Abstract
Background
Hepatic angiomyolipoma is a rare benign mesenchymal tumor. We report an unusual case of a patient with multiple hepatic angiomyolipomas exhibiting high 18 F-fluorodeoxyglucose (FDG) uptake.
Case presentation
A 29-year-old man with a medical history of tuberous sclerosis was admitted to our hospital for fever, vomiting, and weight loss. Abdominal dynamic computed tomography revealed faint hypervascular hepatic tumors in segments 5 (67 mm) and 6 (10 mm), with rapid washout and clear borders; however, the tumors exhibited no definite fatty density. Abdominal magnetic resonance imaging revealed that the hepatic lesions were slightly hypointense on T1-weighted imaging, slightly hyperintense on T2-weighted imaging, and hyperintense with no apparent fat component on diffusion-weighted imaging. FDG-positron emission tomography (PET) imaging revealed high maximum standardized uptake values (SUVmax) of 6.27 (Segment 5) and 3.22 (Segment 6) in the hepatic tumors. A right hepatic lobectomy was performed, and part of the middle hepatic vein was also excised. Histological examination revealed that these tumors were characterized by the background infiltration of numerous inflammatory cells, including spindle-shaped cells, and a resemblance to an inflammatory pseudotumor. Immunohistochemical evaluation revealed that the tumor stained positively for human melanoma black-45. The tumor was therefore considered an inflammatory pseudotumor-like angiomyolipoma. Although several case reports of hepatic angiomyolipoma have been described or reviewed in the literature, only 3 have exhibited high 18 F-FDG uptake on PET imaging with SUVmax ranging from 3.3–4.0. In this case, increased 18 F-FDG uptake is more likely to appear, particularly if the inflammation is predominant.
Conclusion
Although literature regarding the role of 18 F-FDG-PET in hepatic angiomyolipoma diagnosis is limited and the diagnostic value of 18 F-FDG-PET has not yet been clearly defined, the possibility that hepatic angiomyolipoma might exhibit high 18 F-FDG uptake should be considered.
Keywords: Hepatic angiomyolipoma, FDG, PET, HMB-45
Background
Angiomyolipoma (AML) of the liver is a rare benign mesenchymal tumor with positive human melanoma black-45 (HMB-45) expression and a heterogeneous composition of blood vessels, and smooth muscle cells, and adipose tissue cells, which account for the tumors varying morphological features [1]. Most AMLs contain various amount of fat that enable detection via computed tomography (CT) or magnetic resonance imaging (MRI) [2-5]. However, some hepatic AMLs have low fat contents, posing difficulties for establishing a definitive preoperative diagnosis [6,7]. Diagnostic functional positron emission tomography (PET) imaging using an 18 F-fluorodeoxyglucose (FDG) tracer is a useful technique for detecting and differentiating benign and malignant tumors. Similar to benign tumors, which tend to exhibit low FDG uptake, hepatic AMLs have been reported to exhibit similarly low uptake levels in the absence of hemorrhage [8]. However, we encountered a distinct case with multiple AMLs with high FDG uptake in the liver of a 29-year-old man.
Case presentation
A 29-year-old man with a medical history of tuberous sclerosis was admitted to our hospital for fever, vomiting, and weight loss (4 kg/month). His serum C-reactive protein and hepatic enzyme levels were increased. Tests for all evaluated markers of hepatic viruses and tumors such as alpha-fetoprotein, carcinoembryonic antigen, protein induced by vitamin K absence-2, and cancer antigen 19–9 were negative.
Abdominal dynamic CT revealed faint hypervascular hepatic tumors in segments 5 (S5; 67 mm) and 6 (S6; 10 mm), with rapid washout and clear borders but without definite fatty densities. CT also revealed multiple tumors with fat components in both kidneys, but these did not show definite enhancements (Figure 1).
Figure 1.
