Abstract
Objective
To compare the utility and diagnostic accuracy of the MoCA and MMSE in the diagnosis of Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI) in a clinical cohort.
Method
321 AD, 126 MCI and 140 older adults with healthy cognition (HC) were evaluated using the the MMSE, MoCA, a standardized neuropsychological battery according to the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD-NB) and an informant based measure of functional impairment, the Dementia Severity Rating Scale (DSRS). Diagnostic accuracy and optimal cut-off scores were calculated for each measure, and a method for converting MoCA to MMSE scores is presented also.
Results
The MMSE and MoCA offer reasonably good diagnostic and classification accuracy as compared to the more detailed CERAD-NB; however, as a brief cognitive screening measure the MoCA was more sensitive and had higher classification accuracy for differentiating MCI from HC. Complementing the MMSE or the MoCA with the DSRS significantly improved diagnostic accuracy.
Conclusion
The current results support recent data indicating that the MoCA is superior to the MMSE as a global assessment tool, particularly in discerning earlier stages of cognitive decline. In addition, we found that overall diagnostic accuracy improves when the MMSE or MoCA is combined with an informant-based functional measure. Finally, we provide a reliable and easy conversion of MoCA to MMSE scores. However, the need for MCI-specific measures is still needed to increase the diagnostic specificity between AD and MCI.
Keywords: Alzheimer’s disease, Mild Cognitive Impairment, MMSE, MoCA, Diagnostic accuracy
1. Introduction
Alzheimer’s disease (AD) is a chronic, debilitating condition causing significant disease burden and mortality in adults over the age of 65 (1–4). The health and economic impact of AD has lead to a pressing need to prevent or slow disease onset and progression, and recent research efforts have focused on the transitional period from normal cognitive aging to dementia (5). This transition period, typically associated with Mild Cognitive Impairment (MCI), is signified by a measurable deterioration in cognitive function that is greater than expected based upon an individual’s age and education but which has not meaningfully affected a person’s daily functioning (6). Despite its being a major research focus in recent years (7), establishing the diagnosis of MCI with standard neuropsychological assessment instruments has remained challenging (8).
In the absence of MCI screening measures with established cut-off scores and confidence intervals, more extensive neuropsychological testing is often advised to reliably differentiate MCI from healthy cognitive aging (HC) or AD, although professional availability, time and cost do not often allow this. An efficient battery used at many specialized memory clinics and Alzheimer’s Disease Research Centers is the Consortium to Establish a Registry for Alzheimer’s neuropsychological battery (CERAD-NB) (9–13). While this battery was developed specifically for AD, it also has been used to discriminate between MCI and HC with some success (12–14). While the latter battery is briefer than standard neuropsychological batteries, the CERAD-NB is still lengthy and requires specialized training for proper administration making it less practical for use in the typical neurology or geriatric practices. Thus, brief, validated, practical measures that can differentiate MCI and dementia would be very useful given the increase in the proportion of elderly individuals seeking medical help for memory-related changes.
Brief screening measures, such as the Mini–Mental State Examination (MMSE) (15) and Montreal Cognitive Assessment (MoCA) (16), are easily administered with little training and have demonstrated diagnostic utility (17, 18), particularly in differentiating dementia from normal cognitive aging (19, 20). Recently, the diagnostic accuracy of these two screening measures has received increased attention as the importance of differentiating AD and MCI has grown (17, 21). Both the MMSE and MoCA accurately differentiate cognitive impairment (MCI or AD) from normal cognitive aging (20), but the MoCA has shown more utility in other disorders (e.g. Parkinson’s disease(22). To our knowledge, only one study has directly compared the MoCA and the MMSE in differentiating MCI from AD and healthy aging in a large clinical cohort (19) and one study examined MCI subtypes (23). The latter study, which included AD patients, found improved accuracy for the MoCA, but the study was limited to the amnestic subtype of MCI in a Portuegese sample. There is a dearth of research comparing the diagnostic accuracy of the MoCA to other standardized measures in a well-characterized clinical dementia cohort. Furthermore, if the MoCA is a more accurate screening measure for MCI, as shown in other neurological disorders (24, 25), then a straightforward conversion of MoCA to MMSE scores would be useful given the vast amount of clinical and research that has been collected using the MMSE.
It is doubtful that brief cognitive screening instruments alone would be sufficient to accurately distinguish mild dementia from MCI. Such a distinction is made when cognitive decline is by definition associated with meaningful decline in daily personal, social, or occupational functioning (26–28). Such functional decline may not be reported by the patient and requires report from a family member or other informant. There are many observer-based functional rating scales that together with a brief cognitive measure may significantly improve diagnostic accuracy (29–35).
The aims of this study were: 1) to compare the diagnostic accuracy of the MMSE and MoCA to a more detailed assessment of neuropsychological performance (CERAD-NB); 2) to measure the improvement in diagnostic accuracy when the MMSE or MoCA were used in combination with a measure of functional impairment (DSRS; 35) and 3) to determine a simple and reliable algorithm for conversion of MoCA to MMSE scores in diagnosis of AD and MCI in a large, well-characterized community-dwelling cohort evaluated and diagnosed by clinicians at a specialized memory center.
