Background: The endothelium is centrally involved in acute inflammatory disorders.
Results: WIN55,212-2 and the endocannabinoid N-arachidonoyl dopamine (NADA), but not anandamide nor 2-arachidonoylglycerol, reduce endothelial activation by bacterial Toll-like receptor agonists and TNFα.
Conclusion: NADA is a newly identified endogenous regulator of endothelial inflammation.
Significance: The endothelial endocannabinoid system represents a novel immune regulatory system that could be exploited therapeutically.
Keywords: Cannabinoid Receptors, Cannabinoids, Endocannabinoids, Endothelial Cell, Endothelial Dysfunction, Endothelium, Sepsis, Toll-like Receptors (TLR), TRP Channels
Abstract
Although cannabinoids, such as Δ9-tetrahydrocannabinol, have been studied extensively for their psychoactive effects, it has become apparent that certain cannabinoids possess immunomodulatory activity. Endothelial cells (ECs) are centrally involved in the pathogenesis of organ injury in acute inflammatory disorders, such as sepsis, because they express cytokines and chemokines, which facilitate the trafficking of leukocytes to organs, and they modulate vascular barrier function. In this study, we find that primary human ECs from multiple organs express the cannabinoid receptors CB1R, GPR18, and GPR55, as well as the ion channel transient receptor potential cation channel vanilloid type 1. In contrast to leukocytes, CB2R is only minimally expressed in some EC populations. Furthermore, we show that ECs express all of the known endocannabinoid (eCB) metabolic enzymes. Examining a panel of cannabinoids, we demonstrate that the synthetic cannabinoid WIN55,212-2 and the eCB N-arachidonoyl dopamine (NADA), but neither anandamide nor 2-arachidonoylglycerol, reduce EC inflammatory responses induced by bacterial lipopeptide, LPS, and TNFα. We find that endothelial CB1R/CB2R are necessary for the effects of NADA, but not those of WIN55,212-2. Furthermore, transient receptor potential cation channel vanilloid type 1 appears to counter the anti-inflammatory properties of WIN55,212-2 and NADA, but conversely, in the absence of these cannabinoids, its inhibition exacerbates the inflammatory response in ECs activated with LPS. These data indicate that the eCB system can modulate inflammatory activation of the endothelium and may have important implications for a variety of acute inflammatory disorders that are characterized by EC activation.
Introduction
Cannabinoids are a structurally diverse group of lipophilic compounds that bind the transient receptor potential cation channel vanilloid type 1 (TRPV1)2 and G protein-coupled receptors, including cannabinoid receptors type 1 and 2 (CB1R and CB2R), GPR18, and GPR55 (1). Although some cannabinoids, such as Δ9-tetrahydrocannabinol, have been studied extensively for their psychoactive effects, cannabinoids are also known to possess medicinal properties. However, support for their use as therapeutic agents did not intensify until the discovery of the endocannabinoid (eCB) system in the early 1990s (2). The eCB system is composed of a complex network of metabolic enzymes, arachidonic acid-based lipid messengers (i.e. endocannabinoids), and their receptors (3, 4). N-Arachidonoylethanolamide (AEA; anandamide) and 2-arachidonoylglycerol (2-AG) are the two most thoroughly studied eCBs; however, other eCBs also exist, including 2-arachidonyl glycerol ether (Noladin ether), O-arachidonoylethanolamine (virodhamine), N-arachidonoyl glycine, and N-arachidonoyl dopamine (NADA) (1). Although the functions of AEA and 2-AG in neuronal synaptic plasticity have been substantially documented, the functions of these molecules outside the nervous system, and the roles of the other eCBs, are not as well understood (5–8).
Cannabinoid receptors are expressed in immune cells. In particular, CB2R is expressed at relatively high levels, especially in B-cells, natural killer cells, and monocytes, and its activation can dampen the inflammatory response to infection (9–12). In fact, targeting CB2R in leukocytes has been suggested as a therapy for inflammatory disorders such as sepsis (13–16). Although it is clear that several plant-derived (i.e. phytocannabinoids) and synthetic cannabinoids possess immunomodulatory activity, prior studies have primarily focused on their effects on leukocytes and microglia without assessing their effects on the endothelium, which is known to be centrally involved in acute inflammatory disorders such as sepsis (17–19). In sepsis, the persistent, direct, and unresolved engagement of endothelial Toll-like receptors (TLRs) by microbial components, and the sustained secretion of endogenous inflammatory mediators, such as tumor necrosis factor (TNF)α and interleukin (IL)-1β, lead to endothelial activation and dysfunction (20–24). Ultimately, this contributes to the development of coagulopathy and increased vascular permeability, which subsequently leads to multiorgan failure and death (23). Therefore, therapeutically targeting components of the endothelial eCB system may represent a novel approach to ameliorating the damaging effects that occur during acute inflammatory disorders.
To this end, we sought to identify cannabinoids that are able to modulate EC inflammation and delineate which components of the eCB system are expressed by ECs. Using a panel of synthetic cannabinoids, phytocannabinoids, and eCBs, we found that the synthetic cannabinoid WIN55,212-2 and the eCB NADA have the ability to dampen the activation of primary human lung microvascular ECs (HMVEC-lung) by a variety of inflammatory agonists, including bacterial lipopeptides (TLR2 agonist), lipopolysaccharide (LPS) (TLR4 agonist), and TNFα. Although the anti-inflammatory properties of WIN55,212-2 have previously been described, only a few reports have described the physiological functions of NADA, including its role in inflammation (16, 25–32).
NADA was first described as a putative eCB in 2000, and was subsequently also identified as an endovanilloid (33, 34). In mice, NADA was shown to induce the following tetrad of physiological paradigms associated with cannabinoids: hypothermia, hypo-locomotion, catalepsy, and analgesia (33, 35–37). NADA has been found to play a regulatory role in both the peripheral and central nervous systems and displays antioxidant and neuroprotectant properties (34, 38–41). In addition to its neurological effects, NADA has been implicated in smooth muscle contraction and vasorelaxation in blood vessels (42–45). Additionally, NADA has been observed to suppress inflammatory activation of human Jurkat T-cells and to inhibit the release of prostaglandin E2 (PGE2) from LPS-activated astrocytes, microglia and mouse brain ECs (46–48). NADA also displays inhibitory activity in HIV-1 replication assays (49). Finally, a recent report suggests that NADA can prevent the degranulation and release of TNFα from RBL-2H3 mast cells treated with an IgE-antigen complex (50). Together, these studies show that physiological functions attributed to NADA are multifaceted and include the potential to modulate the immune response.
The binding specificity for the cannabinoid receptors differs between WIN55,212-2 and NADA. Although WIN55,212-2 is a strong agonist of both CB1R and CB2R, NADA displays approximately a 40-fold selectivity for CB1R over CB2R (1, 33, 51). Moreover, the TRPV1 ion channel is activated by NADA but is inhibited by WIN55,212-2 (34, 52–54). Therefore, we sought to determine the expression repertoire of the four G protein-associated CBRs, and TRPV1, in different human primary endothelial cell niches. We found that primary human ECs from multiple vascular beds all express CB1R, GPR18, GPR55, and TRPV1 and differentially express CB2R at relatively low levels. Additionally, we determined that human ECs express all the eCB metabolic enzymes identified to date. We find that CB1R/CB2R are necessary for the anti-inflammatory properties of NADA but not WIN55,212-2. Furthermore, although TRPV1 inhibition further reduces the LPS-induced inflammatory activation of HMVEC-lung in the presence of WIN55,212-2 or NADA, paradoxically, TRPV1 inhibition augments inflammation in the absence of the cannabinoids. Together, these results suggest that human ECs have the machinery to metabolize and respond to NADA and that the endothelial eCB system represents a novel target for inflammatory disorder therapies.
EXPERIMENTAL PROCEDURES
Cells
Human umbilical vein endothelial cells (HUVEC, passage 2–6, multiple donors) (Lonza, Walkersville, MD), human brain microvascular endothelial cells (HMVEC-brain, passage 4–9, unknown donor gender) (Cell Sciences, Canton, MA), human liver sinusoidal microvascular endothelial cells (HMVEC-liver, passage 4–9, unknown donor gender) (Cell Sciences), human lung microvascular endothelial cells (HMVEC-lung, passage 4–9, female and male donors) (Lonza), and human coronary artery endothelial cells (HCAEC, passage 4–9, female donor) (Lonza) were incubated at 37 °C under humidified 5% CO2. HUVEC were grown in EGM-2 and HMVEC-brain, -liver, and -lung, and HCAEC were grown in microvascular EGM-2 (Lonza). Peripheral blood mononuclear cells (PBMCs) and neutrophils were isolated by gradient centrifugation using LymphoprepTM and PolymorphprepTM (Axis-Shield, Oslo, Norway), respectively, from heparinized whole blood collected by venipuncture from healthy human volunteers. PBMCs were isolated according to the manufacturers supplied instructions and neutrophils as we have described previously (55).
Cannabinoid and Inflammatory Agonist Treatments
ECs were grown to confluence before agonist treatment. Unless otherwise noted, in all experiments cells were preincubated with the cannabinoids or the TRPV1 agonist, capsaicin, at the indicated concentrations for 1 h prior to and continuously during the inflammatory agonist treatment. In Fig. 6B, samples were incubated continuously with polymyxin B (Invivogen, San Diego) at 50 μg/ml to minimize effects from contaminating LPS. Compounds used were 2-AG (Tocris, Ellisville, MO), abnormal cannabidiol (Axon Medchem, Groningen, Netherlands), arachidonoyl-2′-chloroethylamide (Tocris), N-arachidonoylethanolamine (Sigma), AEA in Tocrisolve 100 (Tocris), CBD (Axon Medchem), capsaicin (Tocris), HU-308 (Tocris), N-arachidonoyl dopamine (NADA) (Sigma, Tocris, and Cayman Chemical), N-arachidonoyl glycine (Tocris), and WIN55,212-2 (Sigma). The inflammatory agonists used include FSL-1 (EMC Microcollections, Tubingen, Germany), LPS (List Laboratories, Los Gatos, CA), and recombinant human TNFα (PeproTech, Inc., Rocky Hill, NJ) at the indicated concentrations and times. Preparations of FSL-1, TNFα, CAP, and dopamine contained <0.1 EU/ml LPS based on the Limulus amoebocyte lysate chromogenic endotoxin quantitation assay (Thermo-Scientific). Because AA, 2-AG, AEA, N-arachidonoyl glycine, arachidonoyl-2′-chloroethylamide, HU-308, abnormal CBD, and NADA preparations caused the Limulus amoebocyte lysate substrate to become cloudy, the quantitative OD readings were unreliable; however, based on the observed color changes compared with the standards, the levels of LPS appeared qualitatively insignificant.
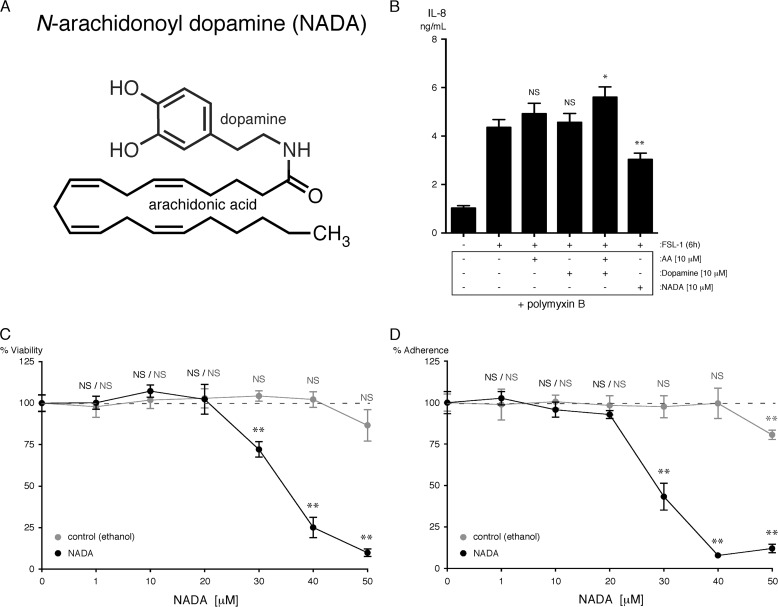
FIGURE 6.
NADA, and not its chemical constituents AA and dopamine, reduce IL-8 secretion from HMVEC-lung activated with FSL-1, although concentrations of NADA less than 20 μm do not affect HMVEC-lung viability. A, NADA is composed of AA and dopamine moieties covalently linked by an amide bond. B, levels of IL-8 were quantified in the supernatants of HMVEC-lung that were pretreated for 1 h with either 10 μm of AA, 10 μm of dopamine, 10 μm of both AA and dopamine, or 10 μm NADA and then treated with 10 μg/ml FSL-1 for an additional 6 h while in the continuous presence of AA, dopamine, or NADA (n = 4). Polymyxin B at 50 μg/ml was added to each sample for the duration of the experiment. NS, not significant; *, p < 0.05; **, p < 0.01, FSL-1 versus FSL-1 plus AA, dopamine, or NADA. C and D, an MTT (C; n = 4) and crystal violet (D; n = 4) assay was performed on HMVEC-lung treated for 7 h at the indicated concentrations of NADA. Control samples were incubated with a concentration of ethanol equivalent to that present in the NADA-treated samples. NS, not significant; **, p < 0.01; NADA versus ethanol control. The IL-8 ELISA was performed three times, and the MTT and crystal violet assays were performed two times.
Immunoblots
Cells were lysed with RIPA-Lysis Buffer (4 mm sodium dihydrogen phosphate, pH 7.0; 6 mm disodium hydrogen phosphate, pH 7.0; 150 mm sodium chloride; 1% Nonidet P-40; 1% sodium deoxycholate; 0.1% SDS; 2 mm EDTA; 50 mm sodium fluoride; 0.1 mm sodium vanadate) plus protease inhibitor mixture (Sigma), and protein concentrations of the lysates were estimated using the RCDC protein assay kit (Bio-Rad). Total proteins were separated by SDS-PAGE (11%) and then transferred to PVDF membranes (Pall Corp, Ann Arbor, MI). Membranes were blocked in 3% BSA for 45 min at room temperature (RT) and then incubated with primary antibody solution overnight at 4 °C. Membranes were washed and then incubated with goat anti-rabbit peroxidase-conjugated secondary antibodies (111-035-045; Jackson ImmunoResearch, West Grove, PA). Immunoblots were developed using SuperSignal West Dura Extended Duration Substrate (34076; Thermo Scientific), and the signal was detected using a Gel Logic 2200 Imaging System (Eastman Kodak, Rochester, NY) run on Carestream imaging software (Carestream Health, Rochester, NY). The antibodies used were as follows: CB1R (2 μg/ml, AP01265PU-N, Acris Antibodies, San Diego); CB2R (1 μg/ml, ARP63486_P050, Aviva Systems Biology, San Diego); GPR18 (2 μg/ml, ab76258, Abcam, Cambridge, MA); GPR55 (0.8 μg/ml, orb101191, Biorbyt, Cambridge, UK); TRPV1 (1 μg/ml, ab111973, Abcam); and β-actin (0.1 μg/ml, A2066, Sigma).
