Abstract
A growing body of evidence supports an association between vitamin D and cardiovascular disease. However, the mechanisms underlying this association are unknown. From 2000 to 2002, we identified 946 participants with stable cardiovascular disease in San Francisco, California, and followed them prospectively for cardiovascular events (heart failure, myocardial infarction, stroke, or cardiovascular death). We then examined the extent to which the association was attenuated by adjustment for poor health behaviors, comorbid health conditions, and potential biological mediators. During a median follow-up period of 8.0 years (through August 24, 2012), 323 subjects (34.1%) experienced a cardiovascular event. Following adjustment for sociodemographic factors, season of blood measurement, health behaviors, and comorbid conditions, 25-hydroxyvitamin D levels under 20 ng/mL remained independently associated with cardiovascular events (hazard ratio = 1.30, 95% confidence interval: 1.01, 1.67). However, after further adjustment for potential biological mediators, the independent association was no longer present (hazard ratio = 1.11, 95% confidence interval: 0.85, 1.44). Parathyroid hormone, a potentially modifiable biological factor downstream from 25-hydroxyvitamin D, was responsible for the majority of this attenuation. These findings highlight the need for randomized controlled trials to determine whether vitamin D supplementation in persons with deficiency could be beneficial for the primary or secondary prevention of cardiovascular events.
Keywords: cardiovascular events, coronary heart disease, nutrition, vitamin D, vitamin D deficiency
Editor's note:An invited commentary on this article appears on page 1288, and the authors’ response appears on page 1291.
Vitamin D deficiency is highly prevalent; approximately 25%–57% of the US population (1) and more than 1 billion persons worldwide (2) are either insufficient (defined as serum 25-hydroxyvitamin D (25(OH)D) levels of 20–29.9 ng/mL) or deficient (defined as serum 25(OH)D levels under 20 ng/mL) in vitamin D. Several lines of evidence support the hypothesis that vitamin D deficiency may contribute to cardiovascular disease (CVD) (3). Ecological studies have demonstrated a higher burden of hypertension and CVD at greater distances from the equator (4, 5). Observational studies have shown inverse associations between 25(OH)D levels and hypertension (6, 7), body mass index (8), congestive heart failure (9, 10), myocardial infarction (11–13), stroke (12, 14), peripheral arterial disease (15, 16), mortality (13, 17) and combined cardiovascular events (18, 19). A recent meta-analysis suggested that vitamin D supplementation at moderate-to-high doses may reduce CVD risk (20).
However, it is unclear whether vitamin D deficiency is simply a marker for poor health or whether this association is mediated by biological changes that increase risk of cardiovascular events. For example, persons with vitamin D deficiency may have comorbid health conditions or poor health behaviors which cause them to stay indoors. Therefore, vitamin D deficiency could simply be a surrogate for poor health. Alternatively, however, a variety of plausible biological mechanisms (blood pressure elevation (21), insulin resistance (22), inflammation (23), obesity (8), endothelial dysfunction (24), or vascular remodeling due to hyperparathyroidism (25)) have been proposed by which vitamin D deficiency may cause cardiovascular events.
A better understanding of the mechanisms underlying the association between vitamin D deficiency and CVD would be informative for determining the potential benefit of vitamin D supplementation. If low 25(OH)D levels simply identify persons with poor overall health, vitamin D supplementation is unlikely to be effective in the primary or secondary prevention of CVD. Alternatively, if low vitamin D levels produce biological changes which mediate CVD risk, repletion of vitamin D could have substantial public health benefit. Such relationships may be particularly beneficial in secondary prevention, as the risks of recurrent CVD and death are much higher in populations with established CVD. Therefore, in a population of outpatients with stable coronary heart disease (CHD), we evaluated the association between 25(OH)D levels and cardiovascular events and sought to elucidate the potential mediators underlying this association.
METHODS
The Heart and Soul Study is a prospective cohort study originally designed to investigate psychosocial factors and health outcomes in patients with stable CHD. Details regarding recruitment methods and study design have been published previously (26, 27). In brief, 1,024 outpatients with stable CHD were recruited from 12 outpatient clinics in the San Francisco Bay Area. Eligible participants met 1 or more of the following criteria: 1) history of myocardial infarction; 2) evidence of at least 50% stenosis in one or more coronary vessels upon cardiac catheterization; 3) evidence of exercise-induced ischemia by treadmill electrocardiogram or nuclear perfusion stress imaging; or 4) a history of coronary revascularization. Subjects were excluded if they had a history of myocardial infarction in the previous 6 months, were unable to walk 1 block, or were planning to move out of the local area within 3 years. The study was approved by the institutional review boards of the University of California, San Francisco, and the San Francisco VA Medical Center, and all participants provided written informed consent.
