Abstract
The pathophysiological consequences of caregiving have not been fully elucidated. We evaluated how caregiving, stress, and caregiver strain were associated with shorter relative telomere length (RTL), a marker of cellular aging. Caregivers (n = 240) and some noncaregivers (n = 98) in the 2008–2010 Survey of the Health of Wisconsin, comprising a representative sample of Wisconsin adults aged 21–74 years, reported their sociodemographic, health, and psychological characteristics. RTL was assayed from blood or saliva samples. Median T and S values were used to determine the telomere-to-single copy gene ratio (T/S) for each sample, and log(T/S) was used as the dependent variable in analyses. Multivariable generalized additive models showed that RTL did not differ between caregivers and noncaregivers (difference in log(T/S) = −0.03; P > 0.05), but moderate-to-high levels of stress versus low stress were associated with longer RTL (difference = 0.15; P = 0.04). Among caregivers, more hours per week of care, caring for a young person, and greater strain were associated with shorter RTL (P < 0.05). Caregivers with discordant levels of stress and strain (i.e., low perceived stress/high strain) compared with low stress/low strain had the shortest RTL (difference = −0.24; P = 0.02, Pinteraction = 0.13), corresponding to approximately 10–15 additional years of aging. Caregivers with these characteristics may be at increased risk for accelerated aging. Future work is necessary to better elucidate these relationships and develop interventions to improve the long-term health and well-being of caregivers.
Keywords: caregivers; caregiver strain; population-based studies; stress, psychological; Survey of the Health of Wisconsin
Informal caregiving (i.e., providing unpaid care to a family member or friend with an illness or disability) is critical to the care of the aging and disabled in the United States (1). Such caregivers, however, are themselves at increased risk of poor health outcomes (2–13). As the number of adults and children requiring informal care rises (14), it is increasingly important to understand the health implications for caregivers.
Telomere length, a putative biological marker of cellular aging, may provide valuable information about the pathophysiological consequences of caregiving. Telomeres are DNA-protein complexes that cap the ends of chromosomes, protecting them from degradation during cell division (15). Telomeres generally shorten with age (16–24), and short telomere length has been associated with numerous health conditions (18, 25–34) and earlier mortality (35–37), although evidence is conflicting (38–41). Short telomere length may be indicative of a poor biological state or higher disease risk (35).
Previous work has provided conflicting evidence for an association between caregiving and shortened telomere length (42, 43). However, perceived stress has consistently been associated with shorter telomeres or reduced telomere maintenance (42, 44–49). Stress theory and recent research suggest that the environment may play a critical role in explaining why some caregivers, but not others, experience elevated stress or adverse health outcomes (50). The details of a caregiver's role and experiences may be important environmental factors that might influence these outcomes and can be readily monitored in the clinical setting. However, aside from 1 study examining duration of caregiving (42), the associations among caregiving characteristics, caregiver strain, and telomere length have yet to be examined. Elucidating these relationships will improve our understanding of the physiological impact of caregiving and help identify high-risk caregivers and potential points of intervention for improving caregiver outcomes.
In this study, we sought to determine the association between caregiving and telomere length in a population-based sample. Specifically, we aimed to determine whether and to what extent 1) caregivers had shorter telomeres than noncaregivers, 2) global perceived stress in the past year was associated with telomere length, and 3) caregiving characteristics and caregiver strain were associated with telomere length. We further evaluated whether this association differed by level of global stress. Findings from this study should help to clarify the pathophysiological impact of caregiving, improving our ability to identify, monitor, and track high-risk caregivers. Further, the findings will suggest points of intervention to prevent or ameliorate the adverse consequences of caregiving, potentially improving the long-term health of both caregivers and their families.
METHODS
Data source
Data were from the 2008–2010 Survey of the Health of Wisconsin (SHOW). The SHOW is an annual statewide survey of civilian noninstitutionalized adults aged 21–74 years, representative of the state of Wisconsin. A description of SHOW procedures is available elsewhere (51). Briefly, participants were selected from a random sample of Wisconsin households using a 2-stage cluster sampling approach. Participants completed face-to-face interviews, self-administered questionnaires, and a physical examination and provided a blood (venipuncture) or saliva (Oragene; DNA Genotek Inc., Kanata, Ontario, Canada (www.dnagenotek.com)) sample. All informal caregivers who provided samples (n = 240) were included in the present study. We randomly selected a subset of noncaregivers with samples (n = 98), frequency-matched to the caregivers on age and sex. This study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin-Madison.
Measures
Independent variables
Identification of caregivers
Informal caregivers were identified by the following question: “There are situations in which people provide regular unpaid care or assistance to a family member (including children) or a friend who has a long-term illness or a disability. In the past 12 months, did you provide any such care or assistance to a family member or friend living with you or living elsewhere?” (52).
Global stress
The Global Perceived Stress Scale from the Jackson Heart Study (53) was used to measure global stress over the last 12 months. This scale assesses perceptions of ongoing stressful conditions in 8 domains (i.e., job, relationships, neighborhood, caring for others, legal problems, medical problems, racism/discrimination, and meeting basic needs). Participants rated each domain on a 4-point Likert scale (ranging from not stressful (0) to very stressful (3)), and scores for the items were summed (possible range, 0–24). Higher scores indicated greater stress.
Caregiving characteristics
Caregivers reported their duration of caregiving (years); number of hours of care provided per week; travel distance from the care recipient (co-resident, ≤20 minutes away, or >20 minutes away); relationship to the care recipient (spouse, adult child (caring for a parent), parent (caring for a child), or other friend/relative); the care recipient's condition (dementia, recovery from surgery, injury, acute illness, or other condition), age, and sex; number of care recipients in the past year; and whether they were currently providing care.
Caregiver strain
A 12-item version of the Caregiver Strain Index (54) was used to evaluate perceived strain among caregivers. This version of the index asked respondents whether 12 statements related to caregiving applied to them (e.g., “It is inconvenient for you”). The number of items endorsed was summed (possible range, 0–12). Higher scores indicated greater strain. Cronbach's α was 0.81.
Dependent variable: telomere length
Telomere length assays were conducted using stored DNA extracted from blood using phenol/chloroform (55) or from saliva (n = 45; 32 caregivers (13.3%) and 13 noncaregivers (13.3%)) using the DNA Genotek protocol (www.dnagenotek.com). Relative telomere length was assayed using quantitative real-time polymerase chain reaction (56). This assay uses separate primer pairs to hybridize and amplify 1) telomere hexamer repeats and 2) single-copy gene (β2-globin) DNA. All polymerase chain reactions were performed on the Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Inc., Foster City, California). The data were then analyzed with Applied Biosystems SDS software (Applied Biosystems, Inc.) to generate the standard curve for each plate. The intraassay coefficients of variation of the threshold cycle values for the telomere and single-gene reactions were 1.27% and 1.41%, respectively. The interplate variations were 4.79% and 4.06%, respectively. All samples were analyzed in triplicate. The median T value and the median S value were used to determine the telomere-to-single copy gene ratio (T/S) for each sample, and the natural logarithm of the ratio, log(T/S), was used in the analyses to account for skewness. A linear transformation of the T/S ratio was used to estimate telomere length in base pairs: base pairs = (T/S) × 1,470.8 + 7,674.5, based on comparisons between telomere restriction fragment lengths, as determined by Southern blot, and T/S ratios among samples previously examined by the laboratory (n = 16; r = 0.57, P = 0.02).
Covariates
Sociodemographic characteristics
Participants reported their age, sex, race/ethnicity (non-Hispanic white vs. other), annual income, educational attainment, employment status (employed in the past week vs. unemployed), type of health insurance (none, public, private, or mixed), marital status (married/partnered, divorced/widowed/separated, or never married), and the number of adults and children in the household. Participants reported their combined annual family income categorically (e.g., $25,000–$29,999). These data were recoded to the midpoint to approximate a continuous measure; the highest category (≥$200,000) was recoded to $392,396 by assuming a Pareto distribution of income (57). Educational attainment was reported as the highest grade or level of schooling completed and was recoded as years of education.
