Background: IRS1/2 is a critical component of insulin signaling, but it remains unclear whether IRS1/2 functions on Wnt signaling.
Results: IRS1/2 interacts with and stabilizes Dvl2 by suppressing its autophagic degradation, leading to promotion of Wnt-mediated EMT and cell proliferation.
Conclusion: IRS1/2 positively regulates Wnt/β-catenin signaling through Dvl2.
Significance: The IRS1/2-Dvl2 node might be implicated in tumorigenesis and metastasis.
Keywords: Autophagy, β-Catenin, Cell Proliferation, EMT, Wnt Signaling, Dishevelled2, IRS
Abstract
Wnt signaling plays a pivotal role in cell proliferation, tissue homeostasis, and tumorigenesis. Dishevelled (Dvl) is a central node of Wnt signaling. Insulin receptor substrates (IRSs), as a critical component of insulin signaling, are involved in cell proliferation, metabolism, and cancer development. In this study, we report that IRS1/2 promotes Wnt/β-catenin signaling by stabilizing Dvl2. We found that IRS1/2 interacts with Dvl2. Overexpression of IRS1/2 increased the protein level of Dvl2 and promoted canonical Wnt signaling, as evidenced by the increased T cell-specific factor 4 transcriptional activity and the up-regulation of expression of CYCLIN D1 and c-MYC, two Wnt target genes critical for cell growth, whereas depletion of IRS1/2 reduced the level of Dvl2 and attenuated Wnt/β-catenin signaling. Biochemical analyses revealed that IRS1/2 decreased Lys-63-linked ubiquitination of Dvl2 and stabilized Dvl2 protein via suppressing its autophagy-mediated degradation. We further revealed that IRS1/2 blocks autophagy-induced formation of the Dvl2-p62/SQSTM1 complex, resulting in disabled association of Dvl2 to autophagosomes. We demonstrated that IRS1/2 promoted the induction of epithelial-mesenchymal transition (EMT) and cell proliferation in response to Wnt stimulation, whereas depletion of Dvl2 impaired the IRS1/2-mediated EMT and cell growth. Our findings revealed that IRS1/2 promotes EMT and cell proliferation through stabilizing Dvl2.
Introduction
Wnt/β-catenin signaling transduces from the activation of Dishevelled proteins (Dvls)3 by receptor Frizzled and low density lipoprotein receptor-related protein 5/6 co-receptor after Wnt ligand binding. Dvls mediate the association of the receptors and a destruction complex containing adenomatous polyposiscoli (APC)-Axin-GSK3β-CK1, resulting in the inactivation of β-catenin degradation and leading to the accumulation and translocation of β-catenin into the nucleus, where β-catenin interacts with T cell-specific factor/LEF to initiate the transcription of Wnt target genes (1, 2).
Dvl2, a cytoplasm-nucleus shuttling protein (3), has been demonstrated to be a hub in Wnt signaling (4); therefore, its stability is tightly regulated by a variety of factors. The ubiquitin-proteasome and autophagy-lysosomal pathways are known to regulate the stability of Dvl2 (5–10). Our group showed that GABARAPL1, a member of the ATG8 family, binds to Dvl2 and promotes its degradation through autophagy (5). It has been demonstrated that autophagy-mediated degradation of Dvl2 was through a complex formed with p62/SQSTM1 and LC3 after ubiquitination of Dvl2 by pVHL (6). Interestingly, Malin was reported to enhance both Lys-48- and Lys-63-linked ubiquitination of Dvl2, resulting in its degradation through both the ubiquitin-proteasome and the autophagy-lysosomal pathways (7).
Insulin receptor substrates (IRSs), as adaptor proteins to mediate insulin-like growth factor I/insulin signaling, are involved in various biological processes including diabetes, metastasis, and adipocyte and bone differentiation (11–17). IRSs, activated by insulin/insulin-like growth factor I receptors, recruit intracellular proteins containing Src homology-2 domains and lead to transduction of intracellular cascades, such as the activation of the PI3K-AKT and Ras-MAPK pathways (12). Recent studies demonstrated that overexpression of IRS1/2 promoted mammary tumorigenesis and metastasis, and constitutive activation of IRS1 was found in a variety of solid tumors, including breast cancers, leiomyomas, Wilms tumors, leiomyosarcomas, and adrenal cortical carcinomas (13, 15, 18–21). In addition, down-regulation of IRS1 by microRNA-145 was shown to inhibit the growth of colon cancer cells (17), and IRS2 was reported to function as a positive regulator for tumor metastasis because knock-out of IRS2 blocked mammary tumor metastasis (18, 21).
IRS1/2 has been identified as a major player in the regulation of cell proliferation and EMT, a feature characterized by the loss of epithelial markers such as E-cadherin and acquisition of mesenchymal markers such as N-cadherin (22). It appeared that IRS1/2 regulates the expression of E-cadherin during EMT (14, 16) and CyclinD1 during cell growth (20). However, Wnt/β-catenin signaling has been reported to regulate various biological processes including embryonic development, tissues regeneration, and tumorigenesis (1, 2). Wnt/β-catenin signaling plays an important role in regulation of cell proliferation (by regulating the expression of cell cycle-related genes including CyclinD1 and c-Myc) and EMT (by up-regulating N-cadherin and down-regulating E-cadherin) (23–26). Here we demonstrate that IRS1/2 interacts with Dvl2 and suppresses its degradation mediated by autophagy, leading to the activation of Wnt/β-catenin signaling. IRS1/2 also positively regulates EMT and cell proliferation in response to Wnt signals and depletion of Dvl2 impairs the IRS1/2-mediated EMT and cell proliferation.
EXPERIMENTAL PROCEDURES
Plasmids and RNA Interference
The pcDNA4/Myc-IRS1, pcDNA4/Myc-IRS2, pLVX-IRS1, and pLVX-IRS2 plasmids were constructed by inserting PCR-amplified fragments encoding IRS1 or IRS2 into the indicated vectors. FLAG-β-catenin, FLAG-Dvl2, GFP-LC3, GFP-p62, and pGL3/LEF-1-Luc plasmids were kept in our laboratory. shRNAs for depletion of IRS1, IRS2, and Dvl2 were synthesized as two single-strand fragments: 5-TGTCAGTCTGTCGTCCAGTATTCAAGAGATACTGGACGACAGACTGACTTTTTTC-3 and 5-TCGAGAAAAAAGTCAGTCTGTCGTCCAGTATCTCTTGAATACTGGACGACAGACTGACA-3 (targeting human IRS1); 5-TGGTGACGCTGCAGCTCATGATTCAAGAGATCATGAGCTGCAGCGTCACCTTTTTTC-3 and 5-TCGAGAAAAAAGGTGACGCTGCAGCTCATGATCTCTTGAATCATGAGCTGCAGCGTCACCA-3 (targeting human IRS2); 5-TAACTTTGAGAACATGAGCAACTTCAAGAGAGTTGCTCATGTTCTCAAAGTTTTTTTTC-3 and 5-TCGAGAAAAAAAACTTTGAGAACATGAGCAACTCTCTTGAAGTTGCTCATGTTCTCAAAGTTA-3 (targeting human Dvl2); 5-TACAAGACCTAAGTGCACTGTTCAAGAGACAGTGCACTTAGGTCTTGTTTTTTTC-3 and 5-TCGAGAAAAAAACAAGACCTAAGGCACTGTCTCTTGAACAGTGCACTTAGGTCTTGTA-3 (NS, nonspecific target sequence as a control), inserted into HpaI-XhoI sites of the pLL3.7 vector.
Reagents and Antibodies
Bafilomycin A1, Earle's balanced salt solution, cycloheximide, and rapamycin were purchased from Sigma. LY294002 was from Cell Signaling Technology. Antibody against β-actin was purchased from Sigma. Anti-Myc (9E10), anti-IRS1, anti-IRS2, anti-β-catenin, β-tubulin, c-Jun, and anti-HA (F-7) antibodies were from Santa Cruz Biotechnology. Antibodies against E-cadherin, N-cadherin, and anti-Dvl2 were purchased from Cell Signaling Technology. Fluorescent secondary antibodies (goat anti-rabbit IgG and goat anti-mouse IgG) were purchased from Jackson ImmunoResearch Laboratories.
