Background: Keratinocytes cease proliferation during differentiation, and the mechanism that mediates these events is not well understood.
Results: PKCδ increases p38δ activity, which increases p53 transcription and acts to increase p21Cip1 promoter activity.
Conclusion: PKCδ drives a MAPK cascade to increase p53 to control keratinocyte proliferation.
Significance: This study provides detailed information regarding the mechanisms that control cell proliferation.
Keywords: Cell Differentiation, Cell Proliferation, Cell Signaling, MAP Kinases (MAPKs), p38 MAPK
Abstract
PKCδ suppresses keratinocyte proliferation via a mechanism that involves increased expression of p21Cip1. However, the signaling mechanism that mediates this regulation is not well understood. Our present studies suggest that PKCδ activates p38δ leading to increased p21Cip1 promoter activity and p21Cip1 mRNA/protein expression. We further show that exogenously expressed p38δ increases p21Cip1 mRNA and protein and that p38δ knockdown or expression of dominant-negative p38 attenuates this increase. Moreover, p53 is an intermediary in this regulation, as p38δ expression increases p53 mRNA, protein, and promoter activity, and p53 knockdown attenuates the activation. We demonstrate a direct interaction of p38δ with PKCδ and MEK3 and show that exogenous agents that suppress keratinocyte proliferation activate this pathway. We confirm the importance of this regulation using a stratified epidermal equivalent model, which mimics in vivo-like keratinocyte differentiation. In this model, PKCδ or p38δ knockdown results in reduced p53 and p21Cip1 levels and enhanced cell proliferation. We propose that PKCδ activates a MEKK1/MEK3/p38δ MAPK cascade to increase p53 levels and p53 drives p21Cip1 gene expression.
Introduction
Protein kinase C (PKC) isoforms play a key role as regulators of cell differentiation (1). PKCs include three families. The novel PKCs (δ, ϵ, η, and θ) are activated by diacylglycerol and phospholipids, but they do not respond directly to calcium; classical PKCs (α, β and γ) are calcium-, phospholipid-, and diacylglycerol-dependent; and atypical PKCs (ζ and λ) are calcium- and diacylglycerol-independent and undergo allosteric activation (2, 3). PKCα, βII, δ, ϵ, η, and ζ are expressed in human epidermal keratinocytes (4–10). PKCδ stimulates keratinocyte differentiation (11–16, 18) by activating MAPK signaling to increase nuclear levels of key transcription factors that act to increase target gene transcription (19–21). PKC isoforms also regulate keratinocyte proliferation by altering cell cycle control protein expression (11, 15, 22–25). Because increased keratinocyte differentiation is associated with cessation of proliferation; it makes mechanistic sense that a common signaling cascade may control these processes. p21Cip1 is an important suppressor of cell cycle progression and cell proliferation (26) and is a key PKCδ target in keratinocytes (27). Moreover, increased p21Cip1 expression suppresses keratinocyte proliferation (28–33).
However, despite this progress, we have a limited understanding of the mechanisms whereby PKCδ increases p21Cip1 level. Our previous study indicates that Kruppel-like factor 4 is a downstream mediator of PKCδ action that increases p21Cip1 expression and that this involves KLF42 interaction at DNA sites located in the proximal promoter of the p21Cip1 gene (27). Our present studies identify a second pathway that mediates PKCδ action. This involves PKCδ-dependent activation of the p38δ kinases to activate p53 expression, which interacts via the p53 sites in the distal p21Cip1 promoter to drive transcription. Moreover, we confirm that this regulation is physiologically meaningful using a stratifying epidermal equivalent culture model that mimics in vivo epidermal differentiation. Knockdown of p38δ in this model results in reduced p21Cip1 expression, enhanced cell proliferation, and reduced differentiation.
EXPERIMENTAL PROCEDURES
Chemicals, Reagents, and Antibodies
Rabbit polyclonal antibodies for MEK3 (sc-961), PKCδ (sc-937), p53 (sc-6243), goat anti-p38δ (sc-7587), and mouse monoclonal antibodies for p38δ (sc-271292), p38α (sc-7972), and anti-MEK3-P (sc-8407) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-MEKK1 (ab69533) and anti-MEKK1-P (Thr-1381) (ab138662) were purchased from Abcam (Cambridge, MA).
Rabbit polyclonal antibodies against p21Cip1 (2947) and PKCδ-P(Tyr-311) were obtained from Cell Signaling Technology (Danvers, MA) and mouse monoclonal antibody against β-actin (A-5441) and anti-FLAG M2 (F3165) were purchased from Sigma Aldrich. Peroxidase-conjugated anti-mouse IgG (NXA931) and peroxidase-conjugated anti-rabbit IgG (NA934V) were obtained from GE Healthcare. Phorbol ester, 12-O-tetradecanoylphorbol-13-acetate (TPA) (524400) and rottlerin (557370) were obtained from Calbiochem (Billerica, MA).
We report results using control (sc-37007), p38δ (sc-36456), PKCδ (sc-36253), p53 (sc-44218), and MEK3 (sc-35907) siRNA reagents obtained from Santa Cruz Biotechnology. Key findings were confirmed using additional siRNA that targets p38δ (D-003591-01-0005 and D-003591-03-0005), PKCδ (D-003524-03-0005 and D-003524-05-0005), MEK3 (D-003509-02-0005 and D-003509-06-0005), and p53 (D-003329-05-0005 and D-003329-07-0005), which were purchased from Dharmacon, Inc. (Lafayette, CO).
Cell Culture, Viruses, and Plasmids
Keratinocyte serum-free medium (KSFM), trypsin, and Hank's balanced salt solution were purchased from Invitrogen. Primary cultures of human epidermal keratinocytes were obtained by separation of epidermis from the dermis with dispase followed by cell dispersion in trypsin. Cells were cultured in KSFM supplemented with epidermal growth factor and bovine pituitary extract (34, 35). Adenoviruses encoding MEK3, HA-p38δ, PKCδ, and empty control virus (Ad5-MEK3, tAd5-HA-p38δ, Ad5-PKCδ, Ad5-FLAG-p38δ, and Ad5-EV) were prepared by propagation in HEK293 cells followed by cesium chloride gradient centrifugation. For experiments, keratinocytes were incubated with 5 to 15 MOI of adenovirus in KSFM containing 6 μg/ml polybrene. Tetracycline-inducible viruses (tAd-EV, tAd5-PKCδ, tAd5-HA-38δ) were co-infected with Ad5-TA encoding virus to induce PKCδ and p38δ expression (13).
The human p21Cip1 promoter luciferase plasmid, p21-2326, was a gift from Dr. Bert Vogelstein (36). p21-124, p21-101, and p21-60 were obtained from Dr. Toshiyuki (37). The other p21Cip1 truncation plasmids (p21-251, p21-501, p21-1001, and p21-2001) and the p53 site mutants, p21-2326 p53(Δ1), p21-2326 p53(Δ2), and p21-2326 p53(Δ1-2), were constructed in pBluescript II KS(+) (38). The p38δ expression vector was pcDNA3.1-HA-p38δ. p38α, MEK3, PKCδ, and FLAG-DNp38α expression plasmids were described previously (34, 39). PG13-Luc was obtained from Dr. Nancy Colburn (40).
Statistical Methods
All experiments were performed a minimum of three times, and significant difference was determined using the Student's t test.
p21Cip1 Promoter Activity Assay
p21Cip1 promoter reporter plasmid (1 μg) was mixed with 2 μl of FuGENE 6 (Roche Applied Science) diluted with 98 μl of KSFM. The mixture was incubated for 25 °C for 20 min and added to a 50% confluent culture of human epidermal keratinocytes maintained in KSFM in a 9.6-cm2 dish. For co-transfection experiments, 1 μg of p21Cip1 promoter reporter plasmid and 1 μg of pcDNA3.1 or pcDNA3.1-HA-p38δ were mixed with FuGENE 6 and added to the cells. After 24 h, the cells were harvested for luciferase activity assay, and data were normalized based on protein content.
siRNA-mediated Knockdown
Keratinocytes were electroporated with control or p38δ siRNA using an Amaxa electroporator and the VPD-1002 nucleofection kit (Amaxa, Cologne, Germany). Keratinocytes were harvested with trypsin and replated 1 day prior to electroporation. After 24 h, the keratinocytes were harvested with trypsin, and 1 × 106 cells were centrifuged at 2000 rpm, washed in 1 ml of sterile 1× phosphate-buffered saline (pH 7.5), and suspended in 100 μl of keratinocyte nucleofection solution. Control or p38δ siRNA (3 μg) was added to the cell suspension, mixed by gentle pipetting, and electroporated using the T-018 setting. KSFM (500 μl) was added, and the suspension was transferred to a 60-mm dish containing 4 ml of KSFM medium. The cells were maintained for various times before extracts were prepared for preparation of RNA or protein or activity assay. This electroporation method delivers nucleic acid reagents to cells with greater than 90% efficiency (13).
