Background: Global interferon-β deficiency causes osteoporosis. Lack of interferon-β production by osteoclast precursors is considered to induce excess osteoclastogenesis.
Results: Isolated osteocytes express higher amount of interferon-β mRNA than osteoclast precursors and inhibit osteoclastogenesis partially in interferon-β-dependent manner.
Conclusion: Osteocytes produce interferon-β as an inhibitor of osteoclastogenesis.
Significance: Osteocytic interferon-β might be involved in the regulation of bone homeostasis.
Keywords: Bone, Cytokines/Interferon, Osteoclast, Osteocyte, Osteoporosis, Osteoclastogenesis
Abstract
Osteoclastogenesis is controlled by osteocytes; osteocytic osteoclastogenesis regulatory molecules are largely unknown. We searched for such factors using newly developed culture methods. Our culture system mimics the three-dimensional cellular structure of bone, consisting of collagen gel-embedded osteocytic MLO-Y4 cells, stromal ST2 cells on the gel as bone lining cells, and bone marrow cells. The gel-embedded MLO-Y4 cells inhibited the osteoclastogenesis induced by 1,25(OH)2D3 without modulating receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) production by ST2 cells, despite MLO-Y4 cells supported osteoclastogenesis in the absence of ST2 cells. In the bone marrow cell culture, the conditioned medium from MLO-Y4 cells decreased the capability of osteoclastic differentiation from the cells induced by macrophage colony-stimulating factor. This decreased capability was concomitant with an increase in protein kinase R mRNA expression and an inhibition of c-Fos translation. These changes were partially normalized by the simultaneous addition of an anti-interferon (IFN)-β neutralizing antibody to MLO-Y4 cell conditioned medium. To study primary osteocytes, we prepared non-osteocytic cell-free osteocyte-enriched bone fragments (OEBFs). When osteoclast precursors were induced by macrophage colony-stimulating factor in the presence of OEBFs, the generated cells exhibited a diminished capacity for osteoclastogenesis. OEBFs prepared from OPG-knock-out mice exhibited a similar effect, indicating OPG-independent inhibition. The addition of anti-IFN-β neutralizing antibody during the co-culture with OEBFs partially recovered the osteoclastogenic potential of the generated cells. The MLO-Y4 cells and OEBFs expressed IFN-β mRNA. Although osteocytic RANKL is known to be important for osteoclastogenesis, our data suggest that osteocytes also produce IFN-β as an inhibitor of osteoclastogenesis.
Introduction
Bone is continuously remodeled to maintain its architecture and strength and calcium homeostasis in the serum. Bone remodeling is primarily performed in a tightly regulated manner at the bone surface by bone-resorbing osteoclasts and bone-forming osteoblasts (1, 2).
Osteoclasts are hematopoietic in origin, and osteoclast precursors of the monocyte/macrophage lineage differentiate during stimulation with macrophage colony-stimulating factor (M-CSF)3 (3, 4). The osteoclastic differentiation of precursor cells is positively or negatively controlled by two critical factors: receptor activator of NF-κB ligand (RANKL) and its decoy receptor osteoprotegerin (OPG) (5–7), respectively. It has been reported that the RANKL-dependent differentiation of osteoclasts is negatively controlled by interferon-β (IFN-β), which is produced by the osteoclast precursors themselves in a feedback-regulated manner (8, 9). IFN-β inhibits osteoclastogenesis through the induction of double-stranded RNA-activated protein kinase (also known as protein kinase R, PKR) in the precursors (8). PKR has been shown to inhibit the translation of viral proteins in virus-infected cells (10). In the case of RANKL-stimulated osteoclast precursors, however, IFN-β-induced PKR inhibits the translation of c-Fos protein (8), an indispensable component of AP-1 for osteoclastogenesis, thereby negatively regulating osteoclastic differentiation (11, 12). In fact, global IFN-β-knock-out (KO) mice exhibited an osteoporotic phenotype due to augmented osteoclastogenesis, indicating that IFN-β plays a critical role in the negative regulation of osteoclastogenesis for bone homeostasis (8).
Bone-forming osteoblasts and bone marrow (BM) stromal cells are known to produce both RANKL and OPG (7). Osteoblasts terminally differentiate into osteocytes, which are individually embedded in the lacunae of the mineralized matrix (13). Osteocytes communicate with each other and with cells on the bone surface, such as bone lining cells, including osteoblasts and possibly cells of the osteoclast lineage. This communication occurs via prominent cell processes running through the canaliculi, thereby forming a three-dimensional network throughout the bone (13, 14). Although osteocytes are known to negatively regulate osteoblastogenesis by secreting sclerostin (15), recent in vivo experiments using osteocyte-specific transgenic mouse models have also revealed a crucial role for osteocytes in the regulation of osteoclastogenesis. The osteocyte-specific disruption of RANKL demonstrated that osteocytic RANKL is indispensable for osteoclastogenesis during bone remodeling but not during bone modeling/development (16, 17). Osteocyte-specific β-catenin-deficient mice exhibit increased osteoclastogenesis due to the down-regulation of OPG production by osteocytes (18). In addition, osteocyte-specific RANKL-deficient mice (17) and mice with specific osteocyte ablation (19) were resistant to the acceleration of osteoclastogenesis induced by the mechanical unloading of the hind limbs by tail suspension. These results indicated that osteocytes sense local changes in the mechanical strains evoked by unloading and provide RANKL to up-regulate osteoclastogenesis. In contrast, the osteocyte-specific ablation model mouse demonstrated an acceleration of osteoclastogenesis and a concomitant increase in RANKL mRNA expression in long bones, presumably by osteoblasts and/or the remaining living osteocytes under ambulatory conditions (19). In addition, the bone of osteocyte ablation model mice expressed a similar level of OPG mRNA as “normal” bone containing osteocytes (19), indicating that cells other than osteocytes compensate for OPG mRNA expression when osteocytes are disrupted, although it could not rule out the possibility that the remaining osteocytes produce higher amount of OPG mRNA. These data suggested that osteocytes regulate osteoclastogenesis by affecting RANKL and/or OPG production by other cell types. Furthermore, these findings raise the intriguing possibility that osteocyte-derived factor(s) other than RANKL or OPG also regulate osteoclastogenesis. However, only a few molecules produced by osteocytes such as transforming growth factor-β (TGF-β) (20) have been identified as being involved in the regulation of osteoclastogenesis.
Functional and molecular analyses of osteocytes have been hampered by the inaccessibility of osteocytes in the mineralized matrix. Although several isolation methods have been established for osteocytes (16, 21–23) and the clonal osteocytic cell line MLO-Y4 (24), culture systems suitable for the in vitro analysis of the intrinsic function of osteocytes are lacking. In this study, we employed a culture system that mimics a three-dimensional cellular network and consists of osteocytic MLO-Y4 cells embedded in type I collagen gel, a layer of stromal ST2 cells on the gel, representing bone lining cells, and BM cells on the ST2 cell layer, serving as a source of osteoclast precursors. We also developed a culture method using osteocyte-enriched bone fragments (OEBFs), consisting of mineralized bone matrix containing osteocytes but free of non-osteocytic cells, e.g. osteoblasts and BM cells. Using these systems, we investigated the functions of osteocytes in osteoclastogenesis and found that osteocytes produce IFN-β as an inhibitory factor of osteoclastogenesis.
EXPERIMENTAL PROCEDURES
Growth Factors and Reagents
Fetal bovine serum (FBS) was purchased from Nichirei Biosciences (Chuo, Tokyo, Japan), and calf serum (CS) was obtained from Thermo Fisher Scientific (Yokohama, Kanagawa, Japan). Recombinant mouse M-CSF and recombinant mouse soluble RANKL (sRANKL) were purchased from R&D Systems (Minneapolis, MN), and recombinant mouse IFN-β and rabbit anti-mouse IFN-β neutralizing antibody (α-IFN-β-Ab) were obtained from PBL Interferon Source (Piscataway, NJ). Normal rabbit IgG, rabbit anti-c-Fos antibody (α-c-Fos-Ab), and mouse anti-β-actin antibody (α-β-actin-Ab) were purchased from R&D Systems, Santa Cruz Biotechnologies (Dallas, TX), and Sigma-Aldrich, respectively. Mouse anti-signal transducer and activation of transcription (STAT)-1-antibody (α-STAT-Ab) and anti-phosphorylated STAT-1 (pSTAT-1)-antibody (α-pSTAT-1-Ab) were from Cell Signaling Technology (Beverly, MA).
Mice
Five-week-old male ddy mice were purchased from Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan). OPG-KO mice from a C57BL/6J background (25) were kindly provided by Dr. Nobuyuki Udagawa (Matsumoto Dental University). Wild-type C57BL/6J mice were obtained from CLEA Japan, Inc. (Meguro, Tokyo, Japan). All experimental animal procedures were reviewed and approved by the Meikai University School of Dentistry Animal Care Committee.
Cells
Immortalized osteocytic MLO-Y4 cells (24) from transgenic mice were kindly provided by Dr. Lynda F. Bonewald (University of Missouri), and mouse BM-derived stromal ST2 cells were obtained from the RIKEN BioResource Center (Tsukuba, Ibaraki, Japan). Primary osteoblasts were isolated by sequential collagenase treatment (26) and confirmed their abilities to produce calcified bone-like matrix and to support osteoclastogenesis from BM cells in the presence of dexamethasone (Dex) and 1,25-dihydroxyvitamin D3 (1,25D3).
