Background: Apolipoprotein E (apoE) isoforms influence the risk for Alzheimer disease (AD).
Results: All-trans-retinoic acid (RA), 9-cis-RA, and 13-cis-RA were identified as compounds that increase apoE expression and lipidation in astrocytes.
Conclusion: RA isomers are effective modulators of apoE production through the retinoid X receptor (RXR) and RA receptor (RAR).
Significance: RXR/RAR agonists can be explored for AD therapy.
Keywords: Alzheimer Disease, Amyloid, ApoE, Cholesterol, Retinoid, Retinoic Acid
Abstract
Apolipoprotein E (apoE) is the major cholesterol transport protein in the brain. Among the three human APOE alleles (APOE2, APOE3, and APOE4), APOE4 is the strongest genetic risk factor for late-onset Alzheimer disease (AD). The accumulation of amyloid-β (Aβ) is a central event in AD pathogenesis. Increasing evidence demonstrates that apoE isoforms differentially regulate AD-related pathways through both Aβ-dependent and -independent mechanisms; therefore, modulating apoE secretion, lipidation, and function might be an attractive approach for AD therapy. We performed a drug screen for compounds that modulate apoE production in immortalized astrocytes derived from apoE3-targeted replacement mice. Here, we report that retinoic acid (RA) isomers, including all-trans-RA, 9-cis-RA, and 13-cis-RA, significantly increase apoE secretion to ∼4-fold of control through retinoid X receptor (RXR) and RA receptor. These effects on modulating apoE are comparable with the effects recently reported for the RXR agonist bexarotene. Furthermore, all of these compounds increased the expression of the cholesterol transporter ABCA1 and ABCG1 levels and decreased cellular uptake of Aβ in an apoE-dependent manner. Both bexarotene and 9-cis-RA promote the lipidation status of apoE, in which 9-cis-RA promotes a stronger effect and exhibits less cytotoxicity compared with bexarotene. Importantly, we showed that oral administration of bexarotene and 9-cis-RA significantly increases apoE, ABCA1, and ABCG1 levels in mouse brains. Taken together, our results demonstrate that RXR/RA receptor agonists, including several RA isomers, are effective modulators of apoE secretion and lipidation and may be explored as potential drugs for AD therapy.
Introduction
Alzheimer disease (AD)3 is the most common form of dementia in the elderly population. The accumulation and deposition of amyloid-β (Aβ) peptides cleaved from amyloid precursor protein (APP) in the brain are thought to be the central events in AD pathogenesis (1–3). Although multiple genetic and environmental factors are involved in AD pathogenesis (4, 5), the ϵ4 allele of the apolipoprotein E (APOE) gene is the strongest genetic risk factor for late-onset AD compared with the common allele APOE3 or the protective allele APOE2 (6–8). ApoE isoforms differentially regulate AD pathogenesis through Aβ-dependent (9) and Aβ-independent pathways (10). ApoE4 suppresses Aβ clearance in the brain compared with apoE3, which results in accelerated Aβ deposition as senile plaques and cerebral amyloid angiopathy (8, 11–13). In the central nervous system (CNS), apoE transports cholesterol from astrocytes to neurons through cell surface receptors, including the low density lipoprotein receptor (LDLR) and LDLR-related protein 1 (LRP1) (6, 8, 9). When apoE is secreted by astrocytes, cholesterol and other lipids are loaded into lipoprotein particles by the plasma membrane ATP-binding cassette transporters ABCA1 and ABCG1 (14). Because cholesterol is an essential component of membranes and myelin sheaths (15), apoE plays a critical role in maintaining synaptic plasticity and neuronal function by controlling cholesterol homeostasis with the apoE3 allele having a superior function than apoE4 (8, 11, 12).
Interestingly, apoE protein levels in cerebrospinal fluid (CSF) and plasma are reduced in APOE4 carriers and patients with AD (16). Furthermore, low levels of total cholesterol also increase the risk of AD (17). Therefore, increasing apoE and cholesterol levels in the brain may be an effective therapeutic strategy for AD. In fact, treatment of AD mice with liver X receptor (LXR) agonists or retinoid X receptor (RXR) agonists consistently improves memory and reduces Aβ levels by increasing the apoE level and lipidation in amyloid model mice (18–21). However, despite their efficacy and tolerability in rodents, the controversy on drug toxicity still precludes their translation into human clinical trials (22). To identify novel compounds that can regulate apoE production, we performed a drug screen using a known bioactives library from the Institute of Chemistry and Cell Biology Bioactives, which contains 480 compounds with defined biological activity and a culture system of immortalized astrocytes from human APOE3-targeted replacement (TR) mice. Our analyses led to the identification of several retinoic acid (RA) family members, including all-trans-RA, 9-cis-RA, and 13-cis-RA, which can increase apoE production and lipidation by modulating the expression of ABCA1 and ABCG1 through RXR and RAR.
EXPERIMENTAL PROCEDURES
Small Compounds Libraries
The screened compounds were from a commercially available known bioactives library from the Institute of Chemistry and Cell Biology Bioactives (Enzo Life Sciences) containing 480 compounds with defined biological activity and targets.
Cell Lines and Reagents
Immortalized apoE3 astrocytes were a kind gift from Dr. David M. Holtzman. This cell line was generated from primary astrocytes derived from human APOE-TR mice, in which human APOE3 gene is knocked in the mouse Apoe locus (23). All-trans-RA, 9-cis-RA, 13-cis-RA, bexarotene, all-trans-retinal, 9-cis-retinal, 13-cis-retinal, TO901317, HX531, AGN193109, and geranylgeranyl pyrophosphate (GGPP) were purchased from Sigma. All compounds were dissolved into 10 mm stock solution in dimethyl sulfoxide (DMSO). Rabbit polyclonal anti-LRP1 and anti-LDLR antibodies were produced in our laboratory (24). Mouse monoclonal anti-ABCA1 (ab18180), rabbit polyclonal anti-ABCG1 (ab36969), mouse anti-LXRα (ab41902), and anti-TATA-binding protein (ab818) antibodies were purchased from Abcam. Mouse monoclonal anti-actin antibody was purchased from Sigma (A5316). Rabbit polyclonal anti-RARα (2554) and rabbit monoclonal anti-RXRα (3085S) antibodies were obtained from Cell Signaling.
Cell Culture and Drug Treatment
Immortalized apoE3 astrocytes were cultured in DMEM/F-12 (Invitrogen) with 20% fetal bovine serum (Invitrogen), 2 mm l-glutamine, glutamine, 1× nonessential amino acids, 1 mm sodium pyruvate, and 1% penicillin/streptomycin (Invitrogen). Cells were cultured at 37 °C in humidified air containing 5% CO2. For primary screening and dose dependence experiments, immortalized astrocytes were seeded and cultured overnight in 96-well plates at 10,000 cells/well density. For immunoblotting and mRNA analyses, cells were seeded on the day before treatments in 12-well plates (200,000 cells/well) and 6-well plates (400,000 cells/well), respectively. Cells were washed once with serum-free DMEM/F-12 media and then treated with DMSO as a negative control or the selected compounds in DMEM/F-12 containing N2 supplement (Invitrogen).
Lentivirus-mediated Short Hairpin RNA (shRNA) Gene Silencing
MISSION shRNA lentiviral particles were purchased from Sigma. Lentiviral knockdown of immortalized apoE3 astrocytes with viral particles for shRNA targeting RXRα (SHVRS-NM_011305), RARα (SHVRS-NM_009024), LXRα (SHCLNV-NM_009473), or a nontarget negative control (SHC016V) was performed according to manufacturer's protocol.
