Abstract
The availability of weekly Web-based participatory surveillance data on self-reported influenza-like illness (ILI), defined here as self-reported fever and cough/sore throat, over several influenza seasons allows for estimation of the incidence of influenza infection in population cohorts. We demonstrate this using syndromic data reported through the Influenzanet surveillance platform in the Netherlands. We used the 2011–2012 influenza season, a low-incidence season that began late, to assess the baseline rates of self-reported ILI during periods of low influenza circulation, and we used ILI rates above that baseline level from the 2012–1013 season, a major influenza season, to estimate influenza attack rates for that period. The latter conversion required estimates of age-specific probabilities of self-reported ILI given influenza (Flu) infection (P(ILI | Flu)), which were obtained from separate data (extracted from Hong Kong, China, household studies). For the 2012–2013 influenza season in the Netherlands, we estimated combined influenza A/B attack rates of 29.2% (95% credible interval (CI): 21.6, 37.9) among survey participants aged 20–49 years, 28.3% (95% CI: 20.7, 36.8) among participants aged 50–60 years, and 5.9% (95% CI: 0.4, 11.8) among participants aged ≥61 years. Estimates of influenza attack rates can be obtained in other settings using analogous, multiseason surveillance data on self-reported ILI together with separate, context-specific estimates of P(ILI | Flu).
Keywords: attack rate, influenza, influenza-like illness, participatory surveillance
Estimates of age-specific attack rates of influenza infection can aid in planning a response to an influenza epidemic. They provide information on the age-specific risks of becoming infected and of transmitting infection, which may be used to guide mitigation efforts, such as vaccination and antiviral treatment. However, such estimates are difficult to obtain, as many influenza virus infections are associated with subclinical disease, while acute upper respiratory tract infections can have a variety of etiologies other than influenza virus infection.
Traditional surveillance systems used in many countries to characterize influenza epidemics are 1) sentinel surveillance systems tracking rates of medical consultations associated with influenza-like symptoms and 2) virological surveillance systems reporting subtype-specific influenza positivity rates for influenza among the tested respiratory specimens (1, 2). Those 2 data streams may be combined to form a multiplicative proxy for the influenza epidemic curve (3). However, absolute rates of influenza infection are difficult to estimate from these data streams because of uncertainties about the age- and strain-specific likelihoods of consulting a physician upon contracting an influenza infection, the sensitivity of the laboratory testing of respiratory specimens, and the lack of patient age recording for influenza-positive specimens in many countries, including the United States. Influenza infection attack rates during an epidemic (referring to both symptomatic and asymptomatic infections; hereafter called “influenza attack rates”) can potentially be estimated in a transmission modeling framework calibrated against various data sources, such as data on contacts between different age strata, data on medical consultations, virological testing of respiratory specimens, etc. (4–6). While such a framework can shed light on additional issues that are not accessible through analysis of surveillance data alone, such as the impact of vaccination and school closures on an epidemic, it may be subject to uncertainties associated with the assumptions used in the modeling approach. Serological surveillance can potentially overcome some of these difficulties; however, it can be costly and has rarely been employed in the United States (7, 8). It has been used mostly in the pandemic context in recent times (9), largely for the estimation of whole-season attack rates, although midyear serological surveys can be profitable for extracting real-time information about influenza attack rates (10). In addition, serological data are imperfect for measuring influenza incidence, particularly among population strata with higher initial titers (11).
We utilized participatory, survey-based data on self-reported symptoms to estimate influenza attack rates. Such data have previously been used to describe trends in influenza circulation (12) and to estimate influenza attack rates based on changes in the frequencies of certain combinations of symptoms among survey participants (13). In this paper, we demonstrate an approach similar to that of Goldstein et al. (13). We considered weekly rates of self-reported influenza-like illness (ILI), defined as the presence of self-reported fever and cough or sore throat, among participants in the Influenzanet surveillance platform in the Netherlands during the 2011–2012 and 2012–2013 influenza seasons. Our goal was to estimate influenza incidence during the 2012–2013 season, a major influenza season, with the 2011–2012 season, a low-incidence season that started late, being used to examine rates of self-reported ILI not associated with influenza. We found (see Web Appendix 1, available at http://aje.oxfordjournals.org/) that weekly rates of self-reported ILI were stable in winter periods of low influenza circulation during the 2011–2012 season (Web Table 1). This stability allows one to define age-specific baseline (noninfluenza) ILI rates in the survey data. Changes in the ILI rates above the baseline level during periods of active influenza circulation, as indicated by the virological data (14), can then be used to gauge influenza attack rates, provided that one has estimates of the probability of self-reported ILI for influenza cases in different age groups. The latter estimates require a separate data source, which in our case consisted of data on reported symptoms of polymerase chain reaction (PCR) influenza-positive household contacts of clinical cases from Hong Kong, China, studies (15–17). In this paper, we present a detailed description of the above inference method, exhibit its results for the Dutch Influenzanet 2011–2013 data, and include a discussion of data needs for performing analogous estimation in other settings.