Three-dimensional Culture System
Type I collagen gel solution (Cellmatrix Type I-A; Nitta Gelatin, Osaka, Japan), containing the indicated number of MLO-Y4 cells, 1.5 mg/ml type I collagen, 2.5% FBS, 2.5% CS, and α-minimal Eagle's medium (α-MEM), was prepared for each experiment. A 50-μl aliquot of this cell suspension was poured into each well of a 96-well plate and allowed to solidify at 37 °C for 20 min. Subsequently, 50 μl of α-MEM containing 2.5% FBS and 2.5% CS was added to each well, and the plate was incubated in a humidified incubator at 37 °C and 5% CO2. After culturing for 2–3 days, 50 μl of the culture medium was removed from each well, and 50 μl of ST2 cell suspension (1 × 104 cells in 10% FBS/α-MEM) was added onto the gel, which was further cultured for 2–3 days to form an ST2 cell layer. Thereafter, BM cells were prepared from the femora of 5-week-old ddy mice and 50 μl of the culture medium in each well was replaced with an equal volume of BM cell suspension (5 × 103 cells in 10% FBS/α-MEM) containing a 2× concentration of the indicated factors. In some experiments, three-dimensional cultures were performed without the ST2 cell layer. After culturing for 3–4 days, 50 μl of the culture medium was replaced with fresh medium and cultured for an additional 3 days, followed by an evaluation of osteoclastogenesis, as described below.
Ultraviolet Light Exposure
The gel solution containing MLO-Y4 cells, prepared as described above, was poured into 35-mm Petri dishes on ice (1.0 ml/dish) and immediately exposed to UV light (50 cm below a UV lamp; wavelength of 253.7 nm; 15 watts; Panasonic, Kadoma, Osaka, Japan) for the indicated times. The solution was then poured into each well of a 96-well plate for construction of the three-dimensional culture and to assay cell viability and mRNA expression.
Cell Viability Assay
Cell viability was assessed using a Cell Counting kit-8 (Dojindo, Kamimashiki, Kumamoto, Japan) according to the manufacturer's instructions.
Three-dimensional Culture with Cell Culture Inserts
The gel solution containing the MLO-Y4 cells was poured into each well of a 24-well plate (600 μl/well), and ST2 cells were seeded onto each cell culture insert (1 × 104 cells/insert; 0.4 μm pore size; Coaster, NY) to allow attachment to the top of the gel. After culturing for 3 days in 2.5% FBS and 2.5% CS/α-MEM, the cells were further cultured in 10% FBS/α-MEM containing the indicated factors. After culturing for 2 days, the inserts were removed from the gel, and the ST2 cells on the inserts were harvested for real-time reverse transcription (RT)-PCR analysis.
Co-cultures with Cell Culture Inserts Containing MLO-Y4 Cells
MLO-Y4 cells (5 × 103) in 50 μl of the gel were seeded into each cell culture insert (pore size, 0.4 μm) in a 24-well plate, and ST2 cells (6 × 104) or MLO-Y4 cells (6 × 104) were seeded into each well of the 24-well plate. The cells were cultured for 3 days in 2.5% FBS, 2.5% CS/αMEM. Next, BM cells (6 × 104) were seeded onto ST2 cells or MLO-Y4 cells in each well and cultured in 10% FBS/α-MEM containing Dex (10 nm) and 1,25D3 (10 nm) for 7 days, followed by an evaluation of osteoclastogenesis.
BM Cell Culture System
BM cells (1.5 × 105 cells/cm2), obtained as described above, were cultured for 3 days in 10% FBS/α-MEM containing 10 ng/ml M-CSF and/or the indicated factors. After washing the cells with α-MEM, fresh medium containing 10 ng/ml M-CSF, 10 ng/ml sRANKL, and/or the indicated factors was added, and the cells were further cultured for 2–3 days, followed by an evaluation of osteoclastogenesis. For Western blotting, the BM cells were cultured for 3 days in 10% FBS/α-MEM containing 10 ng/ml M-CSF and the indicated factors and used for Western blotting.
Evaluation of Osteoclastogenesis
Osteoclastogenesis was evaluated based on the tartrate-resistant acid phosphatase (TRAP) activity in the culture medium as described previously (27). In several experiments, the cells were stained for TRAP activity using a leukocyte acid phosphatase kit (Sigma-Aldrich). TRAP+ multinucleated cells with more than three nuclei were counted as osteoclastic cells using an inverted microscope, and we confirmed that TRAP activity in the culture medium reflected the number of TRAP+ multinucleated cells in each well.
Enzyme-linked Immunosorbent Assay (ELISA) for OPG
The OPG concentration in the culture medium was determined using a Mouse OPG/TNFRSF11B Quantikine ELISA Kit (R&D Systems).
Real-time RT-PCR
Total RNA was purified using an RNeasy Plus mini kit (Qiagen), and cDNA was synthesized using a high capacity RNA-to-cDNA kit (Invitrogen). Real-time RT-PCR was performed using TaqMan probe/primer mixture (Invitrogen) and a GeneAmp 5700 Sequence Detection System (Invitrogen). The relative quantification of the target mRNA expression was calculated and normalized to the amount of 18 S rRNA.
Preparation of Conditioned Medium from MLO-Y4 Cell Cultures and Primary Osteoblasts
MLO-Y4 cells were cultured for 3 days in the collagen gel, as described above except that 60-mm dishes were used. The culture medium was then replaced with 10% FBS/α-MEM, followed by culture for 2 days; the culture medium was then harvested and designated as MLO-Y4 cell-conditioned medium (MLO-Y4-CM). Culture medium prepared without MLO-Y4 cells in the gel was harvested and designated as control CM. We confirmed that control CM had no effects on osteoclastogenesis. Primary osteoblasts were cultured in 10% FBS/αMEM until they were confluent. The culture medium was then replaced with 10% FBS/α-MEM, followed by culture for 2 days; the culture medium was then harvested as primary osteoblast-CM (pOB-CM).
Western Blot Analysis
After cultivation, cells were washed with PBS and lysed in cell lysis buffer (10 mm sodium phosphate (pH 7.5), 150 mm NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1.0 mm EDTA, 1.0 mm p-aminoethyl-benzenesulfonyl fluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 10 μg/ml aprotinin), and Western blot analysis was performed as described previously (28).
Preparation of OEBFs
OEBFs were prepared from the femora of 5-week-old mice from the indicated strains. BM cells were removed from the isolated femora, and each diaphysis of the femora was divided longitudinally into two pieces. Each piece of diaphysis was further cut into two pieces, and these bone fragments were incubated in α-MEM containing 0.2% collagenase and 1.0% FBS for 30 min with stirring (collagenase treatment). After washing the bone fragments with PBS, the fragments were incubated in PBS containing 5.0 mm EDTA and 0.1% BSA for 20 min with stirring (EDTA treatment). After three sets of sequential treatment with collagenase and EDTA, the bone fragments were used as OEBFs. To prepare OEBFs without vital cells, OEBFs were subjected to repeated freeze/thaw treatment using liquid nitrogen and PBS. In several experiments, a portion of bone fragments at each step of collagenase/EDTA treatment was used for real-time RT-PCR and cytological analyses. For the cytological analysis, the bone fragments were stained for alkaline phosphatase activity, an enzymatic marker of osteoblasts, or stained with hematoxylin and eosin (H&E). Using a stereomicroscope, we confirmed that nearly no cells existed on the surface of the OEBFs after three sets of sequential treatment. In some experiments, after the first collagenase treatment of bone fragments, the cells removed by the subsequent collagenase/EDTA treatments were harvested for mRNA expression analysis.
Culture of OEBFs
Approximately 10 OEBFs placed on a cell culture insert (pore size, 0.4 μm) in a 24-well plate were cultured for the indicated number of days. The cell viability of the OEBFs was measured as described above. To examine the effects of OEBFs on osteoclastogenesis by BM cells, BM cells (3 × 105) were seeded into each well of 24-well plates and cultured with the inserts containing OEBFs in 10% FBS/α-MEM supplemented with 10 ng/ml M-CSF and the indicated factors. The inserts were removed after co-culturing for 3 days. The cells in each well were washed with α-MEM and further cultured for 2–3 days in 10% FBS/α-MEM containing 10 ng/ml M-CSF and 10 ng/ml sRANKL in the absence of OEBFs, followed by an evaluation of osteoclastogenesis.
Statistical Analysis
The group means were compared by a t test, one-way analysis of variance or two-way analysis of variance, and the significance of differences was determined by post hoc testing using Bonferroni's method.