ELISAs, MTT Assay, and Crystal Violet Assay
IL-6 and IL-8 levels were quantified in culture supernatants by ELISA (R&D Systems, Minneapolis, MN, and BD Biosciences, respectively) according to the manufacturer's instructions. For the MTT and crystal violet (CV) assays, the CBR agonist or antagonist was added at the indicated concentrations for 7 h in the absence of inflammatory agonists. MTT Assays (Biotium, Hayward, CA) were performed in 96-well plates according to the manufacturer's instructions. Because NADA induces the formation of formazan crystal from tetrazolium salt in the absence of cells, in the MTT assay graph (Fig. 5C), background ODs of NADA in the absence of cells were subtracted from ODs in the presence of cells at the same NADA concentration. For CV assays, after the indicated treatments, the cells in 48-well plates were washed twice with PBS and then stained with 0.5% CV in methanol for 10 min (100 μl). The plates were then dunked several times in tap water to remove the excess CV solution, after which time the plates were gently tapped upside down on a paper towel to remove all traces of liquid. 1% SDS in water (300 μl) was then added to each well, and the remaining stained cells were allowed to solubilize for several hours. One well in the plate was left without cells to control for background staining. Absorbance was read at 570 nm in a FLUOstar OPTIMA fluorescent plate reader (BMG Labtech, Cary, NC); the background staining was subsequently subtracted, and the percent adherence relative to the control was calculated.
FIGURE 5.
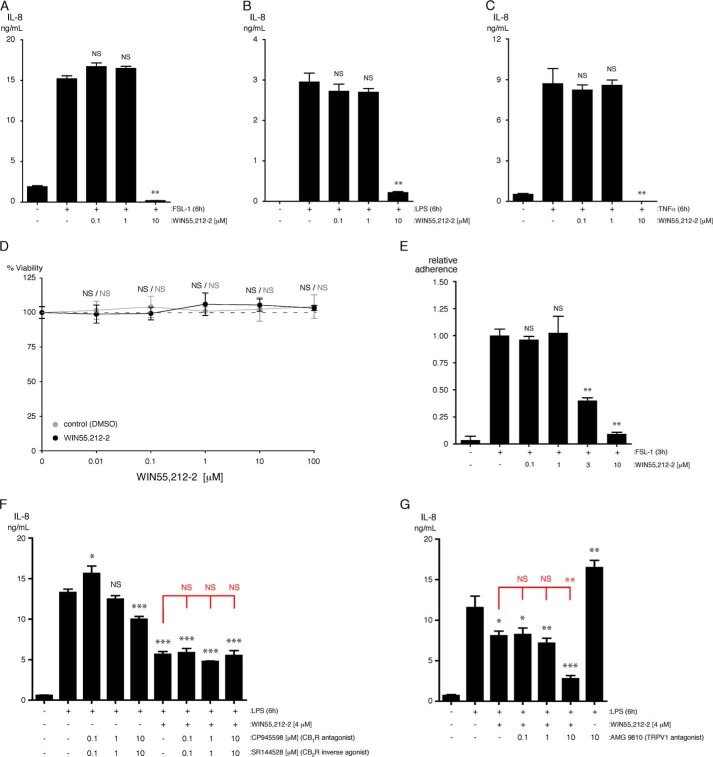
WIN55,212-2 reduces the inflammatory activation of HMVEC-lung without affecting viability. A–C, levels of IL-8 were quantified in the supernatants of HMVEC-lung that were pretreated for 1 h with the indicated concentrations of WIN55,212-2 and then treated with either 10 μg/ml FSL-1 (A) or LPS (B) or 100 ng/ml TNFα (C) for an additional 6 h while in the continuous presence of WIN55,212-2 (n = 3). NS, not significant; **, p < 0.01; FSL-1 versus FSL-1 plus WIN55,212-2. D, MTT assay was performed on HMVEC-lung that were treated for 7 h at the indicated concentrations of WIN55,212-2. Control samples were incubated with a concentration of DMSO equivalent to that present in the WIN55,212-2-treated samples. NS, not significant. E, HMVEC-lung were pretreated for 1 h with the indicated concentrations of WIN55,212-2 and then treated with 10 μg/ml FSL-1 for an additional 3 h while in the continuous presence of WIN55,212-2. The HMVEC-lung were then washed, and calcein AM-labeled primary human neutrophils were allowed to adhere for 20 min. Nonadherent neutrophils were subsequently removed, and the percentage of remaining adherent neutrophils was calculated (n = 3). **, p < 0.01; ANOVA. F, levels of IL-8 were quantified in the supernatants of HMVEC-lung that were pretreated for 30 min with either 0.1, 1, or 10 μm CP945598/SR144528 and then incubated for another 30 min with 4 μm WIN55,212-2 in the presence of CP945598/SR144528. ECs were then treated with 10 μg/ml LPS for an additional 6 h while in the continuous presence of CP945598/SR144528 and/or WIN55,212-2 (n = 3). Crystal violet readings between samples were not significantly different. G, cells were treated and analyzed similarly as F, except using the TRPV1 inhibitor AMG9810 (n = 4). Crystal violet readings for the samples treated with AMG9810 (10 μm) displayed a significant decrease in cell adherence (p < 0.01). F and G, t tests (black): NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001; LPS versus LPS plus CP945598/SR144528 or AMG9810 and/or WIN55,212-2. ANOVA (red): NS, not significant; **, p < 0.01, LPS plus WIN55,212-2 versus LPS plus WIN55,212-2 and CP945598/SR144528 or AMG9810. WIN55,212-2 ELISAs were performed three times, and the MTT and neutrophil adhesion assays were performed two times.
Flow Cytometry
HUVEC were detached using Accutase Cell Detachment Solution (Innovative Cell Technologies, San Diego). HUVEC were passed through a 70-μm filter, counted, and aliquoted at 5 × 105 cells per sample. The cells were then washed using Flow Cytometry Staining Buffer (FCSB) (R&D Systems) and incubated with 10 μg/ml human IgG (R&D Systems) in FCSB for 15 min at 4 °C. Next, the cells were incubated for 30 min at 4 °C with either 0.25 μg/sample of FITC-mouse anti-human CD62E (E-selectin) (BBA21; R&D Systems) or FITC-mouse IgG1 (IC002F; R&D Systems). The samples were washed once more with FCSB and then analyzed by flow cytometry (LSRII Flow Cytometer, BD Biosciences). A far-red fluorescent dye (L10119; Invitrogen) was used to assess cell viability.
Quantitative Real Time PCR (qPCR)
Specific gene expression assays (HPRT1 (Hs01003267_m1), GUSB (Hs99999908_m1), CNR1 (Hs00275634_m1), CNR2 (Hs00275635_m1; assay 1), CNR2 (Hs00952005_m1; assay 2), GPR18 (Hs00245542_m1), GPR55 (Hs00995276_m1; assay 1), GPR55 (Hs00271662_s1; assay 2), TRPV1 (Hs00218912_m1), ABHD4 (Hs01040459_m1), ABHD6 (Hs00977889_m1), ABHD12 (Hs01018047_m1), DAGLA (Hs00391374_m1), FAAH1 (Hs01038660_m1), FAAH2 (Hs00398732_m1), GDE-1 (Hs00213347_m1), MGLL (Hs00200752_m1), NAPE-PLD (Hs00419593_m1), PTPN-22 (Hs01587518_m1), DDC (Hs01105048_m1), TH (Hs00165941_m1), and the manufacturer's suggested assay reagents were purchased from Applied Biosystems (Foster City, CA). HUVEC, HMVEC, HCAEC, and PBMCs were lysed, and mRNA was isolated using TRIzol according to the manufacturer's supplied protocol (Invitrogen). mRNA concentrations were determined with an ND-1000 (NanoDrop®/Thermo Fisher Scientific), and mRNA was reverse-transcribed to cDNA using the High Capacity RNA-to-cDNA kit using 2 μg of mRNA per reaction (Invitrogen). Human adrenal tissue cDNA was purchased from Zyagen (San Diego). An input of either 5 or 80 ng of cDNA in 10 μl of total reaction volume per well containing TaqMan® Fast Advanced Master Mix (Applied Biosystems) was used in all qPCR experiments, and qPCR was performed using the StepOnePlusTM System (Applied Biosystems). For the run method, PCR activation at 95 °C for 20 s was followed by 40 cycles of 1 s at 95 °C and 20 s at 60 °C. The average Ct value of two technical replicates was used in all calculations. The average Ct value of the internal controls HPRT1 and GUSB were used to calculate ΔCt values. For single donor assays (HMVEC-liver, HMVEC-lung, and HCAEC (n = 1)), data analysis was performed using the 2−ΔΔCt method (56). For multiple donor assays (HUVEC (n = 2 lots), HMVEC-brain (n = 2), and PBMCs (n = 3)), the initial data analysis was performed using the 2−ΔΔCt method, and the data were corrected using log transformation, mean centering, and auto scaling to ensure appropriate scaling between biological replicates (57). The methods of calculation utilized assume an amplification efficiency of 100% between successive cycles.
Neutrophil Adhesion Assay
The neutrophil adhesion assay was performed as described previously (55). Briefly, neutrophils were resuspended in RPMI 1640 medium at 2 × 106 cells/ml and labeled with 3 μm calcein-AM (Invitrogen). Just prior to adding labeled neutrophils, 48-well plates containing HMVEC-lung monolayers were washed three times with RPMI 1640 medium containing 3% BSA. Labeled neutrophils were then added at 6 × 105 cells/300 μl/well and allowed to incubate for 20 min at 37 °C in the dark before washing five times with PBS (with Ca2+ and Mg2+) to remove nonadherent cells. Pre-washing and post-washing fluorescence was read at an excitation of 485 nm and an emission of 520 nm in a FLUOstar OPTIMA fluorescent plate reader. The relative adherence was calculated by first subtracting the background fluorescence of the ECs from the pre- and post-wash readings and then dividing the post-wash reading by the pre-wash reading to quantitate the fluorescence decrease for each well. The ratio of adherence of each well is then divided by the average ratio of adherence for the positive control samples. Neutrophil adhesion was visualized using a Zeiss Axio Imager D1 microscope.
Electric Cell-Substrate Impedance Sensing (ECIS)
ECIS Z Theta system (Applied Biophysics, Troy, NY) was used to measure the transendothelial electrical resistance (TER), a measure of endothelial barrier integrity, of HMVEC-lung monolayers, as described in detail by Giaever and Keese (58). Briefly, HMVEC-lung (1.4 × 105 cells/cm2) were seeded into wells containing 10 small gold electrodes and a larger counter electrode on a 96-well plate (Applied Biophysics) and were allowed to grow to confluence. Inflammatory agonists (FSL, LPS, and TNFα) and either NADA (2 or 10 μm) or WIN55,212-2 (1 or 2 μm) were then added to the wells. Resistance was measured repeatedly in each well for 8 h. Data were normalized to the resistance measured before the addition of inflammatory agonists and NADA.
Antagonist/Inverse Agonist Assays
Confluent monolayers of HMVEC-lung were pretreated for 30 min with either the combination of CP945598 (CB1R antagonist; Tocris) and SR144528 (CB2R inverse agonist; Cayman Chemical) or with AMG9810 (TRPV1 antagonist; Tocris) (0.1, 1, or 10 μm) and then incubated for another 30 min with WIN55,212-2 (4 μm) or NADA (10 μm) in the continued presence of either CP945598 plus SR144528 or AMG9810 (0.1, 1, or 10 μm). ECs were then treated with LPS (10 μg/ml) for an additional 6 h while in the continuous presence of the combination of CP945598 and SR144528 or AMG9810 and WIN55,212-2 or NADA. IL-6 and IL-8 were quantified in culture supernatants.
Calcium Influx Assay
HMVEC-lung were grown to confluency in 96-well black-walled TC plates. Endothelial Ca2+ influx was determined using the FLIPR Calcium 6 assay (Molecular Devices) according to the manufacturer's supplied instructions. Cells were treated with either ethanol, ATP (10 μm), or NADA at the indicated concentrations, and fluorescence output (i.e. Ca2+ influx) was measured for 3 min with a Flex Station fluorometer (Molecular Devices). The data were then normalized to the background readings before treatments for each condition.
LC-MS/MS to Detect NADA in HMVEC-Lung
An analytical LC-MS/MS method to detect endocannabinoids in cell lysates was developed at Cayman Chemical Co. (Ann Arbor, MI). Confluent monolayers of HMVEC-lung were grown in 15-cm tissue culture dishes and left untreated or treated either with FSL-1 (10 μg/ml), LPS (10 μg/ml), or TNFα (100 ng/ml) for 3 h, and then lysed with 2.25 ml of RIPA lysis buffer (see above for formulation and procedures) per plate. For each sample, the lysate from 2 × 15-cm tissue culture dishes were combined (4.5 ml total). After an initial extraction with chloroform/methanol (2:1), both 1 and 3 ml of the lysate from each sample were concentrated and analyzed by LC-MS/MS. NADA standard was prepared at Cayman Chemical using RIPA buffer as the matrix.
Statistics
The data were analyzed using t-tests (unequal variance two-tail) or ANOVA using a Dunnett's post-test. GraphPad Prism or Excel® 2008 was used for statistical analyses. p values ≤0.05 were considered significant for all data except crystal violet assays, where p values ≤0.01 were considered significant. Data were expressed as mean ± S.D., except for the data compiled in Fig. 4, which are expressed as the mean ± S.E.
FIGURE 4.
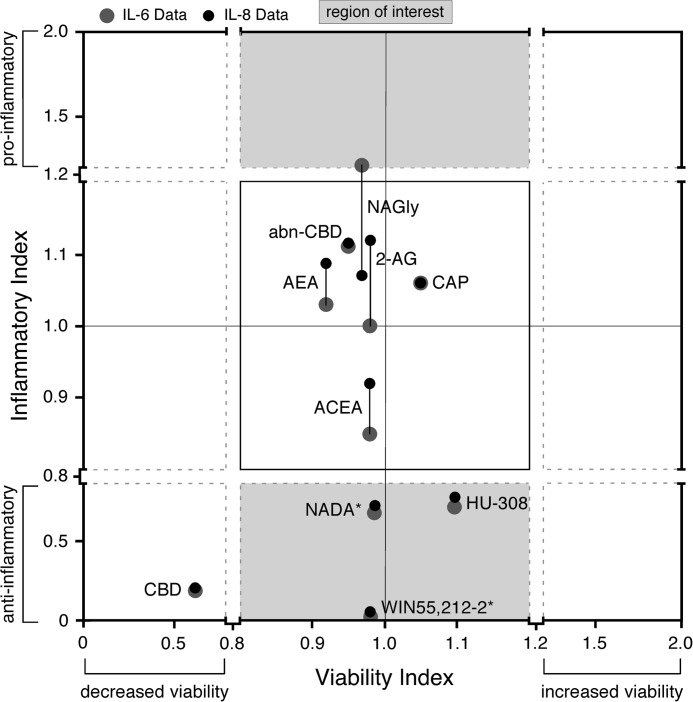
NADA and WIN55,212-2 significantly decrease IL-6 and IL-8 secretion from ECs without affecting cell viability. Levels of IL-6 and IL-8 were quantified in supernatants of HMVEC-lung that were pretreated for 1 h with 10 μm of the indicated CBR agonist/antagonist and then treated with either 10 μg/ml FSL-1 or LPS or with 100 ng/ml TNFα for an additional 6 h while in the continuous presence of inhibitor. The relative viability of HUVEC or HMVEC-lung was determined by an MTT assay after incubation with 10 μm of the indicated CBR agonist/antagonist, DMSO, or ethanol control for 7 h. To calculate the inflammatory indices, the relative inflammatory index in each independent experiment for each CBR agonist/antagonist was first calculated (i.e. the average cytokine concentration for the samples treated with both the inflammatory agonist (FSL-1, LPS, or TNFα) and CBR agonist/antagonist divided by the average cytokine concentration for the samples treated with inflammatory agonist alone; n = 3 minimum). Then, the means of these relative indices for each CBR agonist/antagonist were plotted on the graph (n = 4–5; see supplemental Table S3 for the n value breakdowns for the different inflammatory agonists). The MTT viability indices were calculated in the same manner. The region of interest (shaded gray area) indicates CBR agonists/antagonists that decrease or increase cytokine expression by more than 20% versus control (those compounds with inflammatory indices <0.8 and >1.2, respectively) and do not decrease or increase viability by more than 20% versus control (those compounds with viability indices between 0.8 and 1.2). *, CBR agonists/antagonists that have a significant (p < 0.05) effect on IL-6 and IL-8 secretion, without significantly (p > 0.05) affecting viability (e.g. NADA and WIN55,212-2). Note: solutions of AEA in ethanol and Tocrisolve® were tested with similar results. The calculated results, including mean, S.E., and p values, are shown in supplemental Table S3. abnCBD, abnormal CBD.