Between September 2000 and December 2002, all participants attended a baseline study appointment which included completion of a medical history, a physical examination, and a comprehensive health status questionnaire. Of the 1,024 original study participants, we excluded 78 with missing covariate data. The remaining 946 patients were followed for cardiovascular events through August 24, 2012.
25(OH)D levels
The night prior to the baseline examination, participants completed an overnight (12-hour) fast, except for taking their regularly prescribed medications. Blood samples were drawn into chilled tubes containing ethylenediaminetetraacetic acid; plasma was aliquoted and stored at −70°C until April 2011. We used an API 5000 LC/MS/MS mass spectrometer (Ab Sciex, Framingham, Massachusetts) to measure 25-hydroxyvitamin D3 and D2 levels. The analytical measuring range for the 25(OH)D3 and 25(OH)D2 assays was 5–100 ng/mL. The intraassay coefficient of variation of both assays at a concentration of 40 ng/mL was 3.6%; the interassay coefficient of variation for both assays was 8.9%. Total 25(OH)D levels (referred to throughout this article as 25(OH)D) were calculated by the addition of 25(OH)D2 and 25(OH)D3 levels.
Cardiovascular events
The primary outcome was any cardiovascular event, which was defined as a composite of hospitalization for myocardial infarction, stroke, heart failure, or cardiovascular mortality. We also evaluated the individual outcomes of myocardial infarction, stroke, heart failure, cardiovascular mortality, and all-cause mortality as secondary outcomes. We conducted annual follow-up interviews with participants or their proxies to inquire about interval death or hospitalization for “heart trouble.” For any reported event, we retrieved medical records, which 2 independent and blinded physician adjudicators reviewed. If the adjudicators agreed on the outcome classification, their classification was binding. In the event of a disagreement, a third blinded adjudicator was consulted.
Myocardial infarction was defined using the American Heart Association diagnostic criteria (28). Stroke was defined as a new neurological deficit not known to be secondary to brain trauma, tumor, infection, or other cause. Heart failure was defined as hospitalization for a clinical syndrome based on the Framingham heart failure criteria (28). Cardiovascular mortality was defined as a death which occurred as a result of a cardiovascular cause. Cardiovascular mortality and all-cause mortality were determined by review of death certificates.
Other patient characteristics
Age, sex, race/ethnicity, and medical history were self-reported. Season of the year in which the blood draw occurred was recorded. Weight and height were measured, and body mass index (weight (kg)/height (m)2) was calculated. Estimated glomerular filtration rate was calculated using the combined creatinine-cystatin C equation (29). Medication use was recorded by study personnel. Depression was assessed by means of Patient Health Questionnaire 2. Tobacco use, multivitamin use, educational level, and physical activity (defined as exercise performed at least 1–2 times per week) were self-reported. Medication nonadherence was defined as a self-report of taking prescribed medications 75% of the time or less (30).
Potential biological mediators
Blood pressure was measured in the supine position after 5 minutes of rest. Lipids (mg/dL) and levels of hemoglobin A1c (%), C-reactive protein (mg/dL), parathyroid hormone (mg/dL), calcium (mg/dL), albumin (g/dL), and phosphorus (mg/dL) were measured from the fasting blood samples. Fibroblast growth factor 23 (relative units/mL) was also measured from fasting blood samples, as previously described in detail (31, 32). Urinary albumin:creatinine ratio (mg/g) was measured from 24-hour urine specimens as previously described (33).
Statistical analyses
We first performed exploratory analyses using an age-adjusted cubic spline, which revealed that the association was nonlinear, with a sharp increase in CVD risk at 25(OH)D levels less than 20 ng/mL. Therefore, we chose a cutpoint of 20 ng/mL for our analysis. This value also corresponds to a clinically relevant cutpoint (deficiency is currently defined by most experts as a 25(OH)D level less than 20 ng/mL) (2).
We compared baseline differences in participant characteristics by 25(OH)D level across categories using χ2 tests for dichotomous variables and t tests for continuous variables. Data on triglycerides, C-reactive protein, parathyroid hormone, urinary albumin:creatinine ratio, and fibroblast growth factor 23 were log-transformed for the analysis because they were not normally distributed. We used Cox models to evaluate the association of 25(OH)D with time to cardiovascular events, adjusting for potential confounding and mediating factors which met our a priori criteria of either face validity (age, sex, race/ethnicity, season) or an association across categories of vitamin D at P < 0.1. The reference category was a 25(OH)D level greater than or equal to 20 ng/mL. We developed sequential models: Model 1 adjusted for sociodemographic factors; model 2 adjusted for model 1 variables plus health behaviors; model 3 adjusted for model 2 variables plus comorbid health conditions; and model 4 adjusted for all model 3 variables plus potential biological mediators. We assessed proportional hazards assumptions by visual inspection of Schoenfeld residuals and log-minus-log plots, and found no evidence of violation.