Primary Astrocyte Cultures
Primary astrocytes were prepared from newborn mice (P1–P2) of APOE3-TR, APOE4-TR, and Apoe knock-outs as described previously (25). In brief, the brain was removed from the skull, and the meninges were discarded. Subsequently, the cortices were minced and incubated with 0.05% trypsin in a water bath at 37 °C for 15 min. Enzyme-digested dissociated cells were triturated with astrocyte growth medium and centrifuged at 300 × g for 5 min. The pellet was resuspended, passed through a 70-μm nylon mesh, washed, and centrifuged at 300 × g for 5 min. The cells were plated on poly-d-lysine-coated 75-cm2 flask in astrocyte growth media, and the media were changed every 3 days. Cells were grown until confluence and then re-plated for the experiments at a density of 2.0 × 105 cells/well on 12-well plates coated with poly-d-lysine.
Western Blotting
Samples were lysed in PBS containing 1% Triton X-100 and protease inhibitor mixture from Roche Applied Science or radioimmune precipitation assay buffer. Protein concentration was determined using a protein assay kit (Bio-Rad), and an equal amount of samples was analyzed by SDS-PAGE. Immunoreactive bands were quantified using Odyssey Infrared Imaging System (LI-COR Biosciences). For determining silencing efficiency, nuclear proteins were extracted from cells using NE-PER (Pierce) according to the manufacturer's protocol. The ECL detection reagent from Amersham Biosciences was used for immunodetection.
Reverse Transcription and PCR
Total RNA was isolated from tissues or cells using RNeasy mini kit (Qiagen) and subjected to DNase I digestion to remove contaminating genomic DNA. Total RNA was dissolved in nuclease-free water and stored at −80 °C. Reverse transcription was performed using a SuperScript II RNase H-reverse transcriptase (Invitrogen), and the reaction mixture was subjected to quantitative real time PCR to detect levels of the corresponding mouse actin, human apoE, mouse ABCA1, ABCG1, LRP1, and LDLR (Qiagen). The set of actin primers (Qiagen) was used as an internal control for each specific gene amplification. The relative levels of expression were quantified and analyzed by using Bio-Rad iCycler iQ software (Bio-Rad). The real time value for each sample was averaged and compared using the CT method, where the amount of target RNA (2 −ΔΔCT) was normalized to the endogenous actin reference (ΔCT) and related to the amount of target gene in tissue cells, which was set as the calibrator at 1.0.
ApoE Concentration Measured by ELISA
After incubation with compounds in serum-free medium for 24 h at 37 °C, the conditioned media from immortalized or primary astrocytes were analyzed using an in-house developed sandwich ELISA. Briefly, 96-well plates were coated overnight with an apoE monoclonal antibody WU-E4 (26), previously employed for similar purposes (27), in carbonate buffer at room temperature. The plates were thereafter washed with PBS, blocked with 1% milk in PBS, and then washed again. Recombinant ApoE (Fitzgerald) and samples were diluted using 1% milk/PBS and added at a volume of 100 μl/well, incubated for 4 h at room temperature, and then detected with biotin-conjugated goat anti-apoE antibody (Meridian Life Science). After incubation with streptavidin-polyHRP40 (Fitzgerald), the plate was developed with tetramethylbenzidine super-slow (Sigma) for 10 min, stopped by the addition of o-phosphoric acid, and read at 450 nm with a BioTek 600 plate reader. The inter- and intra-assay variation of coefficients determined for this assay were <15 (n = 10 individual assays) and <10, respectively.
Aβ ELISA
Cells were incubated with compounds for 18 h in serum-free media at 37 °C and then treated with 125 nm soluble Aβ42 for another 18 h in the presence of tested compounds. Cells were harvested by incubating with trypsin for 5 min at 37 °C. Cell pellets were collected by centrifugation at 1,000 × g for 5 min. After washing two times with PBS, cells were dissolved in 5 m guanidine in 50 mm Tris-HCl (pH 8.0). To measure Aβ42, samples were captured with mAb 2.1.3 followed by detection with HRP-conjugated Ab5 (28).
Size-exclusion Chromatography of ApoE Particles and Cholesterol Assay
Thirty ml of serum-free conditioned medium from immortalized astrocyte was concentrated 60-fold with a 10-kDa cutoff filter (Millipore Corp., Bedford, MA) prior to fractionation. The concentrated serum-free conditioned medium (500 μl) was isolated by size-exclusion chromatography using fast protein liquid chromatography (FPLC) (GE Healthcare) with tandem Superose-6, 10/300 GL columns (GE Healthcare) in PBS containing 50 mm sodium phosphate (pH 7.4), with 150 mm NaCl, 1 mm EDTA, and 0.02% sodium azide at a flow rate of 0.4 ml/min (29, 30). Fractions were collected and analyzed for the concentrations of apoE and total cholesterol. Total cholesterol was quantified with the Amplex Red cholesterol assay kit (Invitrogen) according to the manufacturer's protocol.
In Vivo Effects of Tested Compounds
Both male and female APOE3-TR mice were orally gavaged daily for 10 days with 100 mg/kg/day bexarotene, 100 mg/kg/day 9-cis-RA, or vehicle (corn oil) at the age of 6–7 months. Bexarotene and 9-cis-RA were suspended in corn oil and vortexed each time before administration. One hour after the final administrations, the mice were perfused with PBS, and the brain tissues were dissected and kept frozen at −80 °C until further analysis. All animal procedures were approved by the Animal Study Committee at Mayo Clinic and in accordance with the regulations of the American Association for the Accreditation of Laboratory Animal Care.
Cytotoxicity Assays and Aspartate Aminotransferase Assay
The in vitro cytotoxic effects of compounds were evaluated with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kits (Roche Applied Science) following the manufacturer's instructions. Aspartate aminotransferase in the serum was quantified with the aspartate aminotransferase activity assay kit (Sigma) according to the manufacturer's protocol.
Statistical Analysis
All quantified data were analyzed by one-way analysis of variance with a Tukey's post test. Error bars represent standard deviation, and p < 0.05 was considered significant.
RESULTS
RA Isomers Increase ApoE Production in Immortalized Astrocytes
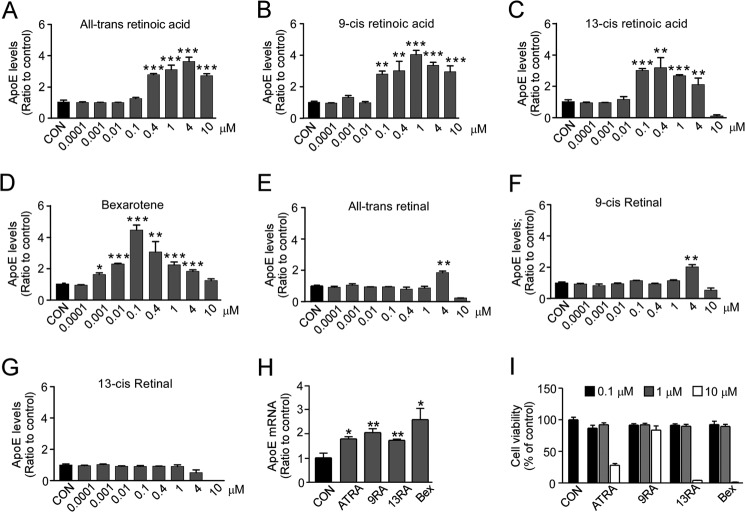
To identify modulators of apoE, we screened a bioactives library containing compounds with defined biological activity and targets using a culture system of immortalized astrocytes from human APOE3-TR mice (23). Upon primary screening of 480 compounds in the library, 14 compounds were found to increase secreted apoE levels, whereas 27 compounds decreased the apoE levels in the media. The 14 compounds that increased apoE secretion include all-trans-RA, 9-cis-RA, and 13-cis-RA, which are RA family members. To further assess the concentration-dependent effects of the compounds, we treated immortalized apoE3 astrocytes with all-trans-RA, 9-cis-RA, 13-cis-RA, an RXR agonist bexarotene, all-trans-retinal, 9-cis-retinal, and 13-cis-retinal at concentrations between 0.01 and 10 μm for 24 h and measured apoE levels in conditioned media by ELISA. All-trans-RA, 9-cis-RA, 13-cis-RA, and bexarotene significantly increased apoE secretion in a concentration-dependent manner mostly followed a bell-shaped pattern (Fig. 1, A–D). Although all-trans-RA, 9-cis-RA, and 13-cis-RA had a peak effect at the concentration range of 0.4–4 μm, bexarotene showed the strongest effect at 0.1 μm (Fig. 1D). In addition, we examined the effects of all-trans-retinal, 9-cis-retinal, and 13-cis-retinal to assess whether RA analogues stimulate apoE secretion. Although 4 μm of all-trans-retinal (Fig. 1E) and 4 μm of 9-cis-retinal (Fig. 1F) significantly increased apoE levels, the effects were in general less than those of RA isomers. There were no significant effects at lower dosages of all-trans-retinal and 9-cis-retinal treatment and in all examined concentrations of 13-cis-retinal (Fig. 1G). These results indicate that RA isomers, including all-trans-RA, 9-cis-RA, and 13-cis-RA, specifically increase apoE levels in the media by targeting a specific pathway.
