Abstract
Perineural tumor invasion of intrapancreatic nerves, neurogenic inflammation, and tumor metastases along extrapancreatic nerves are key features of pancreatic malignancies. Animal studies show that chronic pancreatic inflammation produces hypertrophy and hypersensitivity of pancreatic afferents and that sensory fibers may themselves drive inflammation via neurogenic mechanisms. Whereas genetic mutations are required for cancer development, inflammation has been shown to be a precipitating event that can accelerate the transition of precancerous lesions to cancer. These observations led us to hypothesize that inflammation that accompanies early phases of PDAC would produce pathologic changes in pancreatic neurons and innervation. Using a lineage labeled genetically engineered mouse model of PDAC we found that pancreatic neurotrophic factor mRNA expression and sensory innervation increased dramatically when only pancreatic intraepithelial neoplasia (PanIN) were apparent. These changes correlated with pain-related decreases in exploratory behavior and increased expression of nociceptive genes in sensory ganglia. At later stages, cells of pancreatic origin could be found in the celiac and sensory ganglia along with metastases to the spinal cord. These results demonstrate that the nervous system participates in all stages of PDAC, including those that precede appearance of cancer.
Keywords: neurotrophic factors, pain, peripheral nervous system, perineural invasion, metastases
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is associated with significant morbidity, mortality and pain that can significantly impact quality of life and survival time (1). Both PDAC-related pain and local tumor spread to retroperitoneal structures are thought to be related to perineural tumor invasion (1). Tumor invasion of intrapancreatic nerves facilitates local and distant tumor spread and exposes nerves to a complex inflammatory milieu. Inflammatory mediators and neurotrophic factors identified in resected PDAC specimens, mostly from patients with advanced disease, are known to produce nerve hypertrophy, enhance excitability, and promote perineural invasion (2–6). Furthermore, these neuroplastic changes correlate strongly with the severity of PDAC-associated pain (1,6).
Neuroplastic changes and their sequelae are thought to be consequences of pancreatic cancer due to factors released in the tumor. However, a recent study of neuroplastic changes related to the development of prostate cancer suggests that the peripheral nervous system plays an early, active role in tumor development and invasion (7). Furthermore, neuroplastic changes in the pancreas have been described in pancreatitis (8,9) and we hypothesized that similar changes may occur during PanIN-only stages of PDAC development as well. To address this issue we examined the time course of neuroplastic changes throughout PDAC progression in a genetically engineered mouse model of PDAC to determine whether changes in pancreatic nerves begin during pre-cancer stages. Our results demonstrate that changes in innervation and sensory neuron properties parallel disease progression and begin prior to the appearance of cancer, suggesting that a better understanding of early changes in the peripheral nervous system is important for our understanding of PDAC biology.
Materials and Methods
Mouse Strains
The KPC mouse strain was generously provided by Dr. Ronald DePinho (MD Anderson Cancer Center, Houston, TX). KPC mice express a conditional mutant Kras allele (LSL-KrasG12D), a conditional Trp53 allele with LoxP sites (p53Lox/+) and p48-Cre (p48-Cre), as previously described (10–13). Mice with LSL-KrasG12D; p53+/+, LSL-KrasG12D; p53lox/+, LSL-KrasG12D; p53lox/lox genotypes or either conditional allele alone were used as age- and sex-matched littermate controls. To visualize tumor cells of pancreatic origin, PDAC mice and littermate controls were crossed with the ROSA-LSL-tdTomato reporter strain (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J; The Jackson Laboratory, Bar Harbor, ME) to produce KPCT mice and tdTomato controls. KPC and KPCT mice of both sexes were analyzed at four time points during tumor development: 3-4 weeks, 6-8 weeks, 10-12 weeks, and greater than16 (>16) weeks.
KPC mice were weighed weekly to monitor sickness, although no significant difference in weight between KPC mice and age- and sex-matched littermates was observed (data not shown). While age provided an approximation of disease progression, significant variability in tumor size, tumor location, and disease severity was observed among KPC mice. Disease progression and sickness severity were also assessed using a mouse hunching scale adapted from a previous study of pancreatic acinar carcinoma in the mouse (14). Animals were scored weekly starting at post-natal day 28 using the following criteria: 0= no detectable sickness-related behavior, 1= slight notch visible in the animals’ back, near the shoulders, 2= a noticeably hunched posture and mild piloerection, 3= moderately hunched posture and increased piloerection, 4= severe hunching, piloerection, and very limited voluntary movement. The average age that a hunching score of 1 was first detected in KPC mice was 10 weeks, which correlates with the PanIN stage of tumor development described above. A hunching score of 2 or 3 correlates with significant sickness and was detected as early as 19 weeks and as late as 26 weeks, at which point animals have significant tumor burden.
All animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Division of Laboratory Animal Resources at the University of Pittsburgh with ad libitum access to water and food. Animals were cared for and used in accordance with guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Tissue Immunolabeling
Tissues were harvested from KPCT mice and tdTomato controls perfused with 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB). Pancreata/tumors were embedded in OCT compound and sectioned on a cryostat at 30μm thickness. Celiac ganglia and the spinal cord with associated dorsal root ganglia (DRG) from one KPCT mouse with tumor encasement of the spinal cord were embedded in 10% gelatin in 0.1M PB and sectioned on a sliding microtome at 20μm thickness. Tissue sections were washed, blocked (5% normal horse serum and 0.25% Triton X-100 in 0.1M PB), incubated in primary antibody overnight at room temperature, washed, incubated in secondary antibody (donkey anti-rabbit Cy2 1:500; Jackson ImmunoResearch) for 2 hours at room temperature, washed, dehydrated, and coverslipped with DPX mounting media (pancreas sections) or Vectashield mounting media (celiac ganglion and spinal cord). Primary antibodies used were rabbit anti-protein gene product 9.5 (PGP 9.5; 1:1000, UltraClone), rabbit anti-tyrosine hydroxylase (TH; 1:200, Cell Signaling Technology), and rabbit anti-activating transcription factor 3 (ATF3; 1:200, Santa Cruz Biotechnology). Sections of pancreas were also hematoxylin and eosin stained and coverslipped with DPX mounting medium. Sections were viewed and photographed on a LEICA DM 4000B microscope (Leica Microsystems, Wetzlar, Germany) using Leica Application Suite software.