To better define the individual contributions of each of the potential mediators, we also evaluated how much each potential mediator changed the strength of the association between 25(OH)D and cardiovascular events. For each covariate in models 3 and 4, we calculated the percent change in the magnitude of the association (log hazard ratio, adjusted for all model 2 covariates) before and after adjustment for the potential mediator of interest. We then performed stratified analyses to determine whether the association differed by age (≥65 years), sex, race/ethnicity, hypertension, diabetes, obesity (body mass index ≥30), chronic kidney disease (defined as an estimated glomerular filtration rate of ≥60 mL/minute/1.73 m2), albuminuria (urinary albumin:creatinine ratio ≥30 mg/g), or hyperparathyroidism (parathyroid hormone concentration ≥65 mg/dL). For all analyses, including interactions, P values less than 0.05 were considered significant. All analyses were conducted using STATA, version 11.0 (StataCorp LP, College Station, Texas).
RESULTS
Baseline characteristics of the 946 study participants are displayed in Table 1. The mean 25(OH)D concentration in this sample was 25.8 ng/mL. The prevalence of vitamin D deficiency (25(OH)D level <20 ng/mL) was 32%. Compared with participants with 25(OH)D levels greater than or equal to 20 ng/mL, those with levels under 20 ng/mL were younger, less likely to be male, and less likely to have graduated from college. Participants with vitamin D deficiency were more likely to use tobacco, less likely to take multivitamins, and less likely to engage in physical activity. Consistent with prior studies, participants with vitamin D deficiency were more likely to have hypertension, diabetes, and depression. They had higher systolic and diastolic blood pressures, higher levels of hemoglobin A1c, C-reactive protein, parathyroid hormone, fibroblast growth factor 23, and serum phosphorus, and higher urinary albumin:creatinine ratios. Serum calcium levels did not differ across categories (Table 1).
Table 1.
Baseline Characteristics of 946 Participants in the Heart and Soul Study, by 25-Hydroxyvitamin D Status, 2000–2002
| Characteristic | 25-Hydroxyvitamin D Level, ng/mL |
P Value | |||||
|---|---|---|---|---|---|---|---|
| <20 (n = 304) |
≥20 (n = 642) |
||||||
| % | Mean (SD) | Median (IQR)a | % | Mean (SD) | Median (IQR) | ||
| Demographic factors | |||||||
| Age, years | 65 (11) | 67 (11) | 0.001 | ||||
| Male sex | 77 | 83 | 0.02 | ||||
| Race/ethnicity | <0.001 | ||||||
| White | 49 | 66 | |||||
| Black | 33 | 8 | |||||
| Hispanic | 9 | 9 | |||||
| Asian | 7 | 13 | |||||
| Other | 2 | 4 | |||||
| Season of year | |||||||
| Spring | 24 | 24 | 0.14 | ||||
| Summer | 18 | 24 | |||||
| Fall | 33 | 28 | |||||
| Winter | 25 | 24 | |||||
| College graduation | 19 | 42 | <0.001 | ||||
| Health behaviors | |||||||
| Tobacco use | 28 | 16 | <0.001 | ||||
| Alcohol use | 27 | 30 | 0.27 | ||||
| Medication nonadherence | 10 | 7 | 0.17 | ||||
| Multivitamin use | 16 | 45 | <0.001 | ||||
| Physical activity | 35 | 54 | <0.001 | ||||
| Comorbid conditions | |||||||
| Hypertension | 76 | 68 | 0.007 | ||||
| Diabetes | 23 | 33 | 0.001 | ||||
| Myocardial infarction | 53 | 55 | 0.41 | ||||
| Left ventricular ejection fraction | 62 | 61 | 0.11 | ||||
| eGFR, mL/minute/1.73 m2 | 71.3 (1.4) | 70.4 (0.8) | 0.56 | ||||
| Body mass indexb | 29.2 (0.4) | 28.0 (0.2) | 0.001 | ||||
| Depression | 24 | 17 | 0.01 | ||||
| Medication use | |||||||
| Aspirin | 73 | 73 | 0.99 | ||||
| Statins | 63 | 66 | 0.34 | ||||
| Beta blockers | 61 | 57 | 0.22 | ||||
| Angiotensin inhibitors | 52 | 52 | 0.88 | ||||
| Potential biological mediators | |||||||
| Systolic blood pressure, mm Hg | 136 (23) | 132 (20) | 0.01 | ||||
| Diastolic blood pressure, mm Hg | 76 (12) | 74 (11) | 0.02 | ||||
| Hemoglobin A1c, % | 6.1 (1.3) | 5.9 (1.1) | 0.004 | ||||
| LDL cholesterol, mg/dL | 103 (32) | 105 (34) | 0.42 | ||||
| HDL cholesterol, mg/dL | 44 (13) | 46 (14) | 0.08 | ||||
| Triglycerides, mg/dL | 109 (78–174) | 110 (72–159) | 0.07 | ||||
| C-reactive protein, mg/dL | 2.7 (1.2–6.6) | 2.0 (0.8–4.1) | <0.001 | ||||
| Parathyroid hormone, mg/dL | 61 (45–82) | 50 (39–65) | <0.001 | ||||
| Calcium, mg/dL | 9.5 (0.5) | 9.5 (0.5) | 0.21 | ||||
| Corrected calcium,c mg/dL | 9.6 (0.5) | 9.6 (0.5) | 0.17 | ||||
| Phosphorus, mg/dL | 3.8 (0.7) | 3.7 (0.6) | 0.01 | ||||
| Urinary albumin:creatinine ratio, mg/g | 9.4 (5.3–23.5) | 8.3 (5.0–15.8) | 0.01 | ||||
| Fibroblast growth factor 23, RU/mL | 50 (31–103) | 41(28–62) | 0.004 | ||||
Abbreviations: eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; RU, relative units; SD, standard deviation.