RESULTS
Osteocytic MLO-Y4 Cells Inhibited Osteoclastogenesis in a Three-dimensional Culture
In a three-dimensional culture system, BM cells were cultured on ST2 cell layer formed on collagen gel containing osteocytic MLO-Y4 cells in the presence of Dex and 1,25D3 (Fig. 1A). Using this culture system, we evaluated the effect of the osteocytes in the gel on osteoclastogenesis. The MLO-Y4 cells in the gel significantly inhibited osteoclastogenesis in a cell number-dependent manner (Fig. 1B). When the MLO-Y4 cells in the gel were pre-exposed to UV light, the viability of the cells was decreased in an exposure time-dependent fashion (Fig. 1C, left panel). RANKL mRNA expression stimulated by 1,25D3 was also similarly decreased (Fig. 1C, right panel). When these MLO-Y4 cells were used in the three-dimensional cultures with ST2 cells, the inhibition of osteoclastogenesis was partially reversed in an exposure time-dependent fashion (Fig. 1D), suggesting that osteoclastogenesis in this culture condition is supported by other than RANKL of MLO-Y4 cells, presumably by that of ST2 cells. However, when BM cells were directly cultured on the gel containing MLO-Y4 cells without ST2 cells, osteoclastogenesis was supported in a cell number-dependent manner (Fig. 1E), in agreement with other reports (29, 30). Therefore, our data suggested that MLO-Y4 cells in the gel exhibit an inhibitory effect on osteoclastogenesis from BM cells seeded on ST2 cell layer that primarily contact with BM cells to support osteoclastogenesis.
FIGURE 1.
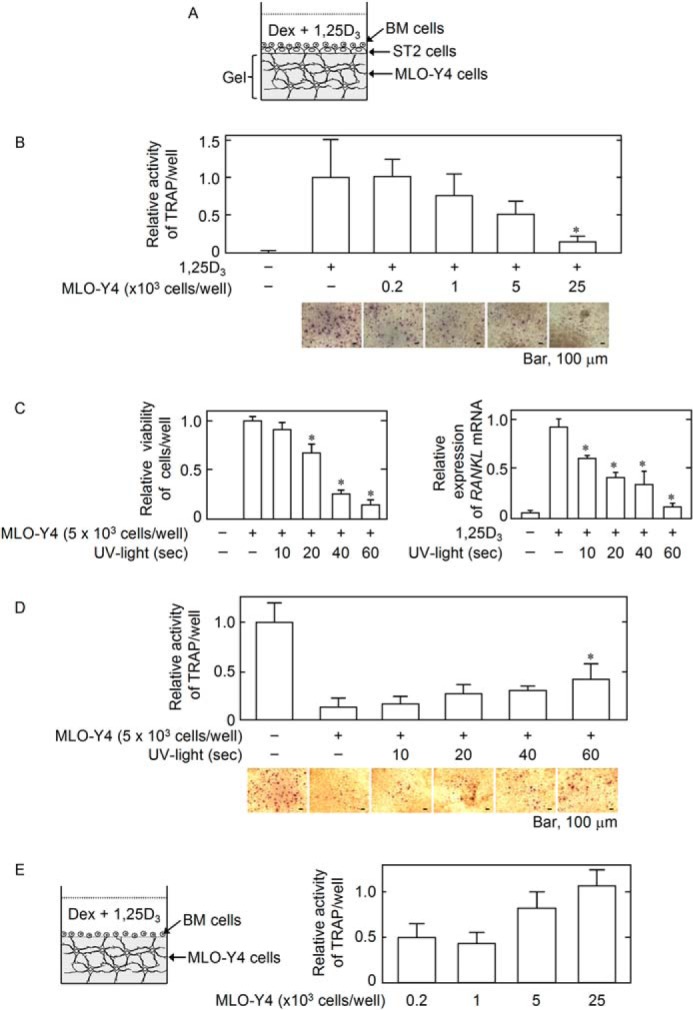
Illustration of the three-dimensional culture system. A, the BM cells on the ST2 cell layer formed on the collagen gel (Gel) containing the MLO-Y4 cells were co-cultured. A combination of Dex (10 nm) and 1,25D3 (10 nm) was added to the culture for the induction of RANKL-dependent osteoclastogenesis. B, effect of the MLO-Y4 cells in the gel on osteoclastogenesis. The indicated number of MLO-Y4 cells were seeded into the three-dimensional culture system. At the end of the culture, osteoclastogenesis was evaluated based on the relative TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 6). *, p < 0.01 versus MLO-Y4 (−) 1,25D3 (+) group. Microphotographs of TRAP+ cells on gels containing the indicated number of MLO-Y4 cells are shown. Bar, 100 μm. C, effects of UV light exposure on MLO-Y4 cell viability and RANKL mRNA expression. MLO-Y4 cells in the collagen gel solution were exposed to UV light for the indicated time and culture for 2 days. Cell viability (left panel) and RANKL mRNA expression (right panel) were evaluated by Cell Counting kit-8 and real-time RT-PCR, respectively. The data are expressed as the mean ± S.E. (n = 6). *, p < 0.01 versus MLO-Y4 (+) UV light exposure (−) group (left panel) and as the mean ± S.E. (n = 3). *, p < 0.01 versus 125D3 (+) UV light exposure (−) group (right panel). D, effect of MLO-Y4 cell death induced by UV light exposure on osteoclastogenesis. MLO-Y4 cells exposed to UV light were used in the three-dimensional culture system. Osteoclastogenesis was evaluated based on the TRAP activity. The data are expressed as the mean ± S.E. (n = 6). *, p < 0.05 versus MLO-Y4 (+) UV light exposure (−) group. Microphotographs of TRAP+ cells on gels containing MLO-Y4 cells exposed to UV light for the indicated times are shown. Bar, 100 μm. E, effect of the MLO-Y4 cells in the gel on osteoclastogenesis in the absence of the ST2 cell layer. The indicated number of MLO-Y4 cells was seeded into the gel, and BM cells were seeded directly onto the gel. The osteoclastogenesis was induced by Dex and 1,25D3. Osteoclastogenesis was evaluated based on the relative TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 6).
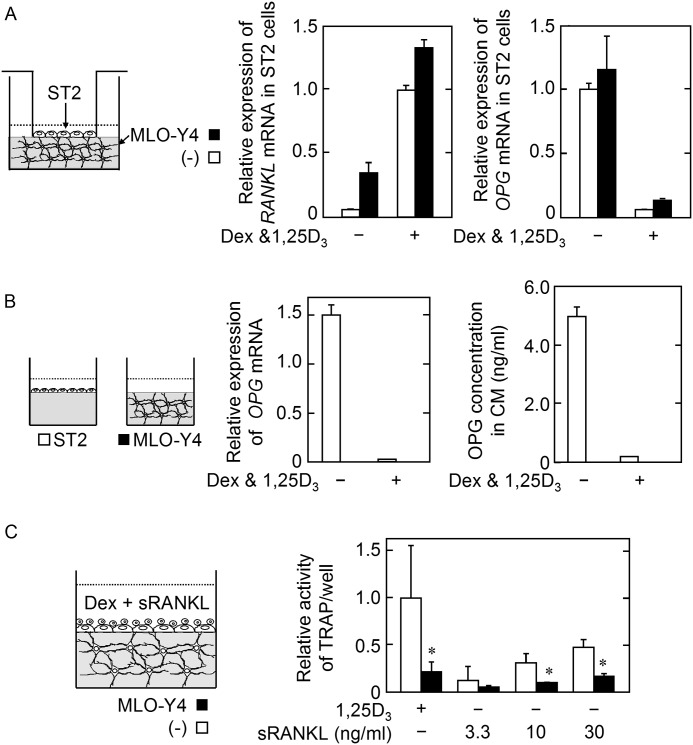
MLO-Y4 Cells Embedded in the Gel Did Not Affect RANKL or OPG mRNA Expression by the Stromal ST2 Cells on the Gel in the Presence of Dex and 1,25D3
We next investigated whether the MLO-Y4 cells in the gel regulate the functions of the ST2 cells on the gel toward anti-osteoclastogenic properties, i.e. down-regulation of RANKL and/or up-regulation of OPG production to inhibit osteoclastogenesis. The cell culture inserts on which ST2 cells were seeded were set on the gel containing MLO-Y4 cells and cultured in the presence of Dex and 1,25D3. After cultivation, the inserts were removed, and the production of RANKL and OPG by the ST2 cells was analyzed. The presence of MLO-Y4 cells in the gel did not significantly modulate RANKL or OPG mRNA expression by ST2 cells in the presence of Dex and 1,25D3 (Fig. 2A). In addition, OPG production by the MLO-Y4 cells in the gel was undetectable at both the protein and mRNA levels, irrespective of the presence of Dex and 1,25D3 (Fig. 2B). To confirm that the inhibitory effect of the MLO-Y4 cells in the gel on osteoclastogenesis is independent of the modulation of RANKL/OPG production by ST2 cells, osteoclastogenesis was stimulated by the exogenous addition of sRANKL in the presence of Dex in the three-dimensional culture. It should be noted that the addition of Dex is necessary to decrease the high level of OPG production by ST2 cells for osteoclastogenesis induction by exogenous sRANKL in three-dimensional culture (data not shown). The results showed that the presence of MLO-Y4 cells in the gel inhibited the osteoclastogenesis induced by exogenous sRANKL with Dex in the ST2 cell layer on the gel (Fig. 2C). These data suggested that MLO-Y4 cells inhibit osteoclastogenesis independently of the modulation of RANKL/OPG production by ST2 cells.
FIGURE 2.