METHODS
Weekly ILI incidence rates in the surveillance data
We used data on self-reported symptoms among participants in the Influenzanet surveillance platform in the Netherlands during the 2012–2013 and 2011–2012 influenza seasons. An influenza season is defined as starting in calendar week 47 of a given year and ending in week 20 of the subsequent year. For each season, 4 age-group-specific “main cohorts” (ages ≤19, 20–49, 50–60, and ≥61 years) were defined as the set of persons in each age group who 1) had filled out a report by calendar week 50 and 2) completed reports for at least 50% of the weeks from the date of their first report through week 20 of the subsequent year, with lower thresholds for the number of reports examined in Web Appendix 2 (Web Figures 1 and 2). The week 50 cutoff was chosen because of evidence from virological surveillance data (14) that little influenza had circulated prior to calendar week 51 in either season; different choices might be appropriate in other seasons. There were 379, 3,114, 2,846, and 2,790 participants in the 4 main cohorts, respectively, during the 2012–2013 season. For weeks 48–50, the cohorts included only the subset of persons in the main cohorts who had filled out a report by the preceding calendar week (the week of the first report was discarded because of a potential correlation between symptom presence and willingness to join Influenzanet).
For each age group, the ILI prevalence in this cohort, ILIPr(t), was defined as the number of self-reported ILI cases in week t divided by the number of persons in the cohort (not the number of persons filling out a report during that week—see Discussion). Because an ILI episode may overlap with each of a pair of consecutive weeks (see Discussion), we defined ILI incidence in week t, ILI(t), analogously, except that we removed people from the numerator if they were in their second consecutive week of reporting ILI. In the few cases where people reported ILI for 3 consecutive weeks, we did not remove the third week from the incidence calculation, only the second.
Probability of reporting ILI for influenza cases
Because estimates of the age-specific probabilities of self-reported ILI given influenza (Flu) infection, designated P(ILI | Flu), were not available for the Netherlands, we used data on reported symptoms from participants in community-based studies of influenza transmission within households in Hong Kong (15–17) to estimate P(ILI | Flu) for both adults and children. Index cases were recruited from outpatient clinics if they had acute respiratory illness with recent onset, and a rapid influenza test was used to identify cases with influenza for further follow-up. Households of these cases were visited 3 times (with a 3-day interval between visits), and information on respiratory specimens was collected from members of those households regardless of illness. Data on age, symptoms, and measured temperature of PCR influenza-positive household contacts who had reported on their symptoms and temperature for at least 6 days were utilized. People who tested positive for seasonal influenza A/H1N1 were excluded (because of lack of seasonal A/H1N1 circulation in the Netherlands between 2011 and 2013). The remaining data set represented 99 adults (ages ≥18 years) and 64 children (ages ≤17 years).
The presence of self-reported ILI could not be ascertained directly for the Hong Kong household contacts due to lack of information on self-reported fever. Instead, data on recorded temperature for those household contacts were used, and the probability of reporting fever was related to observed temperature via additional data on the persons who visited outpatient clinics in Hong Kong (2,405 children and 2,139 adults). For those persons, temperature was recorded and the people were independently asked whether they had a recent history of febrile illness at the time of the visit (15, 16). We first stratified the clinic patients who had information on measured and self-reported fever into 22 temperature categories: <36°C, 36°C–36.1°C, … , 39.8°C–39.9°C, and ≥40°C. For each category T, the probability of reporting fever in that temperature category, P(Fever|T), was estimated from data on the presence of self-reported fever among clinical cases with measured temperature in the category T.
