Abstract
We report that PGE2 promotes Smad2-Smad4 complex formation and this phenomenon could be blocked by DIDS, an anion transporter inhibitor. Our data suggest that PGE2 had no effects on Smad2 phosphorylation, suggesting that PGE2-mediated Smad2-Smad4 complex formation is independent of TGF-β signaling and that PGE2 induced Smad2 modification which is different from TGF-β-mediated phosphorylation. We demonstrate that in primary human glomerular mesangial cells PGE2 caused modification of Smad2 as detected by Smad2N antibody, raised against a peptide near the N-terminus of Smad2. We hypothesize that Smad2 protein is post-translationaly modified by PGE2. Direct evidence of Smad2 modification by PGE2 was achieved by avidin pulldown assay which showed that endogenous Smad2 and recombinant Smad2 protein were attached by biotin-labeled PGE2. Taken together, our results provided evidence that post-translational modification of Smad2 could be a mechanism for the action of PGE2 in the pathogenesis of human pathologies.
Introduction
Prostaglandins (PGs) are a group of lipid mediators derived from cyclooxygenase (COX) metabolites of arachidonic acid [24]. The COX-derived PGs are critical modulators of numerous physiological and pathophysiological conditions including cardiovascular homeostasis, inflammation and immune regulation [29]. Cyclooxygenase-2 (COX-2) is the inducible form of COX which is implicated in progression of multiple types of cancer [6;8;18;30]. MAPK cascades are the convergence point of the diverse stimuli which induce COX-2 expression [23;35]. Often, the biological role of COX-2 is associated with antiapoptotic action of COX-2 derived PGs [5;15].
PGs actions are likely mediated through their specific receptors. However, some effects of PGs may be non-receptor-mediated. A number of studies implied that PGs exerted their diverse effects through post-translational modification of cellular proteins [17;19;34]. Since PGs possess anionic moieties at physiological pH and diffuse poorly through the lipid bilayer [2;4] the covalent modification of proteins by PGs should be a carrier-mediated transport process. Several PGs carriers have been cloned and characterized [28].
Smad proteins are key signal effectors for TGF-β signaling. When binding to and activating the signaling receptors, TGF-β phosphorylates R-Smads (Smad2 or Smad3) in their C-terminal SXS motif. The activated R-Smads undergo conformational changes and form a complex with Smad4, which then translocates into the nucleus and regulates gene transcription in combination with other transcriptional cofactors [13]. Besides phosphorylation, Smads can be post-translationaly modified through other mechanisms, such as ubiquitination, SUMOylation and acetylation [14;20;21]. Some of these covalent modifications are induced by non-TGF-β signals, thus providing ways for other signals to affect TGF-β signal transduction.
PGE2, the most abundant PGs detected in the kidney, plays an important role in the development of many inflammatory renal glomerular diseases [11;12;22]. Intracellular signaling pathways initiated by PGE2 contribute to the manifestation of glomerulonephritis [31]. In this study, we report that the Smad2 protein can be post-translationaly modified by PGE2. Furthermore, PGE2 promotes the Smad2-Smad4 complex formation, which is independent of TGF-β stimulation. Meanwhile, we also found that PGE2-mediated Smad2-Smad4 complex formation can be blocked by DIDS, an anion transporter inhibitor. Therefore, post-translational modification of Smad2 protein by PGE2 may be a mechanism for the action of PGE2 in the pathogenesis of renal disease.
Materials and methods
Cell culture
Isolation of primary human renal glomerular mesangial cells (HMC) was done previously [37;38]. For this study cells were taken from previously prepared frozen stock and were maintained in RPMI 1640 containing 16.7% FBS and 100ug/ml penicillin and streptomycin as previously described [37;38]. HMC at passages 5 through 8 were used.
Plasmid constructs and protein purification
Human Smad2 cDNA was subcloned into pGEX-2T. Plasmids encoding Smad2 were transformed into Escherichia coli BL21 (DE3) RIL (Stratagene). Protein expression was achieved by culturing E. coli at 30 °C for 2h with shaking (250rpm), 1mM IPTG was added, and the incubation was continued for an additional 2h. GST-Smad2 was purified using glutathione-Sepharose 4B affinity chromatography (Amersham Biosciences).
