Abstract
Many current pharmacological treatments for neuropsychiatric disorders, such as anxiety and depression, are limited by a delayed onset of therapeutic effect, adverse side effects, abuse potential or lack of effect in many patients. These off-target effects highlight the need to identify novel mechanisms and targets for treatment. Recently, modulation of glyoxalase 1 (GLO1) activity was shown to regulate anxiety-like behavior and seizure susceptibility in mice. These effects are likely mediated through the regulation of methylglyoxal (MG) by GLO1, as MG acts as a competitive partial agonist at GABAA receptors (GABAARs). Thus, modulation of MG by GLO1 represents a novel target for treatment. Here, we evaluate the therapeutic potential of indirectly modulating MG concentrations through GLO1 inhibitors for the treatment of neuropsychiatric disorders.
Keywords: GLO1 Inhibitor, Glyoxalase 1, Methylglyoxal, neuropsychiatric disorders, epilepsy, treatment
Introduction
Anxiety and depressive disorders affect one in four adults at some point in their lifetime, while epilepsy affects one in fifty[1,2]. Although a variety of pharmaceuticals are available to treat these neuropsychiatric disorders, illness remains refractory in a significant portion of patients and many currently used drugs have adverse side effects and high abuse potential. Thus, identification of new biological targets and novel pharmaceuticals remains an important goal in treating these disorders [3,4].
Recent studies have identified glyoxalase 1 (GLO1) as a new target for neurological and psychiatric conditions. Increased Glo1 gene-expression is associated with anxiety- and depression-like behavior as well as seizure susceptibility in mice[5–9]. GLO1 is a ubiquitous cytosolic enzyme that catalyzes the reaction between glutathione and acyclic α-oxoaldehydes, particularly methylglyoxal (MG)[10–13]. MG is formed as a byproduct during photosynthesis, protein and fatty acid catabolism and glycolysis; principally by the non-enzymatic degradation of acetone, aminoacetone and the glycolytic intermediates dihydroxyacetone phosphate and glyceraldehyde-3-phosphate[14]. In vitro studies have demonstrated a critical role for GLO1 in clearing MG; indeed, overexpression of Glo1 prevents MG accumulation, while GLO1 inhibition results in MG accumulation [10–13].
Historically, most research on GLO1 has focused on the importance of detoxification of MG to prevent cellular damage due to the glycation of proteins and nucleic acids[15,16]. These studies have implicated high concentrations of MG and/or low GLO1 activity in the etiology of metabolic disorders, such as diabetes and in the development of cellular pathologies including aging[13,17]. Thus strategies to reduce MG concentrations and/or enhance GLO1 activity have therapeutic potential. In contrast, many cancers exhibit enhanced GLO1 activity; it has been suggested that inhibition of GLO1 would therefore have anticancer properties [15,18–20].
In addition, recent studies from several labs indicated that modulation of MG concentrations and GLO1 activity can alter anxiety, depression, seizure, sleep, and pain phenotypes in mice [6,7,21–23]. Therefore, increasing MG concentrations by inhibiting GLO1 may also represent a novel strategy for the treatment of neuropsychiatric and epileptic disorders. In this review, we will focus on evaluating the therapeutic potential of utilizing GLO1 inhibitors to indirectly modulate neurophysiology by reducing the rate of MG clearance in the CNS.
Glo1 and methylglyoxal in neuropsychiatric disorders and epilepsy
In mice, a positive correlation between Glo1 expression and anxiety-like behavior was first reported among a panel of inbred mouse strains, and has since been corroborated by numerous studies[24–28]. Subsequent studies confirmed a causal role for Glo1 in anxiety-like behavior using viral vectors and transgenic mice to show that Glo1 overexpression increased anxiety-like behavior, while knockdown decreased anxiety-like behavior[24]. However, human genetic studies have yielded discrepant results regarding the association between Glo1 and anxiety[29,30]. Interpretation of these data in humans is limited by small sample sizes and potential population stratification. Larger, well-controlled human genetic studies are required to elucidate the role of Glo1 in human anxiety disorders.
In addition to anxiety, there is strong evidence that Glo1 regulates other neuropsychiatric phenotypes in mice, including epilepsy, depression and neuropathic pain. For example, increased seizure susceptibility was associated with high Glo1 expression among recombinant inbred mice and transgenic mice overexpressing Glo1[6]. Also, there is a clear, positive correlation between GLO1 protein levels and depression-phenotype as assessed by the tail-suspension test (TST)[9]. While some studies have also suggested a role for Glo1 in human neuropsychiatric diseases the evidence is usually less compelling and is limited by small sample size and a lack of replication. For example, one study reported a negative correlation between Glo1 expression and depression; additional studies have reported negative correlation between Glo1 expression and neuropathic pain, as well as associations between Glo1 expression and autism, schizophrenia, and restless legs syndrome[21,31–44]. At this time, rigorous analysis to determine the impact of Glo1 expression levels, copy number variants or polymorphisms on the etiology or pathogenesis of human neuropsychiatric disorders is lacking.