On-line Supplemental Material
The supplemental Tables S1 and S2 display the mean ΔCt calculations for the CBR and eCB metabolic enzyme qPCR data in Figs. 1 and 3, respectively. The supplemental Table S3 gives a description and shows the breakdown of the calculated data for the mean viability and inflammatory indices depicted in the graph in Fig. 4. The supplemental Figs. S1 and S2 depict the WIN55,212-2 and NADA, respectively, IC50 values, and pre-/post-treatment graphs. The supplemental Fig. S3 displays the flow cytometry gating schematic for the data depicted in Fig. 8. The supplemental Fig. S4 describes two anabolic pathways proposed for NADA and the qPCR RQ graphs for DDC and TH expression in ECs, PBMCs, and human adrenal tissue.
FIGURE 1.
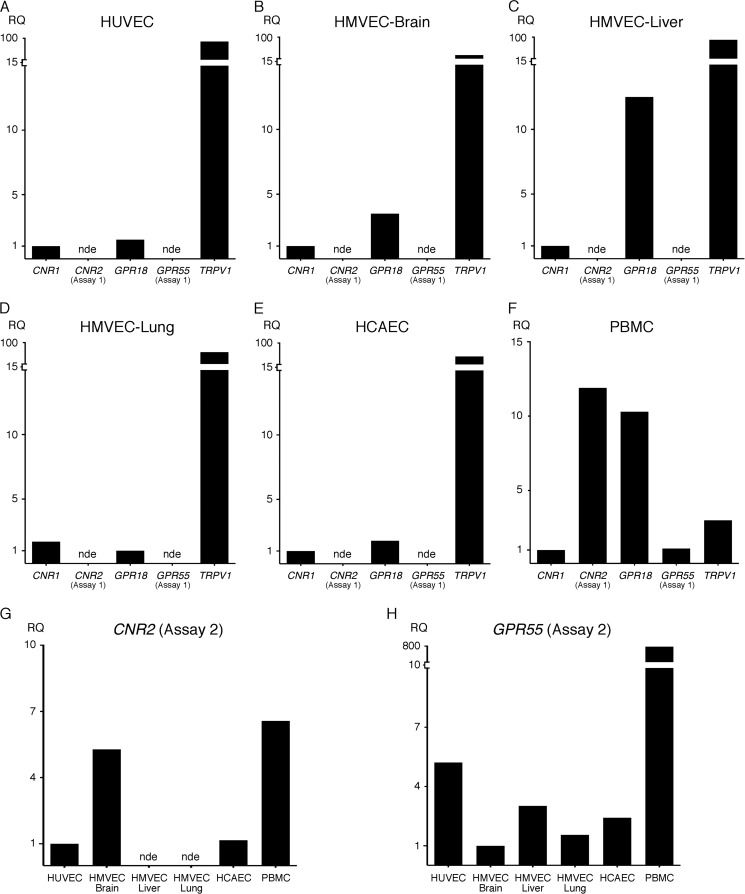
ECs express a similar subset of CBR mRNA transcripts. HUVEC (multiple donors) (A, G, and H), HMVEC-brain (two donors) (B, G, and H), HMVEC-liver (one donor) (C, G, and H), HMVEC-lung (one donor) (D, G, and H), and HCAEC (one donor) (E, G, and H) were grown in their respective media and lysed with TRIzol upon confluency. PBMCs (three donors) (F, G, and H) were immediately lysed upon isolation. Three biological replicates were analyzed for each donor. The mean Ct value for HPRT1 and GUSB was used as the reference in calculating the ΔCt values (supplemental Fig. S1) for each biological replicate as described under “Experimental Procedures.” 80 ng of cDNA was analyzed for each sample. The relative quantification (RQ) values shown in the graphs are relative to lowest detectable expressing CBR gene for each cell type (A–F) or lowest detectable expressing cell type for each gene (G and H). When gene expression was not detected in more than half of the technical replicates, it was defined as not expressed (nde, not detectably expressed). Because CNR1 and GPR55 were not initially detected using assay 1, a second gene assay (assay 2) was performed using different primers to re-assess expression in the same samples (G and H).
FIGURE 3.
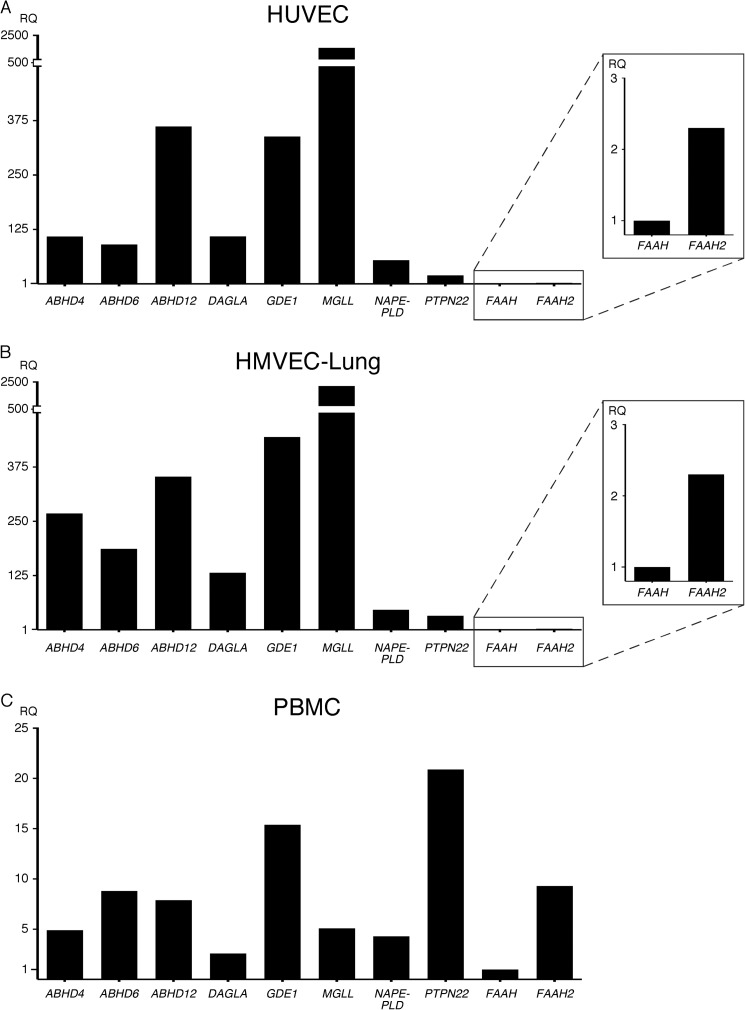
ECs express the same subset of eCB metabolic enzymes. HUVEC (multiple donors) (A) and HMVEC-lung (one donor) (B) were grown in their respective media and lysed with TRIzol upon confluency. PBMCs (one donor) (C) were immediately lysed upon isolation. The samples were analyzed as described in Fig. 1. RQ, relative quantification.
FIGURE 8.
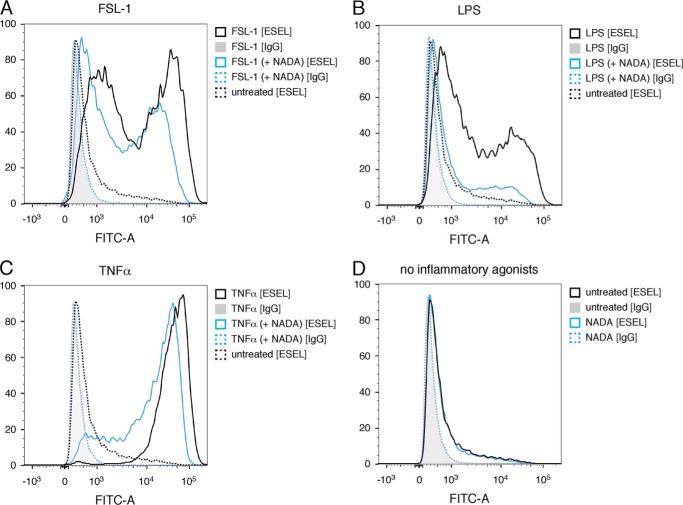
NADA reduces the surface expression of E-selectin on ECs activated by inflammatory agonists. HUVEC were pretreated for 1 h with 10 μm NADA and then treated with either 10 μg/ml FSL-1 (A) or LPS (B) or 100 ng/ml TNFα (C) for an additional 3 h while in the continuous presence of NADA or treated with NADA only for 4 h (D). FITC-labeled mouse IgG was used an antibody specificity control. Flow cytometry experiments were performed two times.
RESULTS
Endothelial Cells from Different Vascular Beds Express a Similar Subset of Cannabinoid Receptors and eCB Metabolic Enzymes
To determine which cannabinoid receptors are expressed in primary human endothelial cells, mRNA expression levels from HUVEC, HCAEC, brain, liver, and lung microvascular ECs (HMVEC-brain, -liver, and -lung) were measured. cDNA derived from human peripheral blood mononuclear cells (PBMCs) was used for comparison. Commercially available assays designed to detect the presence of CNR1 (CB1 receptor/CB1R), CNR2 (CB2 receptor/CB2R), GPR18 and GPR55, and the ion channel TRPV1 were utilized. Initial results showed that ECs express CNR1, GPR18, and TRPV1 but not CNR2 nor GPR55 (Fig. 1, A–E, and supplemental Table S1). In contrast, PBMCs expressed CNR2 and GPR55 in addition to the other receptors detected in ECs (Fig. 1F and supplemental Table S1). Although detectable in all cell lines, endothelial CNR1 expression was relatively low and, in fact, was not detected in any of the ECs when an input of 5 ng of cDNA was used (supplemental Table S1). To confirm the expression profile of CNR2 and GPR55 in ECs, qPCR was repeated using different assays (assay 2) containing primers that recognize different exons. Using the assay 2 mixes, GPR55 was detected in all the ECs tested, and CNR2 transcripts were detected in HUVEC, HMVEC-brain, HCAEC, and PBMCs, although the levels were extremely low (Fig. 1, G and H). CNR2 was not detected with assay 2 in any of the ECs, or the PBMCs, when an input of 5 ng of cDNA was used (supplemental Table S1). These results suggest that alternative splice variants of GPR55 and CNR2 may be expressed in different tissues.
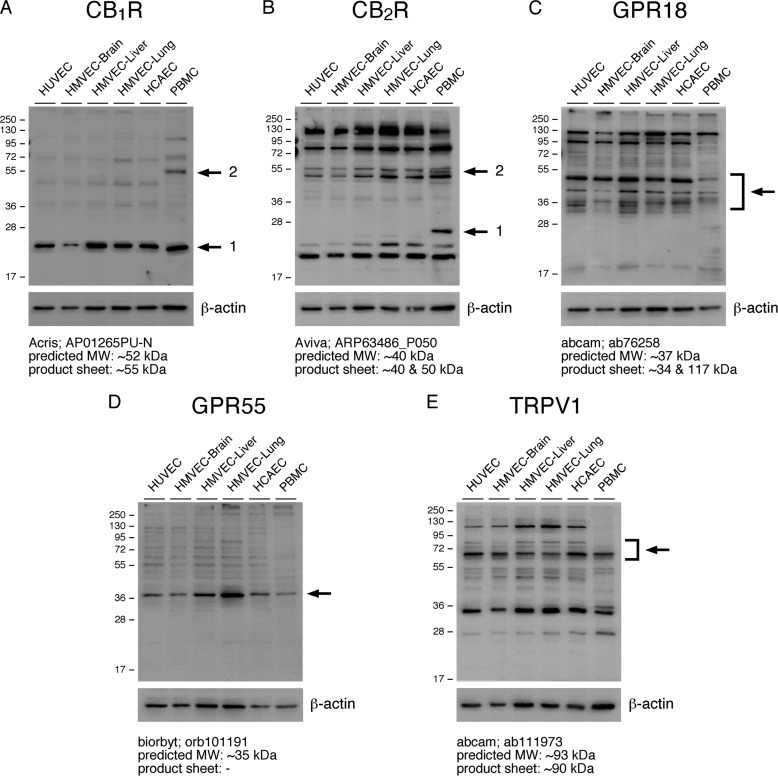
To verify that CB1R, CB2R, GPR18, GPR55, and TRPV1 are expressed at the protein level in the aforementioned cell types, immunoblotting was performed using lysates derived from the same primary cells used in qPCR expression analysis. The results appear to corroborate the qPCR expression analysis, although it was difficult to determine the correct protein band in some blots due to excessive background protein staining and to discrepancies between the observed and predicted or described molecular weights (Fig. 2). Nonetheless, we are confident that CB1R (protein band 1), GPR18, GPR55, and TRPV1 protein bands were definitively detected in ECs (Fig. 2). However, in the CB1R immunoblot, a fainter protein band is detected at ∼55 kDa in the PBMC lysates (protein band 2), which is not present in ECs (Fig. 2A). Because this protein band more closely corresponds to the predicted molecular weight for CB1R and is consistent with the relatively low expression of CNR1 in ECs, as observed in the qPCR data, it may represent CB1R, suggesting this receptor is not detectable by immunoblot in ECs (Fig. 2 and supplemental Table S1). Additionally, in Fig. 2B, if the protein band observed either at ∼25 kDa (protein band 1) or ∼52 kDa (i.e. the middle band of the triplet found between 50 and 55 kDa; protein band 2) in the PBMC lane is in fact CB2R, the results indicate that ECs do not express detectable quantities of this G protein-coupled receptor, which would be consistent with our qPCR results using assay 1 (Figs. 1 and. 2B and supplemental Table S1). Furthermore, several protein bands are in the predicted or previously observed molecular weight ranges for TRPV1; however, in comparison with ECs, some of these are not highly expressed in PBMCs (59, 60). Taken together, the results from our CBR expression studies indicate that ECs express CB1R, GPR18, GPR55, and TRPV1 and possibly CB2R at very low levels.
FIGURE 2.
ECs express CBR and TRPV1 proteins. HUVEC, HMVEC-brain, -liver, and -lung, and HCAEC were grown to confluency in 6-well plates and lysed with RIPA-LB. CB1R (A), CB2R (B), GPR18 (C), GPR55 (D), and TRPV1 (E) protein levels were assessed by immunoblot using the indicated antibodies. PBMCs were immediately lysed in RIPA-LB upon isolation. β-Actin levels were assessed as a loading control (bottom panels) (A–E). Arrows indicate candidate CBR or TRPV1 protein bands. For reference, the predicted molecular weight and the molecular weight indicated in the respective products sheets are shown.
