Abstract
Aggressively managing low-density lipoprotein cholesterol (LDL-C) after myocardial infarction (MI) is a cornerstone of secondary prevention. The changes in LDL-C after MI and the factors associated with LDL-C levels are unknown. Therefore, we directly measured fasting LDL-C levels in 797 MI patients from 24 US hospitals from 2005 to 2008. Mean LDL-C levels at discharge, 1 month, and 6 months were 95.1, 81.9, and 87.1 mg/dL, respectively. In a hierarchical, multivariable, repeated measures model, older age, male sex, and hypertension were associated with lower LDL-C levels, whereas self-reported avoidance of health care because of cost was associated with higher LDL-C. Both the presence and intensity of statin therapy at discharge were strongly associated with LDL-C levels, with adjusted mean 6-month changes of −3.4 mg/dL (95% confidence interval (CI): −12.1, 5.3) for no statins; 1.7 mg/dL (95% CI: −4.7, 8.1) for low statins; −10.2 mg/dL (95% CI: −14.5, −6.0) for moderate statins; and −13.9 mg/dL (95% CI: −19.7, −8.0) for intensive statins (P < 0.001). In conclusion, we found that greater reductions in LDL-C levels after MI were strongly associated with the presence and intensity of statin therapy, older age, male sex, hypertension, and better socioeconomic status. These findings support the use of intensive statin therapy in post-MI patients and provide estimates of the expected LDL-C changes after MI in a real-world population.
Keywords: cholesterol, cholesterol-lowering drugs, myocardial infarction, statins
Among survivors of myocardial infarction (MI), aggressive secondary prevention measures are needed to reduce the risk of recurrent ischemic events and death. A cornerstone of that treatment strategy is lipid management—specifically with statins, if they can be tolerated by the patient (1). Multiple clinical trials have demonstrated a reduction in morbidity and mortality rates when patients are treated with statins after an MI (2, 3), with even greater benefit obtained from more intensive statin therapy (4–6). Much of the benefit of statins is thought to be mediated through the reduction of low-density lipoprotein cholesterol (LDL-C) (7), and in clinical trials of patients randomized to intensive or moderate statins during hospitalization for MI, patients achieved average LDL-C declines of 45 and 22 mg/dL, respectively, after 8 months of treatment (4–6). However, the impact of statins on lipid levels over time in routine clinical practice is less well known. Furthermore, it is possible there are several factors—both patient- and treatment-related—that contribute to changes in lipid levels over time. A better understanding of post-MI trajectories in LDL-C levels and of the patient characteristics, beyond statin treatment, that are associated with changes in LDL-C could help optimize management of patients after acute MI.
To address these gaps in knowledge, we investigated the LDL-C levels at discharge and at 1 month and 6 months after hospitalization among 797 patients from 24 hospitals within the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) prospective cohort study (8). By defining the changes in LDL-C levels over time and identifying the factors associated with these changes, we hope to provide insights into the changes in lipid levels over time with the goal of identifying high-risk patients who might benefit from more intensive monitoring after MI.
METHODS
Study population
Details of the TRIUMPH study, including the design, patient selection, site characteristics, and follow-up assessments, have been previously published (8). Between April 2005 and December 2008, 4,340 patients from 24 US hospitals were enrolled into the TRIUMPH registry. Inclusion criteria included biomarker evidence of myocardial necrosis and additional clinical evidence supporting the diagnosis of MI, such as prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes during the initial 24 hours of admission. Patients not presenting initially to an enrolling institution were eligible only if transferred within 24 hours of presentation.
Study protocol
Baseline sociodemographic and clinical data were obtained through chart abstraction and a detailed structured interview within 24–72 hours following admission. Lipid-lowering medications (type and dose) at hospital discharge were categorized as intensive statin therapy (expected LDL-C lowering of >50%; i.e., 80 mg of atorvastatin or 20–40 mg of rosuvastatin daily), moderate statin therapy (expected LDL-C lowering of 40%–50%; i.e., 20–40 mg of atorvastatin, 10 mg of rosuvastatin, or 80 mg of pravastatin, lovastatin, or simvastatin daily), or low statin therapy (all other statins and doses) (9). Data on any allergies or other contraindications to lipid-lowering therapy were prospectively abstracted from the hospital record. Consenting patients had fasting blood specimens collected prior to discharge, which were processed, refrigerated, and sent by overnight mail daily to the core laboratory (Clinical Reference Laboratory, Lenexa, Kansas). Blood was then analyzed for lipid levels using the VAP test (Atherotech Diagnostics Lab, Atlanta, Georgia), which directly measures LDL-C levels.
