Background: A GTPase-deficient Rab27A(Q78L) mutation caused perinuclear melanosome aggregation by an unknown mechanism.
Results: Forcible targeting of Rab27A(Q78L) to melanosomes by a novel melanosome-targeting tag restored peripheral melanosome distribution in Rab27A-deficient cells.
Conclusion: The GTPase activity of Rab27A is required for its melanosome localization, not for melanosome transport.
Significance: These findings provide new insights into the mechanism underlying the spatiotemporal regulation of Rab27A activity.
Keywords: Low Molecular Weight G Proteins, Melanogenesis, Membrane Trafficking, Protein Targeting, Rab, Small GTPases, Subcellular Organelles
Abstract
The small GTPase Rab27A is a crucial regulator of actin-based melanosome transport in melanocytes, and functionally defective Rab27A causes human Griscelli syndrome type 2, which is characterized by silvery hair. A GTPase-deficient, constitutively active Rab27A(Q78L) mutant has been shown to act as an inhibitor of melanosome transport and to induce perinuclear aggregation of melanosomes, but the molecular mechanism by which Rab27A(Q78L) inhibits melanosome transport remained to be determined. In this study, we attempted to identify the primary cause of the perinuclear melanosome aggregation induced by Rab27A(Q78L). The results showed that Rab27A(Q78L) is unable to localize on mature melanosomes and that its inhibitory activity on melanosome transport is completely dependent on its binding to the Rab27A effector Slac2-a/melanophilin. When we forcibly expressed Rab27A(Q78L) on mature melanosomes by using a novel melanosome-targeting tag that we developed in this study and named the MST tag, the MST-Rab27A(Q78L) fusion protein behaved in the same manner as wild-type Rab27A. It localized on mature melanosomes without inducing melanosome aggregation and restored normal peripheral melanosome distribution in Rab27A-deficient cells. These findings indicate that the GTPase activity of Rab27A is required for its melanosome localization but is not required for melanosome transport.
Introduction
The small GTPase Rab family constitutes the largest group of membrane trafficking proteins that are conserved in all eukaryotes (1, 2). Rabs function as a molecular switch by cycling between two nucleotide-bound states, a GDP-bound inactive state and a GTP-bound active state, and regulate various steps or types of membrane traffic, including vesicle budding, vesicle movement along cytoskeletons, vesicle docking to acceptor membranes, and vesicle fusion (1, 2). The GDP-GTP cycling of Rab proteins is generally regulated by two classes of key regulatory enzymes, guanine nucleotide exchange factors, which promote the release of GDP from Rabs and the binding of GTP to Rabs (3), and GTPase-activating proteins, which promote the GTPase activity of Rabs (3–5). Because fixation in either the active state or inactive state by a point mutation (a Gln-to-Leu mutation in the switch-2 region or a Thr/Ser-to-Asn mutation in the conserved nucleotide binding motifs) has often been shown to affect specific membrane traffic events, overexpression of either a constitutively active (CA)3 mutant or a constitutively negative (CN) mutant of Rab is regarded as one of the most effective strategies for analyzing Rab-mediated membrane traffic (6). For example, overexpression of the CA form of Rab5A(Q79L) induces enlarged early endosomes by accelerating homotypic fusion of early endosomes, whereas overexpression of the CN form of Rab5A(S34N) induces the accumulation of small early endosomes by inhibiting early endosome fusion (7).
However, Rab(CA) mutants do not always promote membrane traffic, and some of them have been shown to inhibit membrane traffic, the same as their corresponding Rab(CN) mutant, and Rab27A is a representative example. Rab27A regulates various secretory pathways, including melanosome transport in melanocytes and secretory granule exocytosis in secretory cells (reviewed in Ref. 8 and the references therein), and it is well known that overexpression of the CA form of Rab27A(Q78L) in melanocytes has been shown to cause inhibition of actin-based melanosome transport (i.e. to cause induction of perinuclear melanosome aggregation as a result) rather than to promote melanosome transport (9). One hypothesis to explain this inhibitory effect of Rab27A(Q78L) is that the GTPase activity (or proper GDP-GTP cycling) of Rab27A is essential to melanosome transport in melanocytes. However, this hypothesis has never been tested experimentally, and the exact molecular mechanism by which Rab27A(Q78L) inhibits Rab27A-dependent membrane traffic has remained unknown.
In this study, we analyzed the impact of Rab27A(Q78L) expression on melanosome transport in melanocytes, and the results showed that Rab27A(Q78L) is not targeted to mature melanosomes and that its inhibitory effect depends on binding activity toward Slac2-a (also called melanophilin), a specific Rab27A effector molecule (10–14). Our development of a novel melanosome-targeting tag that we named the MST tag enabled us to show that MST-Rab27A(Q78L), which forcibly targets to mature melanosomes, supports normal peripheral melanosome distribution the same as wild-type Rab27A does. On the basis of our findings, we discuss the possible molecular mechanism of the Rab27A(Q78L)-induced inhibition of melanosome transport.
EXPERIMENTAL PROCEDURES
Materials
The following antibodies used in this study were obtained commercially: anti-GFP rabbit polyclonal antibody (MBL, Nagoya, Japan), anti-FLAG tag rabbit polyclonal antibody (Sigma-Aldrich), HRP-conjugated anti-T7 tag mouse monoclonal antibody and anti-T7 tag antibody-conjugated agarose beads (Merck Biosciences Novagen, Darmstadt, Germany), and Alexa Fluor 488-conjugated anti-rabbit IgG goat antibody (Invitrogen). Anti-Slac2-a rabbit polyclonal antibody was prepared as described previously (15).