Cell Culture, Transfection, Luciferase Assay, and Immunofluorescent Analysis
HEK293T, HEK293, MCF-7, and HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin (100 units/ml)/streptomycin (100 units/ml). All cells were grown in atmosphere at 37 °C with 5% CO2. Cells were transfected with plasmids as indicated using Vigofect (Vigorous Inc., Beijing, China), according to the manufacturer's instructions. Lentivirus was generated using pLVX and pLL3.7 vectors to establish stable cell lines to stably overexpress the indicated genes in 293, MCF-7, and HeLa cells through screening the GFP-positive cells by FACS. Luciferase and immunofluorescent assays were performed as described previously (27).
Co-immunoprecipitation (Co-IP) and Western Blotting (WB)
For the Co-IP assay, HEK293T cells were plated in a 60-mm dish transfected with the indicated plasmids. After a 24–48-h transfection, cells were incubated with 800 μl of cell lysis buffer (80 mmol/liter KCl, 10 mmol/liter Na2HPO4, 1 mmol/liter EDTA (pH 8.0), 0.5% Nonidet P-40, 10% glycerol, 1 mmol/liter DTT, 0.1 mmol/liter Na3VO4, 1 mmol/liter phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin). 600 μl of whole cell lysates was incubated with 1–2 μg of the indicated antibody and 30 μl of protein G/A-Sepharose beads at 4 °C overnight. The beads were washed four times with cell lysis buffer, and precipitates were eluted with 2× SDS-PAGE sample buffer and analyzed by WB with the indicated antibody.
Quantitative Real-time RT-PCR and Autophagy Analysis
Total RNA was prepared with TRIzol (Invitrogen), and cDNA was synthesized with Quant cDNA (Tiangen). Quantitative real-time RT-PCR was performed with the SYBR Green detection method with Mx3000p Quantitative PCR system (Stratagene). The primers used were synthesized as: human β-actin, 5-GTACCACTGGCATCGTGATGGACT-3 and 5-CCGCTCATTGCCAATGGTGAT-3; human Dvl2, 5-GCTTCCACATGGCCATGGGC-3 and 5-TGGCACTGCTGGTGAGAGTCACAG-3. For autophagy induction, cells were washed with PBS three times and then starved with 1× Earle's balanced salt solution for 4–6 h or incubated in completed medium containing 2 μm rapamycin. To block starvation-induced autophagy, cells were treated with 1× Earle's balanced salt solution containing 0.1 μm bafilomycin A1, which blocks the function of lysosome.
Cell Proliferation Assay and Wound Healing Assay
For the cell proliferation assay, 1 × 103 cells were seeded in triplicate in plates, and cell numbers were counted every day over a 6-day period (28). For the wound healing assay, monolayer cells were wounded with a sterile plastic tip. Cell migration was observed by microscopy 36 h later.
Statistic Analysis
The relative protein levels measured by WB were quantified by ImageJ software. Data are presented as means ± S.D. from three independent experiments. Significance between groups was assessed by a Student's t test, and p < 0.05 was considered as significant.
RESULTS
IRS1/2 Interacts with Dvl2
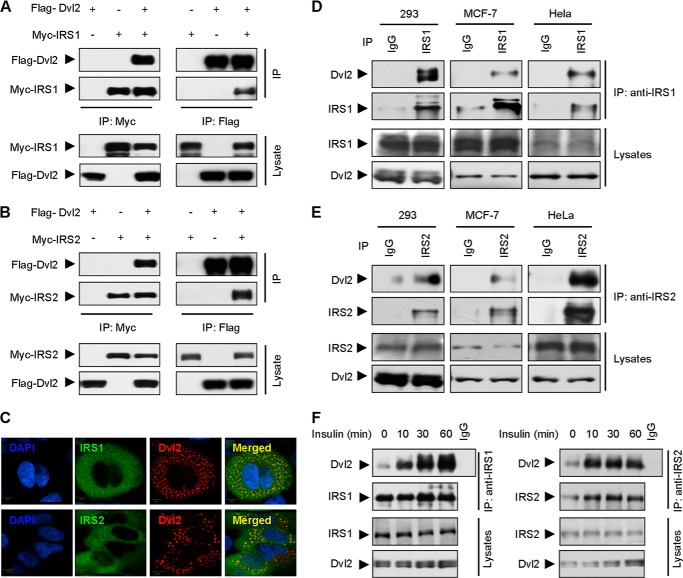
Dvl2 is a hub of Wnt signaling to transduce the Wnt signaling cascades (1, 2, 4). To address whether IRS1/2 is involved in the canonical Wnt signaling pathway, we performed a Co-IP experiment to examine the ability of IRS1/2 and Dvl2 to form a complex, Myc-IRS1 or Myc-IRS2 was co-expressed with FLAG-Dvl2 in HEK293T cells. The cell extracts were immunoprecipitated with an anti-Myc antibody and immunoblotted with an anti-FLAG antibody to determine the presence of FLAG-Dvl2. We detected FLAG-Dvl2 in the immunoprecipitates of cells expressing both Myc-IRS and FLAG-Dvl2, but not in the cells expressing either FLAG-Dvl2 or Myc-IRS alone (Fig. 1, A and B, left panels). Reciprocally, FLAG-Dvl2 precipitated down Myc-IRS1/2 using an anti-FLAG antibody (Fig. 1, A and B, right panels). We next examined whether IRS1/2 co-localizes with Dvl2. MCF-7 cells were transiently transfected with plasmids encoding IRS1 or IRS2 along with Dvl2. Previous studies demonstrated that ectopic expression of Dvl2 was mainly localized in cytoplasmic puncta (6, 7). Consistently, immunofluorescence assay revealed that IRS1/2 mainly co-localized with cytoplasm puncta of Dvl2 (Fig. 1C). These data indicated that IRS1/2 interacts with Dvl2.
FIGURE 1.
IRS1/2 interacts with Dvl2. A and B, Dvl2 interacts with IRS1 or IRS2. Cell lysates from 293T cells transfected with FLAG-Dvl2 and Myc-IRS1 (A) or IRS2 (B) were immunoprecipitated with an anti-Myc antibody or an anti-FLAG antibody. The immunoprecipitates were analyzed by WB. The total proteins used for IP were adjusted to equal levels of FLAG-Dvl2 in each experiment. C, IRS1/2 co-localizes with Dvl2. MCF-7 cells were transfected with FLAG-Dvl2 and Myc-IRS1 or Myc-IRS2, and stained with an anti-Myc (green) and anti-FLAG (red) antibody. D and E, IRS1/2 interacts with Dvl2 in vivo. IP assays were performed by an anti-IRS1 or anti-IRS2 antibody using 293, MCF-7, or HeLa cells. Endogenous proteins were analyzed for both immunoprecipitates and cell lysates. F, interaction of IRS1/2 and Dvl2 is regulated by insulin. The interaction of endogenous IRS1/2 and Dvl2 was demonstrated by IP assays in 293 cells stimulated with insulin (1 μm) for different times.
To further address whether the interaction of IRS1/2 with Dvl2 occurs under physiological conditions, we examined the interaction of endogenous IRS1/2 and Dvl2 in cells. The IP experiments with an antibody against IRS1 showed that IRS1 formed a complex with Dvl2 in 293, MCF-7, and HeLa cells (Fig. 1D). Similarly, IRS2 precipitated down Dvl2 in the different cells using an anti-IRS2 antibody (Fig. 1E). These results firmly suggested that IRS1/2 specifically interacts with Dvl2 under physiological conditions. To examine whether the interaction between IRS1/2 and Dvl2 could be regulated by insulin stimulation, we performed IP experiments using 293 cells treated with insulin for different times; the results demonstrated that insulin enhanced the association of IRS1 (Fig. 1F, left panels) or IRS2 (Fig. 1F, right panels) with Dvl2 in a time-dependent manner. These results confirmed that IRS1/2 and Dvl2 interact with each other in mammalian cells.