The eCB system uses a complex network of metabolic enzymes to regulate the synthesis and breakdown of the arachidonic acid-based eCBs (3, 4). Through literature review, we identified 10 enzymes involved in eCB metabolism. We tested for the presence of these enzymes through qPCR expression profiling in HUVEC and HMVEC-lung, again using PBMCs for comparison. Our results show that HUVEC and HMVEC-lung express very similar levels of all the eCB metabolic enzymes tested, including α/β-hydrolase-4, -6, and -12 (ABHD4, ABHD6, and ABHD12), diacylglycerol lipase α (DAGLA), glycerophosphodiesterase (GDE1), monoacylglycerol lipase (MGLL), N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD), and protein-tyrosine phosphatase, nonreceptor type 22 (PTPN22) (Fig. 3, A and B, and supplemental Table S2). Additionally, fatty acid amide hydrolase 1 and 2 (FAAH and FAAH2) were expressed in ECs but at relatively low levels. We also found that PBMCs express all the enzymes (Fig. 3C and supplemental Table S2). These results suggest that ECs and PBMCs are able to regulate eCB metabolism.
NADA and WIN55,212-2 Negatively Regulate EC Inflammatory Activation
Cannabinoids have been reported to modulate the inflammatory activation of leukocytes, but their effects on endothelial inflammation have not been thoroughly investigated (16, 17). Because the inflammatory activation of ECs also plays a central role in recruiting leukocytes and mounting immune responses, we decided to test a panel of synthetic cannabinoids, phytocannabinoids and eCBs, including the TRPV1-activating compound CAP, for their ability to regulate the secretion of IL-6 and IL-8 from HMVEC-lung activated with the pro-inflammatory agonists FSL-1 (TLR2/6 ligand), LPS (TLR4 ligand), or TNFα. Specific cannabinoids and CAP were chosen due to their ability to activate or inhibit the known cannabinoid receptors (Table 1). Based on previous studies, we chose to preincubate HMVEC-lung with 10 μm of each cannabinoid or CAP for 1 h and then treat for 6 h with the inflammatory agonists while in the continuous presence of the compounds. After the 7 h, we quantified IL-6 and IL-8 levels in culture supernatants. MTT assays were used to ensure that changes in cytokine expression were not due differences in cell viability. In the MTT assays, the ECs were incubated with 10 μm of the different compounds for 7 h. Fig. 4 summarizes the combined inflammatory index versus viability index for each cannabinoid tested. A detailed description on how the indices were calculated can be found in the legends of Fig. 4 and supplemental Table S3. Of the compounds tested, only the eCB NADA (IL-6, p < 0.05; IL-8, p < 0.01) and synthetic cannabinoid WIN55,212-2 (IL-6, p < 0.001; IL-8, p < 0.001) significantly reduced IL-6 and IL-8 secretion from ECs after inflammatory agonist treatment without significantly affecting EC viability (MTT, p > 0.05) (Fig. 4 and supplemental Table S3). We therefore decided to further analyze the anti-inflammatory properties of these two compounds in more detail. Of note, although the CB2R agonist HU-308 appears to display anti-inflammatory activity without grossly affecting the viability of ECs, we did not follow up on this cannabinoid in this report because its effect on IL-6 and IL-8 secretion was not statistically significant by our criteria (p > 0.05) and because of our inconclusive results regarding CB2R expression in ECs (Figs. 1, 2, and 4 and supplemental Tables S1 and S3). Also, although 10 μm CBD strongly inhibited cytokine secretion from all ECs tested, we determined that this reduction was due to a substantial increase in cell death (Fig. 4 and supplemental Table S3). Lower concentrations of CBD (≤1 μm) did not cause cell death but also did not have a significant effect on cytokine secretion, and therefore, we did not further pursue this phytocannabinoid (data not shown).
TABLE 1.
Cannabinoid receptor agonists/antagonists used in experiments
++, agonist activity <100 nm; +, agonist activity 100 nm to 10 μm; 0, no effect; −, antagonist activity; =, inverse agonist; (in), indirect; (?), uncertain. Note: the discrepancies in binding specificities reported for individual compounds are due primarily to the different assays utilized in the studies (1). Abn-CBD, abnormal cannabidiol; NAGly, N-arachidonoylglycine; ND, no data (reported in good faith); ACEA, arachidonoyl-2'-chloroethylamide; CAP, capsaicin; CBD, cannabidiol.
| CBR |
Refs. | |||||
|---|---|---|---|---|---|---|
| CB1R | CB2R | GPR18 | GPR55 | TRPV1 | ||
| 2-AG | ++/+ | + | + | ++/0 | +/0 | 134–140 |
| Abn-CBD | 0 | 0 | + | ++/+/0 | 0 | 134, 137, 138, 141, 142 |
| ACEA | ++ | + | ND | ND | + | 40, 143, 144 |
| AEAa | ++ | + | +/0 | ++/+/0 | + | 134, 137, 138, 140, 145 |
| CBDb | − (in) | = | + (?)/− | 0/− | +/0 | 134, 138, 146–148 |
| CAP | ND | ND | ND | ND | ++ | 149 |
| HU-308 | 0 | ++ | ND | ND | ND | 150 |
| NADAc | + | +/0 | ND | ND | ++ | 33, 34 |
| NAGly | 0 | 0 | ++/0 | 0 | 0 | 135, 151–155 |
| WIN55,212-2d | ++ | ++ | 0/− | 0 | 0/− (in) | 134, 137, 138, 154, 156 |
a AEA is the inhibitor of TRPM8 (115).
b CBD is the agonist for the 5-HT1A (serotonin) receptor (157).
WIN55,212-2 Inhibits HMVEC-Lung Activation by Inflammatory Agonists
WIN55,212-2 is a well studied synthetic compound and has been shown previously to have anti-inflammatory activity in a variety of cells (26–30). We first analyzed the effects of WIN55,212-2 (0.1, 1, and 10 μm) on the FSL-1-, LPS-, and TNFα-induced up-regulation of IL-8 in HMVEC-lung. We found that 10 μm WIN55,212-2 strongly inhibits the up-regulation of IL-8 expression in HMVEC-lung treated with all three of the inflammatory agonists (Fig. 5, A–C). More refined concentration curves of WIN55,212-2 between 1 and 10 μm in FSL-1- and LPS-treated HMVEC-lung indicated that the IC50 for WIN55,212-2 inhibition of IL-6 and IL-8 secretion is between 2.96 and 3.94 μm (supplemental Fig. S1, A–D). Importantly, we also observed that WIN55,212-2 concentrations of up to 100 μm do not effect cell viability (Fig. 5D). Additionally, we found that initiating treatment of HMVEC-lung with WIN55,212-2 anytime from 2 h before to 2 h after the addition of FSL-1 strongly reduced IL-6 and IL-8 secretion (supplemental Fig. S1, E and F). We next used a neutrophil adhesion assay to test the ability of WIN55,212-2 to inhibit the binding of neutrophils to HMVEC-lung activated with either FSL-1, LPS, or TNFα. We found that WIN55,212-2 dose-dependently reduces the adhesion of primary human neutrophils to HMVEC-lung monolayers activated by all three inflammatory agonists (Fig. 5E shown for FSL-1). These results all suggest that WIN55,212-2 robustly inhibits the inflammatory activation of HMVEC-lung without affecting cell viability.
As mentioned previously, WIN55,212-2 has high affinity for both CB1R and CB2R and is reported to inhibit TRPV1 (1, 51, 53, 54). We first analyzed the effects of the CB1R antagonist CP945598 and CB2R inverse agonist SR144528 together (0.1, 1, and 10 μm) on the LPS-induced up-regulation of IL-6 and IL-8 in HMVEC-lung in the presence and absence of WIN55,212-2. We decided to use these inhibitors in combination to exclude any possibility of compensation by one over the other, given the uncertainty of CB2R expression in ECs. We found that the effects of WIN55,212-2 on IL-6 secretion are only significantly abrogated in the presence of 0.1 μm (p < 0.05) CP945598/SR144528, whereas IL-8 secretion is not significantly affected by the inhibitors, suggesting that CB1R and CB2R may have only minimal effects on its anti-inflammatory properties in ECs (Fig. 5F, shown for IL-8). Note that we used 4 μm WIN55,212-2 in these assays to more closely match its IC50 value (supplemental Fig. S1). We next analyzed the effects of the TRPV1 antagonist AMG9810 (0.1, 1, and 10 μm) on the LPS-induced up-regulation of IL-6 and IL-8 in HMVEC-lung pretreated with WIN55,212-2. We found that in the presence of 10 μm AMG9810, the WIN55,212-2-dependent decreases in IL-6 and IL-8 secretion were further reduced in LPS-treated HMVEC-lung, but surprisingly, in the absence of WIN55,212-2, the LPS-induced inflammatory response was exacerbated in the presence of 10 μm AMG9810 (Fig. 5G, shown for IL-8). This suggests that TRPV1 promotes inflammation in the presence of WIN55,212-2 but inhibits inflammation in its absence. Importantly, we did not observe significant effects of AMG9810 at concentrations <10 μm, suggesting that these outcomes may be due to the inhibition of another endothelial TRP channel such as TRPA1, -M8, -V3, and -V4 (61, 62).
NADA, and Not Its Chemical Constituents Arachidonic Acid nor Dopamine, Reduces HMVEC-Lung Activation
We next focused on refining the anti-inflammatory properties of NADA in ECs that were observed in our initial experiments (Fig. 4). NADA is composed of AA and dopamine moieties covalently linked through an amide bond (Fig. 6A). Because several eCB hydrolase enzymes, such as FAAH1/2, MAGL, and ABHD-4, -6, and -12, are expressed in ECs (Fig. 3), it is possible that either AA or dopamine, or a combination of the two, are responsible for the observed anti-inflammatory properties. To explore this possibility, we compared the effects of AA or dopamine alone, the two compounds in combination, or NADA alone on FSL-1 and TNFα activation of HMVEC-lung. We found that in the presence of AA, either alone or in combination with dopamine, it tends to increase the expression of IL-8 by HMVEC-lung when treated with either agonist, in line with previous observations, whereas NADA again decreased cytokine secretion (Fig. 6B, shown for FSL-1) (63). Polymyxin B was used to show that the additional increase in IL-8 levels after FSL-1 addition in the presence of AA was not due to LPS contamination. These results suggest that NADA itself is directly responsible for the observed anti-inflammatory effects and not its breakdown products AA or dopamine. We also tested ethanol preparations of NADA from three different sources (Sigma, Tocris, and Cayman), and we found similar decreases in IL-8 secretion in LPS-treated HMVEC-lung when preincubated with either 10 or 20 μm NADA (supplemental Fig. S2A). Additionally, we found that NADA has no effect on cell viability or cell adherence as measured by MTT and CV assays, respectively, up to 20 μm in vitro, but that higher concentrations did cause significant cell death within 7 h (Fig. 6, C and D). It is possible that at higher concentrations NADA may cause EC death via oxidative stress, similar to results reported for hepatic stellate cells (64).
NADA Reduces Pro-inflammatory Cytokine Secretion and Neutrophil Adherence to HMVEC-Lung Activated by Inflammatory Agonists
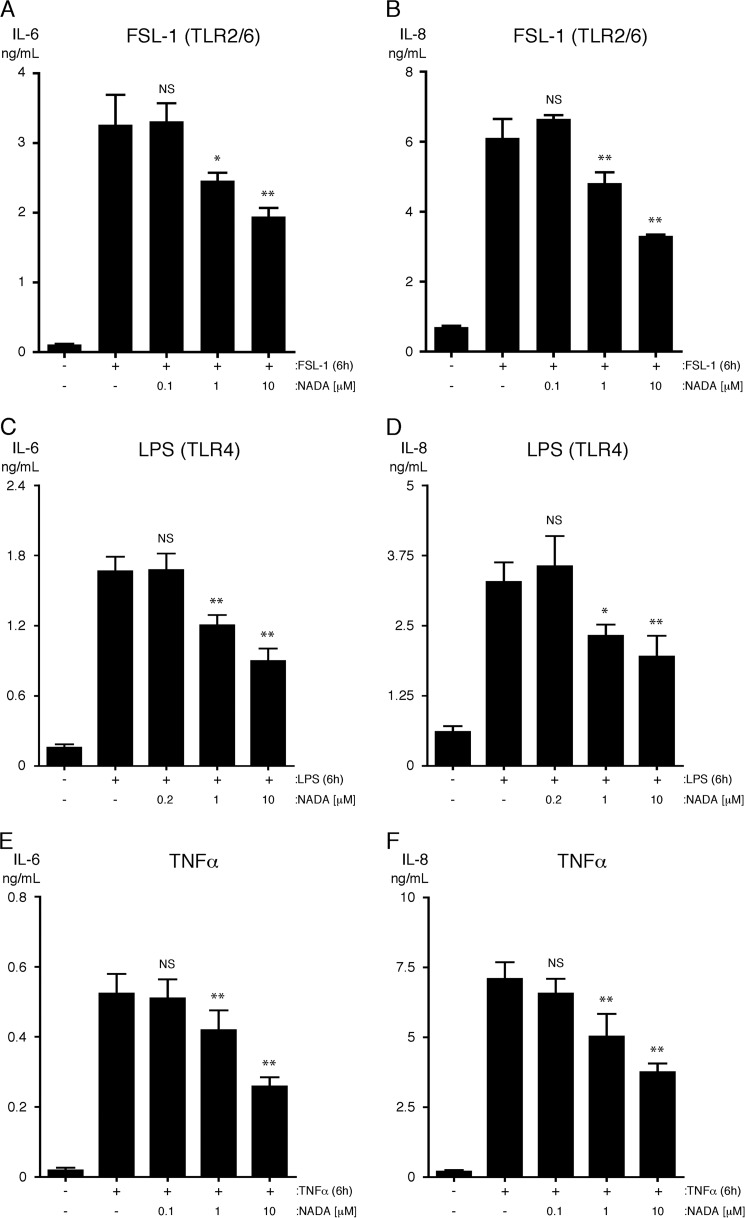
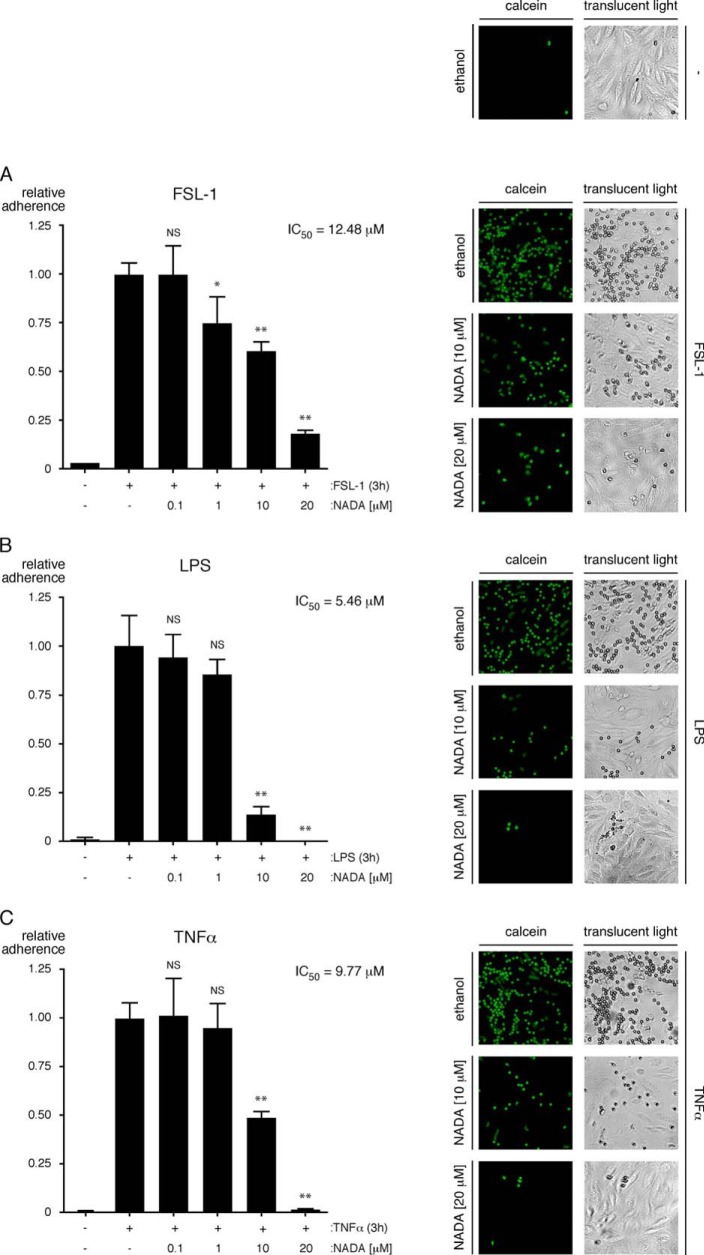
Next, we found that NADA dose-dependently inhibits the up-regulation of IL-6 and IL-8 expression in HMVEC-lung treated with FSL-1, LPS, or TNFα (Fig. 7, A–F). The IC50 values for NADA's inhibitory effects on LPS-induced secretion of IL-6 and IL-8 from HMVEC-lung were 12.79 and 18.00 μm, respectively (supplemental Fig. S2, B and C). Additionally, like WIN55,212-2, we found that initiating treatment of HMVEC-lung with NADA anytime starting 2 h before and up to 2 h after adding FSL-1 strongly reduced the up-regulation of both IL-6 and IL-8 (supplemental Fig. S2, D and E). Using flow cytometry, we also determined that NADA reduces the surface expression of E-selectin on HUVEC activated with either FSL-1, LPS, or TNFα, suggesting that NADA may reduce the adhesion of leukocytes to the endothelium (Fig. 8 and supplemental Fig. S3). To test this, we performed a neutrophil adhesion assay (55). We observed that NADA dose-dependently reduces the adhesion of primary human neutrophils to HMVEC-lung monolayers activated by any of the inflammatory agonists (Fig. 9, A–C). These results indicate that NADA can directly dampen the activation of human ECs by both exogenous and endogenous inflammatory mediators.