Follow-up was attempted on all survivors at 1 month and 6 months after MI by telephone (interview only) or an in-home visit (for consenting patients), during which additional clinical data (e.g., vital signs, weight) and laboratory data were collected. Patients were asked to be fasting, although blood was also obtained on nonfasting patients (4% of patients), in which case the sample was designated as a random blood sample and categorized as such in subsequent analyses. Only patients who had both baseline and either a 1-month or 6-month laboratory assessments were included in the analyses for this study (Figure 1; n = 797). Each participating hospital obtained institutional research board approval, and all patients provided written informed consent for baseline and follow-up assessments.
Figure 1.
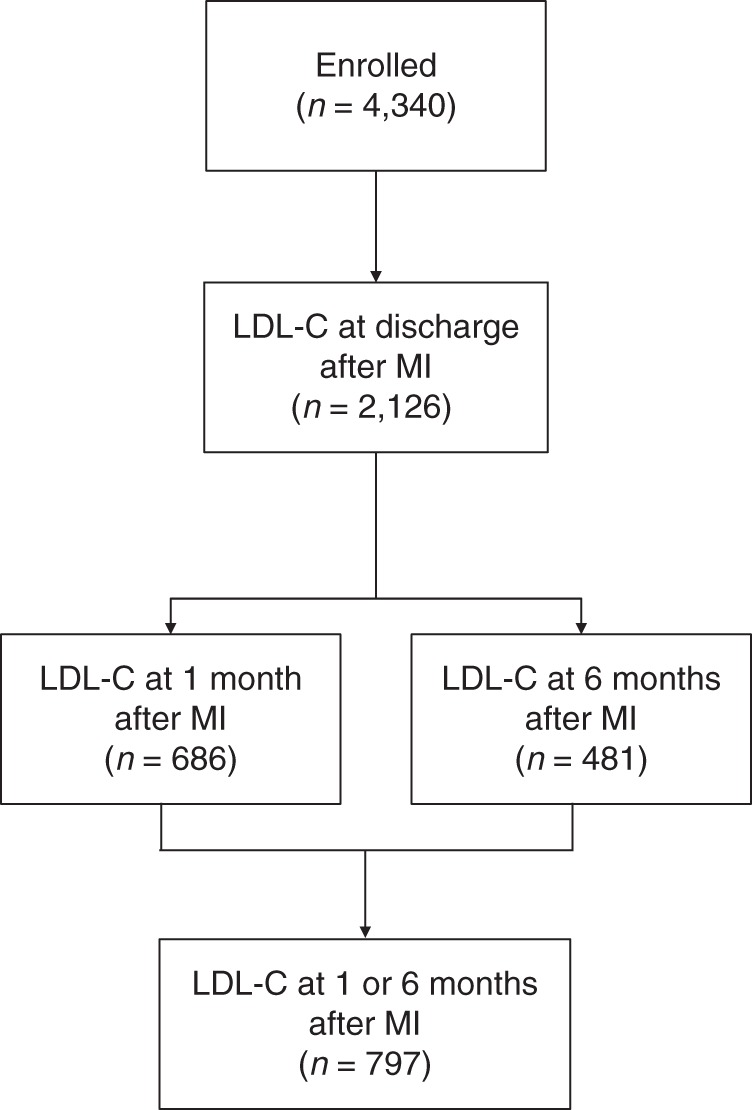
Flowchart of the analytical cohort in the TRIUMPH Study, 2005–2008. LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction.
Statistical analysis
The primary goals of these analyses were to describe the change in LDL-C levels after discharge for an MI and to examine patient and treatment factors associated with these changes in LDL-C levels. First, the patient and treatment characteristics of those who did and did not have an LDL-C level less than 100 mg/dL at 6 months after MI were compared using the χ2 test for categorical variables and t tests for continuous variables. One-month LDL-C levels were used for patients missing 6-month laboratory assessments.