IRS1/2 Stabilizes Dvl2
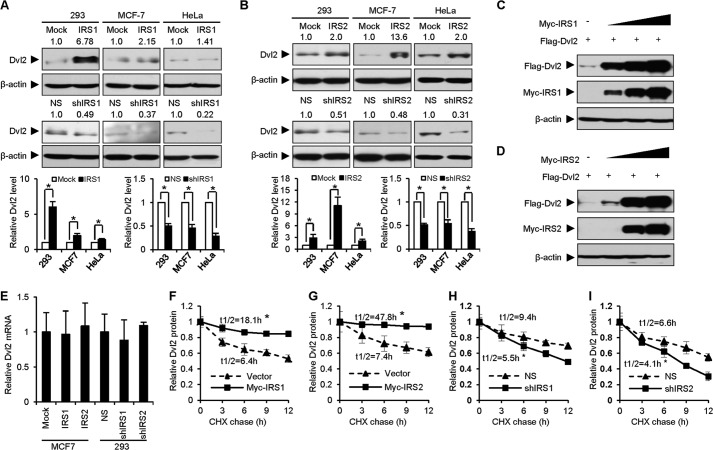
Based on the result that IRS1/2 interacts and co-localizes with Dvl2, we further examined the effect of IRS1/2 on Dvl2 and Wnt/β-catenin signaling. To study the role of IRS1/2 on Dvl2 protein, we generated stable cell lines overexpressing IRS1/2 or an shRNA against endogenous IRS1/2 in 293, HeLa, and MCF-7 cells. We observed that the protein level of Dvl2 increased when IRS1 was stably overexpressed (Fig. 2A, upper panels) but decreased when IRS1 was stably depleted (Fig. 2A, bottom panels). Similar results were observed when IRS2 was overexpressed or depleted in the same cells (Fig. 2B). Quantitative analyses from three independent repeats validated the significant role of IRS1/2 on Dvl2 protein (bottom graphs in Fig. 2, A and B). The protein level of Dvl2 increased in a dose-dependent manner when IRS1 (Fig. 2C) or IRS2 (Fig. 2D) was overexpressed in 293T cells. These results suggested that IRS1/2 regulates the protein level of Dvl2. Because the mRNA level of Dvl2 was not changed by IRS1/2 (Fig. 2E), we speculated that IRS1/2 might regulate the stability of Dvl2 protein.
FIGURE 2.
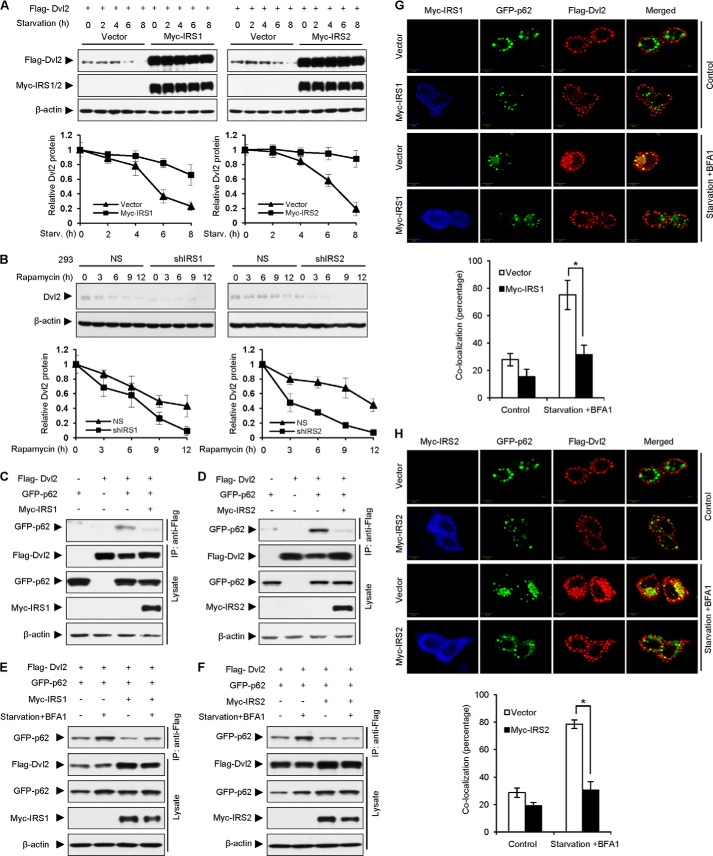
IRS1/2 stabilizes Dvl2. A and B, IRS1/2 regulates the protein level of Dvl2. IRS1 (A) or IRS2 (B) was stably overexpressed (upper panels) or depleted (bottom panels) in 293, MCF-7, and HeLa cells. NS, nonspecific shRNA; shIRS1 or shIRS2, shRNA against IRS1 or IRS2. Dvl2 protein levels were examined by WB and quantified using ImageJ software (bottom graphs). Data are presented as means ± S.D. (error bars). *, p < 0.05. C and D, IRS1/2 increases Dvl2 protein level in a dose-dependent manner. 293T cells were transfected with FLAG-Dvl2 and increasing amounts of Myc-IRS1 (C) or Myc-IRS2 (D). E, overexpression of IRS1/2 or depletion of IRS1/2 has no effect on the Dvl2 mRNA level. Total RNA was extracted from MCF-7 cells stably expressing IRS1/2, or 293 cells stably expressing an shRNA against IRS1/2, and subjected to a quantitative real-time RT-PCR analysis. Results are shown as means ± S.D. (n = 3). F–I, IRS1/2 influences the degradation rate of Dvl2 protein. 293T cells overexpressing Myc-IRS1 (F) or Myc-IRS2 (G) and 293 cells stably expressing an shRNA against IRS1 (H) or IRS2 (I) were treated with 25 μg/ml cycloheximide (CHX) for different times as indicated. Quantified results were obtained from three independent experiments. The half-life was calculated using t½ = −0.693/ke where ke is the regression slope of the Dvl2 level to times. Data are presented as means ± S.D. *, p < 0.05.
To examine whether IRS1/2 regulates Dvl2 protein turnover, we performed cycloheximide assays using 293T cells (where IRS1/2 was weakly expressed) transfected with FLAG-Dvl2 and Myc-IRS1/2 and 293 cells (where IRS1/2 was abundant) stably expressing an shRNA against IRS1/2. Cells were treated with cycloheximide for different times, which blocks new protein synthesis. The results showed that overexpression of IRS1 (Fig. 2F) or IRS2 (Fig. 2G) in 293T cells enhanced, whereas depletion of IRS1 (Fig. 2H) or IRS2 (Fig. 2I) in 293 cells reduced, the half-life of Dvl2 compared with the controls. These results suggested that IRS1/2 stabilizes the Dvl2 protein.