RNA isolation and RT-PCR
HMC total cellular RNA was isolated using TRIzol (Invitrogen). Amplification of transcripts was performed using 200 ng of total RNA and one-step RT-PCR system (Qiagen) according to the manufacturing protocol. The PCR primer sequences used in this study were designed using Primer3 software and the forward and reverse primers are presented as follows: EP1 (350bp), 5’-CTTGTCGGTATCATGGTGGTGTC-3’ and 5’-GGTTGTGCTTAGAAGTGGCTGAGG-3’; EP2 (111bp), 5’-CTGTTCTGAGACTAATGCGTTCA-3’ and 5’-GGTCAGCCTGTTTACTGGCA-3’; EP3 (298bp), 5’-GGGCTGACCATGACTGTTTT-3’ and 5’-CAGAGGCGAAGAAAAGGTTG-3’; EP4 (120bp), 5’-AGCTGGGACTCGTCTTTGAA-3’ and 5’-GGTCAGAGTTGCCAGCTTTC-3’. The cycling parameters consisted of one cycle of 50°C for 30 min, one cycle of 95°C for 15 min then 30 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1 min followed by a single 10 min cycle at 72°C for extension. RT-PCR products were electrophoresed on a 2% agarose gel using DNA markers (Promega) as a standard to determine the molecular size.
Co-immunoprecipitation and immunoblotting
HMC were lysed in immunoprecipitation buffer (20mM Tris-Cl, 150mM NaCl, 1% Triton X-100 and 10% glycerol; pH 7.5) and precipitated with the Smad2 antibody conjugated with agarose for 2 h at 4°C. The beads were then washed, boiled, and subjected to SDS-PAGE. For immunoblotting, the proteins were electrophoretically transferred onto PVDF membranes and blotted with primary antibodies. The primary antibodies were then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies and detected by chemiluminescence.
Avidin pulldown assay
HMC grown on 150mm plates were incubated with 1uM of biotin-labeled PGE2 (bio-PGE2) for 2h at 37°C. The cells were rinsed with PBS and lysed in 1% NP-40 lysis buffer. After centrifugation, supernatants were incubated with 50ul of 50% slurry of immobilized NeutrAvidin agarose for 1h at 4 °C. The avidin agarose was then washed with lysis buffer, boiled, and subjected to SDS-PAGE. In experiments with purified Smad2 protein, 2ug of GST or GST-Smad2 protein was incubated with bio-PGE2 in 1% NP-40 lysis buffer for 2h at room temperature.
Materials
PGE2 and AH6809 were purchased from Sigma. Biotin-labeled PGE2 and GW627368X were purchased from Cayman Chemical. SC51322 was purchased from BIOMOL International. Phospho-Smad2 antibody, Smad2 antibody and Smad4 antibody were purchased from Cell Signaling Technology and Santa Cruz Biotechnology, respectively. Neutravidin agarose resin was purchased from Thermo Scientific. The Smad2 cDNA was a gift from Dr. Heldin (Ludwig Institute for Cancer Research, Sweden).
Statistics
All data are expressed as means ± s.e.m. from at least three separate experiments. Effect of PGE2 was compared to a normal level 1 with the one sample t-test. The effect of PGE2 versus the effect of PGE2 with tested antagonists was compared with the two sample t-test. Values were considered statistically significant at P < 0.05.
Results
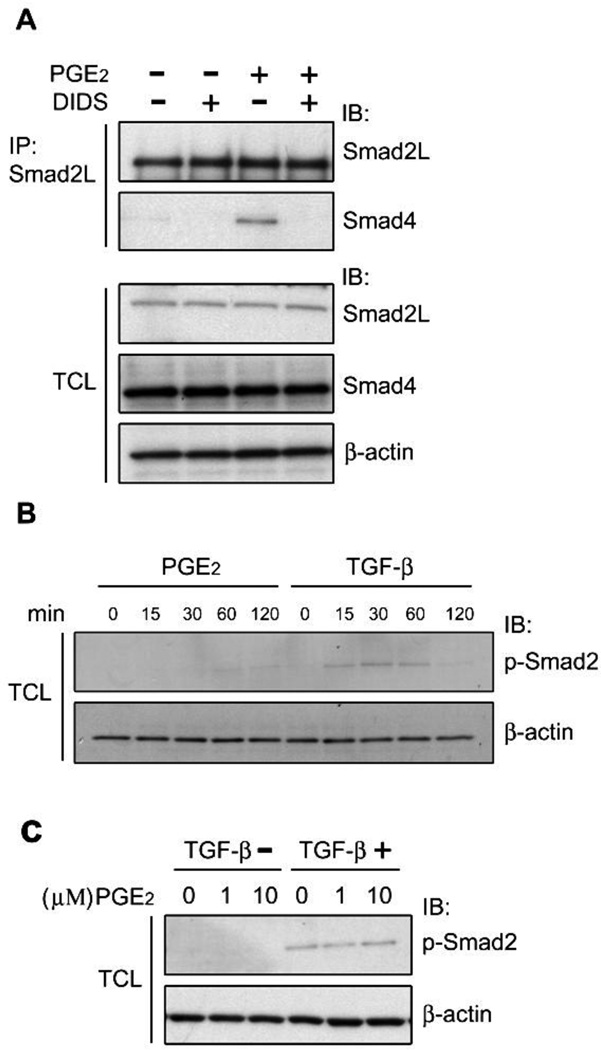
In TGF-β signaling, the phosphorylated/activated Smad2 exerts its transcriptional activity through forming a complex with Smad4 [7]. To investigate effect of PGE2 on Smad2-Smad4 complex formation, we performed Co-IP experiments. Smad2 protein from cell lysates of human mesangial cells (HMC) was immunoprecipitated with Smad2L antibody. Co-IP demonstrated that Smad4 was precipitated along with Smad2 following PGE2 treatment; DIDS, an anion transporter inhibitor, blocked PGE2-mediated Smad2-Smad4 complex formation (Fig. 1A). Meanwhile, we found that PGE2 had no effects on Smad2 phosphorylation and TGF-β-mediated Smad2 phosphorylation (Fig. 2B, 2C). These data suggested that PGE2 induced Smad2 modification which is different from TGF-β-mediated phosphorylation and that PGE2-mediated Smad2 post-translational modification is likely to contribute to Smad2-Smad4 complex formation.