Second, to examine LDL-C changes over time, we constructed hierarchical repeated measures regression models, which allowed us to take advantage of all available data. Multiple covariates were added to the model to examine the patient-level factors associated with LDL-C changes. Covariates were selected a priori based on clinical judgment and included sociodemographic factors, cardiac and noncardiac comorbidities, clinical factors, and treatments (Table 1). In a second model, statin treatment at discharge (categorized as none, low, moderate, or intensive) was included, as well as the interaction of treatment × time. All analyses were conducted using SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina), and statistical significance was determined by a 2-sided P value of less than 0.05.
Table 1.
Baseline Characteristics by LDL-C Level at 6 Months After Myocardial Infarction in the TRIUMPH Study, 2005–2008
| Characteristic | LDL-C <100 mg/dL (n = 604) |
LDL-C ≥100 mg/dL (n = 193) |
P Value | ||
|---|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | ||
| Age, years | 59.8 (11.2) | 55.8 (11.7) | <0.001 | ||
| Male sex | 70.0 | 62.2 | 0.041 | ||
| White race | 77.3 | 63.2 | <0.001 | ||
| Married | 60.3 | 45.3 | <0.001 | ||
| Low social support | 16.2 | 23.0 | 0.033 | ||
| High school or greater education | 93.0 | 89.6 | 0.122 | ||
| Avoids health care because of cost | 24.2 | 31.1 | 0.059 | ||
| Hypertension | 67.1 | 62.2 | 0.214 | ||
| Prior bypass graft surgery | 10.9 | 10.4 | 0.826 | ||
| Diabetes mellitus | 26.5 | 32.1 | 0.129 | ||
| Current/former smoking | 58.4 | 66.8 | 0.038 | ||
| Chronic lung disease | 7.1 | 5.2 | 0.347 | ||
| Prior heart failure | 7.1 | 9.3 | 0.315 | ||
| Chronic kidney diseasea | 20.1 | 20.8 | 0.818 | ||
| Body mass indexb | 29.7 (6.1) | 29.3 (6.2) | 0.480 | ||
| Prior stroke or transient attack | 5.5 | 8.8 | 0.095 | ||
| Depressive symptoms | 21.9 | 21.8 | 0.978 | ||
| In-hospital coronary angiogram | 95.5 | 92.2 | 0.074 | ||
| In-hospital angioplasty | 71.0 | 61.7 | 0.015 | ||
| In-hospital bypass graft surgery | 9.6 | 8.8 | 0.742 | ||
| ST elevations | 48.2 | 43.5 | 0.259 | ||
| GRACE risk scorec | 100.0 (27.1) | 96.1 (31.2) | 0.089 | ||
| Length of stay, days | 5.1 (6.1) | 5.3 (5.1) | 0.677 | ||
| Statins at discharge | 0.205 | ||||
| No statin | 9.7 | 13.7 | |||
| Low statin | 19.8 | 19.5 | |||
| Moderate statin | 48.3 | 41.1 | |||
| Intensive statin | 22.1 | 25.8 | |||
| Aspirin at discharge | 97.8 | 98.4 | 0.773 | ||
| β-blocker at discharge | 94.4 | 92.4 | 0.324 | ||
| ACE inhibitor/ARB at discharge (for patients with ventricular dysfunction) | 83.2 | 96.7 | 0.072 | ||
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; GRACE, Global Registry of Acute Coronary Events; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation; TRIUMPH, Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status.
a Estimated glomerular filtration rate <60 mL/minute/1.73 m2.
b Weight (kg)/height (m)2.
c A risk score calculated at hospital discharge to predict long-term mortality risk after myocardial infarction (10).