Plasmid Construction
pEGFP-C1-Rab27A and its mutant expression plasmids (Y6F, Q78L, and C219A/C221A) used in this study were prepared essentially as described previously (16–18) and as summarized in Fig. 1A. The cDNA insert of pEGFP-C1-Rab27A(Q78L) was also transferred to the pmStr (monomeric Strawberry)-C1 vector (19). cDNA fragments of full-length melanoregulin (Mreg) lacking a stop codon and of an N-terminal portion of Mreg (MregΔC/MST, amino acid residues 1–139) were amplified by PCR with pEGFP-C1-Mreg (19) as a template and the following pairs of oligonucleotides (restriction enzyme sites and a kozak sequence are identified by underlining and by italics, respectively): 5′-GGATCCGCCACCATGGGGCTGCGCCGCTGG-3′ (Mreg-Met primer, sense) and 5′-GTCGACGGGCTTGGAAATGGAAGGC-3′ (MregΔstop primer, antisense) and the Mreg-Met primer and 5′-GTCGACGTGTTTTTAGAATCACTCATG-3′ (MregΔC primer, antisense). The fragments amplified were then inserted into the BglII/SalI site of the pmStr-N1 vector (20) and named pmStr-N1-Mreg and pmStr-N1-MregΔC/MST, respectively (see Fig. 2A). The MST tag cassette vector (pmStr-N1-MST-Gly-linker) was constructed by the following procedure. cDNA of the MST fragment was reamplified by PCR with the Mreg-Met primer and an Mreg-Gly-linker primer (5′-AGATCTACCACCGGTACCACCGGAACCACCTGTGTTTTTAGAATCACTCATG-3′ (the BglII site is underlined and the Gly linker sequence is in boldface)). After digestion with BamHI/BglII, the fragment was subcloned into the BglII site of the pmStr-N1 vector. cDNA of Rab27A(Q78L/C219A/C221A) lacking a stop codon that was amplified by PCR with a Rab27A-Met primer (5′-CGGATCCATGTCGGATGGAGATTACGT-3′, sense; the BamHI site is underlined), a Rab27A-C219A/C221A primer (5′-GTCGACGCGCCAGCCAACCCCTTCTC-3′, antisense; the SalI site is underlined), and pEGFP-C1-Rab27A(Q78L) as a template was inserted into the BglII/SalI site of the pmStr-N1-MST-Gly-linker vector. The resulting pmStr-N1-MST-Gly-linker-Rab27A(Q78L/C219A/C221A) expresses MST-Rab27A(Q78L)-mStr (see Fig. 2A, bottom panel). Similarly, a cDNA fragment of Rab5AΔCys (amino acid residues 1–211 of mouse Rab5A) was subcloned into the BglII/SalI site of the above pmStr-N1-MST-Gly-linker to express MST-Rab5A-mStr. The BamHI/NotI fragment of Slac2-aΔSHD-FLAG (amino acid residues 147–590 of mouse Slac2-a) (21) was subcloned into the BglII/NotI site of the above pmStr-N1-MST-Gly-linker vector to express MST-Slac2-aΔSHD-FLAG (the mStr tag was replaced by a FLAG tag). The Slac2-aΔSHD-FLAG fragment was also subcloned into the pEF-Myc expression vector (22). cDNA of enhanced GFP (EGFP) was amplified by PCR with pEGFP-C-1 as a template and subcloned into the pEF-FLAG expression vector (23) by conventional molecular biology techniques.
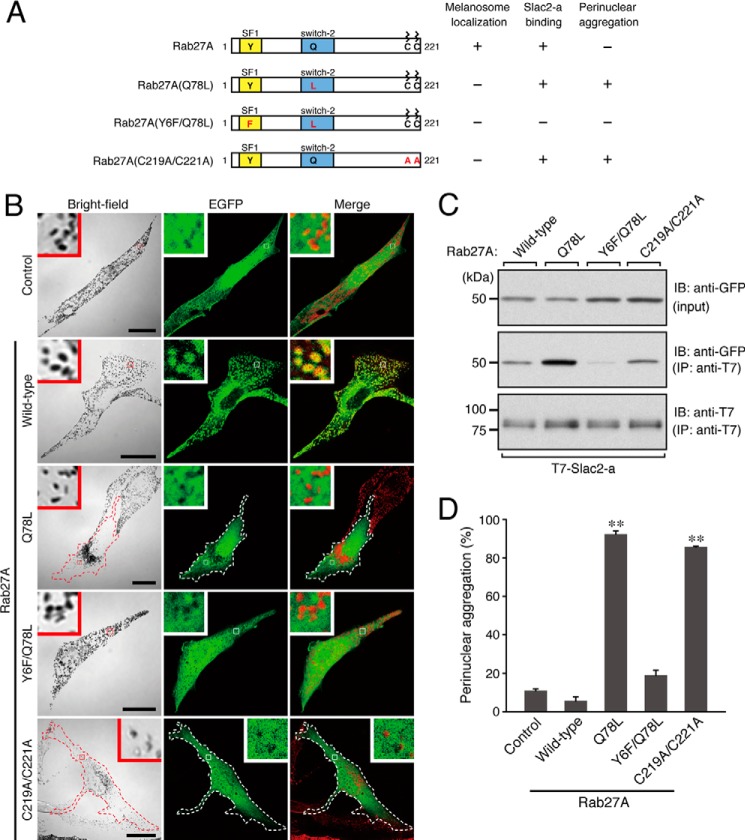
FIGURE 1.
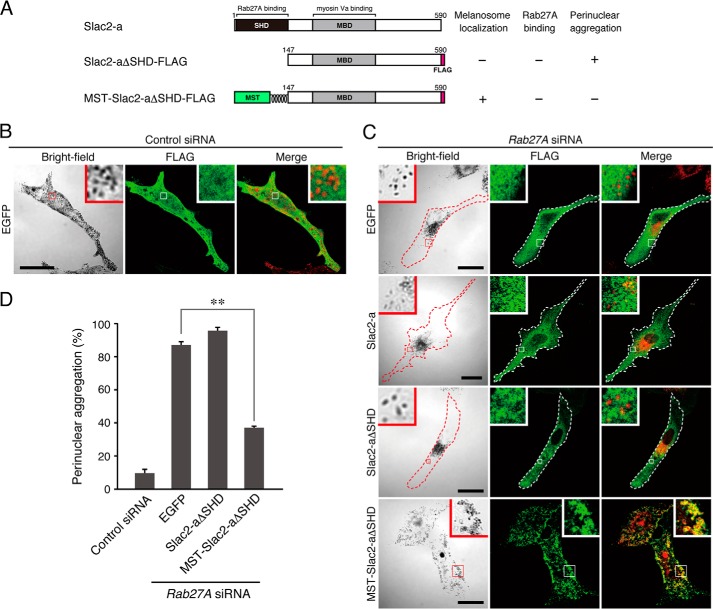
Effect of expression of Rab27A mutants on melanosome distribution in melanocytes. A, schematic of the Rab27A mutants used in this study. The melanosome localization activity, Slac2-a binding activity, and perinuclear aggregation activity of each mutant are shown in the right panel. The SF1 region is one of the conserved regions of a certain Rab subfamily (28), and the switch-2 region is thought to be sensitive to the nucleotide-binding state of Rab proteins. B, typical images of melanocytes expressing EGFP-tagged Rab27A mutants and EGFP alone (EGFP fluorescence images and their corresponding bright-field images). Cells exhibiting perinuclear melanosome aggregation are outlined with a dashed line. The melanosomes in the merged images (right column) are pseudocolored in red. The insets show magnified views of the boxed areas. Note that wild-type Rab27A alone, and not any of the Rab27A mutants, is present on mature melanosomes (second row, yellow signals in the inset). Scale bars = 20 μm. C, Slac2-a binding activity of the Rab27A mutants. Testing for associations between T7-tagged Slac2-a and EGFP-tagged Rab27A (wild-type and mutants) was performed by coimmunoprecipitation assays with anti-T7 tag antibody-conjugated agarose beads as described previously (23, 27). Coimmunoprecipitated EGFP-Rab27A (center panel) and immunoprecipitated T7-Slac2-a (IP) (bottom panel) were detected with the antibodies indicated. Input means 1/80 volume of the reaction mixture used for immunoprecipitation (top panel). The positions of the molecular mass markers (in kilodaltons) are shown at the left. IB, immunoblot. D, the number of melanocytes showing perinuclear melanosome aggregation is expressed as a percentage of the number of transfected melanocytes shown in B. **, p < 0.01, unpaired Student's t test.