To estimate P(ILI | Flu) in a given age group, suppose that there are N influenza-positive household contacts in that age group and that CS is the portion who reported cough or sore throat. For each individual i in CS (i ∈ CS), let Tmax(i) be that individual's maximal recorded temperature. Then the probability of self-reported ILI given influenza infection in that age group is estimated as
 |
(1) |
Inference of influenza attack rates in the adult cohorts
We estimated the influenza attack rate (cumulative incidence) between calendar weeks 51 (in 2012) and 15 (in 2013) of the 2012–2013 season in the Netherlands in each adult age group (with youths aged ≤19 years being excluded because of a small cohort size). We first estimated influenza-associated excess ILI above the non-influenza-associated baseline (Base) for the full season as
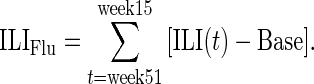 |
Here, the baseline rate of self-reported ILI among noninfluenza cases, Base = P(ILI | non-Flu), is assumed to be constant throughout the influenza season and is estimated as the average weekly ILI incidence during weeks 48–5 of the 2011–2012 season:
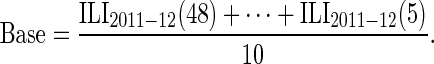 |
(2) |
Selection of the above baseline period during the 2011–2012 season was based on virological data (14) which indicated very little influenza circulation prior to week 6 of 2012 in the Netherlands. Sensitivity with respect to the choice of baseline estimate is explored in Web Appendix 3 (Web Table 2).
Using the estimates derived from equations 1 and 2, one can estimate the influenza attack rate (AR), ARFlu, between weeks 51 and 15 in each age group as
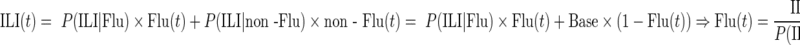 |
Hence,
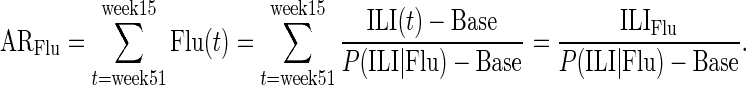 |
(3) |
We extracted posterior samples for each of the quantities in equation 3 (e.g., weekly population ILI incidence) using the observed counts estimating those quantities (e.g., weekly ILI reports and cohort sizes), binomial likelihoods (and, in the case of P(ILI | Flu), where binning of observed temperatures into 22 categories was used, multinomial likelihoods), and flat priors. We combined those independently extracted posterior samples to obtain a posterior sample of estimates for ARFlu via equation 3 (using the first elements of each sample above, the second elements, etc.), for which the mean values and 95% credible intervals are reported.
Statistical analyses were performed using R, version 2.15.2 (R Development Core Team, Vienna, Austria).
RESULTS
Figure 1 shows the probability of self-reported fever, P(Fever|T), at various levels of measured temperature estimated from the Hong Kong clinic data (see Methods section).
Figure 1.
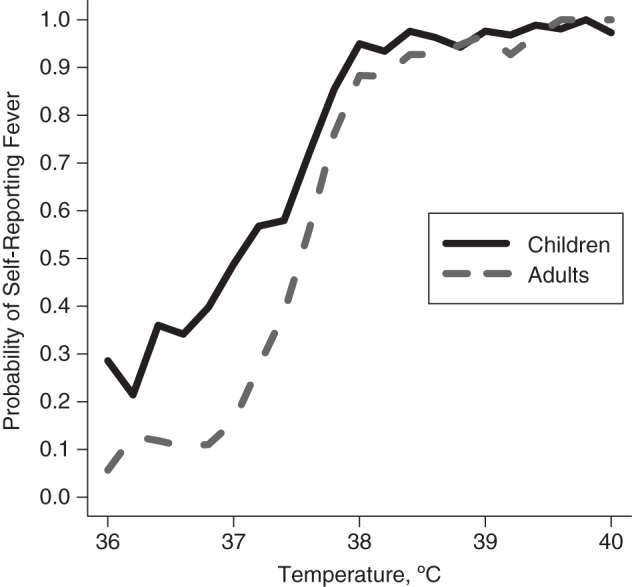
Probability of self-reported fever, P(Fever|T), at given measured temperature levels among children (ages ≤17 years) and adults (ages ≥18 years), 2008. Data were derived from Hong Kong, China, clinic studies (15–17).