Quantification of Pancreatic Innervation
Changes in pancreatic innervation were quantified in sections of pancreas from KPCT mice >16 and 10-12 weeks of age and tdTomato controls (n=4 for each group). Nerve fibers were visualized using an antibody for PGP 9.5, as described above, and at least 40 images were captured per animal, spanning across approximately 36 sections (about 1mm thickness). Images were captured at 20× magnification and nerve fibers were traced and analyzed using the ImageJ plugin, NeuronJ (15). The total number of fibers, average fiber density (fibers/mm2), and average fiber length were calculated for each animal. For each parameter, statistical significance between cancer and control groups was determined using t-test comparisons (SPSS Statistics, IBM Corporation).
Open-Field Exploratory Behavior
To assess pain-related behavior in KPC mice open-field exploratory behavior was analyzed at time points ranging from 7-31 weeks of age. As previously described (8,9), mice were placed in plexiglass boxes and their activity in the horizontal and vertical planes was measured photoelectrically at a 0.75 cm spatial resolution for a period of 15 minutes. TruScan software (Coulbourn Instruments) analyzed movements, speed, distance travelled, time spent moving. Both horizontal movement along the floor of the behavior arena and vertical movement in which animals were extending upward, were measured. The monitoring period was divided into 3 blocks of 5 minutes and data for each movement parameter was analyzed using a linear mixed effects model with age, genotype and time block treated as fixed effects and each individual animal treated as a random effect to account for intra-animal correlation. All analyses were performed using the R package lme4 and p-values for the fixed effects were based on likelihood ratio tests. Data from the second time block are presented as representative.
qRT-PCR Analysis
Animals were deeply anesthetized with 0.1-0.2cc ketamine/xylazine and either the pancreas/tumor was removed and immediately homogenized in 2mL Trizol reagent (Invitrogen) or mice were transcardially perfused with 0.9% saline and DRG 9-12 were removed bilaterally and frozen on dry ice. RNA was Trizol/chloroform extracted, precipitated in isopropanol, washed with 75% ethanol, resuspended in RNase-free water, and further purified using RNeasy columns (Qiagen, Valencia, CA). RNA from DRG was isolated using RNeasy columns and reconstituted in RNase-free water. All samples were treated with DNAse (Invitrogen) and 300ng-1μg was reverse-transcribed using Superscript II (Invitrogen). SYBR Green-labeled PCR amplification was performed using an ABI 7000 real-time thermal cycler controlled by Prism 7000 SDS software (Applied Biosystems, Foster City, CA) and threshold cycle (Ct) values were recorded as a measure of initial template concentration. Primers for nerve growth factor (Ngf), brain-derived neurotrophic factor (Bdnf), glial cell line-derived neurotrophic factor (Gdnf), neurturin (Nrtn), and artemin (Artn), their respective receptors, neurotrophic tyrosine kinase receptor, type 1 and type 2 (Trka and Trkb), and glycosylphosphatidylinositol-linked receptor α1, α2, and α3 (Gfrα1, Gfrα2, and Gfrα3), the nociception-related genes transient receptor potential cation channel vanilloid 1 (Trpv1), transient receptor potential cation channel subfamily A member 1 (Trpa1), sodium voltage-gated channel 1.8 (Nav. 1.8), potassium voltage-gated channel 4.3 (Kv 4.3), calcitonin-related polypeptide α (Cgrp), and neurokinin1 (Nk1), and the injury marker, ATF (Atf3) are listed in Supplementary Table S1. Two housekeeping genes, ribosomal protein L13A (Rpl13a) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were used for normalization (Supplementary Table S1). Additional housekeeping genes were tested using primers from a mouse housekeeping gene primer set (MHK-1; RealTimePrimers.com, Elkins Park, PA; data not shown). Relative fold change in RNA was calculated using the ΔΔCt method (16) with Rpl13a as a reference standard for pancreas samples and Gapdh as a reference standard for DRG samples. Significance was determined using Mann-Whitney U-tests (SPSS Statistics, IBM Corporation).
PDAC Cell Lines
Transcriptional profiling of human tumor-derived cell lines (MiaPaCa2 and Panc1) and mouse tumor-derived cell lines (Kpc1 and Kpc2) was carried out. Mouse cell lines were derived from tumors from two different KPC mice (p48-Cre; LSL-KRASG12D; p53lox/lox) and cultured in RPMI media with 10% fetal bovine serum (FBS) and 1% Penicillin/Streptomycin (Pen/Strep; Invitrogen). MiaPaCa2 cells (ATCC, CRL-1420) were cultured in MEM with 10% FBS, 2.5% horse serum (HS) and 1% Pen/Strep. Panc1 cells (ATCC, 1469) were cultured in MEM with 10% FBS and 1% Pen/Strep. Cell lines were grown to confluence and RNA was extracted using Trizol reagent. 1μg of DNAsed RNA was reverse-transcribed and PCR amplified using GoTaq DNA polymerase (Qiagen) using primer sets listed in Supplementary Table S1 (murine) and Supplementary Table S2 (human).
Results
Hypertrophied nerve bundles and perineural invasion accompany PDAC progression
Neuroplastic changes associated with PDAC were studied using genetically engineered mice that express a conditional mutant Kras allele and a Trp53 allele with LoxP sites under control of a pancreas-specific promoter driving Cre, p48-Cre. While disease course was variable among KPC and KPCT mice, PanIN lesions and fibrosis typically were present by 6-8 weeks of age, more advanced PanIN lesions and more extensive fibrosis were evident by 10-12 weeks, and ductal adenocarcinoma was observed in most mice by 16 weeks of age (Supplementary Figure S1). This time course of tumor development is consistent with prior reports using related mouse strains (11,13).