FIGURE 1.
RA isomers increase apoE production in immortalized astrocytes. Immortalized astrocytes were cultured and treated with increasing concentrations of ATRA (A), 9-cis-RA (9RA) (B), 13-cis-RA (13RA) (C), bexarotene (Bex) (D), all-trans-retinal (E), 9-cis-retinal (F) and 13-cis-retinal (G). After incubation for 24 h, apoE levels in media were measured by ELISA. H, cells were cultured with ATRA (1 μm), 9-cis-RA (1 μm), 13-cis-RA (0.4 μm), and bexarotene (0.1 μm) for 24 h, and then the mRNA levels of apoE were measured by qRT-PCR (I). Cell viability was assessed by MTT assay after cultures were treated with the compounds at the concentration range of 0.1–10 μm for 24 h. Controls (CON) indicate vehicle treatments only. Data represent mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
To characterize the mechanism by which RA isomers modulate apoE levels in astrocytes, we treated immortalized astrocytes with all-trans-RA, 9-cis-RA, 13-cis-RA, and bexarotene for 24 h, and then analyzed the apoE mRNA levels by qRT-PCR. Consistent with the results from ELISA, apoE mRNA levels were significantly increased as a result of treatments with RA isomers (Fig. 1H), indicating that RA isomers and bexarotene facilitate apoE production at least partially by increasing apoE expression.
To determine the cytotoxicity of each compound, we performed MTT assay at increasing dosages of tested compounds (0.1, 1, and 10 μm). We found that none of them had a toxic effect at or below the concentration of 1 μm, which is sufficient to increase apoE levels. Interestingly, 9-cis-RA at 10 μm did not have significant cytotoxicity, whereas bexarotene, all-trans-RA, and 13-cis at this concentration did (Fig. 1I). These results suggest that 9-cis-RA might be a safer compound than bexarotene when explored at high concentrations as an apoE modulator.
RA Isomers Increase ApoE Secretion in Primary Culture of Astrocytes from ApoE3- and ApoE4-TR Mice
To confirm the effects of RA isomers on apoE production, we next used primary cultures of astrocytes from both apoE3-TR and apoE4-TR mice. Primary astrocytes were treated with all-trans-RA (1 μm), 9-cis-RA (1 μm), 13-cis-RA (0.4 μm), and bexarotene (0.1 μm) for 24 h, followed with measurements of apoE concentrations in the media by ELISA. Consistent with results in immortalized astrocytes, all tested compounds significantly increased apoE levels (Fig. 2). Interestingly, the effects of these compounds were stronger in primary astrocytes from apoE3-TR mice (Fig. 2A) than those from apoE4-TR mice (Fig. 2B). Although further experiments are necessary to assess their apoE isoform-dependent effects, these results confirm that all-trans-RA, 9-cis-RA, and 13-cis-RA have an ability to increase apoE levels in the media of primary astrocyte cultures.
FIGURE 2.
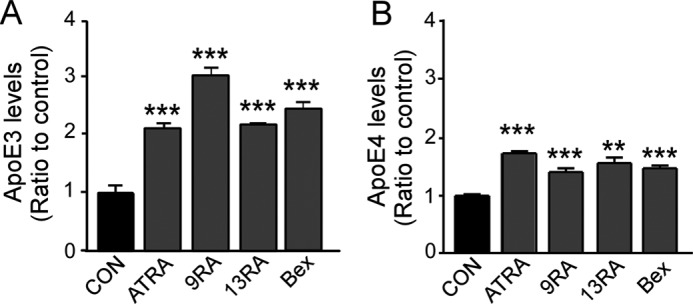
RA isomers increase apoE secretion in primary astrocyte cultures. Primary cultures of astrocytes from apoE3-TR (A) and apoE4-TR mice (B) were treated with ATRA (1 μm), 9-cis-RA (9RA; 1 μm), 13-cis-RA (13RA; 0.4 μm) and bexarotene (Bex; 0.1 μm) for 24 h. ApoE levels in media were measured by ELISA. Data represent mean ± S.D. from three independent experiments. **, p < 0.01; ***, p < 0.001. Con, control.
RA Isomers Promote ApoE Production through RXR/RAR Pathway
RA isomers function through the RXR/RAR pathway, where 9-cis-RA predominantly binds to RXR and all-trans-RA interacts with RAR. Thus, to confirm the roles of the RXR/RAR pathway in apoE production, we assessed the effects of specific RXR antagonist HX531 and RAR antagonist AGN193109. In the presence of HX531, the increases of apoE by bexarotene and 9-cis-RA were mostly suppressed, and AGN193109 reversed the effect of all-trans-RA (Fig. 3A). To further corroborate the mechanism by which RA isomers facilitate apoE production, lentivirus-mediated delivery of shRNA was used to knock down the expression of RARα and RXRα in immortalized astrocytes. RARα and RXRα were efficiently knocked down after infection with lentivirus carrying RARα shRNA and RXRα shRNA, respectively (Fig. 3B). RXRα knockdown significantly reduced the effects of both all-trans-RA and 9-cis-RA on apoE production. In the case of RARα knockdown, the effect of all-trans-RA was reversed, whereas that of 9-cis-RA was slight (Fig. 3B). These results suggest that all-trans-RA requires both RAR and RXR, whereas 9-cis-RA might mediate the pathway mainly through RXR.
FIGURE 3.