Immunoblot Analysis
Equal amounts of protein were electrophoresed on a 12% denaturing polyacrylamide gel and transferred to nitrocellulose. The membranes were blocked with 5% skimmed milk in Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 for 1 h. Following this, the blots were incubated with primary antibody, washed, and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 2 h. Chemiluminescent detection (Amersham Biosciences) was used to detect antibody binding. β-Actin was used as the loading control in all immunoblot experiments.
Quantitative RT-PCR
Total RNA was isolated using Illustra RNAspin mini kit (GE Healthcare), and 1 μg of RNA was used for cDNA synthesis. Gene expression was measured by real time PCR using Light Cycler 480 SYBR Green I Master Mix (04-707 516 001) from Roche Diagnostics (Indianapolis, IN). The signals were normalized using cyclophilin A control primers. The gene specific primers used for detection of mRNA levels were as follows: p38δ (forward, 5′-TGT GCA GAA GCT GAA CGA CAA AGC; reverse, 5′-AGG GTT CAA AGA AGG GAT GGG TGA), p21Cip1 (forward, 5′-AAG ACC ATG TGG ACC TGT CAC TGT; reverse, 5′-AGG GCT TCC TCT TGG AGA AGA TCA), PKCδ (forward, 5′-GGC CAC ATC AAG ATT GCC GAC TTT; reverse, 5′-ACT GGC CAA TGA GCA TCT CGT ACA), MEK3 (forward, 5′-AGC TCA TGG ACA CAT CCT TGG ACA; reverse, 5′- ACA CAT CTT CAC ATG GCC CTC CTT), and cyclophilin A (forward, 5′-cat ctg cac tgc caa gac tga; reverse, 5′-TTC ATG CCT TCT TTC ACT TTG C).
p38δ Activity Assay
Kinase assays were used to determine the activity of the endogenous p38δ isoform. Keratinocyte cell lysates were prepared under nondenaturing conditions. Equal amounts of total protein (200 μg) were used for each kinase assay. p38δ-specific antibodies (sc-7587 or sc-271292) were used to selectively immunoprecipitate this enzyme. The precipitated kinase was then assayed for ability to phosphorylate ATF-2 in an in vitro kinase reaction performed in the presence of ATP. Phosphorylation of the substrate proteins was analyzed by immunoblot using phosphorylated ATF-2-specific antibody (34, 41).
Epidermal Equivalent Cultures
Keratinocytes, freshly isolated from foreskins, were harvested with trypsin, and 1.5 × 106 cells were electroporated with 3 μg of control or p38δ siRNA and replated. After an additional 72 h, the cells were harvested, and 2 × 106 cells from each group were re-electroporated with 3 μg of control siRNA or p38δ siRNA. They were then allowed to settle overnight onto the membrane in Millicell-PCF chambers (diameter, 12 mm; 0.4-μm pore size) in KSFM (Millipore, Billerica, MA). The next day, the cells were shifted to Epilife medium containing 1.4 mm calcium chloride and 5 μg/ml of vitamin C and cultured at the air-liquid interface with addition of fresh Epilife medium every 2 days. After 4 days, the epidermal equivalents were harvested for preparation of RNA, protein, and histological sections (42). Total RNA was isolated for qRT-PCR using the Illustra RNAspin Mini kit (GE Healthcare). For protein lysates, the inserts were washed twice with PBS, and the cells were harvested in 0.0625 m Tris-HCl, pH 7.5, containing 10% glycerol, 5% SDS, and 5% β-mercaptoethanol. They were sonicated and centrifuged at 10,000 rpm for 5 min, and the supernatant was collected for immunoblot.
RESULTS
PKCδ, MEK3, and p38δ Regulate p21Cip1 Level
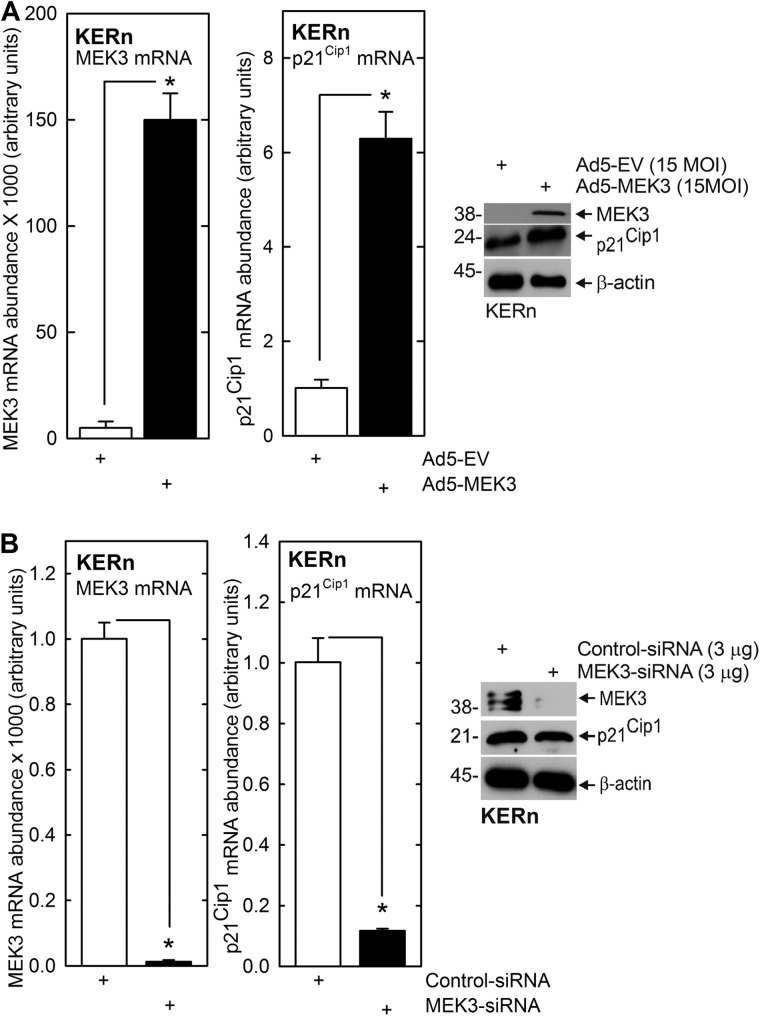
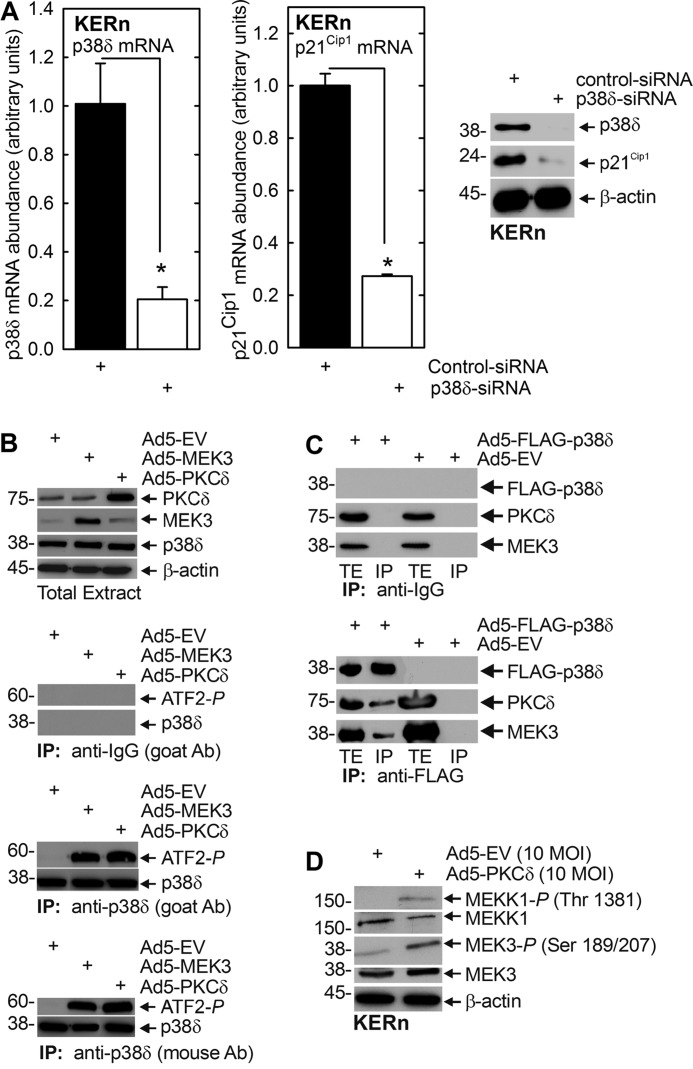
PKCδ regulates keratinocyte proliferation by increasing p21Cip1 expression via mechanisms that are not well understood (27). We propose that MEK3 and p38δ may be the intervening signaling kinases that mediate this regulation. To test this, we expressed MEK3 and p38δ and monitored the impact on p21Cip1 expression. Fig. 1, A and B, show that p21Cip1 expression is directly correlated with MEK3 level. MEK3 expression increases and MEK3 knockdown reduces p21Cip1 mRNA and protein level. The impact on p21Cip1 mRNA level is particularly dramatic. We next examined the role of p38δ. Three p38 MAPK isoforms, p38α, β, and δ, are expressed in keratinocytes (43, 44), and MEK3 activates p38δ to stimulate differentiation-associated gene expression (21, 41). We therefore assessed whether p38δ plays a role in regulating p21Cip1 level. Fig. 2A shows that siRNA-dependent knockdown of p38δ results in a marked reduction in p21Cip1 encoding mRNA and p21Cip1 protein. If p38δ is a mediator in this pathway, we would expect that PKCδ and MEK3 should increase p38δ activity. This was studied by measuring the ability of p38δ to phosphorylate ATF2 transcription factor on threonine 71 (21, 34, 41). Cells were infected with empty-, PKCδ-, or MEK3-encoding adenovirus. After 24 h, endogenous p38δ was immunoprecipitated, and ability of precipitated p38δ to phosphorylate ATF2 was measured. We used two antibodies to pull down p38δ: one prepared in goats and a second in mice. This analysis revealed that expression of PKCδ or MEK3 enhances p38δ activity (Fig. 2B). No precipitation was observed when anti-IgG was used in the pulldown as a negative control.