IRS1/2 Positively Regulates Wnt/β-Catenin Signaling
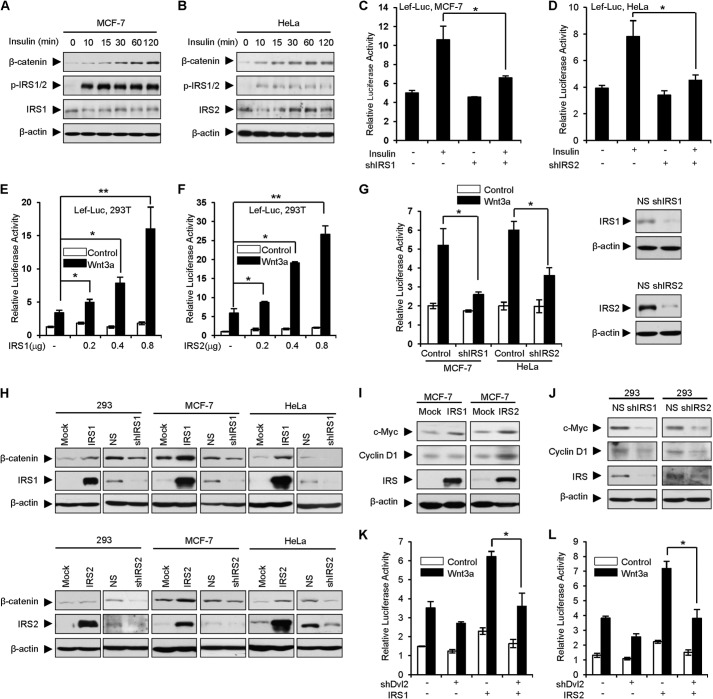
To explore the role of IRS1/2 on Wnt/β-catenin signaling, we first examined the effect of insulin on Wnt/β-catenin signaling using MCF-7 cells (with high endogenous levels of IRS1 and low levels of IRS2) and HeLa cells (which highly express IRS2 and weakly express IRS1). The results revealed that insulin treatment increased β-catenin levels in a time-dependent manner (Fig. 3, A and B). Because insulin initiates signal transduction through activation of IRSs, we examined whether IRS1/2 is required for insulin-stimulated Wnt/β-catenin signaling. A luciferase assay was performed using MCF-7 and HeLa cells which were treated with insulin and transfected with LEF-Luc, a reporter responding to canonical Wnt signaling. The result showed that depletion of IRS1 resulted in impaired activation of Wnt/β-catenin signaling whereas insulin enhanced Wnt stimulation in the control MCF-7 cells (Fig. 3C). Similar results were observed in HeLa cells when IRS2 was depleted (Fig. 3D). These results suggested that IRS1/2 may be involved in regulation of Wnt/β-catenin signaling. To determine whether IRS1/2 modulates Wnt/β-catenin signaling, we performed luciferase assays using HEK293T cells which were transiently transfected with Myc-IRS1 or IRS2 together with LEF-Luc. Overexpression of Myc-IRS1 (Fig. 3E) or Myc-IRS2 (Fig. 3F) dose-dependently enhanced the transcriptional activity of the luciferase reporter activated by Wnt3a, suggesting that IRS1/2 enhances Wnt/β-catenin signaling. To examine whether endogenous IRS1/2 regulates Wnt/β-catenin signaling, we depleted IRS1 in MCF-7 cells (where endogenous IRS1 remains at a high level) or IRS2 in HeLa cells (where the endogenous IRS2 level is high) using an shRNA against IRS1 or IRS2. The luciferase assay demonstrated that depletion of IRS1 or IRS2 impaired activation of Wnt/β-catenin signaling (Fig. 3G). These results suggested that IRS1/2 positively regulates Wnt/β-catenin signaling.
FIGURE 3.
IRS1/2 positively regulates Wnt/β-catenin signaling. A and B, insulin increases β-catenin protein levels. MCF-7 (A) or HeLa (B) cells were treated with insulin (1 μm) for the indicated times and harvested for WB. p-IRS1/2 was used as an indicator of insulin signaling activation. C and D, depletion of IRS1 or IRS2 impairs insulin-induced Wnt/β-catenin signaling. Luciferase assays were performed using MCF-7 (C) and HeLa (D) cells transfected with LEF-Luc, LEF-1, pRL-TK Renilla, and an shRNA against IRS1 (C) or IRS2 (D). Relative luciferase activities were normalized to Renilla and are presented as means ± S.D. (error bars) from three independent experiments. *, p < 0.05. E and F, IRS1 or IRS2 enhances Wnt3a-mediated transcriptional activity in a dose-dependent manner. Luciferase assays were performed using 293T cells transfected with LEF-Luc, LEF-1, pRL-TK, and increasing amounts of IRS1 (E) or IRS2 (F). Wnt3a conditioned medium was used to activate the luciferase activity. Data are presented as means ± S.D. *, p < 0.05; **, p < 0.01. G, depletion of IRS1 or IRS2 suppresses the Wnt/β-catenin signaling. IRS1 or IRS2 was depleted by an shRNA in MCF-7 or HeLa cells treated or not with Wnt3a conditional medium. Luciferase assay was performed as in E and F. NS, nonspecific shRNA as a control; the efficiency of shRNA against IRS1 or IRS2 was examined by WB (right panels). H, overexpression of IRS1/2 increases, depletion of IRS1/2 reduces, the protein levels of β-catenin. IRS1/2 was stably overexpressed or depleted in the 293, MCF-7, and HeLa cells. β-Catenin protein levels were examined by WB. I and J, IRS1/2 regulates the downstream targets of Wnt/β-catenin signaling. The protein levels of Wnt signaling targeted genes including c-Myc and CyclinD1 were examined. K and L, depletion of Dvl2 abolishes the role of IRS1/2 on Wnt/β-catenin signaling. Luciferase assays were performed using MCF-7 cells transfected with LEF-Luc, LEF-1, pRL-TK, Myc-IRS1 (K), or Myc-IRS2 (L) along with an shRNA against Dvl2 (shDvl2). Data are presented as means ± S.D. *, p < 0.05.
To further confirm the activation of Wnt/β-catenin signaling by IRS1/2, we examined the protein level of β-catenin, which accumulates when Wnt triggers signal transduction (1, 2). The result indicated that overexpression of IRS1 resulted in an enhanced level of β-catenin, whereas depletion of IRS1 decreased the level of β-catenin (Fig. 3H, upper panels). Overexpression or depletion of IRS2 has a similar effect on β-catenin levels (Fig. 3H, bottom panels). Consistently, the endogenous levels of two downstream target genes (c-Myc and CyclinD1) were increased in MCF-7 cells stably expressing IRS1/2 (Fig. 3I), whereas depletion of IRS1/2 reduced the expression levels of c-Myc and CyclinD1 using 293 cells which highly express IRS1 and IRS2 (Fig. 3J).
To address whether IRS1/2 regulates Wnt/β-catenin signaling through Dvl2, we depleted endogenous Dvl2 by an shRNA. The luciferase assay showed that depletion of Dvl2 abolished the role of IRS1/2 on Wnt/β-catenin signaling (Fig. 3, K and L). These data, together with Fig. 2, suggested that IRS1/2 positively regulates Wnt/β-catenin signaling through Dvl2.
IRS1/2 Suppresses Degradation of Dvl2 Mediated by Autophagy
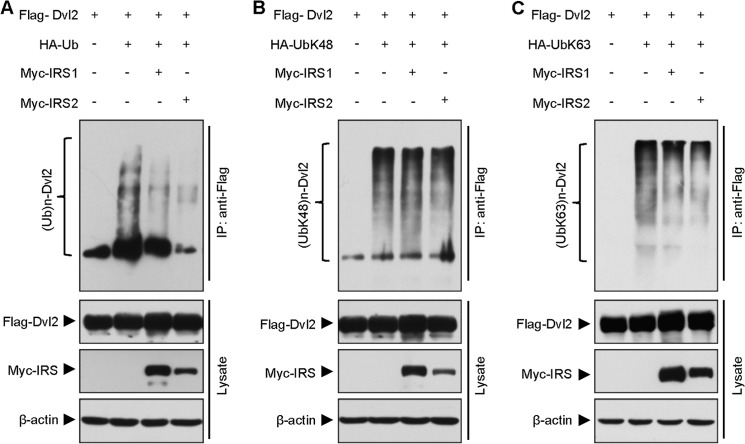
Dvl2 has been shown to be regulated by proteasome and autophagy (6, 7, 10). To explore the mechanism by which IRS1/2 enhances Dvl2 stability and canonical Wnt signaling, we first determined whether IRS1/2 regulates ubiquitination of Dvl2. An IP result showed that overexpression of either Myc-IRS1 or Myc-IRS2 significantly inhibited polyubiquitination of FLAG-Dvl2 protein (Fig. 4A), suggesting that IRS1/2 regulates ubiquitination of Dvl2. In general, polyubiquitination occurs in a manner of Lys-48-linked or Lys-63-linked polyubiquitin chain formation in regulation of different cellular functions (29–31). To better understand the role of IRS1/2 in the regulation of Dvl2 ubiquitination, we examined both Lys-48- and Lys-63-linked polyubiquitin chains on the Dvl2 protein. The IP analyses indicated that neither Myc-IRS1 nor Myc-IRS2 affected Lys-48-linked ubiquitination on the FLAG-Dvl2 protein (Fig. 4B). Interestingly, overexpression of Myc-IRS1/2 significantly reduced Lys-63-linked ubiquitination on the FLAG-Dvl2 protein (Fig. 4C). These results suggested that the inhibitory role of IRS1/2 on polyubiquitination is mainly on the Lys-63-linked ubiquitin chain formation.