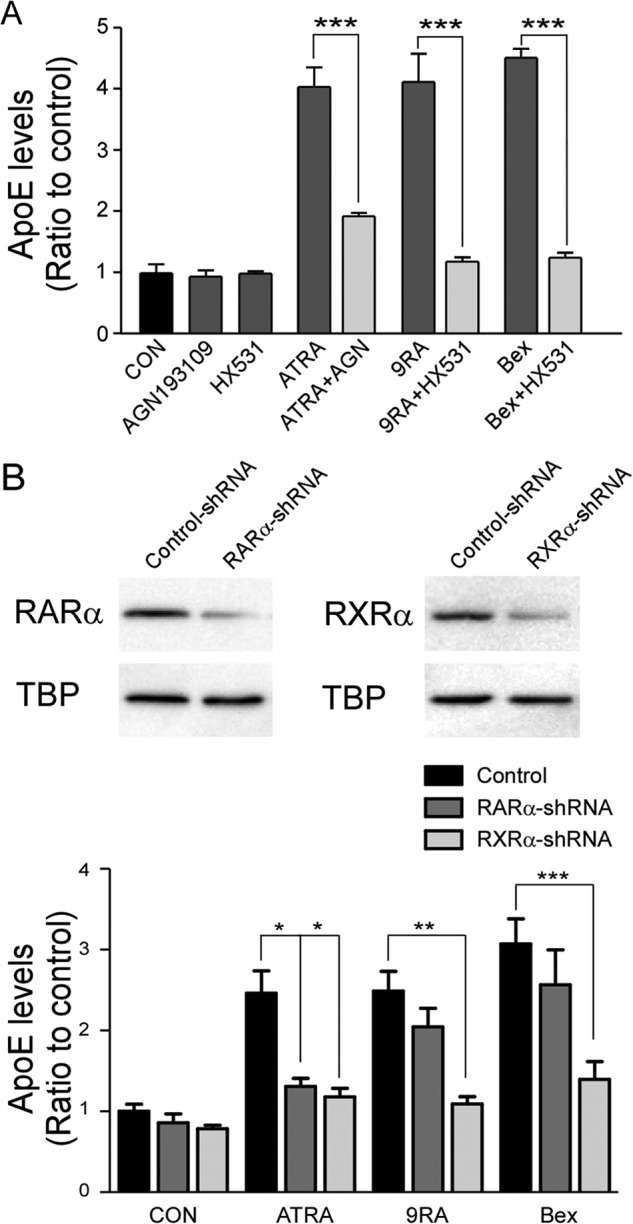
RXRα and RARα are involved in the regulation of apoE production by RA isomers. A, apoE levels in culture media were measured by ELISA after treatments with ATRA (1 μm), 9-cis-RA (9RA; 1 μm), and bexarotene (Bex; 0.1 μm) in the presence or absence of RAR antagonist AGN193109 (0.1 μm) or RXR antagonist HX531 (0.1 μm). B, Western blot showing that expression of RARα and RXRα was effectively suppressed by their respective lentiviral shRNA. After 24 h infection, cells were treated with ATRA (1 μm), 9-cis-RA (9RA; 1 μm), or bexarotene (Bex; 0.1 μm), and apoE levels in the media were measured by ELISA. Data represent mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. CON, control; TBP, TATA-binding protein.
Because LXR signaling also regulates apoE production, we assessed whether LXR is involved in apoE regulation by RA isomers. A specific LXR agonist TO901317 significantly increased apoE levels, an effect that was reversed by the presence of a specific LXR antagonist GGPP (Fig. 4A) or when LXRα expression was knocked down by shRNA (Fig. 4B). In addition, we found that the effects of increased apoE by all-trans-RA, 9-cis-RA, and bexarotene were suppressed when the LXR pathway was blocked by either GGPP treatment (Fig. 4A) or LXRα knockdown (Fig. 4B). Taken together, these results indicate that RA isomers and bexarotene facilitate apoE production through activation of the RXR/RAR pathways, but they might also require LXR function.
FIGURE 4.

LXR pathways are involved in regulating apoE production by RA isomers. A, apoE levels in culture media were measured by ELISA after treatments with TO901317 (TO; 2 μm), ATRA (1 μm), 9-cis-RA (9RA; 1 μm), and bexarotene (Bex; 0.1 μm) in the presence or absence of LXR antagonist GGPP (0.5 μm). B, Western blot showing that LXRα expression was effectively suppressed by its specific lentiviral shRNA. After 24 h of infection, cells were treated with TO901317 (TO; 2 μm), ATRA (1 μm), 9-cis-RA (9RA; 1 μm), or bexarotene (Bex; 0.1 μm), and apoE levels in the media were measured by ELISA. Data represent mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Con, control.
In addition to apoE, RXR/RAR are known to regulate ABCA1 and ABCG1 expression (31). Western blotting demonstrated that these tested compounds indeed increased the levels of ABCA1 (Fig. 5, A and B) and ABCG1 (Fig. 5, A and C) in immortalized astrocytes. The increased mRNA levels of ABCA1 (Fig. 5D) and ABCG1 (Fig. 5E) were also confirmed by qRT-PCR.
FIGURE 5.

RA isomers increase ABCA1 and ABCG1 levels in immortalized astrocytes. A–C, cells were treated with ATRA (1 μm), 9-cis-RA (9RA; 1 μm), 13-cis-RA (13RA; 0.4 μm), or bexarotene (Bex; 0.1 μm) for 24 h, and then the protein expression levels of ABCA1 (B) and ABCG1 (C) in the cells were analyzed by Western blot and quantified. The mRNA levels of ABCA1 (D) and ABCG1 (E) were measured by qRT-PCR. Data represent mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Con, control.
To rule out the possibility that all-trans-RA, 9-cis-RA, 13-cis-RA, and bexarotene increase apoE levels in the conditioned media by suppressing its cellular clearance, we examined the levels of two major apoE metabolic receptors LDLR and LRP1 (32). We found that receptor expression at both protein and mRNA levels was not changed by tested compounds (Fig. 6, A–E), indicating that cellular apoE clearance pathways are unlikely involved in the mechanisms.
FIGURE 6.
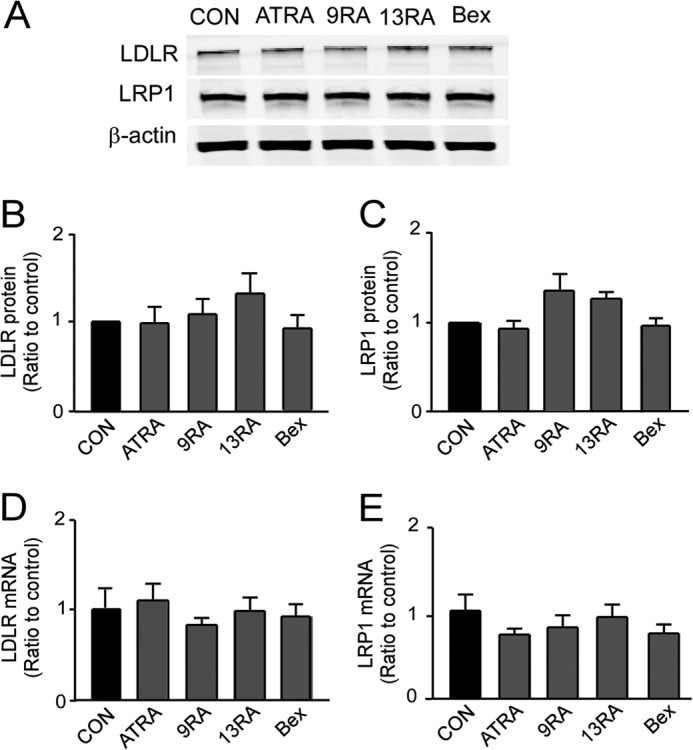
RA isomers do not affect LDLR and LRP1 levels in immortalized astrocytes. A–C, cells were treated with ATRA (1 μm), 9-cis-RA (9RA; 1 μm), 13-cis-RA (13RA; 0.4 μm), or bexarotene (Bex; 0.1 μm) for 24 h. The protein levels of LDLR (B) and LRP1 (C) in the cells were analyzed by Western blotting and quantified. The mRNA levels of LDLR (D) and LRP1 (E) were measured by qRT-PCR. Data represent mean ± S.D. from three independent experiments. Con, control.
Bexarotene and 9-cis-RA Increase ApoE Lipidation Status
ABCA1 and ABCG1 promote cholesterol efflux to lipidate apoE; therefore, we next investigated whether 9-cis-RA and bexarotene affect the lipidation of apoE in immortalized astrocytes. Cells were treated with 9-cis-RA or bexarotene, and the conditioned medium was fractionated by size-exclusion chromatography using FPLC. When the apoE level of each fraction was quantified by ELISA, a major peak was detected in the fraction eluting at a molecular size of ∼160-kDa under each condition. This size is consistent with lipidated apoE/lipoprotein particles found in plasma, CSF, and primary astrocyte cultures (29, 30). In the presence of bexarotene, the height of this peak was dramatically increased (Fig. 7A), indicating that this compound increases the astrocytic secretion of apoE particles. Interestingly, 9-cis-RA treatment induced another peak of apoE close to the 670-kDa molecular size marker (Fig. 7A), suggesting that this compound has a different effect on secreted apoE particles than bexarotene. When the cholesterol level of each fraction was analyzed, the peaks were similar to apoE peaks with increased peak heights in samples treated with bexarotene and 9-cis-RA compared with control (Fig. 7B), although it is possible that the peaks may be derived from other lipoproteins in addition to apoE. We did not detect any cholesterol in fractions representing the unique peak at around the 670-kDa marker upon treatment with 9-cis-RA (Fig. 7B), suggesting that this apoE peak might contain apoE aggregates, rather than larger apoE particles.