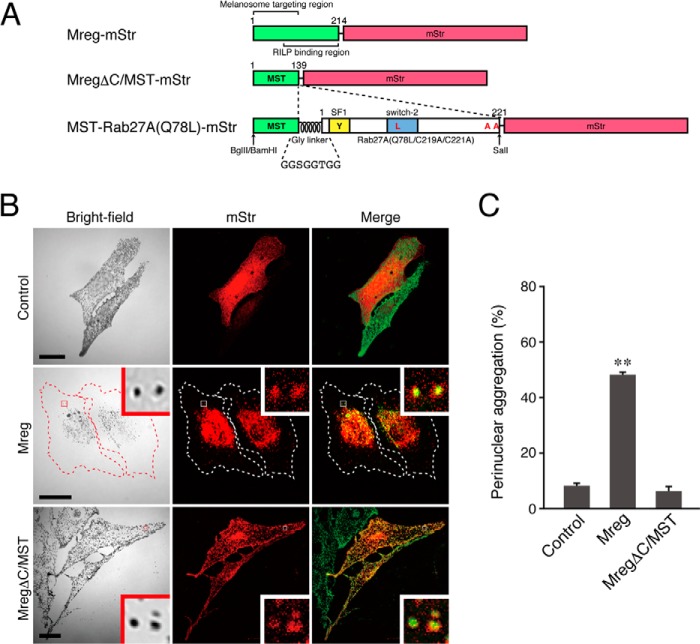
FIGURE 2.
The N-terminal portion of Mreg is necessary and sufficient for melanosome targeting in melanocytes. A, schematic of the Mreg mutants and MST tag used in this study. The N-terminal portion of Mreg is required for its melanosome localization (19, 40), whereas its C-terminal portion binds Rab-interacting lysosomal protein, which forms a retrograde melanosome transport complex (19). MST-Rab27A(Q78L)-mStr was produced by insertion of Gly-linker+Rab27A(Q78L/C219A/C221A) into the site between MST and mStr. BamHI, BglII, and SalI are restriction enzyme sites that were used for the construction of MST-Rab27A(Q78L)-mStr (see “Experimental Procedures” for details). RILP, Rab-interacting lysosomal protein. B, typical images of melanocytes expressing mStr-tagged Mreg mutants and mStr alone (mStr fluorescence images and their corresponding bright-field images). Cells exhibiting perinuclear melanosome aggregation are outlined with a dashed line. The melanosomes in the merged images (right column) are pseudocolored green. The insets show magnified views of the boxed areas. Note that the full-length Mreg with mStr-tag was clearly targeted to mature melanosomes and that Mreg-mStr-expressing cells often exhibited perinuclear melanosome aggregation (center row). MregΔC/MST-mStr, on the other hand, was able to target mature melanosomes without altering peripheral melanosome distribution (bottom row). Scale bars = 20 μm. C, the number of melanocytes exhibiting perinuclear melanosome aggregation expressed as a percentage of the number of transfected melanocytes shown in B. **, p < 0.01; unpaired Student's t test.
Rab27A siRNA was prepared as described previously (24) (19-base target site, 5′-AAGAGAGTGGTGTACAGAG-3′). An siRNA-resistant (SR) form of Rab27A (wild-type and mutants) was constructed by conventional PCR techniques using the following oligonucleotides (substituted nucleotides are shown in italics): 5′-AGGGAAAAACGGGTAGTATATAGGGCCAAT-3′ (Rab27A-SR sense primer) and 5′-ATTGGCCCTATATACTACCCGTTTTTCCCT-3′ (Rab27A-SR antisense primer).
Immunofluorescence Analysis and Melanosome Distribution Assay
The black mouse-derived immortal melanocyte cell line melan-a (a gift from Dorothy C. Bennett) was cultured as described previously (25, 26). Plasmids were transfected into melan-a cells by using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. Three days after transfection, cells were fixed with 10% (w/v) TCA, permeabilized with 0.3% Triton X-100, stained with anti-FLAG tag antibody (1/750 dilution) in 1% BSA/PBS or with anti-Slac2-a antibody (0.04 μg/μl) in Can Get Signal immunostain solution B (Toyobo, Osaka, Japan), and then visualized with Alexa Fluor 488-conjugated anti-rabbit IgG secondary antibody. Cells were examined for immunostaining signals with a confocal laser-scanning microscope (Fluoview, Olympus, Tokyo, Japan) as described previously (26). The images were processed with Adobe Photoshop software (CS5). Melanosome distribution assays (normal peripheral distribution versus perinuclear aggregation) were performed as described previously (n > 50 from three independent dishes) (26), and the data are expressed as means + S.E. Statistical analyses were performed by unpaired Student's t test. p <0.05 was considered statistically significant.
Coimmunoprecipitation Assay and Immunoblotting
Coimmunoprecipitation assays in COS-7 cells were performed with anti-T7 tag antibody-conjugated agarose beads as described previously (23, 27). Immunoprecipitated T7-Slac2-a and coimmunoprecipitated EGFP-Rab27A were analyzed by immunoblotting with specific antibodies and detected by ECL (GE Healthcare).
RESULTS
Rab27A(Q78L) Is Not Targeted to Mature Melanosomes in Melanocytes
When EGFP-tagged Rab27A(Q78L) was expressed in melan-a cells, it strongly induced perinuclear melanosome aggregation (Fig. 1B, third row, and D), consistent with a previous report (9). However, in contrast to the melanosome localization of wild-type Rab27A (Fig. 1B, second row), Rab27A(Q78L) did not localize on mature melanosomes and appeared to be dispersed throughout the cytosol, the same as EGFP alone (Fig. 1B, first and third rows). A similar cytosolic localization pattern of Rab27A(C219A/C221A) (Fig. 1A, and B, fifth row) as a result of mutations of geranylgeranylation sites has also been observed (18). Intriguingly, the Rab27A(C219A/C221A) mutant also strongly induced perinuclear melanosome aggregation (Fig. 1B, fifth row, and D), despite not containing a Q78L mutation.