a 25th–75th percentiles.
b Weight (kg)/height (m)2.
c Corrected calcium = total calcium + (4.0 – serum albumin) × 0.8.
During a median follow-up period of 8.0 years, 323 subjects (34.1%) experienced a cardiovascular event. We observed a nonlinear association, with a sharp increase in cardiovascular events at 25(OH)D levels less than 20 ng/mL (Figure 1). The question of whether 25(OH)D levels of 20–29.9 ng/mL (often referred to as vitamin D insufficiency) also confer adverse health consequences is currently controversial. Therefore, we performed additional exploratory analyses to compare annual cardiovascular event rates at 3 different 25-OH levels: <20 ng/mL, 20–29.9 ng/mL, and ≥30 ng/mL. These analyses confirmed that the cardiovascular event rates observed in participants with 25(OH)D levels of 20–29.9 ng/mL were similar to the rates observed in participants with 25(OH)D levels greater than or equal to 30 ng/mL (Figure 2).
Figure 1.
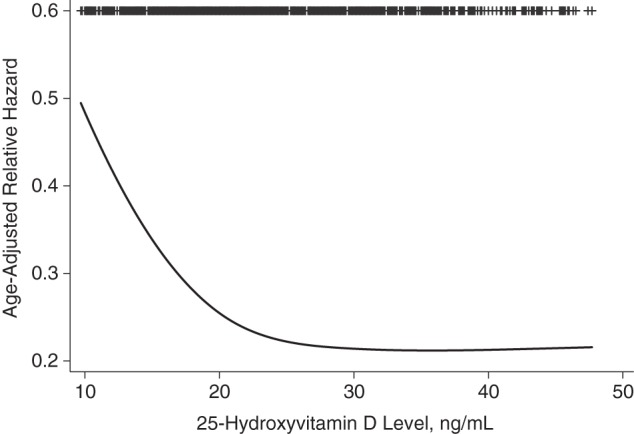
Nonlinearity of the association between 25-hydroxyvitamin D levels and subsequent cardiovascular events among 946 participants in the Heart and Soul Study, 2000–2012.
Figure 2.
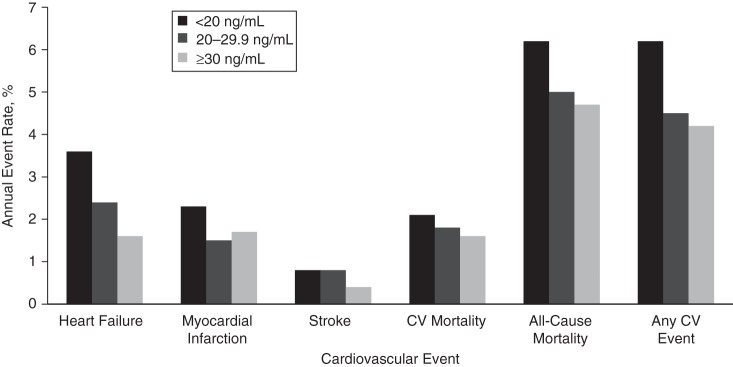
Incidence of cardiovascular (CV) events during a median follow-up period of 8.0 years, by 25-hydroxyvitamin D status, among 946 participants in the Heart and Soul Study, 2000–2012.