Lifestyle factors
Information on smoking (current, former, or never smoker), alcohol consumption (nondrinker, moderate drinker, or risky drinker), leisure-time and transportation-related physical activity (metabolic equivalent of task (MET)-minutes per week), and diet (fruit/vegetable consumption and percentage of calories derived from fat (58)) was obtained via personal interview. Sleep quality (single-item report on a 5-point Likert scale; dichotomized to excellent/very good/good vs. fair/poor), sleep problems, and nightly sleep duration (hours; continuous nonlinear term) were reported using the self-administered questionnaires.
Health factors
Respondents reported their history of 47 health conditions (see the Web Appendix, available at http://aje.oxfordjournals.org/), and the number of health conditions was calculated. An inventory of prescription medications taken by the participant was conducted during the home interview using a standardized protocol (59), and the total number of prescription medications used in the past 30 days was calculated. Height and weight were measured during the examination visit, and body mass index (weight (kg)/height (m)2) was calculated.
Analytical approach
All analyses were conducted in R 2.15.0 (60). Multiple imputation with predictive mean matching was used to predict the values of missing data (61); 5 imputations were conducted. Nonlinear transformations were allowed when predicting missing values for continuous variables. All analyses were conducted using the imputed data sets. Estimates and standard errors were combined using Rubin's rules (62, 63).
Caregiver and noncaregiver characteristics were compared using cross-tabulations with χ2 tests, t tests, or Wilcoxon tests. All tests of statistical significance were 2-sided. Generalized additive models with thin-plate regression splines were constructed to examine whether 1) caregivers had shorter telomeres than noncaregivers, 2) global stress was associated with shorter telomere length, and 3) caregiving characteristics and caregiver strain were associated with telomere length. Multivariable analyses controlled a priori for caregiver age, sex, race, number of chronic conditions, and prescription medication use. Manual backward selection was used to determine whether additional sociodemographic characteristics were included in the model: All covariates were included simultaneously, and each variable with P < 0.20 was removed individually. If removing the variable did not substantially change the β coefficient of the primary independent variable (>10%), it was not included in the final model. The models were also tested while controlling for lifestyle factors that may lie in the pathway between caregiving or stress and telomere length—including diet, exercise, smoking, alcohol consumption, and sleep—and body mass index. Because these factors did not substantively influence the findings, only the results from the parsimonious models are reported. All continuous variables were tested as nonlinear terms in the models; generalized cross-validation (64) was used.
Follow-up analyses were conducted among caregivers in order to determine whether the association of global stress and telomere length differed by the amount of reported caregiver strain. The variables were dichotomized at the median value among caregivers (global stress: 6; strain: 4), and mutually exclusive groups were created. The association between these stress-strain groups and telomere length was evaluated as above.
In order to account for the complex survey design, we then conducted these analyses using sampling weights. Nonlinear terms were included in the models as natural cubic splines, with fixed degrees of freedom estimated from the generalized additive models.
In addition, because mean telomere length differed by tissue source (blood: mean T/S = 0.91; saliva: mean T/S = 1.16 (P < 0.05)), we performed a sensitivity analysis dropping the saliva samples.
RESULTS
Table 1 gives the characteristics of caregivers and noncaregivers in this study. Caregivers did not differ significantly from noncaregivers with regard to any of the characteristics.
Table 1.
Characteristics of Caregivers and Noncaregivers in the Survey of the Health of Wisconsin, 2008–2010a
| Noncaregivers (n = 246,927)b |
Caregivers (n = 546,156)b |
|||
|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | |
| Sociodemographic Factors | ||||
| Age, years | 46.7 (13.5) | 51.6 (12.7) | ||
| Sex | ||||
| Female | 55.8 | 63.6 | ||
| Male | 44.2 | 36.4 | ||
| Race/ethnicity | ||||
| White (non-Hispanic) | 90.5 | 88.0 | ||
| Other | 9.5 | 12.0 | ||
| Annual income (in thousands), dollars | 71.8 (37.2) | 71.9 (81.8) | ||
| Educational attainment, years | 14.6 (2.1) | 14.2 (2.5) | ||
| Employment status | ||||
| Employed | 81.4 | 69.3 | ||
| Unemployed | 18.6 | 30.7 | ||
| Type of health insurance | ||||
| None | 7.2 | 5.7 | ||
| Public | 6.0 | 8.8 | ||
| Private | 77.9 | 66.1 | ||
| Mixed | 9.0 | 19.4 | ||
| Marital status | ||||
| Married/partnered | 80.6 | 74.1 | ||
| Divorced/widowed/separated | 7.2 | 16.8 | ||
| Never married | 12.1 | 9.1 | ||
| No. of adults in the household | 2.1 (0.6) | 2.1 (0.8) | ||
| No. of children in the household | 1.0 (1.1) | 0.7 (1.1) | ||
| Lifestyle Factors | ||||
| Smoking status | ||||
| Current smoker | 8.7 | 18.3 | ||
| Former smoker | 30.8 | 32.9 | ||
| Never smoker | 60.5 | 48.8 | ||
| Alcohol consumption statusc | ||||
| Nondrinker | 46.6 | 43.3 | ||
| Moderate drinker | 45.8 | 41.7 | ||
| Risky drinker | 7.6 | 15.0 | ||
| Physical activity, MET-minutes/week | 1,761.7 (2,830.5) | 2,030.6 (3,011.6) | ||
| Housework, minutes/month | 727.5 (1,934.4) | 879.4 (1,652.5) | ||
| Fruit/vegetable consumption, servings/day | 2.5 (1.6) | 2.7 (1.6) | ||
| Percentage of calories derived from fat | 34.7 (0.7) | 34.9 (0.7) | ||
| Sleep time, hours/night | 7.1 (1.2) | 6.8 (1.1) | ||
| Health Factors | ||||
| Total no. of health conditions | 2.5 (2.0) | 2.8 (2.0) | ||
| No. of prescription medications | 2.3 (2.7) | 2.8 (3.5) | ||
| Global stressd | 6.2 (3.9) | 6.7 (4.3) | ||
| Telomere length (estimated no. of base pairs) | 9,121.4 (771.2) | 9,015.9 (677.8) | ||
| Caregiving Characteristics | ||||
| Duration of caregiving, years | 4.8 (6.1) | |||
| Amount of caregiving, hours/week | 17.7 (31.1) | |||
| Travel distance from care recipient, minutes | ||||
| Co-resident | 32.3 | |||
| ≤20 | 50.6 | |||
| >20 | 17.0 | |||
| Relationship with care recipient | ||||
| Spouse | 15.9 | |||
| Adult child (caring for a parent) | 44.8 | |||
| Parent (caring for a child) | 10.5 | |||
| Other friend/relative | 28.7 | |||
| Care recipient's condition | ||||
| Dementia | 14.9 | |||
| Recovery from surgery | 8.3 | |||
| Other | 76.8 | |||
| No. of care recipients | 1.2 (0.5) | |||
| Caregiver status | ||||
| Current caregiver | 59.5 | |||
| Caregiving in the past 12 months | 40.5 | |||
| Caregiver straine | 4.8 (3.2) | |||
| Age of care recipient, years | 63.8 (25.8) | |||
| Sex of care recipient | ||||
| Male | 37.4 | |||
| Female | 62.6 | |||
Abbreviations: MET, metabolic equivalent of task; SD, standard deviation.
a No differences reached statistical significance (P < 0.05; χ2 tests, t tests, and Wilcoxon tests were used).
b Weighted values. Unweighted totals were 98 for noncaregivers and 240 for caregivers.
c Moderate drinking was defined as fewer than 8 drinks per week in the past year for women and fewer than 15 drinks per week in the past year for men. Risky drinking was defined as 8 or more drinks per week in the past year for women and 15 or more drinks per week in the past year for men. Persons who did not report any alcohol consumption in the past year were classified as nondrinkers.
d Global stress over the last 12 months was self-reported using the Global Perceived Stress Scale from the Jackson Heart Study (53). Eight items were measured on a 4-point Likert scale (ranging from not stressful (0) to very stressful (3)), and scores were summed (possible range, 0–24); higher scores indicate greater stress.
e A 12-item version of the Caregiver Strain Index (54) was used to evaluate perceived strain among caregivers. Respondents were asked whether 12 statements related to caregiving applied to them (e.g., “It is inconvenient for you”). The number of items endorsed was summed (possible range, 0–12); higher scores indicate greater strain.