Fig. 1.
Effects of PGE2 treatment upon Smad2-Smad4 complex formation. A, Serum-starved HMC were pretreated with DIDS for 30min, followed by stimulation with 1µM PGE2 for 2h at 37°C. Cell lysates were collected and immunoprecipitated with the Smad2L antibody. Precipitated Smad2 and Smad4 were analyzed by immunoblotting. Expression of endogenous proteins was detected by immunoblotting. β-actin was used as a loading control. B, HMC were treated with 1 µM of PGE2 or 10ng/ml TGF-β for the indicated time. TCL were collected and phosphorylated Smad2 was examined by immunoblotting. C, HMC were pretreated with 1 or 10 µµ of PGE2 for 45min, followed by stimulation with 10ng/ml TGF-β for another 30min. Cell lysates were collected and phosphorylated Smad2 was examined by immunoblotting. β-actin was used as a loading control.
Fig. 2.
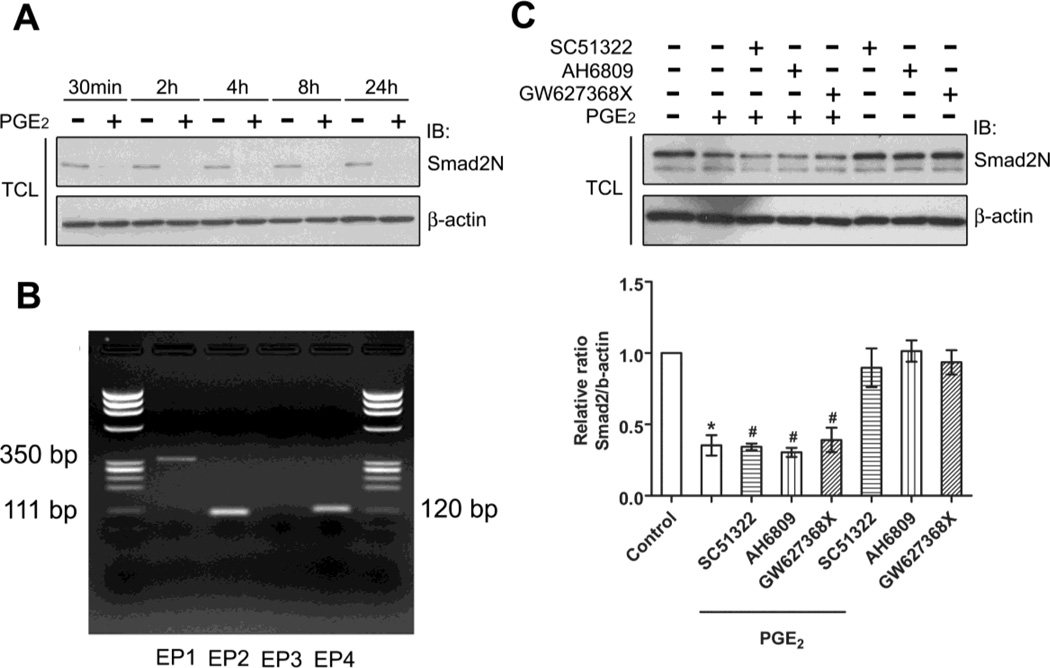
The epitope of endogenous Smad2 protein is changed after PGE2 stimulation. A, Serum-starved HMC were stimulated with 1µM of PGE2 for the indicated time. The total cell lysates (TCL) were collected and endogenous Smad2 protein was examined by immunoblotting with the Smad2N antibody, raised against a peptide near the N-terminus of Smad2 protein. B, Total RNA was isolated from HMC, and subjected to RT-PCR for EP1, EP2, EP3, and EP4 mRNAs using specific primers. C, Serum-starved HMC were pretreated with selective antagonists of EPs, SC51322 (EP1), AH6809 (EP2), or GW627368X (EP4) for 30min followed by stimulation with PGE2 for 60min, and endogenous Smad2 was examined by immunoblotting with the Smad2N antibody. The lower panel shows the results of three independent experiments (means± s.e.m). * , p <0.001 versus control; #, p> 0.05 versus PGE2-treated group.