Mechanism of action - GABA receptors and MG
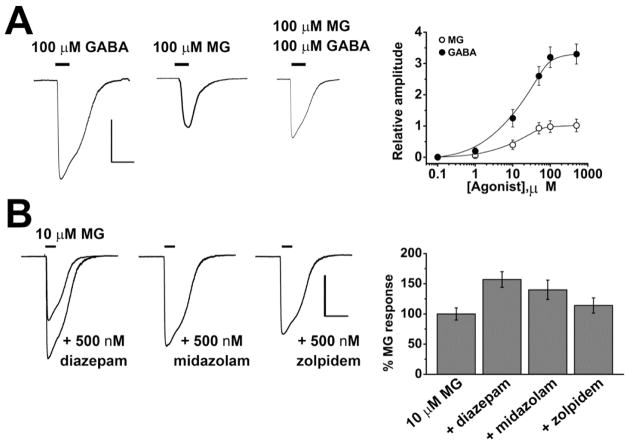
We recently reported that physiological levels of MG (low μM) are anxiolytic in mice by a simple mechanism: MG is a specific, partial, reversible agonist of GABAARs in central neurons[7]. GABAARs are pentameric, ligand-gated ion channels, and are comprised of two α-subunits (α1-6), two β-subunits (β1-4) and one γ1-4, δ, ε, θ, π or ρ1-3 subunit. The namesake ligand for GABAARs is γ-aminobutyric acid (GABA). In the adult brain GABA serves as an inhibitory neurotransmitter. Binding of GABA to specific pockets at the interface of α and β-subunits opens a channel in the center of GABAARs, this hyperpolarizes the membrane potential by passing Cl− ions. GABAARs are present both at synapses and on the soma of neurons, and produce phasic and tonic currents, respectively[45–47]. Application of MG to cerebellar granule (CGN) or hippocampal neurons (HN) evokes Cl− currents that modulate the membrane potential and are blocked by the GABAA specific antagonist SR-95531[7]. MG evoked currents are ~ ⅓of the magnitude of those evoked by GABA in the same cells and co-application with GABA is competitive, not additive, suggesting that both ligands act at the same binding site[7]. Importantly, the concentration of MG required to evoke currents in neurons is in the physiological range and the EC50 measured from the concentration-response relationship is ~10 μM, suggesting that small changes in concentration of MG will produce marked effects in the current magnitude. Based on these observations, MG can be described as an endogenously produced competitive partial agonist at GABAARs at physiologically relevant concentrations (Figure 1A).
Figure 1. MG is an endogenous, partial agonist at neuronal GABAA receptors.
(A) The application of 100 μM MG to hippocampal neurons evokes Cl− currents through GABAA receptors that are ~ ⅓ the magnitude of those evoked by 100 μM GABA in the same cells. The EC50 of the currents evoked by MG was 9.5 ± 1 μM and the physiological concentration of MG in rodent brain was measured at 5 μM. MG has a similar efficacy when applied to cerebellar granule neurons. (B) MG evoked currents in hippocampal neurons are augmented by co-application of classical anxiolytics that act as positive allosteric modulators of GABAARs, such as the benzodiazapenes diazepam and midazolam and the imidazopyridine zolpidem. Scale bars represent 1 nA and 10 s. Adapted from Distler et al., (2012)[7].
Alterations in GABAergic signaling are implicated in numerous neurological and psychiatric disorders, including depression, panic, schizophrenia, Huntington’s, Parkinson’s, Alzheimer’s, epilepsy, sleep, and chronic pain syndromes[45]. Many commonly prescribed anxiolytic agents, such as the benzodiazepine, midazolam, target extrasynaptic GABAARs with the aim of augmenting tonic inhibition[47,48]. Extracellular GABAARs frequently contain α5/α6 and δ subunits; assemblies that are prominent in hippocampal and neocortical pyramidal neurons (α5βγ2) and CGN (α6βδ)[49]. The action of MG at extrasynaptic GABAARs may be of particular relevance to pathophysiology because the concentration of GABA at extrasynaptic receptors is low (<μM), while MG has been measured at ~5 μM in mouse brain[7,46].