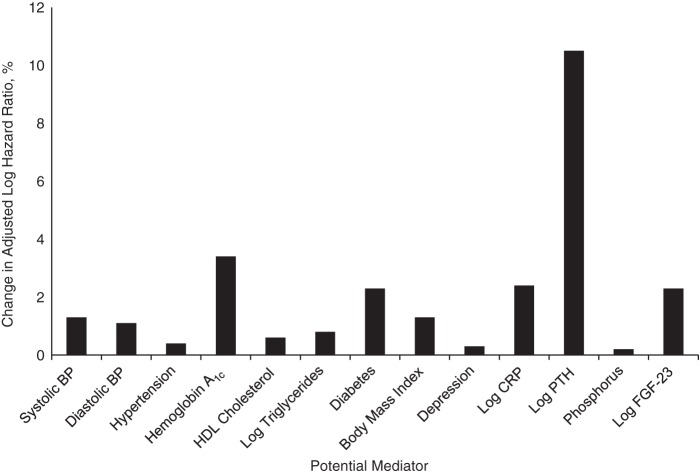
After adjustment for age, sex, race/ethnicity, and season of blood draw, participants with 25(OH)D levels less than 20 ng/mL had a 50% greater rate of cardiovascular events (hazard ratio (HR) = 1.50, 95% confidence interval (CI): 1.19, 1.90) than participants with 25(OH)D levels of 20 ng/mL or higher. Further adjustment for poor health behaviors (tobacco use, no multivitamin use, low physical activity) modestly attenuated the association (HR = 1.31, 95% CI: 1.02, 1.69). Adjustment for comorbid health conditions (diabetes, hypertension, depression, higher body mass index) did not materially alter findings (HR = 1.30, 95% CI: 1.01, 1.67). Following further adjustment for potential biological mediators (systolic and diastolic blood pressure, high-density lipoprotein cholesterol, triglycerides, hemoglobin A1c, C-reactive protein, parathyroid hormone, phosphorus, and fibroblast growth factor 23), the association was no longer significant (HR = 1.11, 95% CI: 0.85, 1.44) (Table 2). These associations were similar across individual outcomes (Table 3). Results revealed that parathyroid hormone most strongly attenuated the association, resulting in a 10.5% change in the size of the association (Figure 3).
Table 2.
Association Between Baseline 25-Hydroxyvitamin D Level (<20 ng/mL vs. ≥20 ng/mL) and Subsequent Cardiovascular Eventsa (n = 323) After Multivariate Adjustment Among 946 Participants in the Heart and Soul Study, 2000–2012
| Model | Hazard Ratio |
95% Confidence Interval |
P Value |
|---|---|---|---|
| Model 1b | 1.50 | 1.19, 1.90 | 0.001 |
| Model 2c | 1.31 | 1.02, 1.69 | 0.03 |
| Model 3d | 1.30 | 1.01, 1.67 | 0.04 |
| Model 4e | 1.11 | 0.85, 1.44 | 0.43 |
a Subsequent cardiovascular events were defined as heart failure, myocardial infarction, stroke, or cardiovascular mortality.
b Results were adjusted for sociodemographic factors (age, sex, white race/ethnicity, season of blood draw, and college graduation).
c Results were adjusted for all model 1 covariates plus likely confounders, including health behaviors (tobacco use, multivitamin use, and physical activity).
d Results were adjusted for all model 2 covariates plus comorbid health conditions (diabetes, hypertension, depression, and body mass index).
e Results were adjusted for all model 3 covariates plus potential biological mediators (systolic blood pressure, diastolic blood pressure, hemoglobin A1c, triglycerides, high-density lipoprotein cholesterol, C-reactive protein, phosphorus, parathyroid hormone, and fibroblast growth factor 23).
Table 3.
Association Between Baseline 25-Hydroxyvitamin D Level (<20 ng/mL vs. ≥20 ng/mL) and Subsequent Cardiovascular Events,a With Multivariate Adjustment, Among 946 Participants in the Heart and Soul Study, 2000–2012
| Model | Heart Failure (n = 172) |
Myocardial Infarction (n = 127) |
Stroke (n = 49) |
Cardiovascular Mortality (n = 141) |
All-Cause Mortality (n = 369) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Model 1b | 1.75 | 1.27, 2.41 | 1.43 | 0.99, 2.09 | 1.27 | 0.69, 2.34 | 1.45 | 1.01, 2.08 | 1.39 | 1.11, 1.74 |
| Model 2c | 1.56 | 1.11, 2.19 | 1.34 | 0.90, 1.99 | 1.42 | 0.74, 2.72 | 1.21 | 0.83, 1.77 | 1.22 | 0.97, 1.55 |
| Model 3d | 1.44 | 1.02, 2.02 | 1.27 | 0.85, 1.90 | 1.39 | 0.72, 2.68 | 1.23 | 0.84, 1.80 | 1.25 | 0.98, 1.58 |
| Model 4e | 1.25 | 0.87, 1.79 | 1.19 | 0.78, 1.82 | 1.08 | 0.54, 2.18 | 1.13 | 0.76, 1.70 | 1.18 | 0.92, 1.52 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Subsequent cardiovascular events were defined as heart failure, myocardial infarction, stroke, or cardiovascular mortality.
b Results were adjusted for sociodemographic factors (age, sex, white race/ethnicity, season of blood draw, and college graduation).
c Results were adjusted for all model 1 covariates plus likely confounders, including health behaviors (tobacco use, multivitamin use, and physical activity).
d Results were adjusted for all model 2 covariates plus comorbid health conditions (diabetes, hypertension, depression, and body mass index).
e Results were adjusted for all model 3 covariates plus potential biological mediators (systolic blood pressure, diastolic blood pressure, hemoglobin A1c, triglycerides, high-density lipoprotein cholesterol, C-reactive protein, phosphorus, parathyroid hormone, and fibroblast growth factor 23).