Missing data analysis
Of the 4,340 patients enrolled in the TRIUMPH study, 2,126 patients (50%) had LDL-C levels obtained prior to hospital discharge. Among these patients, 797 had LDL-C levels recorded at 1 month and/or 6 months after MI (Figure 1). There were 3,469 patients who did not participate in the follow-up lipid substudy but who survived at least 1 month after MI (and thus had the opportunity to participate). Patients who did not participate were more likely to be nonwhite, unmarried, and less educated, and to have chronic kidney disease than those who participated. Although nonparticipants were less likely to present with ST elevations and had lower mean troponin levels than participating patients, Global Registry of Acute Coronary Events (GRACE) discharge scores (10) were similar between groups. In addition, baseline LDL-C levels and the frequency and intensity of statin therapy were also similar between groups (Appendix Table 1).
RESULTS
Patient population
Of the 4,340 patients enrolled in the TRIUMPH study, 797 survived at least 1 month after MI and participated in the lipid substudy (Figure 1). The mean age of the population was 59 years, and 68% were male, 74% were white, 28% had diabetes, and 60% were current or former smokers. Forty-seven percent of patients presented with ST elevations, and 95% underwent invasive management of their MIs. Eighty-nine percent of patients were prescribed statins at discharge (26% of which were intensive statins).
LDL-C levels over time
The mean LDL-C levels at discharge, 1 month, and 6 months (from the repeated measures model) were 95.1, 81.9, and 87.1 mg/dL, respectively. At 6 months after MI, 76% of patients had LDL-C levels of less than 100 mg/dL. The prescription of statins at hospital discharge was similar between groups (for 6-month LDL-C levels <100 vs. ≥100 mg/dL, 90% vs. 86%; P = 0.12), as was the prescription of intensive statin therapy (for 6-month LDL-C levels <100 vs. ≥100 mg/dL, 22% vs. 26%; P = 0.30). Patients with low LDL-C levels were more likely to be male, white, and married, and they were less likely to be current or former smokers (Table 1).
In a hierarchical, repeated measures model, older age, male sex, and hypertension were associated with lower LDL-C levels over time, whereas avoiding care because of cost was associated with higher LDL-C levels over time (Table 2). In a second model that also considered statin therapy at hospital discharge, along with the interaction between statin therapy and follow-up time (both of which were highly significant at P < 0.001), patients not discharged with statin prescriptions and those discharged with low-potency statin prescriptions had no significant changes in their LDL-C levels over time (Figure 2). In contrast, the LDL-C levels of patients discharged with moderate statin therapy were, on average, 13.6 mg/dL lower at 1 month and 10.2 mg/dL lower at 6 months after hospital discharge. Patients discharged with intensive statin therapy had the largest declines in LDL-C levels over time, with mean LDL-C declines of 25.1 mg/dL at 1 month and 13.9 mg/dL at 6 months after hospital discharge (Table 2, Figure 2). After adjustment for statin therapy at discharge, older age, male sex, and hypertension remained significantly associated with lower LDL-C levels over time. Self-reported avoidance of medical care because of costs was significantly associated with higher LDL-C levels over time.