2. Methods
2.1 Study population
All participants were recruited from the Penn Memory Center and Clinical Core of the University of Pennsylvania’s Alzheimer’s Disease Center. Participants included 321 individuals with clinical consensus diagnoses of AD, 126 individuals with MCI and 140 HC adults (Table 1). Diagnostic assessments included history, physical, and neurologic examinations conducted by experienced clinicians, including the review of neuroimaging, psychometric and laboratory data. On the basis of all this data, a consensus diagnosis was established using standardized clinical criteria for AD, MCI, or other neurological or psychiatric conditions presenting with cognitive impairment (5, 6, 26, 36).
Table 1.
Demographic characteristics and performance for Alzheimer’s disease (AD), Mild Cognitive Impairment (MCI) and healthy comparison (HC) participants.
| Characteristic | AD (n=321) | MCI (n=126) | HC (n=140) |
|---|---|---|---|
| Age, years | 75.69 (8.21)d,e | 72.29 (8.12)e | 71.19 (9.20) |
| Age Range, years | 53–93 | 52–88 | 50–88 |
| Sex, Men/Women | 122/199 | 64/62f | 46/94 |
| Race, (AA/As/C/M/O) | 45/3/232/37/4 | 12/5/93/15/0 | 22/2/107/9/0 |
| Education, years | 13.33 (4.15)d,e | 14.86 (4.20)e | 15.91 (3.03) |
| GDSa | 3.07 (2.92)d,e | 2.69 (2.68)e | 0.99 (1.64) |
| Ethnicity (% Latino) | 14% | 14% | 11% |
| MMSE | 19.04 (5.88)d,e | 26.00 (3.46)e | 29.30 (0.87) |
| MoCA | 13.04 (6.05)d,e | 20.94 (4.50)e | 26.83 (2.64) |
| Neuropsychological Score (CERAD)b | 44.89 (13.71)d,e | 64.21 (11.66)e | 86.87 (9.02) |
| Adjusted CERADc | 58.29 (13.72) | 76.08 (10.93) | 97.28 (8.43) |
| DSRS | 16.01 (8.50)d,e | 6.20 (4.22)e | 0.28 (0.69) |
AA= African American, As= Asian, C= Caucasian, M=Multiple Races reported, O=Other (e.g. Native American); GDS= Geriatric Depression Scale; MMSE= Mini Mental Status Examination; MoCA= Montreal Cognitive Assessment; CERAD= Consortium to Establish a Registry for Alzheimer’s Disease; DSRS= Dementia Severity Rating Scale.
AD: n=283; MCI: n=119, HC: n=137;
AD: n=275; MCI: n=122, HC: n=138;
adjusted for age, education and gender based on Chandler et al., 2005; dAD: n=318; MCI: n=124, HC: n=137
p<.01 as compared to MCI;
p<.01 as compared to HC;
p<.01, larger proportion of men as compared to AD and HC
Screening and cognitive assessments included the MMSE, MoCA and the CERAD-NB. A composite score for the CERAD battery was derived using a method previously described by Chandler et al. (12). We used the method from this paper that excluded the MMSE from the CERAD composite total score, and also provided age-, education-, and gender corrected scores (12). The MoCA was administered on the same day as the clinical and neuropsychological evaluation. In addition, the Dementia Severity Rating Scale (DSRS) was administered to assess functional capacity. The DSRS is a reliable and simple 12-item multiple-choice questionnaire that collects information from a knowledgeable informant on impairment severity in twelve cognitive and functional domains (37). The MMSE, CERAD-NB and DSRS were available during consensus diagnosis, but the MoCA was not. MCI subtypes and dementias other than AD were not analyzed in this study. HC subjects were also recruited and assessed identically to the patients with AD and MCI. Informed consent for the use of all data was obtained from all persons, in accord with university institutional review board–approved protocols. All participants completed the MMSE and MoCA. Most, but not all, participants completed the CERAD-NB and DSRS. Most participants also received the Geriatric Depression Scale (GDS) as a screening measure for mood disturbances (38) (See Table 1).
2.2 Statistical analysis
Demographic characteristics were compared across diagnostic groups using Pearson χ2 or one-way ANOVAs with post-hoc t-tests. The diagnosis accuracy for each measure (or combination of measures) was calculated as the area under the receiver operating characteristic (ROC) curve (AUC). The AUC measure represents the mean sensitivity value for all possible values of specificity. A cut-off score for each measure that best differentiated diagnostic groups was determined using the Youden Index (39), which maximizes the tradeoffs between sensitivity and specificity. The classification accuracy (probability of correct classification of subject with or without impairment at a given cut-off score) was calculated based upon these cut-off scores (Table 2). Diagnostic accuracy of the MMSE, MoCA and DSRS (or combination of measures) was compared to the CERAD-NB using Chi-Square analysis. Classification accuracy of each measure was compared using the Wilcoxon Signed Rank test. These statistical analyses were performed using SAS Program software (v 9.1, SAS Institute, Cary, NC).
Table 2.