Figure 2 shows the probability of self-reported ILI, P(ILI | Flu), for influenza cases in children and adults estimated from the Hong Kong data (equation 1).
Figure 2.
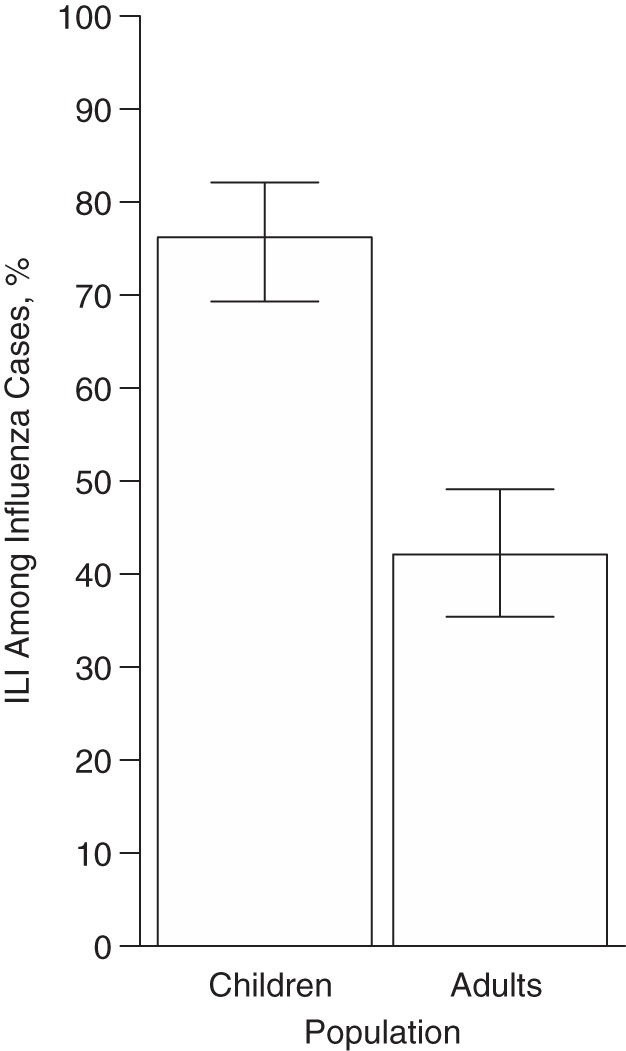
Estimated probability of self-reported influenza-like illness (ILI), P(ILI | Flu), among child (ages ≤17 years) and adult (ages ≥18 years) influenza (Flu) cases, 2008–2009. Data were derived from Hong Kong, China, household studies (15–17). Bars, 95% credible intervals.
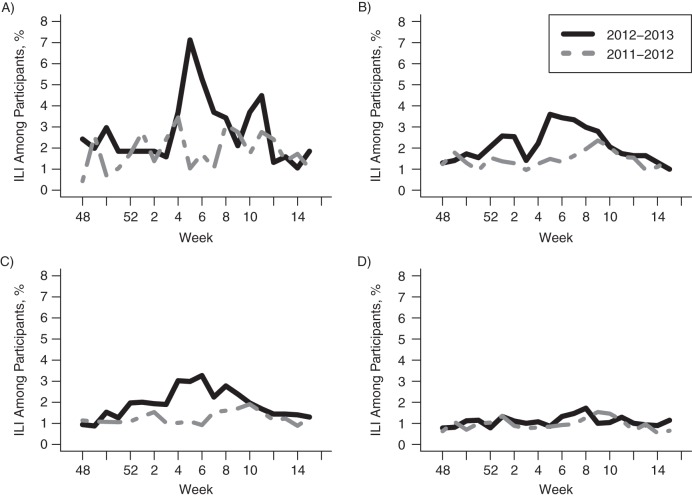
Figure 3 shows the weekly ILI prevalence (ILIPr(t); see Methods section) in the Influenzanet data in the 4 main age groups (≤19, 20–49, 50–60, and ≥61 years) during the 2011–2012 and 2012–2013 seasons. The initial peak of influenza incidence during the 2012–2013 season was dominated by influenza A, followed by a fairly large influenza B peak (see Graphs for The Netherlands, Season 2012/2013 (14), panel 3), which may explain the protracted decline in ILI levels in the age groups 20–49 years and 50–60 years in Figure 3. We also note that for the 2011–2012 season, there seemed to be a good temporal correspondence between the bulk of influenza incidence (Graphs for The Netherlands (14), panel 4) and excess ILI in Figure 3.