FIGURE 7.

Bexarotene and 9-cis-RA increase apoE lipidation. Immortalized astrocytes were cultured in serum-free conditioned media with 9-cis-RA (9RA; 1 μm) or bexarotene (Bex; 0.1 μm). The condition media were concentrated and then fractioned by size-exclusion chromatography on FPLC using tandem Superose-6 columns. Concentrations of apoE (A) in each fraction and cholesterol (B) in selective fractions were analyzed by ELISA or fluorometric assay, respectively. Graphs are an average of three independent experiments. Con, control.
Bexarotene and 9-cis-RA Increase ABCA1, ABCG1, and ApoE Levels in the Brains of ApoE3-TR Mice
Next, we investigated whether 9-cis-RA could increase apoE levels in vivo. ApoE3-TR mice were fed through oral administration with bexarotene (100 mg/kg/day), 9-cis-RA (100 mg/kg/day), and vehicle (corn oil) for 10 days, and apoE, ABCA1, and ABCG1 levels in the brains were analyzed by Western blotting (Fig. 8A). ApoE levels were increased by 34 ± 8 and 66 ± 19% versus controls upon treatment with 9-cis-RA and bexarotene, respectively (Fig. 8B). Furthermore, 9-cis-RA and bexarotene treatments also significantly increased the expression levels of ABCA1 (Fig. 8C) and ABCG1 (Fig. 8D). These results indicate that similar to bexarotene (21), 9-cis-RA can also increase apoE production and cholesterol efflux in the brain. In these experiments, the drug treatments did not lead to obvious deleterious effects, including loss of body weight. Although the average body weights of each group were 26.8 ± 1.3 (control), 26.9 ± 1.2 (9-cis-RA), and 27.1 ± 1.5 g (bexarotene) before the treatments, their body weights after the treatments were 28.7 ± 2.0, 28.9 ± 1.0, and 28.4 ± 1.9 g, respectively. In addition, we also measured a marker of hepatic function, the aspartate aminotransferase level in the serum by ELISA, as a major side effect of bexarotene is hepatic toxicity (33) We found that there was no significant difference in serum aspartate aminotransferase levels between control and the drug-treated groups (Fig. 8E).
FIGURE 8.

Bexarotene and 9-cis-RA increase ABCA1, ABCG1, and apoE expression in mouse brains. ApoE3-TR mice (6–7 months old) were orally gavaged daily for 10 days with bexarotene (Bex; 100 mg/kg/day), 9-cis-RA (9RA; 100 mg/kg/day), or vehicle (corn oil). A, Western blotting for the protein levels of apoE, ABCA1, and ABCG1 in the brain. B–D, results from Western blotting were quantified for apoE (B), ABCA1 (C) and ABCG1 (D). E, serum aspartate aminotransferase (AST) levels were analyzed for each treatment group. Data represent mean ± S.D. from seven mice. **, p < 0.01. Con, control.
RA Isomers Suppress Cellular Aβ Uptake in an ApoE-dependent Manner in Immortalized Astrocytes
Bexarotene has been shown to stimulate the apoE-dependent cellular clearance of Aβ through the LXR/peroxisome proliferator-activated receptor γ pathway (21). To investigate the effects of RA isomers on cellular Aβ uptake, immortalized astrocytes were incubated with 125 nm soluble Aβ42 for 18 h in the presence of RA isomers or bexarotene, and the amounts of cell-associated Aβ were analyzed (Fig. 9A). ELISA demonstrated that RA isomers or bexarotene significantly decreased cellular Aβ uptakes, consistent with recent studies demonstrating that lipidated apoE particles compete with Aβ for cellular uptake (23, 34). These effects were eliminated in the presence of the RAR antagonist AGN193109 or RXR antagonist HX531 (Fig. 9A). When primary astrocytes from apoE knock-out mice were similarly analyzed for cellular Aβ uptake, these compounds did not have any significant effects on cellular Aβ uptake (Fig. 9B), suggesting that RA isomers regulate Aβ metabolism in an apoE-dependent manner.
FIGURE 9.

RA isomers decrease cell-associated Aβ levels in immortalized astrocytes. A, immortalized astrocytes were incubated with ATRA (1 μm), 9-cis-RA (9RA; 1 μm), 13-cis-RA (13RA; 0.4 μm), and bexarotene (Bex; 0.1 μm) for 18 h in serum-free media. In selected experiments, cells were treated with RAR antagonist AGN193109 (0.1 μm) or RXR antagonist HX531 (0.1 μm). They were then treated with 125 nm soluble Aβ42 for another 18 h in the presence of tested compounds. Cell-associated Aβ levels were quantified by ELISA. B, similarly, primary astrocyte cultures from apoE knock-out mice were analyzed for cellular Aβ uptake upon treatments. Data represent mean ± S.D. from three independent experiments. **, p < 0.01; ***, p < 0.001. Con, control.
DISCUSSION
With the recent failures of AD clinical trials targeting Aβ alone, there is an urgent need to define alternative targets. Toward this effort, we performed a drug library screening and identified several RA isomers, which are converted from vitamin A (retinol), as modulators that increase secreted apoE levels in astrocytes. We demonstrated that all-trans-RA, 9-cis-RA, and 13-cis-RA significantly increased apoE production and lipidation through the RXR/RAR pathway in astrocytes and mouse brains. RA isomers interact with two major groups of nuclear receptors, RAR and RXR. All-trans-RA efficiently activates the RAR pathway, although 13-cis-RA binds to RAR with a lower affinity (35, 36). Although 9-cis-RA is known to be a ligand for both RAR and RXR, it predominantly binds to RXR (37). RXR forms a heterodimer with several nuclear receptors, including RAR, LXR, peroxisome proliferator-activated receptor γ, and the vitamin D receptor, through which the transcription of a variety of genes are regulated (37–39). Interestingly, we found that treatment with the LXR-specific antagonist GGPP and LXRα knockdown suppressed the effects of retinoid isomers. Consistent with our findings, a previous report has also shown that 9-cis-RA recruits LXR and initiates the interaction with RXR to function as an active ligand-binding heterodimeric receptor (40). Thus, RA isomers may facilitate apoE production through RXR/RAR in an LXR-dependent manner. Further studies are needed to clarify the roles of the individual nuclear receptors.
RA isomers play important roles in cell proliferation, differentiation, and apoptosis (41, 42). In the CNS, they are also known to regulate neuronal patterning, differentiation, axonal outgrowth, nerve regeneration, and neuronal plasticity (42, 43). The deprivation of vitamin A was shown to disturb hippocampal long term potentiation in rats (44). In addition, RA isomers can function as immunomodulators, which are required for the maintenance of the immune systems (45). All-trans-RA has been reported to protect neurons from oxidative damage and apoptosis (46), whereas 9-cis-RA has protective effects on neurons after stroke by increasing the secretion of growth factors (47). In fact, there are several studies indicating an involvement of vitamin A and RA isomers in AD pathogenesis. In AD patients, serum concentrations of vitamins A, C, E, and β-carotene are significantly lower than in normal individuals (48). All-trans-RA inhibits APP processing and τ hyperphosphorylation and rescues the impairment of spatial learning and memory in amyloid model mice (49). Furthermore, 9-cis-RA suppresses Aβ production in vitro (50). Thus, RA isomers are likely to have beneficial effects to ameliorate AD pathology through several pathways as follows: inductions of neurogenesis, suppression of inflammatory responses, and reduction of APP processing to Aβ.