Diagnostic parameters for MMSE, MoCA, CERAD-NB and DSRS across AD, MCI and healthy older groups.
| A. | |||
|---|---|---|---|
| Parameter | HC and AD | HC and MCI | MCI and AD |
| CERAD total scorea | |||
| AUC (+/− 95%CI) | 0.99 (0.98–1.00) | 0.93c,e (0.90–0.96) | 0.84 (0.82–0.89) |
| Cut-offb | 83 | 89 | 69 |
| Sensitivity/Specificity | 96/97 | 87/90 | 74/73 |
| Youden Index | 0.94 | 0.77 | 0.47 |
| Classification Accuracy | 0.97f | 0.90f,g | 0.76 |
| MMSE total score | |||
| AUC(+/− 95%CI) | 0.99 (0.98–0.99) | 0.85 (0.80–0.89) | 0.86 (0.82–0.90) |
| Cut-off | 28 | 29 | 25 |
| Sensitivity/Specificity | 96/97 | 82/73 | 77/83 |
| Youden Index | 0.93 | 0.55 | 0.60 |
| Classification Accuracy | 0.93 | 0.70 | 0.81h |
| MoCA total score | |||
| AUC(+/− 95%CI) | 0.99 (0.97–0.99) | 0.89d (0.85–0.92) | 0.85 (0.81–0.89) |
| Cut-off | 23 | 25 | 19 |
| Sensitivity/Specificity | 94/96 | 84/79 | 77/80 |
| Youden Index | 0.91 | 0.63 | 0.57 |
| Classification Accuracy | 0.95 | 0.80f | 0.79i |
| B. | |||
| DSRS total score | |||
| AUC(+/− 95%CI) | 0.99c,e (0.99–1.00) | 0.96c,e (0.94–0.98) | 0.86 (0.82–0.90) |
| Cut-off | 3 | 2 | 10 |
| Sensitivity/Specificity | 99/98 | 89/93 | 74/78 |
| Youden Index | 0.97 | 0.82 | 0.52 |
| Classification Accuracy | 0.98f,g | 0.91f,g | 0.75 |
AUC = area under the curve; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; HC= healthy older controls; MCI = mild cognitive impairment; AD= Alzheimer’s disease
age-, education-, gender-adjusted score (Chandler et al., 2005)
Cut-off scores are greater than or equal to
AUC was better than MMSE AUC (p<.001)
AUC was marginally better than MMSE (p=.07)
AUC was better than MoCA AUC (p<.005)
Classification accuracy better than MMSE
Classification accuracy better than MoCA
Classification accuracy better than DSRS
Classification accuracy marginally better than DSRS (p=.07).
MoCA scores were equated to MMSE scores using the equipercentile equating method (40). Equipercentile equating has been used to equate numerous standardized tests including equating of the Telephone Interview for Cognitive Status (TICS) scores to MMSE scores in older adults (41). This statistical method allows for the determination of comparable test scores from two different measures on the basis of their corresponding percentile ranks. The advantage of equipercentile equating method is that the equated scores always fall within the range of possible scores, which is not always true when using traditional mean and linear equating methods. However, this method can lead to an irregular distribution of scores, thus a log-linear transformation (42) was used to smooth the raw scores of MoCA and MMSE into a regular distribution. Equipercentile equating and log-linear smoothing was performed using the ‘equate’ library in the R statistical package (43–46).
3. Results
Demographic characteristics along with significance values are presented in Table 1. The diagnostic groups differed in age, education, and sex distribution, but not race or ethnicity. The groups differed in GDS score [F(2, 536)=30.14, p<.01], with the AD group reporting the highest score on this measure followed by the MCI group and the NC group. GDS mean scores were, however, below five for all three groups and for the majority of individual participants, suggesting that no subject suffered from clinically significant depression (Table 1). The AD group performed significantly poorer than the MCI and HC group on each task; the MCI group performed significantly poorer on each cognitive task than the HC group (Table 1). Across all participants age was weakly, but significantly, correlated with each screening and cognitive measure: age and MMSE [r=−0.19, p<.001, n=587]; age and MoCA [r=−0.20, p<.001, n=587]; age and CERAD-NB [r=−0.25, p<.001, n=534]; age and DSRS [r=0.23, p<.001, n=579].
3.1 ROC analysis of standard screening measures: a comparison of CERAD-NB, MMSE and MoCA
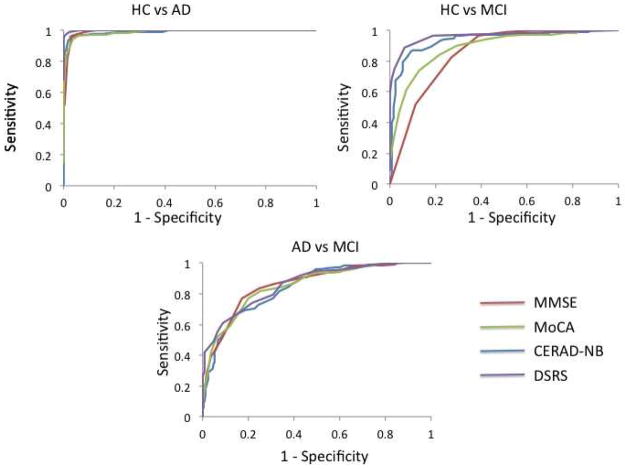
The ROC curve analysis was used to evaluate the diagnostic accuracy of each measure (CERAD-NB, MMSE and MoCA) to discriminate AD and MCI from each other and from healthy cognitive aging. Graphic representations of the ROC curves are provided in Figure 1. Table 2A shows clinically relevant cut-offs for the CERAD-NB, MMSE and MoCA. The diagnostic accuracies of the CERAD-NB, MMSE and MoCA were all excellent for HC vs. AD, with AUCs > 0.99. Diagnostic accuracies were lower, but still very good in the CERAD-NB (0.93), MMSE (0.85) and MoCA (0.89) for HC vs. MCI. The CERAD-NB resulted in a significantly higher AUC than either the MoCA [χ2(1)=6.18, p<.05] or MMSE [χ2(1)=11.40, p<.05], and the MoCA was nominally better at differentiating HC from MCI than the MMSE [χ2(1)=3.14, p=.07]. Diagnostic accuracies of all three cognitive measures were the lowest when differentiating AD from MCI, yet the AUCs were still good and equally high for the three measures: CERAD-NB (0.85), MMSE (0.86) and MoCA (0.85).