Benzodiazepines are positive allosteric modulators of GABAARs, augmenting inhibitory currents when GABA binds[47]. Two such benzodiazepines (midazolam and diazepam) also augment GABAergic Cl− currents when MG binds to GABAAR in HNs. Similarly, the effects of MG are augmented by zolpidem, a non-benzodiazepine, imidazopyridine-based positive allosteric modulator of GABAARs[7] (Figure 1B). It is not yet known whether the activity or efficacy of benzodiazepines at specific GABAAR subtypes differs between MG- and GABA-induced activation. However, the studies described above suggest that MG can activate GABAARs that contain diazepam- and midazolam-sensitive α1-3 and α5 subunits as well as receptors with zolpidem- sensitive α1 and γ2 subunits. This array of subunits is common in areas of the brain associated with anxiety and depressive disorders, including hippocampal and cortical interneurons (α1β2γ2 receptors) and the limbic system (α2βXγ1 receptors).
GABA analogues have also been considered as potential therapeutics, particularly for acute conditions, such as seizure or mania. However, this strategy has been hampered by significant challenges; principally, that GABA is highly polar and flexible and activates GABAB and GABAC receptors in addition to GABAARs. In contrast to GABA, MG does not activate neuronal GABABRs; the effects of MG at GABACRs have yet to be characterized. MG can easily cross the blood-brain barrier[7]; thus, MG precursors or MG bioisosteres might be clinically useful compounds.
In summary, activation of GABAARs by MG is a promising approach for treatment of neuropsychiatric disorders and other diseases linked to GABA signaling. Possible approaches include GLO1 inhibition or administration of MG precursors or bioisosteres.
Therapeutic potential of GLO1 inhibitors
Current drug-therapies for depression are limited by negative side effects, including sexual dysfunction, weight gain and insomnia, and require several weeks to produce their full therapeutic effect. Similarly, anxiolytic and anti-epileptic drugs are limited by their sedating effects and abuse potential. Identification of novel molecular targets may provide alternatives with fewer or different side effects. Additionally, the identification of targets with applications in multiple disorders is particularly beneficial as drug development is time consuming and expensive. Given its role in multiple neuropsychiatric disorders, agents that modulate MG levels might be of benefit as next generation treatments.
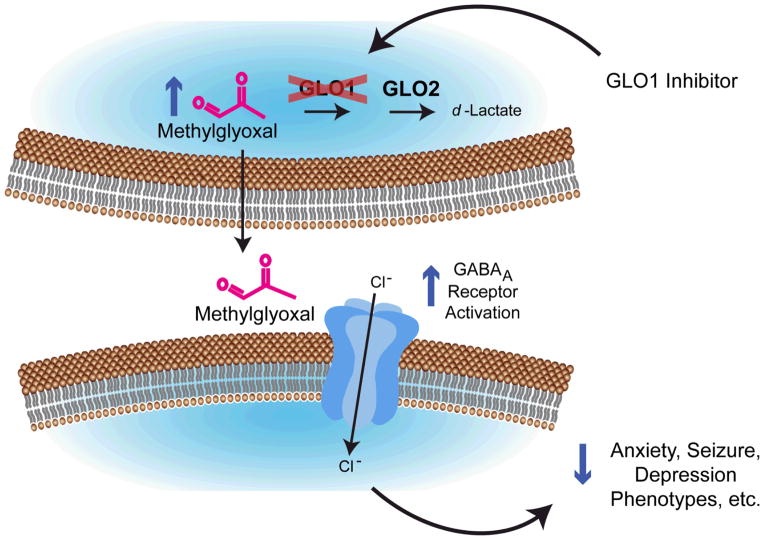
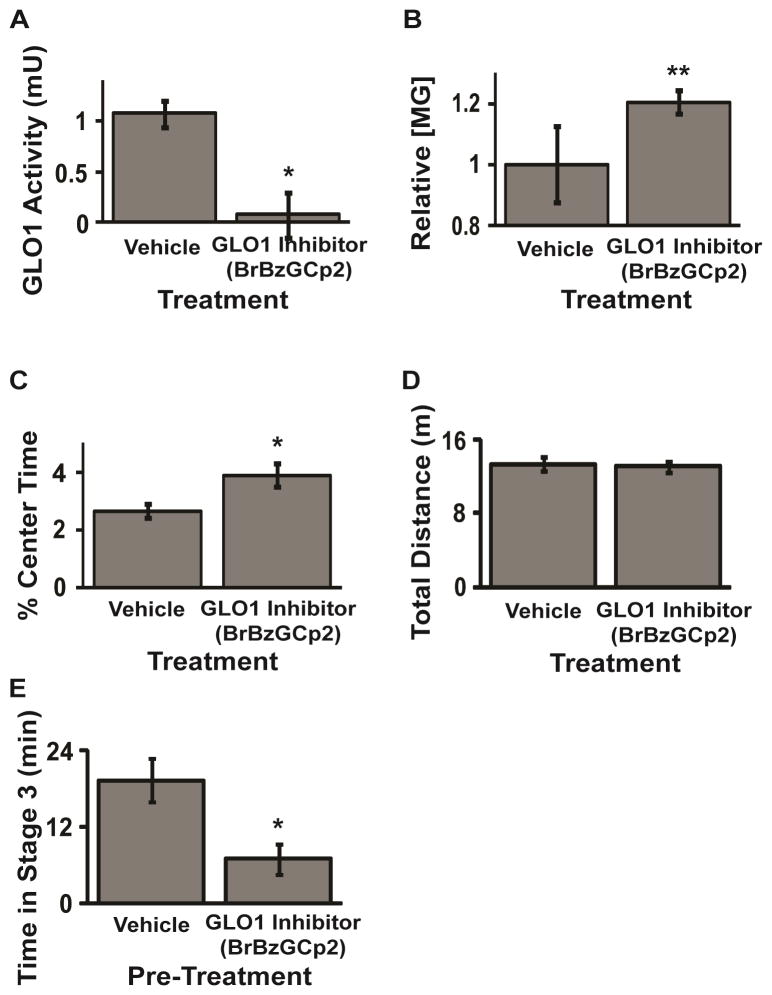
However, MG is highly bioreactive, modifying arginine and lysine residues in proteins and has been shown to be directly toxic to cells in vitro, inducing apoptosis when applied at concentrations > 100 μM. Thus, instead of direct administration of MG, an alternative and perhaps more promising strategy is to raise MG levels by inhibiting the GLO pathway. Application of a GLO1 inhibitor is expected to potentiate the activity of GABAARs by reducing the degradation of MG to augment basal levels in the brain (Figure 2). This mechanism of action is fundamentally different to the action