Our findings support additional beneficial effects of RA isomers in ameliorating AD pathogenesis through up-regulation of apoE, ABCA1, and ABCG1 in the brain. Increasing evidence suggests that cholesterol metabolism is important in the pathogenesis of AD (8, 11, 12, 51). Because cholesterol is essential for synaptic function and neuronal repair, efficient cholesterol transport is likely to be required for maintenance of neuronal integrity. A cholesterol transporter ABCA1 regulates cholesterol efflux into lipid-poor apolipoproteins, including apoE, to produce nascent lipoproteins (52, 53). Another cholesterol acceptor, ABCG1, further induces cholesterol efflux into nascent lipoproteins, which leads to the maturation and/or remodeling of lipoproteins (54, 55). In fact, deletion of ABCA1 in APP transgenic mice exacerbates Aβ deposition by suppressing apoE lipidation and accelerating its turnover (56–58). ABCA1 overexpression increases lipidated apoE levels and reduces the formation of amyloid plaques in amyloid model mice (59, 60). LXR agonists or RXR agonists also reduce Aβ deposition by increasing apoE and ABCA1 levels in amyloid model mice (18–21). Interestingly, apoE levels in CSF appear lower in APOE4 carriers than non-APOE4 carriers however, with APOE genotype only accounting for 8.2% of the variability in ApoE concentrations (16). Thus, the suppressed cholesterol homeostasis in APOE4 carriers may be one of the contributing factors to the AD risk. Although it has been reported that RA isomers increase the levels of ABCA1 and ABCG1, and enhance cholesterol efflux in primary rat astrocytes (31), we have directly demonstrated that RA isomers can increase apoE production as well as ABCA1 and ABCG1 levels. More importantly, 9-cis-RA increases these levels in mouse brains, indicating that RA isomers have potential as a therapeutic compound for AD.
Although clearance of soluble Aβ in brain interstitial fluid is likely to be increased in apoE knock-out mice (61), apoE has also been shown to promote Aβ clearance and degradation through several pathways (62–66). Thus, although cellular Aβ clearance through lysosomal degradation is a major pathway, the specific role of apoE in this pathway is complex. ApoE and Aβ bind to each other and also to their common receptors, including LRP1, LDLR, and heparan sulfate proteoglycan (11, 12). Thus, apoE might compete with Aβ for receptor binding (67), although if it forms complexes with Aβ, it might also facilitate cellular Aβ binding and uptake (28). Thus, the specific effects of apoE on Aβ clearance may be depending on their concentrations, apoE isoforms, apoE lipidation status, Aβ aggregation status, and specific cell types. In this study, we found that RA isomers or bexarotene significantly suppressed cellular Aβ uptake by increasing apoE levels in immortalized astrocytes, which is consistent with previous reports demonstrating decreased astrocytic Aβ uptake as a result of apoE interference (21, 34). Although further studies are necessary to determine whether the suppression of cellular Aβ uptake in astrocytes facilities or prevents Aβ clearance, our findings indicate that RA isomers can modulate the Aβ metabolic pathway.
Bexarotene is a specific ligand for RXR, whereas 9-cis-RA can interact with both RXR and RAR. Gene expression profiles following in vivo treatment with bexarotene or 9-cis-RA in rats revealed that these compounds induce different gene expression in various tissues. Bexarotene modulated 316 transcripts in the mammary gland, 155 in the liver, and 120 in the lung. Conversely, 9-cis-RA modulated 48 transcripts in the mammary gland, 73 in the liver, and 24 in the lung, where 21 of 48 genes in the mammary gland, 39 of 73 in the liver, and 10 of 24 in the lung significantly overlapped with those of bexarotene treatments (68). Although the effects of these drugs on gene expression profile in the brain remain unclear, the treatment of bexarotene and 9-cis-RA is likely to induce different effects in astrocytes despite the fact that they activate the same RXR. We also found that their effective concentrations to enhance apoE production were different. Although 9-cis-RA showed the strongest effect at 1 μm, bexarotene could induce a similar effect at a lower concentration of 0.1 μm. However, the effective range of bexarotene on apoE was narrower compared with 9-cis-RA, with effects dramatically decreased at higher concentrations (≥1 μm). Thus, it might be critical to maintain an effective concentration of bexarotene when treated in vivo. Although the administration of bexarotene has been reported to suppress Aβ deposition and improve cognitive function in amyloid model mice (21), conflicting results are also reported, and the effects of bexarotene on AD pathology remain controversial (69–73). Our results suggest that one reason for these conflicting results might be due to the difficulty of maintaining effective concentrations of bexarotene in the brain.
In summary, we have demonstrated that all-trans-RA, 9-cis-RA, and 13-cis-RA promote apoE production and lipidation by increasing ABCA1 and ABCG1 through RXR/RAR pathways, which may influence Aβ clearance. The oral administration of 9-cis-RA or bexarotene increases apoE, ABCA1, and ABCG levels in the brain of mice without obvious toxic effects. Given that RA isomers are known to promote neurogenesis, suppress neuroinflammation, and inhibit Aβ production, they may hold therapeutic potentials. Moreover, our findings suggest the RXR/RAR pathway might be a critical target for the development of novel therapeutic strategies for AD.
This work was supported, in whole or in part, by National Institutes of Health Grants R01AG046205, R01AG035355, R01AG027924, P01AG030128, and P01NS074969 (to G. B.). This work was also supported by a grant from the Alzheimer's Drug Discovery Foundation, Alzheimer's Association New Investigator Research Grant, and Mayo Clinic Center for Regenerative Medicine Career Development Award (to T. K.).
- AD
- Alzheimer disease
- RAR
- retinoic acid receptor
- ABCA1
- adenosine triphosphate-binding cassette subfamily A1
- ABCG1
- adenosine triphosphate-binding cassette subfamily G1
- Aβ
- amyloid-β
- APP
- amyloid precursor protein
- apoE
- apolipoprotein E
- LDLR
- low density lipoprotein receptor
- LRP1
- low density lipoprotein receptor-related protein 1
- LXR
- liver X receptor
- RA
- retinoic acid
- RXR
- retinoid X receptor
- TR
- targeted replacement
- GGPP
- geranylgeranyl pyrophosphate
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- qRT-PCR
- quantitative RT-PCR
- ATRA
- all-trans-RA.