Figure 1.
ROC curve analysis of the MMSE, MoCA, CERAD-NB, and DSRS. HC=Healthy Controls; AD=Alzheimer’s disease; MCI= Mild Cognitive Impairment
3.2 Cut-off scores: CERAD, MMSE and MoCA
3.3 HC vs. AD
A cut-off score of 83 on the adjusted CERAD-NB, 28 on the MMSE, and 23 on the MoCA yielded the highest Youden Index for discriminating between HC and AD (Table 2A). The classification accuracy of a score of 83 on CERAD-NB was nominally better at classifying healthy and AD individuals than the optimal MoCA score (Z=1.71, p=.08) and significantly better than the optimal MMSE score (Z=2.53, p=.01). The classification accuracy of the MMSE and MoCA were comparable (Z=1.33, p=.18).
3.4 HC vs. MCI
A cut-off score of 89 on the CERAD-NB, 29 on the MMSE, and 25 on the MoCA yielded the highest Youden Index for the discriminating between HC and MCI (Table 2A). The classification accuracy of the optimal CERAD-NB score was better at classifying healthy and AD individuals than either the optimal MMSE (Z=5.74, p<.001) or MoCA (Z=2.94, p<.01) score. Classification accuracy of the MoCA was superior to the MMSE (Z=3.56, p<.001).
3.5 AD vs. MCI
A cut-off score of 69 on the CERAD-NB, 25 on the MMSE, and 19 on the MoCA yielded the highest Youden index for discriminating between AD and MCI (Table 2A). The classification accuracy of the cut-off score was equally good for the CERAD-NB, the MMSE, and the MoCA.
3.6 ROC analysis of informant based measure: the DSRS
Area under the ROC curve, the optimal cut-off score, sensitivity, specificity, and classification accuracy for the DSRS is presented in Table 2B. The DSRS provided comparably good discrimination between each group as the cognitive and screening measures. The diagnostic accuracy of the DSRS for HC and AD, was equal to the CERAD-NB, but better than the MMSE [χ2(1)=6.26, p<.05] or MoCA, [χ2 (1)=8.59, p<.01], albeit these increases in AUC were very small (<0.5%). The diagnostic accuracy of the DSRS for HC and MCI was superior to the MMSE [χ2 (1)=21.67, p<.0001] or MoCA [χ2 (1)=13.52, p<.001]. The DSRS was equally discriminant as the CERAD-NB, MMSE and MoCA when differentiating AD and MCI.
3.7 Cut-off scores: DSRS
A cut-off score of below 3 on the DSRS, yielded the highest Youden index for discriminating between HC and AD, a score of 2 was best for discriminating HC and MCI, and a score of 10 best differentiated AD from MCI. When discriminating between HC and AD or HC and MCI the DSRS yielded higher classification accuracy than the MMSE [HC vs AD: Z=3.89, p<.001; HC vs MCI: Z=5.90, p<.001] or MoCA [HC vs AD: Z=3.02, p<.01; HC vs MCI: Z=3.56, p<.001]. In contrast, the DSRS was poorer than the MMSE [z=2.25, p=.03], nominally poorer than the MoCA [Z=1.84, p=.07], but equally as accurate as the CERAD-NB when discriminating MCI and AD (Table 2B).
3.8 ROC analysis of combining an informant-based measure with cognitive screening tasks
Combining the DSRS with the MMSE or MoCA significantly improved diagnostic accuracy as compared to using either screening measure alone (Table 3). Diagnostic accuracy of the MMSE improved by 13% when differentiating HC from MCI [χ2 (1)=32.53, p<.0001], and 5% when differentiating AD from MCI [χ2 (1)=7.90, p<.0005]. Using the combination of optimal cut-off scores (a score below either cut-off score) of the MMSE and/or DSRS to classify individuals resulted in an increase of 27% for HC and MCI and 12% for MCI and AD. Diagnostic accuracy (AUC) of the MoCA was improved by 9% when differentiating HC from MCI [χ2 (1)=25.12, p<.001], and 5% when differentiating AD from MCI [χ2 (1)=9.84, p<.001]. Using the combination of optimal cut-off scores of the MoCA and/or DSRS to classify individuals resulted in an increase of 17% for HC and MCI and 13% for MCI and AD. Changes in diagnostic accuracy were not assessed for HC vs. AD, as diagnostic accuracy of each test alone was already excellent (>0.99).
Table 3.