FIGURE 1.
MEK3 regulates p21Cip1 mRNA level. A, KERn were infected with 15 MOI of Ad5-EV or Ad5-MEK3, and after 24 h, RNA was isolated, and MEK3 and p21Cip1 mRNA levels were assessed by qRT-PCR. The values are mean ± S.E. (n = 3). In parallel identically treated cultures, protein extracts were prepared for assay of p21Cip1 and MEK3 protein level by immunoblot. MEK3 is also present in Ad5-EV-infected cells but is only visible at higher film exposures (not shown). B, KERn were electroporated with 3 μg of control siRNA or MEK3 siRNA. After 24 h, RNA was prepared, and MEK3 and p21Cip1 mRNA level of was measured by qRT-PCR. The values are mean ± S.E. (n = 3). The asterisks indicate significant differences (*, p < 0.005). In parallel identically treated cultures, protein extracts were prepared for assay of p21Cip1 and MEK3 protein level by immunoblot. Similar results were observed with other MEK3 siRNA, indicating that these responses are not due to off-site effects (not shown).
FIGURE 2.
p38δ regulates p21Cip1 protein and mRNA level and promoter activity. A, KERn were electroporated with 3 μg of control or p38δ siRNA, and after 24 and 48 h, respectively, extracts were prepared for detection of p38δ and p21Cip1 mRNA and protein. The values are mean ± S.E. (n = 3). The asterisks indicate significant differences (*, p < 0.005). Similar results were observed with other p38δ siRNA, indicating that these changes are not due to off-site actions (not shown). B, KERn were infected with 10 MOI EV, Ad5-EV, Ad5-MEK3, or Ad5-PKCδ. After 48 h, 200 μg of protein extract was used to immunoprecipitate p38δ for use in an in vitro p38 kinase activity assay using ATF2 as substrate (44). Immunoprecipitation was achieved using anti-p38δ antibody (Ab) produced in goat or mouse, or anti-IgG, as a negative control. The level of precipitated p38δ and ATF2 phosphorylation was monitored by immunoblot. Similar results were observed in each of three experiments. C, KERn were infected with 10 MOI of Ad5-EV or Ad5-FLAG-p38δ and 2.5 MOI of Ad5-TA encoding virus, and after 24 h, total extract was prepared for electrophoresis or precipitation with anti-IgG or anti-FLAG. Immunoprecipitate (IP) and total extract (TE) were electrophoresed and for immunoblot with anti-FLAG, anti-PKCδ, and anti-p38δ. Similar results were observed in each of three experiments. D, PKCδ activates MEKK1 and MEK3. KERn were infected with empty or PKCδ encoding adenovirus, and after 24 h, extracts were prepared for immunoblot to detect the indicated epitopes. Similar results were observed in each of three experiments.
The ability of these kinases to produce a change in p21Cip1 expression predicts a physical interaction. To assess this, we infected cells with empty or FLAG-p38δ encoding vector and prepared extracts for immunoprecipitation with anti-FLAG. Fig. 2C shows that PKCδ and MEK3 co-precipitate with FLAG-p38δ, suggesting an interaction of p38δ with PKCδ and MEK3. No precipitation was observed when anti-IgG was substituted for anti-FLAG (Fig. 2C). We also monitored the activity status of MEKK1 and MEK3. In the presence of increased levels of PKCδ, there is a substantial increase in MEKK1 and MEK3 activity as evidence by enhanced phosphorylation (Fig. 2D).
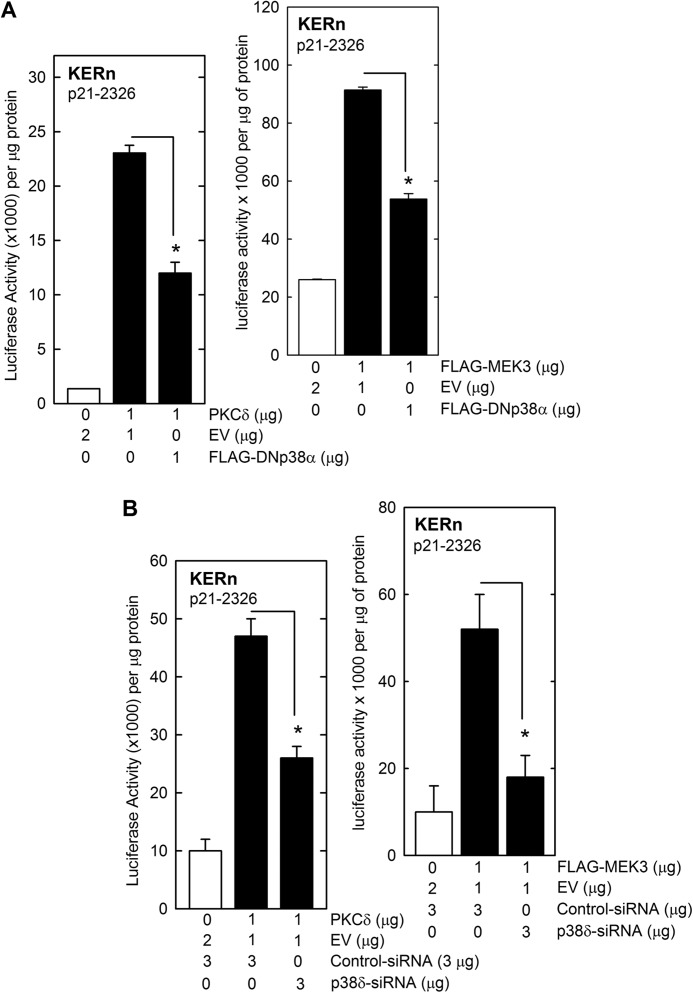
To further confirm a role for this cascade, we expressed PKCδ or MEK3 in the presence of DNp38α. DNp38α inhibits function of all p38 MAPK isoforms (41). Fig. 3A shows that DNp38α inhibits PKCδ- and MEK3-dependent p21Cip1 promoter activity, confirming that p38δ activity is required for activation of p21Cip1. We also examined the impact of p38δ knockdown and found that this also reduces the ability of PKCδ and MEK3 to activate p21Cip2 promoter activity (Fig. 3B). These findings suggest that a PKCδ/MEK3/p38δ pathway regulates p21Cip1 expression.
FIGURE 3.
p38δ is required for PKCδ and MEK3-dependent activation of p21Cip1 promoter transcription. A, KERn were transfected with 1 μg of p21-2326 luciferase reporter plasmid in the presence of 1 μg of empty vector or vector encoding PKCδ, MEK3, or DNp38α. Levels were adjusted to a total of 3 μg per transfection by addition of empty vector (EV). At 24 h post-transfection, cell extracts were prepared and assayed for promoter activity. B, KERn (1 million cells per group) were electroporated with 3 μg of control or p38δ siRNA. At 48 h post electroporation, the cells were harvested and counted, and 0.5 million cells from each group were re-electroporated with 1 μg of endotoxin-free p21-2326 luciferase reporter plasmid in the presence of 2 μg of PKCδ or MEK3 encoding endotoxin-free plasmid or the empty vector. After an additional 24 h, cell extracts were prepared for luciferase assay. The values are mean ± S.E. (n = 3). The asterisks indicate significant differences (*, p < 0.005).