REFERENCES
- 1. Zheng H., Koo E. H. (2011) Biology and pathophysiology of the amyloid precursor protein. Mol. Neurodegener. 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 3. Blennow K., de Leon M. J., Zetterberg H. (2006) Alzheimer's disease. Lancet 368, 387–403 [DOI] [PubMed] [Google Scholar]
- 4. Thies W., Bleiler L. (2013) 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 9, 208–245 [DOI] [PubMed] [Google Scholar]
- 5. Rayaprolu S., Mullen B., Baker M., Lynch T., Finger E., Seeley W. W., Hatanpaa K. J., Lomen-Hoerth C., Kertesz A., Bigio E. H., Lippa C., Josephs K. A., Knopman D. S., White C. L., 3rd, Caselli R., Mackenzie I. R., Miller B. L., Boczarska-Jedynak M., Opala G., Krygowska-Wajs A., Barcikowska M., Younkin S. G., Petersen R. C., Ertekin-Taner N., Uitti R. J., Meschia J. F., Boylan K. B., Boeve B. F., Graff-Radford N. R., Wszolek Z. K., Dickson D. W., Rademakers R., Ross O. A. (2013) TREM2 in neurodegeneration: Evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson's disease. Mol. Neurodegener. 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 7. Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. (1993) Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu C. C., Liu C. C., Kanekiyo T., Xu H., Bu G. (2013) Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kanekiyo T., Xu H., Bu G. (2014) ApoE and Aβ in Alzheimer's disease: Accidental encounters or partners? Neuron 81, 740–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang Y., Mucke L. (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148, 1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J., Basak J. M., Holtzman D. M. (2009) The role of apolipoprotein E in Alzheimer's disease. Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song H., Bu G. (2009) MicroRNA-205 inhibits tumor cell migration through down-regulating the expression of the LDL receptor-related protein 1. Biochem. Biophys. Res. Commun. 388, 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liraz O., Boehm-Cagan A., Michaelson D. M. (2013) ApoE4 induces Aβ42, tau, and neuronal pathology in the hippocampus of young targeted replacement apoE4 mice. Mol. Neurodegener. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vance J. E., Hayashi H. (2010) Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta 1801, 806–818 [DOI] [PubMed] [Google Scholar]
- 15. Pfrieger F. W. (2003) Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 60, 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cruchaga C., Kauwe J. S., Nowotny P., Bales K., Pickering E. H., Mayo K., Bertelsen S., Hinrichs A., Alzheimer's Disease Neuroimaging Initiative, Fagan A. M., Holtzman D. M., Morris J. C., Goate A. M. (2012) Cerebrospinal fluid APOE levels: An endophenotype for genetic studies for Alzheimer's disease. Hum. Mol. Genet. 21, 4558–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reitz C., Tang M. X., Luchsinger J., Mayeux R. (2004) Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 61, 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donkin J. J., Stukas S., Hirsch-Reinshagen V., Namjoshi D., Wilkinson A., May S., Chan J., Fan J., Collins J., Wellington C. L. (2010) ATP-binding cassette transporter A1 mediates the beneficial effects of the liver X receptor agonist GW3965 on object recognition memory and amyloid burden in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 285, 34144–34154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitz N. F., Cronican A., Pham T., Fogg A., Fauq A. H., Chapman R., Lefterov I., Koldamova R. (2010) Liver X receptor agonist treatment ameliorates amyloid pathology and memory deficits caused by high-fat diet in APP23 mice. J. Neurosci. 30, 6862–6872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koldamova R. P., Lefterov I. M., Staufenbiel M., Wolfe D., Huang S., Glorioso J. C., Walter M., Roth M. G., Lazo J. S. (2005) The liver X receptor ligand T0901317 decreases amyloid β production in vitro and in a mouse model of Alzheimer's disease. J. Biol. Chem. 280, 4079–4088 [DOI] [PubMed] [Google Scholar]
- 21. Cramer P. E., Cirrito J. R., Wesson D. W., Lee C. Y., Karlo J. C., Zinn A. E., Casali B. T., Restivo J. L., Goebel W. D., James M. J., Brunden K. R., Wilson D. A., Landreth G. E. (2012) ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groot P. H., Pearce N. J., Yates J. W., Stocker C., Sauermelch C., Doe C. P., Willette R. N., Olzinski A., Peters T., d'Epagnier D., Morasco K. O., Krawiec J. A., Webb C. L., Aravindhan K., Jucker B., Burgert M., Ma C., Marino J. P., Collins J. L., Macphee C. H., Thompson S. K., Jaye M. (2005) Synthetic LXR agonists increase LDL in CETP species. J. Lipid Res. 46, 2182–2191 [DOI] [PubMed] [Google Scholar]
- 23. Morikawa M., Fryer J. D., Sullivan P. M., Christopher E. A., Wahrle S. E., DeMattos R. B., O'Dell M. A., Fagan A. M., Lashuel H. A., Walz T., Asai K., Holtzman D. M. (2005) Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-β. Neurobiol. Dis. 19, 66–76 [DOI] [PubMed] [Google Scholar]
- 24. Liu Q., Zerbinatti C. V., Zhang J., Hoe H. S., Wang B., Cole S. L., Herz J., Muglia L., Bu G. (2007) Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron 56, 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinstein D. E. (2001) Isolation and purification of primary rodent astrocytes. Curr. Protoc. Neurosci. 10.1002/0471142301.ns0305s00 [DOI] [PubMed] [Google Scholar]
- 26. Krul E. S., Tikkanen M. J., Schonfeld G. (1988) Heterogeneity of apolipoprotein E epitope expression on human lipoproteins: Importance for apolipoprotein E function. J. Lipid Res. 29, 1309–1325 [PubMed] [Google Scholar]
- 27. Wahrle S. E., Shah A. R., Fagan A. M., Smemo S., Kauwe J. S., Grupe A., Hinrichs A., Mayo K., Jiang H., Thal L. J., Goate A. M., Holtzman D. M. (2007) Apolipoprotein E levels in cerebrospinal fluid and the effects of ABCA1 polymorphisms. Mol. Neurodegener. 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J., Kanekiyo T., Shinohara M., Zhang Y., LaDu M. J., Xu H., Bu G. (2012) Differential regulation of amyloid-β endocytic trafficking and lysosomal degradation by apolipoprotein E isoforms. J. Biol. Chem. 287, 44593–44601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fagan A. M., Holtzman D. M., Munson G., Mathur T., Schneider D., Chang L. K., Getz G. S., Reardon C. A., Lukens J., Shah J. A., LaDu M. J. (1999) Unique lipoproteins secreted by primary astrocytes from wild type, apoE (−/−), and human apoE transgenic mice. J. Biol. Chem. 274, 30001–30007 [DOI] [PubMed] [Google Scholar]
- 30. Fagan A. M., Younkin L. H., Morris J. C., Fryer J. D., Cole T. G., Younkin S. G., Holtzman D. M. (2000) Differences in the Aβ40/Aβ42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann. Neurol. 48, 201–210 [PubMed] [Google Scholar]
- 31. Chen J., Costa L. G., Guizzetti M. (2011) Retinoic acid isomers up-regulate ATP binding cassette A1 and G1 and cholesterol efflux in rat astrocytes: Implications for their therapeutic and teratogenic effects. J. Pharmacol. Exp. Ther. 338, 870–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu C. C., Pearson C., Bu G. (2009) Cooperative folding and ligand-binding properties of LRP6 β-propeller domains. J. Biol. Chem. 284, 15299–15307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Väkevä L., Ranki A., Hahtola S. (2012) Ten-year experience of bexarotene therapy for cutaneous T-cell lymphoma in Finland. Acta Derm. Venereol. 92, 258–263 [DOI] [PubMed] [Google Scholar]
- 34. Nielsen H. M., Mulder S. D., Beliën J. A., Musters R. J., Eikelenboom P., Veerhuis R. (2010) Astrocytic Aβ 1–42 uptake is determined by Aβ-aggregation state and the presence of amyloid-associated proteins. Glia 58, 1235–1246 [DOI] [PubMed] [Google Scholar]
- 35. Idres N., Marill J., Flexor M. A., Chabot G. G. (2002) Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J. Biol. Chem. 277, 31491–31498 [DOI] [PubMed] [Google Scholar]
- 36. Blaner W. S. (2001) Cellular metabolism and actions of 13-cis-retinoic acid. J. Am. Acad. Dermatol. 45, S129–135 [DOI] [PubMed] [Google Scholar]
- 37. Bastien J., Rochette-Egly C. (2004) Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene 328, 1–16 [DOI] [PubMed] [Google Scholar]