Comparison of AUCs for MMSE or MoCA alone and when combined with DSRS for differentiating Healthy from MCI and MCI from AD.
| Parameter | HC and MCI | MCI and AD |
|---|---|---|
| MMSE total score | ||
| AUC | 0.84 (0.80–0.89) | 0.86 (0.83–0.90) |
| Classification Accuracy | 0.70 | 0.81 |
| MMSE + DSRS | ||
| AUC | 0.97a (0.95–0.99) | 0.91a (0.88–0.94) |
| Δ AUC | +0.12 | +0.05 |
| Classification Accuracy | 0.97c | 0.93c |
| Δ Classification Accuracy | +0.27 | +0.12 |
| MoCA | ||
| AUC | 0.88 (0.84–0.92) | 0.85 (0.82–0.90) |
| Classification Accuracy | 0.80 | 0.79 |
| MoCA + DSRS | ||
| AUC | 0.97b (0.95–0.99) | 0.90b (0.87–0.93) |
| Δ AUC | +0.09 | +0.05 |
| Classification Accuracy | 0.97c | 0.92c |
| Δ Classification Accuracy | +0.17 | +0.13 |
AUC = area under the curve; Δ AUC = change in AUC; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; HC= healthy older controls; MCI = mild cognitive impairment; AD= Alzheimer’s disease
Score was better than MMSE AUC (p<.0001);
Score was better than MoCA AUC (P<.001);
Classification accuracy higher than single measure
3.9 Equating MMSE and MoCA scores
The plot of equipercentile equivalent scores on the MMSE and MoCA is presented in Figure 2. As an example, Figure 2 indicates that a score of 18 on the MoCA is equivalent to a score of 24 on the MMSE and, both of these scores fall at approximately the 50th percentile within a clinical sample with a wide range of cognitive impairment. Table 4 shows scores on the MoCA and their respective equivalents on the MMSE. In general, lower MoCA scores are equal to higher MMSE scores. For example, scores of 28–30 on the MoCA are equivalent to the highest score of 30 on the MMSE.
Figure 2.
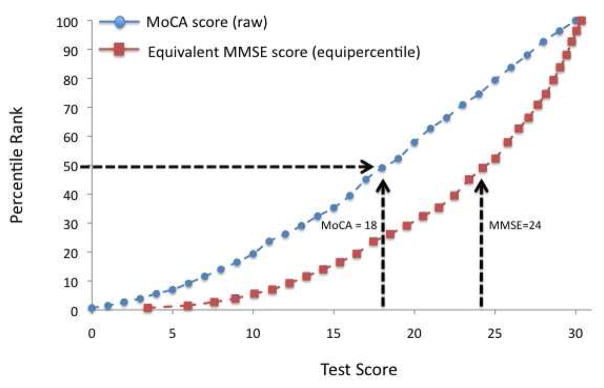
Corresponding raw scores and raw percentile ranks for the MMSE and MoCA. As an example, a score of 18 on the MoCA is equivalent to a score of 24 on the MMSE.
Table 4.
Conversion table for MMSE and MoCA screening measures based upon equipercentile equating in 321 AD, 126 MCI and 140 HC. Equivalent scores were derived from equipercentile equating with log-linear smoothing.
| Raw MoCA Score | Equivalent MMSE Score |
|---|---|
| 0 | 3 |
| 1 | 6 |
| 2 | 8 |
| 3 | 9 |
| 4 | 10 |
| 5 | 11 |
| 6 | 12 |
| 7 | 13 |
| 8 | 14 |
| 9 | 15 |
| 10 | 16 |
| 11 | 17 |
| 12 | 19 |
| 13 | 20 |
| 14 | 21 |
| 15 | 22 |
| 16 | 22 |
| 17 | 23 |
| 18 | 24 |
| 19 | 25 |
| 20 | 26 |
| 21 | 26 |
| 22 | 27 |
| 23 | 28 |
| 24 | 28 |
| 25 | 29 |
| 26 | 29 |
| 27 | 29 |
| 28 | 30 |
| 29 | 30 |
| 30 | 30 |
4. Discussion
Overall, our findings in a relatively large, community-dwelling cohort evaluated at a specialty memory clinic suggest that the individual screening measures (MMSE and MoCA) typically used in clinical practice to aid in the diagnosis of AD or MCI offer reasonably high classification and diagnostic accuracy when compared to a more detailed neuropsychological evaluation (CERAD-NB). However, as a brief, stand-alone cognitive screening measure, the MoCA appears to be more sensitive than the older, but more widely used MMSE. Furthermore, using the optimal cut-off score, the classification accuracy of the MoCA exceeded that of the MMSE when differentiating MCI from HC. This finding is consistent with a recent study that indicates the MoCA is more sensitive than the MMSE at detecting patients with mild cognitive impairment in Parkinson’s disease (PD) from those patients with a diagnosis of PD alone. Additionally, using an informant-based questionnaire of functional impairment (DSRS) resulted in comparable, or higher, overall diagnostic accuracy for most comparisons. However, accurately differentiating MCI from AD proved the most difficult regardless of the measure used. Finally, we provide a simple, yet reliable, method for equating MoCA scores to traditional MMSE scores.