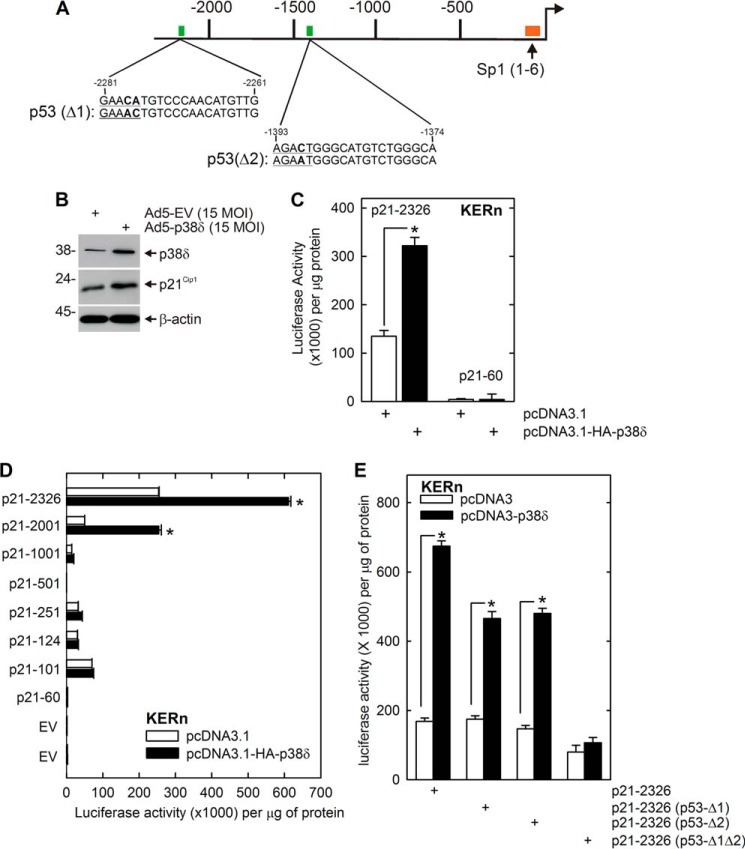
p38δ Response Elements in p21Cip1 Promoter
We hypothesized that p38δ may regulate p21Cip1 gene promoter activity to increase p21Cip1 expression. To examine this, we transfected keratinocytes with p21-2326 and monitored the impact of p38δ on promoter activity. p21-2326 encodes the full-length p21Cip1 promoter and upstream regulatory region (Fig. 4A) (27, 38). Fig. 4, B and C, shows that p38δ overexpression increases p21-2326 activity and confirms that this is associated with an increase in p21Cip1 protein. Moreover, we confirm that this activity is enhancer element-mediated, as p21-60, which encodes only the minimal promoter, is not regulated by p38δ. These findings implicate p38δ as regulating p21Cip1 gene expression.
FIGURE 4.
p38δ acts via p53 response elements on the p21Cip1 promoter. A, p21Cip1 promoter schematic showing Sp1 and p53 DNA response elements. The numbers indicate distance in nucleotides relative to the transcription start site. B, p38δ increases p21Cip1 level. KERn were infected with 15 MOI of Ad5-EV or Ad5-p38δ. After 24 h, extracts were prepared for detection of p38δ and p21Cip1 protein. β-Actin is used as the loading control. C, KERn were transfected with 1 μg each of p21-2326 or p21-60 p21Cip1 promoter reporter plasmids and 1 μg of pcDNA3.1 or pcDNA3.1-HA-p38δ, and after 24 h, extracts were prepared for assay of luciferase activity. Values are mean ± S.E. (n = 3). The asterisk indicates a significant difference (*, p < 0.005). D, KERn were transfected with 1 μg of the indicated p21Cip1 luciferase reporter plasmid and 1 μg of pcDNA3.1 or pcDNA3.1-HA-p38δ, and after 24 h, extracts were prepared for assay of luciferase activity. The values are mean ± S.E. (n = 3), and the asterisks indicate significant differences in activity (*, p < 0.005). E, p53 response elements are required for p38δ activation of p21Cip1 expression. KERn were transfected with 1 μg of the indicated p21Cip1 promoter plasmid along with 1 μg of pcDNA3.1 or pcDNA3.1-HA-p38δ, and after 24 h, extracts were prepared for luciferase activity assay. The values are mean ± S.E. (n = 3). The asterisks indicate significant difference (*, p < 0.005).
To locate p21Cip1 promoter elements that mediate this regulation, we measured the impact of p38δ on activity of a series of promoter deletion constructs. Fig. 4D shows that activity of the p21-2326 and p21-2001 constructs are increased in response to p38δ, but that the shorter constructs are not. This suggests that DNA elements, located between nucleotides −2326/−1001, mediate this regulation. This region encodes two p53 response elements (Fig. 4A). To determine whether these elements are required for this regulation, we transfected keratinocytes with empty- or p38δ-encoding expression plasmid and monitored the impact on activity of p21-2326 constructs encoding wild-type and mutant p53 binding sites. Fig. 4E shows that mutation of the p53 transcription factor binding sites produces a substantial reduction in promoter response to p38δ.
PKCδ, MEK3, and p38δ Suppress Keratinocyte Proliferation
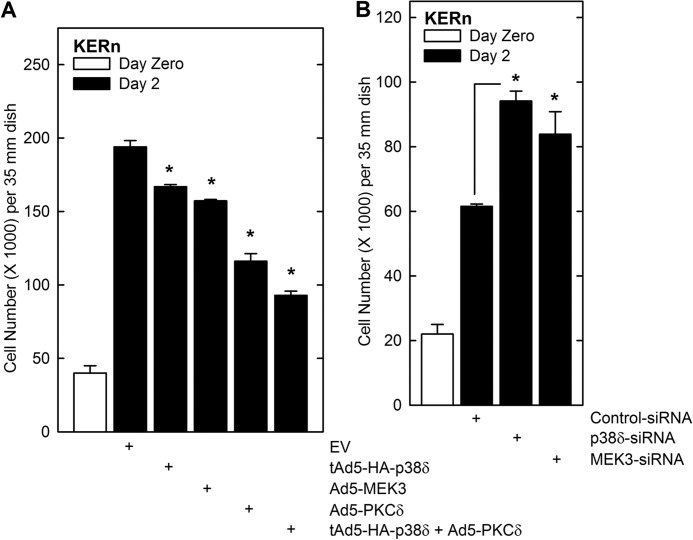
Our findings suggest that PKCδ, MEK3, and p38δ activate p21Cip1 expression, and we predict that this should be associated with reduced cell proliferation. To test this, KERn were plated at low density and on day zero infected with empty (EV) adenovirus or virus encoding PKCδ, MEK3, or p38δ. After an additional 2 days, the cells were harvested and counted. Fig. 5A shows that PKCδ and MEK3 suppress proliferation, and a more dramatic reduction is observed when both PKCδ and p38δ are present. These findings are consistent with a role for these kinases as proliferation suppressors. We also tested the inverse experiment and determined whether p38δ- or MEK3 knockdown enhances proliferation. Indeed, as shown in Fig. 5B, loss of either kinase resulted in a 15 to 20% increase in cell number.
FIGURE 5.
PKCδ, MEK3, and p38δ alter keratinocyte proliferation. A, KERn were seeded at 15,000 cells per well in triplicate 35-mm dishes and permitted to attach. At time zero, the cells were infected with 15 MOI of the indicated adenovirus. After an additional 48 h, the cells were harvested and counted. The white bar indicates the cell count at time zero, and the black bars indicate the 48-h counts. Ad5-TA encoding virus (2.5 MOI) was included in each treatment (27). The values are mean ± S.E. (n = 3). The asterisks indicate a significant difference, p < 0.05. B, KERn (1 million) were twice electroporated with 3 μg of the indicated siRNA. After the second electroporation, 15,000 cells were seeded into six-well cluster wells, and cell number was assessed at time zero and 48 h later. The values are mean ± S.E. (n = 3). The asterisks indicate a significant difference (*, p < 0.05).