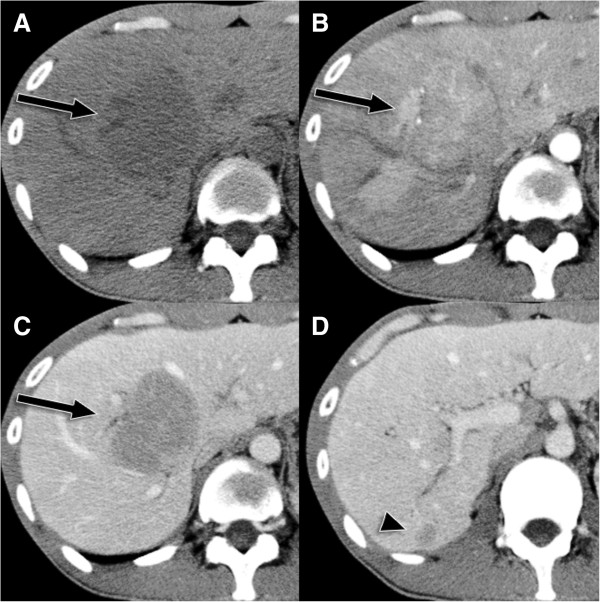
Abdominal computed tomography. (A) Axial computed tomography (CT) imaging shows a liver lesion (arrow) with low attenuation. (B) On early-phase axial contrast-enhanced CT imaging, the lesion exhibits heterogeneous enhancement. (C) On portal-phase axial contrast-enhanced CT imaging, the lesion shows rapid washout in segment 5. (D) The segment 6 lesion exhibits a similar pattern (arrowhead).
Abdominal MR imaging (1.5 T Magnetom Symphony; Siemens Medical Solutions, Erlangen, Germany) revealed that the hepatic lesions were slightly hypointense on T1-weighted imaging (T1WI), slightly hyperintense on T2-weighted imaging (T2WI), and hyperintense on diffusion-weighted imaging (DWI) with no apparent fat component (Figure 2). The tumors in both kidneys were mildly hypointense on both T1WI and T2WI and mildly hyperintense with fat components on DWI.
Figure 2.
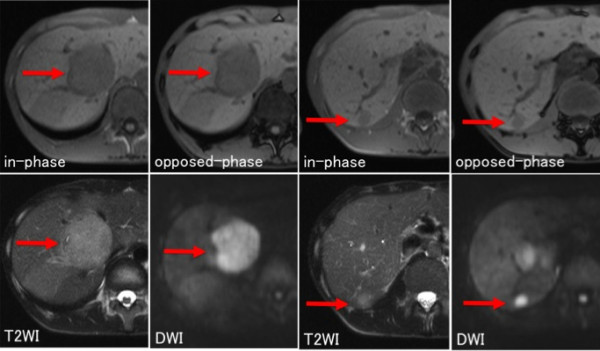
Abdominal magnetic resonance (MR) imaging. Abdominal magnetic resonance (MR) imaging revealed that the hepatic tumors (segment 5: arrow, segment 6: arrowhead) were slightly hypointense on T1-weighted imaging (WI), slightly hyperintense on T2WI, and hyperintense without an apparent fat component on diffusion-weighted imaging.
18 F-FDG PET/CT (Biograph 16; Siemens Medical Solutions, Erlangen, Germany) imaging revealed that the hepatic tumors exhibited high maximum standard uptake values (SUVmax) of 6.27 (S5) and 3.22 (S6; Figure 3). No definite FDG-uptake was observed in the renal AMLs.
Figure 3.
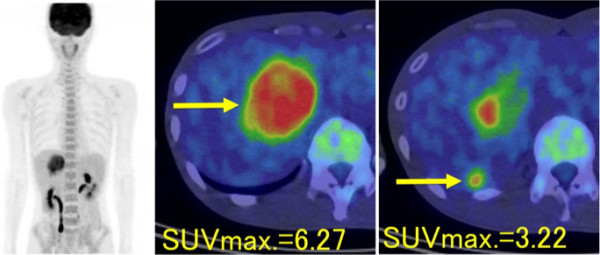
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) imaging of hepatic angiomyolipomas. The fused PET/CT image demonstrates the markedly increased FDG uptake in segments 5 and 6 of the liver.
Although a preoperative percutaneous biopsy was performed and a histological examination of the specimens suggested the diagnosis of angiomyolipoma, the high FDG uptake and increasing tumor sizes made it difficult to exclude a malignant transformation of angiomyolipoma. A right hepatic lobectomy was performed and a part of the middle hepatic vein was also excised. The patient recovered well after surgery. His initial symptoms resolved completely, and his serological parameters of inflammation returned to normal by the time of release from hospital. Currently, the patient remains in a healthy condition and has exhibited no signs of recurrence at 4 years after surgery.
Histological examination revealed that these tumors were characterized by the background infiltration of numerous inflammatory cells, including spindle-shaped cells, and an absence of fat (Figure 4) and thus resembled inflammatory pseudotumors. On immunohistochemical analysis, the tumors stained positively for HMB-45, CD31, vimentin, and α-smooth muscle actin. However, the tumor cells were negative for keratin, epithelial membrane antigen, hepatocyte paraffin1, c-kit, CD34, CD56, and S-100. The tumor was hence diagnosed as an inflammatory pseudotumor-like AML.
Figure 4.