FIGURE 4.
IRS1/2 regulates the ubiquitination of Dvl2. A, IRS1 or IRS2 reduces the polyubiquitination of Dvl2. 293T cells were transfected with HA-ubiquitin (HA-Ub), FLAG-Dvl2, Myc-IRS1, or Myc-IRS2, and cell lysates were immunoprecipitated with an anti-FLAG antibody. The immuno-complexes were subjected to an WB with an anti-HA antibody to show the HA-Ub-conjugated Dvl2 proteins. B, IRS1 or IRS2 has no effect on Dvl2 Lys-48-linked polyubiquitination. HA-UbLys-48 was co-expressed with the indicated plasmids, and Lys-48Ub-conjugated Dvl2 proteins are shown. C, IRS1 or IRS2 attenuates Dvl2 Lys-63-linked polyubiquitination. HA-UbLys-63 was used to demonstrate the Lys-63Ub-conjugated Dvl2 proteins.
Lys-63-linked polyubiquitination boosts protein degradation through the autophagy pathway whereas Lys-48-linked polyubiquitination initiates protein degradation through the proteasome pathway (30, 32). Our results that IRS1/2 reduced Lys-63-linked polyubiquitination of Dvl2 promoted us to examine whether IRS1/2 affects the autophagy-mediated degradation of Dvl2. To test this hypothesis, we examined the level of Dvl2 under starvation, which induces the occurrence of autophagy. The result showed that the Dvl2 protein level was decreased upon autophagy induction by nutrient deprivation, whereas overexpression of Myc-IRS1 (Fig. 5A, left panels) or Myc-IRS2 (Fig. 5A, right panels) dramatically restored the level of Dvl2 protein. Quantitative analyses from three independent experiments indicated that overexpression of Myc-IRS1/2 prolonged the half-life of FLAG-Dvl2 (Fig. 5A, bottom graphs). In contrast, the protein level of Dvl2 was significantly decreased when IRS1/2 was stably depleted in 293 cells maintained with rapamycin which induced autophagy (Fig. 5B). These data suggested that IRS1/2 reverses the role of autophagy in promoting Dvl2 degradation.
FIGURE 5.
IRS1/2 suppresses Dvl2 degradation mediated by the autophagic pathway. A, overexpression of IRS1/2 blocks Dvl2 degradation mediated by autophagy. Cells were subjected to starvation for the indicated durations and prepared for WB. Quantitative results (bottom graphs) are from three independent experiments and presented as means ± S.D. (error bars). B, depletion of IRS1/2 promotes Dvl2 degradation induced by rapamycin. 293 cells stably expressing an shRNA against IRS1 (shIRS1) or IRS2 (shIRS2) were incubated with rapamycin (2 μm) to induce autophagy for different times. Data are presented as means ± S.D. C and D, IRS1/2 induces the dissociation of Dvl2 from p62. Cell lysates from 293T cells transfected with FLAG-Dvl2, GFP-p62, and Myc-IRS1 (C) or Myc-IRS2 (D) were immunoprecipitated with an anti-FLAG antibody. Immunoprecipitates and cell lysates were analyzed by WB. E and F, IRS1/2 reduces the interaction between Dvl2 and p62 under autophagy. Cells transfected with GFP-p62, FLAG-Dvl2, and Myc-IRS1 (E) or Myc-IRS2 (F) were treated with nutrient-rich medium or under starvation and addition of BFA1, an inhibitor of autophagy, for 4 h. Cell lysates were immunoprecipitated with an anti-FLAG antibody and examined by an WB. G and H, IRS1/2 blocks the co-localization of Dvl2 and p62 during autophagy. Immunostaining assays were performed in HeLa cells transfected with Myc-IRS1 (G) or Myc-IRS2 (H), FLAG-Dvl2 and GFP-p62. Cells were maintained in nutrient-rich medium or under starvation condition and with addition of BFA1 for 4 h, and stained with an anti-Myc antibody (blue) and an anti-FLAG (red) antibody. The percentage of dots with co-localized FLAG-Dvl2 and GFP-p62 was calculated using ImageJ software from at least 50 cells randomly selected from the immunostaining slides. Results are presented as means ± S.D. *, p < 0.05.
Recent studies demonstrated that p62/SQSTM1 recruits Dvl2 to autophagosomes for degradation (5, 6). To reveal whether IRS1/2 is involved in the regulation of the p62-Dvl2 interaction mediated by autophagy, we assessed the effect of IRS1/2 on the interaction between Dvl2 and p62. The results showed that FLAG-Dvl2 associated with GFP-p62 (Fig. 5C, third lane) in control cells and the interaction of GFP-p62 and FLAG-Dvl2 was significantly reduced when Myc-IRS1 was expressed (Fig. 5C, fourth lane). The interaction of FLAG-Dvl2 and GFP-p62 was also impaired by overexpression of Myc-IRS2 (Fig. 5D). These results suggested that IRS1/2 blocks the formation of the Dvl2-p62 complex.
To reveal the role of IRS1/2 on the interaction between Dvl2 and p62 during autophagy (6), we next examined whether IRS1/2 affects the autophagy-induced formation of the Dvl2-p62 complex. The results showed that overexpression of Myc-IRS1 (Fig. 5E) or Myc-IRS2 (Fig. 5F) reduced the formation of the Dvl2-p62 complex under the condition of starvation and BFA1 (an inhibitor of lysosome). The confocal microscopy experiments demonstrated that overexpression of Myc-IRS1 (Fig. 5G) or Myc-IRS2 (Fig. 5H) blocked the co-localization of GFP-p62 and FLAG-Dvl2 under the starvation treatment, whereas starvation significantly induced the co-localization of GFP-p62 and FLAG-Dvl2. Quantitative analyses confirmed the significance of the role of Myc-IRS1 (Fig. 5G, bottom graphs) or Myc-IRS2 (Fig. 5H, bottom graphs) on co-localized puncta of p62 and Dvl2. These results indicated that Dvl2 was localized to the autophagosomes under starvation and IRS1/2 blocked the interaction of Dvl2 and p62 during autophagy, leading to disabled localization of Dvl2 to autophagosomes and enhancement of Dvl2 stability. Taken together, these findings suggested that IRS1/2 stabilizes Dvl2 via inhibition of Dvl2-p62 association.
IRS1/2 Up-regulates EMT
During the study, we observed that the morphology of the cells was changed into a fibroblast-like phenotype when IRS1 or IRS2 was overexpressed (Fig. 6A), implying that IRS1/2 is involved in the regulation of EMT. To test this possibility, we examined the levels of E-cadherin, N-cadherin, and β-catenin, well established markers during EMT. The results showed that overexpression of IRS1/2 decreased the level of E-cadherin, a feature of epithelial cells, and increased the levels of N-cadherin and β-catenin, markers of mesenchymal cells (Fig. 6B). These results suggested that IRS1/2 promotes EMT.
FIGURE 6.