Figure 3.
Weekly prevalence of influenza-like illness (ILI), ILIPr(t), among Influenzanet participants in the Netherlands during the 2011–2012 and 2012–2013 influenza seasons, by age group. A) ≤19 years; B) 20–49 years; C) 50–60 years; D) ≥61 years.
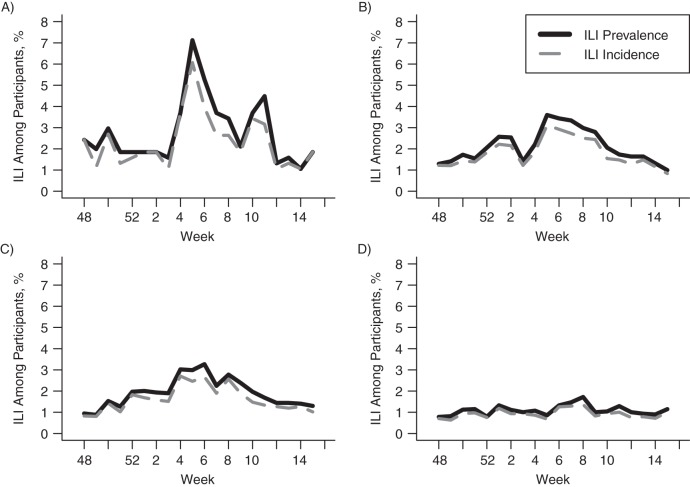
Figure 4 shows the course of ILI incidence, ILI(t), and ILI prevalence, ILIPr(t), by week for the 4 age groups during the 2012–2013 season. Removal of the ILI data from consecutive-week ILI reports results in reductions in ILI incidence of 17.1%, 15.1%, 15.9%, and 15.3%, as compared with prevalence, between weeks 51 and 15 of the 2012–2013 season for the age groups ≤19, 20–49, 50–60, and ≥61 years, respectively.
Figure 4.
Weekly influenza-like illness (ILI) incidence, ILI(t), and prevalence, ILIPr(t), among Influenzanet participants in the Netherlands during the 2012–2013 influenza season, by age group. A) ≤19 years; B) 20–49 years; C) 50–60 years; D) ≥61 years.
Table 1 shows estimates of the influenza attack rate (ARFlu (equation 3)) between weeks 51 and 15 of the 2012–2013 season in the Netherlands for the age groups 20–49, 50–60, and ≥61 years.
Table 1.
Estimates of the Influenza Attack Rate in Different Age Groups in the Netherlands Between Weeks 51 and 15 of the 2012–2013 Influenza Season
| Age Group, years | Influenza Attack Rate |
|
|---|---|---|
| Estimate, % | 95% CI | |
| 20–49 | 29.2 | 21.6, 37.9 |
| 50–60 | 28.3 | 20.7, 36.8 |
| ≥61 | 5.9 | 0.4, 11.8 |
Abbreviation: CI, credible interval.
DISCUSSION
Here we have presented a method for inference of influenza attack rates based on syndromic surveillance data and the assumption that increases in the incidence of self-reported ILI in a defined cohort during periods of known influenza circulation (excess ILI) can be fully attributed to incident influenza cases in the cohort. We chose excess ILI as the correlate of influenza used to estimate influenza incidence in the Dutch Influenzanet data because we found weekly ILI incidence to be stable during periods of low influenza circulation in the winter (Web Appendix 1). We estimated fairly high influenza attack rates in nonelderly adults in the Netherlands during the 2012–2013 season, a season in which influenza A/H1N1pdm, A/H3N2, and B were all circulating actively (14). Those estimates appeared to be noticeably higher than those for the 2009 pandemic in the Netherlands, when incidence was strongly dominated by infections among children (18). In principle, our method can be used to assess influenza attack rates during any period of influenza circulation, not merely the annual attack rate, provided that enough appropriate data exist for such estimation. Real-time information about the impact of an influenza epidemic on different age groups may play a role in promoting extra vaccination efforts, deciding on prioritization strategies for distribution of scarce vaccine (19), emphasizing the need for antiviral treatment in different population groups, etc., and we believe that syndromic surveillance data may play a role in obtaining such information.