FIGURE 7.
NADA reduces the secretion of IL-6 and IL-8 from HMVEC-lung activated with inflammatory agonists. Levels of IL-6 (A, C, and E) and IL-8 (B, D, and F) were quantified in the supernatants of HMVEC-lung that were pretreated for 1 h with the indicated concentrations of NADA and then treated with either 10 μg/ml FSL-1 (A and B) or LPS (C and D) or 100 ng/ml TNFα (E and F) for an additional 6 h while in the continuous presence of NADA (n = 4). NS, not significant; *, p < 0.05; **, p < 0.01; inflammatory agonist versus inflammatory agonist plus NADA. All experiments were performed four times.
FIGURE 9.
NADA reduces the binding of neutrophils to activated ECs. A–C, HMVEC-lung were pretreated for 1 h with the indicated concentrations of NADA and then treated with 10 μg/ml FSL-1 (A) or LPS (B) or 100 ng/ml TNFα (C) for an additional 3 h while in the continuous presence of NADA. The HMVEC-lung were then washed, and calcein AM-labeled primary human neutrophils were allowed to adhere for 20 min. Nonadherent neutrophils were subsequently removed, and the percentage of remaining adherent neutrophils was calculated (n = 3). NS, not significant; *, p < 0.05; **, p < 0.01, inflammatory agonist versus inflammatory agonist plus NADA. Representative images of calcein-labeled neutrophils bound to HMVEC-lung are shown to the right of the bar graphs. Fluorescence images taken with a fluorescein filter set are shown in the left-hand column, and translucent light microscopy images of the same field of reference are shown in the right-hand column. Images were taken at ×10 magnification on an Olympus IX51 inverted microscope equipped with a Retiga 2000R camera (Q imaging). The neutrophil adhesion assays were performed two times.
NADA and WIN55,212-2 Do Not Reduce the Permeability of HMVEC-Lung Monolayers
Increased permeability of the vascular endothelium is a hallmark of acute inflammatory disorders such as sepsis, and it is believed to contribute to the pathogenesis of organ injury and failure (19, 23). We utilized ECIS to quantify the effects of NADA and WIN55,212-2 on endothelial permeability induced either by FSL-1, LPS, or TNFα. ECIS uses TER to measure electrical current as a surrogate for endothelial permeability. A reduction in TER is consistent with increased permeability. We did not find that treatment of HMVEC-lung monolayers either with NADA or WIN55,212-2 prevents FSL-1-, LPS-, or TNFα-induced decreases in TER (data not shown). Together, these results suggest that NADA and WIN55,212-2 may not modulate inflammation-induced endothelial permeability. It is also possible that we were unable to detect NADA- or WIN55,212-2-induced changes in TER under our chosen experimental conditions.
Effects of CB1R/CB2R and TRPV1 Inhibition on the Anti-inflammatory Properties of NADA
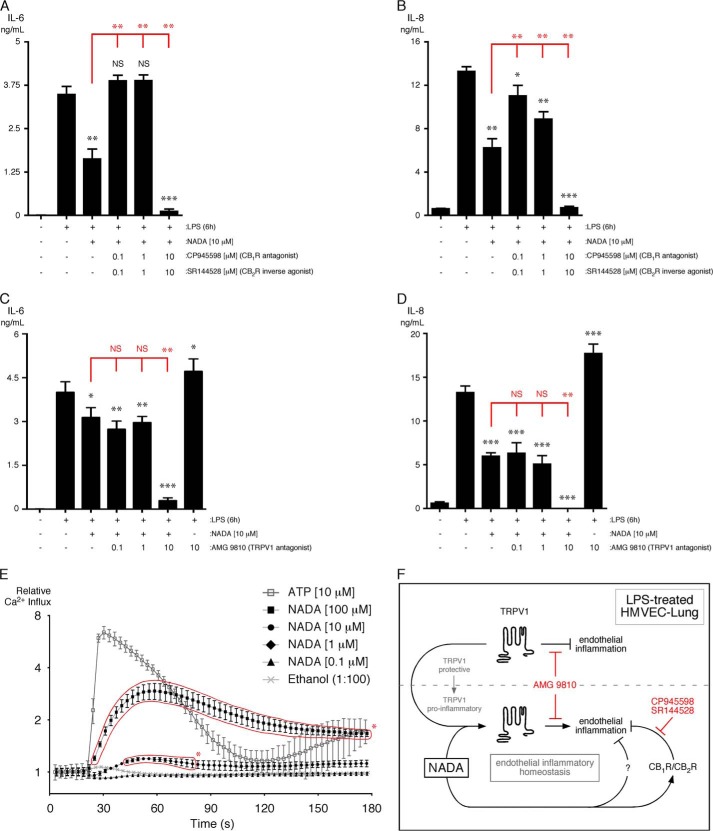
NADA is an agonist for CB1R and CB2R with differing affinities and can activate the TRPV1 channel (i.e. induce cation influx) (33, 34, 52). We analyzed the effects of the CB1R/CB2R inhibitors CP945598/SR144528 (0.1, 1, and 10 μm) on the LPS-induced up-regulation of IL-6 and IL-8 in HMVEC-lung in the presence of NADA. We found that 0.1 and 1 μm CP945598/SR144528 strongly abated the NADA-dependent decrease in IL-6 and IL-8 secretion in LPS-treated HMVEC-lung, whereas at the higher concentrations (10 μm) they actually facilitated the NADA-dependent reduction in cytokine secretion in LPS-activated ECs (Fig. 10, A and B). Because CP945598 and SR144528 display subnanomolar potency in binding and functional assays, the effects at 0.1 and 1 μm may reflect specific inhibition of CB1 and CB2 receptors, whereas the outcomes observed at 10 μm may represent off-target effects (65–67). Therefore, the anti-inflammatory effects of NADA may be at least partially due to agonism at CB1R and/or CB2R. We next analyzed the effects of the TRPV1 antagonist AMG9810 (0.1, 1, and 10 μm) on the LPS-induced up-regulation of IL-6 and IL-8 in HMVEC-lung pretreated with NADA. We found that in the presence of 10 μm AMG9810, the NADA-dependent decreases in IL-6 and IL-8 secretion were further reduced in LPS-treated HMVEC-lung. Conversely, in the absence of NADA, the LPS-induced inflammatory response was exacerbated in the presence of 10 μm AMG9810 (Fig. 10, C and D). This suggests that TRPV1 promotes inflammation in the presence of NADA but inhibits inflammation in its absence. Like our studies with WIN55,212-2, we did not observe significant effects of AMG9810 at concentrations <10 μm, again suggesting that these outcomes may be due to the inhibition of another endothelial TRP channel such as TRPA1, -M8, -V3, and -V4 (61, 62). However, we found that NADA dose-dependently induces Ca2+ influx into HMVEC-lung (Fig. 10E). In these experiments, 10 μm ATP was used as a positive control (68). This indicates that NADA is an agonist for endothelial TRPV1 because to date NADA has not been shown to activate any other ion channels.
FIGURE 10.
CB1R/CB2R mediate the anti-inflammatory effects of NADA, whereas TRPV1 counters the anti-inflammatory properties of NADA in ECs. A and B, levels of IL-6 (A) and IL-8 (B) were quantified in the supernatants of HMVEC-lung that were pretreated for 30 min with either 0.1, 1, or 10 μm of CP945598/SR144528 and then incubated for another 30 min with 10 μm NADA in the presence of CP945598/SR144528. ECs were then treated with 10 μg/ml LPS for an additional 6 h while in the continuous presence of CP945598/SR144528 and/or NADA (n = 3). Crystal violet readings between samples were not significantly different. C and D, cells were treated and analyzed similarly as A and B, except using the TRPV1 inhibitor AMG9810 (n = 4). Crystal violet readings between samples were not significantly different. A–D, t tests (black): NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001; LPS versus LPS plus CP945598/SR144528 or AMG9810 and/or NADA. ANOVA (red): NS, not significant; **, p < 0.01, LPS plus NADA versus LPS plus NADA and CP945598/SR144528 or AMG9810. E, NADA induces Ca2+ influx into HMVEC-lung. ECs were grown to confluency, and Ca2+ influx was measured as described under “Experimental Procedures.” Cells were treated with either ethanol, ATP (10 μm), or NADA at the indicated concentrations, and Ca2+ influx was measured for 3 min. The time points at which NADA induced significant influx are enclosed with a red border: t test, *, p < 0.05; NADA versus ethanol for each time point. F, proposed model depicting the role of CB1R/CB2R and TRPV1 in modulating LPS-induced endothelial inflammation by NADA. In the absence of NADA, TRPV1 displays protective (i.e. anti-inflammatory) properties in LPS-treated HMVEC-lung. However, in the presence of NADA, TRPV1 appears to promote inflammation (i.e. TRPV1 switches from a protective to a pro-inflammatory role). NADA can reduce inflammation via CB1R/CB2R, but the downstream pathways have not yet been fully delineated, nor has the existence of parallel anti-inflammatory pathways induced by NADA been determined in ECs (as indicated by a question mark). Together with TRPV1, NADA may serve to regulate endothelial inflammatory homeostasis. CP945598, CB1R antagonist; SR144528, CB2R inverse agonist; AMG 9810, TRPV1 antagonist. ELISAs and Ca2+ influx experiments were performed two times.
Human ECs Express the Dopamine Biosynthetic Enzyme DDC but Endogenous NADA Was Not Detected in HMVEC-Lung
Two potential biosynthetic routes have been postulated to be responsible for the production of NADA in cells (34, 69, 70). One route requires the direct ligation of dopamine to AA. The other route first requires the ligation of tyrosine to AA to form N-arachidonoyl tyrosine. The tyrosine moiety is subsequently converted to l-DOPA by TH to generate N-arachidonoyl-DOPA, and then the l-DOPA moiety is converted to dopamine by DDC to produce NADA (supplemental Fig. S4A). Using qPCR expression analysis, we determined that both HUVEC and HMVEC-lung express DDC, but not TH, although PBMCs express neither and, as expected, adrenal tissue express high levels of both DDC and TH (supplemental Fig. S4, B and C). These results suggest that in human ECs only the first biosynthetic route for NADA is possible. However, because TH has been detected in murine liver sinusoidal ECs, the mechanisms of NADA synthesis may vary in different EC niches or species (64). We next attempted to quantify endogenous NADA in lysates of HMVEC-lung. Using an LC-MS/MS method developed at Cayman Chemical Co., NADA was not detected in HMVEC-lung lysates either before or after activation by FSL-1, LPS, or TNFα (data not shown). This result indicates either that HMVEC-lung do not endogenously express NADA, as the levels were below the detection limit of ∼0.25 ng/ml, or NADA was not efficiently isolated from HMVEC-lung lysates, degraded prior to LC-MS/MS analysis, or was not detected for alternative technical reasons.
DISCUSSION
In this report, we have identified the endocannabinoid/endovanilloid NADA and the synthetic cannabinoid WIN55,212-2 as potent modulators of the inflammatory activation of the endothelium. Other eCBs, including AEA and 2-AG, did not exhibit this immunomodulatory property. Both NADA and WIN55,212-2 reduce the secretion of pro-inflammatory cytokines and the adherence of neutrophils to HMVEC-lung activated by bacterial TLR agonists or TNFα. Importantly, our expression profiling data show that primary human ECs express CBRs, TRPV1, and all of the eCB metabolic enzymes that have been identified to date. These results strongly support the hypothesis that the endothelial eCB system represents a novel immune regulatory system that could be exploited therapeutically to ameliorate the deleterious effects of dysregulated inflammation in a variety of disorders, including sepsis and noninfectious inflammatory syndromes.
We transcriptionally detected the expression of CNR1, GPR18, GPR55, and TRPV1 in all five EC types tested. CNR2, however, was only detected at low levels in HUVEC, HMVEC-brain, and HCAEC by only one of the two primer sets tested (assay 2). However, we were unable to detect CB2R in ECs by immunoblot, suggesting that if present the CB2R is expressed at extremely low levels. The expression of CB1R in ECs is fairly well documented, but the expression of CB2R in ECs is more controversial (71–76). For example, other investigators have reported the expression of CB2R in HCAEC and HMVEC-brain but not in HUVEC, and interestingly, inflammatory agonists and 2-AG have been observed to induce CB2R up-regulation in HMVEC-brain (73, 76–79). Therefore, the detection of CB2R in EC cultures may depend on several factors, including the EC niche, detection method, and inflammatory state, and on the health, passage number, or growth conditions of the ECs. Nonetheless, the synthetic CB2R agonists JWH133, HU-308, and O-1966 have been reported to reduce the LPS- and TNFα-induced inflammatory activation of both HCAEC and HMVEC-brain and protect barrier function in LPS-treated HMVEC-brain (73, 77). Consistent with these results, we observed an anti-inflammatory trend with HU-308 when administered to activated HMVEC-lung. Based upon this information, an anti-inflammatory role for CB2R in ECs cannot be excluded.
