Background: Modulation of integrin/kindlin interactions can lead to human disease pathogenesis.
Results: Interaction between integrin β1 and the kindlin-2 is mainly governed by the β1 C-terminal carboxylate moiety.
Conclusion: The interaction chemistry identified here between integrin β1 and kindlin-2 appears to represent a novel protein-protein interaction mode.
Significance: The unusual integrin β1/kindlin-2 association chemistry sheds new light on this aspect of integrin biology.
Keywords: Cell-Cell Interaction, Development, Integrins, Protein-Protein Interactions, Zebrafish, FERM, PDZ, cd29, cd51, Kindlin
Abstract
Protein-protein interactions are driving forces in cellular processes. As a prime example, transmembrane integrins link extracellular matrix and intracellular proteins, resulting in bidirectional signaling that regulates cell migration, proliferation, differentiation, and survival. Here we provide the first evidence that interaction between the integrin β1 cytoplasmic tail and kindlin-2, a member of a family of adapters implicated in human disease pathogenesis, is mainly governed by the β1 C-terminal carboxylate moiety and is required for laterality organ development in zebrafish. Affinity measurements indicate that this unusual protein-protein interaction mode is coordinated by a putative carboxylate-binding motif in the kindlin-2 FERM subdomain F3. Contrary to the C terminus of proteins that engage PDZ domains, the C-terminal three residues of β1, per se, do not contribute to kindlin-2 binding or to laterality organ development. Thus, by employing zebrafish as an in situ physiological tool to correlate protein structure and function, we have discovered an unexpected association chemistry between an integrin and a key adapter involved in integrin signaling.
Introduction
Protein sequence motifs are the basic building blocks of protein-protein interactions, where the identity of amino acid side chains in these motifs and their three-dimensional configurations provide the specificity required for complex cellular signaling networks to function (1–3). Integrins represent a large family of adhesion and signaling receptors that interact with a multitude of extracellular and intracellular proteins by using various sequence motifs (4). Modulation of these interactions can impact physiological events such as angiogenesis, hemostasis, and immune cell function (5). Recently, the interaction of integrins with members of the kindlin family of adapter proteins has gained considerable attention because kindlins can cooperate with other intracellular proteins to activate integrins and to mediate adhesion-dependent cellular responses, in part through interactions of the kindlin FERM2 (four point one, ezrin, radixin, moesin) subdomain F3 with integrin β cytoplasmic tails (6–8). To date, there is no available high-resolution structure of an integrin β tail/kindlin F3 interaction, and consequently, the molecular basis of integrin/kindlin interactions remains incompletely understood (9, 10). Therefore, in the present study, we utilized zebrafish as a physiological platform in parallel with in vitro biochemical analyses to provide the first insights into the interaction chemistry between the integrin β1 tail and the ubiquitously expressed kindlin family member, kindlin-2.
EXPERIMENTAL PROCEDURES
Zebrafish Stocks and Cell Lines
Zebrafish were housed in the University of California San Diego (UCSD) animal facility, and embryos from natural matings were staged according to Ref. 11. Experiments were performed in accordance with the guidelines of UCSD Institutional Animal Care and Use Committee. Human umbilical vein endothelial cells (HUVECs) were acquired from ScienCell (Carlsbad, CA) and grown as adherent monolayer cultures in EC media supplemented with 5% fetal bovine serum + penicillin/streptomycin + endothelial cell growth supplement (all from ScienCell) and with antibiotic-antimycotic solution (Invitrogen).
Antisense Depletion of Integrins αV, β1b, β3a, and Kindlin-2
MOs targeting zebrafish integrin αV, β1b, and kindlin-2 transcripts have been reported elsewhere (12, 13). To assess nonspecific effects of morpholino (MO) injections for kindlin-2 knockdown injections, we used a morpholino targeting integrin β3a transcripts as a control (ctl MO; 5′-ATTATGTGTGTTTGCAGCTTCACCT-3′). This was chosen because β3a expression is absent during gastrulation (14). All MOs were obtained from Gene-Tools, LLC, Philomath, OR. MOs were injected between 1–4 cell stage blastulae except where noted.
mRNA and Peptide Injections
Synthetic peptides were injected into 1–4 cell stage blastulae. Co-expression of mCherry and β1b, or kindlin-2, proteins in vivo was achieved by using Thosea asigna virus 2A peptide (T2A) linked vectors, which mediate protein cleavage by a ribosomal “skip” mechanism (15). T2A was chosen because of its cleavage efficiency in zebrafish (16). All mRNAs, including T2A bicistronic constructs, were synthesized with the mMESSAGE mMACHINE T7 Ultra kit (Ambion, Austin, TX). Either mRNAs were injected alone into 1–4 cell stage blastulae or in rescue experiments 250 pg of respective mRNAs were co-injected with either β1bEI10 or Kin2b morpholinos to a final concentration of ∼6 ng/embryo.
Immunoprecipitation, Affinity Chromatography, Western Blotting, and Direct Binding Measurements
Expression and purification of His6-recombinant integrin cytoplasmic tail model proteins and His6/HA-tagged FERM domains, their purification from Escherichia coli extracts, and affinity chromatography of cell lysates were similar to Refs. 17 and 18. Briefly, purified His6-tagged fusion proteins coupled to NeutrAvidin-agarose beads (29200, Thermo Scientific) were incubated at 4 °C with cell lysates in radioimmunoprecipitation assay buffer (50 mm Tris, pH 7.4, 75 mm NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 5 mm EDTA, 1 mm sodium vanadate, and protease inhibitor mixture). Precipitates were resolved with SDS-polyacrylamide gel electrophoresis, and specific proteins were detected by Western blotting. Antibodies to His6 (sc-804), Hck (sc-72), and Lyn (sc-15) were from Santa Cruz Biotechnology (Dallas, TX); antibody to c-Yes was from BD Transduction Laboratories (610375); monoclonal antibody 327 is specific for the c-Src (19); monoclonal antibody against HA tag was from Invitrogen (32-6700). Immunoreactive signals were detected and quantified by infrared emission spectrometry (Odyssey, LI-COR Biosciences, Lincoln, NE).
For ELISA binding assays, biotinylated recombinant integrin tails (100 ng) were bound at saturating concentrations (20 μg/ml) to wells of NeutrAvidin-coated microtiter plates (Thermo Scientific) (17, 18). Purified FERM proteins were diluted in PBS with 1% BSA, added to wells in duplicates or triplicates, and incubated overnight at 37 °C. After three washes with PBS + 0.2% Tween 20 and subsequent incubations with mouse anti-HA antibody and horseradish peroxidase-conjugated anti-mouse IgG, bound proteins were quantified in an ELISA reader at 490 nm. Competition assays were performed in the presence of 10 μm synthetic Q-peptides with varying concentrations of FERM domains. Binding isotherms were generated by nonlinear regression to a single-site binding model with PRISM (GraphPad, San Diego, CA).