Our data suggest Smad2 modification caused by PGE2 involves alteration of Smad2 epitope recognized by Smad2N antibody, which was raised against a peptide near the N-terminus of Smad2 protein. Smad2N antibodies failed to recognize endogenous Smad2 protein in HMC treated with PGE2. This alteration of epitope recognized by Smad2N antibody occurred as early as 30 min and lasted till 24 h after stimulation (Fig. 2A.). Recognition of Smad2 by antibodies raised against other epitope was not affected (Fig.3). PGE2 exerts its actions by acting on a group of G-protein-coupled receptors, designated as EP1, EP2, EP3, and EP4 [33]. To investigate the role of EPs subtype receptors in PGE2-mediated Smad2 modulation, we first examined the expression profile of EPs subtype receptors in HMC by RT-PCR. The mRNAs of EP1, EP2, and EP4 subtype receptors could be detected in HMC, whereas the transcript of EP3 subtype receptor remained undetected (Fig. 2B). We next examined whether EP1, EP2 and EP4 subtype receptors were involved in the PGE2-mediated Smad2 recognition. We found that SC51322 (EP1 antagonist, 10µM), AH6809 (EP2 antagonist, 10µM) and GW627368X (EP4 antagonist, 10µM) failed to block PGE2-mediated Smad2 protein apparent decline (Fig. 2C). In addition, we excluded the possibility that protein degradation contributed to the PGE2-mediated Smad2 protein recognition by using proteasome inhibitors (UBEI-41, MG-132 and lactacystin), protease inhibitors (chloroquine, leupeptin and NH4Cl) and QVD-OPH, a pan caspase inhibitor (data not shown). Based on these results, it appears that a new mechanism participates in PGE2-mediated Smad2 modification which prevents detection of protein with Smad2N antibodies.
Fig. 3.
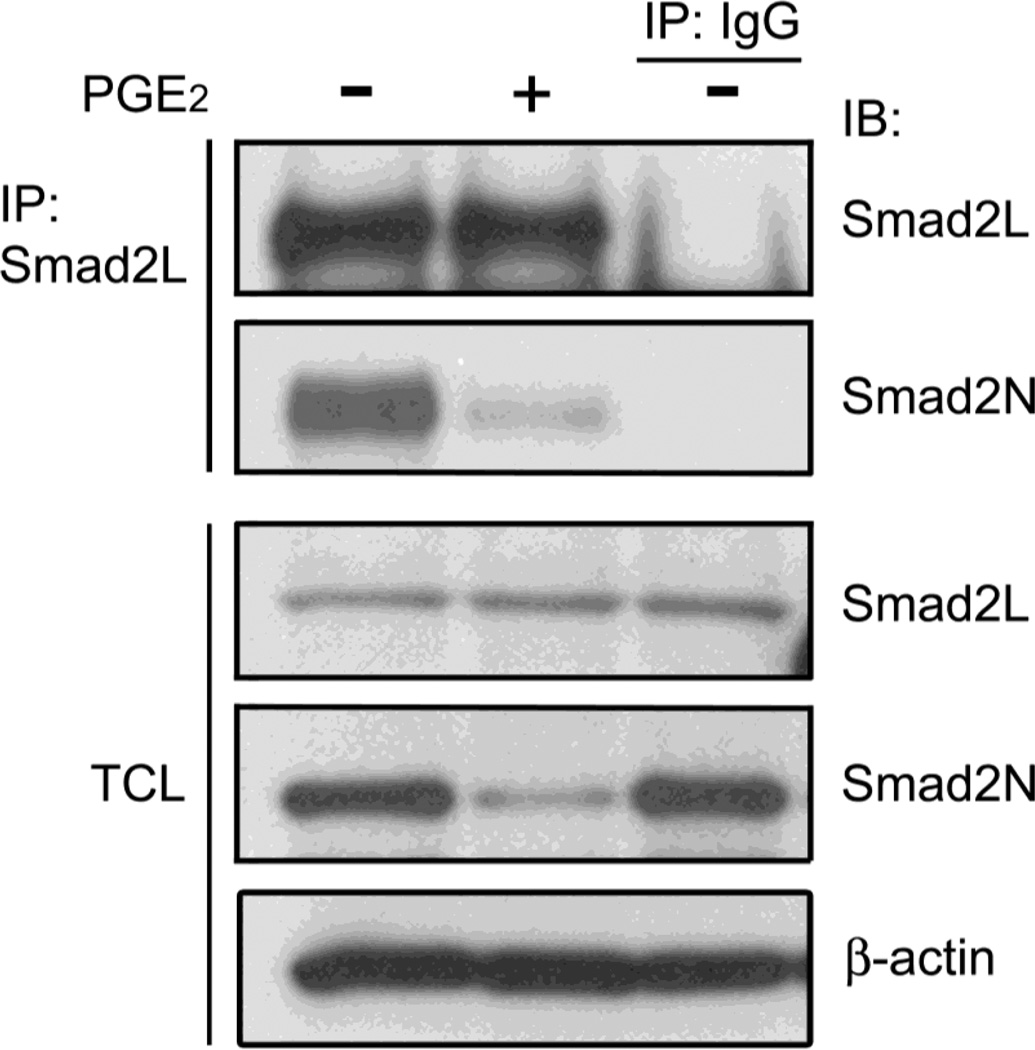
The level of Smad2 protein is not affected by PGE2. Serum-starved HMC were stimulated with PGE2 for 2h. The TCL were collected and endogenous Smad2 protein was pulled down with by the Smad2L antibody, raised against a peptide near the link region of Smad2 protein. The precipitated Smad2 protein and Smad2 protein in TCL were examined by the Smad2L and Smad2N antibodies, respectively. β-actin was used as a loading control.