Figure 3.
Change in the strength of the association between 25-hydroxyvitamin D level (<20 ng/mL vs. ≥20 ng/mL) and cardiovascular events after adjustment for potential mediators (expressed as percent change in the age-adjusted log hazard ratio) among 946 participants in the Heart and Soul Study, 2000–2012. All models adjusted for sociodemographic factors (age, sex, white race/ethnicity, season of blood draw, and college graduation) and health behaviors (tobacco use, multivitamin use, and physical activity). BP, blood pressure; CRP, C-reactive protein; FGF-23, fibroblast growth factor 23; HDL, high-density lipoprotein; PTH, parathyroid hormone.
Stratified analyses revealed that the association between 25(OH)D levels and cardiovascular events was stronger in participants with diabetes (n = 249; HR = 1.62, 95% CI: 1.06, 2.48) than in participants without diabetes (n = 695; HR = 1.09, 95% CI: 0.79, 1.51 (P for interaction < 0.001)). The association was also stronger in participants with albuminuria (n = 207; HR = 1.66, 95% CI: 1.07, 2.57) than in those without albuminuria (n = 731; HR = 1.07, 95% CI: 0.77, 1.47 (P for interaction < 0.001)). The association was modestly greater in participants with chronic kidney disease (n = 290; HR = 1.44, 95% CI: 0.99, 2.08) than in those without chronic kidney disease (n = 656; HR = 1.27, 95% CI: 0.88, 1.81 (P for interaction < 0.001)). The association did not differ by age, sex, race/ethnicity, hypertension, obesity, or hyperparathyroidism.
DISCUSSION
In this ambulatory population of persons with stable CHD, we found that participants with vitamin D deficiency (25(OH)D level <20 ng/mL) had a 50% greater rate of cardiovascular events than participants who were sufficient in vitamin D (25(OH)D level ≥20 ng/mL). Even following adjustment for sociodemographic factors, poor health behaviors, and comorbid health conditions, 25(OH)D levels less than 20 ng/mL remained associated with a 30% greater rate of cardiovascular events. However, after further adjustment for parathyroid hormone level and other potentially mediating biological factors, the association was no longer significant. These findings raise the possibility that correction of vitamin D deficiency could reduce CVD risk by preventing elevations in parathyroid hormone levels and other biological changes.
Two prior prospective studies have examined the association between vitamin D and cardiovascular events in participants with established CVD, both with differing conclusions. In a large study of over 3,000 patients who were referred for cardiac catheterization, Dobnig et al. (34) found inverse associations between 25(OH)D levels and cardiovascular mortality. However, in a German study of 1,000 patients who were referred for cardiac rehabilitation after an acute cardiovascular event, Grandi et al. (35) found no association between 25(OH)D levels and secondary cardiovascular events or mortality. The authors noted that the inconsistencies in the two studies could be attributable to the differences in the patient populations. In the Grandi study, all participants had suffered a recent acute event (35), whereas the Dobnig study was comprised of patients both with and without CHD, and a stratified analysis revealed a greater association in nondiseased participants (34).
In this large sample of outpatients, all of whom had established CHD, we observed an inverse association between 25(OH)D levels and subsequent cardiovascular events. In contrast to the Grandi population of patients who had recently suffered an acute event (35), our population was comprised entirely of patients with stable CHD (those who had had a myocardial infarction in the prior 6 months were excluded). Thus, our results suggest that in patients who have established, stable CHD, vitamin D deficiency is associated with subsequent cardiovascular events.
Poor health behaviors explained a portion of the association between 25(OH)D and cardiovascular events, but they did not explain the entire association. The association was mostly explained by biological mediators; in particular, parathyroid hormone, which is downstream of 25(OH)D. Hyperparathyroidism has been proposed as one mechanism by which vitamin D deficiency could mediate cardiovascular events, since it may promote cardiac hypertrophy (25), vascular remodeling (36, 37), and inflammation (38). In some studies, parathyroid hormone has also been identified as an independent risk factor for cardiovascular events both in the general population (39) and in patients with existing CVD (40). Our findings suggest that the elevations in parathyroid hormone which accompany vitamin D deficiency could mediate cardiovascular events. Moreover, the association between 25(OH)D and cardiovascular outcomes was similar in persons with and without overt hyperparathyroidism, indicating that even small elevations in parathyroid hormone may mediate CVD risk.