Slac2-a Binding Ability of Rab27A(Q78L) Is Required for Induction of Perinuclear Melanosome Aggregation
The inhibitory effect of Rab27A(C219A/C221A) on melanosome transport led us to hypothesize that cytosolic localization of Rab27A(Q78L) is the primary cause of the inhibition of melanosome transport and that it traps the Rab27A effector Slac2-a, which functions as a linker protein between Rab27A on melanosomes and an actin-based motor myosin-Va (12–14), in the cytosol. Because the Y6F mutation in the SF1 region of Rab27A (17, 28) has been shown to specifically impair Slac2-a binding ability but to have no effect on binding to another Rab27 effector, Slp2-a, in melanocytes (17, 29, 30), we tested our hypothesis by introducing an additional mutation (Y6F) into Rab27A(Q78L). It should be noted that the resulting Rab27A(Y6F/Q78L) mutant exhibited cytosolic localization, the same as Rab27A(Q78L) did (Fig. 1B, fourth row), and that it did not induce perinuclear aggregation (Fig. 1D). Thus, there were good correlations between the Slac2-a binding activity of Rab27A mutants and their melanosome aggregation activity. Both Rab27A(Q78L) and Rab27(C219A/C221A), but not Rab27A(Y6F/Q78L), were found to interact with Slac2-a in coimmunoprecipitation assays (Fig. 1C) and to induce perinuclear melanosome aggregation.
Melanoregulin Was Used to Develop a Novel Melanosome-targeting Tag
If the cytosolic localization of Rab27A(Q78L) is the primary cause of perinuclear melanosome aggregation, then forced targeting of Rab27A(Q78L) to mature melanosomes should restore peripheral melanosome distribution. Several signals that target certain organelles, including the nucleus, mitochondria, and peroxisomes, e.g. a nuclear localization signal of certain transcription factors (31), a mitochondrial targeting signal of mitochondrial outer membrane proteins (32), and a peroxisomal targeting signal 1 (SKL) of catalase (33), have been reported, and because their fusion with a target protein enables it to be targeted to a specific organelle, they are often used as specific organelle-targeting tags. Although the cytoplasmic region of several melanosomal membrane proteins, e.g. tyrosinase, Tyrp1 (tyrosinase-related protein 1), and OA1 (ocular albinism type 1), contains a dileucine-based motif that is necessary for melanosome targeting (34–37), no melanosome-targeting tag whose fusion with a target protein enables its melanosomal localization has ever been reported. To develop the novel mature melanosome-targeting tag that we named the MST tag, we searched the literature for proteins that are specifically localized on mature melanosomes. Among those that we found in our search, we focused our attention on Mreg, a dilute suppressor gene product (38, 39), because EGFP-tagged Mreg has been shown to target mature melanosomes via its N-terminal palmitoylation (19, 40). Because expression of the full-length Mreg in melanocytes induced perinuclear melanosome aggregation by activation of retrograde melanosome transport through its interaction with Rab-interacting lysosomal protein (RILP)–p150Glued (a dynactin subunit) via its C-terminal domain (19) (Fig. 2B, center row, and C), we decided to use the N-terminal domain of Mreg as the MST tag (Fig. 2A). To visualize the MST tag, we fused monomeric Strawberry (mStr) to the C terminus of MST rather than to its N terminus because the palmitoylation site was located near the N terminus of Mreg (40). As anticipated, in contrast to the cytosolic localization of mStr alone (Fig. 2B, top row), C-terminally mStr-tagged MST (MST-mStr) clearly targeted mature melanosomes without altering their normal peripheral distribution (Fig. 2B, bottom row, and C).
Next, we attempted to evaluate whether additional fusion to MST-mStr affects its melanosome-targeting activity. To do so, we turned our attention to Rab5A, an early endosome-resident Rab that is not normally associated with mature melanosomes (Fig. 3, center row; the red mStr-Rab5A signals and green melanosome signals were completely separate). When Rab5A was fused to MST-mStr (named MST-Rab5A-mStr) and expressed in melanocytes, it was clearly localized on mature melanosomes (Fig. 3, bottom row). These results indicate that the MST tag is likely to function as a melanosome-targeting tag.
FIGURE 3.
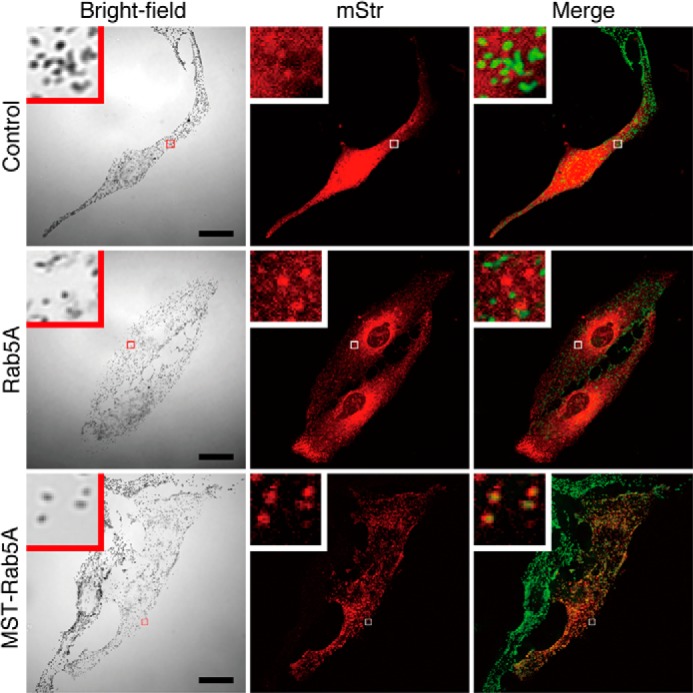
Forcible targeting of Rab5A, an early endosome-resident Rab, to mature melanosomes in melanocytes by the MST tag. Typical images of melanocytes expressing mStr-tagged Rab5A and MST-Rab5A (mStr fluorescence images and their corresponding bright-field images). The melanosomes in the merged images (right column) are pseudocolored green. The insets show magnified views of the boxed areas. Note that MST-Rab5A was clearly targeted to mature melanosomes without altering peripheral melanosome distribution (bottom row). Scale bars = 20 μm.