PGE2 contains a long-chain fatty acid portion that could bind covalently to proteins by an ester bond between its carboxyl group and a hydroxyl amino acid of a protein [34]. The peptide used for generation Smad2N antibody includes a hydroxyl amino acid threonine. Thus, it is possible that direct attachment of PGE2 to the threonine takes place and that alters the epitope of the Smad2 antigen, resulting in the Smad2N antibody failing to detect endogenous Smad2 protein. As shown in Fig. 3, the Smad2L antibody, which recognize the linker region of Smad2, could precipitate equal amount of endogenous Smad2 from HMC cell lysates in both PGE2-treated and untreated conditions, while the Smad2N antibody failed to detect the precipitated Smad2 in the PGE2-treated condition by immunoblotting. Correspondingly, the Smad2 protein in total cell lysate (TCL) of HMC could be detected by the Smad2L antibody, but not by the Smad2N antibody following PGE2 treatment. These results argue that Smad2 might be post-translationaly modified by PGE2 through interaction with hydroxyl amino acids, such as threonine located near the N-terminus.
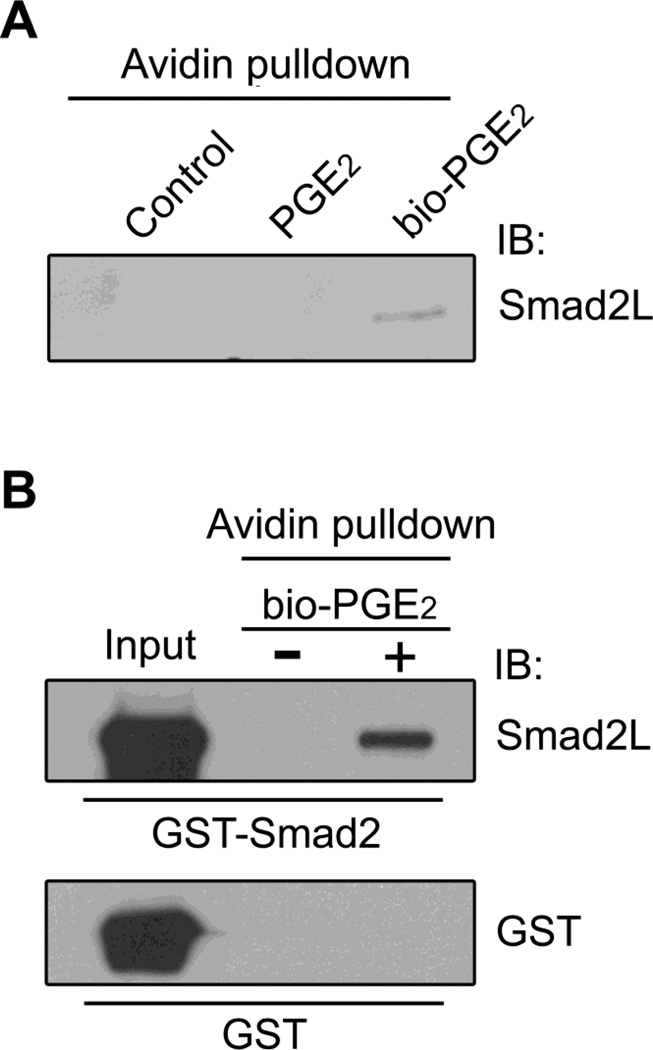
To further investigate Smad2 post-translational modification by PGE2, we performed avidin pulldown experiments using biotin-labeled PGE2 (bio-PGE2). In HMC, after treatment with bio-PGE2, endogenous Smad2 protein was precipitated by Neutravidin agarose beads, as demonstrated with immunoblotting (Fig. 4A). Direct attachment of PGE2 to Smad2 was further confirmed by avidin pulldown assay using purified recombinant Smad2 protein. As shown in Fig. 4B, the recombinant GST-Smad2 protein was precipitated by bio-PGE2, whereas the GST was not. Smad2 modification by PGE2 was therefore confirmed.