Our results confirm and extend prior findings on the diagnostic utility of cognitive tests in AD and MCI. First, we find that an efficient but multi-dimensional neuropsychological inventory, the CERAD-NB, is more accurate at distinguishing MCI or AD from healthy individuals than brief screening measures, but not more accurate than the MMSE or MoCA at differentiating AD from MCI. The diagnostic accuracy of the CERAD-NB (93%) when differentiating MCI from the HC group in our study is very similar to a recent, multinational study of the diagnostic accuracy of the total CERAD score (13). Better diagnostic accuracy using the CERAD-NB is expected as it is more comprehensive, encompasses multiple cognitive domains and is highly correlated with the MMSE and MoCA (12). Thus it can serve as a reliable standard to which the MMSE and MoCA can be compared.
We extend upon previous findings (19, 20, 47, 48) that the MoCA is a superior screen than the MMSE for detecting AD and MCI. Both measures easily identified AD compared to HC and were comparable to the CERAD-NB. More striking was the accuracy of the MoCA at differentiating MCI from HC as compared to the MMSE. These data suggest that the MoCA may be more sensitive to early changes in cognitive ability as it includes more robust measures of visuospatial and executive function (16). Diagnostic accuracy declined when using these instruments to distinguish between AD and MCI. Although we found that the MMSE and MoCA were comparably accurate in differentiating AD from MCI, and the accuracy of both screens was similar to the CERAD-NB, this transition in clinical status remains a challenge for psychometric instruments. In particular, difficulty in differentiating AD from MCI is likely due to several factors including, but not limited to: 1) the heterogeneity of the MCI diagnosis; 2) progression rates of 10–15% from MCI to AD per year (5); 3) limited research that has focused on cross-sectional differentiation of MCI from AD and healthy older adults (see (8)),and 4) the relative dearth of MCI-specific screening measures. The importance of this last point cannot be understated as the real clinical value of any test will be in its prediction of further decline, and its relationship with disease-specific biomarkers that lead to significantly improved diagnostic accuracy.
We extend upon previous findings by assessing the diagnostic accuracy of the DSRS, an informant-based measure of functional impairment. Notably, the DSRS provided better classification accuracy than the MMSE or MOCA, and comparable accuracy to the CERAD-NB, for AD and MCI as compared to HC. Yet, the diagnostic and classification accuracy of the DSRS fell, like the other measures, when differentiating AD from MCI. The DSRS may provide additional insight into the functional levels of disease severity or subtypes of MCI especially in the absence of overt dementia. Given the capability of the DSRS to correctly discriminate dementia from healthy cognitive aging, we found that combining this measure with either the MMSE or MoCA increased diagnostic accuracy. This increase is of note as the DSRS is a reliable tool for indicating the severity of AD throughout the course of the disease (37). Furthermore, the DSRS, unlike many cognitive measures, changes at a steady predictable rate (35) making it an ideal measure to monitor changes in the disease course and may provide a useful metric to measure response to treatment. Previous studies have found that combining cognitive and informant-based screens (e.g. Informant Questionnaire on Cognitive Decline in the Elderly) have improved diagnostic accuracy (30, 31) and suggest that measures of instrumental activities of daily living be incorporated into the diagnostic criteria for MCI (49). To our knowledge, this is the first study to combine the DSRS with other screening measures to differentiate AD from MCI from HC. Given these results, further studies should consider including the DSRS during diagnostic assessment.
Finally, we used equipercentile equating to develop a conversion score between the MoCA and MMSE. Here, we provide a table of conversion scores that will enable the widely recognized cut-off scores on the MMSE to be reliably compared with scores on the MoCA. While the MMSE is the most commonly used clinical screening for monitoring acute or declining cognitive impairment, it has several limitations such as marked ceiling effects in younger, well-educated individuals and inconsistent performance in differentiating MCI from healthy older adults as many individuals with MCI score above the recommended MMSE cut-off (score of 26) for impairment (for review see (8)). Thus the MMSE has limited utility in detecting subtle changes in cognition that may signal pending impairment (50, 51). The MoCA does not overcome all of the shortcomings of the MMSE, but recent data, including ours, indicate that the MoCA is a superior screening measure for classifying MCI. Equating scores on these two measures provides a straightforward way of comparing the MoCA to the MMSE, thus allowing for continuity of cognitive tracking in the clinic and comparability of data in longitudinal studies of MCI or dementing illness.
