
- 43.Colditz GA, Coakley E. Weight, weight gain, activity, and major illnesses: the Nurses' Health Study. Int J Sports Med. 1997;18(suppl 3):S162–S170. doi: 10.1055/s-2007-972709. [DOI] [PubMed] [Google Scholar]
- 44.Juonala M, Magnussen CG, Berenson GS, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 45.Tamakoshi K, Yatsuya H, Kondo T, et al. The accuracy of long-term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord. 2003;27(2):247–252. doi: 10.1038/sj.ijo.802195. [DOI] [PubMed] [Google Scholar]
- 46.Perry GS, Byers TE, Mokdad AH, et al. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6(1):61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 48.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 2007;15(1):188–196. doi: 10.1038/oby.2007.504. [DOI] [PubMed] [Google Scholar]
- 49.Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23(6):603–611. doi: 10.1038/sj.ijo.0800875. [DOI] [PubMed] [Google Scholar]
- 50.The NS, Suchindran C, North KE, et al. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304(18):2042–2047. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patton GC, Coffey C, Carlin JB, et al. Overweight and obesity between adolescence and young adulthood: a 10-year prospective cohort study. J Adolesc Health. 2011;48(3):275–280. doi: 10.1016/j.jadohealth.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 52.Lawlor DA, Benfield L, Logue J, et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010;341:c6224. doi: 10.1136/bmj.c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tybor DJ, Lichtenstein AH, Dallal GE, et al. Independent effects of age-related changes in waist circumference and BMI z scores in predicting cardiovascular disease risk factors in a prospective cohort of adolescent females. Am J Clin Nutr. 2011;93(2):392–401. doi: 10.3945/ajcn.110.001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freedman DS, Katzmarzyk PT, Dietz WH, et al. Relation of body mass index and skinfold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr. 2009;90(1):210–216. doi: 10.3945/ajcn.2009.27525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361(23):2252–2260. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.