of commonly prescribed GABAergic drugs because it depends on the local accumulation of a competitive partial agonist rather that positive allosteric modulation of GABAARs. Therefore, GLO1 inhibition is likely to cause anatomically and pharmacologically distinct responses to those observed following treatment with benzodiazepines and barbiturates. Early studies already support a role for GLO1 inhibition in modulating behavioral phenotypes. For instance, GLO1 inhibition by S-bromobenzylglutathione cyclopentyl diester (BrBzGCp2) increased MG concentration and reduced anxiety-like behavior in mice [7] (Figure 3A–D). Similarly, BrBzGCp2 attenuated epileptic seizures in mice [6](Figure 3E). To date we have not observed undesirable side effects following treatment with anxiolytic and anti-epileptic doses of BrBzGCp2 (unpublished data), however more work is needed to address this obvious concern.
Figure 2. A model for GLO1 Inhibition in the treatment of neuropsychiatric disorders and epilepsy.
Treatment with GLO1 inhibitors will increase concentrations of methylglyoxal due to decreased clearance by GLO1. Increased methylglyoxal will result in increased activation of GABAA receptors and subsequently, a decrease in neuropsychiatric disorder phenotypes (ie. reduced anxiety, depression and seizure). Adapted from Distler and Palmer (2013)[67].
Figure 3. Systemic administration of a GLO1 inhibitor regulates MG concentration, reduces anxiety-like behavior and attenuates seizure in mice.
Pharmacological inhibition of GLO1 by BrBzGCp2 (A) reduces GLO1 enzymatic activity; (B) increases concentrations of MG in whole brain of mice 2 hrs after i.p. treatment; (C) reduces anxiety-like behavior in the open field test (C57BL6/J mice) without affecting total distance traveled (D); and (E) attenuates pilocarpine-induced seizures (50 mg/kg prior to pilocarpine (250mg/kg)). Adapted from Distler et al. (2012) and (2013)[6,7].
Using the TST, Benton and colleagues reported a positive correlation between Glo1 expression and depression-like behavior in mice[27,50]. This observation appears surprising in light of the link between Glo1, MG and GABA, since no other GABAAR agonists (e.g. barbiturates and benzodiazepines) reduce immobility on the TST[50]. While GLO1 inhibitors have not been evaluated for their efficacy in depression-like behaviors, these data suggest that GLO1 inhibition may have antidepressant activity, likely by increasing MG levels. While anxiolytic drugs that modulate GABAergic signaling, such as benzodiazepines, are not effective for treatment of depression, recent evidence shows that co-administration of the serotonin-selective reuptake inhibitor fluoxetine with eszopiclone (a partial agonists at GABAARs that contain α1, α2 or α3 subunits) has a greater antidepressant effect than fluoxetine alone, suggesting a role for GABAergic drugs in the treatment of depression[51,52]. In conjunction with the correlation between Glo1 and depression-like behavior on the TST, these data reflect a role for GABAARs in the treatment of depression and highlight the potential utility of GLO1 inhibition versus classical anxiolytics for regulating GABAergic signaling.