We found that the hydrolases FAAH, FAAH2, MAGL, and ABHD4, -6, and -12 are expressed in ECs. Because these enzymes are involved in catabolism of the eCBs AEA and 2-AG, both of which have been reported to reduce inflammation, and NADA has been reported to inhibit both FAAH and MAGL, the observed anti-inflammatory properties of NADA may be indirectly due to increases in either AEA or 2-AG levels (26, 33, 80–86). Furthermore, these hydrolases may catabolize NADA to its constituents AA and dopamine, which themselves could potentially diminish EC activation. However, we did not observe a significant change in IL-6 and IL-8 secretion from ECs treated with inflammatory mediators in the presence of either AEA or 2-AG (Fig. 4). In fact, 2-AG exhibited a pro-inflammatory trend with IL-8, which is consistent with findings in several published reports (87–89). Additionally, AA also appears to augment inflammation, suggesting that the breakdown of NADA is not a prerequisite for the observed inflammatory reduction. Therefore, it is unlikely that NADA exerts its immunomodulatory effects via the production of AEA and 2-AG or through its catabolism to AA and dopamine, although it is possible that the exogenous application of AA, AEA, or 2-AG to ECs in vitro does not replicate their in vivo effects on the endothelium.
We observed that NADA concentrations of ≥1 μm were necessary to elicit its immunomodulatory properties in vitro. Although these levels of NADA are unlikely to be present in the circulation, the observed activity of NADA at this concentration may be functionally significant for several reasons. First, many of NADA's targets are intracellular, including its binding site on TRPV1 (39, 90–92). This suggests that NADA must be imported into the cell prior to acting on its receptors when added exogenously. The anandamide (or endocannabinoid) membrane transporter has been reported to facilitate NADA transport into the cell. Therefore, higher exogenous NADA concentrations may be necessary to efficiently overcome this rate-limiting step or, alternatively, the activity of the anandamide membrane transporter may not be optimal in vitro (52, 93–95). Second, the lack of a concurrently activated allosteric pathway (e.g. PKC) or allosteric mediators (e.g. ATP or low pH), or low expression level of the effectors themselves, may reduce the efficacy of NADA in vitro (96–99). These instances indicate that higher concentrations of NADA would be necessary to induce activation of its effectors. Third, relatively high exogenous concentration of NADA may be necessary to overcome its metabolism to a less active form, such as a 3-O-methyl derivative (34). Finally, the concentrations of NADA used in our studies are consistent with, or even lower than, those typically used to elicit the effects of histamine on ECs in vitro, a compound known to directly activate and induce permeability of the endothelium in vivo (100–105).
NADA and WIN55,212-2 have been reported to activate CB1R and CB2R on cells (1, 33, 51). Therefore, we determined the contribution of these receptors to the anti-inflammatory properties of NADA and WIN55,212-2 in ECs. The combination of the CB1R antagonist and CB2R inverse agonist abrogated the anti-inflammatory properties of NADA, which suggests that CB1R and/or CB2R are necessary to elicit the anti-inflammatory properties of NADA in LPS-activated HMVEC-lung. In contrast, the inhibitors did not reduce the anti-inflammatory properties of WIN55,212-2, which is unexpected given the high affinity of WIN55,212-2 for both CB1R and CB2R (106). Possible explanations are that these inhibitors do not prevent the high affinity binding of WIN55,212-2 to CB1R and/or CB2R or that WIN55,212-2 modulates inflammatory activation of ECs via alternative mechanisms.
To determine the role of TRPV1 in EC inflammation, we utilized AMG9810, a competitive antagonist of TRPV1. Although AMG9810 further reduces the LPS-induced inflammatory activation of ECs in the presence of either WIN55,212-2 or NADA, AMG9810 augments inflammation in their absence. This suggests that cannabinoids may switch the role of TRPV1 from an anti-inflammatory to a pro-inflammatory receptor in ECs, and in conjunction with NADA, TRPV1 may regulate endothelial inflammatory homeostasis. Indeed, we observed that NADA causes a dose-dependent transient increase in calcium levels in ECs, which may promote inflammatory signaling pathways, consistent with its role in activating endothelial TRPV1, the only known ion channel known to be ligand-gated by NADA (34, 52, 107–110). Our results suggest that WIN55,212-2 does not exert its endothelial anti-inflammatory properties via its reported inhibition of TRPV1, as the TRPV1 antagonist augments the anti-inflammatory effects of WIN55,212-2 (53, 54). However, these previous studies were performed primarily in neurons, and the modulation of TRPV1 by WIN55,212-2 may differ between neurons and ECs. It is also possible that our observations were due to the effects of AMG9810 on other TRP channels (61, 62).
Targets other than the endocannabinoid and endovanilloid receptors have also been described for both NADA and WIN55,212-2 (29, 33, 45, 48, 52–54, 86, 106, 111–116). Many of these, including PPAR-α, PPAR-γ, and calcineurin, are expressed in ECs and can themselves modulate endothelial inflammatory outputs (48, 53, 54, 117–119). In fact, WIN55,212-2 has been reported to reduce inflammation in murine brain ECs through PPAR-γ, independent of CB1R and CB2R, which is consistent with our observations using combined CB1R antagonist and CB2R inverse agonist (29, 117, 118). Furthermore, NADA has also been reported to down-regulate secretion of the inflammatory mediator PGE2 from LPS-treated cells, unlike AEA, whose catabolism to AA enhances PGE2 release (46, 47, 120, 121). Therefore, in addition to CB1R/CB2R and TRPV1, NADA and WIN55,212-2 may modulate endothelial inflammation by additional mechanisms.
Studies in neurons have shown that the NADA-dependent activation of CB1R/CB2R and TRPV1 can exhibit disparate outcomes within the same cell (91). The canonical pathway activated downstream of CB1R/CB2R leads to the inhibition of protein kinase A (PKA) through the Gi/o proteins (122). However, the PKA-dependent phosphorylation of TRPV1 augments its sensitization, and correspondingly, a reduction in PKA activity will lead to its inactivation, opposing the agonist activity of NADA at TRPV1 (123). Therefore, NADA may diametrically regulate the influx of cations into the cell by either inducing or reducing cation influx via TRPV1 and CB1R/CB2R, respectively, to modulate neuronal homeostasis (91). This dual modulatory effect of NADA has previously been proposed to fine-tune the transmission of glutamate onto dopamine neurons and explain such observations that NADA has both pro- and anti-nociceptive properties (39, 41). Based on our observations that NADA may reduce inflammation through CB1R/CB2R, but promote inflammation through TRPV1, a logical conclusion is that NADA may also fine-tune the inflammatory activation of ECs (124). A model based on our observations with NADA in LPS-treated HMVEC-lung is shown in Fig. 10F, and a summation can be found in the figure legend.
Recent findings using TRPV1 knock-out mice in sepsis models indicate a protective role for TRPV1, which is consistent with our observations that inhibition of TRPV1 in ECs augments the LPS-induced inflammatory response in the absence of cannabinoids (Figs. 5G and 10, C, D, and F) (125, 126). In these published studies, the loss of TRPV1 exacerbates the inflammatory response to infection. This is analogous to the reported effects of deleting the nicotinic acetylcholine receptor α7 subunit (α7nAChR), another cation channel (127). The vagus nerve transmits action potentials via the ganglia to peripheral nerves that terminate in organs, where they stimulate the release of the neurotransmitter acetylcholine (ACh) from T-cells (128). The ACh-induced activation of α7nAChR on leukocytes inhibits pro-inflammatory signaling pathways in these cells (129). Because endogenous NADA has thus far only been detected in various parts of the central and peripheral nervous systems, it is conceivable that it may be a component of the inflammatory reflex arc that regulates EC inflammation, analogous to the role of ACh and α7nAChR in leukocyte inflammatory regulation (34, 39, 130). Interestingly, in the brain there is a close association of ECs and perivascular nerves within the neurovascular unit (131, 132). In such areas, the secretion of NADA from perivascular nerves onto brain microvascular ECs may directly modulate neuroinflammation. We speculate that a similar process may occur within visceral organs, whereby the release of NADA from varicosities on innervating efferent nerve axons directly regulates the inflammatory activation of microvascular ECs. Analogous to neuromuscular junctions on smooth muscle, these putative neuroendothelial junctions would not require specialized structures on the endothelial cells (133). This highly speculative model additionally predicts that localized, physiologically relevant concentrations of NADA may be achieved at neuroendothelial junctions.
In conclusion, our studies indicate that the eCB/endovanilloid NADA and the synthetic cannabinoid WIN55,212-2 potently reduce the activation of human ECs in response to endogenous and exogenous inflammatory agonists. Furthermore, our results identify the endothelial eCB system as a novel regulator of endothelial inflammatory activation. Although further studies are required to thoroughly elucidate the mechanisms of eCB function in ECs, our observations may have important implications for a variety of acute inflammatory disorders that are characterized by endothelial activation and dysfunction and cause organ injury and failure, such as sepsis and ischemia-reperfusion injury.
Supplementary Material
Acknowledgments
We thank Dr. Arun Prakash for critical reading of the manuscript and Steven Joonil Chung for insightful discussions.
This work was supported by the Department of Anesthesia and Perioperative Care, University of California at San Francisco, National Science Foundation Graduate Research Fellowship Program Grant 1144247, and the University of California at San Francisco Research Evaluation and Allocation Committee Program (Huntington Fund).

This article contains supplemental Figs. S1–S4 and Tables S1–S3.
- TRPV1
- transient receptor potential cation channel vanilloid type 1
- EC
- endothelial cell
- CBR
- cannabinoid receptor
- NADA
- N-arachidonoyl dopamine
- eCB
- endocannabinoid
- HUVEC
- human umbilical vein endothelial cell
- PBMC
- peripheral blood mononuclear cell
- HMVEC
- human microvascular EC
- HCAEC
- human coronary artery endothelial cell
- TRP
- transient receptor potential
- TLR
- Toll-like receptor
- CBD
- cannabidiol
- AEA
- N-arachidonoylethanolamide
- 2-AG
- 2-arachidonoylglycerol
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- CV
- crystal violet
- AA
- arachidonic acid
- TER
- transendothelial electrical resistance
- TH
- tyrosine hydroxylase
- α7nAChR
- nicotinic acetylcholine receptor α7 subunit
- ACh
- acetylcholine
- qPCR
- quantitative real time PCR
- ANOVA
- analysis of variance
- CAP
- capsaicin
- FCSB
- Flow Cytometry Staining Buffer
- DDC
- l-DOPA decarboxylase.