Unadjusted analyses
In the unadjusted analyses (Table 2), there was no mean difference in telomere length between caregivers and noncaregivers (difference = −0.07; P = 0.33). Global stress was nonlinearly associated with telomere length, such that persons reporting moderate global stress (approximately 7–11 points on the global stress scale; data not shown) had longer telomeres. When stress was examined categorically, persons with stress scores of 7–11 had significantly longer telomeres than those with stress scores of 6 or less (difference = 0.14; P = 0.04). Among caregivers, providing more hours of care per week and reporting greater strain were associated with shorter telomeres (difference in log(T/S) per doubling of hours = −0.04 (P = 0.04); difference in log(T/S) per doubling of strain = −0.06 (P = 0.05)).
Table 2.
Bivariate Associations Between Caregiving and Stress Factors and Telomere Length (Log(T/S)) in the Survey of the Health of Wisconsin, 2008–2010
| βa | 95% CI | P Value | |
|---|---|---|---|
| Caregiver status | |||
| Caregiver | −0.07 | −0.20, 0.07 | 0.33 |
| Noncaregiver | 0 | Reference | |
| Global stress scoreb | |||
| ≤6 | 0 | Reference | |
| 7–11 | 0.14 | 0.00, 0.28 | 0.04 |
| ≥12 | −0.04 | −0.21, 0.14 | 0.67 |
| Duration of caregiving (per doubling), years | −0.02 | −0.05, 0.02 | 0.30 |
| Amount of caregiving (per doubling), hours/week | −0.04 | −0.07, 0.00 | 0.04 |
| Relationship with care recipient | |||
| Spouse | 0 | Reference | |
| Adult child (caring for a parent) | 0.03 | −0.11, 0.18 | 0.64 |
| Parent (caring for a child) | −0.13 | −0.33, 0.07 | 0.20 |
| Other friend/relative | 0.15 | −0.05, 0.35 | 0.13 |
| Travel distance from care recipient, minutes | |||
| Co-resident | 0 | Reference | |
| ≤20 | 0.01 | −0.15, 0.17 | 0.92 |
| >20 | −0.12 | −0.27, 0.03 | 0.12 |
| Care recipient's condition | |||
| Dementia | 0.05 | −0.10, 0.20 | 0.51 |
| Recovering from surgery | −0.08 | −0.56, 0.41 | 0.75 |
| Other | 0 | Reference | |
| Care recipient's age, years | |||
| <25 | −0.15 | −0.37, 0.06 | 0.16 |
| 25–44 | −0.02 | −0.24, 0.21 | 0.87 |
| 45–64 | 0.03 | −0.19, 0.25 | 0.80 |
| 65–84 | −0.03 | −0.24, 0.18 | 0.77 |
| ≥85 | 0 | Reference | |
| Caregiver strain,c per doubling | −0.06 | −0.12, 0.00 | 0.05 |
Abbreviations: CI, confidence interval; T/S, telomere-to-single copy gene ratio.
a For continuous covariates, the estimate represents the change in log(T/S) per doubling of the covariate; for categorical covariates, the estimate represents the difference from the reference group.
b Global stress over the last 12 months was self-reported using the Global Perceived Stress Scale from the Jackson Heart Study (53). Eight items were measured on a 4-point Likert scale (ranging from not stressful (0) to very stressful (3)), and scores were summed (possible range, 0–24); higher scores indicate greater stress.
c A 12-item version of the Caregiver Strain Index (54) was used to evaluate perceived strain among caregivers. Respondents were asked whether 12 statements related to caregiving applied to them (e.g., “It is inconvenient for you”). The number of items endorsed was summed (possible range, 0–12); higher scores indicate greater strain.
Adjusted analyses
Caregiver status
In the adjusted analyses, there was no mean difference in telomere length between caregivers and noncaregivers after controlling for covariates (difference = −0.03; P = 0.64 (Table 3)). As expected, older age was associated with shorter telomeres (P = 0.004; Figure 1).
Table 3.
Association Between Caregiver Status and Telomere Length (Log(T/S)) in the Survey of the Health of Wisconsin, 2008–2010a
| βb | 95% CI | P Value | |
|---|---|---|---|
| Intercept | −0.21 | −0.59, 0.17 | |
| Caregiver status | |||
| Caregiver | −0.03 | −0.16, 0.10 | 0.64 |
| Noncaregiver | 0 | Reference | |
| Sex | |||
| Female | 0.04 | −0.08, 0.16 | 0.52 |
| Male | 0 | Reference | |
| Race/ethnicity | |||
| White (non-Hispanic) | 0.05 | −0.11, 0.20 | 0.56 |
| Other | 0 | Reference | |
| Employment status | |||
| Employed | −0.14 | −0.26, −0.01 | 0.03 |
| Unemployed | 0 | Reference | |
| Educational attainment, years | |||
| <12 | 0 | Reference | |
| ≥12 | 0.17 | 0.03, 0.31 | 0.02 |
| No. of adults in the household | 0.07 | 0.01, 0.13 | 0.02 |
| No. of health conditions | 0.02 | −0.01, 0.05 | 0.21 |
| No. of prescription medications | |||
| 0 | 0 | Reference | |
| ≥1 | −0.13 | −0.28, 0.03 | 0.11 |
Abbreviations: CI, confidence interval; T/S, telomere-to-single copy gene ratio.
a The model also controlled for age as a nonlinear term (P < 0.01).
b For continuous covariates, the estimate represents the change in log(T/S) per unit increase in the covariate; for categorical covariates, the estimate represents the difference from the reference group.
Figure 1.
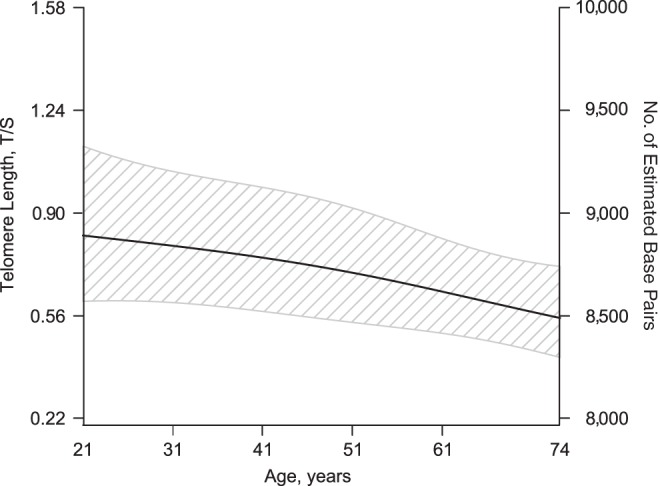
Association between age and telomere length among caregivers and noncaregivers in Wisconsin, Survey of the Health of Wisconsin, 2008–2010. The model controlled for caregiver status, sex, race/ethnicity, employment status, educational attainment, household composition (numbers of adults and children in the household), number of health conditions, and prescription medication use (P = 0.004 (generalized additive model)). T/S, telomere-to-single copy gene ratio.