Neuroplastic changes are common in sections of resected tumor from PDAC patients (1) and these changes in innervation frequently include perineural tumor invasion (17–22). To determine if similar changes in innervation and perineural invasion occur in the mouse model of PDAC, we used the pan-neuronal marker PGP 9.5 to examine the density and distribution of pancreatic nerve fibers in KPCT mice (Figure 1). At 6-8 weeks of age, KPCT pancreata exhibited focal areas of hyperinnervation (Figure 1E-H) not present in control pancreata (Figure 1A-D). These areas were restricted to relatively small regions of the pancreas containing fibrosis, acinar atrophy, and/or PanIN lesions, whereas innervation in histologically normal pancreas was similar to that of controls. In the pancreata of KPCT mice at 10-12 weeks of age, multifocal PanIN lesions ranging from PanIN 1a to PanIN 3 were seen and hyperinnervation accompanied this expansion of pancreas pathology (Figure 1I-L).
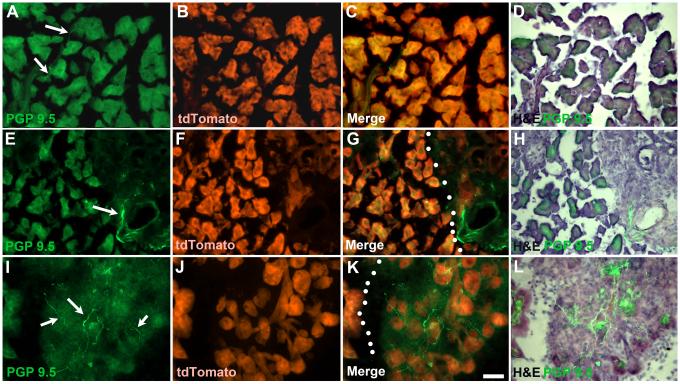
Figure 1.
Distribution of PGP 9.5–positive fibers during development of PDAC. A-D) In control mice, only occasional thin PGP 9.5-positive fibers are observed (arrows) within the parenchyma of the pancreas (as indicated by expression tdTomato). E-H) By 6-8 weeks, fibrosis begins to develop and numerous PGP-positive fibers can be seen associated with blood vessels and dilated ducts (arrow). Dotted lines indicate border between fibrotic region and normal appearing pancreas (G). I-L) Significant areas of fibrosis containing dense innervation by PGP-positive fibers are present in the pancreas of 10-12 week old tPDAC mice. Dotted lines in K indicate region with developing fibrosis with atrophied acinar tissue identified based on tdTomato expression. Calibration bar = 50μm.
Large intrapancreatic nerve bundles were observed in PDAC pancreata/tumors at >16 weeks of age (Figure 2). Hypertrophied nerves were often associated with areas of fibrosis and acinar cell atrophy (2D, H, L). PGP+ fibers were visualized extending from atrophied/aggregated islets (Figure 2A-D), near ducts and vasculature (Figure 2E-H), and adjacent to areas of PanIN lesions or tumor cells (Figure 2I-L). While intra-pancreatic PNI was not definitively observed, areas of focal fibrosis with hyperinnervation often featured branching of PGP9.5+ fibers near tdTomato+ cells or PGP9.5+ fibers appearing to engulf tdTomato+ cells, suggesting a mutual tropism between the two (Figure 2 C).
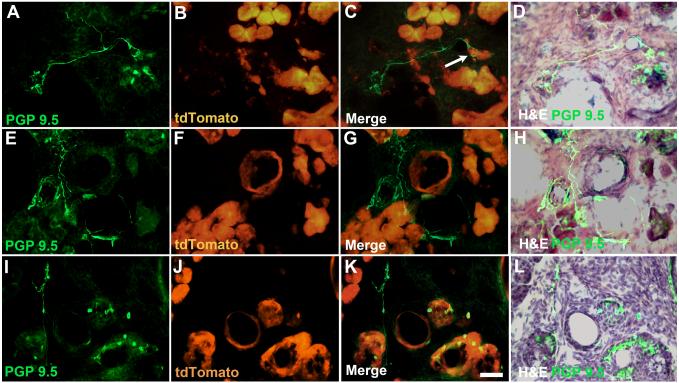
Figure 2.
Examples of abnormal innervation in tPDAC mice with identifiable tumors. Hypertrophied nerves with multiple fibers were present, primarily in regions that had developed fibrosis as indicated by overlap of PGP 9.5 and loss of tdTomato-expressing cells. A-D) In some cases fiber bundles can be seen extending from atrophied/aggregated islets, E-H) near ducts or vasculature, I-L) and adjacent to areas of PanIN lesions or tumor cells. Arrow in (C) shows nerve fibers appearing to engulf tdTomato-positive cells. Calibraion bar = 50μm
PGP+ fibers in the pancreas were traced using NeuronJ software to quantify the changes in pancreatic innervation observed in KPCT mice at 10-12 and >16 weeks of age. Total number of fibers (mean +/− standard deviation) traced in the pancreas/tumor of KPCT mice at 10-12 weeks (570.5 ±151.4) and >16 weeks (585.3±118.6) of age was significantly increased compared with tdTomato controls (348 ± 52.39; p=0.032 and p=0.011 respectively). Similarly, the average fiber density in the pancreas/tumor of KPCT mice at 10-12 weeks (3.828 ± 1.043 fibers/mm2) and >16 weeks (3.943 ± 0.7990 fibers/mm2) was significantly increased compared with tdTomato controls (2.275 ± 0.4203 fibers/mm2; p=0.03 and p=0.0086 respectively). Consistent with the observed hypertrophied pancreatic afferents described above, the average fiber length in KPCT mice at 10-12 weeks (54.72 ± 8.021um) and >16 weeks (45.61 ± 5.977um) was also significantly increased compared with tdTomato controls (33.38 ± 4.69um; p=0.0037 and p=0.018 respectively).