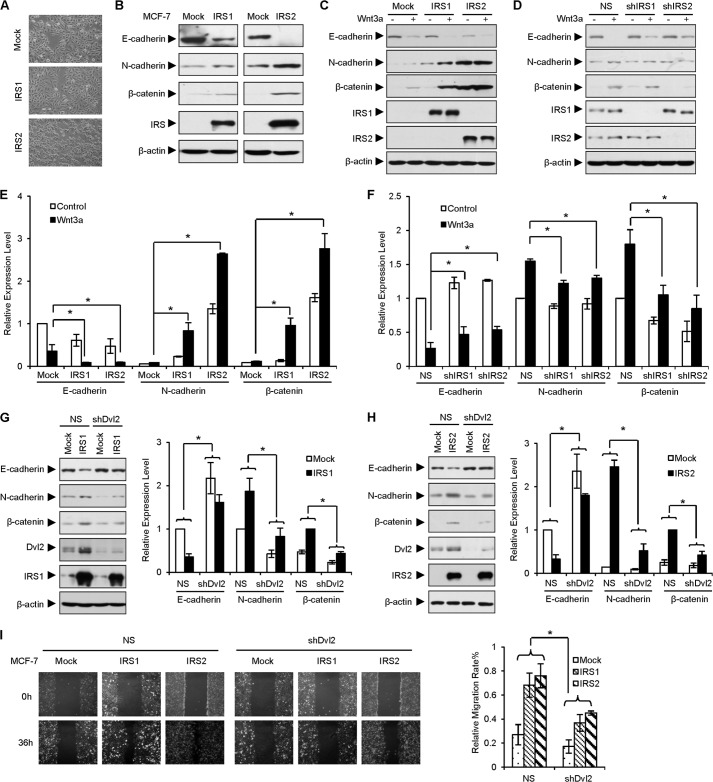
IRS1/2 up-regulates EMT. A, MCF-7 cells stably expressing IRS1/2 gain a fibroblast-like phenotype. Cell morphology was examined under a microscope. B, IRS1/2 regulates EMT. MCF-7 cells stably expressing IRS1 or IRS2 were cultured, and EMT-related gene expression was examined by WB. C–F, overexpression of IRS1/2 induces, but depletion of IRS1/2 reduces, EMT mediated by Wnt3a stimulation. MCF-7 cells stably expressing IRS1/2 (C) or an shRNA against IRS1/2 (D) were incubated with Wnt3a for 48 h. Quantitative analyses are from three independent experiments (E and F). Data are presented as means ± S.D. (error bars). *, p < 0.05. G and H, depletion of endogenous Dvl2 impairs the effect of IRS1/2 on the EMT. Cell lysates of MCF-7 stably expressing IRS1 (G) or IRS2 (H) were transfected with an shRNA against Dvl2. The changes of protein levels (right graphs) were quantified and are presented as means ± S.D. *, p < 0.05. NS, nonspecific shRNA as a control. I, depletion of Dvl2 impairs the effect of IRS1/2 on cell migration. Cells were seeded in triplicate and wounded with a sterile plastic tip. Cell migration was observed by microscopy 36 h later. Relative migration rates were measured by ImageJ and are presented as means ± S.D. *, p < 0.05.
We then investigated the role of IRS1/2 on EMT induced by Wnt/β-catenin signaling. The results demonstrated that Wnt3a reduced the expression of E-cadherin but strongly promoted the expression of N-cadherin in the presence of IRS1 or IRS2 (Fig. 6C). Consistently, we observed that the expression of E-cadherin was elevated and Wnt3a failed to induce the expression of N-cadherin when IRS1/2 was depleted by shRNA (Fig. 6D), suggesting that overexpression of IRS1/2 promoted, whereas depletion of IRS1/2 reduced, the process of EMT mediated by Wnt/β-catenin signaling. Quantitative analyses from triplicate experiments validated the significant role of IRS1/2 on Wnt3a-induced EMT (Fig. 6, E and F). Overall, these results indicated that IRS1/2 positively regulates EMT.
Because IRS1/2 increased the protein level of Dvl2, we questioned whether the elevated expression of N-cadherin induced by IRS1 or IRS2 was attributed to the enhancement of Dvl2 stability. To examine this hypothesis, we depleted Dvl2 by an shRNA and examined the levels of E-cadherin and N-cadherin. The results showed that IRS1/2 was efficient to promote the EMT induction featured by loss of E-cadherin and gain of N-cadherin and β-catenin. Depletion of Dvl2 decreased the level of N-cadherin and up-regulated the E-cadherin expression level. Importantly, depletion of Dvl2 impaired the IRS1/2-induced expression of N-cadherin and reversed the expression of E-cadherin inhibited by IRS1/2 (Fig. 6, G and H), suggesting that the role of IRS1/2 in regulation of EMT is dependent on Dvl2. EMT gives cell the ability of migration, we therefore examined the effect of IRS1/2 on cell migration, a wound healing assay showed that overexpression of IRS1/2 promoted the cell migration in MCF-7, but depletion of endogenous Dvl2 blocked the effect of IRS1/2 on cell migration (Fig. 6I). Taken together, these results suggested that IRS1/2 promotes the EMT process and depletion of Dvl2 inhibits the EMT induced by IRS1/2.
IRS1/2 Promotes Cell Proliferation
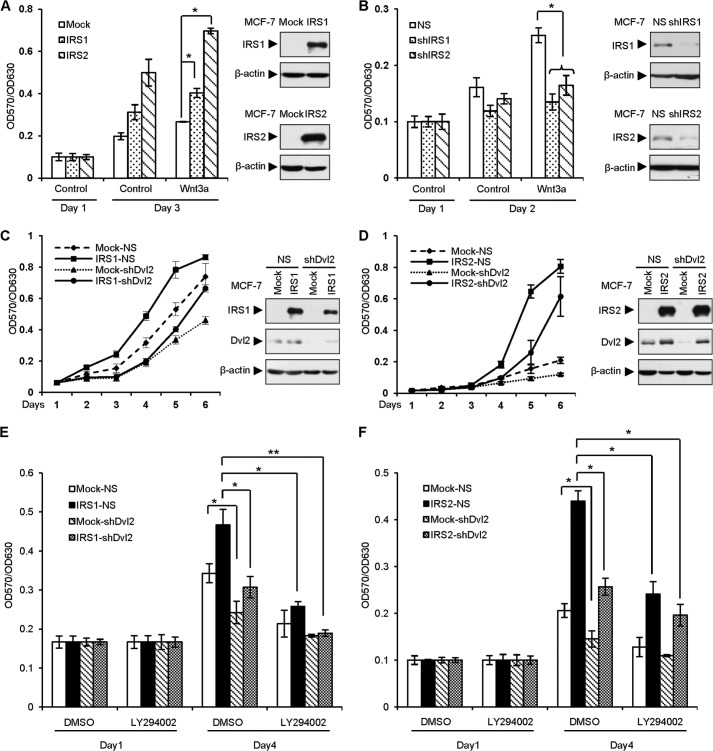
IRS1/2 and Wnt/β-catenin signaling are critical for cell growth. To examine the role of IRS1/2 on cell proliferation, we first evaluated the effect of IRS1/2 on cell proliferation mediated by Wnt/β-catenin signaling in MCF-7 cells stably overexpressing or depleting IRS1/2. The results showed that overexpression of IRS1/2 promoted Wnt3a-mediated cellular growth (Fig. 7A). Consistently, depletion of IRS1/2 attenuated Wnt3a-induced cell proliferation (Fig. 7B), suggesting that IRS1/2 is important for Wnt-induced cell proliferation. Based on the effect of IRS1/2 on the protein level of Dvl2, we speculated that Dvl2 may be critical for IRS1/2-mediated cell growth. We observed that depletion of endogenous Dvl2 dramatically reduced the cellular growth of MCF-7 cells mediated by IRS1/2 (Fig. 7, C and D), suggesting that Dvl2 is important for cell proliferation driven by IRS1/2.
FIGURE 7.
