Background: The Rieske protein QcrA was recently shown to be exported by twin-arginine translocation (Tat) in Bacillus subtilis.
Results: QcrA has disulfide bond and co-factor requirements for effective Tat-dependent translocation.
Conclusion: A hierarchy exists between disulfide bonding and co-factor insertion in QcrA quality control and translocation.
Significance: First studies on Tat-dependent translocation where oxidative folding and co-factor attachment were addressed in a single native molecule.
Keywords: Bacillus, Cytochromes, Disulfide, Iron-Sulfur Protein, Protein Translocation, QcrA, Rieske Protein, TatAy, Twin-arginine Translocation
Abstract
The twin-arginine translocation (Tat) pathway can transport folded and co-factor-containing cargo proteins over bacterial cytoplasmic membranes. Functional Tat machinery components, a folded state of the cargo protein and correct co-factor insertion in the cargo protein are generally considered as prerequisites for successful translocation. The present studies were aimed at a dissection of these requirements with regard to the Rieske iron-sulfur protein QcrA of Bacillus subtilis. Notably, QcrA is a component of the cytochrome bc1 complex, which is conserved from bacteria to man. Single amino acid substitutions were introduced into the Rieske domain of QcrA to prevent either co-factor binding or disulfide bond formation. Both types of mutations precluded QcrA translocation. Importantly, a proofreading hierarchy was uncovered, where a QcrA mutant defective in disulfide bonding was quickly degraded, whereas mutant QcrA proteins defective in co-factor binding accumulated in the cytoplasm and membrane. Altogether, these are the first studies on Tat-dependent protein translocation where both oxidative folding and co-factor attachment have been addressed in a single native molecule.
Introduction
The Tat pathway is unique in that it is able to translocate correctly folded (1–3) and cofactor-attached cargo proteins across bacterial and chloroplast thylakoidal membranes (4–6). Apart from the globular nature of the Tat-dependent cargo, a second distinctive feature of the Tat system is the eponymous twin-arginine motif within its signal peptide (7–9). The current interpretation of the mechanism associated with Tat translocation is that the cargo proteins are first recognized by a docking complex composed of so-called TatC and TatA-like proteins (10–13). Importantly, it is thought that the cargo protein undergoes proofreading and quality control at this point (1–3, 8, 14–17). After docking, the complex and cargo protein interacted, more TatA-like components are recruited such that multiple TatA-like components form a translocation pore (18). The proton-motive force then drives translocation (19, 20).
Bacillus subtilis is a non-pathogenic Gram-positive bacterium with a high capacity for protein secretion. Accordingly, it has become an important model organism for fundamental studies on protein export and secretion (7, 21–23). The Tat system in B. subtilis is composed of three TatA-like proteins (TatAd, TatAy, and TatAc) and two TatC proteins (TatCd and TatCy). TatAd-TatCd and TatAy-TatCy combine to form two independent parallel protein transport pathways, each with its own substrate specificities (24–26). The third TatA component, TatAc, has the intrinsic ability to form active Tat translocases both with TatCd and TatCy (27). The tatAy-tatCy operon is expressed under a broad range of conditions, which corresponds to the broad expression profile of the known substrates of the TatAy-TatCy translocase, namely the Dyp-type peroxidase EfeB (YwbN), the alkaline phosphatase YkuE, and the Rieske iron-sulfur protein QcrA (also known as PetC) (24, 28–31).
Rieske iron-sulfur proteins, in short Rieske proteins, form part of the cytochrome bc1 complex in B. subtilis. Cytochrome bc1 is a main component of the electron transport chain and, as a homolog of the complex III in mitochondria, it is structurally and functionally highly conserved in all three kingdoms of life (32, 33). In B. subtilis, the cytochrome bc1 complex is a menaquione:cytochrome c reductase composed of the Rieske protein (QcrA), cytochrome b (QcrB), and a larger than normal cytochrome c1 (QcrC) (34). Notably, cytochrome bc1 is a membrane protein complex that faces the extracellular side of the cytoplasmic membrane. The Sec pathway individually translocates the cytochrome b and cytochrome c1 components across the membrane before they are processed, matured, and combined (32, 35–39). It is believed that cytochrome b and cytochrome c1 form a primary protease-resistant complex, where the final step in complex maturation is the incorporation of the Rieske protein (32).
All Rieske proteins contain a well defined Rieske domain. Within this domain there are specific residues involved in 2Fe-2S co-factor binding. In some Rieske proteins, like QcrA of B. subtilis, the Rieske domain contains Cys residues involved in disulfide bond formation (33, 40–42)(Fig. 1). Apart from QcrA of B. subtilis, other Rieske proteins also share a strong association with the Tat pathway as was demonstrated in various bacteria and chloroplasts of green plants (43–47). Notably, the Gram-negative bacterium Escherichia coli does not contain a cytochrome bc1 complex, which precludes studies on Rieske protein assembly in this important model organism for research on Tat-dependent protein translocation (32, 33). B. subtilis on the other hand is an attractive organism to explore the requirements and Tat dependence of Rieske protein translocation. First, the electron transport chain in B. subtilis has two branches, creating a functional redundancy that allows for cytochrome bc1 mutation studies (32, 48, 49). Second, the qcrABC operon is highly expressed under a wide range of tested conditions and, consequently, it does not need to be artificially induced to investigate QcrA export in tat mutant strains (31, 50). The present studies were therefore aimed at analyzing the requirements for Tat-dependent export of QcrA in B. subtilis. For this purpose, QcrA was mutated to assess the roles of co-factor assembly and disulfide bonding in membrane translocation. Altogether, these studies reveal a hierarchy in the folding events needed for productive QcrA translocation. Although folding and co-factor insertion of native Tat substrates have been investigated previously, this is the first example of an investigation into the importance of folding and co-factor assembly for Tat-dependent membrane translocation of a single native (non-engineered) cargo protein.
FIGURE 1.
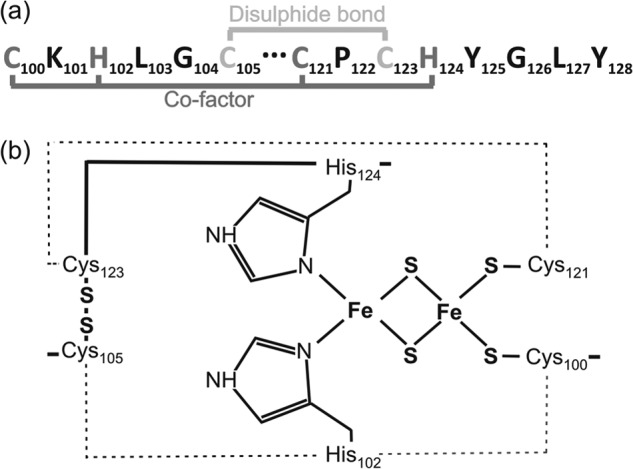
The Rieske domain of B. subtilis QcrA. a, annotated amino acid residues involved in co-factor attachment and disulfide bond formation. b, schematic representation of 2Fe-2S co-factor-binding by Cys-100, His-102, Cys-123, and His-124.