Forcible Expression of Rab27A(Q78L) on Mature Melanosomes by the MST Tag
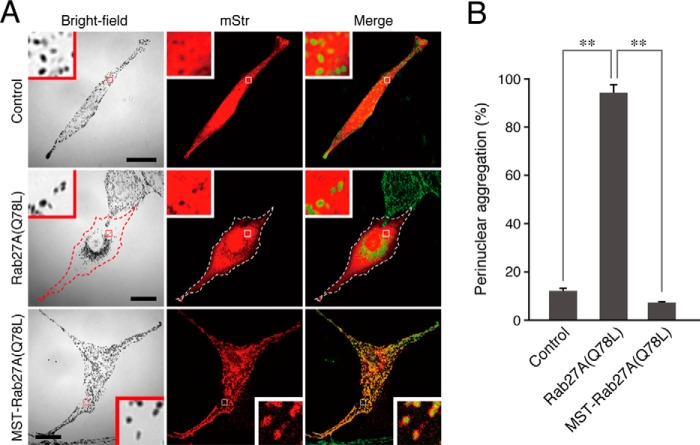
To forcibly target Rab27A(Q78L) to mature melanosomes, Rab27A(Q78L/C219A/C221A), which lacks C-terminal geranylgeranylation sites, was introduced into the above MST-mStr cassette (named MST-Rab27A(Q78L)-mStr, Fig. 2A). In contrast to the cytosolic localization of Rab27A(Q78L) (Fig. 4A, center row), MST-Rab27A(Q78L)-mStr was clearly targeted to mature melanosomes (Fig. 4A, bottom row). Most importantly, MST-Rab27A(Q78L)-mStr did not induce any perinuclear melanosome aggregation at all (Fig. 4B), indicating that the GTPase activity of Rab27A is not essential for peripheral melanosome distribution.
FIGURE 4.
Forcible targeting of Rab27A(Q78L) to mature melanosomes in melanocytes by the MST tag. A, typical images of melanocytes expressing mStr-tagged Rab27A(Q78L) and MST-Rab27A(Q78L) (mStr fluorescence images and their corresponding bright-field images). Cells exhibiting perinuclear melanosome aggregation are outlined with a dashed line. The melanosomes in the merged images (right column) are pseudocolored green. The insets show magnified views of the boxed areas. In contrast to Rab27A(Q78L), MST-Rab27A(Q78L) was clearly targeted to mature melanosomes without inducing perinuclear melanosome aggregation (bottom row). Scale bars = 20 μm. B, the number of melanocytes exhibiting perinuclear melanosome aggregation expressed as a percentage of the number of transfected melanocytes shown in A. **, p < 0.01, unpaired Student's t test.
MST-Rab27A(Q78L)-mStr Supports Normal Peripheral Melanosome Distribution in Rab27A-deficient Melanocytes
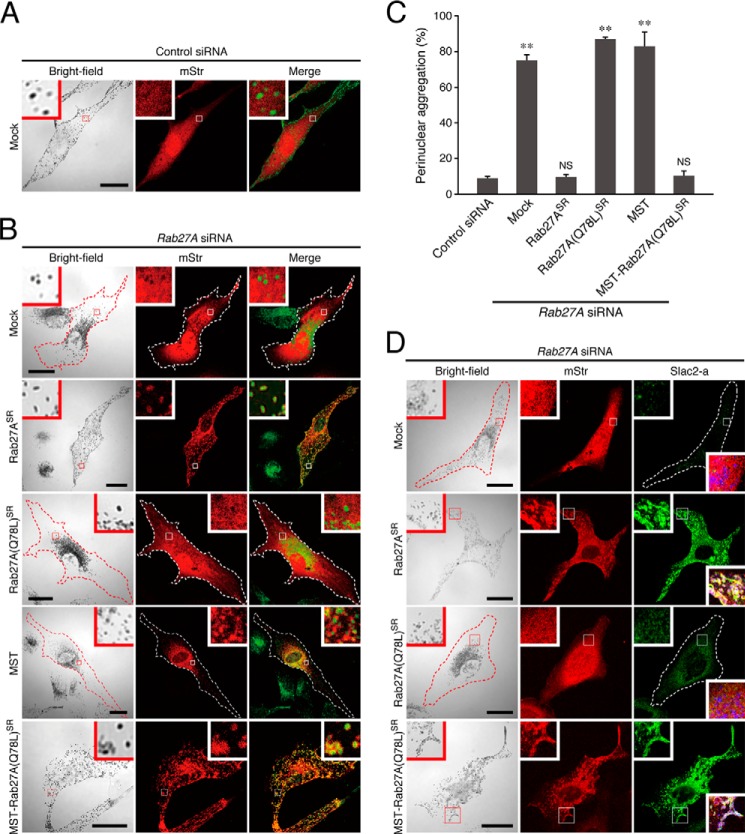
Because MST-Rab27A(Q78L)-mStr was able to target mature melanosomes (Fig. 4A, bottom row, insets), we next investigated whether MST-Rab27A(Q78L)-mStr was able to functionally replace endogenous Rab27A in actin-based melanosome transport. To do so, we coexpressed MST-Rab27A(Q78L)-mStr with an siRNA against Rab27A in melanocytes (24) and evaluated their effect on melanosome distribution. When Rab27A was depleted by the specific Rab27A siRNA, actin-based melanosome transport was impaired (41–43), and melanosomes that had not been transferred to actin filaments were returned to the center of the cell because of increased retrograde melanosome transport activity on microtubules, thereby resulting in melanosome aggregation around the nucleus (19, 44) (Fig. 5A, and B, first row). This aggregation phenotype was clearly rescued by coexpression of an SR form of wild-type Rab27A (mStr-Rab27ASR) with the Rab27A siRNA because the mStr-Rab27ASR in the Rab27A-deficient cells localized to the melanosomes, and they exhibited normal peripheral melanosome distribution (Fig. 5B, second row, and C). By contrast, mStr-Rab27A(Q78L)SR was mainly present in the cytosol, and its expression did not restore peripheral melanosome distribution in Rab27A-deficient cells (Fig. 5B, third row, and C). However, although MST-mStr alone did not restore peripheral melanosome distribution, when the MST tag was added to the N terminus of Rab27A(Q78L)SR, MST-Rab27A(Q78L)SR-mStr was clearly targeted to mature melanosomes and completely rescued Rab27A deficiency in relation to melanosome distribution (Fig. 5B, fourth and fifth rows, and C). Consistent with the normal peripheral melanosome distribution of MST-Rab27A(Q78L)SR-mStr-expressing cells, endogenous Slac2-a molecules were clearly present on mature melanosomes, the same as in wild-type mStr-Rab27ASR-expressing cells (Fig. 5D, second and fourth rows). By contrast, no clear melanosomal localization of Slac2-a was observed in mStr-Rab27A(Q78L)SR-expressing cells (Fig. 5D, third row). These results, taken together, indicate that MST-Rab27A(Q78L)SR-mStr actually functions in actin-based melanosome transport by recruiting Slac2-a to mature melanosomes, the same as the endogenous Rab27A protein does.
FIGURE 5.