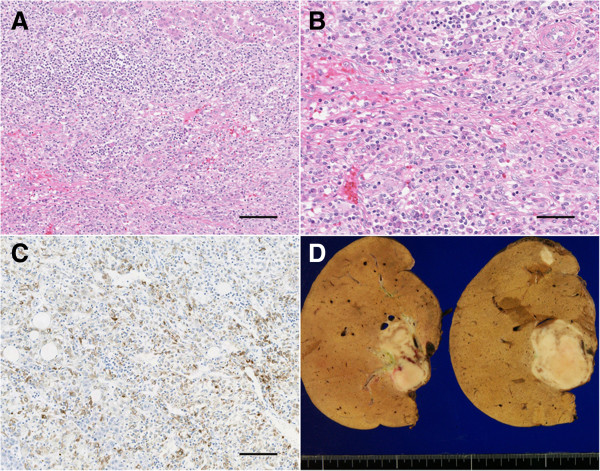
Histological features of hepatic angiomyolipomas. (A, B) Histological examination revealed the background infiltration of numerous inflammatory cells, including spindle-shaped cells (A: original magnification = 20×, scale bar = 50 μm, B: 40×, scale bar = 100 μm; hematoxylin–eosin staining). (C) The immunohistochemical analysis revealed positive staining for human melanoma black-45 (HMB-45; original magnification = 20×, scale bar = 50 μm). (D) In a gross examination, the cut AML surfaces revealed well-delineated borders and slightly variegated appearances.
Discussion
AML is a benign mesenchymal tumor that has reportedly occurred frequently in the kidney but rarely in the liver. Approximately 5%–10% of hepatic AML are associated with renal AML and tuberous sclerosis (Bourneville’s disease), and this was true for our patient. The first case of hepatic AML was described by Ishak in 1976 [9]. Although fatty tissue is a characteristic of AML, the fat content within the tumor can range from 5% to 90% of the tumor volume, thus resulting in varying radiological appearances [2,5,6,10]. Preoperative diagnosis relies on imaging studies, which include CT and MRI. However, it is often difficult to distinguish these lesions from other hepatic fatty tumors such as fat-containing hepatic adenomas or hepatocellular carcinomas with fatty metamorphosis.
Although AMLs are considered benign, malignant transformation is not a rare complication associated with renal AML [11]. In recent years, the malignant transformation of hepatic AML has also been reported in the literature [12,13]. 18 F-FDG PET/CT is useful for detecting malignant transformation [14]. Moreover, because hepatic AML has been reported to exhibit low 18 F-FDG-uptake [8], 18 F-FDG PET/CT might be highly valuable for excluding malignancies among hepatic lesions suspected to be AML.
We performed a Medline search using the search terms “liver”, “angiomyolipoma”, and “FDG”. Although several case reports of hepatic AML have been described or reviewed in the literature, only 3 cases exhibited high 18 F-FDG uptake on PET imaging, with SUVmax ranging from 3.3–4.0 [8,13,15-20] (Table 1).
Table 1.
Reports of FDG uptake in hepatic AMLs to the present date
| Author | Age | Gender | Tumor size (cm) | SUVmax |
|---|---|---|---|---|
| Takanami [8] |
74 |
Female |
20 |
Low |
| 62 |
Female |
15 |
4.0 |
|
| Awane [15] |
48 |
Female |
6 |
Low |
| Kubo [16] |
70 |
Female |
3.2 |
1.9 |
| Kinugasa [17] |
42 |
Female |
1.4 |
3.9 |
| Sakaguchi [18] |
80 |
Female |
4.3 |
3.3 |
| Kawaoka [19] |
41 |
Female |
3.9 |
1.9 |
| Lhommel R [20] |
64 |
- |
7 |
Low |
| Lee JH [6] | 47 | Female | 4 | Low |
FDG, 18 F-fluorodeoxyglucose; AML, angiomyolipoma; SUVmax, maximum standard uptake value.
Kinugasa et al. [17] suggested that glucose hypermetabolism in smooth muscle cells and a high cell density due to the lack of a fat component might increase the 18 F-FDG uptake. Takanami et al.[8] suggested that hepatic AMLs might exhibit increased 18 F-FDG uptake in the presence of hemorrhage or an inflammatory response. In such cases, increased 18 F-FDG uptake is more likely to appear, particularly if the inflammatory tissue is predominant. Because the average mean SUV of the normal liver is approximately 3.5 ± 3.1 and the malignant lesion-to-liver SUV ratio is approximately 1.9 ± 1.4 [21], the FDG uptakes of the angiolipomas described in Table 1 were easily obscured by the FDG uptake of the normal liver tissue. In our case, the angiolipomas exhibited apparently high levels of FDG uptake.