Defining influenza incidence is not simple. Our methodology was devised to estimate the proportion of individuals in a cohort who would have been PCR-positive for influenza had they (possibly contrary to fact) been swabbed during the week in question. Because we divided the excess proportion with ILI by P(ILI | Flu), our estimate included both those who were asymptomatic and those who were symptomatic but did not have ILI symptoms. The incidence of PCR-detectable influenza is different from (and in particular, probably larger than) the incidence of seroconversion to influenza, especially for people with high initial titers (11). We note in this regard that our estimates of influenza attack rates in adults were somewhat higher than the estimates of seasonal influenza attack rates in adults obtained by Monto et al. (7, 8) using seroconversion as a criterion for infection, with the differences potentially stemming in part from differences in influenza circulation patterns and in part from the aforementioned difference in the definition of infection. Further work is required to understand how PCR positivity for influenza (and viral shedding more generally), symptoms, and seroconversion (all of which can be measured) relate to the key variables in models of transmission dynamics: infectiousness and acquisition of short- (3) or long-term immunity.
Our assumption that the weekly incidence of ILI among noninfluenza cases was stable throughout the winter period is supported by the demonstrated stability of weekly ILI incidence during known low-influenza periods in our data set (Web Appendix 1) and by the fact that ILI incidence in different age groups appeared to return to the initial baseline levels following periods of active influenza circulation (Figure 3). This assumption may or may not hold in other surveillance systems and time periods, and it should be reexamined in other contexts. We also assumed that stability of ILI incidence during low-influenza winter periods implies stability of non-influenza-associated ILI during the same calendar winter periods for high-influenza seasons, such as weeks 51–5 of the 2012–2013 season. This assumption may also be questioned. For example, panel 4 in Graphs for The Netherlands (14) shows apparently higher levels of respiratory syncytial virus circulation between weeks 50–3 during the 2012–2013 season than during the 2011–2012 season, and the impact of this on ILI in the age groups considered is uncertain. However, overall incidence of ILI during the 2011–2012 season, reflecting ILI associated with respiratory syncytial virus and other causes, suggests that excess ILI caused by respiratory syncytial virus in 2012–2013 was limited compared with baseline ILI levels.
We defined ILI incidence (with the second of 2 consecutive ILI reports removed) for inference of influenza attack rates for the following reason: Given that the probability of reporting ILI is relatively low (peaking at 3.9% for the age group 20–49 years at the peak of influenza incidence) and that 2 consecutive influenza episodes in successive weeks are rather unlikely biologically, the probability of having a second independent ILI case during the following week is quite small, and removing consecutive ILI episodes should have largely eliminated double-reporting of the same disease episode.
For the definition of weekly ILI incidence rates, ILI(t), we used whole cohorts rather than the number of survey participants in each week as the denominator. We believe that our definition of ILI(t) is better suited for estimation of ILI attack rates, because Influenzanet participants are asked to list the symptoms they have experienced since their last survey. Thus, participants (particularly those who report frequently enough) are, in principle, expected to report all ILI episodes they have experienced during the study period (even if they did not fill out a survey during certain weeks). Correspondingly, the average number of ILI episodes a cohort member has experienced is expected to be the total number of ILI episodes reported in the cohort (with double-reporting removed) divided by the cohort size, which is the cumulative rate of incidence (attack rate) for ILI(t).
The extent to which Influenzanet participants are representative of the general Dutch population is uncertain. A detailed comparison of the Influenzanet cohort with the Dutch population was performed by Friesema et al. (12) and Marquet et al. (20). Certain key parameters (percent working, vaccination coverage, underlying health conditions) in the different age groups were found to be similar for Influenzanet participants and the general Dutch population (12, 20). While the age distributions of Influenzanet participants and the Dutch population are rather different, particularly because of the small number of participants under age 20 years in Influenzanet, we applied our estimation of attack rates to adults aged ≥20 years only and stratified them into 3 age groups, improving upon the correspondence in the age distributions.