EXPERIMENTAL PROCEDURES
Strains, Plasmids, and Growth Conditions
Bacterial strains and plasmids used in this study are listed in Table 1. Lysogeny Broth (LB) was composed of 1% tryptone, 0.5% yeast extract, and 1% NaCl. Bacterial cultures were grown in LB at 37 °C under vigorous shaking, or on LB agar plates incubated at 37 °C. B. subtilis cells were made competent in Paris medium (51). When appropriate, the cultures were supplemented with antibiotics: Escherichia coli cultures with 100 μg/ml of ampicillin (Ap) and B. subtilis cultures with 2 μg/ml of erythromycin (Em), 5 μg/ml of chloramphenicol (Cm), 10 μg/ml of tetracycline (Tc), 100 μg/ml of spectinomycin (Sp), or 20 μg/ml of kanamycin (Km).
TABLE 1.
Strains and plasmids used in this study
| Relevant properties | Ref. | |
|---|---|---|
| Strains | ||
| B. subtilis 168 | trpC2 | 76 |
| B. subtilis 168 tatAdCd | trpC2, tatAd-tatCd::Km; Kmr | 55 |
| B. subtilis 168 tatAyCy | trpC2, tatAy-tatCy::Sp; Spr | 24 |
| B. subtilis 168 tatAc1-tatAyCy | trpC2; tatAc::Em, tatAy-tatCy::Sp; Emr; Spr | 55 |
| B. subtilis 168 tatCd-tatCy | trpC2; tatCd::Km; tatCy::Sp; Kmr; Spr | 25 |
| B. subtilis 168 total-tat2 | trpC2, tatAd-tatCd::Km, tatAy-tatCy::Sp; tatAc::Emr; Spr, Kmr, Emr | 24 |
| B. subtilis qcrA | trpC2, qcrA::pPP435 | 28 |
| B. subtilis ATCC6633 | Subtilin producer | 57 |
| L. lactis MG1363 | Plasmid-free derivative of NCDO 712 | 77 |
| Plasmids | ||
| pHB-201 | B. subtilis-E. coli expression vector; ori-pBR322; ori-pTA1060; cat86::lacZa; Emr; Cmr | 78 |
| pHB-AyCy | pHB201 derivative carrying the tatAy-tatCy operon; Emr; Cmr | 79 |
| pHB-TatCaa | pHB201 derivatives carrying tatCy genes that specify mutant TatCy proteins with specific single amino acid substitutions or C-terminal deletions; Emr; Cmr | 65 |
| pHB-TatAaaTatCyWT | pHB201 derivatives carrying a wild-type copy of the tatCy gene plus tatAy genes that specify mutant TatAy proteins with specific single amino acid substitutions; Emr; Cmr | 53 |
| pHB-QcrA | pHB201 derivative carrying wild-type qcrA; Emr; Cmr | 28 |
| pHB-QcrAH102L | pHB201 derivative carrying a mutant qcrA gene that specifies QcrA-H102L; Emr; Cmr | This study |
| pHB-QcrAH124L | pHB201 derivative carrying a mutant qcrA gene that specifies QcrA-H124L; Emr; Cmr | This study |
| pHB-QcrAC123S | pHB201 derivative carrying a mutant qcrA gene that specifies QcrA-C123L; Emr; Cmr | This study |
| pNZ8910 | SURE expression vector, PspaS; Emr | 57 |
| pNZ-QcrAH102L | pNZ8910 derivative carrying a mutant qcrA gene that specifies QcrA-H102L; Emr | This study |
| pNZ-QcrAC123S | pNZ8910 derivative carrying a mutant qcrA gene that specifies QcrA-C123L; Emr | This study |
Cloning and DNA Techniques
Cloning and ligation reactions were performed as described previously (52) using products from New England Biolabs. PCR were performed using the Phusion (New England Biolabs) or Pwo (Roche Applied Sciences) polymerases. Primers are listed in Table 2. The methodology for site-directed mutagenesis has been described previously in detail (53). Briefly, although external primers were the same for constructing all qcrA mutants, the internal primers included nucleotide changes that translated into single amino acid mutations in the full QcrA sequence. External primers included sequences specifying restriction sites for BamHI and SpeI, which allowed for cloning into the E. coli-B. subtilis shuttle vector pHB-201. To overproduce mutant QcrA proteins, the respective mutated qcrA genes were PCR amplified from pHB-201-based plasmids, and cloned into plasmid pNZ8910 digested with NcoI and HindIII. Ligation mixtures were introduced into Lactococcus lactis by transformation, and pNZ8910-derivative plasmids thus obtained were then introduced into B. subtilis as previously described (29, 30).
TABLE 2.
Primers used in this study
| Primer sequence | Underlined region | |
|---|---|---|
| External primers | ||
| QcrALnew | CGGAAGGTTACTAGTAGGGGTGACTTAGAGGGGG | SpeI |
| QcrARnew | CCG GGATCC GTGTAATATCAAGCCGCTCG | BamHI |
| Internal primers | ||
| QcrAH102L-left | GTACACCCTAAAAGCTTACAAATTG | CAT → CTT (His → Leu) |
| QcrAH102L-right | CAATTTGTAAGCTTTTAGGGTGTAC | CAT → CTT (His → Leu) |
| QcrAH124L-left | CCGTAAAGGCATGGACAAAAGAATTTAT | CAT → CTT (His → Leu) |
| QcrAH124L-right | ATAAATTCTTTTGTCCATGCCTTTACGG | CAT → CTT (His → Leu) |
| QcrAC123S-left | CCGTAATGGGATGGACAAAAGAATTTAT | GCC-CCC (Cys → Ser) |
| QcrAC123S-right | ATAAATTCTTTTGTCCATCCCATTACGG | GCC-CCC (Cys → Ser) |
Crude Cell Fractionations
Crude cell protein extractions were performed after cultures were grown to early stationary phase. Culture aliquots (2 ml) were treated with Complete protease inhibitor (Roche Applied Science) and pelleted. The extracellular fraction (1.5 ml) was removed, and proteins in this fraction were precipitated overnight with trichloroacetic acid (TCA; final concentration 10%). The cell pellet was resuspended in 100 μl of lithium dodecyl sulfate gel loading buffer and reducing agent (NuPAGE, Invitrogen) before disruption by bead-beating three times with glass beads at 6500 rpm for 3 s with 30-s intervals (Precellys 24 lysis and homogenization, Bertin Technologies). The TCA-precipitated extracellular fraction was acetone washed and resuspended in 50 μl of lithium dodecyl sulfate gel loading buffer and reducing agent. Samples were heated at 95 °C and, if necessary, stored at −20 °C. Crude cell extract aliquots of 10 μl and growth medium aliquots of 20 μl corresponding to 2 A600 units were used for NuPAGE and Western blotting.
Subcellular Fractionations
Subcellular fractionation studies were performed as described previously (28). However, for the present experiments cultures were grown to early stationary phase. The protein concentration of samples was estimated using the DCTM Bio-Rad Assay (Bio-Rad) and samples were stored at −20 °C until further use. Aliquots of 10 μg of protein were used for sample analysis by NuPAGE and Western blotting.
Pulse-Chase Labeling Experiments
Pulse-chase labeling experiments were performed as described previously with minor adaptions (54, 55). Cells were grown overnight in an adapted MBD medium (56) in which the Soytone was replaced with 3.5% maltodextrin and the glucose concentration was reduced to 2.1%. Overnight 2-ml cultures with an optical density at 600 nm (A600) of ∼1 were labeled with 40 μCi of [35S]methionine/cysteine (PerkinElmer Life Sciences). After 2 min labeling of proteins (i.e. the pulse), any further incorporation of [35S]methionine/cysteine was stopped by addition of a excess non-radioactive methionine/cysteine mixture (i.e. the chase; 2.5 mg/ml final concentration). Next, samples were withdrawn and immediately mixed with ice-cold TCA (10% final concentration). Upon 30 min incubation on ice, the precipitated proteins were collected by centrifugation and QcrA was immunoprecipitated with specific antibodies and protein A-Sepharose beads as previously described (54). The immunoprecipitated QcrA was analyzed by NuPAGE and subsequent autoradiography on a PhosphorImager (Cyclone® Plus storage phosphor system, PerkinElmer Life Sciences).