Recently, some hepatic epithelioid AMLs have been reported to have features similar to those of renal AMLs reported previously. Epithelioid AMLs either lack or contain only a minimal amount of adipose tissue and are thus more difficult to distinguish from other hypervascular tumors. The imaging features of epithelioid AMLs include an absent capsule and hypervascularity with central punctiform or filiform vessels that exhibit characteristic enhancement [22,23]. No literature reports have described the FDG-PET imaging features of epithelioid AMLs. In our case, although the CT and MRI imaging features were similar to those of epithelioid AML, no epithelial cells were observed in a histopathological examination.
The presence of HMB-45-positive smooth muscle cells in an immunostaining analysis is the definitive criterion for diagnosing hepatic AML [24]. Although AML was already suggested by the findings the preoperative percutaneous biopsy, we could not completely rule out the possibility of malignancy because of the atypical MRI findings and high 18 F-FDG uptake in the lesions.
Conclusion
We report herein a case of multiple hepatic angiomyolipomas with high 18 F-FDG uptake. Although the literature reports on the role of 18 F-FDG PET/CT in hepatic AML diagnosis are limited and the diagnostic value of 18 F-FDG PET/CT has not yet been clearly defined, the possibility that hepatic AML might exhibit high 18 F-FDG uptake should be considered.
Consent
Written informed consent for the publication of this case report and any accompanying images was obtained from the patient. A copy of this written consent is available for review by this journal.
Abbreviations
PET: Positron emission tomography; CT: Computed tomography; FDG: Fluorodeoxyglucose; MRI: Magnetic resonance imaging; SUV: Standardized uptake values; SUVmax: Maximum standardized uptake values; T1WI: T1-weighted imaging; T2WI: T2-weighted imaging; DWI: Diffusion-weighted imaging; AML: Angiomyolipoma; HMB-45: Human melanoma black-45.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SK, YA, and TN drafted the manuscript. SK, YA, AT, and TH contributed to the diagnosis. YA and TN reviewed the radiological findings and interpreted the data. YA conceived the study. YA, TN, and YT reviewed the manuscript. All authors approved the final version of the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Soma Kumasaka, Email: kumasaka88@gmail.com.
Yukiko Arisaka, Email: yarisaka12022808@gmail.com.
Azusa Tokue, Email: azusana45@yahoo.co.jp.
Tetsuya Higuchi, Email: tetsuyah92md@gmail.com.
Takahito Nakajima, Email: gunma_radtech@yahoo.co.jp.
Yoshito Tsushima, Email: tyoshito@showa.gunma-u.ac.jp.
Acknowledgment
The authors thank Arifudin Achmad, MD, PhD, for his assistance with the preparation of this manuscript. The authors would like to thank Enago (http://www.enago.jp) for the English language review.
References
- Yang C-Y, Ho M-C, Jeng Y-M, Hu R-H, Wu Y-M, Lee P-H. Management of hepatic angiomyolipoma. J Gastrointest Surg. 2007;11:452–7. doi: 10.1007/s11605-006-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte JM, Do RK, Shia J, Gönen M, D’Angelica MI, Getrajdman GI, Allen PJ, Fong Y, Dematteo RP, Klimstra DS, Jarnagin WR. Liver angiomyolipomas: a clinical, radiologic, and pathologic analysis of 22 patients from a single center. Surgery. 2011;150:557–67. doi: 10.1016/j.surg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, Heiken FP. Fat-containing lesions of the liver: radiologic-pathologic correlation1. Radiographics. 2005;25:321–331. doi: 10.1148/rg.252045083. [DOI] [PubMed] [Google Scholar]
- Blasiak B, Barnes S, Foniok T, Rushforth D, Matyas J, Ponjevic D, Weglarz WP, Tyson R, Iqbal U, Abulrob A, Sutherland GR, Obenaus A, Tomanek B. Comparison of T2 and T2*-weighted MR molecular imaging of a mouse model of glioma. BMC Med Imaging. 2013;13:20. doi: 10.1186/1471-2342-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T-A, Zhao Q-Y, Chen M-Y, Wang L-J, Ao J-Y. Diagnostic analysis of hepatic angiomyolipoma. Hepatobiliary Pancreat Dis Int. 2005;4:152–5. [PubMed] [Google Scholar]