Global stress
Perceived global stress was significantly nonlinearly associated with telomere length: Persons reporting moderate global stress (about 7–11 points on the Global Perceived Stress Scale) had longer telomeres (P = 0.01; Figure 2). When this variable was examined categorically, persons with stress scores of 7–11 had significantly longer telomeres than those with scores of 6 or less (difference = 0.15 (about 184 base pairs); P = 0.04 (Table 4)).
Figure 2.
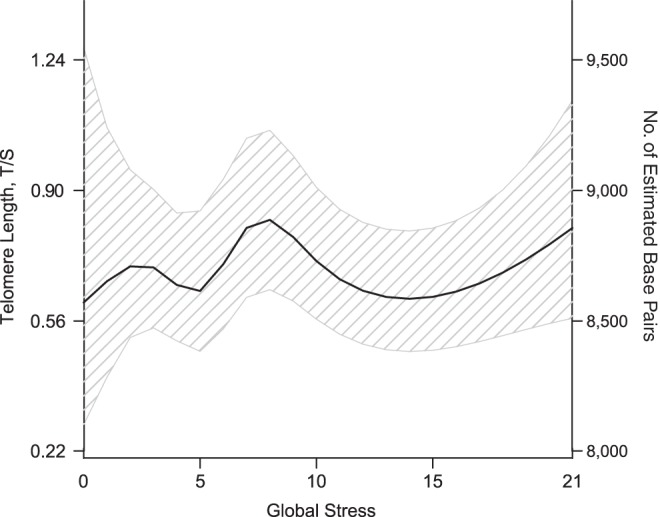
Association between global stress and telomere length among caregivers and noncaregivers in Wisconsin, Survey of the Health of Wisconsin, 2008–2010. The model controlled for caregiver status, age, sex, race/ethnicity, employment status, educational attainment, number of adults in the household, number of health conditions, and prescription medication use (P = 0.01 (generalized additive model)). Global stress over the last 12 months was self-reported using the Global Perceived Stress Scale from the Jackson Heart Study (53). Eight items were measured on a 4-point Likert scale (ranging from not stressful (0) to very stressful (3)), and scores were summed; higher scores indicate greater stress. T/S, telomere-to-single copy gene ratio.
Table 4.
Association Between Global Stress and Telomere Length (Log(T/S)) Among Caregivers and Noncaregivers in the Survey of the Health of Wisconsin, 2008–2010
| βa | 95% CI | P Value | |
|---|---|---|---|
| Intercept | −0.24 | −0.64, 0.15 | |
| Global stress scoreb | |||
| ≤6 | 0 | Reference | |
| 7–11 | 0.15 | 0.01, 0.29 | 0.04 |
| ≥12 | −0.08 | −0.27, 0.11 | 0.42 |
| Age, per 10 years | −0.05 | −0.10, −0.01 | 0.03 |
| Sex | |||
| Female | 0.04 | −0.08, 0.16 | 0.51 |
| Male | 0 | Reference | |
| Race/ethnicity | |||
| White (non-Hispanic) | −0.01 | −0.17, 0.14 | 0.87 |
| Other | 0 | Reference | |
| Educational attainment, years | |||
| <12 | 0 | Reference | |
| ≥12 | 0.19 | 0.07, 0.32 | <0.01 |
| No. of adults in the household | 0.08 | 0.02, 0.15 | <0.01 |
| Caregiver status | |||
| Caregiver | −0.04 | −0.17, 0.10 | 0.60 |
| Noncaregiver | 0 | Reference | |
| No. of health conditions | 0.02 | −0.01, 0.05 | 0.19 |
| No. of prescription medications | |||
| 0 | 0 | Reference | |
| ≥1 | −0.12 | −0.27, 0.04 | 0.13 |
Abbreviations: CI, confidence interval; T/S, telomere-to-single copy gene ratio.
a For continuous covariates, the estimate represents the change in log(T/S) per unit increase in the covariate; for categorical covariates, the estimate represents the difference in log(T/S) from the reference group.
b Global stress over the last 12 months was self-reported using the Global Perceived Stress Scale from the Jackson Heart Study (53). Eight items were measured on a 4-point Likert scale (ranging from not stressful (0) to very stressful (3)), and scores were summed (possible range, 0–24); higher scores indicate greater stress.
Caregiving characteristics
Among caregivers, those who provided more hours of care per week had significantly shorter telomeres (difference in log(T/S) per doubling of hours = −0.04 (about 54 fewer base pairs); P = 0.004) (see Table 5, bivariate-adjusted results). In addition, those providing care to persons under the age of 25 years had significantly shorter telomeres than those caring for older persons, equating to as much as a 342-base-pair difference. Finally, greater caregiver strain was associated with shorter telomeres (difference in log(T/S) per doubling of strain = −0.07 (about 99 fewer base pairs); P = 0.03). When all of the caregiving characteristics were included in the model simultaneously (see Table 5, multivariate-adjusted results), these findings remained. In addition, persons who did not co-reside with the care recipient had shorter telomeres than those who did. Full regression results are shown in Web Tables 1–7.
Table 5.
Association Between Caregiving Characteristics and Telomere Length (Log(T/S)) Among Caregivers in the Survey of the Health of Wisconsin, 2008–2010
| Bivariate-Adjusted Results (Caregiving Characteristics Modeled Separately) |
Multivariate-Adjusted Resultsa (Caregiving Characteristics Modeled Jointly) |
|||||
|---|---|---|---|---|---|---|
| βb | 95% CI | P Value | βb | 95% CI | P Value | |
| Intercept | 0.52 | −0.05, 1.10 | ||||
| Caregiving characteristics | ||||||
| Duration of caregiving (per doubling), yearsc | −0.01 | −0.04, 0.01 | 0.34 | −0.02 | −0.04, 0.01 | 0.21 |
| Amount of caregiving (per doubling), hours/weekc | −0.04 | −0.07, −0.01 | <0.01 | −0.05 | −0.08, −0.02 | <0.01 |
| Relationship with care recipientd | ||||||
| Spouse | 0 | Reference | 0 | Reference | ||
| Adult child (caring for a parent) | 0.07 | −0.06, 0.19 | 0.30 | 0.11 | −0.10, 0.33 | 0.29 |
| Parent (caring for a child) | −0.12 | −0.32, 0.08 | 0.25 | 0.00 | −0.20, 0.20 | 0.98 |
| Other friend/relative | 0.09 | −0.11, 0.29 | 0.37 | 0.15 | −0.07, 0.38 | 0.18 |
| Travel distance from care recipient, minutese | ||||||
| Co-resident | 0 | Reference | 0 | Reference | ||
| ≤20 | 0.06 | −0.07, 0.19 | 0.39 | −0.19 | −0.34, −0.04 | 0.01 |
| >20 | −0.02 | −0.17, 0.13 | 0.84 | −0.24 | −0.45, −0.03 | 0.02 |
| Care recipient's conditiond | ||||||
| Dementia | 0.07 | −0.11, 0.25 | 0.45 | 0.06 | −0.09, 0.22 | 0.43 |
| Recovering from surgery | −0.13 | −0.54, 0.28 | 0.54 | −0.20 | −0.58, 0.19 | 0.32 |
| Other | 0 | Reference | 0 | Reference | ||
| Care recipient's age, yearsf | ||||||
| <25 | −0.29 | −0.44, −0.13 | <0.01 | −0.26 | −0.46, −0.06 | <0.01 |
| 25–44g | −0.12 | −0.31, 0.07 | 0.21 | −0.02 | −0.25, 0.21 | 0.88 |
| 45–64g | −0.08 | −0.25, 0.09 | 0.37 | −0.06 | −0.29, 0.18 | 0.64 |
| 65–84g | −0.08 | −0.24, 0.09 | 0.38 | −0.02 | −0.16, 0.13 | 0.84 |
| ≥85g | 0 | Reference | 0 | Reference | ||
| Caregiver strain (per doubling)h | −0.07 | −0.13, −0.01 | 0.03 | −0.07 | −0.13, 0.00 | 0.05 |
Abbreviations: CI, confidence interval; T/S, telomere-to-single copy gene ratio.