Overproduction of QcrA Mutant Proteins
B. subtilis cells containing pNZ-derivative plasmids with a subtilin-inducible mutated qcrA gene were grown in LB medium at 37 °C until an A600 of 0.6 at which point the cultures were induced by addition of subtilin-containing supernatant from B. subtilis ATCC 6633 prepared as described by Bongers et al. (57). After 3 h of induction, cells were harvested and subjected to subcellular fractionation, NuPAGE, and Western blotting.
AMS Cross-linking
Cross-linking experiments with 4-acetamido-4′-maleimidyl-stilbene-2,2′-disulfonate (AMS)2 were performed as described previously (58). Samples were analyzed by non-reducing NuPAGE and Western blotting with specific antibodies against QcrA and TrxA.
Gel Electrophoresis and Western Blotting
Proteins were separated using NuPAGE gels (Invitrogen) and transferred onto nitrocellulose membranes (Protran, Schleicher & Schuell) by semi-dry blotting. Polyclonal antibodies specific for BdbD, LipA, QcrA, and TrxA have been described previously (28, 59, 60). Bound antibodies were detected with fluorescent IgG secondary antibodies (IRDye 800 CW goat anti-rabbit from LiCor Biosciences) and visualized at 700 or 800 nm with the Odyssey Infrared Imaging System (LiCor Biosciences).
RESULTS
Proofreading Hierarchy with Regard to Co-factor Binding and Disulfide Bond Formation
The Rieske domain is highly conserved in all known Rieske iron-sulfur proteins and, therefore, the residues responsible for both the formation of the disulfide bond and those associated with co-factor attachment are well defined (Fig. 1) (32, 33, 40–42). To assess the roles of co-factor binding and disulfide bonding in QcrA export in B. subtilis, site-specific mutations were introduced into the Rieske domain of QcrA. These involved single amino acid changes altering the co-factor insertion site (H102L or H124L), or a cysteine residue involved in disulfide bond formation (C123S). The qcrA genes specifying these amino acid mutations were cloned into the pHB-201 expression plasmid and introduced into a B. subtilis strain with a mutated chromosomal qcrA gene. As illustrated in Fig. 2a, expression of the wild-type qcrA gene from pHB-201 in the qcrA mutant strain (lanes labeled qcrA + qcrA) resulted in the production of cellular and extracellular QcrA levels that are comparable with those of the parental strain 168 (lanes labeled wild-type).
FIGURE 2.
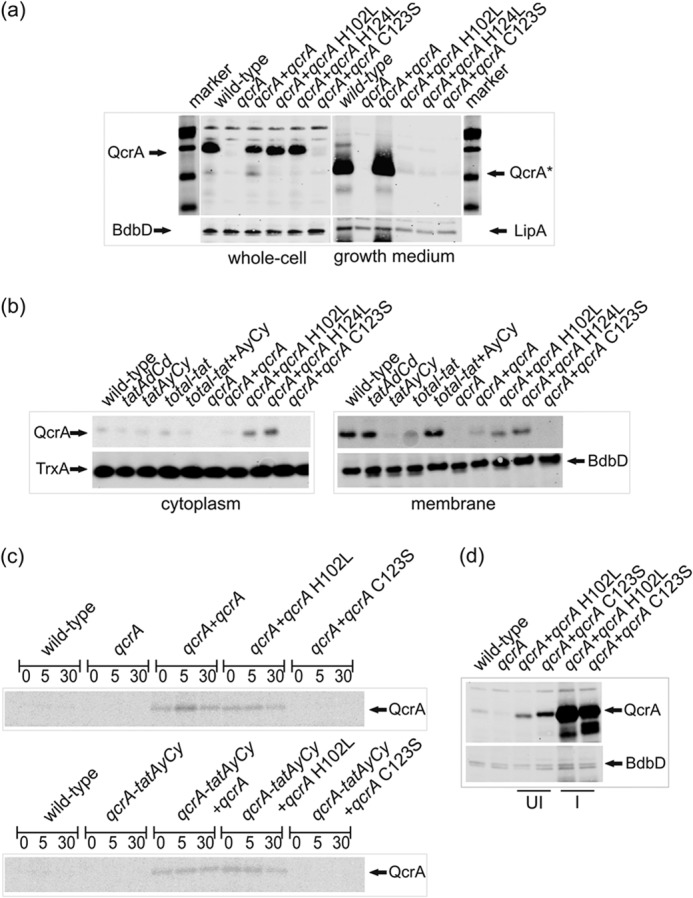
Translocation and quality control of QcrA mutant proteins defective in co-factor binding or disulfide bond formation. a, translocation of QcrA in strains with a deleted chromosomal qcrA gene that ectopically express wild-type qcrA or site specifically mutated qcrA genes from pHB-201. The mutant qcrA genes specify the H102L or H124L mutant QcrA proteins impaired in co-factor binding, or the C123S mutant QcrA protein defective in disulfide bond formation. All strains were grown in LB with 1% NaCl. Cells were separated from the growth medium, and crude whole cell extracts and growth medium fractions were analyzed by Western blotting using specific antibodies against QcrA, BdbD, or LipA. The positions of the 18-kDa full-size QcrA protein in the whole cell fraction and the 14-kDa processed QcrA protein (QcrA*) in the growth medium fraction, both separated on the same gel, are marked with arrows. The membrane protein BdbD and the secreted protein LipA were used as positive controls. b, subcellular fractionation was performed to separate the cytoplasmic and membrane proteins of tat mutant cells and qcrA mutant cells producing site specifically mutated QcrA proteins as indicated. The positions of QcrA, the cytoplasmic marker protein TrxA, and the membrane protein BdbD are marked with arrows. c, pulse-chase labeling of wild-type QcrA and QcrA-H102L or QcrA-C123S mutant proteins in tat-proficient cells (upper panel) or tatAy-tatCy-deficient cells (lower panel). Cells were labeled for 2 min with a [35S]methionine/cysteine mixture prior to chase with an excess of non-radioactive methionine/cysteine. At the time of chase (t = 0) and 5 and 30 min after the chase, samples were collected in which the presence of labeled QcrA was assessed by immunoprecipitation, NuPAGE, and autoradiography. The position of QcrA is marked with an arrow. d, the possibility to severely overexpress QcrA-C123S was assessed with the subtilin-inducible “SURE” expression plasmid pNZ8910. In this case, wild-type cells and cells over-expressing QcrA-H102L were used as controls. The positions of QcrA and the control protein BdbD are marked with arrows. UI, uninduced; I, subtilin-induced expression.