- Lee SJ, Kim SY, Kim KW, Shin YM, Kim HJ, Lee JS, Kim SA. Hepatic angiomyolipoma with minimal fat, mimicking hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:330–5. doi: 10.3350/cmh.2012.18.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha I, Cartwright D, Guis M, Miller TR, Ferrell LD. Angiomyolipoma of the liver in fine-needle aspiration biopsies: its distinction from hepatocellular carcinoma. Cancer. 1999;87:25–30. doi: 10.1002/(SICI)1097-0142(19990225)87:1<25::AID-CNCR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Takanami K, Kaneta T, Hitachi S, Yamada T, Ishida K, Rikiyama T, Katayose Y, Unno M, Yamada S, Takahashi S. F-18 FDG PET/CT FINDINGS in two patients with hepatic angiomyolipoma with and without intratumoral hemorrhage. Clin Nucl Med. 2010;35:18–21. doi: 10.1097/RLU.0b013e3181c3611f. [DOI] [PubMed] [Google Scholar]
- Ishak K. In: Hepatocell carcinoma. Okuda K, Peters RL, editor. 1976. Mesenchymal tumors of the liver; pp. 247–304. [Google Scholar]
- Guidi G, Catalano O, Rotondo A. Spontaneous rupture of a hepatic angiomyolipoma: CT findings and literature review. Eur Radiol. 1997;7:335–7. doi: 10.1007/s003300050162. [DOI] [PubMed] [Google Scholar]
- Brimo F, Robinson B, Guo C, Zhou M, Latour M, Epstein JI. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34:715–22. doi: 10.1097/PAS.0b013e3181d90370. [DOI] [PubMed] [Google Scholar]
- Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443–50. doi: 10.1046/j.1365-2559.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol. 2008;32:793–8. doi: 10.1097/PAS.0b013e3181607349. [DOI] [PubMed] [Google Scholar]
- Macheda M, Rogers S, Best J. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- Awane M, Naito M, Matsusue S, Honjo G, Youichiro Kobashi HM. A case of hepatic angiomyolipoma presented with rupture and intraabdominal hemorrhage. Japanese J Gastroenterol Surg. 2010;43:724–729. doi: 10.5833/jjgs.43.724. [DOI] [Google Scholar]
- Takafumi K. A case of rapid growth of hepatic angiomyolipoma. J Japan Surg Assoc. 2012;73:648–653. doi: 10.3919/jjsa.73.648. [DOI] [Google Scholar]
- Kinugasa H. A case of ring-like enhanced angiomyolipoma on CT and MRI with abnormal FDG uptake. Liver cancer. 2008;14:97–104. [Google Scholar]
- Tatsuma S. A case of hepatic angiomyolipoma showing high 18f-fluorodeoxyglucose uptake on positron emission tomography. J Japan Surg Assoc. 2012;74:947–951. [Google Scholar]
- Toru K. Hepatic angiomyolipoma originating in the caudate process: a case report. Yamaguchi Igaku. 2013;62:21–26. doi: 10.2342/ymj.62.21. [DOI] [Google Scholar]
- Lhommel R, Annet L, Bol A, Gigot J-F, Sempoux C, Mathieu I, Seret M, Lonneux M. PET scan with 11C-acetate for the imaging of liver masses: report of a false positive case. Eur J Nucl Med Mol Imaging. 2005;32:629. doi: 10.1007/s00259-004-1698-3. [DOI] [PubMed] [Google Scholar]
- Al-Nabhani KZ, Syed R, Michopoulou S, Alkalbani J, Afaq A, Panagiotidis E, O’Meara C, Groves A, Ell P, Bomanji J. Qualitative and quantitative comparison of PET/CT and PET/MR imaging in clinical practice. J Nucl Med. 2014;55:88–94. doi: 10.2967/jnumed.113.123547. [DOI] [PubMed] [Google Scholar]
- Ahmadi T, Itai Y, Takahashi M, Onaya H, Kobayashi T, Tanaka YO, Matsuzaki Y, Tanaka N, Okada Y. Abdominal imaging angiomyolipoma of the liver: significance of CT and MR dynamic study. Abdom Imaging. 1998;23:520–526. doi: 10.1007/s002619900391. [DOI] [PubMed] [Google Scholar]
- Ji J, Lu C, Wang Z, Xu M, Song J. Epithelioid angiomyolipoma of the liver: CT and MRI features. Abdom Imaging. 2013;38:309–14. doi: 10.1007/s00261-012-9911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf HR, Ishak KG, Shekar R, Sesterhenn IA, Young DY, Fanburg-Smith JC. Melanoma markers in angiomyolipoma of the liver and kidney: a comparative study. Arch Pathol Lab Med. 2002;126:49–55. doi: 10.5858/2002-126-0049-MMIAOT. [DOI] [PubMed] [Google Scholar]