Current GLO1 inhibitors, such as BrBzGCp2, are frequently based on the glutathione scaffold and have been patented for a variety of disorders[53–57]. Flavonoids, curcumin and other non-peptidic reagents have also been evaluated for their GLO1 inhibitory activity[58–62]. Although these compounds generally inhibit GLO1 activity with therapeutically useful Ki, utilizing native structures such as glutathione as a scaffold a priori increases the risk of interaction with other signaling pathways and could result in undesired off-target effects or limited bioavailability [58,62,63]. Poor cell permeability has also hampered the utility of some glutathione analogs and flavonoids in vitro, while poor absorption and bioavailability have limited the success of curcumin in human trials [60,62,63]. Further, many existing inhibitors of GLO1 were intended as anti-tumor agents and as such, have frequently been evaluated in vitro for their ability to inhibit cellular proliferation and induce apoptosis in tumor cells at high concentrations [58–61]. Thus, a key question is whether doses of GLO1 inhibitors can be identified produce therapeutic effects without also producing undesired effects such as increases in neuropathic pain. Identification or synthesis of novel GLO1 inhibitors could address the limitations of current inhibitors by reducing off target effects and minimizing side effects. Ultimately, the therapeutic viability of GLO1 inhibitors requires the identification of an inhibitor with excellent oral availability, a favorable pharmacokinetics and dynamics and negligible toxicity after chronic treatment.
Although mounting evidence shows that GLO1 inhibitors may have applications in the treatment of anxiety, depression and epilepsy, a negative correlation was observed between Glo1 copy number and sensitivity to neuropathic pain in diabetic mice[64,65]. Subsequent mechanistic studies demonstrated that overexpression of human GLO1 reduced hyperalgesia in diabetic mice[21]. Although a correlation between a SNP in Glo1 and diabetic neuropathy among type 2 diabetics has been reported in humans, the effect was not statistically significant when corrected for multiple comparisons [34]. Recent work has demonstrated decreased GLO1 activity in patients with painful diabetic neuropathy as compared to those with painless diabetic neuropathy, suggesting a role for GLO1 in pain [33]. The mechanism of MG-induced hyperalgesia has been attributed to protein modification and activation of TRPA1 receptors [21,66]. Such studies underscore the need to assess the potential cytotoxic consequences of GLO1 inhibition and suggest that GLO1 inhibitors may be contraindicated in diabetic patients[21,66].
Conclusions
While effective in many cases, current drug therapies for neuropsychiatric disorders and epilepsy are plagued by confounding off-target effects and often carry a risk for addiction in patients, generating the need for novel pharmaceuticals to treat these debilitating disorders. Therapeutic treatment by GLO1 inhibition/MG accumulation would provide a pharmacological avenue for anxiety and other mental health issues that is fundamentally distinct from the current pharmacopeia, such as positive allosteric modulators of GABAARs. The neuroanatomical distribution of MG production will influence the effects of GLO1 inhibition as local MG production would dictate regions of accumulation. MG is cell-permeable, and as such, it is possible that MG preferentially acts at extrasynaptic GABAARs where concentrations of GABA are low. Insufficient inhibition of neuronal excitability in amygdala-prefrontal cortex circuitry could explain GLO1/MG control over anxiety and depression-like behavior and would suggest a role for GLO1/MG in the regulation of behaviors associated with anxiety and depression.
Thus, GLO1 inhibition has the potential to improve efficacy, reduce side effects and ultimately treat multiple highly comorbid disorders. While evidence in mice suggests that GLO1 inhibition alters behavior, concerns about neuropathic pain and cytotoxicity mandate further exploration and characterization of lead compounds to properly evaluate the therapeutic efficacy of GLO1 inhibitors for the treatment of neuropsychiatric disorders and epilepsy.