As previously shown by subcellular fractionation, the intact QcrA is an 18-kDa membrane-associated protein with Nin-Cout topology (28). Importantly, the translocated QcrA is exposed to signal peptidase activity, resulting in the release of a 14-kDa processed form (QcrA*) into the growth medium (Fig. 1a). In fact, the processing of QcrA was only abolished when four of the five type I signal peptidase-encoding genes of B. subtilis were deleted (28). Because the catalytic site of signal peptidase is positioned on the extracytoplasmic side of the membrane, QcrA processing and release of QcrA* into the medium can be used as a read-out for the translocation of this protein. Hence, membrane translocation of QcrA was visualized by Western blotting of crude whole cell extracts and growth medium fractions. As shown in Fig. 2a, QcrA* was absent from the growth media of all strains expressing mutated forms of QcrA (C123S, H102L, or H124L). Interestingly, in the crude whole cell extract, the presence of full-size QcrA was observed only for the co-factor-binding site mutants (H102L and H124L) and the wild-type protein, but not for the mutant impaired in disulfide bonding (C123S).
Strains producing mutated QcrA proteins were further investigated by subcellular fractionation. Samples were taken from cultures in the early stationary phase. Mutant proteins defective in the co-factor-binding site (QcrA-H102L or -H124L) were detectable in the membrane fraction (Fig. 2b). As signal peptidase-processed forms of QcrA-H102L and QcrA-H124L were completely absent from the growth medium (Fig. 2a), these findings imply that correct co-factor binding by QcrA is a prerequisite for productive membrane translocation. In contrast, the QcrA-C123S mutant protein defective in disulfide bond formation was neither observed in the cytoplasmic, nor the membrane or extracytoplasmic fractions (Fig. 2, a and b). Because QcrA-C123 was produced from exactly the same expression plasmid (pHB-201) as QcrA-H102L and QcrA-H124L, we performed pulse-chase labeling analyses with tat-proficient and tatAy-tatCy mutant cells to detect any short-lived intermediate forms of QcrA-C123. However, such short-lived forms remained undetectable, whereas the full-size forms of wild-type QcrA and QcrA-H102L were readily detectable, albeit only after labeling for 2 min (Fig. 2c). Since the C terminally truncated QcrA protein encoded by the qcrA mutant strain (29) remained undetectable in both our Western blotting and pulse-chase labeling analyses (Fig. 2), we conclude that the QcrA-C123 mutant protein with the inability to form the correct disulfide bond is degraded shortly after synthesis and does neither build up in the cytoplasm nor reach the membrane. Nevertheless, this protein can accumulate in B. subtilis when heavily overexpressed from the very strong subtilin-inducible promoter on pNZ8910 (Fig. 2d), confirming our sequencing data that the qcrA-C123 mutant gene is intact. Notably, this promoter is leaky, which is also why uninduced cells overproduced QcrA-C123S. Together, our observations on the behavior of the QcrA mutant proteins imply that there is a proofreading hierarchy where non-disulfide bonded QcrA is rapidly degraded, whereas QcrA defective in co-factor binding is stable.
The fast degradation of the non-disulfide bonded QcrA-C123S protein is in line with the previously documented degradation of other extracytoplasmic proteins, such as ComEC and ComGC, lacking an essential disulfide bond (60, 61). It should, however, be noted that, unlike ComEC or ComGC, the presence of QcrA was not affected by any of the bdb mutations that are known to interfere with oxidative protein folding in B. subtilis (51, 61–63). Specifically, we tested QcrA production directed from the chromosomal qcrA gene in previously analyzed bdbA-bdbB, bdbB-BdbC, or bdbC-bdbD mutant strains (Fig. 3a). Because none of these mutations had a reproducible impact on the cellular and extracellular QcrA levels, we conclude that oxidative folding of QcrA is independent of the BdbABCD thiol-disulfide oxidoreductases of B. subtilis. Notably, the Bdb proteins are active at the extracytoplasmic side of the membrane, which could mean that disulfide bonding in QcrA takes place in a membrane-protected environment. We therefore investigated the localization of potentially cross-linkable Cys residues in QcrA-H102L and wild-type QcrA with the membrane-impermeable thiol-specific cross-linking reagent AMS. This analysis was aided by the fact that QcrA contains only four Cys residues, all of which are located at the 2Fe-2S co-factor binding site (Fig. 1). Therefore, protoplasts of cells producing either QcrA-H102L or wild-type QcrA were treated with AMS. As shown in Fig. 3b, AMS cross-linking of QcrA-H102L and wild-type QcrA, which is reflected by a QcrA band up-shift upon NuPAGE and Western blotting, was only observed when the protoplast membrane was disrupted with 1% Triton X-100. This would suggest that the AMS cross-linkable Cys-100 and Cys-121 in QcrA-H102L (Fig. 1b), which lacks the co-factor, are located in a membrane-protected environment. Accordingly the adjacent disulfide-bonded residues Cys-105 and Cys-123 could also be located in this membrane-protected environment. The observed AMS cross-linking of wild-type QcrA would imply that AMS competes with the 2Fe-2S cofactor for binding to Cys-100 and Cys-121, eventually resulting in covalent AMS binding to these residues. Together, these findings would thus be consistent with the proposed proofreading hierarchy in disulfide bonding and co-factor binding.
FIGURE 3.
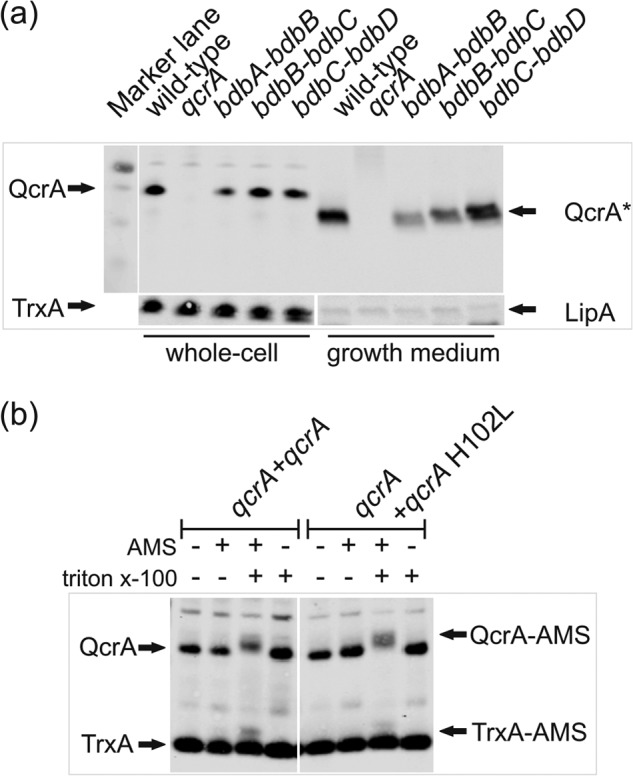
Bdb-independent biogenesis of QcrA and cross-linking with AMS. a, translocation of QcrA by bdbA-bdbB, bdbB-bdbC, or bdbC-bdbD mutant cells was assessed by Western blotting as described in the legend to Fig. 2a. All protein samples were loaded on the same gel. The positions of QcrA, QcrA*, TrxA, and LipA are marked with arrows. b, AMS cross-linking of QcrA in protoplasts of B. subtilis 168. AMS cross-linking was detectable by an up-shift of the QcrA band upon NuPAGE and Western blotting only if Triton X-100 was present during the incubation of protoplasts with AMS. TrxA was used as a cytoplasmic control protein. Note that under the tested conditions only a minor fraction of TrxA was cross-linkable with AMS upon protoplast lysis with Triton X-100. The positions of QcrA, QcrA-AMS, TrxA, and TrxA-AMS are indicated.