Fig. 4.
Smad2 protein post-translational modification by PGE2. A, HMC were treated with 1µM of PGE2 or bio-PGE2 for 2h at 37°C. NeutrAvidin pulldown was then performed as described in experimental procedures. Resin-bound proteins were analyzed by immunoblotting with the Smad2L antibody. B, Purified 2 µg of GST or GST-Smad2 protein was incubated with 1µM bio-PGE2 for 2h at room temperature and then precipitated by NeutrAvidin-agarose. Resin-bound proteins were analyzed by immunoblotting with GST antibody and the Smad2L antibody, respectively.
Discussion and Conclusions
The covalent binding of PGs to proteins has been detected in microsomal cell fractions and in intact platelets [1;9;36]. Takahashi and his colleagues demonstrated that proteins in HL-60 cells were labeled by PGE2 [34]. PGE2 possesses a long-chain fatty acid portion that could bind covalently to proteins by an ester bond between its carboxyl group and either a hydroxyl amino acid or a cysteine of a protein. It is possible that PGE2 attaches the peptide through forming an ester bond between its carboxyl group and the hydroxyl group of the threonine. The PGE2-modified threonine alters the epitope of the peptide, thus resulting in the Smad2N antibody failing to detect the Smad2 protein. The fact that the Smad2L antibody can recognize Smad2 protein after PGE2 treatment further confirms our hypothesis and also rules out the possibility that protein degradation mechanisms play a role in the process.
There are four principal bioactive PGs in vivo, including PGE2, PGI2, PGD2, and PGF2α. It has been reported that many effects of PGs are mediated through their specific receptors. However, some effects of PGs may be non-receptor-mediated. Recent studies implied that PGs exert their diverse actions through direct covalent modification of proteins. For example, 15d-PGJ2, a derivative of PGD2, has been reported to covalently modify a variety of cellular proteins such as transcription factors NF-κB and AP-1, translational initiation factor eIF4A, and proteins that are regulated by oxidative stress [3;17;27;32]. These 15d-PGJ2-mediated modifications were reported to be closely associated with the anti-inflammatory effect of 15d-PGJ2. In this study, we demonstrated that PGE2 promoted Smad2-Smad4 complex formation. It appeared that it happens as a direct effect of intracellular PGE2 because PGE2-mediated Smad2-Smad4 complex formation was blocked by an anion transporter inhibitor, DIDS. It was also demonstrated that PGE2 had no effects of Smad2 phosphorylation, excluding the possibility that TGF-β signaling played a role in the process. Therefore, it is possible that PGE2-modified Smad2 mediates Smad2-Smad4 complex formation. The attachment of PGE2 to Smad2 may alter Smad2 conformation, activate Smad2 and promote Smad2-Smad4 complex formation. The underlining mechanism merits further investigation.
We were able to observe the effect of PGE2 on Smad2 modification with concentrations of PGE2 as low as 0.01uM. The transient accumulation of cyclooxygenase-derived prostanoids in kidney tissue could be as high as 10 pg PGE2 per ug of rat inner medulla tissue [26] with local concentration of PGE2 at the cell membrane being significantly higher. PGE2 concentrations 0.5–1.0 uM are routinely used in cell culture studies [5;10;15].
The covalent modification of proteins by PGs is considered a transporter-mediated process, as PGs diffuses poorly through the lipid bilayer. It is further confirmed by our findings that anion transporters participates in PGE2-mediated Smad2-Smad4 complex formation. Anion transporters facilitate PGE2 traversing the plasma membrane, which promotes Smad2-Smad4 complex formation. Recently, specific prostaglandin transporter PGT has been cloned [16]. It has been thought that PGT takes up and oxidizes PGE2 to the inactive metabolites, thus terminating PGE2 actions [25]. However, our findings imply that PGE2 take-up by PGT is the first step in novel intracellular function of PGE2.