A, effect of MLO-Y4 cells on the Dex/1,25D3-dependent expression of RANKL mRNA (left panel) or on the repression of OPG mRNA (right panel) by ST2 cells. ST2 cells on cell culture inserts were allowed to attach to the gel containing (black bar) or not containing (open bar) MLO-Y4 cells and were cultured in the absence or presence of Dex (10 nm) and 1,25D3 (10 nm) for 2 days. RANKL and OPG mRNA expression by the ST2 cells was evaluated. The data are expressed as the mean ± S.E. (n = 3). B, OPG production by MLO-Y4 cells. The MLO-Y4 cells in the gel were cultured in the absence or presence of Dex (10 nm) and 1,25D3 (10 nm) for 2 days. The MLO-Y4 cells and the culture medium were harvested to evaluate the production of OPG (black bar). The ST2 cells on the gel were used as a positive control (open bar). OPG mRNA expression (left panel) and the OPG concentration in the culture medium (right panel) are shown. The data are expressed as the mean ± S.E. (n = 3). C, effect of MLO-Y4 cells on sRANKL-induced osteoclastogenesis in the three-dimensional culture system. Osteoclastogenesis in three-dimensional cultures using gel containing (black bar) or not containing (open bar) MLO-Y4 cells was induced with the indicated concentration of sRANKL in the presence of 10 nm Dex. The data are expressed as the mean ± S.E. (n = 6). *, p < 0.01 versus MLO-Y4 (−) group at each sRANKL concentration.
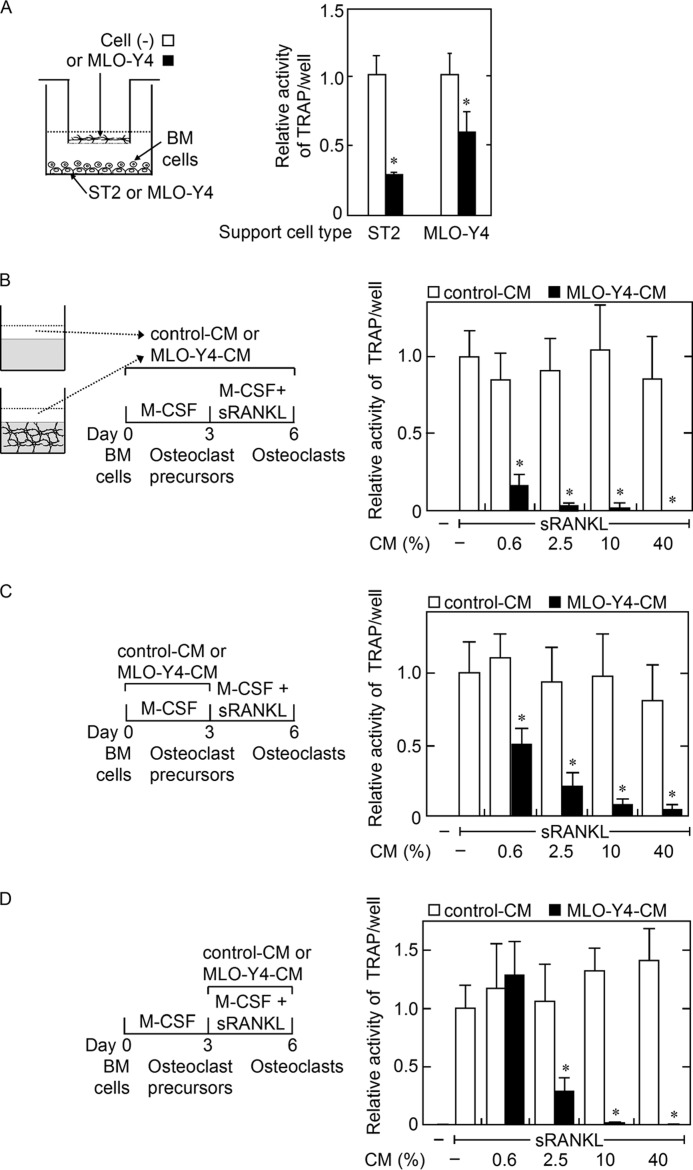
Humoral Factor(s) Produced by MLO-Y4 Cells Inhibited both Osteoclast Precursor Generation and Osteoclastic Differentiation of Precursors in BM Cell Cultures
We next examined whether co-culture of MLO-Y4 cells in culture inserts inhibit osteoclastic differentiation of BM cells supported by ST2 cells or MLO-Y4 cells. The presence of MLO-Y4 cells in the cell culture inserts inhibited osteoclastogenesis supported by either ST2 cells or MLO-Y4 cells (Fig. 3A), suggesting that although MLO-Y4 cells are capable of supporting osteoclastogenesis through cell contact with BM cells, MLO-Y4 cells also produce humoral inhibitory factor(s) of osteoclastogenesis. To examine the effects of humoral factor(s) produced by MLO-Y4 cells on osteoclastogenesis, we utilized a BM cell culture system and MLO-Y4-CM. The addition of MLO-Y4-CM through two phases of osteoclastogenesis, i.e. osteoclast precursor generation by M-CSF and the subsequent differentiation of the precursors into osteoclasts by sRANKL, inhibited osteoclastogenesis in an MLO-Y4-CM concentration-dependent manner (Fig. 3B). However, the addition of MLO-Y4-CM only during the phase of M-CSF-induced osteoclast precursor generation decreased the capability of the M-CSF-induced cells to differentiate into osteoclasts in this MLO-Y4-CM concentration-dependent manner (Fig. 3C). The addition of MLO-Y4-CM and sRANKL also inhibited the osteoclastic differentiation of the osteoclast precursors generated by M-CSF without MLO-Y4-CM (Fig. 3D). These results suggested that MLO-Y4 cells produce humoral factor(s) capable of inhibiting both the M-CSF-induced generation of osteoclast precursors from BM cells and the sRANKL-induced osteoclastic differentiation of these precursors.
FIGURE 3.
A, effects of MLO-Y4 cells in cell culture inserts on osteoclastogenesis from BM cells supported by ST2 cells or MLO-Y4 cells. MLO-Y4 cells in cell culture inserts were cultured with BM cells and ST2 cells or MLO-Y4 cells in the presence of Dex (10 nm) and 1,25D3 (10 nm) (black bar). Cell culture inserts without cells were used as control (open bar). Osteoclastogenesis was evaluated based on the TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 4). *, p < 0.01 versus the insert cell (−) group. B–D, effects of MLO-Y4-CM on osteoclastogenesis in BM cell cultures. The effects of MLO-Y4-CM or control (Cont) CM on osteoclastogenesis were examined using a BM cell culture system. The cells were treated with the indicated concentration of CM during total culture period (B), during the first 3 days only (C) or during the latter 3 days only (D). Osteoclastogenesis was evaluated based on the TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 6). *, p < 0.01 versus control CM group at each CM concentration.
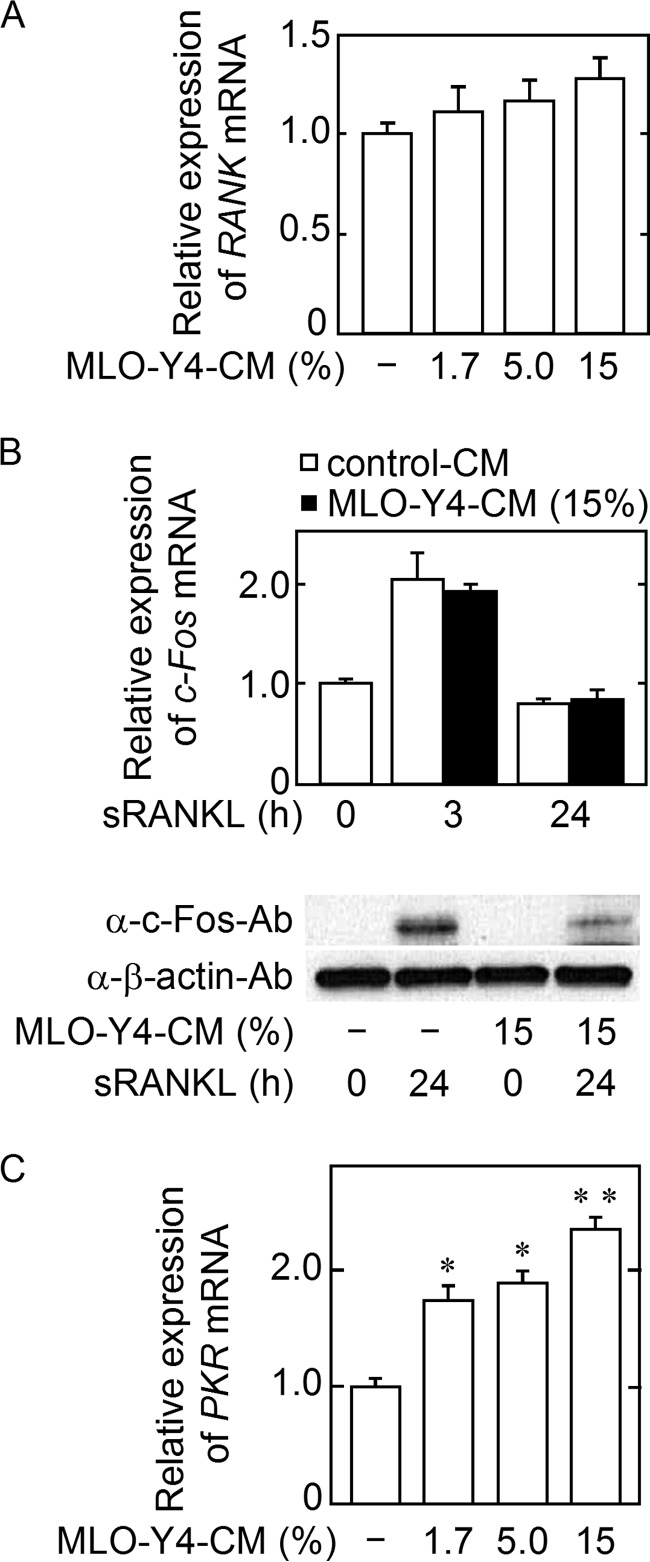
PKR mRNA Expression Increased, and sRANKL-induced c-Fos Protein Synthesis (but Not c-Fos mRNA Expression) Was Inhibited in the Cells Generated by M-CSF in the Presence of MLO-Y4-CM
To address how MLO-Y4-CM treatment with M-CSF decreases the osteoclastic potential of M-CSF-induced cells in BM cell culture, we compared the characteristics of the cells generated by M-CSF with or without MLO-Y4-CM. The RANK mRNA expression level in the cells generated with MLO-Y4-CM was comparable with the level in the cells generated without MLO-Y4-CM (Fig. 4A). When the cells were induced by M-CSF with control CM or MLO-Y4-CM and were subsequently stimulated with sRANKL in the absence of CM, sRANKL-induced c-Fos protein production, but not c-fos mRNA expression, was remarkably reduced in the cells generated with MLO-Y4-CM (Fig. 4B). Because the inhibition of c-Fos mRNA translation is known to be partially mediated by PKR in osteoclast precursors (8), we examined PKR mRNA expression and found that PKR mRNA expression increased in the cells generated with MLO-Y4-CM in an MLO-Y4-CM concentration-dependent manner (Fig. 4C). These data suggested that the increase in PKR in the cells generated by M-CSF with MLO-Y4-CM decreases sRANKL-induced c-Fos protein translation, thereby inhibiting osteoclastogenesis.