a In the multivariate-adjusted analysis, the model controlled for all variables in the table and all variables listed in footnote c, as well as marital/partner status, employment status, and sex of the care recipient.
b For continuous covariates, the estimate represents the change in log(T/S) per doubling of the covariate; for categorical covariates, the estimate represents the difference from the reference group.
c In the bivariate-adjusted analysis, the model controlled for age, sex, race/ethnicity, educational attainment ≥12 years, number of adults in the household, number of health conditions, prescription medication use, current caregiving status, and having more than 1 care recipient.
d In the bivariate-adjusted analysis, the model controlled for all of the variables listed in footnote c, as well as marital/partner status and number of children in the household.
e In the bivariate-adjusted analysis, the model controlled for all of the variables listed in footnote c, as well as employment status, annual income (log-transformed), marital/partner status, and number of children in the household.
f In the bivariate-adjusted analysis, the model controlled for all of the variables listed in footnote c, as well as marital/partner status.
g Significantly different from <25 years of age (P < 0.05) in both bivariate-adjusted and multivariate-adjusted analyses.
h In the bivariate-adjusted analysis, the model controlled for age, sex, race/ethnicity, educational attainment ≥12 years, number of adults in the household, number of health conditions, prescription medication use, and current caregiving status.
In addition, the association between caregiver strain and telomere length differed by the level of stress reported by caregivers. As Figure 3 shows, persons who reported high caregiver strain but low global perceived stress had significantly shorter telomeres than those reporting both low strain and low stress (about 316 fewer base pairs; P = 0.02) and borderline significantly shorter telomeres than those with high strain and high stress (about 250 fewer base pairs; P = 0.08) or low strain and high stress (about 302 fewer base pairs; P = 0.05). The overall interaction did not reach statistical significance (overall P = 0.13).
Figure 3.
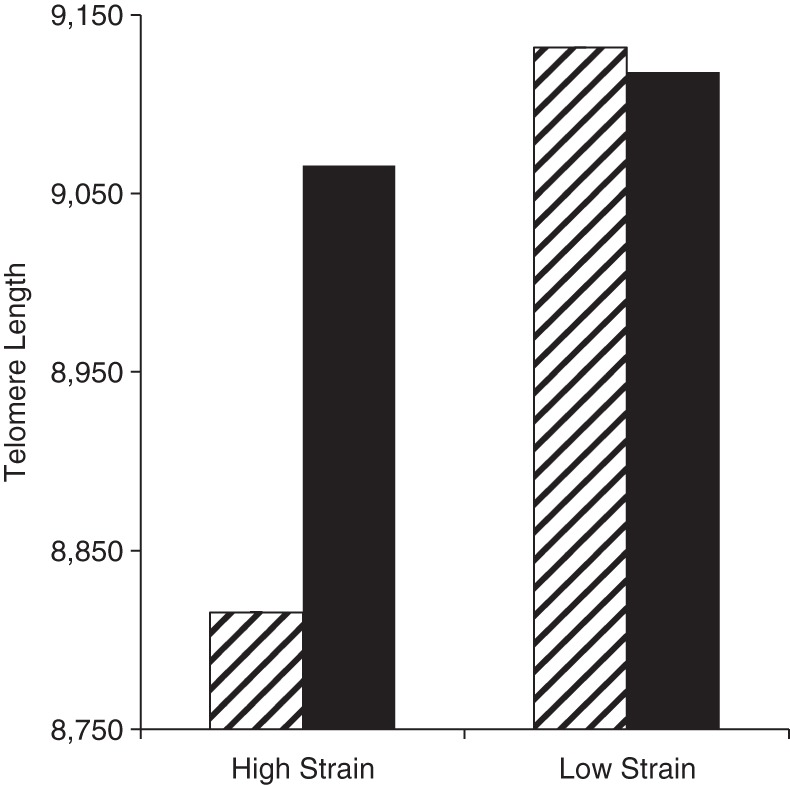
Average telomere length (number of estimated base pairs) according to level of caregiver strain (score of ≤4 vs. >4) and global stress (score of ≤6 vs. >6), Survey of the Health of Wisconsin, 2008–2010. Black bars indicate high stress; striped bars indicate low stress. The model controlled for age, sex, employment status, marital/partner status, race/ethnicity, educational attainment, annual income (log-transformed), household composition (numbers of adults and children in the household), number of health conditions, prescription medication usage, current caregiving status, and having more than 1 care recipient. Average adjusted telomere length in the high-strain/low-stress group was significantly lower than that in the low-strain/low-stress group (P = 0.02) and was borderline significantly lower than that in the low-strain/high-stress and high-strain/high-stress groups (P < 0.10); the overall P value for the interaction was 0.13 (generalized additive model).
When saliva samples were dropped from the analyses, the results were largely unchanged. However, the association between moderate-to-high stress and telomere length was attenuated slightly (difference = 0.13; P = 0.11 (data not shown)). In addition, the estimate for caring for a person under 25 years of age was attenuated (difference = −0.16; P = 0.11), and the association between strain and telomere length was of borderline significance (difference = −0.09; P = 0.07).
DISCUSSION
To our knowledge, this was the first population-based study to examine the association between caregiving and telomere length. The findings from this study highlight subgroups of caregivers who have shorter telomeres (i.e., those providing more hours of care, caring for a child or young adult, or reporting greater strain) and may therefore be at particularly high risk of poor health outcomes. Further, the findings suggest that stress and caregiver strain have complex, nonlinear associations with cellular aging that are in need of further examination.
Previous studies of the association between caregiving and telomere length have been conducted in small convenience samples, and results have been conflicting (42, 43). The present study provides evidence against overall differences in telomere length by caregiver status. This null result may be attributable to caregiver heterogeneity (7, 65–73). Although subgroup sizes limited our ability to examine this, future work should more closely examine the role of heterogeneity in telomere attrition among caregivers.
While several previous studies have provided evidence that greater psychological stress is associated with shorter telomeres (42, 44–49), we found that participants who reported a moderate-to-high level of global stress in the past year had longer telomeres than those with low levels of stress. It is possible that our population-based sample captured a broader swath of the stress distribution than previous convenience-based studies, which may have identified only the associations at the higher end of the stress distribution. In addition, it is possible that psychological stress over short, intermediate, and long terms has differential associations with cellular aging (21, 74). Interestingly, the “U-shaped” curve observed in this study is reminiscent of the association between stress and other factors, such as resiliency (75) and physiological and mental function (76): While a large amount of stress is deleterious, a small amount may improve fitness. Future work is clearly needed to confirm and further explore this finding.
In the only previous study to have examined the association between caregiving characteristics and telomere length, Epel et al. (42) reported that longer duration of care was associated with shorter telomeres. While we found no evidence of this association in our sample, we did find that more hours per week of caregiving, younger age of the care recipient, and greater caregiver strain were associated with shorter telomeres. Psychological aspects, such as feeling more uncertainty or less choice (77, 78) or perceiving a higher level of care recipient disability/need (79), may contribute to poor health outcomes. Interestingly, the association between number of hours per week of providing care and shorter telomere length was not attributable to caregivers' time use (e.g., reduced time spent exercising or sleeping) and was also independent of stress, strain, health factors, and caregivers' health behaviors (data not shown).
Caregivers of persons under the age of 25 years also had significantly shorter telomeres than caregivers of older persons (although this effect was attenuated when saliva samples were dropped). Providing care for a child who is ill or disabled may be considered “off-time” or nonnormative (80) and has been associated with feelings of uncertainty and depression (81, 82), and may therefore be more deleterious to the caregiver. These changes may accumulate over time and may not manifest as health problems until later in life (71), plausibly due to accelerated telomere attrition.