IRS1/2 promotes cell proliferation. A, overexpression of IRS1/2 promotes Wnt3a-induced cell proliferation. 1 × 103 MCF-7 cells stably expressing IRS1 or IRS2 were seeded in a 96-well plate. Cells were grown with or without Wnt3a conditioned medium. A570/A630 was measured to determine cell numbers. Data are presented as means ± S.D. (error bars). *, p < 0.05. The indicated proteins are shown by WB (right panels). B, depletion of IRS1/2 attenuates cell growth mediated by Wnt3a. MCF-7 cells stably expressing an shRNA against IRS1 or IRS2 were incubated with or without Wnt3a, and the numbers were determined by A570/A630. Data are presented as means ± S.D. NS, nonspecific shRNA as a control. The indicated proteins are shown by WB (right panels). C and D, depletion of Dvl2 impairs cell proliferation mediated by IRS1/2. Cell proliferation assay was performed using MCF-7 cells stably expressing IRS1 (C) or IRS2 (D) transfected with an shRNA against Dvl2. Data are presented as means ± S.D. The indicated proteins are shown by WB (right panels). E and F, IRS1/2-induced cell proliferation is impaired by depletion of Dvl2 with or without inhibition of PI3K/AKT signaling. Cell proliferation assays were performed using MCF-7 cells stably expressing IRS1/2 transfected with an shRNA against Dvl2. Cells were maintained with or without PI3K-specific inhibitor LY294002. Data are presented as means ± S.D. *, p < 0.05; **, p < 0.01.
To clarify the effect of IRS1/2 on Dvl2 in regulation of cell proliferation, we inhibited the PI3K/AKT signaling using PI3K-specific inhibitor LY294002 as IRS1/2 also functions through the PI3K/AKT signaling to promote cell proliferation (33). The results demonstrated that either Dvl2 silencing alone or LY294002 treatment alone inhibited cell proliferation (Fig. 7, E and F). Interestingly, depletion of Dvl2 and LY294002 treatment almost abolished the ability of cell growth (Fig. 7E, 3rd column in the last group), and IRS1 had no longer ability to recover cell growth when Dvl2 was depleted and LY294002 was added (Fig. 7E, last column). These results suggested that IRS1 promotes cell proliferation through Dvl2 beside the PI3K/AKT pathway. In the case of IRS2 overexpression, we observed a similar effect of depletion of Dvl2 and addition of LY294002 on the cell proliferation (Fig. 7F, 3rd column in the last group), and overexpression of IRS2 could not completely recover the effect of Dvl2 depletion and LY294002 addition (Fig. 7F, last column). These results suggested that both IRS1/2-Dvl2 and IRS1/2-PI3K/AKT, which are distinct signaling, are important for cell proliferation.
DISCUSSION
In this study, we identified IRS1/2 as a positive regulator to promote Wnt/β-catenin signaling. Intriguingly, we found that IRS1/2 interacts with Dvl2 and suppresses its autophagy-mediated degradation. The effect of IRS1/2 on Dvl2 stability contributes to the regulation of EMT and cell proliferation. Our findings reveal a novel molecular mechanism by which IRS1/2 regulates Wnt/β-catenin signaling via stabilizing Dvl2 to promote EMT and cell proliferation.
Dvl2 plays an critical role in Wnt/β-catenin signaling (4). Our study provided strong evidence that IRS1/2 interacts with Dvl2. In prevailing models, IRS1/2 functions mainly as an adaptor to transduce the signals. In presence of insulin ligand, IRS1/2 is recognized and phosphorylated by insulin receptor, then activated IRS1/2 recruits Src homology-2 domain-containing protein to mediate the signal transduction (12). In this study, we have observed that insulin promoted the interaction between IRS1/2 and Dvl2. However, we did not confirm which phosphorylation sites of IRS1/2 affect the interaction. This was because of a technical difficulty for generating mutation of IRS1/2 as it has >100 phosphorylation residues. Further studies are needed to elucidate the molecular mechanism underlying the functions of IRS1/2 on Dvl2, such as how Dvl2 is recruited by IRS1/2 and which IRS1/2 phosphorylation or other post-translation modifications affect the interaction with Dvl2 to activate Wnt/β-catenin signaling.
We, and colleagues, have shown that the Dvl2 stability is regulated through the ubiquitin-proteasome and autophagy-lysosomal pathways (5, 6, 8–10). In this study, we observed that IRS1/2 blocked the degradation of Dvl2 by inhibiting the ubiquitination of Dvl2. Our results showed that overexpression of IRS1/2 reduced the total ubiquitination of Dvl2, but not Lys-48-linked polyubiquitination. However, we observed that the level of Lys-63-linked polyubiquitination of Dvl2 was decreased by IRS1/2. In general, Lys-48-linked polyubiquitination promotes protein degradation via the proteasome pathway whereas Lys-63-linked polyubiquitination boosts protein degradation through the autophagy pathway (29–32). Therefore, we speculated that IRS1/2 might stabilize Dvl2 through inhibiting the autophagy pathway but not the proteasome pathway. Indeed, our data demonstrated that IRS1/2 stabilized the Dvl2 protein through blocking its degradation mediated by the autophagy pathway.
p62, a major protein that initiates autophagy-mediated protein turnover, is involved in Dvl2 degradation mediated by autophagy (5, 6, 34). In this study, we revealed that IRS1/2 blocked the interaction of Dvl2 with p62. However, it remains unclear whether the role of IRS1/2 on Lys-63-linked polyubiquitination of Dvl2 was through blocking the interaction with p62. In general, p62 is regarded as a key regulator for autophagy-induced protein degradation mediated by targeting ubiquitinated substrates to autophagosomes and Lys-63-linked polyubiquitination should occur before the interaction of a substrate with p62 (6, 34). Based on our finding that IRS1/2 blocked Lys-63-linked polyubiquitination of Dvl2, we envision that IRS1/2 might block the Lys-63-linked polyubiquitination of Dvl2 by impairing the activity of an E3 ligase or promoting the activity of a deubiquitinating enzyme. Further study is needed to identify such an E3 ligase and deubiquitinating enzyme in the regulation of Dvl2 stability by IRS1/2.
It has been documented that IRS1/2 and Wnt/β-catenin signaling, tightly linked with each other (33, 35, 36), are involved in regulation of cell proliferation and EMT (2, 11, 12, 21, 23, 37). However, the role of IRS1/2 on regulation of cell proliferation and EMT mediated by Wnt/β-catenin signaling remains unclear. In this study, we found that IRS1/2 promoted EMT and cell proliferation. We provided evidence that IRS1/2 promoted Wnt/β-catenin signaling-induced EMT and cell proliferation. Moreover, depletion of Dvl2 inhibited the EMT and cell growth induced by IRS1/2. Therefore, we concluded that both IRS1/2 and Dvl2 are important for EMT and cell proliferation.
In conclusion, our findings show that IRS1/2 regulates Wnt/β-catenin signaling through stabilizing Dvl2. The findings extend our understanding of the molecular mechanism of IRS1/2 and offer new insight into the role of IRS1/2 on regulation of EMT and cell proliferation mediated by Wnt/β-catenin signaling.
Acknowledgment
We thank Dr. David M. Irwin from the University of Toronto for a critical reading the manuscript.
This work was supported by 973 Project Grants 2011CB910502 and 2011ZX08011-006, National Natural Science Foundation of China Grants 81372167, 81301700, 30871286, 31071225, and 31030040, Tsinghua Science Foundation Grant 20121080018, and 863 Project Grants 2012AA021730 and 2012AA021703 in China.
- Dvl
- dishevelled
- EMT
- epithelial-mesenchymal transition
- IP
- immunoprecipitation
- IRS
- insulin receptor substrate
- IRS1/2
- IRS1 or IRS2
- LEF-1
- lymphoid enhancer-binding factor 1
- Luc
- luciferase
- WB
- Western blotting.