In summary, we examined the hypotheses that Smad2 is post-translationaly modified by PGE2 and that PGE2-mediated Smad2 modification might interfere with Smad2-Smad4 complex formation. We found that epitope at the N-terminus of Smad2 is masked by PGE2. Smad2 modification by PGE2 was further confirmed in both endogenous Smad2 of HMC and in purified Smad2 protein by bio-PGE2-avidin pulldown assay. Co-IP demonstrated that PGE2 promoted the interaction of Smad2 with Smad4 and that DIDS, an anion transporter inhibitor, inhibited PGE2-mediated Smad2-Smad4 complex formation. In addition, we showed that PGE2 had no effects on Smad2 phosphorylation. These data suggested that post-translational modification of Smad2 may be a mechanism for the action of PGE2 and could contribute to PGE2 action in the pathogenesis of renal disease.
Acknowledgements
We thank Dr Joseph T. Barbieri (Medical College of Wisconsin) for discussion of the manuscript and technical advice.
Sources of support:
Grants from Wisconsin Breast Cancer Showhouse, Cancer Center of Medical College of Wisconsin and National Institutes of Health (NIH/NIDDK 1R21DK088018 and NIH/NIDDK 1R01DK098159) to A. Sorokin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Anderson MW, Crutchley DJ, Chaudhari A, et al. Studies on the covalent binding of an intermediate(s) in prostaglandin biosynthesis to tissue macromolecules. Biochim Biophys Acta. 1979;573:40–50. doi: 10.1016/0005-2760(79)90171-1. [DOI] [PubMed] [Google Scholar]
- 2.Baroody RA, Bito LZ. The impermeability of the basic cell membrane to thromboxane-B2' prostacyclin and 6-keto-PGF 1 alpha. Prostaglandins. 1981;21:133–142. doi: 10.1016/0090-6980(81)90203-3. [DOI] [PubMed] [Google Scholar]
- 3.Cernuda-Morollon E, Pineda-Molina E, Canada FJ, Perez-Sala D. 15-Deoxy-Delta 12,14-prostaglandin J2 inhibition of NF-kappaB-DNA binding through covalent modification of the p50 subunit. J Biol Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 4.Chan BS, Satriano JA, Pucci M, Schuster VL. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter "PGT". J Biol Chem. 1998;273:6689–6697. doi: 10.1074/jbc.273.12.6689. [DOI] [PubMed] [Google Scholar]
- 5.Chang YW, Jakobi R, McGinty A, et al. Cyclooxygenase 2 promotes cell survival by stimulation of dynein light chain expression and inhibition of neuronal nitric oxide synthase activity. Mol Cell Biol. 2000;20:8571–8579. doi: 10.1128/mcb.20.22.8571-8579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dempke W, Rie C, Grothey A, Schmoll HJ. Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol. 2001;127:411–417. doi: 10.1007/s004320000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- 8.DuBois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 9.Eling TE, Wilson AG, Chaudhari A, Anderson MW. Covalent binding of an intermediate(s) in prostaglandin biosynthesis to guinea pig lung microsomal protein. Life Sci. 1977;21:245–251. doi: 10.1016/0024-3205(77)90308-3. [DOI] [PubMed] [Google Scholar]
- 10.Fabre JE, Nguyen M, Athirakul K, et al. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J Clin Invest. 2001;107:603–610. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao CM, Breyer MD. Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol. 2008;70:357–377. doi: 10.1146/annurev.physiol.70.113006.100614. [DOI] [PubMed] [Google Scholar]
- 12.Hartner A, Pahl A, Brune K, Goppelt-Struebe M. Upregulation of cyclooxygenase-1 and the PGE2 receptor EP2 in rat and human mesangioproliferative glomerulonephritis. Inflamm Res. 2000;49:345–354. doi: 10.1007/PL00000215. [DOI] [PubMed] [Google Scholar]
- 13.Heldin CH, Miyazono K, ten DP. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 14.Inoue Y, Itoh Y, Abe K, et al. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- 15.Ishaque A, Dunn MJ, Sorokin A. Cyclooxygenase-2 inhibits tumor necrosis factor alpha-mediated apoptosis in renal glomerular mesangial cells. J Biol Chem. 2003;278:10629–10640. doi: 10.1074/jbc.M210559200. [DOI] [PubMed] [Google Scholar]
- 16.Kanai N, Lu R, Satriano JA, et al. Identification and characterization of a prostaglandin transporter. Science. 1995;268:866–869. doi: 10.1126/science.7754369. [DOI] [PubMed] [Google Scholar]