Synthetic Peptides
To assess carboxylate function at the C terminus of the β1 cytoplasmic tail, we used synthetic peptides originating from the β1 tail membrane-distal 15-amino acid segment (see Fig. 4). These peptides either had free acid at the C terminus (acid peptides) or were amidated (amide peptides). Synthetic Q-acid and Q-amide peptides were obtained from NeoBioLab (Cambridge, MA), and WTsht-amide was from Selleckchem (Houston, TX). All three peptides were biotinylated at the N terminus with a mini PEG linker, and their molecular identities were confirmed by mass spectroscopy.
FIGURE 4.

Kindlin-specific β1b tail mutants show differences in their kindlin-2 F3 binding profiles, and these behaviors can be phenocopied in zebrafish. A, the sequence alignments of human and zebrafish kindlin-2 F3 and talin-1 F3 subdomains. Eight residues that have been identified as essential for talin-1 F3 binding and/or activation of αIIbβ3 are indicated with a red asterisk. Two out of the eight seem to be conserved in kindlin-2; Ile-654 is not essential for kindlin-2/β1 interaction (green asterisk) (6). B and C, direct binding of purified zebrafish kindlin-2 F3 (B) and talin-1 F2,3 (C) to wild-type and mutant integrin β1b tails assessed by enzyme-linked immunosorbent assay using NeutrAvidin-bound biotinylated β1b cytoplasmic tails. WT, black filled circle; Y795A, blue filled circle; T788A, green filled circle; ΔEGK, red filled circle. Nonspecific binding was assessed by αIIb tail binding (gray cross). Binding isotherms were generated by nonlinear regression to a single-site binding model (solid lines). D, WT and mutant β1b mRNA titrations on DFC clustering in live zebrafish embryos. Shown is the β1b mRNA dose dependence of mutant DFC clustering. Number of embryos used in this study: WT, 1138; T788A, 600; Y795A, 805; ΔEGK, 329. Error bars indicate mean ± S.E.
WISH Analysis
General methods for whole mount in situ hybridization (WISH) were similar to Ref. 14. Standard molecular cloning techniques were used to prepare antisense riboprobes, and their GenBankTM accession numbers are: cas, AF362749; ntl, NM_131162; kindlin-1, XM_002665785; and kindlin-2, NM_001256215; kindlin-3, NM_200904.
Live Imaging, Immunohistochemistry, and Image Analysis
General methods were similar to Ref. 12. Dorsal forerunner cells (DFCs) in live Tg(sox17:GFP) embryos at 80–90% E were examined under a Zeiss Stemi 2000 fluorescent stereomicroscope at 24 °C. Methodology to classify mutant DFCs was similar to Ref. 12: wild type (WT), ovoid DFC cluster; Mutant, a linear array of DFCs with occasional gaps; Absent, embryos with endoderm GFP(+) and GFP(−) DFCs.
After live DFC scoring, the embryos were allowed to develop to 6–9 somite stage at 28.5 °C, and some live embryos were scored for the presence/absence of Kupffer's vesicle (KV) with a Zeiss stereo microscope, and/or remaining embryos were fixed in 4% paraformaldehyde and used for immunohistochemistry. Phenotypic classification of KVs was also similar to Ref. 12: WT, under bright field a visible circular, single vesicle, with a diameter larger than notochord, was located at the ventral-caudal tip of notochord, or by confocal imaging, GFP(+) cells were located on the lining of KV, and its inflated lumen was GFP(−); Mutant, under bright field a visible circular, single vesicle, or occasionally multiple vesicles, with a diameter equal or smaller than notochord, or by confocal imaging inflated GFP(−) KV lumen was not identifiable and GFP(+) cells at the ventral-caudal tip of notochord were in disarray; Absent, embryos with GFP(+) endoderm with no visible KV, and/or embryos with no axis.
Primary antibodies were used at 1:100 dilutions: mouse anti-ZO-1 (339100, Invitrogen); rabbit anti-aPKC-ζ (sc-216, Santa Cruz Biotechnology); mouse anti-acetylated tubulin (T-6793, Sigma); rabbit anti-zebrafish αV (ab18001, Millipore, Temecula, CA); goat anti-GFP-FITC (ab6662, Abcam); and rabbit polyclonal antibody to DsRed, which recognizes DsRed variants including mCherry (632397, Clontech). Secondary antibodies were used at 1:400 dilutions: goat anti-mouse IgG (H+L) Alexa Fluor® 647 (A21237, Invitrogen); goat anti-mouse IgG (H+L) Alexa Fluor® 568 (A11031, Invitrogen); goat anti-Mouse IgG DyLight 649 (115-495-208, Jackson ImmunoResearch Laboratories Inc., West Grove, PA); goat anti-rabbit IgG (H+L) Alexa Fluor® 568 (A11011, Invitrogen); and goat anti-rabbit IgG (H+L) Alexa Fluor® 647 F(ab′)2 (A21246, Invitrogen). Confocal images were captured with a 20× objective on an Olympus FV1000. Multiple focal plane confocal images of DFCs and KVs were acquired at 1.26-μm step size z-series. Three-dimensional immunofluorescence images were assembled using Volocity 6.1.1 (Improvision Ltd.), ImageJ (National Institutes of Health), and Photoshop (Adobe) software. Embryonic scoring data were expressed as the number of embryos with DFC phenotype or KV phenotype divided by total number of embryos used per experiment × 100 (%) ± S.E. Samples and images were examined blindly and analyzed by Student's t test.
RESULTS
mRNAs That Encode Mutant Integrin β1b Cytoplasmic Tails Perturb Laterality Organ Development
In a recent study we showed that antisense MO-mediated depletion of the integrin αV and β1b subunits leads to defective DFC migration during zebrafish gastrulation (12). DFCs are the precursor cells of the KV, a ciliated organ involved in zebrafish left-right body axis specification (20, 21) Disorganized DFC migration in β1b morphants impedes KV formation, resulting in left-right defects in zebrafish (12). Because integrin β cytoplasmic tails represent a major signaling hub for integrin interaction with cellular proteins involved in integrin signaling (22), we first explored the signaling potential of the integrin β1b tail in the DFC-to-KV organization processes by taking advantage of the dominant-negative effects of integrin β1b overexpression (22–24). We hypothesized that a cytoplasmic tail-less version of β1b (“ΔC mutant”), but not wild-type β1b (WT), would phenocopy the gastrulation defects of β1b morphants (Fig. 1, A and B) (12). When WT β1b mRNA was delivered into zebrafish blastulae, over 77% of embryos formed oval-shaped DFC clusters (Fig. 1, C and F), and later the majority developed single, normally inflated KVs (77.4 ± 4.8%) (Fig. 1, D, E, and G). In sharp contrast, only 32.2 ± 4.6% of embryos injected with β1b(ΔC) had a normal DFC phenotype. Instead the majority of β1b(ΔC) DFCs (65.0 ± 5.4%) were confined to a linear domain that had multiple gaps within this cellular cluster (Fig. 1, C and F). Later in development, β1b(ΔC) mutants also showed a major reduction in inflated KVs (44.8 ± 3.7%) (Fig. 1, D, E, and G).