MST-Rab27A(Q78L)-mStr can compensate for the function of endogenous Rab27A in melanosome transport in melanocytes. A and B, typical images of melanocytes expressing mStr together with control siRNA (A) and mStr, mStr-Rab27ASR, mStr-Rab27A(Q78L)SR, MST-mStr, or MST-Rab27A(Q78L)SR-mStr together with Rab27A siRNA (B) (mStr fluorescence images and their corresponding bright-field images). Cells exhibiting perinuclear melanosome aggregation are outlined with a dashed line. The melanosomes in the merged images (right column) are pseudocolored green. The insets show magnified views of the boxed areas. Note that MST-Rab27A(Q78L)SR-mStr was able to restore peripheral melanosome distribution in Rab27A-deficient cells (B, fifth row), the same as wild-type Rab27ASR (B, second row). Scale bars = 20 μm. C, the number of melanocytes exhibiting perinuclear melanosome aggregation expressed as a percentage of the number of transfected melanocytes shown in B. **, p < 0.01, unpaired Student's t test. NS, not significant. D, subcellular localization of endogenous Slac2-a molecules in melanocytes expressing mStr, mStr-Rab27ASR, mStr-Rab27A(Q78L)SR, or MST-Rab27A(Q78L)SR-mStr together with Rab27A siRNA. Cells exhibiting perinuclear melanosome aggregation are outlined with a dashed line. The insets show magnified views of the boxed areas. Note that both wild-type mStr-Rab27ASR and MST-Rab27A(Q78L)SR-mStr recruited Slac2-a to mature melanosomes in Rab27A-deficient cells (second and fourth rows), whereas mStr-Rab27A(Q78L)SR did not (third row). The lower insets in the right column are merged images of mStr fluorescence, Slac2-a, and melanosomes (pseudocolored in blue). Scale bars = 20 μm.
Forcible Expression of Slac2-aΔSHD on Mature Melanosomes by the MST Tag
To assess the MST tag as a general tool for melanosome targeting, we finally focused our attention on a Rab27A binding-deficient Slac2-a mutant. As described above, Slac2-a functions as a linker protein between Rab27A and the actin-based motor myosin Va, which is recruited to mature melanosomes in a Rab27A-dependent manner (12–14) (see also Fig. 5D), and impairment of its Rab27A-binding ability causes the perinuclear melanosome aggregation phenotype in human Griscelli syndrome type 3 (45) and in leaden, its corresponding mouse model (46). When wild-type Slac2-a (FLAG-Slac2-a) was expressed in Rab27A-deficient melanocytes, it failed to target mature melanosomes because of the absence of its binding partner, Rab27A, on mature melanosomes, and it was impossible to restore peripheral melanosome distribution in Rab27A-deficient melanocytes (13) (Fig. 6C, second row). Similarly, a Slac2-aΔSHD mutant (Slac2-aΔSHD-FLAG) lacking a Rab27A-binding SHD (12) mainly localized in the cytosol and had no effect on perinuclear melanosome aggregation (Fig. 6C, third row, and D). However, when the MST tag was added to the N terminus of Slac2-aΔSHD (Fig. 6A), the resulting MST-Slac2-aΔSHD-FLAG mutant localized on mature melanosomes and clearly restored peripheral melanosome distribution even in the absence of Rab27A (Fig. 6C, fourth row, and D). Thus, forcible targeting of Slac2-a to mature melanosomes can rescue the Griscelli syndrome phenotype (i.e. perinuclear melanosome aggregation) induced by Rab27A deficiency. These results indicate that the MST tag functions as a universal melanosome-targeting tag that is capable of recruiting a variety of proteins to melanosomes.
FIGURE 6.
Expression of MST-Slac2-aΔSHD in Rab27A-deficient melanocytes restores peripheral melanosome distribution. A, schematic of the Slac2-a mutants used in this study. Slac2-a contains a Rab27A-binding Slp homology domain (SHD) at the N terminus and a myosin Va-binding domain (MBD) in the middle region (12–14, 16). Slac2-aΔSHD lacks the N-terminal SHD and, as a result, is unable to support peripheral melanosome distribution (26). MST-Slac2-aΔSHD contains the MST tag at the N terminus instead of at the SHD. The melanosome localization activity, Rab27A binding activity, and perinuclear aggregation phenotype of Rab27A-deficient cells expressing each mutant are shown in the right panel. B and C, typical images of melanocytes expressing FLAG-EGFP together with control siRNA (B) and FLAG-EGFP, FLAG-Slac2-a, Slac2-aΔSHD-FLAG, or MST-Slac2-aΔSHD-FLAG together with Rab27A siRNA (C) (fluorescence images and their corresponding bright-field images). Cells exhibiting perinuclear melanosome aggregation are outlined with a dashed line. The melanosomes in the merged images (right column) are pseudocolored red. The insets show magnified views of the boxed areas. Note that MST-Slac2-aΔSHD-FLAG was clearly targeted to mature melanosomes even in the absence of Rab27A and that it was able to restore peripheral melanosome distribution in Rab27A-deficient cells (C, fourth row). Scale bars = 20 μm. D, the number of melanocytes exhibiting perinuclear melanosome aggregation expressed as a percentage of the number of transfected melanocytes shown in B and C. **, p < 0.01, unpaired Student's t test.
DISCUSSION
We previously showed that expression of the GTPase-deficient Rab27A(Q78L) mutant in melanocytes induced perinuclear melanosome aggregation (9). However, the molecular mechanism by which Rab27A(Q78L) inhibits melanosome transport has remained unknown throughout the past decade. The results of this study provide two lines of evidence that Rab27A(Q78L) is unable to target mature melanosomes and that it traps the Rab27A effector Slac2-a in the cytosol, thereby resulting in inhibition of actin-based melanosome transport. The first line of evidence is that the Rab27A(Y6F/Q78L) mutant, which specifically impairs Slac2-a binding activity (17, 29), was unable to induce perinuclear melanosome aggregation (Fig. 1). The second line of evidence is that forcible targeting of Rab27A(Q78L) to mature melanosomes by the novel MST tag both neutralized the inhibitory effect of the Q78L mutation in wild-type melanocytes (Fig. 4) and restored peripheral melanosome distribution in Rab27A-deficient melanocytes (Fig. 5). These lines of evidence allow us to conclude that the GTPase activity of Rab27A is not essential for melanosome transport. However, that does not mean that inactivation of Rab27A is not essential for melanocyte function. Because Rab27A(Q78L) was mainly localized in the cytosol (Fig. 1), proper GDP-GTP cycling of Rab27A must be necessary for melanosome targeting by Rab27A (29). Moreover, Rab27A GTPase activity must also be required for Rab27A dissociation from melanosomes after actin-based melanosome transport to enable the next round of melanosome transport from around the nucleus (48).