DISCUSSION
The Tat system is unique in that it can translocate folded and co-factor-containing cargo proteins over the cytoplasmic membrane (8, 9, 64). There are a number of known requirements for successful Tat-dependent translocation of cargo proteins. These include the presence of an N-terminal twin-arginine signal peptide, a properly folded state of the cargo protein, and a functional Tat pathway (8, 9, 13, 64). In the present studies, the latter two requirements were investigated for the Tat-dependent membrane translocation of the Rieske protein QcrA of B. subtilis. Single amino acid substitutions were introduced into QcrA, which either precludes the oxidative folding of this protein or the insertion of its 2Fe-2S co-factor. In this manner, the importance of folding and co-factor insertion for the productive translocation of QcrA was shown.
Several previous studies have shown that, if cargo proteins of the Tat pathway are not correctly folded or if co-factors are inserted incorrectly, the membrane translocation process is terminated and the cargo degraded (4, 66–69). However, it is noteworthy that studies investigating disulfide bond-assisted folding in the Tat system have, thus far, only been performed on heterologous proteins or with Escherichia coli fusion proteins (1–3, 70, 71). Furthermore, spinach-derived Rieske proteins required a partially folded state to allow for co-factor insertion when expressed in E. coli (72). Our present study is the first to examine the Tat-dependent export of a single native protein, the Rieske protein QcrA of B. subtilis, which requires both co-factor binding and disulfide bond formation for its biological function. Importantly, the Rieske protein domain is highly conserved and residues involved in disulfide bond formation and co-factor insertion are well defined by high-resolution structural analyses (33, 40–42). On this basis, a total of three site-specific mutations in QcrA were generated; in two mutant proteins the His residues involved in co-factor binding were individually replaced (i.e. QcrA-H102L and QcrA-H124L), and in a third mutant protein a Cys residue involved in disulfide bond formation was replaced (i.e. QcrA-C123S). Membrane insertion and translocation of these mutant QcrA forms was assessed by Western blotting, taking advantage of the fact that translocated QcrA is to some extent processed by signal peptidase, which results in the release of a smaller form (QcrA*) into the growth medium (28). It is presently not known why QcrA is processed by signal peptidase and why QcrA* is released into the medium, especially because the known QcrA biological function is electron transfer via the cytochrome bc1 complex the cytoplasmic membrane. Nevertheless, by analyzing QcrA processing and QcrA* release into the medium we were able to show that all three QcrA mutant proteins were defective in complete translocation. Therefore, both the correct folding and co-factor insertion are critical for Tat-dependent translocation of QcrA. Interestingly, QcrA-C123S or fragments thereof remained undetectable by Western blotting or even in pulse-chase labeling analyses, indicating that this mutant protein was degraded within the 2 min needed to sufficiently label the QcrA protein with [35S]methionine/cysteine. Here it should be noted that 2 min is a relatively long period of time when it comes to proteolysis which, depending on the substrate, can happen within seconds. In contrast, QcrA-H102L and QcrA-H124L produced from the same expression plasmid as QcrA-C123S were observed in the cytoplasmic and membrane fractions. These findings suggest a sequential and hierarchical quality control process with regard to the Tat-dependent cargo protein QcrA, where oxidative folding undergoes quality control before co-factor insertion. The subsequent membrane translocation and/or processing of QcrA are rather slow as we did not observe any QcrA* in our pulse-chase labeling experiments even after a 30-min chase period.
The idea of a sequential and hierarchical quality control process would be supported by the finding that disulfide bond formation in QcrA is independent of the extracytoplasmic thiol-disulfide oxidoreductases BdbA, BdbB, BdbC, and BdbD, and it would be consistent with the view that insertion of the 2Fe-2S co-factor takes place in the cytoplasm. In addition, our AMS cross-linking analyses indicate that the four Cys residues of QcrA, two of which form a disulfide bond and the other two coordinating the 2Fe-2S cofactor, are located in a membrane-protected environment. If so, this is the first illustration of a cytoplasmic proofreading or quality control mechanism for a Tat-dependent cargo protein in B. subtilis. In this respect it is noteworthy that, although the majority of cytoplasmic proteins are in a reduced state, some cytoplasmic proteins of B. subtilis have been shown to contain disulfide bonds under normal physiological growth conditions (73). It is thus possible that disulfide bond formation in QcrA can take place in the cytoplasm or, perhaps more likely, at the interface between the cytoplasm and the membrane.
It is conceivable that disruption of the disulfide bond in the QcrA-C123S mutant protein causes a structural change that also affects cofactor insertion even though the side chains of Cys and Ser are relatively similar in size. Therefore, the apparently high instability of the QcrA-C123S protein could relate to defects in both oxidative folding and co-factor binding. Here it is relevant to bear in mind that Rieske proteins are defined by their 2Fe-2S co-factor and the conserved co-factor binding motif. In contrast, not all Rieske proteins have or require the disulfide bond within the co-factor binding motif (33). This suggests that the 2Fe-2S-binding motif may not be dependent on the disulfide bond for co-factor insertion. Unfortunately, it will be extremely hard to further separate the requirements for oxidative folding and co-factor insertion in QcrA of B. subtilis, as these processes are interlinked and both important. Moreover, such studies are further complicated by the production of many proteases in B. subtilis, which will rapidly degrade folding intermediates of QcrA (74, 75). In this respect, it was interesting to observe that the quality control system leading to the degradation of QcrA-C123S can be saturated by high level production of this mutant protein. This finding may eventually allow us to unravel the nature of the respective quality control pathway, which may be localized in the cytoplasm, the membrane, or the cell wall, or even extracellularly.
In summary, our present studies show that residues involved in 2Fe-2S co-factor binding and oxidative folding are essential for productive membrane translocation of the QcrA protein of B. subtilis. Interestingly, a proofreading hierarchy with regard to mutated QcrA was uncovered. The inability to form a specific disulfide bond within its Rieske domain caused the immediate degradation of QcrA, most likely in the cytoplasm, whereas mutant proteins lacking the residues needed for co-factor binding were apparently proofread at the interface of the cytoplasm and membrane. Although stably produced, the latter QcrA mutants were not detectably translocated. Last, these are the first studies in the Tat field where both folding and co-factor attachment have been addressed in a single native molecule. The relevance of these studies on QcrA, the Rieske protein of B. subtilis, is underpinned by the important roles of the cytochrome bc1 complex in oxidative phosphorylation in all three domains of life.
This work was supported in part by CEU projects PITN-GA-2008-215524 (TranSys), LSHG-CT-2006-037469 and -244093 (to V. J. G. and J. M. v. D.), and the transnational SysMO initiative through projects BACELL SysMO1 and -2 with funding from the Research Council for Earth and Life Sciences of the Netherlands Organization for Scientific Research (NWO-ALW).
- AMS
- 4-acetamido-4′-maleimidyl-stilbene-2,2′-disulfonate.