FIGURE 1.
When delivered at the early blastulae stage, mutant β1b mRNAs that code for defective cytoplasmic tails interfere with DFC clustering and disrupt physical properties of KV. A, sequence alignments of WT and mutant β1b cytoplasmic tails. Deletion segments are shown by an empty space; alanine mutations in WT sequence are underlined in red. For clarity, human sequences are used for sequence numbering of their zebrafish orthologs. B, schematic of bicistronic mCherry-T2A-β1b vector that was used to co-express tandem cDNAs of mCherry and β1b in zebrafish. The cleavage point on the T2A peptide, which is indicated by the black arrow, yields a 19-amino acid extension on the C terminus of mCherry protein. C–E, confocal images of Tg(sox17:GFP)-expressing embryos (green) injected with mRNAs that code for WT, ΔC, W777A/I782A/K784A, and ΔEGK β1b. Dorsal views of 80% epiboly (80% E) embryos are shown in panels C, and 6–8 somite stage (SS) embryos are shown in panels D and E; anterior is to the top. C, multiple focal planes at the center of the DFC cluster. ΔC and ΔEGK β1b DFCs had occasional gaps (white arrowheads in ΔEGK). Embryos were immunolabeled with anti-cherry antibody (red) and nucleus-stained (blue); channels are merged. D and E, multiple focal planes at the center of KV immunolabeled with anti-acetylated tubulin antibody (white) and with anti-cherry antibody (red) (D) or with anti-aPKC-ζ antibody (red) (E); channels are merged. F, bar graphs showing effects of β1b mRNA injection on DFC clustering. Number of embryos used in this study: WT, 293; ΔC, 331; W777A/I782A/K784A, 110; ΔEGK, 158. G, bar graphs showing effects of β1b mRNA injection on KV lumen formation. Number of embryos used in this study: WT, 167; ΔC, 67; W777A/I782A/K784A, 103; ΔEGK, 125. Phenotypic classification and data expression analyses were identical for all figures; see “Experimental Procedures” for details. Scale bars = 30 μm. Error bars indicate mean ± S.E.
Because the β1 cytoplasmic tail may engage in direct interactions with many proteins (5), we generated additional β1b tail mutants in an attempt to focus on those whose interaction might be lost in the β1b(ΔC) mutant and responsible for the mutant phenotype. Surprisingly, β1b tail mutations (W775A or W775A/I782A/K784A) known to disrupt interaction of β1b with talin (25), a major mediator of integrin signaling, did not phenocopy the DFC or KV defects observed in β1b(ΔC) mutants (Fig. 1, and data not shown). Instead a β1b mutant that lacks the C-terminal three amino acids (ΔEGK) reproduced the β1b(ΔC) defects (Fig. 1). In reverse experiments where specific β1b tail mutant mRNAs were injected in an attempt to rescue β1b knockdown phenotypes, these embryos showed 34.7 ± 6.8% (n = 135) WT phenotype, and neither β1b(ΔC) nor β1b(ΔEGK) mRNAs rescued the DFC organization defects, which had 30.3 ± 4.9% (n = 112) and 36.7 ± 9.2% (n = 126) WT DFC phenotypes, respectively. In contrast, all other mutant constructs tested (W775A and W775A/I782A/K784A) achieved partial rescue, 51.0 ± 11.3% (n = 118) and 54.2 ± 5.5% (n = 126), respectively. These results suggest that a protein interaction requiring the C-terminal three residues of β1b (EGK) is needed for normal DFC function.
β1b-dependent DFC-to-KV Organization Is Likely to Be Mediated by Kindlin-2
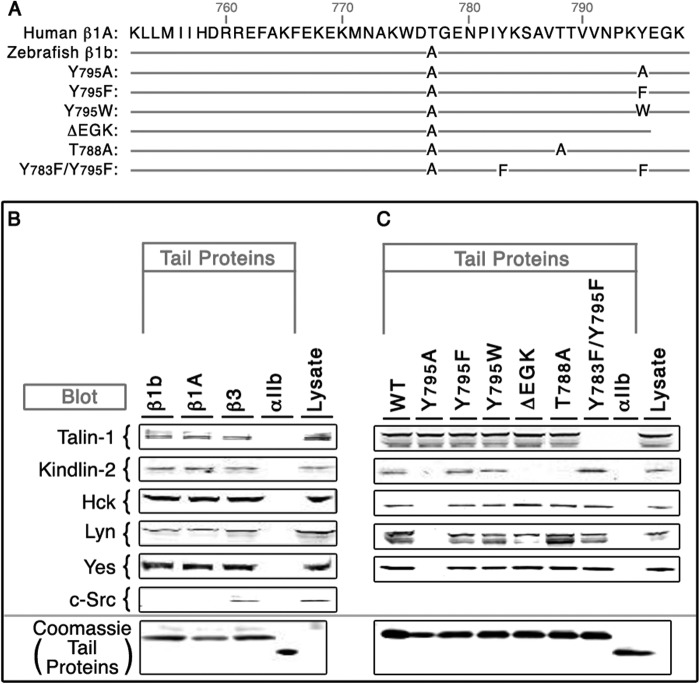
To identify candidate proteins that required the β1b-EGK segment, pulldown assays were employed using recombinant β1b cytoplasmic tail sequences and lysates from human umbilical vein endothelial cells (HUVECs). Since there are very few numbers of DFCs/embryo (12), this approach was chosen because: 1) endothelial cells have been widely used to study integrin signaling (9, 26); 2) vast antibody resources against human proteins are available; and 3) zebrafish β1b and human β1A cytoplasmic tail sequences are 97% identical (Fig. 2A), and many zebrafish and human integrin-binding proteins exhibit high sequence conservation (13, 27, 28). A number of known β1 integrin tail binding proteins were evaluated for which we had antibodies available (Talin-1, Kindlin-2, Hck, Lyn, Yes, and c-Src) (6, 8, 17). The resultant pulldowns demonstrated that only kindlin-2 required the C-terminal β1b EGK segment for binding (Fig. 2, B and C). Therefore, we examined the localization patterns of kindlin transcripts in gastrulating zebrafish. Similar to their human orthologs, there are three kindlins in zebrafish, kindlin-1, kindlin-2, and kindlin-3 (13, 29). Although kindlin-1 and kindlin-2 transcripts showed distinct localization patterns, kindlin-3 transcripts were not evident during gastrulation, and only kindlin-2 was expressed in DFCs (Fig. 3, A–F).