Table 2.
Factors Associated With Change in LDL-C Levels Over 6 Months After MI in the TRIUMPH Study, 2005–2008
| Factor | Change in LDL-C From Discharge to 6 Months |
|||||
|---|---|---|---|---|---|---|
| Adjusted for Patient Factors |
Adjusted for Patient Factors and Statin Therapy at Discharge |
|||||
| Estimate | 95% CI | P Value | Estimate | 95% CI | P Value | |
| Months since MI | <0.001 | |||||
| 1 | −13.2 | −15.5, −10.9 | NA | |||
| 6 | −7.9 | −10.7, −5.0 | NA | |||
| Statin use | <0.001 | |||||
| No statin at 1 month | NA | −5.0 | −12.0, 1.9 | |||
| No statin at 6 months | NA | −3.4 | −12.1, 5.3 | |||
| Low statin at 1 month | −3.1 | −8.2, 2.1 | ||||
| Low statin at 6 months | 1.7 | −4.7, 8.1 | ||||
| Moderate statin at 1 month | NA | −13.6 | −16.9, −10.3 | |||
| Moderate statin at 6 months | NA | −10.2 | −14.5, −6.0 | |||
| Intensive statin at 1 month | NA | −25.1 | −29.9, −20.3 | |||
| Intensive statin at 6 months | NA | −13.9 | −19.7, −8.0 | |||
| Age (per 10 years) | −3.3 | −5.0, −1.6 | <0.001 | −2.8 | −4.5, −1.1 | 0.001 |
| Male | −7.1 | −10.9, −3.2 | <0.001 | −6.8 | −10.7, −3.0 | <0.001 |
| White race | −0.2 | −4.5, 4.1 | 0.931 | −0.7 | −5.0, 3.6 | 0.747 |
| Married | −2.8 | −6.4, 0.9 | 0.135 | −2.4 | −6.0, 1.3 | 0.204 |
| Low social support | 3.7 | −0.9, 8.3 | 0.113 | 4.1 | −0.5, 8.6 | 0.081 |
| High school or greater education | −6.6 | −12.9, −0.2 | 0.044 | −6.6 | −13.0, −0.3 | 0.041 |
| Avoids health care because of cost | 4.3 | 0.3, 8.2 | 0.035 | 4.6 | 0.6, 8.5 | 0.023 |
| Hypertension | −5.4 | −9.2, −1.6 | 0.005 | −5.7 | −9.5, −1.9 | 0.003 |
| In-hospital bypass surgery | −4.6 | −10.9, 1.7 | 0.149 | −3.3 | −9.8, 3.2 | 0.314 |
| In-hospital angioplasty | −3.2 | −7.7, 1.2 | 0.157 | −3.2 | −7.7, 1.3 | 0.162 |
| Diabetes mellitus | 0.7 | −3.4, 4.8 | 0.738 | 0.5 | −3.7, 4.6 | 0.831 |
| Current/former smoking | 0.0 | −3.7, 3.6 | 0.990 | 1.1 | −2.6, 4.8 | 0.558 |
| Chronic lung disease | −4.0 | −11.0, 2.9 | 0.255 | −5.4 | −12.4, 1.5 | 0.124 |
| Prior heart failure | −4.5 | −11.1, 2.2 | 0.190 | −4.9 | −11.6, 1.8 | 0.152 |
| Chronic kidney disease | 0.7 | −4.1, 5.5 | 0.777 | 0.9 | −4.0, 5.7 | 0.729 |
| Cerebrovascular disease | 4.2 | −2.9, 11.2 | 0.840 | 2.6 | −4.5, 9.7 | 0.467 |
| Depression | −3.2 | −7.4, 0.3 | 0.145 | −3.1 | −7.4, 1.1 | 0.147 |
| Body mass indexa | 0.2 | −1.4, 1.7 | 0.840 | 0.4 | −1.1, 2.0 | 0.581 |
| ST elevation MI | −3.5 | −7.3, 0.3 | 0.068 | −3.3 | −7.1, 0.5 | 0.091 |
Abbreviations: CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; NA, not applicable; TRIUMPH, Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status.
a Weight (kg)/height (m)2.
Figure 2.
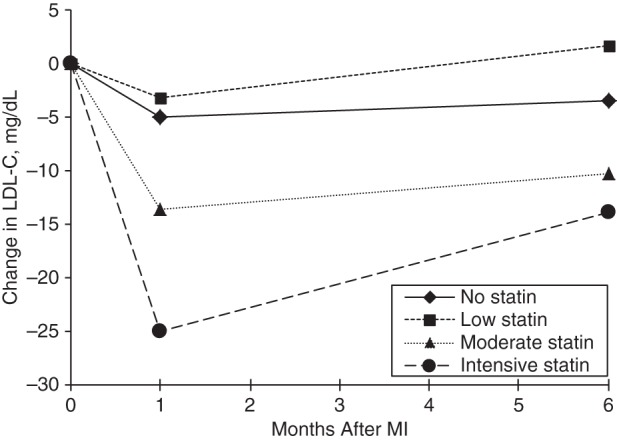
Multivariable-adjusted change in low-density lipoprotein cholesterol (LDL-C) levels over time after myocardial infarction (MI) by statin therapy at hospital discharge in the TRIUMPH study, 2005–2008.