Using KPCT mice allowed tracking of tdTomato+ cells of pancreatic origin in local and distant sensory and sympathetic nerve ganglia. No tdTomato+ cells were ever visualized in the celiac ganglion of tdTomato control mice (Supplementary Figure S2). However, in one KPCT animal with advanced disease tdTomato+ cells invaded into the celiac ganglion (Figure 3), which was surrounded by a large tumor metastasis. In this case many celiac ganglion neurons were ATF3+, a marker of nerve injury only rarely expressed in control celiac ganglion cells (Figure 3D-F, arrow in 3D). In another KPCT case, pancreas-derived cells invaded the dorsal T11 and T10 root ganglia (Figure 4 C,E). This migration of tumor cells into the DRG was associated with a metastatic tumor that surrounded the spinal cord at levels T9-T12, the levels of spinal cord that normally innervate the pancreas (23) (Figure 4A). Some pancreas-derived cells in the DRG assumed complex morphology, exhibiting processes conducive to cell-to-cell adhesion or chemotaxis (inset in Figure 4E). Despite the large number of tdTomato+ cells present in the KPCT DRG, relatively few cells in the ipsi- or contralateral T10 and T11 DRG were ATF3+ (not shown).
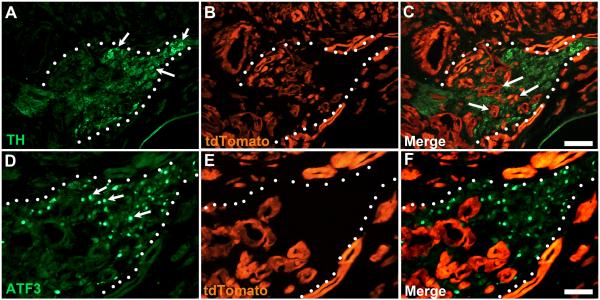
Figure 3.
PDAC induces pathological changes in celiac ganglion (indicated by dotted line). A) Celiac ganglion neurons stained with TH antibody (arrows). B) tdTomato staining reveals extensive tumor growth around and within (arrows in C) the borders of the celiac ganglion. D-F) Same celiac ganglion in A-C stained for expression of ATF3, a marker of neuronal injury. Numerous ATF3-positive neuronal nuclei are seen throughout the ganglion (arrows in A). Calibration bars A-C = 100μm; D-F = 50μm.
Figure 4.
Pancreatic tumor surrounding the spinal cord at vertebral levels that give rise to sensory innervation of the pancreas. A) Right and left arrows indicate T13 and T9 rib, respectively, demarcating the vertebral level of the sensory ganglia giving rise to the majority of sensory innervation to the pancreas. B) Tumor, indicated by tdTomato expression, surrounds both dorsal (DR) and ventral (VR) roots of the right T11 DRG. No tdTomato-positive cells are seen in this DRG. C) Left T11 DRG containing numerous tdTomato-positive cells, presumably representing migrating tumor cells associated with the tumor formation. tdTomato-positive cells were only seen in T10 and T11 DRG on the left side. D) Left T10 DRG stained with PGP 9.5. E) Merged photomicrograph showing numerous tdTomato-positive cells interspersed between PGP 9.5-positive DRG neurons. Insert shows tdTomato–expressing cell appearing to migrating between two DRG neurons. Calibration bars A = 2mm; B&C = 100μm; C&D = 50μm.
Changes in sensory neuron gene expression are associated with PDAC
Prior studies have found that pancreatic nerve hypertrophy is associated with changes in sensory neuron gene expression in mouse models of acute and chronic pancreatitis (8,9). To determine if similar changes are associated with the development and progression of PDAC, the relative level of mRNAs encoding genes related to nociception, neurogenic inflammation, and nerve injury were assessed in DRG 9-12 of KPC mice at ages 10-12 and >16 weeks. Similar to findings reported in mouse models of acute and chronic pancreatitis, expression of Trpa1 and Trpv1 was significantly increased in DRG of KPC mice >16 weeks of age (1.74- and 1.36-fold, respectively, p=0.013 and p=0.043; Table 1). A non-significant trend toward increased Trpa1 expression was also measured in 10-12 week old KPC mice (1.38-fold, p=0.059; Table 1). In rodent models of pancreatitis, TRPV1 and TRPA1 channels are implicated in neurogenic inflammation and increased release of CGRP and NK1 neuropeptides from neural terminals into the pancreas (8,9,24–28). In DRG of KPC mice at 10-12 and >16 weeks of age, Cgrp mRNA expression is significantly increased 1.36- and 1.27- fold, respectively (p=0.043 and p=0.029; Table 1). In contrast, Nk1 expression in the DRG was not significantly different between KPC and control mice at 10-12 weeks and only a non-significant trend toward increased expression was measured at >16 weeks of age (1.44-fold, p= 0.081; Table 1). Similarly, expression of Atf3 was not significantly different between control and KPC DRG at 10-12 weeks, but a non-significant trend toward increased expression was evident at >16 weeks of age (1.92-fold, p= 0.059; Table 1). In addition, mRNAs encoding the voltage-gated ion channels Nav1.8 and Kv4.3 were also not significantly different in the DRG of control and KPC mice at either age (Table 1).
Table 1.
Increased expression of genes related to nociception and neurogenic inflammation in DRG T9-T12 of PDAC mice. Data are normalized using Gapdh as a reference standard and are presented as fold change in expression relative to age-matched controls. For PDAC mice, n= 8, for controls n= 6.