We also observed stronger associations between 25(OH)D and cardiovascular events in participants with diabetes, chronic kidney disease, and albuminuria. The high degree of overlap in these risk factors and the very strong P values for interaction (all P's < 0.001) strengthen the likelihood that these post hoc analyses may be correctly identifying a high-risk subgroup. However, further analyses are warranted for validation of these subgroup results, as well as to parse out which of these factors is the primary contributor.
For each of these 3 subgroups, fairly compelling reasons exist which could explain why the association between 25(OH)D and cardiovascular events may be particularly strong. If vitamin D deficiency causes insulin resistance, these associations may be even more pronounced in persons who already have overt diabetes. Alternatively, it may be that persons with diabetes are at greater risk simply because of their higher prevalence of albuminuria and chronic kidney disease. Patients with chronic kidney disease may be at greater risk because chronic kidney disease is associated with lower 1-α-hydroxylase activity, which results in even lower levels of 1,25-hydroxyvitamin D. Therefore, if vitamin D deficiency leads to cardiovascular events, vitamin D deficiency may be particularly harmful when superimposed on chronic kidney disease. Finally, participants with albuminuria may be at higher risk because albuminuria is associated with endothelial dysfunction and is an independent risk factor for cardiovascular events (41). Randomized controlled trials have demonstrated reductions in albuminuria with vitamin D administration (42, 43). Thus, these data raise the possibility that correction of vitamin D deficiency could modify CVD risk by preventing or reducing albuminuria.
We found no significant mediation by several other commonly proposed biological processes. Inflammation (10, 23) and blood pressure elevation (44) have also been proposed as plausible biological pathways by which vitamin D deficiency could lead to cardiovascular events. However, we found that adjustment for C-reactive protein, hypertension, and blood pressure resulted in no substantial change. Depression has also been proposed as a potential mediator of the association, because vitamin D deficiency is associated with incident depression (45, 46) and depression is known to be an independent risk factor for CVD (26). However, we found no evidence of mediation by depression.
Our study had several important limitations. First, our study was observational in nature. Although we attempted to control for potentially confounding factors prior to performing our mediation analysis, we cannot rule out the possibility that residual confounding may have remained. Second, the study participants were mostly white men, and all had stable CHD. Generalizability to other settings is unknown. Third, the potential mediators we evaluated in this study were measured at the same time as the 25(OH)D levels; therefore, we cannot determine the direction of the association. Finally, over the course of the follow-up period, subjects may have developed interval elevations in blood pressure, insulin resistance, or comorbid conditions which were not accounted for in this study.
In summary, we found that vitamin D deficiency was associated with cardiovascular events in participants with stable CHD, independent of sociodemographic factors, comorbid health conditions, and poor health behaviors. We observed a substantial change in the magnitude of the association after adjustment for parathyroid hormone concentration, indicating that parathyroid hormone could mediate the association. We also discovered that the association was strongest in participants with diabetes, chronic kidney disease, and albuminuria. Elevations in parathyroid hormone and albuminuria are both potentially modifiable by administration of vitamin D; therefore, these findings raise the possibility that correction of vitamin D deficiency could reduce CVD risk. These observations highlight the importance of randomized controlled trials, such as the ongoing Vitamins and Lifestyle (VITAL) Study, in determining whether vitamin D supplementation could play a role in reducing the public health burden of CVD.
ACKNOWLEDGMENTS
Author affiliations: Division of General Internal Medicine, Department of Medicine, School of Medicine, University of California, San Francisco, San Francisco, California (Christine C. Welles, Mary A. Whooley); Department of Family and Preventive Medicine and Division of Nephrology-Hypertension, Department of Medicine, School of Medicine, University of California, San Diego, San Diego, California (Joachim H. Ix); Division of General Medicine and Primary Care (Kenneth J. Mukamal) and Division of Nephrology (Tammy Hod, S. Ananth Karumanchi), Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts; Department of Pathology, Beth Israel Deaconess Medical Center, Boston, Massachusetts (Anders H. Berg); and Division of Nephrology, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts (Ravi Thadhani).
Dr. Christine C. Welles was supported by the Veterans Affairs National Quality Scholars Program. The Heart and Soul Study was supported by the Department of Veterans Affairs, the National Heart, Lung, and Blood Institute, the American Federation for Aging Research, the Robert Wood Johnson Foundation, and the Ischemia Research and Education Foundation. Dr. S. Ananth Karumanchi is an investigator of the Howard Hughes Medical Institute. Dr. Ravi Thadhani was supported by National Institutes of Health grants DK094486 and DK094872.
Conflict of interest: none declared.