FIGURE 2.
Association of talin-1, kindlin-2, and Src family tyrosine kinases with human and zebrafish β integrin tails. A, the sequence alignments of integrin cytoplasmic tails of human β1A and zebrafish β1b. B, pulldown analysis using WT human β1A, zebrafish β1b, human β3, and human αIIb tails with HUVEC proteins. C, pulldown analysis with recombinant zebrafish β1b cytoplasmic domains. NeutrAvidin beads, coated with integrin tail proteins, were incubated with HUVEC lysate. Bound FERM proteins (talin-1 and kindlin-2) and Src family kinases (Hck, Lyn, Yes, and c-Src) were detected by Western blotting. Tail protein loading was assessed by Coomassie Blue staining.
FIGURE 3.
Kindlin-2 morphants phenocopy the DFC-to-KV organization defects of β1b morphants. A–F, WISH staining of wild-type embryos at 80% E hybridized with probes to zebrafish kindlins (dark purple). Double WISH analyses of kindlin expression in DFCs were performed with cas (A, E, and F), or with ntl (B and C) (orange). DFCs are positive for kindlin-2 transcripts (orange arrow) (E and F); however, kindlin-1 transcripts are only present in the non-neural ectoderm (blue arrowhead) (A and B). Note that the opposite location of kindlin-1 in non-neural ectoderm with respect to embryonic axis (black arrow) can be identified with ntl at this stage (B and C). Dorsal views of individual embryos from panels B and F are shown at a higher magnification, and embryos were slightly tilted to visualize ntl or cas expression in DFCs at 80% E. G–J, confocal images of Tg(sox17:GFP)-expressing embryos (green). Dorsal views of 80% E embryos (G) and 6–8 somite stage (SS) embryos (H–J) are shown in all panels; anterior is to the top. G, multiple focal planes at the center of the DFC cluster. Embryos were nucleus-stained (blue); channels are merged. Multiple focal planes at the center of KV immunolabeled with both anti-acetylated tubulin antibody (white) and anti-aPKC-ζ antibody (red) (H); or with anti-zαV antibody (red) and with anti ZO-1 antibody (white) (I–J); channels are merged. Sagittal confocal sections at the position of the dotted lines are shown in panels I′ and J′; embryo surface is to the right. I″ and J″, three-dimensional renderings of anti-ZO-1 labeled embryos of panels I and J. K, bar graphs showing DFC phenotypes at high dose kindlin-2 MO injection. Number of embryos used in this study: ctl MO, 185; Kin2a, 312; Kin2b, 163; Kin2b + kindlin-2 mRNA, 136. L, selective delivery of kindlin-2 MOs into DFCs. Bar graphs show effects of kindlin-2 loss of function specifically in DFCs on DFC clustering phenotypes. When Kin2 MOs were injected into yolk at midblastula stage (512–1000 cells), which targets DFCs specifically, these animals had mutant phenotype. However, when Kin2 MOs were delivered into the yolk cell at an even later stage (dome stage to 30% epiboly, 30% E) when DFC connections with yolk cells are considered to be closed, these yolkKin2 morphants displayed WT DFC clustering. Number of embryos used in this study: yolkKin2a, 163; DFCKin2a, 179; yolkKin2b, 87; DFCKin2b, 110. M, bar graphs showing the effects of low-dose MO co-injections of integrin αV and kindlin-2 on DFC organization. Number of embryos used in this study: αV1, 139; Kin2a, 83; αV1ctl + Kin2a, 73; αV1 + Kin2a, 102; Kin2b, 131; αV1ctl + Kin2b, 126; αV1 + Kin2b, 171; αV1 + Kin2b + αV mRNA, 113; αV1 + Kin2b + kindlin-2 mRNA, 132; αV1 + Kin2b + αV mRNA + kindlin-2 mRNA, 96. Scale bars = 50 μm in panels A–F, 30 μm in panels G–J. Error bars indicate mean ± S.E.
Consequently, we knocked down kindlin-2 with MOs to evaluate kindlin-2 involvement in the process of DFC-to-KV organization. DFCs were properly clustered in over 71% of control morphants, which later developed KVs with fully inflated lumens (Fig. 3, G–K). In contrast, 48.8–51.1% of embryos injected with either of two kindlin-2 MOs (Kin2a; Kin2b) showed DFCs that did not aggregate properly to form inflated KVs. Furthermore, the effects of these MOs could be partially rescued by co-injection of kindlin-2 mRNA (Fig. 3K). When DFC-selective morphants were generated (DFCKin2a, DFCKin2b) (12), they exhibited DFC phenotypes similar to that of the Kin2a and Kin2b morphants (Fig. 3L). As the DFC-to-KV organization processes require integrin αVβ1b (12), we then evaluated αV and kindlin-2 genetic interaction by delivering substantially lower doses of each individual MO with the result that less than 15% of embryos injected with either αV1 or Kin2 MOs had mutant phenotypes (Fig. 3M). This profile was significantly increased (Kin2a, 38.2 ± 5.3%; Kin2b, 51.5 ± 8.3%; p < 0.05) when αV1 and Kin2 MOs were co-injected, whereas no such effect was observed when a negative control MO (αV1ctl) was co-injected with Kin2a or with Kin2b (11.3 ± 2.1%, and 8.8 ± 5.2%, respectively) (Fig. 3M). In addition, the effects of these MO co-injections could be partially rescued by inclusion of αV and/or kindlin-2 mRNAs (Fig. 3M). Collectively these in vivo results are consistent with the idea that kindlin-2 can be recruited to the integrin β1b tail through the latter's EGK segment, and that this interaction is essential in laterality organ development.
Kindlin Binding-defective Integrin Mutants Show Differences in Their Affinity Profiles, and These Behaviors Can Be Phenocopied in Zebrafish
To further elucidate the biochemical nature of the β1b/kindlin-2 interaction, we measured direct binding affinities of recombinant β1b cytoplasmic tail model peptides to purified kindlin-2 F3. This subdomain of kindlin-2 was chosen because: 1) it harbors the β1 tail interaction interface (Fig. 4A) (6, 8); 2) it enables ease of in vitro production and purification; and 3) F3 subdomains can function autonomously (30, 31).