- 38. Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., Gronemeyer H. (2006) International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol. Rev. 58, 760–772 [DOI] [PubMed] [Google Scholar]
- 39. Germain P., Chambon P., Eichele G., Evans R. M., Lazar M. A., Leid M., De Lera A. R., Lotan R., Mangelsdorf D. J., Gronemeyer H. (2006) International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol. Rev. 58, 712–725 [DOI] [PubMed] [Google Scholar]
- 40. Willy P. J., Umesono K., Ong E. S., Evans R. M., Heyman R. A., Mangelsdorf D. J. (1995) LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9, 1033–1045 [DOI] [PubMed] [Google Scholar]
- 41. Chambon P. (1996) A decade of molecular biology of retinoic acid receptors. FASEB J. 10, 940–954 [PubMed] [Google Scholar]
- 42. Maden M., Hind M. (2003) Retinoic acid, a regeneration-inducing molecule. Dev. Dyn. 226, 237–244 [DOI] [PubMed] [Google Scholar]
- 43. Tafti M., Ghyselinck N. B. (2007) Functional implication of the vitamin A signaling pathway in the brain. Arch. Neurol. 64, 1706–1711 [DOI] [PubMed] [Google Scholar]
- 44. Cocco S., Diaz G., Stancampiano R., Diana A., Carta M., Curreli R., Sarais L., Fadda F. (2002) Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience 115, 475–482 [DOI] [PubMed] [Google Scholar]
- 45. Shudo K., Fukasawa H., Nakagomi M., Yamagata N. (2009) Towards retinoid therapy for Alzheimer's disease. Curr. Alzheimer Res. 6, 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ahlemeyer B., Krieglstein J. (2000) Inhibition of glutathione depletion by retinoic acid and tocopherol protects cultured neurons from staurosporine-induced oxidative stress and apoptosis. Neurochem. Int. 36, 1–5 [DOI] [PubMed] [Google Scholar]
- 47. Shen H., Luo Y., Kuo C. C., Deng X., Chang C. F., Harvey B. K., Hoffer B. J., Wang Y. (2009) 9-cis-Retinoic acid reduces ischemic brain injury in rodents via bone morphogenetic protein. J. Neurosci. Res. 87, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grant W. B. (1999) Dietary links to Alzheimer's disease: 1999 update. J. Alzheimers Dis. 1, 197–201 [DOI] [PubMed] [Google Scholar]
- 49. Ding Y., Qiao A., Wang Z., Goodwin J. S., Lee E. S., Block M. L., Allsbrook M., McDonald M. P., Fan G. H. (2008) Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer's disease transgenic mouse model. J. Neurosci. 28, 11622–11634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koldamova R. P., Lefterov I. M., Ikonomovic M. D., Skoko J., Lefterov P. I., Isanski B. A., DeKosky S. T., Lazo J. S. (2003) 22R-Hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid β secretion. J. Biol. Chem. 278, 13244–13256 [DOI] [PubMed] [Google Scholar]
- 51. Walter J., van Echten-Deckert G. (2013) Cross-talk of membrane lipids and Alzheimer-related proteins. Mol. Neurodegener. 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wahrle S. E., Jiang H., Parsadanian M., Legleiter J., Han X., Fryer J. D., Kowalewski T., Holtzman D. M. (2004) ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279, 40987–40993 [DOI] [PubMed] [Google Scholar]
- 53. Oram J. F., Lawn R. M. (2001) ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 42, 1173–1179 [PubMed] [Google Scholar]
- 54. Karten B., Campenot R. B., Vance D. E., Vance J. E. (2006) Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J. Biol. Chem. 281, 4049–4057 [DOI] [PubMed] [Google Scholar]
- 55. Wang N., Lan D., Chen W., Matsuura F., Tall A. R. (2004) ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. U.S.A. 101, 9774–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hirsch-Reinshagen V., Maia L. F., Burgess B. L., Blain J. F., Naus K. E., McIsaac S. A., Parkinson P. F., Chan J. Y., Tansley G. H., Hayden M. R., Poirier J., Van Nostrand W., Wellington C. L. (2005) The absence of ABCA1 decreases soluble apoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J. Biol. Chem. 280, 43243–43256 [DOI] [PubMed] [Google Scholar]
- 57. Koldamova R., Staufenbiel M., Lefterov I. (2005) Lack of ABCA1 considerably decreases brain apoE level and increases amyloid deposition in APP23 mice. J. Biol. Chem. 280, 43224–43235 [DOI] [PubMed] [Google Scholar]
- 58. Wahrle S. E., Jiang H., Parsadanian M., Hartman R. E., Bales K. R., Paul S. M., Holtzman D. M. (2005) Deletion of Abca1 increases Aβ deposition in the PDAPP transgenic mouse model of Alzheimer disease. J. Biol. Chem. 280, 43236–43242 [DOI] [PubMed] [Google Scholar]
- 59. Fan J., Donkin J., Wellington C. (2009) Greasing the wheels of Aβ clearance in Alzheimer's disease: the role of lipids and apolipoprotein E. Biofactors 35, 239–248 [DOI] [PubMed] [Google Scholar]
- 60. Koldamova R., Fitz N. F., Lefterov I. (2010) The role of ATP-binding cassette transporter A1 in Alzheimer's disease and neurodegeneration. Biochim. Biophys. Acta 1801, 824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DeMattos R. B., Cirrito J. R., Parsadanian M., May P. C., O'Dell M. A., Taylor J. W., Harmony J. A., Aronow B. J., Bales K. R., Paul S. M., Holtzman D. M. (2004) ApoE and clusterin cooperatively suppress Aβ levels and deposition: evidence that ApoE regulates extracellular Aβ metabolism in vivo. Neuron 41, 193–202 [DOI] [PubMed] [Google Scholar]
- 62. Ma J., Yee A., Brewer H. B., Jr., Das S., Potter H. (1994) Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature 372, 92–94 [DOI] [PubMed] [Google Scholar]
- 63. Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K. R., Paul S. M. (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat. Med. 10, 719–726 [DOI] [PubMed] [Google Scholar]
- 64. Manelli A. M., Stine W. B., Van Eldik L. J., LaDu M. J. (2004) ApoE and Aβ1–42 interactions: effects of isoform and conformation on structure and function. J. Mol. Neurosci. 23, 235–246 [DOI] [PubMed] [Google Scholar]
- 65. Trommer B. L., Shah C., Yun S. H., Gamkrelidze G., Pasternak E. S., Stine W. B., Manelli A., Sullivan P., Pasternak J. F., LaDu M. J. (2005) ApoE isoform-specific effects on LTP: Blockade by oligomeric amyloid-β1–42. Neurobiol. Dis. 18, 75–82 [DOI] [PubMed] [Google Scholar]
- 66. Stratman N. C., Castle C. K., Taylor B. M., Epps D. E., Melchior G. W., Carter D. B. (2005) Isoform-specific interactions of human apolipoprotein E to an intermediate conformation of human Alzheimer amyloid-β peptide. Chem. Phys. Lipids 137, 52–61 [DOI] [PubMed] [Google Scholar]
- 67. Verghese P. B., Castellano J. M., Garai K., Wang Y., Jiang H., Shah A., Bu G., Frieden C., Holtzman D. M. (2013) ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl. Acad. Sci. U.S.A. 110, E1807–E1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang Y., Yao R., Maciag A., Grubbs C. J., Lubet R. A., You M. (2006) Organ-specific expression profiles of rat mammary gland, liver, and lung tissues treated with targretin, 9-cis retinoic acid, and 4-hydroxyphenylretinamide. Mol. Cancer Ther. 5, 1060–1072 [DOI] [PubMed] [Google Scholar]
- 69. Veeraraghavalu K., Zhang C., Miller S., Hefendehl J. K., Rajapaksha T. W., Ulrich J., Jucker M., Holtzman D. M., Tanzi R. E., Vassar R., Sisodia S. S. (2013) Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924. [DOI] [PubMed] [Google Scholar]
- 70. Tesseur I., Lo A. C., Roberfroid A., Dietvorst S., Van Broeck B., Borgers M., Gijsen H., Moechars D., Mercken M., Kemp J., D'Hooge R., De Strooper B. (2013) Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924. [DOI] [PubMed] [Google Scholar]
- 71. Price A. R., Xu G., Siemienski Z. B., Smithson L. A., Borchelt D. R., Golde T. E., Felsenstein K. M. (2013) Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924. [DOI] [PubMed] [Google Scholar]
- 72. Fitz N. F., Cronican A. A., Lefterov I., Koldamova R. (2013) Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models”. Science 340, 924-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. LaClair K. D., Manaye K. F., Lee D. L., Allard J. S., Savonenko A. V., Troncoso J. C., Wong P. C. (2013) Treatment with bexarotene, a compound that increases apolipoprotein-E, provides no cognitive benefit in mutant APP/PS1 mice. Mol. Neurodegener. 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]