FIGURE 4.
Characterization of the cells generated from BM cells by M-CSF with MLO-Y4-CM. A, BM cells were cultured with M-CSF (10 ng/ml) plus the indicated concentration of MLO-Y4-CM for 3 days, and RANK mRNA expression was evaluated. The data are expressed as the mean ± S.E. (n = 3). B, BM cells were cultured with 15% MLO-Y4-CM or control CM in the presence of M-CSF (10 ng/ml) for 3 days, followed by treatment with M-CSF (10 ng/ml) and sRANKL (10 ng/ml) for the indicated time. c-Fos production was analyzed at mRNA and protein levels. The data are expressed as the mean ± S.E. (n = 3). C, BM cells were cultured as described in A, and PKR mRNA expression was then examined. The data are expressed as the mean ± S.E. (n = 3). **, p < 0.01, *, p < 0.05 versus MLO-Y4-CM (−) group.
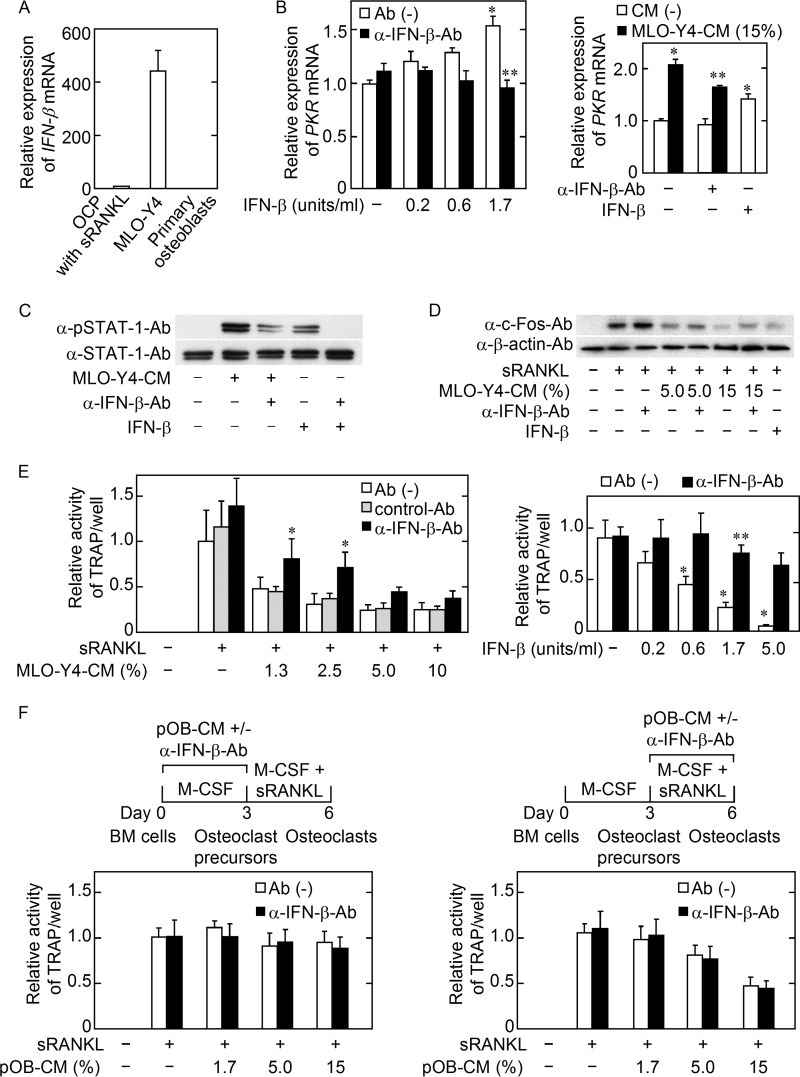
MLO-Y4 Cell Secretion of IFN-β Partially Mediated the Inhibitory Effect of MLO-Y4-CM on Osteoclast Precursor Generation
Because PKR is an IFN-β-inducible gene in osteoclast precursors (8), we examined the possibility that MLO-Y4 cells secrete IFN-β into the culture medium. We detected IFN-β mRNA expression by MLO-Y4 cells and found that the level was much higher than in the osteoclast precursors treated with sRANKL and M-CSF for 3 h. IFN-β mRNA expression by primary osteoblasts was undetectable (Fig. 5A). Next, we examined whether PKR mRNA expression was augmented in the cells induced by M-CSF with authentic IFN-β, and indeed, the presence of recombinant IFN-β during M-CSF-induced osteoclast precursor generation increased PKR mRNA expression, and this increase was normalized to control level by the simultaneous addition of α-IFN-β-Ab (Fig. 5B, left panel). As was the case for authentic IFN-β, MLO-Y4-CM increased the level of PKR mRNA expression, and this increased level was significantly decreased by α-IFN-β-Ab (Fig. 5B, right panel). The phosphorylation of STAT-1 is known to be essential for IFN-β signaling via the IFN-α/β receptor in the osteoclast precursors induced from BM cells by M-CSF (9). When the osteoclast precursors were treated with MLO-Y4-CM or recombinant IFN-β, the ratio of pSTAT-1 to total STAT-1 level in the cells was increased, and the increased phosphorylation was partially reduced by pretreatment of MLO-Y4-CM with α-IFN-β-Ab (Fig. 5C). In addition, a decrease in sRANKL-induced c-Fos protein in the cells generated by M-CSF with MLO-Y4-CM was partially recovered by the simultaneous addition of α-IFN-β-Ab to MLO-Y4-CM (Fig. 5D). Accordingly, the decreased osteoclastogenesis by the cells generated by M-CSF with MLO-Y4-CM was partially recovered by the simultaneous addition of α-IFN-β-Ab to MLO-Y4-CM (Fig. 5E, left panel). When recombinant IFN-β was used instead of MLO-Y4-CM, 1.7 units/ml of IFN-β decreased osteoclastogenesis to ∼20% of the control, and this inhibition was recovered by the simultaneous addition of α-IFN-β-Ab (Fig. 5D, right panel). However, pOB-CM had no effect on the generation of osteoclast precursors, although pOB-CM inhibited osteoclastic differentiation from the precursors IFN-β-independently (Fig. 5F). Taken together, the data suggest that MLO-Y4 cells secrete IFN-β, which partially mediates the inhibitory effect of MLO-Y4-CM on the generation of osteoclast precursors induced by M-CSF.
FIGURE 5.