Binding affinities of the human β1A tail and the zebrafish β1b tail for kindlin-2 F3 were similar (0.23 and 0.24 μm, respectively), and they were in agreement with the reported binding affinity of full-length human kindlin-2 with the β1A tail (9, 32). However, three β1b tail mutants that failed to pull down kindlin-2 (Fig. 2C) exhibited distinct differences in their affinity profiles (Fig. 4B). For example, although β1b(Y795A) tail affinity was reduced 16-fold as compared with wild-type β1b, the β1b(T788A) mutant showed a 110-fold reduction, and β1b(ΔEGK) had no discernable affinity (Fig. 4, B and C). In analogous experiments, when β1b mRNAs were delivered over a wide range of concentrations (8–125 pg/embryo) into zebrafish embryos, all three integrin mutant β1b(ΔEGK), β1b(T788A), and β1b(Y795A) mRNAs caused dose-dependent increases in the malformed DFC phenotype (Fig. 4D). Collectively, these results suggest that under physiological conditions, all three kindlin-2 binding-impaired mutants of integrin β1b (ΔEGK, T788A, and Y795A) could induce DFC organization defects.
The Integrin β1 and Kindlin-2 Association Is Mainly Governed by the β1 C-terminal Carboxylate Moiety and Is Required for Laterality Organ Development in Zebrafish
Because the β1b ΔEGK mutation had the most potent impact on both in vitro and in vivo assays, we investigated further the EGK-mediated kindlin-2 recruitment to the β1b tail by testing an additional series of mutant β1b-EGK sequences in pulldown and direct binding ELISA assays (Fig. 5). Surprisingly, none of these modifications eliminated β1b/kindlin-2 interaction in pulldowns or impaired the affinity of β1b for kindlin-2 F3. Thus, there is no particular amino acid requirement within the EGK segment for β1b binding to kindlin-2.
FIGURE 5.
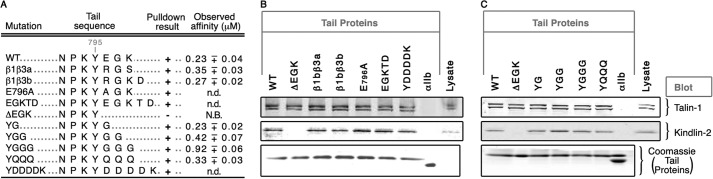
EGK sequence in β1b cytoplasmic tail is not essential. A, summary of amino acid sequences alignments of β1b tail mutants, their associated pulldown results, and their observed direct binding affinities to purified zebrafish kindlin-2 F3. B and C, pulldown analyses with recombinant β1b cytoplasmic tails with HUVEC lysates. Conditions for pulldown analysis were similar to Fig. 2, and analyses of binding isotherms were similar to Fig. 4. N.B., no binding; n.d., not determined.
The only major functional chemical moiety that was left unexplored by these binding experiments is the COOH moiety at the β1b C terminus. To evaluate this, three independent experimental schemes were employed (Fig. 6). First, the impact of gradual β1b C-terminal extensions on kindlin-2 affinity was examined because the effective concentration of peptide tail ends decreases inversely with an increase in the chain length (33), thus making β1b C-terminal COOH less available to interact with a hypothetical carboxylate binding-segment in kindlin-2 F3. Therefore, we introduced 1×, 2×, and 3× FLAG tags at the caudal end of β1b-Y795, resulting in +6, +18, and +30 residue extensions to β1b (Fig. 6A). All three extended peptides pulled down full-length talin-1, and all showed similar affinities to purified talin-1 F2,3 (data not shown). However their affinities for full-length kindlin-2 and its F3 subdomain were reduced as the β1b tail length was increased (Fig. 6, B and C).
FIGURE 6.
The C-terminal COOH of the integrin β1 cytoplasmic tail mediates β1/kindlin-2 association. A, gradual C-terminal extensions of recombinant β1b tails were generated with successive FLAG tags. B and C, pulldown assays with HUVEC lysates (B) and direct binding curves for kindlin-2 F3 (C) with C-terminally extended β1b cytoplasmic tails. Numbers of FLAG tags are indicated in parentheses: WT, black filled circle; Y(1)FLAG, blue filled triangle; Y(2)FLAG, magenta filled triangle; Y(3)FLAG, purple filled triangle; ΔEGK, red filled circle; and αIIb, gray cross. D, sequence alignments of recombinant and synthetic β1b tail peptides used in panels E–G. Either a recombinant 15-amino acid membrane-distal (MD) segment of the WT β1 tail C terminus was bacterially expressed as a fusion protein, or its synthetic derivatives, E796Q mutant (Q), were prepared either as free acid (WTsht-acid, Q-acid) or as amides (WTsht-amide, Q-amide). E and F, direct binding profiles of free acid and amide peptides with purified kindlin-2 F3 (E) and pulldown assays with HUVEC lysates (F). WTsht-acid, red triangle; WTsht-amide, cyan triangle; Q-acid, red square; Q-amide, cyan square; and αIIb, gray cross. G, inhibition of β1b/kindlin-2 F3 association by synthetic Q peptides (10 μm). β1b, full-length β1b tail, black circle; β1b + Q-acid, β1b tail with Q acid peptide, red filled circle; β1b + Q-amide, β1b tail with Q-amide peptide, cyan filled circle; and αIIb, gray cross. H, effects of Q-acid and Q-amide peptide titrations on DFC clustering in live zebrafish embryos. Shown is the effect of synthetic peptide dose on mutant DFC clustering. Number of embryos used in this study: Q-acid, 376; Q-amide, 380. I, schematic of bicistronic mCherry-T2A-β1b vectors that were used to co-express mCherry and β1b proteins. J and K, confocal images of Tg(sox17:GFP)-expressing embryos (green) injected with mRNAs that code for WT and β1b-GATA1a fusion. J and K, dorsal views of 80% E embryos (J) and 6–8 somite stage (SS) embryos (K) are shown in all panels; anterior is at the top. J, multiple focal planes at the center of the DFC cluster. Embryos were immunolabeled with anti-cherry antibody (red) and nucleus-stained (blue); channels are merged. K, multiple focal planes at the center of KV immunolabeled with anti-acetylated tubulin antibody (white). L, bar graphs showing effects of β1b mRNA injection on DFC clustering. Number of embryos used in this study: WT, 163; β1b-GATA1a, 405. M, bar graphs showing effects of β1b mRNA injection on KV lumen formation. Number of embryos used in this study: WT, 151; β1b-GATA1a, 267. MP, membrane-proximal segment of the β1 tail. Conditions for pulldown analysis were similar to Fig. 2, and analyses of binding isotherms were similar to Fig. 4. Scale bars = 50 μm. Error bars indicate mean ± S.E.