Because Rab27A is now widely thought to be a general regulator of secretion (8), in the future it will be very interesting to determine whether the GTPase activity of Rab27A is involved in the exocytosis of secretory vesicles. Consistent with our finding in melanocytes, it has recently been shown that induction of platelet secretion does not require the GTPase activity of Rab27 and that cytosolic expression of Rab27 in platelets strongly inhibits secretion by platelets (49). Furthermore, inactivation of Rab27 by Rab27A-GAP EPI64 in rat parotid acinar cells has been shown to occur only after amylase release from secretory granules (47). Therefore, we speculate that the GTPase activity of Rab27A is not essential for secretory activity in general, just as it is also not essential for melanosome transport.
We also developed a novel melanosome-targeting tag that we named the MST tag (Fig. 2) and succeeded in using it to target three different proteins, i.e. Rab5, Rab27A(Q78L), and Slac2-aΔSHD, to mature melanosomes (Figs. 3, 4, and 6). It should be noted that the MST tag did not seem to interfere with the function of the tagged proteins. That is, MST-Slac2-aΔSHD functioned as a linker protein between melanosomes and myosin-Va (Fig. 6). Thus, the MST tag should be a powerful tool for use in future investigations of the function of proteins involved in melanosome biogenesis and/or transport.
In conclusion, by developing the novel MST tag, we were able to demonstrate that the inhibitory effect of the GTPase activity-deficient Rab27A(Q78L) on melanosome transport is primarily attributable to its cytosolic localization of the Rab27A(Q78L) protein. In other words, proper GDP-GTP cycling of Rab27A is necessary for Rab27A targeting to mature melanosomes. Our findings should provide an important clue to understanding why some GTPase-deficient Rab mutants exhibit an inhibitory effect on membrane traffic.
Acknowledgments
We thank Dr. Dorothy C. Bennett (St. George's Hospital Medical School, London, United Kingdom) for donating melan-a cells, Megumi Aizawa for technical assistance, and members of the Fukuda Laboratory for discussions.
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, and Technology (MEXT) of Japan (to N. O. and M. F.); by a grant from the Tokyo Biochemical Research Foundation (to M. F.); and by the International Advanced Research and Education Organization of Tohoku University (to M. I.).
- CA
- constitutively active
- CN
- constitutively negative
- MST
- melanosome-targeting
- Mreg
- melanoregulin
- EGFP
- enhanced GFP
- SHD
- Slp homology domain
- SR
- siRNA-resistant
- mStr
- monomeric Strawberry.
REFERENCES
- 1. Fukuda M. (2008) Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci. 65, 2801–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 [DOI] [PubMed] [Google Scholar]
- 3. Barr F., Lambright D. G. (2010) Rab GEFs and GAPs. Curr. Opin. Cell Biol. 22, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fukuda M. (2011) TBC proteins. GAPs for mammalian small GTPase Rab? Biosci. Rep. 31, 159–168 [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer S. R. (2013) Rab GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 25, 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukuda M. (2010) How can mammalian Rab small GTPases be comprehensively analyzed? Development of new tools to comprehensively analyze mammalian Rabs in membrane traffic. Histol. Histopathol. 25, 1473–1480 [DOI] [PubMed] [Google Scholar]
- 7. Stenmark H., Parton R. G., Steele-Mortimer O., Lütcke A., Gruenberg J., Zerial M. (1994) Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13, 1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukuda M. (2013) Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 14, 949–963 [DOI] [PubMed] [Google Scholar]
- 9. Bahadoran P., Busca R., Chiaverini C., Westbroek W., Lambert J., Bille K., Valony G., Fukuda M., Naeyaert J.-M., Ortonne J.-P., Ballotti R. (2003) Characterization of the molecular defects in Rab27a, caused by RAB27A missense mutations found in patients with Griscelli syndrome. J. Biol. Chem. 278, 11386–11392 [DOI] [PubMed] [Google Scholar]
- 10. Matesic L. E., Yip R., Reuss A. E., Swing D. A., O'Sullivan T. N., Fletcher C. F., Copeland N. G., Jenkins N. A. (2001) Mutations in Mlph, encoding a member of the Rab effector family, cause the melanosome transport defects observed in leaden mice. Proc. Natl. Acad. Sci. U.S.A. 98, 10238–10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuroda T. S., Fukuda M., Ariga H., Mikoshiba K. (2002) The Slp homology domain of synaptotagmin-like proteins 1-4 and Slac2 functions as a novel Rab27A binding domain. J. Biol. Chem. 277, 9212–9218 [DOI] [PubMed] [Google Scholar]
- 12. Fukuda M., Kuroda T. S., Mikoshiba K. (2002) Slac2-a/melanophilin, the missing link between Rab27 and myosin Va. Implications of a tripartite protein complex for melanosome transport. J. Biol. Chem. 277, 12432–12436 [DOI] [PubMed] [Google Scholar]
- 13. Wu X. S., Rao K., Zhang H., Wang F., Sellers J. R., Matesic L. E., Copeland N. G., Jenkins N. A., Hammer J. A., 3rd (2002) Identification of an organelle receptor for myosin-Va. Nat. Cell Biol. 4, 271–278 [DOI] [PubMed] [Google Scholar]
- 14. Strom M., Hume A. N., Tarafder A. K., Barkagianni E., Seabra M. C. (2002) A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J. Biol. Chem. 277, 25423–25430 [DOI] [PubMed] [Google Scholar]
- 15. Imai A., Yoshie S., Nashida T., Shimomura H., Fukuda M. (2004) The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J. Cell Sci. 117, 1945–1953 [DOI] [PubMed] [Google Scholar]
- 16. Fukuda M., Kuroda T. S. (2002) Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J. Biol. Chem. 277, 43096–43103 [DOI] [PubMed] [Google Scholar]
- 17. Kukimoto-Niino M., Sakamoto A., Kanno E., Hanawa-Suetsugu K., Terada T., Shirouzu M., Fukuda M., Yokoyama S. (2008) Structural basis for the exclusive specificity of Slac2-a/melanophilin for the Rab27 GTPases. Structure 16, 1478–1490 [DOI] [PubMed] [Google Scholar]
- 18. Ohbayashi N., Mamishi S., Ishibashi K., Maruta Y., Pourakbari B., Tamizifar B., Mohammadpour M., Fukuda M., Parvaneh N. (2010) Functional characterization of two RAB27A missense mutations found in Griscelli syndrome type 2. Pigment Cell Melanoma Res. 23, 365–374 [DOI] [PubMed] [Google Scholar]
- 19. Ohbayashi N., Maruta Y., Ishida M., Fukuda M. (2012) Melanoregulin regulates retrograde melanosome transport through interaction with the RILP–p150Glued complex in melanocytes. J. Cell Sci. 125, 1508–1518 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi H., Fukuda M. (2012) Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J. Cell Sci. 125, 2235–2243 [DOI] [PubMed] [Google Scholar]