To our knowledge, this is the first study to have found that greater caregiver strain is associated with shorter telomeres. In addition, we found that persons who had high levels of strain but low levels of stress had substantially shorter telomeres than other caregivers, translating to an estimated 7–10 additional years of aging (17, 24, 83). Some caregivers may have underreported their level of perceived global stress (84), potentially because of their engagement in the caregiver role (i.e., being strong for the family), stress habituation, or avoidant coping or denial, which is deleterious over the long term (85–88). Additionally, the associations between global stress and strain with telomere length may differ, as different types of adversity are associated with different biological cascades (89–92). Further, experiencing stress outside of the caregiving realm may indicate greater connection with the community and engagement outside of caregiving, which may be protective (93–98). However, because the overall interaction was not statistically significant, future work with greater statistical power will be necessary to confirm and clarify these findings.
Several potential biological mechanisms also exist that may explain the observed association between greater global stress and longer telomeres and the potential discordance in telomere length by levels of strain and global stress, including telomerase activation, alternative lengthening of telomeres, and changes in the leukocyte subpopulation (15, 99–103). Psychological stress contributes to a biological cascade resulting in increased cellular stressors (e.g., oxidative stress) that may trigger telomerase activation (104) or alternative lengthening mechanisms. Indeed, some studies have found an association between stress or adverse exposures such as caregiving and increased telomerase activation (43, 48, 105–107), which could contribute to telomere lengthening. Finally, telomere length and dynamics differ across different types of white blood cells (103), and observed lengthening of telomeres may be due to changes in the cell mixture (101, 102). Therefore, if caregiving, stress, or strain contributed to different cell-type proportions, this might contribute to observed telomeric differences.
This study has several important implications. The telomere length differences in our study were in line with the magnitudes seen in relation to chronic disease burden (about 132 base pairs) (108) and myocardial infarction (about 300 base pairs) (109), highlighting the potential clinical significance of these findings. Assessing and monitoring hours per week of caregiving, age of the care recipient, and level of caregiver strain may help clinicians identify high-risk caregivers. Second, interventions that reduce psychological distress have been shown to increase telomere length (101, 110), and those that reduce caregiver strain or hours per week of providing care, such as coping-skills training or respite care, should be tested for their impact on cellular aging among caregivers. Self-care behaviors, such as exercise, may also help to prevent telomere attrition and subsequent poor health outcomes. Finally, this study highlights the need for additional research to better understand the role of both caregiving characteristics and stress in telomere dynamics. The potential interaction between caregiver strain and stress in the relationship with telomere length should also be examined further, in order to better understand how these factors may adversely influence caregivers over time.
This study had potential limitations. The use of telomere length as a biomarker of aging, chronic disease, or mortality risk remains controversial. Studies examining the associations between telomere length and mortality have produced mixed results, and telomere length does not meet all of the criteria for a biomarker of aging (41, 111, 112), although recent meta-analyses have provided evidence supporting associations for at least some disease outcomes (112–116). Second, telomere length was assessed at a single time point, and we could not control for innate individual variation in telomere length. Similarly, we could not evaluate the impact of longitudinal changes in stress or strain on telomere length. Third, our sample size did not permit us to examine heterogeneity in the association between caregiving and telomere length. Fourth, telomere length features that may be important, such as the relative sizes of cell subpopulations (101, 103) or the shortest telomeres in the sample (117), were not examined in this study. Differences in cell-type mixtures may be a source of residual confounding. Finally, the biological samples in this study consisted of both blood and saliva samples. When the saliva samples were excluded, some results were attenuated. Our results should be interpreted in light of the sensitivity analysis, and future work will be needed to replicate and confirm our findings.
This study also had several important strengths. The sample was selected from participants in a large population-based study, improving generalizability. We assessed several caregiving factors and both global stress and caregiver strain. Finally, we were able to measure and test numerous covariates in the models, including health behaviors, body mass index, and sociodemographic factors, that may have confounded the associations of interest.
In conclusion, this population-based study provided evidence that caregiving factors, including hours per week of care, caring for a young person, and greater caregiver strain, were associated with shorter telomere length, a marker of accelerated cellular aging. Further, moderate-to-high stress was associated with longer telomeres. The findings suggest that stress and caregiving situations have adverse consequences on a physiological level that may be predictive of future health problems. Future work is necessary to better elucidate these relationships and to develop interventions that will buffer telomere attrition and improve the long-term health and well-being of caregivers and their families.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Population Health Sciences, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin (Kristin Litzelman, Whitney P. Witt, Ronald E. Gangnon, F. Javier Nieto, Corinne D. Engelman, Halcyon G. Skinner); Department of Biostatistics and Medical Informatics, School of Medicine and Public Health, University of Wisconsin-Madison, Madison, Wisconsin (Ronald E. Gangnon); School of Social Work, University of Wisconsin-Madison, Madison, Wisconsin (Marsha R. Mailick); and Waisman Center, University of Wisconsin-Madison, Madison, Wisconsin (Marsha R. Mailick).
Dr. F. Javier Nieto is the director of the Survey of the Health of Wisconsin (SHOW), and Dr. Corinne D. Engelman is a SHOW investigator. All authors contributed to this work equally.
Funding for SHOW was provided by the Wisconsin Partnership Program (grant 233 PRJ56RV), the National Institutes of Health (Clinical and Translational Science Award 5UL 1RR025011), and the National Heart, Lung, and Blood Institute (grant 1 RC2 HL101468). Funding for this study was provided by a training grant from the National Institute on Aging (grant F31 AG 044073; Principal Investigator: Kristin Litzelman) and the Center for Demography of Health and Aging at the University of Wisconsin-Madison, which supported the caregiving component of the SHOW interviews (Principal Investigator: Whitney P. Witt). The assays of telomere length were supported by National Institutes of Health grant RO-1 CA132718.
We thank Dr. Lisa A. Boardman and Ruth A. Johnson for their assistance in completing the telomere length assays.
Conflict of interest: none declared.