REFERENCES
- 1. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 2. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gan X. Q., Wang J. Y., Xi Y., Wu Z. L., Li Y. P., Li L. (2008) Nuclear Dvl, c-Jun, β-catenin, and TCF form a complex leading to stabilization of β-catenin-TCF interaction. J. Cell Biol. 180, 1087–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao C., Chen Y. G. (2010) Dishevelled: the hub of Wnt signaling. Cell. Signal. 22, 717–727 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y., Wang F., Han L., Wu Y., Li S., Yang X., Wang Y., Ren F., Zhai Y., Wang D., Jia B., Xia Y., Chang Z. (2011) GABARAPL1 negatively regulates Wnt/β-catenin signaling by mediating Dvl2 degradation through the autophagy pathway. Cell. Physiol. Biochem. 27, 503–512 [DOI] [PubMed] [Google Scholar]
- 6. Gao C., Cao W., Bao L., Zuo W., Xie G., Cai T., Fu W., Zhang J., Wu W., Zhang X., Chen Y. G. (2010) Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nat. Cell Biol. 12, 781–790 [DOI] [PubMed] [Google Scholar]
- 7. Sharma J., Mulherkar S., Mukherjee D., Jana N. R. (2012) Malin regulates Wnt signaling pathway through degradation of dishevelled2. J. Biol. Chem. 287, 6830–6839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angers S., Thorpe C. J., Biechele T. L., Goldenberg S. J., Zheng N., MacCoss M. J., Moon R. T. (2006) The KLHL12-Cullin-3 ubiquitin ligase negatively regulates the Wnt-β-catenin pathway by targeting Dishevelled for degradation. Nat. Cell Biol. 8, 348–357 [DOI] [PubMed] [Google Scholar]
- 9. Ding Y., Zhang Y., Xu C., Tao Q. H., Chen Y. G. (2013) HECT domain-containing E3 ubiquitin ligase NEDD4L negatively regulates Wnt signaling by targeting dishevelled for proteasomal degradation. J. Biol. Chem. 288, 8289–8298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wei W., Li M., Wang J., Nie F., Li L. (2012) The E3 ubiquitin ligase ITCH negatively regulates canonical Wnt signaling by targeting dishevelled protein. Mol. Cell. Biol. 32, 3903–3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pollak M. (2012) The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat. Rev. Cancer 12, 159–169 [DOI] [PubMed] [Google Scholar]
- 12. Taniguchi C. M., Emanuelli B., Kahn C. R. (2006) Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 [DOI] [PubMed] [Google Scholar]
- 13. Dalmizrak O., Wu A., Chen J., Sun H., Utama F. E., Zambelli D., Tran T. H., Rui H., Baserga R. (2007) Insulin receptor substrate-1 regulates the transformed phenotype of BT-20 human mammary cancer cells. Cancer Res. 67, 2124–2130 [DOI] [PubMed] [Google Scholar]
- 14. Carew R. M., Browne M. B., Hickey F. B., Brazil D. P. (2011) Insulin receptor substrate 2 and FoxO3a signalling are involved in E-cadherin expression and transforming growth factor-β1-induced repression in kidney epithelial cells. FEBS J. 278, 3370–3380 [DOI] [PubMed] [Google Scholar]
- 15. Chan B. T., Lee A. V. (2008) Insulin receptor substrates (IRSs) and breast tumorigenesis. J. Mammary Gland Biol. Neoplasia 13, 415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorokin A. V., Chen J. (2013) MEMO1, a new IRS1-interacting protein, induces epithelial-mesenchymal transition in mammary epithelial cells. Oncogene 32, 3130–3138 [DOI] [PubMed] [Google Scholar]
- 17. Shi B., Sepp-Lorenzino L., Prisco M., Linsley P., deAngelis T., Baserga R. (2007) Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J. Biol. Chem. 282, 32582–32590 [DOI] [PubMed] [Google Scholar]
- 18. Nagle J. A., Ma Z., Byrne M. A., White M. F., Shaw L. M. (2004) Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol. Cell. Biol. 24, 9726–9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bommer G. T., Feng Y., Iura A., Giordano T. J., Kuick R., Kadikoy H., Sikorski D., Wu R., Cho K. R., Fearon E. R. (2010) IRS1 regulation by Wnt/β-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype. J. Biol. Chem. 285, 1928–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dearth R. K., Cui X., Kim H. J., Kuiatse I., Lawrence N. A., Zhang X., Divisova J., Britton O. L., Mohsin S., Allred D. C., Hadsell D. L., Lee A. V. (2006) Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol. Cell. Biol. 26, 9302–9314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dearth R. K., Cui X., Kim H. J., Hadsell D. L., Lee A. V. (2007) Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle 6, 705–713 [DOI] [PubMed] [Google Scholar]
- 22. Thiery J. P., Acloque H., Huang R. Y., Nieto M. A. (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 [DOI] [PubMed] [Google Scholar]
- 23. Bao X. L., Song H., Chen Z., Tang X. (2012) Wnt3a promotes epithelial-mesenchymal transition, migration, and proliferation of lens epithelial cells. Mol. Vision 18, 1983–1990 [PMC free article] [PubMed] [Google Scholar]
- 24. Yook J. I., Li X. Y., Ota I., Hu C., Kim H. S., Kim N. H., Cha S. Y., Ryu J. K., Choi Y. J., Kim J., Fearon E. R., Weiss S. J. (2006) A Wnt-Axin2-GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat. Cell Biol. 8, 1398–1406 [DOI] [PubMed] [Google Scholar]
- 25. Zhou B. P., Deng J., Xia W., Xu J., Li Y. M., Gunduz M., Hung M. C. (2004) Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell. Biol. 6, 931–940 [DOI] [PubMed] [Google Scholar]
- 26. Yook J. I., Li X. Y., Ota I., Fearon E. R., Weiss S. J. (2005) Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 280, 11740–11748 [DOI] [PubMed] [Google Scholar]
- 27. Rong Y., Cheng L., Ning H., Zou J., Zhang Y., Xu F., Liu L., Chang Z., Fu X. Y. (2006) Wilms' tumor 1 and signal transducers and activators of transcription 3 synergistically promote cell proliferation: a possible mechanism in sporadic Wilms' tumor. Cancer Res. 66, 8049–8057 [DOI] [PubMed] [Google Scholar]
- 28. Wu Y., Zhang Y., Zhang H., Yang X., Wang Y., Ren F., Liu H., Zhai Y., Jia B., Yu J., Chang Z. (2010) p15RS attenuates Wnt/β-catenin signaling by disrupting β-catenin/TCF4 interaction. J. Biol. Chem. 285, 34621–34631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ravid T., Hochstrasser M. (2008) Diversity of degradation signals in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 9, 679–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adhikari A., Chen Z. J. (2009) Diversity of polyubiquitin chains. Dev. Cell 16, 485–486 [DOI] [PubMed] [Google Scholar]
- 31. Tan J. M., Wong E. S., Kirkpatrick D. S., Pletnikova O., Ko H. S., Tay S. P., Ho M. W., Troncoso J., Gygi S. P., Lee M. K., Dawson V. L., Dawson T. M., Lim K. L. (2008) Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum. Mol. Genet. 17, 431–439 [DOI] [PubMed] [Google Scholar]
- 32. Shi C. S., Kehrl J. H. (2010) TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 3, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palsgaard J., Emanuelli B., Winnay J. N., Sumara G., Karsenty G., Kahn C. R. (2012) Cross-talk between insulin and Wnt signaling in preadipocytes: role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5). J. Biol. Chem. 287, 12016–12026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ichimura Y., Kumanomidou T., Sou Y. S., Mizushima T., Ezaki J., Ueno T., Kominami E., Yamane T., Tanaka K., Komatsu M. (2008) Structural basis for sorting mechanism of p62 in selective autophagy. J. Biol. Chem. 283, 22847–22857 [DOI] [PubMed] [Google Scholar]
- 35. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 36. Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C. Y., He X., MacDougald O. A., You M., Williams B. O., Guan K. L. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 [DOI] [PubMed] [Google Scholar]
- 37. Larue L., Bellacosa A. (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′-kinase/AKT pathways. Oncogene 24, 7443–7454 [DOI] [PubMed] [Google Scholar]