Our estimates of the probability of self-reported ILI for influenza cases are limited in several ways. Data on symptoms of influenza cases extracted from relatively small Hong Kong studies (15–17) were applied to the Dutch population. However, the likelihood of self-reported ILI for influenza cases may be dependent on the reporting population; in addition, the proportion of influenza cases with ILI symptoms may potentially be modulated by climatic conditions (21). We were hesitant to use other published studies of symptoms of influenza cases (7, 8, 22) because of differences in case recruitment, differences in outcome measures, and mismatches in age groups. Because of a relatively small sample size, no more finely age-stratified data on the probability of ILI for adult influenza cases were used. The issue might be particularly problematic for older adults, whose probability of experiencing ILI upon influenza infection may be different from the corresponding probability for nonelderly adults. While the conclusion following from our results that the influenza attack rates among older adults (those aged ≥61 years) were much lower than those for adults aged ≤60 years is probably true, the quantification of the attack rate among older adults in our paper may be questionable. The results shown in Web Appendix 4 suggest that no difference in the probability of reporting ILI for influenza A/H3N2 and pandemic influenza A/H1N1 was detected (Web Figure 3); however, this probability appears lower for influenza B, with additional differences being possible for a finer age stratification, while our estimate was averaged over all of the available data for influenza-positive persons. Finally, we had to relate the probability of self-reported fever in the Dutch data to the measured temperatures in the Hong Kong data through an additional data set on clinical cases whose temperature was measured and self-reported fever was documented. All of this suggests that future estimates of influenza attack rates using syndromic data would benefit from reestimation of the probability of reporting ILI for influenza cases in specific contexts.
Overall, we believe that despite certain limitations, our paper lays down a framework for estimation of influenza attack rates using syndromic surveillance data that could be applied in other settings, providing a tool for characterization of influenza epidemics. The principal ingredient in this approach is weekly syndromic data reported by age-stratified cohorts representative of the general population. Persons in those cohorts should be recruited prior to periods of active influenza circulation (to avoid a bias for participation associated with symptoms), and syndromic data should be available for more than 1 season (to obtain as much information as possible on weekly ILI rates not associated with influenza during various calendar periods). Another ingredient that should improve upon the accuracy of our method is context-specific reestimation of the probability of self-reported ILI for influenza cases.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Mathematical, Computational, and Modeling Sciences Center, School of Human Evolution and Social Change, Arizona State University, Tempe, Arizona (Oscar Patterson-Lomba); Center for Communicable Disease Dynamics, Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Edward Goldstein, Marc Lipsitch); Department of Immunology and Infectious Disease, Harvard School of Public Health, Boston, Massachusetts (Marc Lipsitch); Gulbenkian Science Institute, Oeiras, Portugal (Sander Van Noort, M. Gabriela M. Gomes); School of Public Health, Li Ka Shing Faculty of Medicine, University of Hong Kong, Hong Kong Special Administrative Region, China (Benjamin J. Cowling); and Centre for Infectious Disease Control, National Institute of Public Health and the Environment, Bilthoven, the Netherlands (Jacco Wallinga).
This work was supported by the US National Institute of General Medical Sciences (award U54GM088558 to M.L. and E.G.), the US National Institutes of Health (award 1K01AI101010-01 to E.G.), the European Commission (grant EC-ICT-231807 to S.V.N. and M.G.M.G.), and the Hong Kong Special Administrative Region University Grants Committee (Area of Excellence Scheme, grant AoE/M-12/06 to B.J.C.).
We thank Drs. John Brownstein and Rumi Chunara for helpful discussions.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
M.L. has received consulting income from the Avian/Pandemic Flu Registry (Outcome Sciences, Inc., funded in part by F. Hoffmann-La Roche Ltd. (Basel, Switzerland)), from Pfizer/Wyeth (New York, New York), and from Novartis Vaccines and Diagnostics (Basel, Switzerland). B.J.C. has received research funding from MedImmune Inc. (Gaithersburg, Maryland) and consulting income from Crucell N.V. (Leiden, the Netherlands). The other authors declare no competing interests.