p53 Level Is Regulated by p38δ
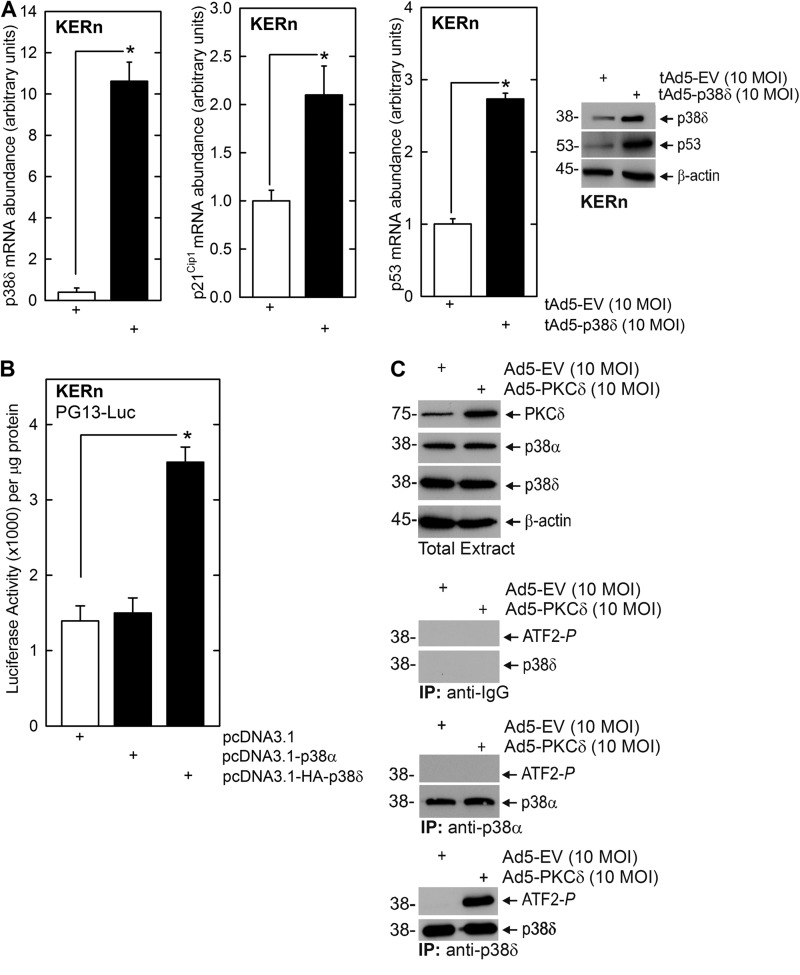
The fact that the p53 transcription factor binding sites appear important for p38δ regulation of p21Cip1 gene expression suggests that p53 level may be regulated by p38δ. Fig. 6A shows that p38δ-expressing keratinocytes produce increased p53 mRNA and protein and increased p21Cip1 mRNA. Fig. 6B shows that the increase in p53 mRNA is associated with increased p53 (PG13-Luc) promoter activity. Moreover, the increase is specific for the p38δ isoform, as the other major p38 isoform present in keratinocytes, p38α, does not cause a significant increase. To further confirm that the regulation is selectively dependent upon p38δ, and not p38α which is abundant in keratinocytes (43, 44), we examined the impact of PKCδ expression on p38α and p38δ phosphorylation (activation). Cells were infected with control or PKCδ-encoding adenovirus and after 24 h p38α or p83δ were precipitated, and ability of the precipitated kinases to phosphorylate ATF2 was monitored. A control precipitation was performed with anti-IgG. These results show that PKCδ does not alter p38δ or p38α level and that only p38δ is activated (phosphorylated) (Fig. 6C).
FIGURE 6.
p53 is a downstream target of p38δ. A, p38δ increases p53 mRNA level. KERn were infected with 10 MOI of empty or p38δ-encoding adenovirus and 2.5 MOI of Ad5-TA and after 24 and 48 h, respectively. Extracts were prepared for qRT-PCR assay of mRNA level or immunoblot to detect the indicated targets. The values are the mean ± S.E. (n = 3). Significant differences are indicated by asterisks (*, p < 0.005). B, p38δ increases p53 promoter activity. KERn were transfected with 1 μg of PG13-Luc in the presence of 1 μg of empty plasmid or plasmid encoding p38δ or p38α, and after 24 h, promoter activity was monitored. The values are mean ± S.E. (n = 4), and the asterisks indicate a significant difference (*, p < 0.005). C, PKCδ activates p38δ but not p38α. KERn were infected with 10 MOI of empty or PKCδ encoding adenovirus. After 48 h, cell extracts were prepared, and 200 μg of protein was immunoprecipitated with anti-p38α or anti-p38δ, and the ability to phosphorylate ATF2 was monitored. Similar results were observed in each of three experiments.
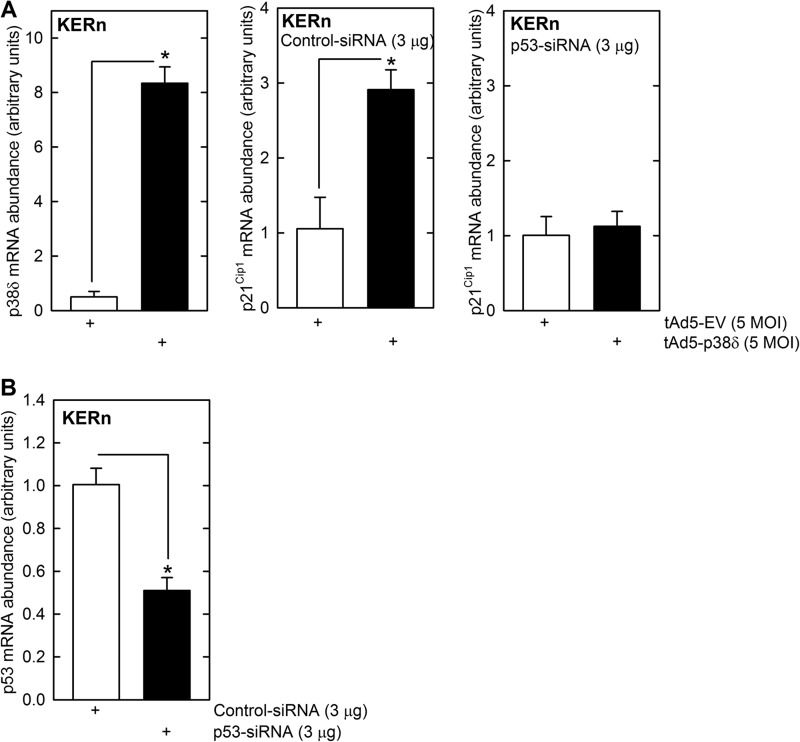
To further assess the role of p53, we electroporated cells with p53 siRNA and then challenged with p38δ encoding or empty virus and monitored the impact on p21Cip1 mRNA level. As shown in Fig. 7A, expression of p38δ substantially increases p21Cip1 mRNA level, and this increase is completely inhibited in the presence of p53 siRNA, confirming that increased p53 is required for the response. Fig. 7B shows that treatment with p53 siRNA reduces p53 encoding mRNA. These findings suggest that increased p53 level is required for PKCδ, MEK3, and p38δ activation of the p21Cip1 promoter.
FIGURE 7.
p53 is required for the p38δ-dependent increase in p21Cip1 expression. A, KERn were electroporated with 3 μg of control (scrambled) or p53-siRNA and after 48 h infected with 5 MOI of tAd5-EV or tAd5-p38δ with 2.5 MOI of Ad5-TA. After an additional 24 h, cells were harvested to monitor mRNA levels by qRT-PCR. Values are mean ± S.E. (n = 4). The asterisks indicate a significant difference (*, p < 0.005). Similar results were observed with other control and p53 siRNA indicating that these responses are not due to off-site actions (not shown). B, p53 siRNA reduces p53 mRNA level. This plot confirms that the p53-specific siRNA, delivered as outlined above, reduces p53 expression. Values are mean ± S.E. (n = 4). The asterisks indicate a significant difference (*, p < 0.005).
p38δ Regulation of p53 and p21Cip1 Expression during Differentiation
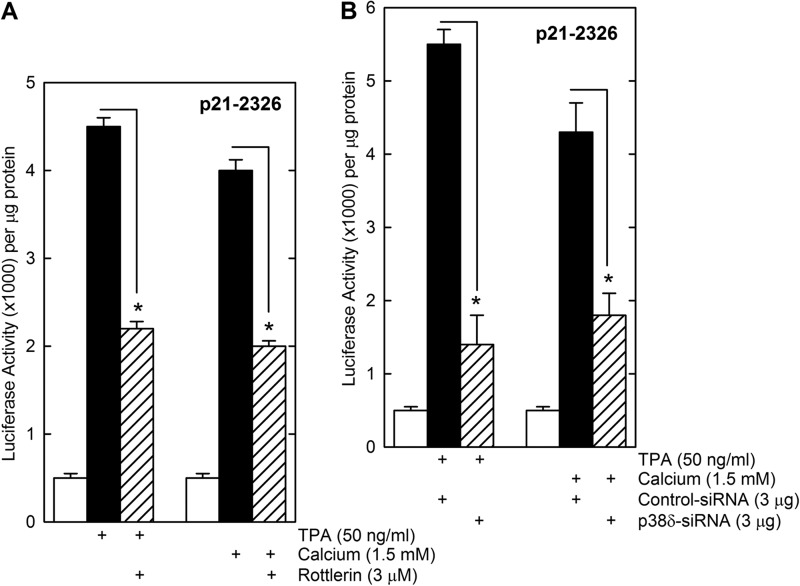
The above studies implicate PKCδ and p38δ in a cascade that activates p21Cip1 expression. We wanted to determine whether TPA and calcium, which activate keratinocyte differentiation and suppress keratinocyte proliferation (45), operate via this cascade. KERn were treated with TPA or calcium in the presence of rottlerin, a PKCδ inhibitor, or p38δ knockdown using p38δ siRNA, and the impact on p21-2326 activity was monitored. Fig. 8, A and B, show that TPA and calcium induce p21-2326 promoter activity and that this can be reduced by treatment with rottlerin or p38δ siRNA.