Our use of a large, well-characterized community-dwelling cohort provides a useful perspective for determining the diagnostic accuracy of standardized global screening measures in AD and MCI. However, this study is not without limitations. The differences in gender distribution across the groups present challenges in interpretation of the current results. As expected, there were more women diagnosed with AD than men (52), however, the gender distribution within the MCI group was essentially a 50/50 split. It is possible the men are overrepresented in the group due to other factors. A comparison of gender-specific diagnostic accuracy was beyond the scope of this study, but should be examined in future investigations. The impact of co-morbid clinical factors, such as depression, were not considered in this study but could impact the utility of some screening measures, however the MoCA appears robust to depression symptoms within healthy cohorts (53) and in the current sample self-reported rates of depression were low. The sample was well-educated, particularly the healthy cognition group, which makes generalization to less well educated cohorts more challenging as education does impact scores MMSE (8) and MoCA (53) performance. Overall, the sample size of this study was adequate, but more precise cut-off values for each diagnostic test could be achieved with an increase in the number of MCI patients. An increase in number and subtypes of MCI, which was not considered in this study, would provide more specificity and may lead to improved diagnostic accuracy and utility. Since the MoCA shows a wider range of performances in AD and MCI than the MMSE, it seems likely that the MoCA can be used to determine differing levels of severity or subtypes of MCI. This is particularly important as the diagnosis of MCI or AD increases with age, thus performance on the MMSE or MoCA may systematically differ in an 80-year-old MCI patient as compared to a 60-year-old MCI patient. Thus, future studies should consider age- and domain-specific performance cut-offs. Finally, the aim of the current study was to compare individuals with a known diagnosis of AD or MCI to a healthy comparison group in specialty memory and aging clinic. As a result, generalizing to other clinical settings should be done with caution. Given that the clinicians were not blinded to the MMSE, CERAD-NB and DSRS during consensus diagnosis it is possible that diagnostic decisions were influenced by the availability of these measures. However, there was no systematic rubric employed at consensus and the data do not suggest that one or more of these measures were used preferentially to achieve diagnosis. Given the paucity of screening tools that are specific to differentiating MCI from AD and normal cognitive aging, we believe that the results of this study provide practical results about well-utilized standardized metrics and their diagnostic effectiveness.
In conclusion, the current results support recent data that the MoCA is superior to the MMSE as a global assessment tool and we provide a reliable way to compare the two screening measures. Furthermore, we find that when these traditional screening measures are used in the diagnosis of AD and MCI with complementary informant-based ratings of functional impairment, overall diagnostic accuracy improves.
Acknowledgments
The authors express appreciation to the research participants and staff of the Penn Memory Center/Clinical Core of the University of Pennsylvania Alzheimer’s Disease Center.
Funding Sources: This work was supported by NIA AG10124 and the Marian S. Ware Alzheimer’s Program/National Philanthropic Trust, and NIH T32 MH019112.
Footnotes
Conflicts: The authors report no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S4–9. [PubMed] [Google Scholar]
- 2.Comas-Herrera A, Northey S, Wittenberg R, Knapp M, Bhattacharyya S, Burns A. Future costs of dementia-related long-term care: exploring future scenarios. Int Psychogeriatr. 2011;23(1):20–30. doi: 10.1017/S1041610210000025. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimo A, Winblad B, Aguero-Torres H, von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord. 2003;17(2):63–7. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 8.Lonie JA, Tierney KM, Ebmeier KP. Screening for mild cognitive impairment: a systematic review. Int J Geriatr Psychiatry. 2009;24(9):902–15. doi: 10.1002/gps.2208. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 10.Welsh-Bohmer KA, Mohs RC. Neuropsychological assessment of Alzheimer’s disease. Neurology. 1997;49(3 Suppl 3):S11–3. doi: 10.1212/wnl.49.3_suppl_3.s11. [DOI] [PubMed] [Google Scholar]
- 11.Collie A, Shafiq-Antonacci R, Maruff P, Tyler P, Currie J. Norms and the effects of demographic variables on a neuropsychological battery for use in healthy ageing Australian populations. Aust N Z J Psychiatry. 1999;33(4):568–75. doi: 10.1080/j.1440-1614.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 12.Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, et al. A total score for the CERAD neuropsychological battery. Neurology. 2005;65(1):102–6. doi: 10.1212/01.wnl.0000167607.63000.38. [DOI] [PubMed] [Google Scholar]
- 13.Paajanen T, Hanninen T, Tunnard C, Mecocci P, Sobow T, Tsolaki M, et al. CERAD neuropsychological battery total score in multinational mild cognitive impairment and control populations: the AddNeuroMed study. J Alzheimers Dis. 2010;22(4):1089–97. doi: 10.3233/JAD-2010-100459. [DOI] [PubMed] [Google Scholar]
- 14.Seo EH, Lee DY, Lee JH, Choo IH, Kim JW, Kim SG, et al. Total scores of the CERAD neuropsychological assessment battery: validation for mild cognitive impairment and dementia patients with diverse etiologies. Am J Geriatr Psychiatry. 2010;18(9):801–9. doi: 10.1097/JGP.0b013e3181cab764. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52(5):329–32. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 18.Damian AM, Jacobson SA, Hentz JG, Belden CM, Shill HA, Sabbagh MN, et al. The Montreal Cognitive Assessment and the mini-mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement Geriatr Cogn Disord. 2011;31(2):126–31. doi: 10.1159/000323867. [DOI] [PubMed] [Google Scholar]