IFN-β mRNA expression by MLO-Y4 cells. A, the osteoclast precursors (OCP) induced from BM cells by M-CSF (10 ng/ml) were treated with M-CSF (10 ng/ml) and sRANKL (10 ng/ml) for 3 h, and MLO-Y4 cells and primary osteoblasts were cultured for 3 days. IFN-β mRNA expression was examined. The data are expressed as the mean ± S.E. (n = 3). B, PKR mRNA expression by the cells generated with M-CSF in the presence of IFN-β (left panel) or MLO-Y4-CM (right panel). The indicated concentration of IFN-β with (black bar) or without 200 units/ml of anti-IFN-β neutralizing antibody (α-IFN-β-Ab) (open bar) was used (left panel). PKR mRNA expression in the cells was then examined. The data are expressed as the mean ± S.E. (n = 3). *, p < 0.01 versus IFN-β (−) group. **, not significant versus IFN-β (−) group (left panel). The cells were cultured with M-CSF (10 ng/ml) in the absence (open bar) or presence (black bar) of 15% MLO-Y4-CM. In addition, α-IFN-β-Ab (200 units/ml) or IFN-β (1.7 units/ml) was added to the culture, as indicated (right panel). *, p < 0.01 versus CM (−) Ab (−) IFN-β (−) group. **, p < 0.01 versus MLO-Y4-CM (15%) Ab (−) IFN-β (−) group (right panel). C, MLO-Y4-CM and culture medium containing IFN-β (0.7 units/ml) were pretreated with or without α-IFN-β-Ab (6000 units/ml) and protein G PLUS-agarose at 4 °C overnight, and the supernatants were harvested by centrifugation. BM cells were cultured with M-CSF (10 ng/ml) for 3 days. Then the cells were incubated with pretreated MLO-Y4-CM or pretreated culture medium for 30 min. The phosphorylation of STAT-1 was analyzed by Western blotting. D, BM cells were cultured with M-CSF (10 ng/ml) plus the indicated concentrations of MLO-Y4-CM or no CM with or without α-IFN-β-Ab (200 units/ml) or IFN-β (1.7 units/ml) for 3 days, followed by treatment with M-CSF (10 ng/ml) and sRANKL (10 ng/ml) for 24 h. c-Fos production was analyzed by Western blotting. E, effect of α-IFN-β-Ab on the inhibitory effect of MLO-Y4-CM (left panel) or IFN-β (right panel) on the osteoclastogenic potential of the cells. The cells were induced with M-CSF (10 ng/ml) in the presence of the indicated concentration of MLO-Y4-CM or IFN-β alone (open bar) or with 200 units/ml α-IFN-β-Ab (black bar) or control (Cont)-Ab (gray bar) and osteoclastogenesis was induced with M-CSF (10 ng/ml) and sRANKL (10 ng/ml). Osteoclastogenesis was evaluated based on the TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 6). *, p < 0.01 versus control Ab group at each concentration of MLO-Y4-CM (left panel). *, p < 0.01 versus IFN-β (−) group. **, not significant versus IFN-β (−) group (right panel). F, effects of pOB-CM on osteoclastogenic potential of the cells. pOB-CM and α-IFN-β-Ab (200 units/ml) were added to BM cell cultures as indicated during the first 3 days only (left panel) or latter 2–3 days only (right panel). Osteoclastogenesis was evaluated based on the TRAP activity of the culture medium.
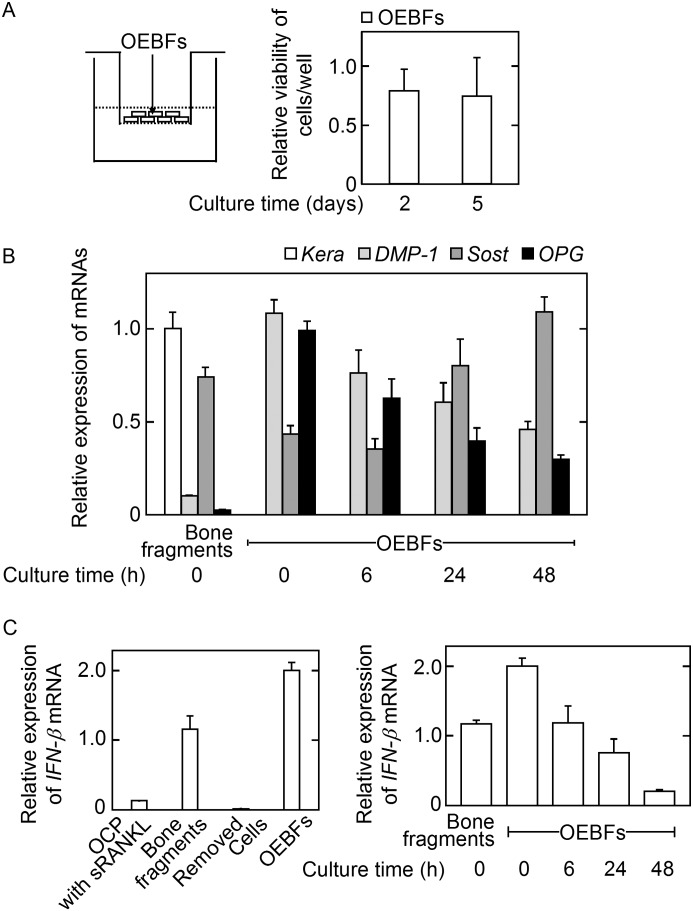
Development of a Culture System for OEBFs
To study whether primary osteocytes produce IFN-β as an inhibitory factor of osteoclastogenesis, we developed a culture system in which non-osteocytic, cell-depleted OEBFs were cultured on cell culture inserts (Fig. 6A, left panel). The OEBF cell viability was evaluated after 2 and 5 days of cultivation and was comparable between these two culture periods, indicating that the OEBF cells were stably cultured for at least 5 days in this culture system (Fig. 6A, right panel). After 2 days of cultivation, mRNA for the osteoblast marker gene keratocan (Kera) (31) was undetectable in OEBFs, whereas significant mRNA levels of the osteocyte marker genes dentin matrix protein 1 (31) and Sost (15) and for OPG were found in comparison with the expression in intact bone fragments. These findings suggested that most cells in OEBFs were osteocytes after 2 days of cultivation (Fig. 6B). Importantly, OEBFs expressed a significantly higher level of IFN-β mRNA compared with the level in intact bone fragments, although this expression decreased during the culture period (Fig. 6C). The removed cells during preparation of OEBFs expressed IFN-β mRNA ∼2000–4000 times less than bone fragments or OEBFs. These results indicated that OEBFs can be successfully cultured as osteocyte-rich, non-osteocytic, cell-free bone fragments that express IFN-β mRNA.
FIGURE 6.
Characterization of OEBFs. OEBFs were prepared by repeated collagenase/EDTA treatment of minced femurs from 5-week-old mice. A, OEBFs were cultured in α-MEM containing 10% FBS for the indicated number of days, and the cell viability was determined using Cell Counting kit-8. The data are expressed as the mean ± S.E. (n = 4). B, OEBFs were cultured for the indicated number of days, and the mRNA expression of the indicated genes was evaluated. Bone fragments prior to collagenase/EDTA treatment were used as the positive control. The data are expressed as the mean ± S.E. (n = 3). C, the expression of IFN-β mRNA was evaluated. Bone fragments were used as the positive control. The data are expressed as the mean ± S.E. (n = 3).
OEBFs Partially Inhibited Osteoclastogenesis in an IFN-β-dependent Manner
To address whether the primary osteocytes residing in mineralized bone matrix inhibit osteoclastogenesis by secreting IFN-β, we first tested the effect of OEBFs on osteoclastogenesis by co-culturing OEBFs on cell culture inserts and BM cells in the culture wells. The OEBFs were co-cultured during the phase of M-CSF-dependent osteoclast precursor generation, followed by washing the cells in each well and culturing in the presence of sRANKL without OEBFs (Fig. 7A, left panel). OEBFs significantly decreased osteoclastogenesis compared with the no OEBF or dead OEBF controls (Fig. 7A, right panel). We used OEBFs prepared from OPG-KO mice (OPG-KO-OEBFs) to confirm that the inhibitory effect of OEBFs on osteoclastogenesis is independent of the OPG produced by OEBFs and found that OPG-KO-OEBFs inhibited osteoclastogenesis to a similar extent as OEBFs prepared from wild-type control mice (WT-OEBFs) (Fig. 7B). To examine whether IFN-β is involved in the inhibitory effect of OEBFs on osteoclastogenesis, α-IFN-β-Ab was simultaneously added to the co-culture of OEBFs and BM cells during the osteoclast precursor generation phase. The presence of α-IFN-β-Ab partially recovered the reduction in osteoclastogenesis in the presence of OEBFs (Fig. 7C). These data suggested that osteocytes in mineralized bone matrix produce IFN-β that partially mediates the inhibitory effect of OEBFs on osteoclastogenesis.
FIGURE 7.
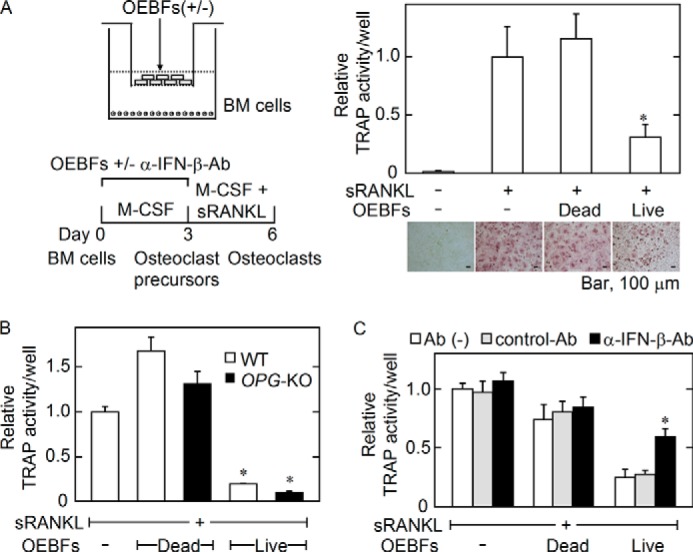
Effects of OEBFs on the capacity of M-CSF-induced cells to differentiate into osteoclasts. A, dead OEBFs were obtained by repeated freeze/thaw cycles. Live or dead OEBFs in culture inserts were co-cultured with BM cells seeded into the wells of 24-well plates in the presence of M-CSF (10 ng/ml) for 3 days. The inserts were then removed, and the cells in the wells were further cultured with M-CSF (10 ng/ml) and sRANKL (10 ng/ml) for 2–3 days. Osteoclastogenesis was evaluated based on the TRAP activity in the culture medium, and the cells were stained for TRAP activity (A). The data are expressed as the mean ± S.E. (n = 4). *, p < 0.01 versus OEBFs (−) with sRANKL group or dead OEBF group. Microphotographs of TRAP+ cells are shown in the lower panel in A. Bar, 100 μm. B, live or dead OEBFs were prepared from WT or OPG knock-out (OPG-KO) mice and co-cultured with BM cells, as described in A. Osteoclastogenesis was evaluated based on the TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 4). *, p < 0.01 versus OEBFs (−) group or dead OEBF group. C, OEBFs and BM cells were co-cultured as described in A except for the presence of the indicated Abs for the first 3 days. Osteoclastogenesis was evaluated based on the TRAP activity in the culture medium. The data are expressed as the mean ± S.E. (n = 4). *, p < 0.01 versus live OEBF control (Cont) Ab group.