REFERENCES
- 1. Pertwee R. G., Howlett A. C., Abood M. E., Alexander S. P., Di Marzo V., Elphick M. R., Greasley P. J., Hansen H. S., Kunos G., Mackie K., Mechoulam R., Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol. Rev. 62, 588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pertwee R. G. (2006) Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 147, S163–S171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Petrocellis L., Di Marzo V. (2009) An introduction to the endocannabinoid system: from the early to the latest concepts. Best Pract. Res. Clin. Endocrinol. Metab. 23, 1–15 [DOI] [PubMed] [Google Scholar]
- 4. Ueda N., Tsuboi K., Uyama T. (2013) Metabolism of endocannabinoids and related N-acylethanolamines: canonical and alternative pathways. FEBS J. 280, 1874–1894 [DOI] [PubMed] [Google Scholar]
- 5. Castillo P. E., Younts T. J., Chávez A. E., Hashimotodani Y. (2012) Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elphick M. R. (2012) The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3201–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luchicchi A., Pistis M. (2012) Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol. Neurobiol. 46, 374–392 [DOI] [PubMed] [Google Scholar]
- 8. Piomelli D. (2014) More surprises lying ahead. The endocannabinoids keep us guessing. Neuropharmacology 76, 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galiègue S., Mary S., Marchand J., Dussossoy D., Carrière D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232, 54–61 [DOI] [PubMed] [Google Scholar]
- 10. Graham E. S., Angel C. E., Schwarcz L. E., Dunbar P. R., Glass M. (2010) Detailed characterisation of CB2 receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int. J. Immunopathol. Pharmacol. 23, 25–34 [DOI] [PubMed] [Google Scholar]
- 11. Lee S. F., Newton C., Widen R., Friedman H., Klein T. W. (2001) Differential expression of cannabinoid CB(2) receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharmacol. 423, 235–241 [DOI] [PubMed] [Google Scholar]
- 12. Rom S., Persidsky Y. (2013) Cannabinoid receptor 2: potential role in immunomodulation and neuroinflammation. J. Neuroimmune Pharmacol. 8, 608–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tschöp J., Kasten K. R., Nogueiras R., Goetzman H. S., Cave C. M., England L. G., Dattilo J., Lentsch A. B., Tschöp M. H., Caldwell C. C. (2009) The cannabinoid receptor 2 is critical for the host response to sepsis. J. Immunol. 183, 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasten K. R., Tschöp J., Tschöp M. H., Caldwell C. C. (2010) The cannabinoid 2 receptor as a potential therapeutic target for sepsis. Endocr. Metab. Immune Disord. Drug Targets 10, 224–234 [DOI] [PubMed] [Google Scholar]
- 15. Gui H., Sun Y., Luo Z. M., Su D. F., Dai S. M., Liu X. (2013) Cannabinoid receptor 2 protects against acute experimental sepsis in mice. Mediators Inflamm. 2013, 741303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagarkatti P., Pandey R., Rieder S. A., Hegde V. L., Nagarkatti M. (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 1, 1333–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein T. W., Newton C., Larsen K., Lu L., Perkins I., Nong L., Friedman H. (2003) The cannabinoid system and immune modulation. J. Leukocyte Biol. 74, 486–496 [DOI] [PubMed] [Google Scholar]
- 18. Kozela E., Pietr M., Juknat A., Rimmerman N., Levy R., Vogel Z. (2010) Cannabinoids Δ(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 285, 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee W. L., Liles W. C. (2011) Endothelial activation, dysfunction and permeability during severe infections. Curr. Opin. Hematol. 18, 191–196 [DOI] [PubMed] [Google Scholar]
- 20. Shin H. S., Xu F., Bagchi A., Herrup E., Prakash A., Valentine C., Kulkarni H., Wilhelmsen K., Warren S., Hellman J. (2011) Bacterial lipoprotein TLR2 agonists broadly modulate endothelial function and coagulation pathways in vitro and in vivo. J. Immunol. 186, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilhelmsen K., Mesa K. R., Lucero J., Xu F., Hellman J. (2012) ERK5 protein promotes, whereas MEK1 protein differentially regulates, the Toll-like receptor 2 protein-dependent activation of human endothelial cells and monocytes. J. Biol. Chem. 287, 26478–26494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilhelmsen K., Mesa K. R., Prakash A., Xu F., Hellman J. (2012) Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response. Innate Immun. 18, 602–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aird W. C. (2003) The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101, 3765–3777 [DOI] [PubMed] [Google Scholar]
- 24. Makó V., Czúcz J., Weiszhár Z., Herczenik E., Matkó J., Prohászka Z., Cervenak L. (2010) Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytometry 77, 962–970 [DOI] [PubMed] [Google Scholar]
- 25. Croxford J. L., Yamamura T. (2005) Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J. Neuroimmunol. 166, 3–18 [DOI] [PubMed] [Google Scholar]
- 26. Facchinetti F., Del Giudice E., Furegato S., Passarotto M., Leon A. (2003) Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lipopolysaccharide. Glia 41, 161–168 [DOI] [PubMed] [Google Scholar]
- 27. Hao M. X., Jiang L. S., Fang N. Y., Pu J., Hu L. H., Shen L. H., Song W., He B. (2010) The cannabinoid WIN55,212-2 protects against oxidized LDL-induced inflammatory response in murine macrophages. J. Lipid Res. 51, 2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y. Y., Yuece B., Cao H. M., Lin H. X., Lv S., Chen J. C., Ochs S., Sibaev A., Deindl E., Schaefer C., Storr M. (2013) Inhibition of p38/Mk2 signaling pathway improves the anti-inflammatory effect of WIN55 on mouse experimental colitis. Lab. Investig. 93, 322–333 [DOI] [PubMed] [Google Scholar]
- 29. Mestre L., Docagne F., Correa F., Loría F., Hernangómez M., Borrell J., Guaza C. (2009) A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol. Cell. Neurosci. 40, 258–266 [DOI] [PubMed] [Google Scholar]
- 30. Sheng W. S., Hu S., Min X., Cabral G. A., Lokensgard J. R., Peterson P. K. (2005) Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1β-stimulated human astrocytes. Glia 49, 211–219 [DOI] [PubMed] [Google Scholar]
- 31. Mormina M. E., Thakur S., Molleman A., Whelan C. J., Baydoun A. R. (2006) Cannabinoid signalling in TNF-α induced IL-8 release. Eur. J. Pharmacol. 540, 183–190 [DOI] [PubMed] [Google Scholar]
- 32. Croxford J. L., Wang K., Miller S. D., Engman D. M., Tyler K. M. (2005) Effects of cannabinoid treatment on Chagas disease pathogenesis: balancing inhibition of parasite invasion and immunosuppression. Cell. Microbiol. 7, 1592–1602 [DOI] [PubMed] [Google Scholar]
- 33. Bisogno T., Melck D., Bobrov MYu, Gretskaya N. M., Bezuglov V. V., De Petrocellis L., Di Marzo V. (2000) N-Acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 351, 817–824 [PMC free article] [PubMed] [Google Scholar]
- 34. Huang S. M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F., Tognetto M., Petros T. J., Krey J. F., Chu C. J., Miller J. D., Davies S. N., Geppetti P., Walker J. M., Di Marzo V. (2002) An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 99, 8400–8405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bezuglov V., Bobrov M., Gretskaya N., Gonchar A., Zinchenko G., Melck D., Bisogno T., Di Marzo V., Kuklev D., Rossi J. C., Vidal J. P., Durand T. (2001) Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg. Med. Chem. Lett. 11, 447–449 [DOI] [PubMed] [Google Scholar]
- 36. Little P. J., Compton D. R., Johnson M. R., Melvin L. S., Martin B. R. (1988) Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J. Pharmacol. Exp. Ther. 247, 1046–1051 [PubMed] [Google Scholar]
- 37. Martin B. R., Compton D. R., Thomas B. F., Prescott W. R., Little P. J., Razdan R. K., Johnson M. R., Melvin L. S., Mechoulam R., Ward S. J. (1991) Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol. Biochem. Behav. 40, 471–478 [DOI] [PubMed] [Google Scholar]
- 38. Huang S. M., Walker J. M. (2006) Enhancement of spontaneous and heat-evoked activity in spinal nociceptive neurons by the endovanilloid/endocannabinoid N-arachidonoyldopamine (NADA). J. Neurophysiol. 95, 1207–1212 [DOI] [PubMed] [Google Scholar]
- 39. Marinelli S., Di Marzo V., Florenzano F., Fezza F., Viscomi M. T., van der Stelt M., Bernardi G., Molinari M., Maccarrone M., Mercuri N. B. (2007) N-Arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology 32, 298–308 [DOI] [PubMed] [Google Scholar]
- 40. Price T. J., Patwardhan A., Akopian A. N., Hargreaves K. M., Flores C. M. (2004) Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br. J. Pharmacol. 141, 1118–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sagar D. R., Smith P. A., Millns P. J., Smart D., Kendall D. A., Chapman V. (2004) TRPV1 and CB(1) receptor-mediated effects of the endovanilloid/endocannabinoid N-arachidonoyl-dopamine on primary afferent fibre and spinal cord neuronal responses in the rat. Eur. J. Neurosci. 20, 175–184 [DOI] [PubMed] [Google Scholar]
- 42. Bobrov M. Y., Lizhin A. A., Andrianova E. L., Gretskaya N. M., Frumkina L. E., Khaspekov L. G., Bezuglov V. V. (2008) Antioxidant and neuroprotective properties of N-arachidonoyldopamine. Neurosci. Lett. 431, 6–11 [DOI] [PubMed] [Google Scholar]
- 43. Harrison S., De Petrocellis L., Trevisani M., Benvenuti F., Bifulco M., Geppetti P., Di Marzo V. (2003) Capsaicin-like effects of N-arachidonoyl-dopamine in the isolated guinea pig bronchi and urinary bladder. Eur. J. Pharmacol. 475, 107–114 [DOI] [PubMed] [Google Scholar]
- 44. O'Sullivan S. E., Kendall D. A., Randall M. D. (2004) Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA). Br. J. Pharmacol. 141, 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Sullivan S. E., Kendall D. A., Randall M. D. (2009) Time-dependent vascular effects of endocannabinoids mediated by peroxisome proliferator-activated receptor γ (PPARγ). PPAR Res. 2009, 425289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Navarrete C. M., Fiebich B. L., de Vinuesa A. G., Hess S., de Oliveira A. C., Candelario-Jalil E., Caballero F. J., Calzado M. A., Muñoz E. (2009) Opposite effects of anandamide and N-arachidonoyl dopamine in the regulation of prostaglandin E and 8-iso-PGF formation in primary glial cells. J. Neurochem. 109, 452–464 [DOI] [PubMed] [Google Scholar]
- 47. Navarrete C. M., Pérez M., de Vinuesa A. G., Collado J. A., Fiebich B. L., Calzado M. A., Muñoz E. (2010) Endogenous N-acyl-dopamines induce COX-2 expression in brain endothelial cells by stabilizing mRNA through a p38-dependent pathway. Biochem. Pharmacol. 79, 1805–1814 [DOI] [PubMed] [Google Scholar]
- 48. Sancho R., Macho A., de La Vega L., Calzado M. A., Fiebich B. L., Appendino G., Muñoz E. (2004) Immunosuppressive activity of endovanilloids: N-arachidonoyl-dopamine inhibits activation of the NF-κB, NFAT, and activator protein 1 signaling pathways. J. Immunol. 172, 2341–2351 [DOI] [PubMed] [Google Scholar]
- 49. Sancho R., de la Vega L., Macho A., Appendino G., Di Marzo V., Muñoz E. (2005) Mechanisms of HIV-1 inhibition by the lipid mediator N-arachidonoyldopamine. J. Immunol. 175, 3990–3999 [DOI] [PubMed] [Google Scholar]
- 50. Yoo J. M., Park E. S., Kim M. R., Sok D. E. (2013) Inhibitory effect of N-Acyl dopamines on IgE-mediated allergic response in RBL-2H3 cells. Lipids 48, 383–393 [DOI] [PubMed] [Google Scholar]
- 51. Felder C. C., Joyce K. E., Briley E. M., Mansouri J., Mackie K., Blond O., Lai Y., Ma A. L., Mitchell R. L. (1995) Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 48, 443–450 [PubMed] [Google Scholar]
- 52. De Petrocellis L., Bisogno T., Davis J. B., Pertwee R. G., Di Marzo V. (2000) Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 483, 52–56 [DOI] [PubMed] [Google Scholar]
- 53. Patwardhan A. M., Jeske N. A., Price T. J., Gamper N., Akopian A. N., Hargreaves K. M. (2006) The cannabinoid WIN 55,212–2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc. Natl. Acad. Sci. U.S.A. 103, 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jeske N. A., Patwardhan A. M., Gamper N., Price T. J., Akopian A. N., Hargreaves K. M. (2006) Cannabinoid WIN 55,212–2 regulates TRPV1 phosphorylation in sensory neurons. J. Biol. Chem. 281, 32879–32890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilhelmsen K., Farrar K., Hellman J. (2013) Quantitative in vitro assay to measure neutrophil adhesion to activated primary human microvascular endothelial cells under static conditions. J. Vis. Exp. 78, e50677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 57. Willems E., Leyns L., Vandesompele J. (2008) Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 379, 127–129 [DOI] [PubMed] [Google Scholar]
- 58. Giaever I., Keese C. R. (1991) Micromotion of mammalian cells measured electrically. Proc. Natl. Acad. Sci. U.S.A. 88, 7896–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zavala K., Lee J., Chong J., Sharma M., Eilers H., Schumacher M. A. (2014) The anticancer antibiotic mithramycin-A inhibits TRPV1 expression in dorsal root ganglion neurons. Neurosci. Lett. 10.1016/j.neulet.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 60. Eilers H., Lee S. Y., Hau C. W., Logvinova A., Schumacher M. A. (2007) The rat vanilloid receptor splice variant VR. 5′sv blocks TRPV1 activation. Neuroreport 18, 969–973 [DOI] [PubMed] [Google Scholar]
- 61. Szallasi A., Cortright D. N., Blum C. A., Eid S. R. (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6, 357–372 [DOI] [PubMed] [Google Scholar]
- 62. Gavva N. R., Tamir R., Qu Y., Klionsky L., Zhang T. J., Immke D., Wang J., Zhu D., Vanderah T. W., Porreca F., Doherty E. M., Norman M. H., Wild K. D., Bannon A. W., Louis J. C., Treanor J. J. (2005) AMG 9810 [(E)-3-(4-t-Butylphenyl)-N-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 313, 474–484 [DOI] [PubMed] [Google Scholar]
- 63. Holladay C. S., Wright R. M., Spangelo B. L. (1993) Arachidonic acid stimulates interleukin-6 release from rat peritoneal macrophages in vitro: evidence for a prostacyclin-dependent mechanism. Prostaglandins Leukot. Essent. Fatty Acids 49, 915–922 [DOI] [PubMed] [Google Scholar]
- 64. Wojtalla A., Herweck F., Granzow M., Klein S., Trebicka J., Huss S., Lerner R., Lutz B., Schildberg F. A., Knolle P. A., Sauerbruch T., Singer M. V., Zimmer A., Siegmund S. V. (2012) The endocannabinoid N-arachidonoyl dopamine (NADA) selectively induces oxidative stress-mediated cell death in hepatic stellate cells but not in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G873–G887 [DOI] [PubMed] [Google Scholar]
- 65. Griffith D. A., Hadcock J. R., Black S. C., Iredale P. A., Carpino P. A., DaSilva-Jardine P., Day R., DiBrino J., Dow R. L., Landis M. S., O'Connor R. E., Scott D. O. (2009) Discovery of 1-[9-(4-chlorophenyl)-8-(2-chlorophenyl)-9H-purin-6-yl]-4-ethylaminopiperidine-4-carboxylic acid amide hydrochloride (CP-945,598), a novel, potent, and selective cannabinoid type 1 receptor antagonist. J. Med. Chem. 52, 234–237 [DOI] [PubMed] [Google Scholar]
- 66. Portier M., Rinaldi-Carmona M., Pecceu F., Combes T., Poinot-Chazel C., Calandra B., Barth F., le Fur G., Casellas P. (1999) SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J. Pharmacol. Exp. Ther. 288, 582–589 [PubMed] [Google Scholar]
- 67. Rinaldi-Carmona M., Barth F., Millan J., Derocq J. M., Casellas P., Congy C., Oustric D., Sarran M., Bouaboula M., Calandra B., Portier M., Shire D., Brelière J. C., Le Fur G. L. (1998) SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 284, 644–650 [PubMed] [Google Scholar]
- 68. Billstrom M. A., Johnson G. L., Avdi N. J., Worthen G. S. (1998) Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J. Virol. 72, 5535–5544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Akimov M. G., Gretskaia N. M., Shevchenko K. V., Shevchenko V. P., Miasoedov N. F., Bobrov MIu, Bezuglov V. V. (2007) New aspects of biosynthesis and metabolism of N-acyldopamines in rat tissues. Bioorganicheskaia khimiia 33, 648–652 [DOI] [PubMed] [Google Scholar]
- 70. Hu S. S., Bradshaw H. B., Benton V. M., Chen J. S., Huang S. M., Minassi A., Bisogno T., Masuda K., Tan B., Roskoski R., Jr., Cravatt B. F., Di Marzo V., Walker J. M. (2009) The biosynthesis of N-arachidonoyl dopamine (NADA), a putative endocannabinoid and endovanilloid, via conjugation of arachidonic acid with dopamine. Prostaglandins Leukot. Essent. Fatty Acids 81, 291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang N. L., Juang J. M., Wang Y. H., Hsueh C. H., Liang Y. J., Lin J. L., Tsai C. T., Lai L. P. (2010) Rimonabant inhibits TNF-α-induced endothelial IL-6 secretion via CB1 receptor and cAMP-dependent protein kinase pathway. Acta Pharmacol. Sin. 31, 1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu J., Gao B., Mirshahi F., Sanyal A. J., Khanolkar A. D., Makriyannis A., Kunos G. (2000) Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 346, 835–840 [PMC free article] [PubMed] [Google Scholar]