- 19.Freitas S, Simoes MR, Alves L, Santana I. Montreal Cognitive Assessment: Validation Study for Mild Cognitive Impairment and Alzheimer Disease. Alzheimer Dis Assoc Disord. 2011 doi: 10.1097/WAD.0b013e3182420bfe. [DOI] [PubMed] [Google Scholar]
- 20.Larner AJ. Screening utility of the Montreal Cognitive Assessment (MoCA): in place of - or as well as - the MMSE? Int Psychogeriatr. 2012;24(3):391–6. doi: 10.1017/S1041610211001839. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Dong Woo L, Cho SJ, Na DL, Hong Jin J, Kim SK, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008;21(2):104–10. doi: 10.1177/0891988708316855. [DOI] [PubMed] [Google Scholar]
- 22.Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–25. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 23.Dong Y, Lee WY, Basri NA, Collinson SL, Merchant RA, Venketasubramanian N, et al. The Montreal Cognitive Assessment is superior to the Mini-Mental State Examination in detecting patients at higher risk of dementia. Int Psychogeriatr. 2012:1–7. doi: 10.1017/S1041610212001068. [DOI] [PubMed] [Google Scholar]
- 24.Ihara M, Okamoto Y, Takahashi R. Suitability of the Montreal Cognitive Assessment versus the Mini-Mental State Examination in Detecting Vascular Cognitive Impairment. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie G, Gould L, Ireland S, LeBlanc K, Sahlas D. Detecting cognitive impairment in clients with mild stroke or transient ischemic attack attending a stroke prevention clinic. Can J Neurosci Nurs. 2011;33(1):47–50. [PubMed] [Google Scholar]
- 26.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision (DSM-IV-TR) ed. [Google Scholar]
- 27.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16(3):275–93. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 30.Flicker L, Logiudice D, Carlin JB, Ames D. The predictive value of dementia screening instruments in clinical populations. Int J Geriatr Psychiatry. 1997;12(2):203–9. doi: 10.1002/(sici)1099-1166(199702)12:2<203::aid-gps603>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Hancock P, Larner AJ. Diagnostic utility of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) and its combination with the Addenbrooke’s Cognitive Examination-Revised (ACE-R) in a memory clinic-based population. Int Psychogeriatr. 2009;21(3):526–30. doi: 10.1017/S1041610209008941. [DOI] [PubMed] [Google Scholar]
- 32.Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual’s complaints of memory impairment in early dementia. Neurology. 2000;55(11):1724–6. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 33.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–9. [PubMed] [Google Scholar]
- 34.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 2):S33–9. [PubMed] [Google Scholar]
- 35.Xie SX, Ewbank DC, Chittams J, Karlawish JH, Arnold SE, Clark CM. Rate of decline in Alzheimer disease measured by a Dementia Severity Rating Scale. Alzheimer Dis Assoc Disord. 2009;23(3):268–74. doi: 10.1097/WAD.0b013e318194a324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24(4):641–52. [PubMed] [Google Scholar]
- 37.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10(1):31–9. [PubMed] [Google Scholar]
- 38.Sheikh J, Yesavage J. Clinical Gerontology: A Guide to Assessment and Intervention. NY: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–73. [Google Scholar]
- 39.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Kolen MJ, Brennan RL. Test equating methods and practice. New York: Springer; 2005. [Google Scholar]
- 41.Fong TG, Fearing MA, Jones RN, Shi P, Marcantonio ER, Rudolph JL, et al. Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement. 2009;5(6):492–7. doi: 10.1016/j.jalz.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moses T, vonDavier AA. An SAS macro for loglinear smoothing: applications and implications. Montreal: American Educational Research Association; 2005. [Google Scholar]
- 43.Braun HI, Holland PW. Observed-score test equating: A mathematical analysis of some ETS equating procedures. In: Holland PW, Rubin DB, editors. Test Equating. New York: Academic; 1982. pp. 9–49. [Google Scholar]
- 44.Livingston SA, Kim S. The circle-arc method for equating in small samples. Journal of Educational Measurement. 2009;46:330–43. [Google Scholar]
- 45.Kolen MJ, Brennan RL. Test Equating, Scaling, and Linking. 2. New York: Springer; 2004. [Google Scholar]
- 46.Lord F. The standard error of equipercentile equating. Journal of Educational Statistics. 1982;7:165–74. [Google Scholar]
- 47.Hancock P, Larner AJ. Test Your Memory test: diagnostic utility in a memory clinic population. Int J Geriatr Psychiatry. 2011;26(9):976–80. doi: 10.1002/gps.2639. [DOI] [PubMed] [Google Scholar]
- 48.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24(2):197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- 49.Brown PJ, Devanand DP, Liu X, Caccappolo E. Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617–26. doi: 10.1001/archgenpsychiatry.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravaglia G, Forti P, Maioli F, Servadei L, Martelli M, Brunetti N, et al. Screening for mild cognitive impairment in elderly ambulatory patients with cognitive complaints. Aging Clin Exp Res. 2005;17(5):374–9. doi: 10.1007/BF03324625. [DOI] [PubMed] [Google Scholar]
- 51.Slavin MJ, Sandstrom CK, Tran TT, Doraiswamy PM, Petrella JR. Hippocampal volume and the Mini-Mental State Examination in the diagnosis of amnestic mild cognitive impairment. AJR Am J Roentgenol. 2007;188(5):1404–10. doi: 10.2214/AJR.06.1052. [DOI] [PubMed] [Google Scholar]
- 52.Vina J, Lloret A. Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis. 2010;20 (Suppl 2):S527–33. doi: 10.3233/JAD-2010-100501. [DOI] [PubMed] [Google Scholar]
- 53.Freitas S, Simoes MR, Alves L, Santana I. Montreal Cognitive Assessment: influence of sociodemographic and health variables. Arch Clin Neuropsychol. 2012;27(2):165–75. doi: 10.1093/arclin/acr116. [DOI] [PubMed] [Google Scholar]