Second, short peptides derived from the membrane-distal, C-terminal 15 amino acids of the β1 tail were tested either in their free acid form (COOH: WT-acid, Q-acid) or in their amide form (CONH2: WT-amide, Q-amide) (Fig. 6D). Two such peptides were designed because there is a glutamate residue (Glu-796) near the C terminus of β1, and we evaluated its potential side chain carboxylate function with an E796Q mutant peptide (Q-acid). Although both acid peptides were able to pull down full-length kindlin-2 and showed considerable binding affinities for kindlin-2 F3 (0.54 ± 0.09 and 1.4 ± 0.34 μm), their amide forms failed to pull down kindlin-2 or to interact with kindlin-2 F3 with discernable affinity (Fig. 6, E and F). As a control, both amide peptides still retained the ability to pull down another β1b-interacting protein, Lyn (Fig. 6F). In addition, although the Q-acid peptide was able to inhibit β1b/kindlin-2 F3 association, its amide form lacked this ability (Fig. 6G), and neither form of the Q peptides blocked β1b tail binding to talin-1 F2,3 (data not shown). In analogous experiments, when the Q-acid peptide was delivered to zebrafish blastulae over a wide range of concentrations (4.4–44 pg/embryo), it was able to induce mutant DFC phenotypes, but its amide form did not seem to have this ability (Fig. 6H).
Third, we studied the effects of overexpression of an irrelevant, large C-terminal β1b fusion construct (β1b-GATA1a) in zebrafish embryos to ascertain the effects of β1b C-terminal blockade in a physiological context (Fig. 6I). Unlike WT β1b, injection of mRNA coding for a large fusion construct caused significantly impaired DFC clustering (p < 0.05) and led to malformed KV development (p < 0.01) (Fig. 6, J–M). Collectively, these results indicate that the carboxylate moiety at the β1b C terminus is essential for β1b/kindlin-2 association.
A Putative Carboxylate-binding Motif in Kindlin-2 F3 May Coordinate Interactions with the β1 C-terminal Carboxylate Moiety
We postulated that kindlin-2 F3 has a corresponding carboxylate-binding segment. To our knowledge, PDZ domain-containing proteins are the only known examples of proteins that possess a C-terminal carboxylate-binding motif (h-Gly-h) (34, 35). In addition, a highly conserved, positively charged amino acid located at the N terminus of the h-Gly-h motif is essential for coordination of electrostatic interactions with the terminal carboxylate (34–36). Indeed, the kindlin-2 F3 subdomain has a potential carboxylate-binding segment located between Lys-581 and Ile-588 (KKDELIGI) (Fig. 7A). Orthologous alignments of kindlin-2 sequences indicated that this sequence is completely conserved between mammalian, avian, amphibian, teleost, and insect lineages (Fig. 7A). Therefore, we generated point mutants of kindlin-2 F3 and evaluated their binding affinities to the β1b tail (Fig. 7B). Of the mutant kindlin-2 F3 subdomains, K581E, G587L, and I586G/I588G completely abrogated binding affinity to the β1b tail (Fig. 7, C and D). Although a conserved tryptophan-to-alanine (W615A) mutant of kindlin-2 is known to disrupt β1 tail/kindlin-2 interaction by a pulldown assay (6, 8), the binding profile of this kindlin-2 F3 mutant revealed a considerable affinity for the β1 tail (11.15 ± 0.35 μm), but less than that of WT kindlin-2 F3 (Fig. 7E). In contrast, conserved mutations in three other positively charged residues (R592E, R595E, and R608E) had no effect on binding affinity (Fig. 7F).
FIGURE 7.
A PDZ-like carboxylate-binding motif exists in kindlin-2 F3. Mutations in this segment phenocopy the DFC and laterality organ defects of kindlin-2 and αV/β1b morphants. A, multiple PDZ carboxylate-binding loop sequence alignments used to identify a putative motif in the kindlin-2 F3 subdomain. Interspecies sequence alignments of the putative kindlin-2 F3 carboxylate-binding loop indicate a complete conservation from mammalian to insect lineages. B, list of kindlin-2 F3 mutants and their respective sequence alignments used in this study. Point mutations in WT sequence are underlined in red. For clarity, human sequences are used for sequence numbering on their zebrafish orthologs. C–E, direct binding of purified zebrafish kindlin-2 F3 mutants (C–E) and talin-1 F2,3 (E) to wild-type recombinant β1b tail assessed by enzyme-linked immunosorbent assay. Kindlin-2 F3 mutants: WT, black filled circle; K581E, red filled triangle; K582E, red circle; K581E/K582E, green filled triangle; G587L, cyan square; I586G/I588G, black filled triangle; R592E, green filled circle; R595E, cyan filled circle; R608E, red filled circle; W615A, black circle; and αIIb, gray cross. Talin-1 F2,3 mutants: WT, magenta filled circle; W359A, magenta circle. F, summary of mutant kindlin-2 F3 direct binding affinities to wild-type integrin β1b tail. G, schematic of bicistronic mCherry-T2A-kindlin-2 vector that was used to co-express mCherry and kindlin-2 proteins. H and I, confocal images of Tg(sox17:GFP)-expressing embryos (green) injected with mRNAs that code for WT, ΔF3, K581E, and R592E. H and I, dorsal views of 80% E embryos (H), and 6–8 somite stage (SS) embryos (I) are shown in all panels; anterior is to the top. H, multiple focal planes at the center of the DFC cluster. Embryos were immunolabeled with anti-cherry antibody (red) and nucleus-stained (blue); channels are merged. I, multiple focal planes at the center of KV immunolabeled with anti-acetylated tubulin antibody (white) and with anti-cherry antibody (red); channels are merged. J, bar graphs showing effects of kindlin-2 mRNA injections on DFC clustering. Number of embryos used in this study: WT, 312; ΔF3, 353; K581E, 267; R592E, 221. K, bar graphs showing effects of kindlin-2 mRNA injections on KV lumen formation. Number of embryos used in this study: WT, 108; ΔF3, 123; K581E, 152; R592E, 122. N.B., no binding. Analyses of binding isotherms were similar to Fig. 4. Scale bars = 50 μm. Error bars indicate mean ± S.E.
To test the physiological impacts of kindlin-2 F3 putative carboxylate-binding domain mutations, mRNAs coding for full-length kindlin-2 (WT), F3-less kindlin-2 (ΔF3), and point mutants of kindlin-2 F3 (K581E, R589E) were injected into zebrafish blastulae and DFC-to-KV organization was evaluated (Fig. 7G). Unlike WT kindlin-2 F3 or the kindlin-2 R589E mutant, both kindlin-2 ΔF3 and K581E disrupted DFC clustering, leading to DFC aggregation defects and malformed KVs (Fig. 7, H–K). Collectively, the biochemical and physiological data suggest that the kindlin-2 F3 possesses a putative carboxylate-binding motif.