- 73. Rajesh M., Mukhopadhyay P., Bátkai S., Haskó G., Liaudet L., Huffman J. W., Csiszar A., Ungvari Z., Mackie K., Chatterjee S., Pacher P. (2007) CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am. J. Physiol. Heart Circ. Physiol. 293, H2210–H2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rajesh M., Mukhopadhyay P., Haskó G., Liaudet L., Mackie K., Pacher P. (2010) Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 160, 688–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sugiura T., Kodaka T., Nakane S., Kishimoto S., Kondo S., Waku K. (1998) Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator? Biochem. Biophys. Res. Commun. 243, 838–843 [DOI] [PubMed] [Google Scholar]
- 76. Waldeck-Weiermair M., Zoratti C., Osibow K., Balenga N., Goessnitzer E., Waldhoer M., Malli R., Graier W. F. (2008) Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 121, 1704–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ramirez S. H., Haskó J., Skuba A., Fan S., Dykstra H., McCormick R., Reichenbach N., Krizbai I., Mahadevan A., Zhang M., Tuma R., Son Y. J., Persidsky Y. (2012) Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 32, 4004–4016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Golech S. A., McCarron R. M., Chen Y., Bembry J., Lenz F., Mechoulam R., Shohami E., Spatz M. (2004) Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 132, 87–92 [DOI] [PubMed] [Google Scholar]
- 79. Lu T. S., Avraham H. K., Seng S., Tachado S. D., Koziel H., Makriyannis A., Avraham S. (2008) Cannabinoids inhibit HIV-1 Gp120-mediated insults in brain microvascular endothelial cells. J. Immunol. 181, 6406–6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alhouayek M., Masquelier J., Muccioli G. G. (2014) Controlling 2-arachidonoylglycerol metabolism as an anti-inflammatory strategy. Drug Discov. Today 19, 295–304 [DOI] [PubMed] [Google Scholar]
- 81. Bátkai S., Rajesh M., Mukhopadhyay P., Haskó G., Liaudet L., Cravatt B. F., Csiszár A., Ungvári Z., Pacher P. (2007) Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am. J. Physiol. Heart Circ. Physiol. 293, H909–H918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chang Y. H., Lee S. T., Lin W. W. (2001) Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J. Cell. Biochem. 81, 715–723 [DOI] [PubMed] [Google Scholar]
- 83. Gallily R., Breuer A., Mechoulam R. (2000) 2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-α production in murine macrophages, and in mice. Eur. J. Pharmacol. 406, R5–R7 [DOI] [PubMed] [Google Scholar]
- 84. Opitz C. A., Rimmerman N., Zhang Y., Mead L. E., Yoder M. C., Ingram D. A., Walker J. M., Rehman J. (2007) Production of the endocannabinoids anandamide and 2-arachidonoylglycerol by endothelial progenitor cells. FEBS Lett. 581, 4927–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ouyang Y., Hwang S. G., Han S. H., Kaminski N. E. (1998) Suppression of interleukin-2 by the putative endogenous cannabinoid 2-arachidonyl-glycerol is mediated through down-regulation of the nuclear factor of activated T cells. Mol. Pharmacol. 53, 676–683 [DOI] [PubMed] [Google Scholar]
- 86. Björklund E., Norén E., Nilsson J., Fowler C. J. (2010) Inhibition of monoacylglycerol lipase by troglitazone, N-arachidonoyl dopamine and the irreversible inhibitor JZL184: comparison of two different assays. Br. J. Pharmacol. 161, 1512–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gokoh M., Kishimoto S., Oka S., Metani Y., Sugiura T. (2005) 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, enhances the adhesion of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes. FEBS Lett. 579, 6473–6478 [DOI] [PubMed] [Google Scholar]
- 88. Sugiura T. (2009) Physiological roles of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Biofactors 35, 88–97 [DOI] [PubMed] [Google Scholar]
- 89. Sugiura T., Oka S., Gokoh M., Kishimoto S., Waku K. (2004) New perspectives in the studies on endocannabinoid and cannabis: 2-arachidonoylglycerol as a possible novel mediator of inflammation. J. Pharmacol. Sci. 96, 367–375 [DOI] [PubMed] [Google Scholar]
- 90. De Petrocellis L., Bisogno T., Maccarrone M., Davis J. B., Finazzi-Agro A., Di Marzo V. (2001) The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 276, 12856–12863 [DOI] [PubMed] [Google Scholar]
- 91. De Petrocellis L., Di Marzo V. (2009) Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium 45, 611–624 [DOI] [PubMed] [Google Scholar]
- 92. Jordt S. E., Julius D. (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108, 421–430 [DOI] [PubMed] [Google Scholar]
- 93. Chu C. J., Huang S. M., De Petrocellis L., Bisogno T., Ewing S. A., Miller J. D., Zipkin R. E., Daddario N., Appendino G., Di Marzo V., Walker J. M. (2003) N-Oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 278, 13633–13639 [DOI] [PubMed] [Google Scholar]
- 94. Millns P. J., Chimenti M., Ali N., Ryland E., de Lago E., Fernandez-Ruiz J., Chapman V., Kendall D. A. (2006) Effects of inhibition of fatty acid amide hydrolase vs. the anandamide membrane transporter on TRPV1-mediated calcium responses in adult DRG neurons; the role of CB receptors. Eur. J. Neurosci. 24, 3489–3495 [DOI] [PubMed] [Google Scholar]
- 95. Price T. J., Patwardhan A. M., Flores C. M., Hargreaves K. M. (2005) A role for the anandamide membrane transporter in TRPV1-mediated neurosecretion from trigeminal sensory neurons. Neuropharmacology 49, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Premkumar L. S., Qi Z. H., Van Buren J., Raisinghani M. (2004) Enhancement of potency and efficacy of NADA by PKC-mediated phosphorylation of vanilloid receptor. J. Neurophysiol. 91, 1442–1449 [DOI] [PubMed] [Google Scholar]
- 97. Vellani V., Mapplebeck S., Moriondo A., Davis J. B., McNaughton P. A. (2001) Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 534, 813–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Olah Z., Karai L., Iadarola M. J. (2001) Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 276, 31163–31170 [DOI] [PubMed] [Google Scholar]
- 99. Kwak J., Wang M. H., Hwang S. W., Kim T. Y., Lee S. Y., Oh U. (2000) Intracellular ATP increases capsaicin-activated channel activity by interacting with nucleotide-binding domains. J. Neurosci. 20, 8298–8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kumar P., Shen Q., Pivetti C. D., Lee E. S., Wu M. H., Yuan S. Y. (2009) Molecular mechanisms of endothelial hyperpermeability: implications in inflammation. Expert Rev. Mol. Med. 11, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tiruppathi C., Ahmmed G. U., Vogel S. M., Malik A. B. (2006) Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 13, 693–708 [DOI] [PubMed] [Google Scholar]
- 102. Talreja J., Kabir M. H., B Filla M., Stechschulte D. J., Dileepan K. N. (2004) Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology 113, 224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Andriopoulou P., Navarro P., Zanetti A., Lampugnani M. G., Dejana E. (1999) Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc. Biol. 19, 2286–2297 [DOI] [PubMed] [Google Scholar]
- 104. Schaefer U., Schneider A., Rixen D., Neugebauer E. (1998) Neutrophil adhesion to histamine stimulated cultured endothelial cells is primarily mediated via activation of phospholipase C and nitric oxide synthase isozymes. Inflamm. Res. 47, 256–264 [DOI] [PubMed] [Google Scholar]
- 105. Jeannin P., Delneste Y., Gosset P., Molet S., Lassalle P., Hamid Q., Tsicopoulos A., Tonnel A. B. (1994) Histamine induces interleukin-8 secretion by endothelial cells. Blood 84, 2229–2233 [PubMed] [Google Scholar]
- 106. Pertwee R. G. (2010) Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 17, 1360–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chang W. C. (2006) Store-operated calcium channels and pro-inflammatory signals. Acta Pharmacol. Sin. 27, 813–820 [DOI] [PubMed] [Google Scholar]
- 108. Li S. W., Westwick J., Poll C. T. (2002) Receptor-operated Ca2+ influx channels in leukocytes: a therapeutic target? Trends Pharmacol. Sci. 23, 63–70 [DOI] [PubMed] [Google Scholar]
- 109. Liu W., Matsumori A. (2011) Calcium channel blockers and modulation of innate immunity. Curr. Opin. Infect. Dis. 24, 254–258 [DOI] [PubMed] [Google Scholar]
- 110. Southall M. D., Li T., Gharibova L. S., Pei Y., Nicol G. D., Travers J. B. (2003) Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J. Pharmacol. Exp. Ther. 304, 217–222 [DOI] [PubMed] [Google Scholar]
- 111. Prusakiewicz J. J., Turman M. V., Vila A., Ball H. L., Al-Mestarihi A. H., Di Marzo V., Marnett L. J. (2007) Oxidative metabolism of lipoamino acids and vanilloids by lipoxygenases and cyclooxygenases. Arch. Biochem. Biophys. 464, 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ross H. R., Gilmore A. J., Connor M. (2009) Inhibition of human recombinant T-type calcium channels by the endocannabinoid N-arachidonoyl dopamine. Br. J. Pharmacol. 156, 740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tseng C. F., Iwakami S., Mikajiri A., Shibuya M., Hanaoka F., Ebizuka Y., Padmawinata K., Sankawa U. (1992) Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-β-phenethylamine and N-acyldopamine derivatives. Chem. Pharm. Bull. 40, 396–400 [DOI] [PubMed] [Google Scholar]
- 114. Sun Y., Alexander S. P., Kendall D. A., Bennett A. J. (2006) Cannabinoids and PPARα signalling. Biochem. Soc. Trans. 34, 1095–1097 [DOI] [PubMed] [Google Scholar]
- 115. De Petrocellis L., Starowicz K., Moriello A. S., Vivese M., Orlando P., Di Marzo V. (2007) Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 313, 1911–1920 [DOI] [PubMed] [Google Scholar]
- 116. Salas M. M., Hargreaves K. M., Akopian A. N. (2009) TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur. J. Neurosci. 29, 1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Reddy A. T., Lakshmi S. P., Kleinhenz J. M., Sutliff R. L., Hart C. M., Reddy R. C. (2012) Endothelial cell peroxisome proliferator-activated receptor γ reduces endotoxemic pulmonary inflammation and injury. J. Immunol. 189, 5411–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sasaki M., Jordan P., Welbourne T., Minagar A., Joh T., Itoh M., Elrod J. W., Alexander J. S. (2005) Troglitazone, a PPAR-γ activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-α. BMC Physiol. 5, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zandbergen F., Plutzky J. (2007) PPARα in atherosclerosis and inflammation. Biochim. Biophys. Acta 1771, 972–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Agro A., Langdon C., Smith F., Richards C. D. (1996) Prostaglandin E2 enhances interleukin 8 (IL-8) and IL-6 but inhibits GMCSF production by IL-1 stimulated human synovial fibroblasts in vitro. J. Rheumatol. 23, 862–868 [PubMed] [Google Scholar]
- 121. Ricciotti E., FitzGerald G. A. (2011) Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Turu G., Hunyady L. (2010) Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 44, 75–85 [DOI] [PubMed] [Google Scholar]
- 123. Bhave G., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W., 4th (2002) cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35, 721–731 [DOI] [PubMed] [Google Scholar]
- 124. Alawi K., Keeble J. (2010) The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol. Ther. 125, 181–195 [DOI] [PubMed] [Google Scholar]
- 125. Clark N., Keeble J., Fernandes E. S., Starr A., Liang L., Sugden D., de Winter P., Brain S. D. (2007) The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 21, 3747–3755 [DOI] [PubMed] [Google Scholar]
- 126. Fernandes E. S., Liang L., Smillie S. J., Kaiser F., Purcell R., Rivett D. W., Alam S., Howat S., Collins H., Thompson S. J., Keeble J. E., Riffo-Vasquez Y., Bruce K. D., Brain S. D. (2012) TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 188, 5741–5751 [DOI] [PubMed] [Google Scholar]
- 127. Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J. (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 128. Rosas-Ballina M., Olofsson P. S., Ochani M., Valdés-Ferrer S. I., Levine Y. A., Reardon C., Tusche M. W., Pavlov V. A., Andersson U., Chavan S., Mak T. W., Tracey K. J. (2011) Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Tracey K. J. (2009) Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Bradshaw H. B., Rimmerman N., Krey J. F., Walker J. M. (2006) Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R349–R358 [DOI] [PubMed] [Google Scholar]
- 131. Hamel E. (2006) Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. 100, 1059–1064 [DOI] [PubMed] [Google Scholar]
- 132. Girouard H., Iadecola C. (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 100, 328–335 [DOI] [PubMed] [Google Scholar]
- 133. Goyal R. K., Chaudhury A. (2013) Structure activity relationship of synaptic and junctional neurotransmission. Auton. Neurosci. 176, 11–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. De Petrocellis L., Di Marzo V. (2010) Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharmacol. 5, 103–121 [DOI] [PubMed] [Google Scholar]
- 135. McHugh D., Hu S. S., Rimmerman N., Juknat A., Vogel Z., Walker J. M., Bradshaw H. B. (2010) N-Arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 11, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N. E., Schatz A. R., Gopher A., Almog S., Martin B. R., Compton D. R. (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50, 83–90 [DOI] [PubMed] [Google Scholar]
- 137. Okuno T., Yokomizo T. (2011) What is the natural ligand of GPR55? J. Biochem. 149, 495–497 [DOI] [PubMed] [Google Scholar]
- 138. Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N. O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P. J. (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sugiura T., Kodaka T., Nakane S., Miyashita T., Kondo S., Suhara Y., Takayama H., Waku K., Seki C., Baba N., Ishima Y. (1999) Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J. Biol. Chem. 274, 2794–2801 [DOI] [PubMed] [Google Scholar]
- 140. Sugiura T., Kondo S., Kishimoto S., Miyashita T., Nakane S., Kodaka T., Suhara Y., Takayama H., Waku K. (2000) Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor. Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 275, 605–612 [DOI] [PubMed] [Google Scholar]
- 141. Járai Z., Wagner J. A., Varga K., Lake K. D., Compton D. R., Martin B. R., Zimmer A. M., Bonner T. I., Buckley N. E., Mezey E., Razdan R. K., Zimmer A., Kunos G. (1999) Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 96, 14136–14141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Johns D. G., Behm D. J., Walker D. J., Ao Z., Shapland E. M., Daniels D. A., Riddick M., Dowell S., Staton P. C., Green P., Shabon U., Bao W., Aiyar N., Yue T. L., Brown A. J., Morrison A. D., Douglas S. A. (2007) The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br. J. Pharmacol. 152, 825–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hillard C. J., Manna S., Greenberg M. J., DiCamelli R., Ross R. A., Stevenson L. A., Murphy V., Pertwee R. G., Campbell W. B. (1999) Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1). J. Pharmacol. Exp. Ther. 289, 1427–1433 [PubMed] [Google Scholar]
- 144. Pertwee R. G. (1999) Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 6, 635–664 [PubMed] [Google Scholar]
- 145. Hillard C. J. (2000) Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 61, 3–18 [DOI] [PubMed] [Google Scholar]
- 146. Costa B., Giagnoni G., Franke C., Trovato A. E., Colleoni M. (2004) Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br. J. Pharmacol. 143, 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Petitet F., Jeantaud B., Reibaud M., Imperato A., Dubroeucq M. C. (1998) Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 63, PL1–6 [DOI] [PubMed] [Google Scholar]
- 148. Thomas A., Baillie G. L., Phillips A. M., Razdan R. K., Ross R. A., Pertwee R. G. (2007) Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 [DOI] [PubMed] [Google Scholar]
- 150. Hanus L., Breuer A., Tchilibon S., Shiloah S., Goldenberg D., Horowitz M., Pertwee R. G., Ross R. A., Mechoulam R., Fride E. (1999) HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U.S.A. 96, 14228–14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Caldwell M. D., Hu S. S., Viswanathan S., Bradshaw H., Kelly M. E., Straiker A. (2013) A GPR18-based signalling system regulates IOP in murine eye. Br. J. Pharmacol. 169, 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., Yasukawa M. (2006) Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem. Biophys. Res. Commun. 347, 827–832 [DOI] [PubMed] [Google Scholar]
- 153. Lu V. B., Puhl H. L., 3rd, Ikeda S. R. (2013) N-Arachidonyl glycine does not activate G protein-coupled receptor 18 signaling via canonical pathways. Mol. Pharmacol. 83, 267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. McHugh D., Page J., Dunn E., Bradshaw H. B. (2012) Δ (9)- Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 165, 2414–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sheskin T., Hanus L., Slager J., Vogel Z., Mechoulam R. (1997) Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 40, 659–667 [DOI] [PubMed] [Google Scholar]
- 156. D'Ambra T. E., Estep K. G., Bell M. R., Eissenstat M. A., Josef K. A., Ward S. J., Haycock D. A., Baizman E. R., Casiano F. M., Beglin N. C. (1992) Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl)indole agonists of the cannabinoid receptor. J. Med. Chem. 35, 124–135 [DOI] [PubMed] [Google Scholar]
- 157. Russo E. B., Burnett A., Hall B., Parker K. K. (2005) Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.