- 21. Fukuda M., Itoh T. (2004) Slac2-a/melanophilin contains multiple PEST-like sequences that are highly sensitive to proteolysis. J. Biol. Chem. 279, 22314–22321 [DOI] [PubMed] [Google Scholar]
- 22. Mori Y., Matsui T., Furutani Y., Yoshihara Y., Fukuda M. (2012) Small GTPase Rab17 regulates dendritic morphogenesis and postsynaptic development of hippocampal neurons. J. Biol. Chem. 287, 8963–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukuda M., Kanno E., Mikoshiba K. (1999) Conserved N-terminal cysteine motif is essential for homo- and heterodimer formation of synaptotagmins III, V, VI, and X. J. Biol. Chem. 274, 31421–31427 [DOI] [PubMed] [Google Scholar]
- 24. Ishida M., Ohbayashi N., Maruta Y., Ebata Y., Fukuda M. (2012) Functional involvement of Rab1A in microtubule-dependent anterograde melanosome transport in melanocytes. J. Cell Sci. 125, 5177–5187 [DOI] [PubMed] [Google Scholar]
- 25. Bennett D. C., Cooper P. J., Hart I. R. (1987) A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer 39, 414–418 [DOI] [PubMed] [Google Scholar]
- 26. Kuroda T. S., Ariga H., Fukuda M. (2003) The actin-binding domain of Slac2-a/melanophilin is required for melanosome distribution in melanocytes. Mol. Cell. Biol. 23, 5245–5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukuda M., Kanno E. (2005) Analysis of the role of Rab27 effector Slp4-a/granuphilin-a in dense-core vesicle exocytosis. Methods Enzymol. 403, 445–457 [DOI] [PubMed] [Google Scholar]
- 28. Pereira-Leal J. B., Seabra M. C. (2001) Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 313, 889–901 [DOI] [PubMed] [Google Scholar]
- 29. Tarafder A. K., Wasmeier C., Figueiredo A. C., Booth A. E. G., Orihara A., Ramalho J. S., Hume A. N., Seabra M. C. (2011) Rab27a targeting to melanosomes requires nucleotide exchange but not effector binding. Traffic 12, 1056–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuroda T. S., Fukuda M. (2004) Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat. Cell Biol. 6, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 31. Lange A., Mills R. E., Lange C. J., Stewart M., Devine S. E., Corbett A. H. (2007) Classical nuclear localization signals. Definition, function, and interaction with importin α. J. Biol. Chem. 282, 5101–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emr S. D., Vassarotti A., Garrett J., Geller B. L., Takeda M., Douglas M. G. (1986) The amino terminus of the yeast F1-ATPase β-subunit precursor functions as a mitochondrial import signal. J. Cell Biol. 102, 523–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gould S. J., Keller G. A., Subramani S. (1988) Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J. Cell Biol. 107, 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sitaram A., Marks M. S. (2012) Mechanisms of protein delivery to melanosomes in pigment cells. Physiology 27, 85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vijayasaradhi S., Xu Y., Bouchard B., Houghton A. N. (1995) Intracellular sorting and targeting of melanosomal membrane proteins. Identification of signals for sorting of the human brown locus protein, gp75. J. Cell Biol. 130, 807–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Calvo P. A., Frank D. W., Bieler B. M., Berson J. F., Marks M. S. (1999) A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 274, 12780–12789 [DOI] [PubMed] [Google Scholar]
- 37. Piccirillo R., Palmisano I., Innamorati G., Bagnato P., Altimare D., Schiaffino M. V. (2006) An unconventional dileucine-based motif and a novel cytosolic motif are required for the lysosomal and melanosomal targeting of OA1. J. Cell Sci. 119, 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Sullivan T. N., Wu X. S., Rachel R. A., Huang J. D., Swing D. A., Matesic L. E., Hammer J. A., 3rd, Copeland N. G., Jenkins N. A. (2004) dsu functions in a MYO5A-independent pathway to suppress the coat color of dilute mice. Proc. Natl. Acad. Sci. U.S.A. 101, 16831–16836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu X. S., Masedunskas A., Weigert R., Copeland N. G., Jenkins N. A., Hammer J. A. (2012) Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 109, E2101–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu X. S., Martina J. A., Hammer J. A., 3rd (2012) Melanoregulin is stably targeted to the melanosome membrane by palmitoylation. Biochem. Biophys. Res. Commun. 426, 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu X., Rao K., Bowers M. B., Copeland N. G., Jenkins N. A., Hammer J. A., 3rd (2001) Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle. J. Cell Sci. 114, 1091–1100 [DOI] [PubMed] [Google Scholar]
- 42. Hume A. N., Collinson L. M., Rapak A., Gomes A. Q., Hopkins C. R., Seabra M. C. (2001) Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J. Cell Biol. 152, 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bahadoran P., Aberdam E., Mantoux F., Buscà R., Bille K., Yalman N., de Saint-Basile G., Casaroli-Marano R., Ortonne J. P., Ballotti R. (2001) Rab27a. A key to melanosome transport in human melanocytes. J. Cell Biol. 152, 843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsui T., Ohbayashi N., Fukuda M. (2012) The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36. Rab36 regulates retrograde melanosome transport in melanocytes. J. Biol. Chem. 287, 28619–28631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ménasché G., Ho C. H., Sanal O., Feldmann J., Tezcan I., Ersoy F., Houdusse A., Fischer A., de Saint Basile G. (2003) Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J. Clin. Invest. 112, 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fukuda M. (2002) Synaptotagmin-like protein (Slp) homology domain 1 of Slac2-a/melanophilin is a critical determinant of GTP-dependent specific binding to Rab27A. J. Biol. Chem. 277, 40118–40124 [DOI] [PubMed] [Google Scholar]
- 47. Imai A., Yoshie S., Ishibashi K., Haga-Tsujimura M., Nashida T., Shimomura H., Fukuda M. (2011) EPI64 protein functions as a physiological GTPase-activating protein for Rab27 protein and regulates amylase release in rat parotid acinar cells. J. Biol. Chem. 286, 33854–33862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Itoh T., Fukuda M. (2006) Identification of EPI64 as a GTPase-activating protein specific for Rab27A. J. Biol. Chem. 281, 31823–31831 [DOI] [PubMed] [Google Scholar]
- 49. Kondo H., Shirakawa R., Higashi T., Kawato M., Fukuda M., Kita T., Horiuchi H. (2006) Constitutive GDP/GTP exchange and secretion-dependent GTP hydrolysis activity for Rab27 in platelets. J. Biol. Chem. 281, 28657–28665 [DOI] [PubMed] [Google Scholar]