Finally, to explore the association of self-reported avoidance of care because of cost and higher LDL-C levels over time, we examined the patterns of statin use among patients who did and who did not report this difficulty over the same time period. At hospital discharge, there was no difference in the frequency of prescription of statins (for “avoids care” vs. “does not avoid care,” 90.3% vs. 88.9%; P = 0.58), nor was there a difference in the frequency of prescription of intensive statin therapy (for “avoids care” vs. “does not avoid care,” 22.7% vs. 23.1%; P = 0.90). At 6 months, slightly fewer patients who expressed an avoidance of care because of costs reported taking statins (77.3% vs. 81.0%, P = 0.34), but the use of intensive statins was identical between groups (16.7% vs. 16.7%, P = 1.000).
DISCUSSION
In a large, contemporary population of real-world patients, we found that LDL-C levels declined by 13 and 8 mg/dL, on average, at 1 month and 6 months after MI, respectively. Not surprisingly, these LDL-C declines are highly dependent on the intensity of statin therapy at hospital discharge, with patients not discharged with statins and those discharged with low-potency statins having no significant changes in LDL-C levels over time. Those discharged with moderate statins saw modest declines in LDL-C, whereas those discharged with intensive statins had LDL-C decreases of 25 and 14 mg/dL at 1 month and 6 months, respectively. These findings demonstrate the importance of intensive statin therapy at hospital discharge in regard to LDL-C lowering and also estimate the changes in LDL-C levels that would be expected in real-world populations.
Our study supports and extends prior analyses investigating LDL-C levels after MI. In the Aggrastat to Zocor (A to Z) Trial, which included only statin-naïve patients with an acute coronary syndrome, those randomized to 80 mg or 20 mg of simvastatin daily had average LDL-C declines of 49 and 34 mg/dL, respectively, at 8 months after hospitalization (5). In the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) Trial of intensive versus moderate statins after acute coronary syndrome, patients randomized to 80 mg of atorvastatin daily had average LDL-C declines of 44 mg/dL, and those randomized to 40 mg of pravastatin daily had average declines of 11 mg/dL after 2 years of treatment (4); in this trial, patients were permitted to be taking lipid-lowering therapy at moderate doses at study entry, and, among those subsequently randomized to intensive statins, LDL-C levels fell by 32%. Thus, although some of the decreased effect of statins in our study compared with the clinical trials can be explained by the fact that approximately one-third of patients were taking a statin on arrival, some of the attenuated effect observed in our analyses is likely due to other factors, such as failure to intensify statin treatment, poorer compliance with both diet and medication over time, and statin discontinuation. This hypothesis is supported in our data by a more marked reduction in LDL-C levels at 1 month versus 6 months for both the moderate and intensive statin groups. Because we were studying an unselected patient population and not the highly motivated patients who enroll in clinical trials, it is not surprising to observe differences such as these between the changes in LDL-C levels in the trials versus our population.
A key strength of our study beyond what is known from clinical trials is the unselected nature of our analytical population. For example, whereas only 12% of the populations of the A to Z and PROVE-IT trials were nonwhite, the lipid substudy population of the TRIUMPH trial had 26% nonwhite patients. Although not all TRIUMPH patients agreed to be in the follow-up lipid substudy, there were few meaningful clinical differences between those who did and those who did not participate (Appendix Table 1); in particular, baseline LDL-C levels and the factors found to most influence follow-up LDL-C levels (i.e., age, sex, avoidance of medical care because of cost, and intensity of statin prescription at discharge) were similar between groups. This provides important support for the generalizability of our findings to other unselected acute MI patients populations.
Although there have been several other studies that have evaluated in a cross-sectional manner the percentage of patients with cardiovascular diseases who are “at goal” (11), the changes that occur after an acute cardiovascular event and the factors associated with these changes have rarely been described outside the setting of clinical trials. In 2 separate study of acute MI patients enrolled in multicenter registries, approximately one-third of patients had LDL-C levels greater than 100 mg/dL at 6–12 months of follow-up (12, 13). Both studies evaluated patient factors associated with LDL-C goal attainment, and both found that good adherence to statins was associated with lower LDL-C levels; however, neither evaluated the effect of intensity of statin therapy on LDL-C, which we found to be exceedingly important.