DISCUSSION
In the present study, we utilized zebrafish as an experimental platform to examine the molecular basis of αVβ1b function in the context of potential interactions with intracellular proteins. By using β1b mRNA overexpression in zebrafish embryos in combination with pulldown assays, we identified kindlins as candidate β1b-binding proteins to participate in αVβ1b signaling in DFCs. Specifically, 1) kindlin-2 transcripts are present in DFCs (Fig. 3); 2) kindlin-2 knockdown causes gastrulation defects identical to those of αVβ1 morphants (Fig. 3) (12); 3) affinity measurements with kindlin-2 F3 indicated that the β1-EGK segment is essential for interaction with kindlin-2 (Fig. 4); and 4) overexpression of β1b(ΔEGK) mRNA induces DFC-to-KV organization defects (Figs. 1 and 4).
Although these observations support a key role for kindlin-2 in laterality organ development through its interaction with the C-terminal EGK residues of integrin β1, to our surprise mutational analyses of the EGK segment revealed that the identity of the amino acid in this segment is not important for β1/kindlin-2 interaction. Rather, the C-terminal carboxylate of β1-EGK is the most essential moiety for this interaction. Specifically, 1) pulldown profiles of full-length kindlin-2 and direct binding affinity of kindlin-2 F3 were inversely correlated with C-terminal extensions onto the β1b tail; 2) chemical modification of the C-terminal carboxylate of synthetic peptides derived from the β1b tail completely abolished affinity for kindlin-2; 3) WT synthetic peptide was able to induce mutant DFC phenotypes, and this ability was lost when its C-terminal amide form was delivered into zebrafish blastulae; and 4) overexpression of a large C-terminal β1b fusion protein led to DFC-to-KV organization defects during zebrafish gastrulation (Fig. 6). It is interesting to note that whereas the last residue of the ΔEGK mutant, Tyr-795, carries two potential binding moieties at the same time (Tyr and backbone COOH), it still cannot interact with kindlin-2. This might arise from a conformational dichotomy caused by a stearic hindrance because F3 domains are known to have a relatively rigid conformation (37), which would not allow tandem attachments to take place at the same time. In addition, lack of β1/kindlin-2 association in β1b ΔEGK could be rescued by inclusion of a single residue (YG peptide, Fig. 5) indicating that flexibility resides in this region of the β1 tail, which was also seen in β3/talin-1 NMR studies (37). Evidence provided here also indicates that this interaction requires a generalized carboxylate-binding motif (h-Gly-h) (34) in the kindlin-2 F3 subdomain, and mutations within this segment both obliterate β1/kindlin-2 F3 affinity in vitro and induce DFC-to-KV organization defects in vivo (Fig. 7).
To date, proteins carrying PDZ domains are the only known example where the C-terminal carboxylate moiety of their ligands contributes to the interaction and where the interaction has an absolute sequence requirement for the C-terminal three (or four) residues of the ligand (1, 3, 36, 38). In contrast, these corresponding residues in integrin β1 do not contribute to discriminative binding. Therefore, we postulate that the interaction chemistry identified here between integrin β1 and kindlin-2 F3 represents a novel protein-protein interaction mode. However, it should be emphasized that such interaction chemistry may not necessarily be a shared feature of other integrin β tail/kindlin complexes, such as that between integrin β2 or β3 and kindlin-2 (9) or that between integrin β6 and kindlin-1 or kindlin-2 (39). Therefore, additional studies with different β integrin/kindlin permutations will be required to fully understand their association chemistries.
Acknowledgments
We thank Dr. Jim Dowling for generously providing kindlin-2 MOs and the UCSD Fish Facility for animal maintenance. Confocal images were gathered at the UCSD Neuroscience Microscopy Shared Facility (supported by National Institutes of Health Grant P30 NS047101)
This work was supported, in whole or in part, by National Institutes of Health Grants HL-57900 and HL-78784 (to S. J. S.).
- FERM
- four point one, ezrin, radixin, moesin
- PDZ
- PSD95, DLG, ZO-1
- DFC
- dorsal forerunner cell
- E
- epiboly
- HUVEC
- human umbilical vein endothelial cell
- KV
- Kupffer's vesicle
- T2A
- Thosea asigna virus 2A peptide
- WISH
- whole mount in situ hybridization
- ctl
- control.
REFERENCES
- 1. Chen J. R., Chang B. H., Allen J. E., Stiffler M. A., MacBeath G. (2008) Predicting PDZ domain-peptide interactions from primary sequences. Nat. Biotechnol. 26, 1041–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pawson T., Nash P. (2003) Assembly of cell regulatory systems through protein interaction domains. Science 300, 445–452 [DOI] [PubMed] [Google Scholar]
- 3. Stiffler M. A., Chen J. R., Grantcharova V. P., Lei Y., Fuchs D., Allen J. E., Zaslavskaia L. A., MacBeath G. (2007) PDZ domain binding selectivity is optimized across the mouse proteome. Science 317, 364–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 5. Cox D., Brennan M., Moran N. (2010) Integrins as therapeutic targets: lessons and opportunities. Nat. Rev. Drug Discov. 9, 804–820 [DOI] [PubMed] [Google Scholar]
- 6. Harburger D. S., Bouaouina M., Calderwood D. A. (2009) Kindlin-1 and -2 directly bind the C-terminal region of β integrin cytoplasmic tails and exert integrin-specific activation effects. J. Biol. Chem. 284, 11485–11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kloeker S., Major M. B., Calderwood D. A., Ginsberg M. H., Jones D. A., Beckerle M. C. (2004) The Kindler syndrome protein is regulated by transforming growth factor-β and involved in integrin-mediated adhesion. J. Biol. Chem. 279, 6824–6833 [DOI] [PubMed] [Google Scholar]
- 8. Shi X., Ma Y. Q., Tu Y., Chen K., Wu S., Fukuda K., Qin J., Plow E. F., Wu C. (2007) The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455–20466 [DOI] [PubMed] [Google Scholar]