REFERENCES
- 1.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Lee JH, Milani RV. Vitamin D and cardiovascular disease: will it live up to its hype? J Am Coll Cardiol. 2011;58(15):1547–1556. doi: 10.1016/j.jacc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30(2):150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 5.Fleck A. Re: “Latitude and ischaemic heart disease” [letter] Lancet. 1989;1(8638):613. doi: 10.1016/s0140-6736(89)91634-6. [DOI] [PubMed] [Google Scholar]
- 6.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49(5):1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 7.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52(5):828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obes Rev. 2013;14(5):393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 9.Zittermann A, Schleithoff SS, Tenderich G, et al. Low vitamin D status: a contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41(1):105–112. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 10.Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 11.Giovannucci E, Liu Y, Hollis BW, et al. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marniemi J, Alanen E, Impivaara O, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005;15(3):188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Brøndum-Jacobsen P, Benn M, Jensen GB, et al. 25-Hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32(11):2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- 14.Poole KES, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37(1):243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 15.Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(6):1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahrleitner A, Dobnig H, Obernosterer A, et al. Vitamin D deficiency and secondary hyperparathyroidism are common complications in patients with peripheral arterial disease. J Gen Intern Med. 2002;17(9):663–669. doi: 10.1046/j.1525-1497.2002.11033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomson J, Emberson J, Hill M, et al. Vitamin D and risk of death from vascular and non-vascular causes in the Whitehall study and meta-analyses of 12 000 deaths. Eur Heart J. 2013;34(18):1365–1374. doi: 10.1093/eurheartj/ehs426. [DOI] [PubMed] [Google Scholar]
- 18.Kilkkinen A, Knekt P, Aro A, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170(8):1032–1039. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Manson JE, Song Y, et al. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152(5):315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 21.Li YC, Qiao G, Uskokovic M, et al. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90(1-5):387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Pittas AG, Harris SS, Stark PC, et al. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes Care. 2007;30(4):980–986. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 23.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79(6):1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chitalia N, Recio-Mayoral A, Kaski JC, et al. Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis. 2012;220(1):265–268. doi: 10.1016/j.atherosclerosis.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Schlüter KD, Piper HM. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol. 1992;263(6):H1739–H1746. doi: 10.1152/ajpheart.1992.263.6.H1739. [DOI] [PubMed] [Google Scholar]
- 26.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruo B, Rumsfeld JS, Hlatky MA, et al. Depressive symptoms and health-related quality of life. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 29.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gehi AK, Ali S, Na B, et al. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the Heart and Soul Study. Arch Intern Med. 2007;167(16):1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152(10):640–648. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominguez JR, Shlipak MG, Whooley MA, et al. Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol. 2013;24(4):647–654. doi: 10.1681/ASN.2012090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ix JH, Shlipak MG, Chertow GM, et al. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115(2):173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 35.Grandi NC, Breitling LP, Vossen CY, et al. Serum vitamin D and risk of secondary cardiovascular disease events in patients with stable coronary heart disease. Am Heart J. 2010;159(6):1044–1051. doi: 10.1016/j.ahj.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Perkovic V, Hewitson TD, Kelynack KJ, et al. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res. 2003;26(1):27–33. doi: 10.1159/000069761. [DOI] [PubMed] [Google Scholar]
- 37.Amann K, Törnig J, Flechtenmacher C, et al. Blood-pressure-independent wall thickening of intramyocardial arterioles in experimental uraemia: evidence for a permissive action of PTH. Nephrol Dial Transplant. 1995;10(11):2043–2048. [PubMed] [Google Scholar]
- 38.Martín-Ventura JL, Ortego M, Esbrit P, et al. Possible role of parathyroid hormone-related protein as a proinflammatory cytokine in atherosclerosis. Stroke. 2003;34(7):1783–1789. doi: 10.1161/01.STR.0000078371.00577.76. [DOI] [PubMed] [Google Scholar]
- 39.Hagström E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119(21):2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 40.Grandi NC, Breitling LP, Hahmann H, et al. Serum parathyroid hormone and risk of adverse outcomes in patients with stable coronary heart disease. Heart. 2011;97(15):1215–1221. doi: 10.1136/hrt.2011.223529. [DOI] [PubMed] [Google Scholar]
- 41.Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2(3):581–590. doi: 10.2215/CJN.03190906. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005;68(6):2823–2828. doi: 10.1111/j.1523-1755.2005.00755.x. [DOI] [PubMed] [Google Scholar]
- 43.de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 44.Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152(5):307–314. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.May HT, Bair TL, Lappé DL, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. Am Heart J. 2010;159(6):1037–1043. doi: 10.1016/j.ahj.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Anglin RES, Samaan Z, Walter SD, et al. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202(2):100–107. doi: 10.1192/bjp.bp.111.106666. [DOI] [PubMed] [Google Scholar]