DISCUSSION
In this study, we showed that osteocytic MLO-Y4 cells embedded in collagen gel inhibit osteoclastogenesis independently of the modulation of RANKL/OPG production by stromal ST2 cells on the gel, although MLO-Y4 cells in the gel supported osteoclastogenesis in the absence of ST2 cell layer. We also revealed that MLO-Y4 cells, but not primary osteoblasts, produce IFN-β to decrease osteoclastogenesis by inhibiting the M-CSF-induced generation of osteoclast precursors coincident with the stimulation of PKR mRNA expression and inhibition of c-Fos translation, downstream signaling molecule of IFN-β and its receptor complex. MLO-Y4-CM has also stimulatory effect of phosphorylation of STAT-1 in osteoclast precursors. In addition, we developed a culture system of OEBFs; using this culture system, we demonstrated that OEBFs produce IFN-β and negatively regulate osteoclastogenesis. OEBFs from OPG-KO mice also shared the capacity of osteocytes to inhibit osteoclastogenesis in an OPG-independent fashion.
A previous three-dimensional culture system of MLO-Y4 cells in collagen gel (on which BM cells were cultured) successfully revealed that the locally induced death of MLO-Y4 cells by mechanical scratching stimulates osteoclastogenesis around the area of dead MLO-Y4 cells (29). However, this system lacked cells representing bone lining cells. Although the nature of bone lining cells remains unclear, it is widely believed that these cells affect osteoclastogenesis by producing RANKL and/or OPG in response to local factors and hormones and also by communicating with osteocytes through cell-cell contact. Therefore, we included an ST2 cell layer, serving as bone-lining cells, on the gel containing MLO-Y4 cells in our three-dimensional culture system and observed the inhibitory effect of living osteocytes on osteoclastogenesis. It is unknown how MLO-Y4 cells in the gel exhibited the opposite effect on osteoclastogenesis, depending on the presence or absence of ST2 cell layer on the gel. When BM cells were seeded on ST2 cell layer, it could be expected that osteoclastic differentiation was induced by primarily RANKL expressed on ST2 cell membrane. In such situations, the inhibitory effect of MLO-Y4 cells on osteoclastogenesis could be predominantly observed at least in our culture system, despite the expression of RANKL on MLO-Y4 cell membrane. As the inhibitory mechanism of MLO-Y4 cells, we expected that the RANKL and/or OPG production by ST2 cells was modulated by gel-embedded MLO-Y4 cells; however, we failed to observe significant changes in expression. This finding is different from the results obtained from osteocyte-specific ablation experiments, suggesting that osteocytes restrain aberrant RANKL expression in the cells of the bone surface under normal loading conditions (19). One possible explanation for this discrepancy is that the functional connection of these two cell types may not be well established in the regulation of RANKL/OPG expression by ST2 cells, albeit cell-cell contact between MLO-Y4 cells embedded in gel has been shown by others (29). We also observed cell-cell contacts between the MLO-Y4 cells and ST2 cells in our three-dimensional culture system using light microscopy. Alternatively, it was recently reported that osteocytes and not bone-lining cells are the major source of RANKL for osteoclastogenesis during bone remodeling (16, 17) and that the role of bone-lining cells is to regulate the contact between BM cells and osteocytes by covering or exposing the bone surface (32, 33). Therefore, it might be necessary to utilize another type of cell to represent bone-lining cells if osteoclastogenesis is dependent on the RANKL produced by osteocytes. The development of another relevant model of the regulation of osteoclastogenesis during bone remodeling based on our three-dimensional culture system will be valuable, allowing the future analysis of the relationship between osteocytes, bone-lining cells, and BM cells.
Although osteocytes are known to produce OPG as a humoral factor inhibiting osteoclastogenesis (18), the MLO-Y4 cells used in this study produced an undetectable level of OPG under our culture conditions but still inhibited osteoclastogenesis via humoral factor(s). Therefore, we took advantage of this clone of MLO-Y4 cells to explore the effect of humoral osteocytic inhibitory factor(s) on osteoclastogenesis. In addition, we used OEBF culture to confirm the results for the MLO-Y4 cells using primary osteocytes in a bone matrix. To exclude the possible effect of OPG acting as a decoy receptor for RANKL, we focused on the inhibitory effect of MLO-Y4-CM on the generation of osteoclast precursors induced by M-CSF, a process that is independent of RANKL (5). We found that IFN-β is produced by MLO-Y4 cells and primary osteocytes in OEBFs. Indeed, IFN-β production in osteocytes is supported by several lines of evidence. IFN-β mRNA expression in MLO-Y4 cells was ∼400 times higher than in osteoclast precursors treated with sRANKL for 3 h, conditions under which osteoclast precursors express IFN-β mRNA, producing a significant amount of IFN-β that negatively regulates the osteoclastic differentiation of the cells (8, 9, 34). IFN-β mRNA was expressed by freshly isolated, intact bone fragments, and, after removing the cells from the surface of the bone fragments the remaining OEBFs expressed an approximately two times higher level of IFN-β mRNA, suggesting that most of the IFN-β mRNA-expressing cells, i.e. osteocytes, existed inside of the bone fragments. In addition, primary osteoblasts and the removed cells during OEBF preparation expressed undetectable or ∼4000 times lower level of IFN-β mRNA compared with OEBFs. Therefore, it is suggested that IFN-β mRNA expression was relatively osteocyte-specific among bone-forming cell and bone-resorbing cell lineage. Although the protein level of IFN-β in MLO-Y4-CM was not measured, the fact that PKR mRNA expression was stimulated in the cells induced by M-CSF with recombinant IFN-β and with MLO-Y4-CM and that these stimulatory effects were partially abolished by the simultaneous addition of α-IFN-β-Ab to MLO-Y4-CM suggested the presence of active IFN-β in MLO-Y4-CM. In addition, MLO-Y4-CM stimulated phosphorylation of STAT-1, which is essential for the IFN-stimulated gene factor-3 complex formation responsible for the signal transduction of type-I IFNs (7), and the increased pSTAT-1 was decreased by the presence of α-IFN-β-Ab. Furthermore, the decreased amount of c-Fos in and the concomitant inhibition of osteoclastogenesis by the cells generated in the presence of MLO-Y4-CM were partially recovered by the simultaneous addition of α-IFN-β-Ab to MLO-Y4-CM. Most importantly, we found that co-culturing BM cells and OPG-KO-OEBFs or the OEBFs from wild-type mice without cell-cell contact during the generation of osteoclast precursors by M-CSF inhibited the ensuing sRANKL-induced osteoclastogenesis. This inhibition was partially diminished by the presence of α-IFN-β-Ab during the co-culture period. Based on the data, we concluded that osteocytes produce several factors that inhibit osteoclastogenesis, including IFN-β. Although α-IFN-β-Ab used in our experiments could neutralize the effects of 1.7 units/ml of recombinant IFN-β on PKR mRNA expression and osteoclastogenesis to the control levels, the recovery of the osteoclastogenesis inhibited by MLO-Y4-CM or OEBFs in the presence of α-IFN-β-Ab was partial. Therefore, it is suggested that osteocytes produce the inhibitory factors of osteoclastogenesis other than IFN-β, which might also stimulate PKR mRNA expression.
Because no osteocyte-specific IFN-β-KO mice have been available until now, it is difficult to compare the significance of the IFN-β produced by osteocytes for in vivo osteoclastogenesis with the IFN-β produced by RANKL-stimulated osteoclast precursors. Osteocytes account for >90% of the total cells in bone, and the humoral factors derived from osteocytes can affect BM cells outside of the bone matrix, as evidenced by the effect of granulocyte CSF produced by osteocytes (35). In addition, our OEBF culture demonstrated that the IFN-β produced by osteocytes in the lacunae is released from the bone matrix to inhibit osteoclastogenesis. It has been hypothesized that bone remodeling under normal loading conditions is initiated by osteoclastic resorption of the locally damaged or microfractured area of the bone tissue, in which osteocytes are dying or apoptotic. Although osteocytic RANKL is indispensable for osteoclastogenesis during bone remodeling (16, 17), the relative contribution of membrane-bound versus soluble RANKL to osteoclastogenesis remains to be established. If RANKL bound to osteocytes, but not soluble RANKL, is significant for osteoclastogenesis, the fact that osteocytes are embedded in calcified matrix suggests that RANKL expressed on only osteocytic cell processes reaching to the bone surface through canaliculi can contact with BM cells to support their osteoclastic differentiation and that RANKL on osteocytes not reaching bone surface cannot contribute for osteoclastogenesis. However, the humoral factors produced by osteocytes even deep inside of the bone matrix are theoretically accessible to BM cells through canaliculi network. It has been speculated that a decrease in the factors that inhibit osteoclastogenesis due to the death of osteocytes is a key event that induces osteoclastogenesis (29). Therefore, there exists a possibility that damaged or aged osteocytes in a distance from bone surface cause a decrease in the humoral inhibitory factor(s) of osteoclastogenesis, resulting in a shift of the balance in favor of osteoclastogenesis supported by RANKL on vital osteocytes near bone surface. Because IFN-β knock-out mice exhibit osteoporosis (8), it is interesting to study the involvement of the IFN-β in this process.