- 9. Bledzka K., Liu J., Xu Z., Perera H. D., Yadav S. P., Bialkowska K., Qin J., Ma Y. Q., Plow E. F. (2012) Spatial coordination of kindlin-2 with talin head domain in interaction with integrin β cytoplasmic tails. J. Biol. Chem. 287, 24585–24594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yates L. A., Lumb C. N., Brahme N. N., Zalyte R., Bird L. E., De Colibus L., Owens R. J., Calderwood D. A., Sansom M. S., Gilbert R. J. (2012) Structural and functional characterization of the kindlin-1 pleckstrin homology domain. J. Biol. Chem. 287, 43246–43261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995) Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- 12. Ablooglu A. J., Tkachenko E., Kang J., Shattil S. J. (2010) Integrin αV is necessary for gastrulation movements that regulate vertebrate body asymmetry. Development 137, 3449–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dowling J. J., Gibbs E., Russell M., Goldman D., Minarcik J., Golden J. A., Feldman E. L. (2008) Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ. Res. 102, 423–431 [DOI] [PubMed] [Google Scholar]
- 14. Ablooglu A. J., Kang J., Handin R. I., Traver D., Shattil S. J. (2007) The zebrafish vitronectin receptor: characterization of integrin αV and β3 expression patterns in early vertebrate development. Dev. Dyn. 236, 2268–2276 [DOI] [PubMed] [Google Scholar]
- 15. Holst J., Vignali K. M., Burton A. R., Vignali D. A. (2006) Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat. Methods 3, 191–197 [DOI] [PubMed] [Google Scholar]
- 16. Kim J. H., Lee S. R., Li L. H., Park H. J., Park J. H., Lee K. Y., Kim M. K., Shin B. A., Choi S. Y. (2011) High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS One 6, e18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. (2003) Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. U.S.A. 100, 13298–13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arias-Salgado E. G., Lizano S., Shattil S. J., Ginsberg M. H. (2005) Specification of the direction of adhesive signaling by the integrin β cytoplasmic domain. J. Biol. Chem. 280, 29699–29707 [DOI] [PubMed] [Google Scholar]
- 19. Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. (2002) Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 157, 265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Essner J. J., Amack J. D., Nyholm M. K., Harris E. B., Yost H. J. (2005) Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development 132, 1247–1260 [DOI] [PubMed] [Google Scholar]
- 21. Schneider I., Houston D. W., Rebagliati M. R., Slusarski D. C. (2008) Calcium fluxes in dorsal forerunner cells antagonize β-catenin and alter left-right patterning. Development 135, 75–84 [DOI] [PubMed] [Google Scholar]
- 22. Lee K. K., de Repentigny Y., Saulnier R., Rippstein P., Macklin W. B., Kothary R. (2006) Dominant-negative β1 integrin mice have region-specific myelin defects accompanied by alterations in MAPK activity. Glia 53, 836–844 [DOI] [PubMed] [Google Scholar]
- 23. Balzac F., Retta S. F., Albini A., Melchiorri A., Koteliansky V. E., Geuna M., Silengo L., Tarone G. (1994) Expression of β1B integrin isoform in CHO cells results in a dominant negative effect on cell adhesion and motility. J. Cell Biol. 127, 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Relvas J. B., Setzu A., Baron W., Buttery P. C., LaFlamme S. E., Franklin R. J., ffrench-Constant C. (2001) Expression of dominant-negative and chimeric subunits reveals an essential role for β1 integrin during myelination. Curr. Biol. 11, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 25. Bouaouina M., Lad Y., Calderwood D. A. (2008) The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J. Biol. Chem. 283, 6118–6125 [DOI] [PubMed] [Google Scholar]
- 26. Weis S. M., Cheresh D. A. (2011) αV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 1, a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Senetar M. A., McCann R. O. (2005) Gene duplication and functional divergence during evolution of the cytoskeletal linker protein talin. Gene 362, 141–152 [DOI] [PubMed] [Google Scholar]
- 28. Tsai W. B., Zhang X., Sharma D., Wu W., Kinsey W. H. (2005) Role of Yes kinase during early zebrafish development. Dev. Biol. 277, 129–141 [DOI] [PubMed] [Google Scholar]
- 29. Pluskota E., Dowling J. J., Gordon N., Golden J. A., Szpak D., West X. Z., Nestor C., Ma Y. Q., Bialkowska K., Byzova T., Plow E. F. (2011) The integrin coactivator kindlin-2 plays a critical role in angiogenesis in mice and zebrafish. Blood 117, 4978–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calderwood D. A., Yan B., de Pereda J. M., Alvarez B. G., Fujioka Y., Liddington R. C., Ginsberg M. H. (2002) The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277, 21749–21758 [DOI] [PubMed] [Google Scholar]
- 31. García-Alvarez B., de Pereda J. M., Calderwood D. A., Ulmer T. S., Critchley D., Campbell I. D., Ginsberg M. H., Liddington R. C. (2003) Structural determinants of integrin recognition by talin. Mol. Cell 11, 49–58 [DOI] [PubMed] [Google Scholar]
- 32. Bledzka K., Bialkowska K., Nie H., Qin J., Byzova T., Wu C., Plow E. F., Ma Y. Q. (2010) Tyrosine phosphorylation of integrin β3 regulates kindlin-2 binding and integrin activation. J. Biol. Chem. 285, 30370–30374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lapidus L. J., Steinbach P. T., Eaton W. A., Szabo A., Hofrichter J. (2002) Effects of chain stiffness on the dynamics of loop formation in polypeptides. Appendix: Testing a 1-dimensional diffusion model for peptide dynamics. J. Phys. Chem. B 106, 11628–11640 [Google Scholar]
- 34. Daniels D. L., Cohen A. R., Anderson J. M., Brünger A. T. (1998) Crystal structure of the hCASK PDZ domain reveals the structural basis of class II PDZ domain target recognition. Nat. Struct. Biol. 5, 317–325 [DOI] [PubMed] [Google Scholar]
- 35. Doyle D. A., Lee A., Lewis J., Kim E., Sheng M., MacKinnon R. (1996) Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell 85, 1067–1076 [DOI] [PubMed] [Google Scholar]
- 36. Skelton N. J., Koehler M. F., Zobel K., Wong W. L., Yeh S., Pisabarro M. T., Yin J. P., Lasky L. A., Sidhu S. S. (2003) Origins of PDZ domain ligand specificity. Structure determination and mutagenesis of the Erbin PDZ domain. J. Biol. Chem. 278, 7645–7654 [DOI] [PubMed] [Google Scholar]
- 37. Wegener K. L., Partridge A. W., Han J., Pickford A. R., Liddington R. C., Ginsberg M. H., Campbell I. D. (2007) Structural basis of integrin activation by talin. Cell 128, 171–182 [DOI] [PubMed] [Google Scholar]
- 38. Luck K., Charbonnier S., Travé G. (2012) The emerging contribution of sequence context to the specificity of protein interactions mediated by PDZ domains. FEBS Lett. 586, 2648–2661 [DOI] [PubMed] [Google Scholar]
- 39. Bandyopadhyay A., Rothschild G., Kim S., Calderwood D. A., Raghavan S. (2012) Functional differences between kindlin-1 and kindlin-2 in keratinocytes. J. Cell Sci. 125, 2172–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]