| Gene | 10-12 wk PDAC DRG | >16wk PDAC DRG |
|---|---|---|
| Atf3 | 1.22 (p=0.950) | 1.92^ (p=0.059) |
| Cgrp | 1.36* (p=0.043) | 1.27* (p=0.029) |
| Kv4.3 | 0.99 (p=0.573) | 1.00 (p=1.000) |
| Nav1.8 | 1.12^ (p=0.081) | 1.19 (p=0.282) |
| Nk1 | 1.11 (p=0.662) | 1.44^ (p=0.081) |
| Trpv1 | 1.42 (p=0.142) | 1.36* (p=0.043) |
| Trpa1 | 1.38^ (p=0.059) | 1.74* (p=0.013) |
p<0.05,
non-significant trend
KPC mice exhibit decreased exploratory behavior
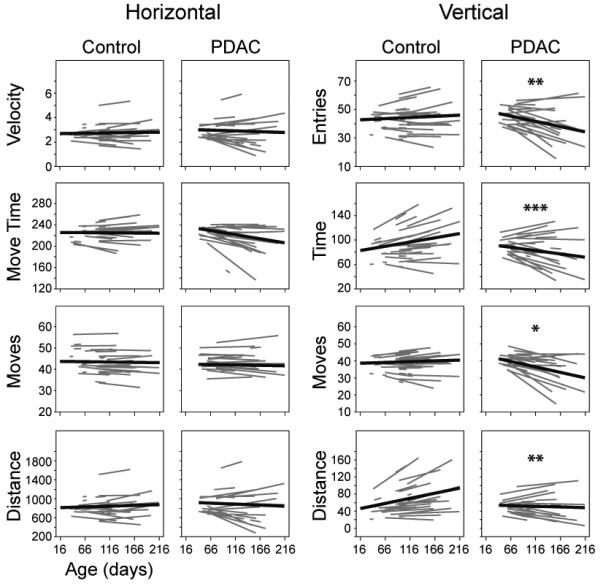
Having observed neuroplastic changes in KPC mice similar to those described in human PDAC, we hypothesized that KPC mice would develop pain related to this condition as well. To measure the impact of PDAC on mouse behavior, we analyzed open-field exploratory activity using photoelectric monitoring throughout disease progression. The change over time in measures of horizontal exploratory activity, as animals moved along the floor of the behavior arena, was not significantly different between cancer and control mice (Figure 5). In contrast, there was a significant difference in the change over time in measures of vertical exploratory activity between KPC and control mice (Figure 5). Vertical movements generally decreased over time in KPC mice but not in controls (Moves, p= 0.0311; Time, p=0.0006; Distance, p=0.0099; Entries, p= 0.0014; Figure 5). Vertical exploratory activity measurements included postural movements such as rearing and more subtle movements in which the mouse dorsiflexed its neck. One aspect that these vertical movements have in common is the elongation of the trunk, which conflicts with the hunched postures adopted by these animals as the disease progresses and with the hunching described in a mouse model of pancreatic acinar carcinoma (14,29).
Figure 5.
Decreased open-field exploratory behavior in PDAC mice with increasing age. PDAC and control mice were photoelectrically monitored in an open-field arena and both horizontal and vertical movement measurements were recorded. Animals were monitored for a total of 15 minutes and data were divided into 3 blocks of 5 minutes each for analysis; data from the second block are presented as representative. * p<0.05, **p<0.01, ***p<0.001
Changes in neurotrophic factor expression occur prior to overt tumor formation
Previous studies have associated increased neurotrophic growth factor expression with PDAC-induced neuronal hypertrophy, perineural invasion and PDAC-related pain (2–6,18,19,28,30,31). To determine if changes occur in neurotrophic factors and their receptors during tumor development and progression, pancreas RNA from KPC and control mice was analyzed at 3-4, 6-8, 10-12, and >16 weeks of age. There were no significant differences in expression of Ngf, Bdnf, Gdnf, Artn, Nrtn or their receptors between KPC and control mice at 3-4 weeks of age (Figure 6). Gfrα2 was significantly increased (3.74 fold; p=0.008) in the pancreas of KPC mice at 6-8 week of age (Figure 6). In the 10-12 week age group (when advanced PanIN lesions are present), Ngf, Trkb, Nrtn, and Gfrα2 were significantly increased (2.25-, 2.18-, 1.39-, and 3.42-fold, respectively; p= 0.007, p= 0.012, p=0 .028, and p= 0.028) in the pancreas of KPC mice (Figure 6). In the pancreas of KPC mice >16 weeks of age, Ngf, Trka, Gdnf, and Gfrα2 were significantly increased (3.6-, 29.9-, 7.11-, and 4.02-fold, respectively; p= 0.03, p= 0.001, p= 0.023, and p= 0.024; Figure 6). In contrast, Gfrα3 expression in the pancreas of >16 week old PDAC mice was significantly decreased (4.30-fold; p= 0.009; Figure 6).
Figure 6.
Expression of neurotrophic factors and neurotrophic factor receptors in the pancreas of PDAC mice was measured at 3-4, 6-8, 10-12, and >16 weeks of age, normalized using Rpl13a as a reference standard, and presented as the percent expression of age-matched controls. Control n= 4 (3-4 weeks), n= 4 (6-8), n=5 (10-12), and n=6 (>16). PDAC n= 6 (3-4), n=7 (6-8), n=7 (10-12), and n=10 (>16). *p<0.05; **p<0.01; ***p<0.001. Data from >16 weeks old mice are also presented as the percent expression of controls normalized to the amount of cDNA amplified (>16Φ) and using this method of comparison, expression all neurotrophic factors and neurotrophic factor receptors except Gfrα3 is significantly increased, p<0.01.
For the analyses above, gene expression was normalized to the housekeeping gene Rpl13a, which did not significantly change in KPC mice at 3-4, 6-8, and 10-12 weeks of age. However, expression of Rpl13a was increased 3-fold in the pancreas of KPC mice >16 weeks of age, and the expression of seven other housekeeping genes was increased 5- to 15-fold (Supplementary Table S3). It should also be mentioned that if mRNA expression is normalized based on the starting cDNA concentration, larger changes in gene expression are apparent for all growth factors and receptors, except Gfrα3 (Figure 6). Thus, regardless of the method used, significant changes in growth factor and receptor mRNA occur, indicating that as cancer progresses, the pancreas produces a milieu that resembles the pro-growth environment experienced by the peripheral nervous system during development (32,33).
Neurotrophic factor and neurotrophic factor receptor is expressed in PDAC cell lines
A variety of cell types including tumor cells, infiltrating immune cells, and cells in the stromal compartment of the tumor could each contribute to the observed changes in growth factor and growth factor expression in the pancreas of KPC mice. Previous studies of human tumor cell lines have demonstrated that many express a variety of growth factors and receptors, suggesting that PDAC cells represent a significant source and target of released intrapancreatic neurotrophic factors. To address the potential contribution of the tumor cells to changes in growth factor expression, RNA from two murine PDAC cell lines derived from KPC mice (Kpc1 and Kpc2) was analyzed to assess tumor-specific neurotrophic factor and neurotrophic factor receptor expression. Kpc1 and Kpc2 expressed Ngf, Artn, Gdnf, Nrtn, and Gfrα2. Kpc2 additionally expressed Gfrα3 and Gfrα1 (Supplementary Table S4). This expression profile is quite similar to the human tumor cell lines, Panc1 and MiaPaCa2, which express ARTN, BDNF, TRKA, GFRα3, and GFRα2 (Supplementary Table S4). Panc1 also expressed NGF, GFRα1 and TRKB while MiaPaCa2 expressed GDNF (Supplementary Table S4).