REFERENCES
- 1.Centers for Disease Control and Prevention. Overview of Influenza Surveillance in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2013. http://www.cdc.gov/flu/weekly/overview.htm. (Accessed November 15, 2013). [Google Scholar]
- 2.Cheng CKY, Lau EHY, Ip DKM, et al. A profile of the online dissemination of national influenza surveillance data. BMC Public Health. 2009;9:339. doi: 10.1186/1471-2458-9-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein E, Cobey S, Takahashi S, et al. Predicting the epidemic sizes of influenza A/H1N1, A/H3N2, and B: a statistical method. PLoS Med. 2011;8(7):e1001051. doi: 10.1371/journal.pmed.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baguelin M, Flasche S, Camacho A, et al. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10(10):e1001527. doi: 10.1371/journal.pmed.1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opatowski L, Fraser C, Griffin J, et al. Transmission characteristics of the 2009 H1N1 influenza pandemic: comparison of 8 Southern Hemisphere countries. PLoS Pathog. 2011;7(9):e1002225. doi: 10.1371/journal.ppat.1002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cauchemez S, Valleron AJ, Boëlle PY, et al. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452(7188):750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 7.Monto AS, Kioumehr F. The Tecumseh Study of Respiratory Illness. IX. Occurrence of influenza in the community, 1966–1971. Am J Epidemiol. 1975;102(6):553–563. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 8.Monto AS, Koopman JS, Longini IM., Jr Tecumseh Study of Illness. XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol. 1985;121(6):811–822. doi: 10.1093/oxfordjournals.aje.a114052. [DOI] [PubMed] [Google Scholar]
- 9.Reed C, Katz JM, Hancock K, et al. Prevalence of seropositivity to pandemic influenza A/H1N1 virus in the United States following the 2009 pandemic. PLoS One. 2012;7(10):e48187. doi: 10.1371/journal.pone.0048187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu JT, Ho A, Ma ES, et al. Estimating infection attack rates and severity in real time during an influenza pandemic: analysis of serial cross-sectional serologic surveillance data. PLoS Med. 2011;8(10):e1001103. doi: 10.1371/journal.pmed.1001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cauchemez S, Horby P, Fox A, et al. Influenza infection rates, measurement errors and the interpretation of paired serology. PLoS Pathog. 2012;8(12):e1003061. doi: 10.1371/journal.ppat.1003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friesema IH, Koppeschaar CE, Donker GA, et al. Internet-based monitoring of influenza-like illness in the general population: experience of five influenza seasons in the Netherlands. Vaccine. 2009;27(45):6353–6357. doi: 10.1016/j.vaccine.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein E, Cowling BJ, Aiello AE, et al. Estimating incidence curves of several infections using symptom surveillance data. PLoS One. 2011;6(8):e23380. doi: 10.1371/journal.pone.0023380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Center for Disease Prevention and Control. Graphs for The Netherlands, Season 2012/2013. Stockholm, Sweden: European Center for Disease Prevention and Control; 2013. http://www.euroflu.org/cgi-files/figures2002.cgi?year=2013&week=20®ion=Netherlands&type=v&pilot=Y. (Accessed November 15, 2013) [Google Scholar]
- 15.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7):437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 16.Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201(10):1509–1516. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362(23):2175–2184. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steens A, Waaijenborg S, Teunis PF, et al. Age-dependent patterns of infection and severity explaining the low impact of 2009 influenza A (H1N1): evidence from serial serologic surveys in the Netherlands. Am J Epidemiol. 2011;174(11):1307–1315. doi: 10.1093/aje/kwr245. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein E, Wallinga J, Lipsitch M. Vaccine allocation in a declining epidemic. J R Soc Interface. 2012;9(76):2798–2803. doi: 10.1098/rsif.2012.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquet RL, Bartelds AI, van Noort SP, et al. Internet-based monitoring of influenza-like illness (ILI) in the general population of the Netherlands during the 2003–2004 influenza season. BMC Public Health. 2006;6:242. doi: 10.1186/1471-2458-6-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Noort SP, Águas R, Ballesteros S, et al. The role of weather on the relation between influenza and influenza-like illness. J Theor Biol. 2012;298:131–137. doi: 10.1016/j.jtbi.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Kilbourne ED. Influenza. New York, NY: Plenum Medical Book Company; 1987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.