There are several potential limitations to our analyses that merit discussion. First, our study cohort represents a substudy of the overall TRIUMPH study. Although all patients were asked to participate in the substudy, patients had to agree to an in-home visit and additional blood sampling to be included in our study. Although there were some differences between patients who did and those who did not allow for in-home visits, our analytical cohort remained fairly representative of a real-world MI population that has survived the first month after hospitalization. In addition, the severity of MI, LDL-C levels, and statin therapy were similar between participants and nonparticipants, further supporting the generalizability of our findings. Second, not all patients had laboratory values at both 1 month and 6 months after MI. We used a repeated measures model, including all available data, an unstructured correlation matrix, and adjustment for patient risk factors, which mitigates missing-data bias attributable to observed factors. There remains the potential for informatively missing data (i.e., missingness related directly to LDL-C levels), but we believe the likelihood of this, and its impact on our findings, are low. Although it is possible that increased LDL-C levels could be a marker of age or other comorbid conditions, which themselves could be associated with completeness of follow-up, we adjusted for many such factors in our models, ameliorating this bias. As such, the inclusion of risk-factor covariates in our model, as well as the repeated measures framework incorporating freely estimated correlations among the 3 time points, mitigates any potential bias associated with those factors or with the patients’ available LDL-C values. Third, although we identified several factors that were associated with LDL-C levels over time, including statin therapy at discharge, there may have been important factors that affect LDL-C levels that we were unable to include in our models. Importantly, we did not adjust for changes in statin therapy over time, which can be altered because of patient preferences or provider titration. However, because the intensity of statin therapy has been shown rarely to change after discharge for MI (12), and given the difficulty in interpretation of changes in treatment as time-varying covariates, we elected to adjust only for the intensity of statin therapy at discharge. Finally, we were unable to deeply explore the underlying basis for most of the significant associations that we identified between various patient factors and LDL-C levels over time. Subsequent studies need to be conducted to further explore the biological or behavioral explanations for these associations.
In conclusion, we found that LDL-C levels decrease modestly over the 6 months after hospitalization for MI. However, the changes in LDL-C levels were strongly associated with the presence and intensity of statin therapy at discharge. Furthermore, older age, male sex, hypertension, and better socioeconomic status were all associated with lower LDL-C levels over time, even after adjustment for statin therapy. Whether these associations are driven by biological differences in LDL-C levels or statin effectiveness, or whether these represent behavioral differences such as higher compliance with diet or statin therapy will require further study. These findings support the importance of intensive statin therapy in these high-risk patients and also estimate the expected LDL-C changes after MI in a real-world population.
ACKNOWLEDGMENTS
Author affiliations: Department of Cardiovascular Research, Saint Luke's Mid America Heart Institute, Kansas City, Missouri (Suzanne V. Arnold, Mikhail Kosiborod, Fengming Tang, John A. Spertus); Department of Medicine, University of Missouri-Kansas City, Kansas City, Missouri (Suzanne V. Arnold, Mikhail Kosiborod, John A. Spertus); and Eli Lilly and Company, Indianapolis, Indiana (Zhenxiang Zhao, Patrick L. McCollam, Julie Birt).
The TRIUMPH study was sponsored by the National Institutes of Health (National Heart, Lung, Blood Institute) (Washington University School of Medicine Specialized Centers of Clinically Oriented Research grant P50HL077113-01).
The funding organization for the overall study did not play a role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The analyses for this study were sponsored by Eli Lilly and Company, Indianapolis, Indiana.
Conflict of interest: Drs. Zhenxiang Zhao, Patrick L. McCollam, and Julie Birt are employees of Eli Lilly and Company. Drs. Suzanne V. Arnold and John A. Spertus received research grant support from Eli Lilly and Company.
Appendix Table 1.