REFERENCES
- 1.Feinberg L, Reinhard SC, Houser A, et al. Valuing the Invaluable: 2011 Update: The Growing Contributions and Costs of Family Caregiving. Washington, DC: AARP Public Policy Institute; 2011. [Google Scholar]
- 2.Cacioppo JT, Poehlmann KM, Kiecolt-Glaser JK, et al. Cellular immune responses to acute stress in female caregivers of dementia patients and matched controls. Health Psychol. 1998;17(2):182–189. doi: 10.1037//0278-6133.17.2.182. [DOI] [PubMed] [Google Scholar]
- 3.Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouin JP, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: a review. Neuroimmunomodulation. 2008;15(4-6):251–259. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovell B, Wetherell MA. The cost of caregiving: endocrine and immune implications in elderly and non elderly caregivers. Neurosci Biobehav Rev. 2011;35(6):1342–1352. doi: 10.1016/j.neubiorev.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord. 2010;40(4):457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull. 2003;129(6):946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 8.Capistrant BD, Moon JR, Berkman LF, et al. Current and long-term spousal caregiving and onset of cardiovascular disease. J Epidemiol Community Health. 2012;66(10):951–956. doi: 10.1136/jech-2011-200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S, Colditz GA, Berkman LF, et al. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am J Prev Med. 2003;24(2):113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 10.Cooper C, Balamurali TB, Livingston G. A systematic review of the prevalence and covariates of anxiety in caregivers of people with dementia. Int Psychogeriatr. 2007;19(2):175–195. doi: 10.1017/S1041610206004297. [DOI] [PubMed] [Google Scholar]
- 11.Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18(2):250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 12.Dura JR, Stukenberg KW, Kiecolt-Glaser JK. Anxiety and depressive disorders in adult children caring for demented parents. Psychol Aging. 1991;6(3):467–473. doi: 10.1037//0882-7974.6.3.467. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 14.Carter R. Addressing the caregiving crisis [editorial] Prev Chronic Dis. 2008;5(1):A02. [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 16.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- 17.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 18.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 19.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 20.Aviv A, Chen W, Gardner JP, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169(3):323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Kimura M, Kim S, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66(3):312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrlenbach S, Willeit P, Kiechl S, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38(6):1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 23.Nordfjäll K, Svenson U, Norrback KF, et al. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5(2):e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farzaneh-Far R, Lin J, Epel E, et al. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the Heart and Soul Study. PLoS One. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Zglinicki T, Serra V, Lorenz M, et al. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80(11):1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Lindquist K, Kluse M, et al. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32(11):2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honig LS, Kang MS, Schupf N, et al. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69(10):1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devore EE, Prescott J, De Vivo I, et al. Relative telomere length and cognitive decline in the Nurses’ Health Study. Neurosci Lett. 2011;492(1):15–18. doi: 10.1016/j.neulet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grodstein F, van Oijen M, Irizarry MC, et al. Shorter telomeres may mark early risk of dementia: preliminary analysis of 62 participants from the Nurses’ Health Study. PLoS One. 2008;3(2):e1590. doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 31.Shen Q, Zhao X, Yu L, et al. Association of leukocyte telomere length with type 2 diabetes in mainland Chinese populations. J Clin Endocrinol Metab. 2012;97(4):1371–1374. doi: 10.1210/jc.2011-1562. [DOI] [PubMed] [Google Scholar]
- 32.Skinner HG, Gangnon RE, Litzelman K, et al. Telomere length and pancreatic cancer: a case-control study. Cancer Epidemiol Biomarkers Prev. 2012;21(11):2095–2100. doi: 10.1158/1055-9965.EPI-12-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 34.Willeit P, Willeit J, Kloss-Brandstätter A, et al. Fifteen-year follow-up of association between telomere length and incident cancer and cancer mortality. JAMA. 2011;306(1):42–44. doi: 10.1001/jama.2011.901. [DOI] [PubMed] [Google Scholar]
- 35.Monaghan P. Telomeres and life histories: the long and the short of it. Ann N Y Acad Sci. 2010;1206(1):130–142. doi: 10.1111/j.1749-6632.2010.05705.x. [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick AL, Kronmal RA, Kimura M, et al. Leukocyte telomere length and mortality in the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deelen J, Beekman M, Codd V, et al. Leukocyte telomere length associates with prospective mortality independent of immune-related parameters and known genetic markers [published online ahead of print January 14, 2014] Int J Epidemiol. doi: 10.1093/ije/dyt267. ( doi:10.1093/ije/dyt267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weischer M, Nordestgaard BG, Cawthon RM, et al. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105(7):459–468. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 39.Bendix L, Thinggaard M, Fenger M, et al. Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol A Biol Sci Med Sci. 2014;69(2):231–239. doi: 10.1093/gerona/glt153. [DOI] [PubMed] [Google Scholar]
- 40.Schaefer C, Sciortino S, Kvale M, et al. B4-3: demographic and behavioral influences on telomere length and relationship with all-cause mortality: early results from the Kaiser Permanente Research Program on Genes, Environment, and Health (RPGEH) [abstract] Clin Med Res. 2013;11(3):146. [Google Scholar]
- 41.Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35(1):112–131. doi: 10.1093/epirev/mxs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damjanovic AK, Yang Y, Glaser R, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puterman E, Lin J, Blackburn E, et al. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One. 2010;5(5):e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geronimus AT, Hicken MT, Pearson JA, et al. Do US black women experience stress-related accelerated biological aging?: a novel theory and first population-based test of black-white differences in telomere length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epel E, Lin J, Wilhelm F, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Epel ES, Lin J, Dhabhar FS, et al. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav Immun. 2010;24(4):531–539. doi: 10.1016/j.bbi.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daubenmier J, Lin J, Blackburn E, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917–928. doi: 10.1016/j.psyneuen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitaliano PP, Strachan E, Dansie E, et al. Does caregiving cause psychological distress? The case for familial and genetic vulnerabilities in female twins. Ann Behav Med. 2014;47(2):198–207. doi: 10.1007/s12160-013-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieto FJ, Peppard PE, Engelman CD, et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010;10:785. doi: 10.1186/1471-2458-10-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Alliance for Caregiving and AARP. Caregiving in the U.S. 2009. Bethesda, MD: National Alliance for Caregiving; 2009. [Google Scholar]
- 53.Jackson Heart Study Coordinating Center. Jackson Heart Study Manual 2: Cohort Procedures. Jackson, MS: Jackson Heart Study Coordinating Center; 2001. pp. 25–26. [Google Scholar]
- 54.Robinson BC. Validation of a caregiver strain index. J Gerontol. 1983;38(3):344–348. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- 55.Ciulla TA, Sklar RM, Hauser SL. A simple method for DNA purification from peripheral blood. Anal Biochem. 1988;174(2):485–488. doi: 10.1016/0003-2697(88)90047-4. [DOI] [PubMed] [Google Scholar]
- 56.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parker RN, Fenwick R. The Pareto curve and its utility for open-ended income distributions in survey research. Soc Forces. 1983;61(3):872–885. [Google Scholar]
- 58.Block Dietary Data Systems. Questionnaires and Screeners. Berkeley, CA: NutritionQuest; 1996. http://nutritionquest.com/assessment/list-of-questionnaires-and-screeners/ ). (Accessed January 10, 2013) [Google Scholar]
- 59.Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 60.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 61.Harrell FE. Hmisc: Harrell Miscellaneous. Nashville, TN: Comprehensive R Archive Network; 2012. [R software package] [Google Scholar]
- 62.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 63.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 64.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 65.Neugaard B, Andresen E, McKune SL, et al. Health-related quality of life in a national sample of caregivers: findings from the Behavioral Risk Factor Surveillance System. J Happiness Stud. 2008;9(4):559–575. [Google Scholar]
- 66.Ho SC, Chan A, Woo J, et al. Impact of caregiving on health and quality of life: a comparative population-based study of caregivers for elderly persons and noncaregivers. J Gerontol A Biol Sci Med Sci. 2009;64(8):873–879. doi: 10.1093/gerona/glp034. [DOI] [PubMed] [Google Scholar]