Abbreviations
- GLO1
Glyoxalase 1
- MG
Methylglyoxal
- GABA
γ-aminobutyric acid
- TST
tail suspension test
- CGN
cerebellar granule
- HN
hippocampal neurons
- BrBzGCp2
S-bromobenzylglutathione cyclopentyl diester
References
- 1.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, Lane MC, Shahly V, Stang PE. Accounting for comorbidity in assessing the burden of epilepsy among US adults: results from the National Comorbidity Survey Replication (NCS-R) Mol Psychiatry. 2012;17:748–58. doi: 10.1038/mp.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–58. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov, Nature Publishing Group. 2011;10:685–97. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams R, Lim JE, Harr B, Wing C, Walters R, Distler MG, Teschke M, Wu C, Wiltshire T, Su AI, et al. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One. 2009;4:e4649. doi: 10.1371/journal.pone.0004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Distler MG, Gorfinkle N, Papale La, Wuenschell GE, Termini J, Escayg A, Winawer MR, Palmer Aa. Glyoxalase 1 and its substrate methylglyoxal are novel regulators of seizure susceptibility. Epilepsia. 2013;54:649–57. doi: 10.1111/epi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA, Palmer AA. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest. 2012;122:2306–15. doi: 10.1172/JCI61319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovatta I, Tennant RS, Helton R, Marr Ra, Singer O, Redwine JM, Ellison Ja, Schadt EE, Verma IM, Lockhart DJ, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–6. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 9.Benton CS, Miller BH, Skwerer S, Suzuki O, Schultz LE, Cameron MD, Marron JS, Pletcher MT, Wiltshire T. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology (Berl) 2012;221:297–315. doi: 10.1007/s00213-011-2574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornalley PJ. The glyoxalase system in health and disease. Mol Aspects Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- 12.Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification--a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–73. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 13.Thornalley PJ. Protecting the genome: defence against nucleotide glycation and emerging role of glyoxalase I overexpression in multidrug resistance in cancer chemotherapy. Biochem Soc Trans. 2003;31:1372–7. doi: 10.1042/bst0311372. [DOI] [PubMed] [Google Scholar]
- 14.Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification--a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–73. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 16.Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–8. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 17.Thornalley PJ, Rabbani N. Glyoxalase in tumourigenesis and multidrug resistance. Semin Cell Dev Biol, Elsevier Ltd. 2011;22:318–25. doi: 10.1016/j.semcdb.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–45. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 19.Fleming TH, Humpert PM, Nawroth PP, Bierhaus A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology. 2011;57:435–43. doi: 10.1159/000322087. [DOI] [PubMed] [Google Scholar]
- 20.Morcos M, Du X, Pfisterer F, Hutter H, Sayed AaR, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–9. doi: 10.1111/j.1474-9726.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 21.Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, Sauer SK, Eberhardt M, Schnölzer M, Lasitschka F, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med. 2012;18:926–33. doi: 10.1038/nm.2750. [DOI] [PubMed] [Google Scholar]
- 22.Hambsch B, Chen BG, Brenndörfer J, Meyer M, Avrabos C, Maccarrone G, Liu RH, Eder M, Turck CW, Landgraf R. Methylglyoxal-mediated anxiolysis involves increased protein modification and elevated expression of glyoxalase 1 in the brain. J Neurochem. 2010;113:1240–51. doi: 10.1111/j.1471-4159.2010.06693.x. [DOI] [PubMed] [Google Scholar]
- 23.Jakubcakova V, Curzi ML, Flachskamm C, Hambsch B, Landgraf R, Kimura M. The glycolytic metabolite methylglyoxal induces changes in vigilance by generating low-amplitude non-REM sleep. J Psychopharmacol. 2013;27:1070–5. doi: 10.1177/0269881113495596. [DOI] [PubMed] [Google Scholar]
- 24.Hovatta I, Tennant RS, Helton R, Marr Ra, Singer O, Redwine JM, Ellison Ja, Schadt EE, Verma IM, Lockhart DJ, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438:662–6. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 25.Loos M, van der Sluis S, Bochdanovits Z, van Zutphen IJ, Pattij T, Stiedl O, Smit aB, Spijker S. Activity and impulsive action are controlled by different genetic and environmental factors. Genes Brain Behav. 2009;8:817–28. doi: 10.1111/j.1601-183X.2009.00528.x. [DOI] [PubMed] [Google Scholar]
- 26.Reiner-Benaim A, Yekutieli D, Letwin NE, Elmer GI, Lee NH, Kafkafi N, Benjamini Y. Associating quantitative behavioral traits with gene expression in the brain: searching for diamonds in the hay. Bioinformatics. 2007;23:2239–46. doi: 10.1093/bioinformatics/btm300. [DOI] [PubMed] [Google Scholar]