Discussion
The histological progression from PanIN to PDAC in KPC mice has been shown previously to closely model histological changes observed in the human disease (12). As such, these mice provide an opportunity to study changes in the nervous system occurring at pre-cancer stages of the disease, which would be difficult to study in patients with PDAC. Here we demonstrate that neuroplastic changes associated with PDAC begin at the histologic pre-cancer stage, suggesting an active role of the nervous system in disease progression. These early neuroplastic changes are likely to contribute to the development of PDAC-related pain and may play a significant role in tumor progression, similar to what has been described in prostate cancer (7). Furthermore, the presence of nerve hypertrophy in the pancreas provides an anatomical substrate for neurogenic inflammation and metastases.
Tumor-nerve interactions have been widely described in human PDAC (17–21,31). Previous studies have shown that intrapancreatic PNI is present in up to 100% of PDAC cases (17–21) and hypertrophied nerve bundles have also been described (1). In the present study, similar changes in pancreas/tumor innervation were observed in KPCT mice >16 weeks old. We hypothesized that PDAC-related changes in pancreas innervation observed in advanced stages of human and mouse PDAC are not simply a consequence of the tumor, but represent a reciprocal relationship between the cancer and the peripheral nervous system that begins early in tumor development. As early as 6-8 and 10-12 weeks of age, areas of hyperinnervation were found in the pancreas of KPCT mice, suggesting that early changes in the microenvironment can, in fact, affect pancreatic nerves.
While intrapancreatic PNI is was difficult to detect in KPCT mice at any age, invasion of local and distant extra-pancreatic nerve ganglia was found. Invasion of extrapancreatic nerve plexuses has been described in PDAC and the presence of tumor invasion of extrapancreatic nerve plexuses is significantly correlated with decreased survival rate following tumor resection (18,21,22). The celiac ganglion of one KPCT mouse was encased by a large tumor metastasis and tdTomato+ cells were present inside the ganglion. This suggests that tumor cells migrated from the pancreas along the sympathetic nerves that synapse in the celiac ganglion or the sensory afferents that run through it, a mode of tumor spread that may occur in human PDAC as well. Similarly, tdTomato+ cells were located inside the T10 and T11 DRG in a different KPCT mouse in which tumor encasement of the spinal cord was grossly visible, again demonstrating the potential for tumor cell migration along sensory afferents. How tumor cells invade and move along peripheral nerve fibers is not well understood. It is likely however, that a variety of secreted factors in the environment and expression of genes in tumor cells such as adhesion molecules play a role.
In human PDAC, pancreatic nerve hypertrophy and PNI are also associated with PDAC-related pain (1,19,28), suggesting that nerves in the pancreas are both damaged by the invading tumor cells and sensitized by changes in the tumor microenvironment. In KPC mice, pain-like behaviors were observed with increasing age. That PDAC-related pain is most evident after the progression from PanIN to PDAC is similar to previous observations made in a genetic mouse model of acinar carcinoma (14,29). Although decreased exploratory behavior could be due to other factors unrelated to pain such as general malaise associated with illness severity, previous studies of acute and chronic pancreatitis pain have shown that pancreatitis-related changes in exploratory behavior can be reversed by morphine treatment (8,9), which supports a role for pain in the observed behavioral changes. Furthermore, the selective inhibition of vertical, but not horizontal, movement is also suggestive of abdominal discomfort. However, it is also true that abdominal pain in KPC mice could be related to mechanical distention of the abdomen due to ascites or somatic pain associated with peritonitis. In the present study, only a minority of mice displayed signs of significant abdominal ascites at the time of testing, but future studies will be necessary to better address the specific contribution of pancreas-related pain to decreased vertical exploratory behavior.
We and others have shown that neurotrophic factors drive sprouting and sensitization of primary sensory afferents and sympathetic neurons in adult systems (34–40). Many of these factors and receptors have also been implicated in human PDAC (2–4,6,30,41). Increased NGF and ARTN is significantly correlated with nerve hypertrophy in human PDAC (2,3) and increased expression of NGF and TRKA in PDAC tissue correlates with patient reports of pain (6,19,30). In KPC mice >16 weeks of age, changes in neurotrophic factor and receptor expression were observed similar to those described in human PDAC. Furthermore, given that both human and murine PDAC cell lines express a variety of neurotrophic factors and receptors, in patients, tumor cells themselves (and not associated tissues like blood vessels or immune cells) probably represent an important source of these molecules. Therefore, neurotrophic factor signaling in PDAC likely plays a prominent role in promoting tumor growth and spread, in addition to affecting pancreatic afferent growth and sensitization.
In KPCT mice, the presence of altered innervation in the pancreas at 6-8 and 10-12 weeks of ages suggests that neurotrophic factor-rich, “pro-growth” microenvironments are also present at pre-cancer stages of disease progression. While detection of changes in growth factor or growth factor receptor expression was limited in KPC mice at 6-8 weeks, at 10-12 weeks, when more pancreas lobules contain pathological changes, the expression of several growth factors and receptors was significantly increased. At pre-cancer time points, neurotrophic factors and receptors may be produced by tumor precursor cells as well as other components of the microenvironment such as infiltrating immune cells. Thus, changes in neurotrophic factor signaling in the pancreas prior to the development of cancer could impact the progression from PanIN to PDAC through direct action on tumor precursor cells and/or via growth factor-mediated changes in pancreatic innervation.