- 67.Hirst M. Carer distress: a prospective, population-based study. Soc Sci Med. 2005;61(3):697–708. doi: 10.1016/j.socscimed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Marks NF, Lambert JD, Choi H. Transitions to caregiving, gender, and psychological well-being: a prospective U.S. national study. J Marriage Fam. 2002;64(3):657–667. [Google Scholar]
- 69.Hoyert DL, Seltzer MM. Factors related to the well-being and life activities of family caregivers. Fam Relat. 1992;41(1):74–81. [Google Scholar]
- 70.Conde-Sala JL, Garre-Olmo J, Turró-Garriga O, et al. Differential features of burden between spouse and adult-child caregivers of patients with Alzheimer's disease: an exploratory comparative design. Int J Nurs Stud. 2010;47(10):1262–1273. doi: 10.1016/j.ijnurstu.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 71.Seltzer MM, Floyd F, Song J, et al. Midlife and aging parents of adults with intellectual and developmental disabilities: impacts of lifelong parenting. Am J Intellect Dev Disabil. 2011;116(6):479–499. doi: 10.1352/1944-7558-116.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seltzer MM, Greenberg JS, Floyd FJ, et al. Life course impacts of parenting a child with a disability. Am J Ment Retard. 2001;106(3):265–286. doi: 10.1352/0895-8017(2001)106<0265:LCIOPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 73.Ory MG, Hoffman RR, 3rd, Yee JL, et al. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39(2):177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- 74.Svenson U, Nordfjall K, Baird D, et al. Blood cell telomere length is a dynamic feature. PLoS One. 2011;6(6):e21485. doi: 10.1371/journal.pone.0021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seery MD, Holman EA, Silver RC. Whatever does not kill us: cumulative lifetime adversity, vulnerability, and resilience. J Pers Soc Psychol. 2010;99(6):1025–1041. doi: 10.1037/a0021344. [DOI] [PubMed] [Google Scholar]
- 76.McEwen BS, Lasley EN. The End of Stress As We Know It. New York, NY: Joseph Henry Press/Dana Press; 2002. [Google Scholar]
- 77.Stetz KM. The relationship among background characteristics, purpose in life, and caregiving demands on perceived health of spouse caregivers. Sch Inq Nurs Pract. 1989;3(2):133–153. [PubMed] [Google Scholar]
- 78.Schulz R, Beach SR, Cook TB, et al. Predictors and consequences of perceived lack of choice in becoming an informal caregiver. Aging Ment Health. 2012;16(6):712–721. doi: 10.1080/13607863.2011.651439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yates ME, Tennstedt S, Chang BH. Contributors to and mediators of psychological well-being for informal caregivers. J Gerontol B Psychol Sci Soc Sci. 1999;54(1):P12–P22. doi: 10.1093/geronb/54b.1.p12. [DOI] [PubMed] [Google Scholar]
- 80.Goldner M, Drentea P. Caring for the disabled: applying different theoretical perspectives to understand racial and ethnic variations among families. Marriage Fam Rev. 2009;45(5):499–518. [Google Scholar]
- 81.Stewart JL, Mishel MH. Uncertainty in childhood illness: a synthesis of the parent and child literature. Sch Inq Nurs Pract. 2000;14(4):299–319. [PubMed] [Google Scholar]
- 82.Singer GHS. Meta-analysis of comparative studies of depression in mothers of children with and without developmental disabilities. Am J Ment Retard. 2006;111(3):155–169. doi: 10.1352/0895-8017(2006)111[155:MOCSOD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 83.Simon NM, Smoller JW, McNamara KL, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 84.Pollack EA, Litzelman K, Wisk LE, et al. Correlates of physiological and psychological stress among parents of childhood cancer and brain tumor survivors. Acad Pediatr. 2013;13(2):105–112. doi: 10.1016/j.acap.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seltzer MM, Greenberg JS, Krauss MW. A comparison of coping strategies of aging mothers of adults with mental illness or mental retardation. Psychol Aging. 1995;10(1):64–75. doi: 10.1037//0882-7974.10.1.64. [DOI] [PubMed] [Google Scholar]
- 86.Suls J, Fletcher B. The relative efficacy of avoidant and nonavoidant coping strategies: a meta-analysis. Health Psychol. 1985;4(3):249–288. doi: 10.1037//0278-6133.4.3.249. [DOI] [PubMed] [Google Scholar]
- 87.Mausbach BT, Aschbacher K, Patterson TL, et al. Avoidant coping partially mediates the relationship between patient problem behaviors and depressive symptoms in spousal Alzheimer caregivers. Am J Geriatr Psychiatry. 2006;14(4):299–306. doi: 10.1097/01.JGP.0000192492.88920.08. [DOI] [PubMed] [Google Scholar]
- 88.Billings DW, Folkman S, Acree M, et al. Coping and physical health during caregiving: the roles of positive and negative affect. J Pers Soc Psychol. 2000;79(1):131–142. doi: 10.1037//0022-3514.79.1.131. [DOI] [PubMed] [Google Scholar]
- 89.Cole SW. Nervous system regulation of the cancer genome. Brain Behav Immun. 2013;30(suppl):S10–S18. doi: 10.1016/j.bbi.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36(4-5):735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 91.Liu H, Hou D, Wu D, et al. Serum pattern profiling for analyzing different types of stress by protein chip technology: a preliminary study. Eur J Mass Spectrom (Chichester, Eng) 2010;16(5):619–623. doi: 10.1255/ejms.1086. [DOI] [PubMed] [Google Scholar]
- 92.Laucht M, Treutlein J, Blomeyer D, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: evidence from a high-risk community sample of young adults. Int J Neuropsychopharmacol. 2009;12(6):737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- 93.Giele JZ, Mutschler P, Orodenker SZ. Stress and Burdens of Caregiving for the Frail Elderly. Waltham, MA: Policy Center on Aging, Brandeis University; 1987. [Google Scholar]
- 94.Scharlach AE. Caregiving and employment: competing or complementary roles? Gerontologist. 1994;34(3):378–385. doi: 10.1093/geront/34.3.378. [DOI] [PubMed] [Google Scholar]
- 95.Stoller EP, Pugliesi KL. Other roles of caregivers: competing responsibilities or supportive resources. J Gerontol. 1989;44(6):S231–S238. doi: 10.1093/geronj/44.6.s231. [DOI] [PubMed] [Google Scholar]
- 96.Stull DE, Bowman K, Smerglia V. Women in the middle: a myth in the making? Fam Relat. 1994;43(3):319–324. [Google Scholar]
- 97.Bachner YG, Karus DG, Raveis VH. Examining the social context in the caregiving experience: correlates of global self-esteem among adult daughter caregivers to an older parent with cancer. J Aging Health. 2009;21(7):1016–1039. doi: 10.1177/0898264309344320. [DOI] [PubMed] [Google Scholar]
- 98.Hong J, Seltzer MM. The psychological consequences of multiple roles: the nonnormative case. J Health Soc Behav. 1995;36(4):386–398. [PubMed] [Google Scholar]
- 99.Chan SR, Blackburn EH. Telomeres and telomerase. Philos Trans R Soc Lond B Biol Sci. 2004;359(1441):109–121. doi: 10.1098/rstb.2003.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 101.Biegler KA, Anderson AK, Wenzel LB, et al. Longitudinal change in telomere length and the chronic stress response in a randomized pilot biobehavioral clinical study: implications for cancer prevention. Cancer Prev Res (Phila) 2012;5(10):1173–1182. doi: 10.1158/1940-6207.CAPR-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1-2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol. 2008;59(suppl 9):169–186. [PubMed] [Google Scholar]
- 104.Epel E. How “reversible” is telomeric aging? Cancer Prev Res (Phila) 2012;5(10):1163–1168. doi: 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- 105.Beery AK, Lin J, Biddle JS, et al. Chronic stress elevates telomerase activity in rats. Biol Lett. 2012;8(6):1063–1066. doi: 10.1098/rsbl.2012.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steptoe A, Hamer M, Butcher L, et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25(7):1292–1298. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 107.Wolkowitz OM, Mellon SH, Epel ES, et al. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol Psychiatry. 2012;17(2):164–172. doi: 10.1038/mp.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sanders JL, Fitzpatrick AL, Boudreau RM, et al. Leukocyte telomere length is associated with noninvasively measured age-related disease: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2012;67(4):409–416. doi: 10.1093/gerona/glr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brouilette S, Singh RK, Thompson JR, et al. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 110.Lavretsky H, Epel ES, Siddarth P, et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry. 2013;28(1):57–65. doi: 10.1002/gps.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mather KA, Jorm AF, Parslow RA, et al. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66(2):202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 112.Boonekamp JJ, Simons MJ, Hemerik L, et al. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell. 2013;12(2):330–332. doi: 10.1111/acel.12050. [DOI] [PubMed] [Google Scholar]
- 113.Albrecht E, Sillanpää E, Karrasch S, et al. Telomere length in circulating leukocytes is associated with lung function and disease. Eur Respir J. 2014;43(4):983–992. doi: 10.1183/09031936.00046213. [DOI] [PubMed] [Google Scholar]
- 114.Zhao J, Miao K, Wang H, et al. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One. 2013;8(11):e79993. doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma H, Zhou Z, Wei S, et al. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6(6):e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wentzensen IM, Mirabello L, Pfeiffer RM, et al. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1238–1250. doi: 10.1158/1055-9965.EPI-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hemann MT, Strong MA, Hao LY, et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