FIGURE 8.
Activation of p21Cip1 expression by keratinocyte differentiating agents. A, KERn (1 million) were transfected with 0.5 μg of p21–2326 and at 24 h after plating treated for 18 h with the indicated agent prior to preparation of extracts for luciferase assay. B, KERn (1 million each group) were electroporated with 3 μg of control or p38δ siRNA. At 48 h post-electroporation, the cells were harvested and counted, and 0.5 million cells from each group were re-electroporated with 1 μg of endotoxin-free p21-2326 luciferase reporter plasmid. At 6-h post electroporation, the cells were treated with the indicated concentration of TPA or calcium. After an additional 24 h, cell extracts were prepared for luciferase assay. The values are mean ± S.E. (n = 3). The asterisks indicate significant differences (*, p < 0.005).
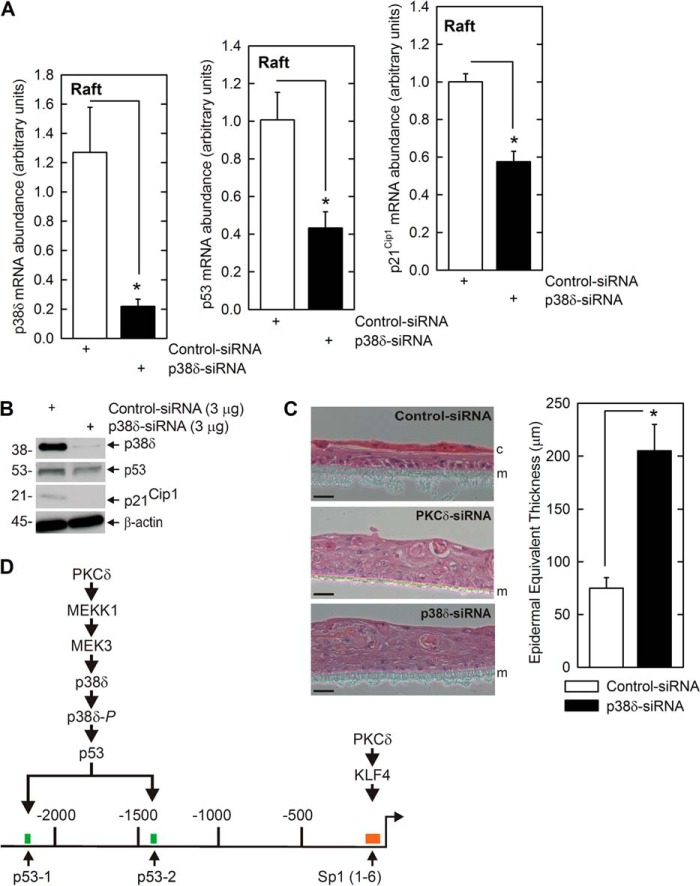
To further assess the biological relevance of this regulation, we examined the impact of altering p38δ level using a keratinocyte epidermal equivalent system. In this model, keratinocytes are grown at the air-liquid interface to produce a stratified, multilayered, and differentiated epidermal equivalent that closely mimics in vivo epidermis. This system can be used to assess biological response under in vivo-like conditions (46). Primary foreskin keratinocytes were electroporated with p38δ or control siRNA and then transferred to Millicell chambers for growth as epidermal equivalent cultures. Fig. 9A shows that the p38δ siRNA treatment reduces p38δ mRNA and that this is associated with reduced mRNA encoding p53 and p21Cip1. Fig. 9B shows that forced reduction in p38δ protein level also reduces p53 and p21Cip1 protein level. To assess the biological impact on differentiation and proliferation, we monitored morphology of the epidermal equivalent cultures. Fig. 9C shows that cultures expressing normal endogenous levels of p38δ undergo appropriate differentiation and produce a multilayered tissue and stable cornified layer. In contrast, p38δ knockdown cells produce a thicker structure comprised of additional layers that is characterized by the absence of a cornified layer, which is indicative of enhanced proliferation/reduced differentiation. The plot quantifies the increase in thickness that is observed in p38δ or PKCδ knockdown cultures. These findings confirm, using an in vivo-like model, that p38δ is required for p21Cip1 expression and differentiation-associated suppression of proliferation.
FIGURE 9.
p38δ regulates p21Cip1 expression and controls proliferation in epidermal equivalent model. A, p38δ is required for expression of p53 and p21Cip1. KERn were twice electroporated with control or p38δ siRNA and then plated in Millicell wells to form stratified and differentiated epidermal equivalent cultures. After 4 days at the air-liquid interface, the cultures were harvested, and extracts were prepared for assay of p38δ, p53, and p21Cip1 mRNA. The values are mean ± S.E. (n = 4), and the asterisks indicate a significant difference (*, p < 0.005). Similar results were observed with other p38δ siRNA, indicating that these responses are not due to off-site actions (not shown). B, p38δ is required for expression of p53 and p21Cip1 proteins. Extracts were prepared from epidermal equivalents, maintained for 4 days at the air-liquid interface as described in A, and the level of the indicated proteins was monitored by immunoblot. Similar results were observed in each of three experiments. C, p38δ is required for appropriate cell proliferation and differentiation. KERn were twice electroporated with control or p38δ siRNA and seeded for epidermal equivalent culture. After 4 days at the air-liquid interface, the cultures were harvested and stained with H&E. The membrane (m) and cornified layer (c) are indicated. Similar results were observed in each of three experiments. The graph shows that loss of p38δ expression results in reduced cornification and production of a thicker epidermal equivalent (increased proliferation). The values are mean ± S.E. (n = 6). The asterisk indicates a significant difference (*, p < 0.005). D, a PKCδ/p53 regulatory pathway controls proliferation. Our studies suggest that PKCδ activates a MEKK1/MEK3/p38δ mitogen-activated protein kinase module to increase p53 levels and that p53 then interacts with p53 response elements in the p21Cip1 promoter to increase p21Cip1 expression and reduce proliferation. Also indicated is PKCδ activation of KLF4 expression, which acts via the Sp1 response elements in the p21Cip1 promoter to drive transcription. This parallel PKCδ-activated pathway was described previously (27).
DISCUSSION
Protein kinase C and p38 MAPK control of cell proliferation has been studied in several systems, and p53 and p21Cip1 have been implicated in some of these studies. In most cases, this regulation involves PKC-dependent covalent modification and stabilization of p53. For example, reovirus infection of target cells increases PKCδ, RAS, and p38 MAPK signaling, leading to increased p53 Ser-1133 phosphorylation and stabilization (47) and treatment of dopamanergic neurons with nitric oxide increases p53 Ser-15 phosphorylation to stabilize p53 against proteasome degradation (48). In vascular smooth muscle cells, PKCδ increases p53 Ser-46 phosphorylation to increase p53 level, and peroxide treatment of aortic endothelial cells results in PKCδ-dependent accumulation of p53 (49). Some studies also implicate p38 MAPK as being important in PKCδ regulation of p53 gene expression. In smooth muscle cells, PKCδ regulation of p53 expression requires p38 MAPK (50). In human endometrial cancer cells, PKCδ activation leads to increased p53 and p21Cip1 expression in a process that is inhibited by GF109203X (51). Very few studies have described PKCδ activation of p53 gene transcription (50). Moreover, in those cases where alteration of p53 function involves p38 MAPK, the p38 isoform involved was not been identified (49).
Novel PKC isoforms are important regulators of keratinocyte function that increase MAPK signaling and the nuclear level of key transcription factors to activate expression of differentiation-associated genes (1, 11–20, 20, 21). PKC isoforms also regulate keratinocyte proliferation (11, 15, 22–25), and p21Cip1 is a key target (27). However, despite this progress, we have a limited understanding of the mechanisms whereby PKCδ regulates p21Cip1 level. Previous studies from our group indicate that Kruppel-like factor 4 and Sp1 transcription factors have a role (27). However, as p21Cip1 is a central controller of cell proliferation, it is expected that the regulation will be complex and that multiple/reinforcing mechanisms may exist.
Our present studies identify a novel PKCδ/MEK3/p38δ/p53/p21Cip1 pathway that regulates PKCδ activation of p21Cip1 expression in keratinocytes. This pathway involves PKCδ-dependent activation of MEK3 and p38δ kinase, which increases p53 expression. p53, in turn, interacts with the p21Cip1 promoter via canonical p53 response elements to activate transcription, which ultimately leads to a reduction in cell proliferation (Fig. 9D). Also shown is a parallel signaling pathway wherein PKCδ acts via KFL4 to drive transcription via the proximal cluster of Sp1 binding sites (Fig. 9D) (27). It is interesting that although keratinocytes express several PKC isoforms (19, 21, 35), the form implicated as controlling proliferation in the present study is PKCδ. This is particularly intriguing, as PKCδ has been implicated as the isoform that drives keratinocyte differentiation (34, 52). This finding suggests that enhanced differentiation and reduced proliferation, which are key simultaneously occurring events during keratinocyte maturation, are controlled by a common pathway that involves PKCδ. It makes sense, in terms of regulatory efficiency, that a common pathway would control both processes.