Neuroplastic changes frequently described in PDAC are similar to changes observed in pancreatitis. Pancreatitis is associated with increased growth factor expression (42), hypertrophied nerve bundles (1,42) and sensitized pancreatic afferents, all of which are thought to increase pancreatitis-related pain (1). In rodent models of pancreatitis, pancreatic sensory afferents up-regulate non-specific cation channels, such as TRPV1 and TRPA1, and demonstrate hypersensitivity (8,9,26). Activation of TRPV1 in sensitized pancreatic afferents can drive neurogenic inflammation in the pancreas through release of peptides such as CGRP and NK1, and importantly, blocking this process attenuates pain and inflammation (8,9,24–28). In the DRG of KPC mice >16 weeks of age, expression of Trpv1, Trpa1 and Cgrp is increased accompanied by a trend toward increased Nk1 expression, suggesting that a similar process of peripheral afferent sensitization and neurogenic inflammation occurs in PDAC. At 10-12 weeks of age, a trend of increased Trpa1 expression and increased Cgrp expression was also measured. While we would expect to see changes in the DRG similar to what has been described in pancreatitis at pre-cancer stages of PDAC progression, the number of DRG neurons in the T9-T12 ganglia that innervate the pancreas is relatively small. And based on the limited distribution of hyperinnervation at early time points, only a subset of pancreatic afferents may be affected early in the disease. Therefore, when analyzing whole DRG gene expression, it is not surprising that we did not detect statistically significant changes of nociception-related molecules until later in the disease process.
Chronic pancreatitis increases the risk of malignancy (43,44) and pancreatic inflammation has been shown to promote disease progression and metastases in mouse models of PDAC (13,45–48). Studies from our laboratories and others show that the peripheral nervous system can drive pancreatic inflammation (8,9,24–28). This raises the possibility that pancreatic nerves play an active role in PDAC development and progression. Even a small population of sensitized pancreatic afferents at early, pre-tumor stages could drive neurogenic inflammation similar to what has been described in animal models of pancreatitis, thereby contributing to and even accelerating the progression from PanIN to PDAC.
Ablation of pancreatic innervation has previously been performed in patients with PDAC, with the hypothesis that pancreatic denervation would inhibit pain and improve survival (49,50). These and other studies have demonstrated at least some reduction in pain following chemical splanchnicectomy or celiac plexus block in patients with unresectable pancreatic cancer. However, the effects on survival time were mixed, with one study demonstrating increased survival time in patients following chemical splanchnicectomy (49) and the other reporting no effect on survival post-celiac plexus block (50). Patients studied in both trials had advanced pancreatic cancer and the results of the present study suggest that if pancreas denervation was performed at an earlier time point (ideally at the pre-cancer stage) a significant inhibitory effect on tumor growth may have been seen. Although it is difficult to perform such a study because it is not possible to routinely identify non-cystic, precancerous PanIN lesions in patients, targeting pancreatic innervation in patients with early, resectable disease could yield benefits in terms of both delayed onset of pain and increased survival time.
In conclusion, early changes in neurotrophic factor expression and pancreatic innervation are important not only for the subsequent development of PDAC-related pain, but also for driving disease progression from pre-malignant stages to cancer via sensory afferent sensitization and neurogenic inflammation. Given that prominent PNI and tumor-nerve interactions have been described in a number of malignancies, early interventions targeting the peripheral nervous system represent a novel tumor treatment strategy for variety of cancers, including PDAC.
Supplementary Material
Acknowledgments
We thank Charlotte Diges and Christopher Sullivan for their technical assistance.
Grant Support
Financial Support: NIH CA177857 (BMD, ADR), NIH NS050758 (BMD), NIH NS075760 (BMD), NIH NS033730 (KMA), NIH DK063922 (RES), NIH DK088945 (ADR), Shirley Hobbs Martin Memorial Fund (BMD)
Footnotes
Conflicts of interest: We have no conflicts of interest to report.
References
- 1.Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186.e1. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Ceyhan GO, Schäfer K-H, Kerscher AG, Rauch U, Demir IE, Kadihasanoglu M, et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann. Surg. 2010;251:923–31. doi: 10.1097/SLA.0b013e3181d974d4. [DOI] [PubMed] [Google Scholar]
- 3.Ceyhan GO, Giese N a, Erkan M, Kerscher AG, Wente MN, Giese T, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann. Surg. 2006;244:274–81. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J, Jiang Y, Jiang Y, Sun Y, Zhao X. Expression of nerve growth factor and tyrosine kinase receptor A and correlation with perineural invasion in pancreatic cancer. J. Gastroenterol. Hepatol. 2008;23:1852–9. doi: 10.1111/j.1440-1746.2008.05579.x. [DOI] [PubMed] [Google Scholar]
- 5.Zeng Q, Cheng Y, Zhu Q, Yu Z, Wu X, Huang K, et al. The Relationship between Over-expression of Glial Cell-derived Neurotrophic Factor and Its RET Receptor with Progression and Prognosis of Human Pancreatic Cancer. J. Int. Med. Res. 2008;36:656–64. doi: 10.1177/147323000803600406. [DOI] [PubMed] [Google Scholar]
- 6.Zhu BZ, Friess H, F F, Zimmermann A, G HU, Korc M, et al. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J. Clin. Oncol. 1999;17:2419–28. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 7.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science. 2013;341:1236361–1236361. doi: 10.1126/science.1236361. (80-.) [DOI] [PubMed] [Google Scholar]