REFERENCES
- 1. DeLisa M. P., Tullman D., Georgiou G. (2003) Folding quality control in the export of proteins by the bacterial twin-arginine translocation pathway. Proc. Natl. Acad. Sci. U.S.A. 100, 6115–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panahandeh S., Maurer C., Moser M., DeLisa M. P., Müller M. (2008) Following the path of a twin-arginine precursor along the TatABC translocase of Escherichia coli. J. Biol. Chem. 283, 33267–33275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maurer C., Panahandeh S., Moser M., Müller M. (2009) Impairment of twin-arginine-dependent export by seemingly small alterations of substrate conformation. FEBS Lett. 583, 2849–2853 [DOI] [PubMed] [Google Scholar]
- 4. Jack R. L., Buchanan G., Dubini A., Hatzixanthis K., Palmer T., Sargent F. (2004) Coordinating assembly and export of complex bacterial proteins. EMBO J. 23, 3962–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oresnik I. J., Ladner C. L., Turner R. J. (2001) Identification of a twin-arginine leader-binding protein. Mol. Microbiol. 40, 323–331 [DOI] [PubMed] [Google Scholar]
- 6. Robinson C., Bolhuis A. (2004) Tat-dependent protein targeting in prokaryotes and chloroplasts. Biochim. Biophys. Acta 1694, 135–147 [DOI] [PubMed] [Google Scholar]
- 7. Tjalsma H., Bolhuis A., Jongbloed J. D., Bron S., van Dijl J. M. (2000) Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol. Rev. 64, 515–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson C., Matos C. F., Beck D., Ren C., Lawrence J., Vasisht N., Mendel S. (2011) Transport and proofreading of proteins by the twin-arginine translocation (Tat) system in bacteria. Biochim. Biophys. Acta 1808, 876–884 [DOI] [PubMed] [Google Scholar]
- 9. Freudl R. (2013) Leaving home ain't easy: protein export systems in Gram-positive bacteria. Res. Microbiol. 164, 664–674 [DOI] [PubMed] [Google Scholar]
- 10. Whitaker N., Bageshwar U. K., Musser S. M. (2012) Kinetics of precursor interactions with the bacterial Tat translocase detected by real-time FRET. J. Biol. Chem. 287, 11252–11260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bolhuis A., Mathers J. E., Thomas J. D., Barrett C. M., Robinson C. (2001) TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J. Biol. Chem. 276, 20213–20219 [DOI] [PubMed] [Google Scholar]
- 12. Fröbel J., Rose P., Lausberg F., Blümmel A. S., Freudl R., Müller M. (2012) Transmembrane insertion of twin-arginine signal peptides is driven by TatC and regulated by TatB. Nat. Commun. 3, 1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goosens V. J., Monteferrante C. G., van Dijl J. M. (2013) The Tat system of Gram-positive bacteria. Biochim. Biophys. Acta 10.1016/j.bbamcr.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 14. Rollauer S. E., Tarry M. J., Graham J. E., Jääskeläinen M., Jäger F., Johnson S., Krehenbrink M., Liu S. M., Lukey M. J., Marcoux J., McDowell M. A., Rodriguez F., Roversi P., Stansfeld P. J., Robinson C. V., Sansom M. S., Palmer T., Högbom M., Berks B. C., Lea S. M. (2012) Structure of the TatC core of the twin-arginine protein transport system. Nature 492, 210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Papish A. L., Ladner C. L., Turner R. J. (2003) The twin-arginine leader-binding protein, DmsD, interacts with the TatB and TatC subunits of the Escherichia coli twin-arginine translocase. J. Biol. Chem. 278, 32501–32506 [DOI] [PubMed] [Google Scholar]
- 16. Santini C. L., Ize B., Chanal A., Müller M., Giordano G., Wu L. F. (1998) A novel sec-independent periplasmic protein translocation pathway in Escherichia coli. EMBO J. 17, 101–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halbig D., Wiegert T., Blaudeck N., Freudl R., Sprenger G. A. (1999) The efficient export of NADP-containing glucose-fructose oxidoreductase to the periplasm of Zymomonas mobilis depends both on an intact twin-arginine motif in the signal peptide and on the generation of a structural export signal induced by cofactor binding. Eur. J. Biochem. 263, 543–551 [DOI] [PubMed] [Google Scholar]
- 18. Alami M., Lüke I., Deitermann S., Eisner G., Koch H. G., Brunner J., Müller M. (2003) Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell 12, 937–946 [DOI] [PubMed] [Google Scholar]
- 19. Mori H., Cline K. (2002) A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid ΔpH/Tat translocase. J. Cell Biol. 157, 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dabney-Smith C., Mori H., Cline K. (2006) Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J. Biol. Chem. 281, 5476–5483 [DOI] [PubMed] [Google Scholar]
- 21. van Dijl J. M., Braun P. G., Robinson C., Quax W. J., Antelmann H., Hecker M., Müller J., Tjalsma H., Bron S., Jongbloed J. D. (2002) Functional genomic analysis of the Bacillus subtilis Tat pathway for protein secretion. J. Biotechnol. 98, 243–254 [DOI] [PubMed] [Google Scholar]
- 22. Graumann P. (2011) Bacillus: Cellular and Molecular Biology, Second Ed., Caister Academic Press, Wymondham, United Kingdom [Google Scholar]
- 23. Tjalsma H., Antelmann H., Jongbloed J. D., Braun P. G., Darmon E., Dorenbos R., Dubois J. Y., Westers H., Zanen G., Quax W. J., Kuipers O. P., Bron S., Hecker M., van Dijl J. M. (2004) Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol. Rev. 68, 207–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jongbloed J. D., Grieger U., Antelmann H., Hecker M., Nijland R., Bron S., van Dijl J. M. (2004) Two minimal Tat translocases in Bacillus. Mol. Microbiol. 54, 1319–1325 [DOI] [PubMed] [Google Scholar]
- 25. Jongbloed J. D., Martin U., Antelmann H., Hecker M., Tjalsma H., Venema G., Bron S., van Dijl J. M., Müller J. (2000) TatC is a specificity determinant for protein secretion via the twin-arginine translocation pathway. J. Biol. Chem. 275, 41350–41357 [DOI] [PubMed] [Google Scholar]
- 26. Jongbloed J. D., van der Ploeg R., van Dijl J. M. (2006) Bifunctional TatA subunits in minimal Tat protein translocases. Trends Microbiol. 14, 2–4 [DOI] [PubMed] [Google Scholar]
- 27. Monteferrante C. G., Baglieri J., Robinson C., van Dijl J. M. (2012) The third TatA subunit TatAc of Bacillus subtilis can form active twin-arginine translocases with the TatCd and TatCy subunits. Appl. Environ. Microbiol. 78, 4999–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goosens V. J., Otto A., Glasner C., Monteferrante C. C., van der Ploeg R., Hecker M., Becher D., van Dijl J. M. (2013) Novel twin-arginine translocation pathway-dependent phenotypes of Bacillus subtilis unveiled by quantitative proteomics. J. Proteome Res. 12, 796–807 [DOI] [PubMed] [Google Scholar]
- 29. Monteferrante C. G., Miethke M., van der Ploeg R., Glasner C., van Dijl J. M. (2012) Specific targeting of the metallophosphoesterase YkuE to the Bacillus cell wall requires the twin-arginine translocation system. J. Biol. Chem. 287, 29789–29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miethke M., Monteferrante C. G., Marahiel M. A., van Dijl J. M. (2013) The Bacillus subtilis EfeUOB transporter is essential for high-affinity acquisition of ferrous and ferric iron. Biochim. Biophys. Acta 1833, 2267–2278 [DOI] [PubMed] [Google Scholar]