It is also interesting that p38δ is involved as a downstream mediator of PKCδ action to increase p53 and p21Cip1. Keratinocyte express three p38 MAPK isoforms, p38α, β, and δ. The major forms are p38α and δ (34, 52). In the present study, we show that p38δ activation, as evidenced by increased phosphorylation, is associated with increased p53 and p21Cip1 expression. In contrast, p38α is not important as a regulator of p53 or p21Cip1, as it produces minimal changes in expression of these genes and is not activated (phosphorylated) in response to PKCδ. In addition, a known downstream mediator of PKCδ-dependent keratinocyte differentiation (17, 41), MEK3, is required for activation of p53 and p21Cip1 expression.
As mentioned above, previous reports in other systems indicate that PKCδ can increase p53 level. In most cases, this is associated with direct PKCδ-dependent phosphorylation of p53 (47, 48, 50), but in other cases, this involves activation of p53 gene transcription (50). However, the mechanism whereby PKCδ increases p53 gene expression is not well understood. In the present study, we show that PKCδ, MEK3, and p38δ form a cascade that increase p53 expression as measured by increased p53 mRNA/protein level and promoter activity. We further show that knockdown of p38δ eliminates the ability of PKCδ to increase p21Cip1 mRNA, indicating that p38δ is an essential upstream regulator of p53 level.
We also studied the link between increased p53 level and p21Cip1 promoter activation. These studies show that a key response element in the p21Cip1 promoter is located within nucleotides −2626/−1001 relative to the transcription start site which is located at nucleotide −1. This region encodes two p53 protein binding elements located at nucleotides −2281/−2261 and −1393/1374. Mutation of these sites results in loss of promoter activity, suggesting that p53 interaction at these sites, in response to PKCδ/MEK3/p38δ activation, drives the increase in transcription.
To assess the physiological relevance of this regulation, we confirmed that this pathway is activated following treatment with agents (TPA and calcium) that suppress keratinocyte proliferation. We also used a stratifying epidermal equivalent model system where cells are grown at the air-liquid interface (42, 46). This is a particularly useful model because these cells closely mimic the in vivo epidermal differentiation process. Our studies show that knockdown of p38δ in this system results in reduced p53 and p21Cip1 expression. In addition, loss of p38δ resulted in formation of a thicker epidermal equivalent that did not include a cornified layer. These studies strongly suggest that the PKCδ/MEK3/p38δ/p53/p21Cip1 cascade is likely to be functional in in vivo epidermis.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AR046494, R01 AR053851, and R21 AR065266 (to R. E.).
- KLF4
- Kruppel-like factor 4
- MEK3
- mitogen activated protein kinase kinase 3
- TPA
- phorbol ester, 12-O-tetradecanoylphorbol-13-acetate
- KSFM
- keratinocyte serum-free medium
- MOI
- multiplicity of infection
- qRT-PCR
- quantitative RT-PCR
- KERn
- normal human keratinocytes
- EV
- empty vector.
REFERENCES
- 1. Newton A. C. (1997) Regulation of protein kinase C. Curr. Opin. Cell Biol. 9, 161–167 [DOI] [PubMed] [Google Scholar]
- 2. Nishizuka Y. (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258, 607–614 [DOI] [PubMed] [Google Scholar]
- 3. Rosse C., Linch M., Kermorgant S., Cameron A. J., Boeckeler K., Parker P. J. (2010) PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 11, 103–112 [DOI] [PubMed] [Google Scholar]
- 4. Gherzi R., Sparatore B., Patrone M., Sciutto A., Briata P. (1992) Protein kinase C mRNA levels and activity in reconstituted normal human epidermis: relationships to cell differentiation. Biochem. Biophys. Res. Commun. 184, 283–291 [DOI] [PubMed] [Google Scholar]
- 5. Matsui M. S., Chew S. L., DeLeo V. A. (1992) Protein kinase C in normal human epidermal keratinocytes during proliferation and calcium-induced differentiation. J. Invest. Dermatol. 99, 565–571 [DOI] [PubMed] [Google Scholar]
- 6. Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. (1990) A phorbol ester receptor/protein kinase, nPKC η, a new member of the protein kinase C family predominantly expressed in lung and skin. J. Biol. Chem. 265, 22434–22440 [PubMed] [Google Scholar]
- 7. Dlugosz A. A., Mischak H., Mushinski J. F., Yuspa S. H. (1992) Transcripts encoding protein kinase C-α, -δ, -ϵ, -ζ, and -η are expressed in basal and differentiating mouse keratinocytes in vitro and exhibit quantitative changes in neoplastic cells. Mol. Carcinog 5, 286–292 [DOI] [PubMed] [Google Scholar]
- 8. Fisher G. J., Tavakkol A., Leach K., Burns D., Basta P., Loomis C., Griffiths C. E., Cooper K. D., Reynolds N. J., Elder J. T. (1993) Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: reduced expression of protein kinase C-beta II in psoriasis. J. Invest. Dermatol. 101, 553–559 [DOI] [PubMed] [Google Scholar]
- 9. Shen S., Alt A., Wertheimer E., Gartsbein M., Kuroki T., Ohba M., Braiman L., Sampson S. R., Tennenbaum T. (2001) PKCδ activation: a divergence point in the signaling of insulin and IGF-1-induced proliferation of skin keratinocytes. Diabetes 50, 255–264 [DOI] [PubMed] [Google Scholar]
- 10. Hara T., Saito Y., Hirai T., Nakamura K., Nakao K., Katsuki M., Chida K. (2005) Deficiency of protein kinase Cα in mice results in impairment of epidermal hyperplasia and enhancement of tumor formation in two-stage skin carcinogenesis. Cancer Res. 65, 7356–7362 [DOI] [PubMed] [Google Scholar]
- 11. Denning M. F. (2004) Epidermal keratinocytes: regulation of multiple cell phenotypes by multiple protein kinase C isoforms. Int. J. Biochem. Cell Biol. 36, 1141–1146 [DOI] [PubMed] [Google Scholar]
- 12. Denning M. F., Dlugosz A. A., Cheng C., Dempsey P. J., Coffey R. J., Jr., Threadgill D. W., Magnuson T., Yuspa S. H. (2000) Cross-talk between epidermal growth factor receptor and protein kinase C during calcium-induced differentiation of keratinocytes. Exp. Dermatol. 9, 192–199 [DOI] [PubMed] [Google Scholar]
- 13. Adhikary G., Chew Y. C., Reece E. A., Eckert R. L. (2010) PKC-δ and -η, MEKK-1, MEK-6, MEK-3, and p38-δ are essential mediators of the response of normal human epidermal keratinocytes to differentiating agents. J. Invest. Dermatol. 130, 2017–2030 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 14. Sonkoly E., Wei T., Pavez Loriè E., Suzuki H., Kato M., Törmä H., Ståhle M., Pivarcsi A. (2010) Protein kinase C-dependent upregulation of miR-203 induces the differentiation of human keratinocytes. J. Invest. Dermatol. 130, 124–134 [DOI] [PubMed] [Google Scholar]
- 15. Papp H., Czifra G., Bodó E., Lázár J., Kovács I., Aleksza M., Juhász I., Acs P., Sipka S., Kovács L., Blumberg P. M., Bíró T. (2004) Opposite roles of protein kinase C isoforms in proliferation, differentiation, apoptosis, and tumorigenicity of human HaCaT keratinocytes. Cell Mol. Life Sci. 61, 1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Szegedi A., Páyer E., Czifra G., Tóth B. I., Schmidt E., Kovács L., Blumberg P. M., Bíró T. (2009) Protein kinase C isoenzymes differentially regulate the differentiation-dependent expression of adhesion molecules in human epidermal keratinocytes. Exp. Dermatol. 18, 122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Balasubramanian S., Efimova T., Eckert R. L. (2002) Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J. Biol. Chem. 277, 1828–1836 [DOI] [PubMed] [Google Scholar]
- 18. Kamioka N., Akahane T., Kohno Y., Kuroki T., Iijima M., Honma I., Ohba M. (2010) Protein kinase C δ and η differently regulate the expression of loricrin and Jun family proteins in human keratinocytes. Biochem. Biophys. Res. Commun. 394, 106–111 [DOI] [PubMed] [Google Scholar]