In conclusion, we found that MLO-Y4 cells and primary osteocytes in bone fragments produce IFN-β as an inhibitory factor of osteoclastogenesis. Although the excessive osteoclastogenesis observed in IFN-β-KO mice has been regarded to be due to the absence of IFN-β production by osteoclast precursors, our findings may provide new insight into the mechanisms for the osteocytic control of bone remodeling.
This work was supported in part by Miyata Research Grant A from the Meikai University School of Dentistry (to T. S.), the Onuki Foundation (to Y. H.), Grant-in-Aid for Scientific Research (KAKENHI) 25293376 from the Japan Society for Promotion of Science (to Y. H.).
- M-CSF
- macrophage-colony stimulating factor
- RANKL
- receptor activator of NF-κB ligand
- OPG
- osteoprotegerin
- 1,25D3
- 1,25-dihydroxyvitamin D3
- PKR
- double-stranded RNA-activated protein kinase (also known as protein kinase R)
- BM
- bone marrow
- Dex
- dexamethasone
- pOB
- primary osteoblast
- OEBF
- osteocyte-enriched bone fragment
- CS
- calf serum
- sRANKL
- soluble RANKL
- Ab
- antibody
- STAT
- signal transducer and activation of transcription
- α-MEM
- α-minimal Eagle's medium
- TRAP
- tartrate-resistant acid phophatase
- CM
- conditioned medium.
REFERENCES
- 1. Karsenty G., Wagner E. F. (2002) Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 [DOI] [PubMed] [Google Scholar]
- 2. Manolagas S. C., Jilka R. L. (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 332, 305–311 [DOI] [PubMed] [Google Scholar]
- 3. Udagawa N., Takahashi N., Akatsu T., Tanaka H., Sasaki T., Nishihara T., Koga T., Martin T. J., Suda T. (1990) Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl. Acad. Sci. U.S.A. 87, 7260–7264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 [DOI] [PubMed] [Google Scholar]
- 5. Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 6. Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M. T., Martin T. J. (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 20, 345–357 [DOI] [PubMed] [Google Scholar]
- 7. Teitelbaum S. L., Ross F. P. (2003) Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 [DOI] [PubMed] [Google Scholar]
- 8. Takayanagi H., Kim S., Matsuo K., Suzuki H., Suzuki T., Sato K., Yokochi T., Oda H., Nakamura K., Ida N., Wagner E. F., Taniguchi T. (2002) RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-β. Nature 416, 744–749 [DOI] [PubMed] [Google Scholar]
- 9. Hayashi T., Kaneda T., Toyama Y., Kumegawa M., Hakeda Y. (2002) Regulation of receptor activator of NF-κB ligand-induced osteoclastogenesis by endogenous interferon-β (INF-β) and suppressors of cytokine signaling (SOCS). The possible counteracting role of SOCSs- in IFN-β-inhibited osteoclast formation. J. Biol. Chem. 277, 27880–27886 [DOI] [PubMed] [Google Scholar]
- 10. García M. A., Gil J., Ventoso I., Guerra S., Domingo E., Rivas C., Esteban M. (2006) Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 70, 1032–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsuo K., Owens J. M., Tonko M., Elliott C., Chambers T. J., Wagner E. F. (2000) Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat. Genet. 24, 184–187 [DOI] [PubMed] [Google Scholar]
- 12. Grigoriadis A. E., Wang Z. Q., Cecchini M. G., Hofstetter W., Felix R., Fleisch H. A., Wagner E. F. (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 [DOI] [PubMed] [Google Scholar]
- 13. Bonewald L. F. (2011) The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamioka H., Honjo T., Takano-Yamamoto T. (2001) A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 28, 145–149 [DOI] [PubMed] [Google Scholar]
- 15. Moester M. J., Papapoulos S. E., Löwik C. W., van Bezooijen R. L. (2010) Sclerostin: current knowledge and future perspectives. Calcif. Tissue Int. 87, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 17. Xiong J., Onal M., Jilka R. L., Weinstein R. S., Manolagas S. C., O'Brien C. A. (2011) Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kramer I., Halleux C., Keller H., Pegurri M., Gooi J. H., Weber P. B., Feng J. Q., Bonewald L. F., Kneissel M. (2010) Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 30, 3071–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 [DOI] [PubMed] [Google Scholar]
- 20. Heino T. J., Hentunen T. A., Väänänen H. K. (2002) Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-β: enhancement by estrogen. J. Cell Biochem. 85, 185–197 [DOI] [PubMed] [Google Scholar]
- 21. van der Plas A., Nijweide P. J. (1992) Isolation and purification of osteocytes. J. Bone Miner. Res. 7, 389–396 [DOI] [PubMed] [Google Scholar]
- 22. Gu G., Nars M., Hentunen T. A., Metsikkö K., Väänänen H. K. (2006) Isolated primary osteocytes express functional gap junctions in vitro. Cell Tissue Res. 323, 263–271 [DOI] [PubMed] [Google Scholar]
- 23. Stern A. R., Stern M. M., Van Dyke M. E., Jähn K., Prideaux M., Bonewald L. F. (2012) Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. BioTechniques 52, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato Y., Windle J. J., Koop B. A., Mundy G. R., Bonewald L. F. (1997) Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 12, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 25. Mizuno A., Amizuka N., Irie K., Murakami A., Fujise N., Kanno T., Sato Y., Nakagawa N., Yasuda H., Mochizuki S., Gomibuchi T., Yano K., Shima N., Washida N., Tsuda E., Morinaga T., Higashio K., Ozawa H. (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem. Biophys. Res. Commun. 247, 610–615 [DOI] [PubMed] [Google Scholar]
- 26. Luben R. A., Wong G. L., Cohn D. V. (1976) Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology 99, 526–534 [DOI] [PubMed] [Google Scholar]
- 27. Sato T., Watanabe K., Masuhara M., Hada N., Hakeda Y. (2007) Production of IL-7 is increased in ovariectomized mice, but not RANKL mRNA expression by osteoblasts/stromal cells in bone, and IL-7 enhances generation of osteoclast precursors in vitro. J. Bone Miner. Metab. 25, 19–27 [DOI] [PubMed] [Google Scholar]
- 28. Okayasu M., Nakayachi M., Hayashida C., Ito J., Kaneda T., Masuhara M., Suda N., Sato T., Hakeda Y. (2012) Low-density lipoprotein receptor deficiency causes impaired osteoclastogenesis and increased bone mass in mice because of defect in osteoclastic cell-cell fusion. J. Biol. Chem. 287, 19229–19241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kurata K., Heino T. J., Higaki H., Väänänen H. K. (2006) Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J. Bone Miner. Res. 21, 616–625 [DOI] [PubMed] [Google Scholar]
- 30. Zhao S., Zhang Y. K., Harris S., Ahuja S. S., Bonewald L. F. (2002) MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Miner. Res. 17, 2068–2079 [DOI] [PubMed] [Google Scholar]
- 31. Paic F., Igwe J. C., Nori R., Kronenberg M. S., Franceschetti T., Harrington P., Kuo L., Shin D. G., Rowe D. W., Harris S. E., Kalajzic I. (2009) Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 45, 682–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chambers T. J., Fuller K. (2011) How are osteoclasts induced to resorb bone? Ann. N.Y. Acad. Sci. 1240, 1–6 [DOI] [PubMed] [Google Scholar]
- 33. Chambers T. J., Fuller K. (1985) Bone cells predispose bone surfaces to resorption by exposure of mineral to osteoclastic contact. J. Cell Sci. 76, 155–165 [DOI] [PubMed] [Google Scholar]
- 34. Zheng H., Yu X., Collin-Osdoby P., Osdoby P. (2006) RANKL stimulates inducible nitric-oxide synthase expression and nitric oxide production in developing osteoclasts. An autocrine negative feedback mechanism triggered by RANKL-induced interferon-β via NF-κB that restrains osteoclastogenesis and bone resorption. J. Biol. Chem. 281, 15809–15820 [DOI] [PubMed] [Google Scholar]
- 35. Fulzele K., Krause D. S., Panaroni C., Saini V., Barry K. J., Liu X., Lotinun S., Baron R., Bonewald L., Feng J. Q., Chen M., Weinstein L. S., Wu J. Y., Kronenberg H. M., Scadden D. T., Divieti Pajevic P. (2013) Myelopoiesis is regulated by osteocytes through Gsα-dependent signaling. Blood 121, 930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]