- 31. Nicolas P., Mäder U., Dervyn E., Rochat T., Leduc A., Pigeonneau N., Bidnenko E., Marchadier E., Hoebeke M., Aymerich S., Becher D., Bisicchia P., Botella E., Delumeau O., Doherty G., Denham E. L., Fogg M. J., Fromion V., Goelzer A., Hansen A., Härtig E., Harwood C. R., Homuth G., Jarmer H., Jules M., Klipp E., Le Chat L., Lecointe F., Lewis P., Liebermeister W., March A., Mars R. A., Nannapaneni P., Noone D., Pohl S., Rinn B., Rügheimer F., Sappa P. K., Samson F., Schaffer M., Schwikowski B., Steil L., Stülke J., Wiegert T., Devine K. M., Wilkinson A. J., van Dijl J. M., Hecker M., Völker U., Bessières P., Noirot P. (2012) Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335, 1103–1106 [DOI] [PubMed] [Google Scholar]
- 32. Thöny-Meyer L. (1997) Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61, 337–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidt C. L., Shaw L. (2001) A comprehensive phylogenetic analysis of Rieske and Rieske-type iron-sulfur proteins. J. Bioenerg. Biomembr. 33, 9–26 [DOI] [PubMed] [Google Scholar]
- 34. Yu J., Hederstedt L., Piggot P. J. (1995) The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J. Bacteriol. 177, 6751–6760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahuja U., Kjelgaard P., Schulz B. L., Thöny-Meyer L., Hederstedt L. (2009) Haem-delivery proteins in cytochrome c maturation System II. Mol. Microbiol. 73, 1058–1071 [DOI] [PubMed] [Google Scholar]
- 36. Sanders C., Wethkamp N., Lill H. (2001) Transport of cytochrome c derivatives by the bacterial Tat protein translocation system. Mol. Microbiol. 41, 241–246 [DOI] [PubMed] [Google Scholar]
- 37. Erlendsson L. S., Acheson R. M., Hederstedt L., Le Brun N. E. (2003) Bacillus subtilis ResA is a thiol-disulfide oxidoreductase involved in cytochrome c synthesis. J. Biol. Chem. 278, 17852–17858 [DOI] [PubMed] [Google Scholar]
- 38. Schiött T., Throne-Holst M., Hederstedt L. (1997) Bacillus subtilis CcdA-defective mutants are blocked in a late step of cytochrome c biogenesis. J. Bacteriol. 179, 4523–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hodson C. T., Lewin A., Hederstedt L., Le Brun N. E. (2008) The active-site cysteinyls and hydrophobic cavity residues of ResA are important for cytochrome c maturation in Bacillus subtilis. J. Bacteriol. 190, 4697–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider D., Schmidt C. L. (2005) Multiple Rieske proteins in prokaryotes: where and why?. Biochim. Biophys. Acta 1710, 1–12 [DOI] [PubMed] [Google Scholar]
- 41. Iwata S., Saynovits M., Link T. A., Michel H. (1996) Structure of a water soluble fragment of the “Rieske” iron-sulfur protein of the bovine heart mitochondrial cytochrome bc1 complex determined by MAD phasing at 1.5-Å resolution. Structure 4, 567–579 [DOI] [PubMed] [Google Scholar]
- 42. Link T. A., Saynovits M., Assmann C., Iwata S., Ohnishi T., von Jagow G. (1996) Isolation, characterisation and crystallisation of a water-soluble fragment of the Rieske iron-sulfur protein of bovine heart mitochondrial bc1 complex. Eur. J. Biochem. 237, 71–75 [DOI] [PubMed] [Google Scholar]
- 43. Molik S., Karnauchov I., Weidlich C., Herrmann R. G., Klösgen R. B. (2001) The Rieske Fe/S protein of the cytochrome b6/f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts?. J. Biol. Chem. 276, 42761–42766 [DOI] [PubMed] [Google Scholar]
- 44. De Buck E., Vranckx L., Meyen E., Maes L., Vandersmissen L., Anné J., Lammertyn E. (2007) The twin-arginine translocation pathway is necessary for correct membrane insertion of the Rieske Fe/S protein in Legionella pneumophila. FEBS Lett. 581, 259–264 [DOI] [PubMed] [Google Scholar]
- 45. Bachmann J., Bauer B., Zwicker K., Ludwig B., Anderka O. (2006) The Rieske protein from Paracoccus denitrificans is inserted into the cytoplasmic membrane by the twin-arginine translocase. FEBS J. 273, 4817–4830 [DOI] [PubMed] [Google Scholar]
- 46. Keller R., de Keyzer J., Driessen A. J., Palmer T. (2012) Co-operation between different targeting pathways during integration of a membrane protein. J. Cell Biol. 199, 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hopkins A., Buchanan G., Palmer T. (2014) The role of the twin arginine protein transport pathway in the assembly of the Streptomyces coelicolor A3(2) cytochrome bc1 complex. J. Bacteriol. 196, 50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Winstedt L., von Wachenfeldt C. (2000) Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa3 or cytochrome bd, is required for aerobic growth. J. Bacteriol. 182, 6557–6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. García-Horsman J. A., Barquera B., Rumbley J., Ma J., Gennis R. B. (1994) The superfamily of heme-copper respiratory oxidases. J. Bacteriol. 176, 5587–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buescher J. M., Liebermeister W., Jules M., Uhr M., Muntel J., Botella E., Hessling B., Kleijn R. J., Le Chat L., Lecointe F., Mäder U., Nicolas P., Piersma S., Rügheimer F., Becher D., Bessieres P., Bidnenko E., Denham E. L., Dervyn E., Devine K. M., Doherty G., Drulhe S., Felicori L., Fogg M. J., Goelzer A., Hansen A., Harwood C. R., Hecker M., Hubner S., Hultschig C., Jarmer H., Klipp E., Leduc A., Lewis P., Molina F., Noirot P., Peres S., Pigeonneau N., Pohl S., Rasmussen S., Rinn B., Schaffer M., Schnidder J., Schwikowski B., Van Dijl J. M., Veiga P., Walsh S., Wilkinson A. J., Stelling J., Aymerich S., Sauer U. (2012) Global network reorganization during dynamic adaptations of Bacillus subtilis metabolism. Science 335, 1099–1103 [DOI] [PubMed] [Google Scholar]
- 51. Kouwen T. R., van der Goot A., Dorenbos R., Winter T., Antelmann H., Plaisier M. C., Quax W. J., van Dijl J. M., Dubois J. Y. (2007) Thiol-disulphide oxidoreductase modules in the low-GC Gram-positive bacteria. Mol. Microbiol. 64, 984–999 [DOI] [PubMed] [Google Scholar]
- 52. Sambrook F., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, New York [Google Scholar]
- 53. van der Ploeg R., Barnett J. P., Vasisht N., Goosens V. J., Pöther D. C., Robinson C., van Dijl J. M. (2011) Salt sensitivity of minimal twin arginine translocases. J. Biol. Chem. 286, 43759–43770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Dijl J. M., de Jong A., Smith H., Bron S., Venema G. (1991) Non-functional expression of Escherichia coli signal peptidase I in Bacillus subtilis. J. Gen. Microbiol. 137, 2073–2083 [DOI] [PubMed] [Google Scholar]
- 55. Jongbloed J. D., Antelmann H., Hecker M., Nijland R., Bron S., Airaksinen U., Pries F., Quax W. J., van Dijl J. M., Braun P. G. (2002) Selective contribution of the twin-arginine translocation pathway to protein secretion in Bacillus subtilis. J. Biol. Chem. 277, 44068–44078 [DOI] [PubMed] [Google Scholar]