- 27.Benton CS, Miller BH, Skwerer S, Suzuki O, Schultz LE, Cameron MD, Marron JS, Pletcher MT, Wiltshire T. Evaluating genetic markers and neurobiochemical analytes for fluoxetine response using a panel of mouse inbred strains. Psychopharmacology (Berl) 2012;221:297–315. doi: 10.1007/s00213-011-2574-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams R, Lim JE, Harr B, Wing C, Walters R, Distler MG, Teschke M, Wu C, Wiltshire T, Su AI, et al. A common and unstable copy number variant is associated with differences in Glo1 expression and anxiety-like behavior. PLoS One. 2009;4:e4649. doi: 10.1371/journal.pone.0004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Politi P, Minoretti P, Falcone C, Martinelli V, Emanuele E. Association analysis of the functional Ala111Glu polymorphism of the glyoxalase I gene in panic disorder. Neurosci Lett. 2006;396:163–6. doi: 10.1016/j.neulet.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Eser D, Uhr M, Leicht G, Asmus M, Länger A, Schüle C, Baghai TC, Mulert C, Rupprecht R. Glyoxalase-I mRNA expression and CCK-4 induced panic attacks. J Psychiatr Res, Elsevier Ltd. 2011;45:60–3. doi: 10.1016/j.jpsychires.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Fujimoto M, Uchida S, Watanuki T, Wakabayashi Y, Otsuki K, Matsubara T, Suetsugi M, Funato H, Watanabe Y. Reduced expression of glyoxalase-1 mRNA in mood disorder patients. Neurosci Lett. 2008;438:196–9. doi: 10.1016/j.neulet.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Jack M, Wright D. Role of advanced glycation endproducts and glyoxalase I in diabetic peripheral sensory neuropathy. Transl Res, Mosby, Inc. 2012;159:355–65. doi: 10.1016/j.trsl.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skapare E, Konrade I, Liepinsh E, Strele I, Makrecka M, Bierhaus A, Lejnieks A, Pirags V, Dambrova M. Association of reduced glyoxalase 1 activity and painful peripheral diabetic neuropathy in type 1 and 2 diabetes mellitus patients. J Diabetes Complications, Elsevier Inc. 2013;27:262–7. doi: 10.1016/j.jdiacomp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Groener JB, Reismann P, Fleming T, Kalscheuer H, Lehnhoff D, Hamann a, Roser P, Bierhaus a, Nawroth PP, Rudofsky G. C332C genotype of glyoxalase 1 and its association with late diabetic complications. Exp Clin Endocrinol Diabetes. 2013;121:436–9. doi: 10.1055/s-0033-1345124. [DOI] [PubMed] [Google Scholar]
- 35.Junaid Ma, Kowal D, Barua M, Pullarkat PS, Sklower Brooks S, Pullarkat RK. Proteomic studies identified a single nucleotide polymorphism in glyoxalase I as autism susceptibility factor. Am J Med Genet A. 2004;131:11–7. doi: 10.1002/ajmg.a.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu YY, Chien WH, Huang YS, Gau SSF, Chen CH. Lack of evidence to support the glyoxalase 1 gene (GLO1) as a risk gene of autism in Han Chinese patients from Taiwan. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1740–4. doi: 10.1016/j.pnpbp.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Rehnström K, Ylisaukko-Oja T, Vanhala R, von Wendt L, Peltonen L, Hovatta I. No association between common variants in glyoxalase 1 and autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:124–7. doi: 10.1002/ajmg.b.30582. [DOI] [PubMed] [Google Scholar]
- 38.Sacco R, Papaleo V, Hager J, Rousseau F, Moessner R, Militerni R, Bravaccio C, Trillo S, Schneider C, Melmed R, et al. Case-control and family-based association studies of candidate genes in autistic disorder and its endophenotypes: TPH2 and GLO1. BMC Med Genet. 2007;8:11. doi: 10.1186/1471-2350-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arai M, Yuzawa H, Nohara I, Ohnishi T, Obata N, Iwayama Y, Haga S, Toyota T, Ujike H, Arai M, et al. Enhanced carbonyl stress in a subpopulation of schizophrenia. Arch Gen Psychiatry. 2010;67:589–97. doi: 10.1001/archgenpsychiatry.2010.62. [DOI] [PubMed] [Google Scholar]
- 40.Toyosima M, Maekawa M, Toyota T, Iwayama Y, Arai M, Ichikawa T, Miyashita M, Arinami T, Itokawa M, Yoshikawa T. Schizophrenia with the 22q11.2 deletion and additional genetic defects: case history. Br J Psychiatry. 2011;199:245–6. doi: 10.1192/bjp.bp.111.093849. [DOI] [PubMed] [Google Scholar]
- 41.Kemlink D, Polo O, Frauscher B, Gschliesser V, Högl B, Poewe W, Vodicka P, Vavrova J, Sonka K, Nevsimalova S, et al. Replication of restless legs syndrome loci in three European populations. J Med Genet. 2009;46:315–8. doi: 10.1136/jmg.2008.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, Palsson S, Sigmundsson T, Sigurdsson AP, Eiriksdottir I, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357:639–47. doi: 10.1056/NEJMoa072743. [DOI] [PubMed] [Google Scholar]
- 43.Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, Fulda S, Pütz B, Eckstein G, Hauk S, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39:1000–6. doi: 10.1038/ng2099. [DOI] [PubMed] [Google Scholar]