- 17.Kim WJ, Kim JH, Jang SK. Anti-inflammatory lipid mediator 15d-PGJ2 inhibits translation through inactivation of eIF4A. EMBO J. 2007;26:5020–5032. doi: 10.1038/sj.emboj.7601920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschenbaum A, Liu X, Yao S, Levine AC. The role of cyclooxygenase-2 in prostate cancer. Urology. 2001;58:127–131. doi: 10.1016/s0090-4295(01)01255-9. [DOI] [PubMed] [Google Scholar]
- 19.Lecomte M, Lecocq R, Dumont JE, Boeynaems JM. Covalent binding of arachidonic acid metabolites to human platelet proteins. Identification of prostaglandin H synthase as one of the modified substrates. J Biol Chem. 1990;265:5178–5187. [PubMed] [Google Scholar]
- 20.Lee PS, Chang C, Liu D, Derynck R. Sumoylation of Smad4, the common Smad mediator of transforming growth factor-beta family signaling. J Biol Chem. 2003;278:27853–27863. doi: 10.1074/jbc.M301755200. [DOI] [PubMed] [Google Scholar]
- 21.Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat Cell Biol. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- 22.Makino H, Tanaka I, Mukoyama M, et al. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol. 2002;13:1757–1765. doi: 10.1097/01.asn.0000019782.37851.bf. [DOI] [PubMed] [Google Scholar]
- 23.McGinty A, Foschi M, Chang YW, et al. Induction of prostaglandin endoperoxide synthase 2 by mitogen-activated protein kinase cascades. Biochem J. 2000;352(Pt 2):419–424. [PMC free article] [PubMed] [Google Scholar]
- 24.Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. 2006;36:37–49. doi: 10.1016/j.semarthrit.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Nomura T, Chang HY, Lu R, et al. Prostaglandin signaling in the renal collecting duct: release, reuptake, and oxidation in the same cell. J Biol Chem. 2005;280:28424–28429. doi: 10.1074/jbc.M408286200. [DOI] [PubMed] [Google Scholar]
- 26.Norregaard R, Jensen BL, Topcu SO, et al. Urinary tract obstruction induces transient accumulation of COX-2-derived prostanoids in kidney tissue. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1017–R1025. doi: 10.1152/ajpregu.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Sala D, Cernuda-Morollon E, Canada FJ. Molecular basis for the direct inhibition of AP-1 DNA binding by 15-deoxy-Delta 12,14-prostaglandin J2. J Biol Chem. 2003;278:51251–51260. doi: 10.1074/jbc.M309409200. [DOI] [PubMed] [Google Scholar]
- 28.Schuster VL. Prostaglandin transport. Prostaglandins Other Lipid Mediat. 2002;68–69:633–647. doi: 10.1016/s0090-6980(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 29.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 30.Sorokin A. Cyclooxygenase-2: potential role in regulation of drug efflux and multidrug resistance phenotype. Curr Pharm Des. 2004;10:647–657. doi: 10.2174/1381612043453117. [DOI] [PubMed] [Google Scholar]
- 31.Sorokin A. Glomerulonephritis and cellular regulation of prostaglandin synthesis, chap. 8. In: Prabhakar Sharma S., editor. An Update on Glomerulopathies - Etiology and Pathogenesis. Rijeka, INTECH; 2011. pp. 141–170. [Google Scholar]
- 32.Stamatakis K, Sanchez-Gomez FJ, Perez-Sala D. Identification of novel protein targets for modification by 15-deoxy-Delta12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J Am Soc Nephrol. 2006;17:89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi N, Breitman TR. Covalent modification of proteins by ligands of steroid hormone receptors. Proc Natl Acad Sci U S A. 1992;89:10807–10811. doi: 10.1073/pnas.89.22.10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Wilson AG, Kung HC, Anderson MW, Eling TE. Covalent binding of intermediates formed during the metabolism of arachidonic acid by human platelet subcellular fractions. Prostaglandins. 1979;18:409–422. doi: 10.1016/s0090-6980(79)80060-x. [DOI] [PubMed] [Google Scholar]
- 37.Yang C, Glass WF. Expression of alpha-actinin-1 in human glomerular mesangial cells in vivo and in vitro. Exp Biol Med (Maywood ) 2008;233:689–693. doi: 10.3181/0710-RM-279. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Patel K, Harding P, et al. Regulation of TGF-beta1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cells. Exp Cell Res. 2007;313:1240–1250. doi: 10.1016/j.yexcr.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]