- 56. Vogtentanz G., Collier K. D., Bodo M., Chang J. H., Day A. G., Estell D. A., Falcon B. C., Ganshaw G., Jarnagin A. S., Kellis J. T., Jr, Kolkman M. A., Lai C. S., Meneses R., Miller J. V., de Nobel H., Power S., Weyler W., Wong D. L., Schmidt B. F. (2007) A Bacillus subtilis fusion protein system to produce soybean Bowman-Birk protease inhibitor. Protein Expr. Purif. 55, 40–52 [DOI] [PubMed] [Google Scholar]
- 57. Bongers R. S., Veening J. W., Van Wieringen M., Kuipers O. P., Kleerebezem M. (2005) Development and characterization of a subtilin-regulated expression system in Bacillus subtilis: strict control of gene expression by addition of subtilin. Appl. Environ. Microbiol. 71, 8818–8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dubois J. Y., Kouwen T. R., Schurich A. K., Reis C. R., Ensing H. T., Trip E. N., Zweers J. C., van Dijl J. M. (2009) Immunity to the bacteriocin sublancin 168 Is determined by the SunI (YolF) protein of Bacillus subtilis. Antimicrob. Agents Chemother. 53, 651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kouwen T. R., van der Ploeg R., Antelmann H., Hecker M., Homuth G., Mäder U., van Dijl J. M. (2009) Overflow of a hyper-produced secretory protein from the Bacillus Sec pathway into the Tat pathway for protein secretion as revealed by proteogenomics. Proteomics 9, 1018–1032 [DOI] [PubMed] [Google Scholar]
- 60. Kouwen T. R., Dubois J. Y., Freudl R., Quax W. J., van Dijl J. M. (2008) Modulation of thiol-disulfide oxidoreductases for increased production of disulfide-bond-containing proteins in Bacillus subtilis. Appl. Environ. Microbiol. 74, 7536–7545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goosens V. J., Mars R. A., Akeroyd M., Vente A., Dreisbach A., Denham E. L., Kouwen T. R., van Rij T., Olsthoorn M., van Dijl J. M. (2013) Is proteomics a reliable tool to probe the oxidative folding of bacterial membrane proteins? Antioxid. Redox Signal. 18, 1159–1164 [DOI] [PubMed] [Google Scholar]
- 62. Meima R., Eschevins C., Fillinger S., Bolhuis A., Hamoen L. W., Dorenbos R., Quax W. J., van Dijl J. M., Provvedi R., Chen I., Dubnau D., Bron S. (2002) The bdbDC operon of Bacillus subtilis encodes thiol-disulfide oxidoreductases required for competence development. J. Biol. Chem. 277, 6994–7001 [DOI] [PubMed] [Google Scholar]
- 63. Draskovic I., Dubnau D. (2005) Biogenesis of a putative channel protein, ComEC, required for DNA uptake: membrane topology, oligomerization and formation of disulphide bonds. Mol. Microbiol. 55, 881–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Palmer T., Berks B. C. (2012) The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 10, 483–496 [DOI] [PubMed] [Google Scholar]
- 65. Eijlander R. T., Kolbusz M. A., Berendsen E. M., Kuipers O. P. (2009) Effects of altered TatC proteins on protein secretion efficiency via the twin-arginine translocation pathway of Bacillus subtilis. Microbiology 155, 1776–1785 [DOI] [PubMed] [Google Scholar]
- 66. Tottey S., Waldron K. J., Firbank S. J., Reale B., Bessant C., Sato K., Cheek T. R., Gray J., Banfield M. J., Dennison C., Robinson N. J. (2008) Protein-folding location can regulate manganese-binding versus copper- or zinc-binding. Nature 455, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 67. Kolkman M. A., van der Ploeg R., Bertels M., van Dijk M., van der Laan J., van Dijl J. M., Ferrari E. (2008) The twin-arginine signal peptide of Bacillus subtilis YwbN can direct either Tat- or Sec-dependent secretion of different cargo proteins: secretion of active subtilisin via the B. subtilis Tat pathway. Appl. Environ. Microbiol. 74, 7507–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hynds P. J., Robinson D., Robinson C. (1998) The sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J. Biol. Chem. 273, 34868–34874 [DOI] [PubMed] [Google Scholar]
- 69. Matos C. F., Robinson C., Di Cola A. (2008) The Tat system proofreads FeS protein substrates and directly initiates the disposal of rejected molecules. EMBO J. 27, 2055–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ribnicky B., Van Blarcom T., Georgiou G. (2007) A scFv antibody mutant isolated in a genetic screen for improved export via the twin arginine transporter pathway exhibits faster folding. J. Mol. Biol. 369, 631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim J. Y., Fogarty E. A., Lu F. J., Zhu H., Wheelock G. D., Henderson L. A., DeLisa M. P. (2005) Twin-arginine translocation of active human tissue plasminogen activator in Escherichia coli. Appl. Environ. Microbiol. 71, 8451–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gubernator B., Króliczewski J., Kallas T., Szczepaniak A. (2006) Iron-sulfur cluster reconstitution of spinach chloroplast Rieske protein requires a partially prefolded apoprotein. Biochim. Biophys. Acta 1764, 735–742 [DOI] [PubMed] [Google Scholar]
- 73. Hochgräfe F., Mostertz J., Albrecht D., Hecker M. (2005) Fluorescence thiol modification assay: oxidatively modified proteins in Bacillus subtilis. Mol. Microbiol. 58, 409–425 [DOI] [PubMed] [Google Scholar]
- 74. Dalbey R. E., Wang P., van Dijl J. M. (2012) Membrane proteases in the bacterial protein secretion and quality control pathway. Microbiol. Mol. Biol. Rev. 76, 311–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krishnappa L., Dreisbach A., Otto A., Goosens V. J., Cranenburgh R. M., Harwood C. R., Becher D., van Dijl J. M. (2013) Extracytoplasmic proteases determining the cleavage and release of secreted proteins, lipoproteins and membrane proteins in Bacillus subtilis. J. Proteome Res. 12, 4101–4110 [DOI] [PubMed] [Google Scholar]
- 76. Kunst F., Ogasawara N., Moszer I., Albertini A. M., Alloni G., Azevedo V., Bertero M. G., Bessières P., Bolotin A., Borchert S., Borriss R., Boursier L., Brans A., Braun M., Brignell S. C., Bron S., Brouillet S., Bruschi C. V., Caldwell B., Capuano V., Carter N. M., Choi S. K., Codani J. J., Connerton I. F., Danchin A. (1997) The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390, 249–256 [DOI] [PubMed] [Google Scholar]
- 77. Gasson M. J. (1983) Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bron S., Bolhuis A., Tjalsma H., Holsappel S., Venema G., van Dijl J. M. (1998) Protein secretion and possible roles for multiple signal peptidases for precursor processing in bacilli. J. Biotechnol. 64, 3–13 [DOI] [PubMed] [Google Scholar]
- 79. van der Ploeg R., Mäder U., Homuth G., Schaffer M., Denham E. L., Monteferrante C. G., Miethke M., Marahiel M. A., Harwood C. R., Winter T., Hecker M., Antelmann H., van Dijl J. M. (2011) Environmental salinity determines the specificity and need for Tat-dependent secretion of the YwbN protein in Bacillus subtilis. PLoS One 6, e18140. [DOI] [PMC free article] [PubMed] [Google Scholar]