- 44.Winkelmann J, Czamara D, Schormair B, Knauf F, Schulte EC, Berger K, Fuhs A, Trenkwalder C, Dauvilliers Y, Polo O, et al. Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 7. 2011:1–10. doi: 10.1371/journal.pgen.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- 46.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev. 2011;91:1009–22. doi: 10.1152/physrev.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron, Elsevier Inc. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeung JYT, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser Ba. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- 49.Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P. Alterations in the expression of GABA A receptor subunits in cerebellar granule cells after the disruption of the α 6 subunit gene. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 50.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Barbui C, Cipriani A, Patel V, Ayuso-Mateos JL, van Ommeren M. Efficacy of antidepressants and benzodiazepines in minor depression: systematic review and meta-analysis. Br J Psychiatry. 2011;198:11–6. doi: 10.1192/bjp.bp.109.076448. sup 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fava M, Schaefer K, Huang H, Wilson A, Iosifescu DV, Mischoulon D, Wessel TC. A post hoc analysis of the effect of nightly administration of eszopiclone and a selective serotonin reuptake inhibitor in patients with insomnia and anxious depression. J Clin Psychiatry. 2011;72:473–9. doi: 10.4088/JCP.09m05131gry. [DOI] [PubMed] [Google Scholar]
- 53.Vince R, Daluge S, Wadd WB. Studies on the inhibition of glyoxalase I by S-substituted glutathiones. J Med Chem. 1971;14:402–4. doi: 10.1021/jm00287a006. [DOI] [PubMed] [Google Scholar]
- 54.Lo TW, Thornalley PJ. Inhibition of proliferation of human leukaemia 60 cells by diethyl esters of glyoxalase inhibitors in vitro. Biochem Pharmacol. 1992;44:2357–63. doi: 10.1016/0006-2952(92)90680-h. [DOI] [PubMed] [Google Scholar]
- 55.Murthy NS, Bakeris T, Kavarana MJ, Hamilton DS, Lan Y, Creighton DJ. S-(N-aryl-N-hydroxycarbamoyl)glutathione derivatives are tight-binding inhibitors of glyoxalase I and slow substrates for glyoxalase II. J Med Chem. 1994;37:2161–6. doi: 10.1021/jm00040a007. [DOI] [PubMed] [Google Scholar]
- 56.More SS, Vince R. Inhibition of glyoxalase I: the first low-nanomolar tight-binding inhibitors. J Med Chem. 2009;52:4650–6. doi: 10.1021/jm900382u. [DOI] [PubMed] [Google Scholar]
- 57.Hamilton DS, Kavarana MJ, Sharkey EM, Eiseman JL, Creighton DJ. A new method for rapidly generating inhibitors of glyoxalase I inside tumor cells using S-(N-aryl-N-hydroxycarbamoyl)ethylsulfoxides. J Med Chem. 1999;42:1823–7. doi: 10.1021/jm980712o. [DOI] [PubMed] [Google Scholar]
- 58.Takasawa R, Takahashi S, Saeki K, Sunaga S, Yoshimori A, Tanuma S. Structure-activity relationship of human GLO I inhibitory natural flavonoids and their growth inhibitory effects. Bioorg Med Chem. 2008;16:3969–75. doi: 10.1016/j.bmc.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 59.Chiba T, Ohwada J, Sakamoto H, Kobayashi T, Fukami Ta, Irie M, Miura T, Ohara K, Koyano H. Design and evaluation of azaindole-substituted N-hydroxypyridones as glyoxalase I inhibitors. Bioorg Med Chem Lett, Elsevier Ltd. 2012;22:7486–9. doi: 10.1016/j.bmcl.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 60.Thornalley PJ, Edwards LG, Kang Y, Wyatt C, Davies N, Ladan MJ, Double J. Antitumour activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and induction of apoptosis. Biochem Pharmacol. 1996;51:1365–72. doi: 10.1016/0006-2952(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 61.Santel T, Pflug G, Hemdan NYa, Schäfer A, Hollenbach M, Buchold M, Hintersdorf A, Lindner I, Otto A, Bigl M, et al. Curcumin inhibits glyoxalase 1: a possible link to its anti-inflammatory and anti-tumor activity. PLoS One. 2008;3:e3508. doi: 10.1371/journal.pone.0003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343:489–99. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 63.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack MM, Ryals JM, Wright DE. Characterisation of glyoxalase I in a streptozocin-induced mouse model of diabetes with painful and insensate neuropathy. Diabetologia. 2011;54:2174–82. doi: 10.1007/s00125-011-2196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jack MM, Ryals JM, Wright DE. Protection from diabetes-induced peripheral sensory neuropathy--a role for elevated glyoxalase I? Exp Neurol, Elsevier Inc. 2012;234:62–9. doi: 10.1016/j.expneurol.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andersson Da, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, Bevan S. Methylglyoxal evokes pain by stimulating TRPA1. PLoS One. 2013;8:e77986. doi: 10.1371/journal.pone.0077986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Distler MG, Palmer Aa. Role of Glyoxalase 1 (Glo1) and methylglyoxal (MG) in behavior: recent advances and mechanistic insights. Front Genet. 2012;3:250. doi: 10.3389/fgene.2012.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]