Sociodemographic and Clinical Characteristics of Patients Who Did and Those Who Did Not Participate in the Lipid Substudy of the TRIUMPH Study, 2005–2008
| Characteristic | Participated (n = 797) |
Did Not Participate (n = 3,469) |
P Value | ||
|---|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | ||
| Sociodemographics | |||||
| Age, years | 58.9 (11.5) | 59.0 (12.5) | 0.791 | ||
| Male sex | 68.1 | 66.3 | 0.331 | ||
| White race | 73.9 | 66.0 | <0.001 | ||
| Married | 56.7 | 51.7 | 0.012 | ||
| Low social support | 17.9 | 17.0 | 0.569 | ||
| High school education | 92.2 | 88.5 | 0.002 | ||
| Avoids health care because of cost | 25.9 | 25.7 | 0.880 | ||
| Comorbidities | |||||
| Hypertension | 65.9 | 66.6 | 0.710 | ||
| Prior bypass graft surgery | 10.8 | 11.4 | 0.615 | ||
| Diabetes mellitus | 27.9 | 31.4 | 0.053 | ||
| Current/former smoking | 60.5 | 59.3 | 0.540 | ||
| Chronic lung disease | 6.6 | 7.3 | 0.508 | ||
| Prior heart failure | 7.7 | 8.5 | 0.419 | ||
| Chronic kidney diseasea | 20.3 | 25.5 | 0.002 | ||
| Body mass indexb | 29.6 (6.1) | 29.5 (6.6) | 0.758 | ||
| Prior stroke or transient attack | 6.3 | 7.1 | 0.382 | ||
| Depressive symptoms | 21.8 | 18.1 | 0.015 | ||
| LDL-C level | 94.3 (30.7) | 95.0 (33.3) | 0.618 | ||
| Clinical presentation and procedures | |||||
| ST elevations | 47.1 | 42.1 | 0.012 | ||
| GRACE risk scorec | 99.1 (28.2) | 100.5 (30.4) | 0.242 | ||
| Ejection fraction, % | 49.3 (12.7) | 48.6 (13.1) | 0.196 | ||
| Length of stay, days | 5.1 (5.9) | 5.5 (6.3) | 0.077 | ||
| Cardiac catheterization | 94.7 | 91.8 | 0.005 | ||
| Angioplasty | 68.8 | 64.6 | 0.026 | ||
| Bypass graft surgery | 9.4 | 9.3 | 0.931 | ||
| Discharge statins | 0.959 | ||||
| No statin | 10.7 | 11.0 | |||
| Submaximal statin | 66.3 | 66.2 | |||
| Maximal statin | 23.0 | 22.8 | |||
Abbreviations: GRACE, Global Registry of Acute Coronary Events; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; SD, standard deviation; TRIUMPH, Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status.
a Estimated glomerular filtration rate less than 60 mL/minute/1.73 m2.
b Weight (kg)/height (m)2.
c A risk score calculated at hospital discharge to predict long-term mortality risk after myocardial infarction (10).
REFERENCES
- 1.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335(14):1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 3.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 4.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 5.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 6.Murphy SA, Cannon CP, Wiviott SD, et al. Effect of intensive lipid-lowering therapy on mortality after acute coronary syndrome (a patient-level analysis of the Aggrastat to Zocor and Pravastatin or Atorvastatin Evaluation and Infection Therapy—Thrombolysis in Myocardial Infarction 22 trials) Am J Cardiol. 2007;100(7):1047–1051. doi: 10.1016/j.amjcard.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 7.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101(2):207–213. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 8.Arnold SV, Chan PS, Jones PG, et al. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4(4):467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong PH. Lack of therapeutic interchangeability of HMG-CoA reductase inhibitors. Ann Pharmacother. 2002;36(12):1907–1917. doi: 10.1345/aph.1C116. [DOI] [PubMed] [Google Scholar]
- 10.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 11.Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation Project Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556–563. doi: 10.1016/j.amjcard.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Melloni C, Shah BR, Ou FS, et al. Lipid-lowering intensification and low-density lipoprotein cholesterol achievement from hospital admission to 1-year follow-up after an acute coronary syndrome event: results from the Medications Applied and Sustained Over Time (MAINTAIN) registry. Am Heart J. 2010;160(6):1121–1129. doi: 10.1016/j.ahj.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Martin SS, Gosch K, Kulkarni KR, et al. Modifiable factors associated with failure to attain low-density lipoprotein cholesterol goal at 6 months after acute myocardial infarction. Am Heart J. 2013;165(1):26–33.e3. doi: 10.1016/j.ahj.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