- 19. Efimova T., Eckert R. L. (2000) Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J. Biol. Chem. 275, 1601–1607 [DOI] [PubMed] [Google Scholar]
- 20. Eckert R. L., Crish J. F., Efimova T., Dashti S. R., Deucher A., Bone F., Adhikary G., Huang G., Gopalakrishnan R., Balasubramanian S. (2004) Regulation of involucrin gene expression. J. Invest. Dermatol. 123, 13–22 [DOI] [PubMed] [Google Scholar]
- 21. Efimova T., Deucher A., Kuroki T., Ohba M., Eckert R. L. (2002) Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38 δ mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein α. J. Biol. Chem. 277, 31753–31760 [DOI] [PubMed] [Google Scholar]
- 22. Bollag W. B. (2009) Protein kinase Cα puts the hand cuffs on epidermal keratinocyte proliferation. J. Invest. Dermatol. 129, 2330–2332 [DOI] [PubMed] [Google Scholar]
- 23. Bollag W. B., Dodd M. E., Shapiro B. A. (2004) Protein kinase D and keratinocyte proliferation. Drug News Perspect. 17, 117–126 [DOI] [PubMed] [Google Scholar]
- 24. Praskova M., Kalenderova S., Miteva L., Poumay Y., Mitev V. (2002) Dual role of protein kinase C on mitogen-activated protein kinase activation and human keratinocyte proliferation. Exp. Dermatol. 11, 344–348 [DOI] [PubMed] [Google Scholar]
- 25. Wheeler D. L., Reddig P. J., Dreckschmidt N. E., Leitges M., Verma A. K. (2002) Protein kinase Cδ-mediated signal to ornithine decarboxylase induction is independent of skin tumor suppression. Oncogene 21, 3620–3630 [DOI] [PubMed] [Google Scholar]
- 26. Gorospe M., Wang X., Holbrook N. J. (1999) Functional role of p21 during the cellular response to stress. Gene. Expr. 7, 377–385 [PMC free article] [PubMed] [Google Scholar]
- 27. Chew Y. C., Adhikary G., Wilson G. M., Reece E. A., Eckert R. L. (2011) PKCδ suppresses keratinocyte proliferation by increasing p21CIP1 level by a KLF4-dependent mechanism. J. Biol. Chem. 286, 28771–28782 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Wong P. P., Pickard A., McCance D. J. (2010) p300 alters keratinocyte cell growth and differentiation through regulation of p21(Waf1/CIP1). PLoS One 5, e8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng F., McLaughlin P. J., Verderame M. F., Zagon I. S. (2009) The OGF-OGFr axis utilizes the p16INK4a and p21WAF1/CIP1 pathways to restrict normal cell proliferation. Mol. Biol. Cell 20, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aliouat-Denis C. M., Dendouga N., Van den Wyngaert I., Goehlmann H., Steller U., van de Weyer I., Van Slycken N., Andries L., Kass S., Luyten W., Janicot M., Vialard J. E. (2005) p53-independent regulation of p21Waf1/Cip1 expression and senescence by Chk2. Mol. Cancer Res. 3, 627–634 [DOI] [PubMed] [Google Scholar]
- 31. Devgan V., Mammucari C., Millar S. E., Brisken C., Dotto G. P. (2005) p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 19, 1485–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okuyama R., LeFort K., Dotto G. P. (2004) A dynamic model of keratinocyte stem cell renewal and differentiation: role of the p21WAF1/Cip1 and Notch1 signaling pathways. J. Investig. Dermatol. Symp. Proc. 9, 248–252 [DOI] [PubMed] [Google Scholar]
- 33. Hauser P., Ma L., Agrawal D., Haura E., Cress W. D., Pledger W. J. (2004) Efficient down-regulation of cyclin A-associated activity and expression in suspended primary keratinocytes requires p21(Cip1). Mol. Cancer Res. 2, 96–104 [PubMed] [Google Scholar]
- 34. Efimova T., Broome A. M., Eckert R. L. (2003) A regulatory role for p38 δ MAPK in keratinocyte differentiation. Evidence for p38 δ-ERK1/2 complex formation. J. Biol. Chem. 278, 34277–34285 [DOI] [PubMed] [Google Scholar]
- 35. Efimova T., Broome A. M., Eckert R. L. (2004) Protein kinase Cδ regulates keratinocyte death and survival by regulating activity and subcellular localization of a p38δ-extracellular signal-regulated kinase 1/2 complex. Mol. Cell Biol. 24, 8167–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 37. Nakano K., Mizuno T., Sowa Y., Orita T., Yoshino T., Okuyama Y., Fujita T., Ohtani-Fujita N., Matsukawa Y., Tokino T., Yamagishi H., Oka T., Nomura H., Sakai T. (1997) Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J. Biol. Chem. 272, 22199–22206 [DOI] [PubMed] [Google Scholar]
- 38. Chew Y. C., Adhikary G., Wilson G. M., Xu W., Eckert R. L. (2012) Sulforaphane induction of p21(Cip1) cyclin-dependent kinase inhibitor expression requires p53 and Sp1 transcription factors and is p53-dependent. J. Biol. Chem. 287, 16168–16178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balasubramanian S., Zhu L., Eckert R. L. (2006) Apigenin inhibition of involucrin gene expression is associated with a specific reduction in phosphorylation of protein kinase Cδ Tyr311. J. Biol. Chem. 281, 36162–36172 [DOI] [PubMed] [Google Scholar]
- 40. Li J. J., Cao Y., Young M. R., Colburn N. H. (2000) Induced expression of dominant-negative c-jun downregulates NFκB and AP-1 target genes and suppresses tumor phenotype in human keratinocytes. Mol. Carcinog. 29, 159–169 [DOI] [PubMed] [Google Scholar]
- 41. Efimova T., LaCelle P., Welter J. F., Eckert R. L. (1998) Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J. Biol. Chem. 273, 24387–24395 [DOI] [PubMed] [Google Scholar]
- 42. Chew Y. C., Adhikary G., Xu W., Wilson G. M., Eckert R. L. (2013) Protein kinase C δ increases Kruppel-like factor 4 protein, which drives involucrin gene transcription in differentiating keratinocytes. J. Biol. Chem. 288, 17759–17768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dashti S. R., Efimova T., Eckert R. L. (2001) MEK7-dependent activation of p38 MAP kinase in keratinocytes. J. Biol. Chem. 276, 8059–8063 [DOI] [PubMed] [Google Scholar]
- 44. Dashti S. R., Efimova T., Eckert R. L. (2001) MEK6 regulates human involucrin gene expression via a p38α- and p38δ-dependent mechanism. J. Biol. Chem. 276, 27214–27220 [DOI] [PubMed] [Google Scholar]
- 45. Eckert R. L., Efimova T., Dashti S. R., Balasubramanian S., Deucher A., Crish J. F., Sturniolo M., Bone F. (2002) Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J. Invest. Dermatol. Symp. Proc. 7, 36–40 [DOI] [PubMed] [Google Scholar]
- 46. Poumay Y., Dupont F., Marcoux S., Leclercq-Smekens M., Hérin M., Coquette A. (2004) A simple reconstructed human epidermis: preparation of the culture model and utilization in in vitro studies. Arch. Dermatol. Res. 296, 203–211 [DOI] [PubMed] [Google Scholar]
- 47. Lin P. Y., Lee J. W., Liao M. H., Hsu H. Y., Chiu S. J., Liu H. J., Shih W. L. (2009) Modulation of p53 by mitogen-activated protein kinase pathways and protein kinase C δ during avian reovirus S1133-induced apoptosis. Virology 385, 323–334 [DOI] [PubMed] [Google Scholar]
- 48. Lee S. J., Kim D. C., Choi B. H., Ha H., Kim K. T. (2006) Regulation of p53 by activated protein kinase C-δ during nitric oxide-induced dopaminergic cell death. J. Biol. Chem. 281, 2215–2224 [DOI] [PubMed] [Google Scholar]
- 49. Niwa K., Inanami O., Yamamori T., Ohta T., Hamasu T., Karino T., Kuwabara M. (2002) Roles of protein kinase C δ in the accumulation of P53 and the induction of apoptosis in H2O2-treated bovine endothelial cells. Free Radic. Res. 36, 1147–1153 [DOI] [PubMed] [Google Scholar]
- 50. Ryer E. J., Sakakibara K., Wang C., Sarkar D., Fisher P. B., Faries P. L., Kent K. C., Liu B. (2005) Protein kinase C δ induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J. Biol. Chem. 280, 35310–35317 [DOI] [PubMed] [Google Scholar]
- 51. Wu H. M., Schally A. V., Cheng J. C., Zarandi M., Varga J., Leung P. C. (2010) Growth hormone-releasing hormone antagonist induces apoptosis of human endometrial cancer cells through PKCδ-mediated activation of p53/p21. Cancer Lett. 298, 16–25 [DOI] [PubMed] [Google Scholar]
- 52. Kraft C. A., Efimova T., Eckert R. L. (2007) Activation of PKCδ and p38δ MAPK during okadaic acid dependent keratinocyte apoptosis. Arch. Dermatol. Res. 299, 71–83 [DOI] [PubMed] [Google Scholar]