- 8.Schwartz ES, Christianson J a, Chen X, La J-H, Davis BM, Albers KM, et al. Synergistic Role of TRPV1 and TRPA1 in Pancreatic Pain and Inflammation. Gastroenterology. 2010:1283–91. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz ES, La J-H, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 Antagonists Prevent the Transition of Acute to Chronic Inflammation and Pain in Chronic Pancreatitis. J. Neurosci. 2013;33:5603–11. doi: 10.1523/JNEUROSCI.1806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuveson D a, Shaw AT, Willis N a, Silver DP, Jackson EL, Chang S, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–87. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 11.Bardeesy N, Aguirre AJ, Chu GC, Cheng K-H, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. U. S. A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 13.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. Elsevier Inc. 2012;148:349–61. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevcik M a, Jonas BM, Lindsay TH, Halvorson KG, Ghilardi JR, Kuskowski M a, et al. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology. 2006;131:900–10. doi: 10.1053/j.gastro.2006.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meijering E, Jacob M, Sarria J-CF, Steiner P, Hirling H, Unser M. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry. A. 2004;58:167–76. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Ishikura H, Motohara T. Perineural Invasion by Ductal Adenocarcinoma of the Pancreas. J. Surg. Oncol. 1997:164–70. doi: 10.1002/(sici)1096-9098(199707)65:3<164::aid-jso4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Liebig C, Ayala G, Wilks J, Berger DH, Albo D. Perineural invasion in cancer: a review of the literature. Cancer. 2009;115:3379–91. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 19.Bapat A a, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer. Nature Publishing Group. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Lu K-Y. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat. Dis. Int. 2002:469–76. [PubMed] [Google Scholar]
- 21.Mitsunaga S, Hasebe T, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. Detail Histologic Analysis of Nerve Plexus Invasion in Invasive Ductal Carcinoma of the Pancreas and Its Prognostic Impact. Am. J. Surg. Pathol. 2007;31:1636–44. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]
- 22.Nakao a, Harada a, Nonami T, Kaneko T, Takagi H. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357–61. doi: 10.1097/00006676-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Fasanella KE, Christianson J a, Chanthaphavong RS, Davis BM. Distribution and neurochemical identification of pancreatic afferents in the mouse. J. Comp. Neurol. 2008;509:42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romac JMJ, McCall SJ, Humphrey JE, Heo J, Liddle R a. Pharmacologic disruption of TRPV1-expressing primary sensory neurons but not genetic deletion of TRPV1 protects mice against pancreatitis. Pancreas. 2008;36:394–401. doi: 10.1097/MPA.0b013e318160222a. [DOI] [PubMed] [Google Scholar]
- 25.Liddle R a, Nathan JD. Neurogenic inflammation and pancreatitis. Pancreatology. 2004;4:551–9. doi: 10.1159/000082180. discussion 559-60. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Colak T, Shenoy M, Liu L, Pai R, Li C, et al. Nerve Growth Factor Modulates TRPV1 Expression and Function and Mediates Pain in Chronic Pancreatitis. Gastroenterology. 2011;141:370–7. doi: 10.1053/j.gastro.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan JD, Peng RY, Wang Y, McVey DC, Vigna SR, Liddle R a. Primary sensory neurons: a common final pathway for inflammation in experimental pancreatitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G938–46. doi: 10.1152/ajpgi.00105.2002. [DOI] [PubMed] [Google Scholar]
- 28.Ceyhan GO, Michalski CW, Demir IE, Müller MW, Friess H. Pancreatic pain. Best Pract. Res. Clin. Gastroenterol. 2008;22:31–44. doi: 10.1016/j.bpg.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Lindsay TH, Jonas BM, Sevcik M a, Kubota K, Halvorson KG, Ghilardi JR, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119:233–46. doi: 10.1016/j.pain.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Dang C, Zhang Y, Ma Q, Shimahara Y. Expression of nerve growth factor receptors is correlated with progression and prognosis of human pancreatic cancer. J. Gastroenterol. Hepatol. 2006;21:850–8. doi: 10.1111/j.1440-1746.2006.04074.x. [DOI] [PubMed] [Google Scholar]
- 31.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Müller MW, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem. Biophys. Res. Commun. 2008;374:442–7. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 33.Huang EJ, Reichardt LF. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molliver DC, Lindsay J, Albers KM, Davis BM. Overexpression of NGF or GDNF alters transcriptional plasticity evoked by inflammation. Pain. 2005;113:277–84. doi: 10.1016/j.pain.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Elitt CM, Malin S a, Albers KM. Effects of the neurotrophic factor artemin on sensory afferent development and sensitivity. Sheng Li Xue Bao. 2008;60:565–70. [PMC free article] [PubMed] [Google Scholar]
- 36.Elitt CM, McIlwrath SL, Lawson JJ, Malin S a, Molliver DC, Cornuet PK, et al. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J. Neurosci. 2006;26:8578–87. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malin S a, Davis BM. Postnatal roles of glial cell line-derived neurotrophic factor family members in nociceptors plasticity. Sheng Li Xue Bao. 2008;60:571–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci. Lett. 1999;274:159–62. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- 39.Ciobanu C, Reid G, Babes A. Acute and chronic effects of neurotrophic factors BDNF and GDNF on responses mediated by thermo-sensitive TRP channels in cultured rat dorsal root ganglion neurons. Brain Res. 2009;1284:54–67. doi: 10.1016/j.brainres.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Schmutzler BS, Roy S, Hingtgen CM. Glial cell line-derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons. Neuroscience. 2009;161:148–56. doi: 10.1016/j.neuroscience.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J. Natl. Cancer Inst. 2010;102:107–18. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ceyhan GO, Bergmann F, Kadihasanoglu M, Erkan M, Park W, Hinz U, et al. The neurotrophic factor artemin influences the extent of neural damage and growth in chronic pancreatitis. Gut. 2007;56:534–44. doi: 10.1136/gut.2006.105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernández-Porras I, Cañamero M, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitcomb DC, Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol. Clin. North Am. 2002;31:663–78. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 45.Carrière C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res. Commun. Elsevier Inc. 2009;382:561–5. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Carrière C, Young AL, Gunn JR, Longnecker DS, Korc M. Acute pancreatitis accelerates initiation and progression to pancreatic cancer in mice expressing oncogenic Kras in the nestin cell lineage. PLoS One. 2011;6:e27725. doi: 10.1371/journal.pone.0027725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fukuda A, Wang SC, Morris JP, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 Contribute to Pancreatic Ductal Adenocarcinoma Initiation and Progression. Cancer Cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt H a, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann. Surg. 1993;217:447–55. doi: 10.1097/00000658-199305010-00004. discussion 456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. Effect of Neurolytic Celiac Plexus Block on Pain Relief, Quality of Life, and Survival in Patients with Unresectable Pancreatic Cancer A Randomized Control Trial. JAMA. 2004;291:1092–9. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.