Background: Bleeding during pregnancy is a risk factor for premature rupture of the fetal membranes.
Results: Thrombin causes preterm birth in mice and activates PAR-1 and TLR4 to increase MMPs and COX-2.
Conclusion: Thrombin acts through multiple mechanisms to increase MMPs and PGE2 in amnion.
Significance: Thrombin plays a pivotal role in the pathogenesis of preterm labor and rupture of the membranes.
Keywords: Cyclooxygenase (COX) Pathway, Fibronectin, Matrix Metalloproteinase (MMP), Prostaglandins, Thrombin, Amnion, Decidua, Fetal Membrane, Protease-activated Receptor-1
Abstract
Here, we investigated the effects of thrombin on matrix metalloproteinases (MMPs) and prostaglandin (PG) synthesis in fetal membranes. Thrombin activity was increased in human amnion from preterm deliveries. Treatment of mesenchymal, but not epithelial, cells with thrombin resulted in increased MMP-1 and MMP-9 mRNA and enzymatic activity. Thrombin also increased COX2 mRNA and PGE2 in these cells. Protease-activated receptor-1 (PAR-1) was localized to amnion mesenchymal and decidual cells. PAR-1-specific inhibitors and activating peptides indicated that thrombin-induced up-regulation of MMP-9 was mediated via PAR-1. In contrast, thrombin-induced up-regulation of MMP-1 and COX-2 was mediated through Toll-like receptor-4, possibly through thrombin-induced release of soluble fetal fibronectin. In vivo, thrombin-injected pregnant mice delivered preterm. Mmp8, Mmp9, and Mmp13, and PGE2 content was increased significantly in fetal membranes from thrombin-injected animals. These results indicate that thrombin acts through multiple mechanisms to activate MMPs and PGE2 synthesis in amnion.
Introduction
Amnion and chorion, a contiguous structure of cells and extracellular matrix, comprise the fetal membranes. Preterm premature rupture of membranes (PPROM)2 is associated with 30–40% of preterm deliveries and occurs in ∼1–3% of all pregnancies (1). Currently, there is no effective prevention or treatment for PROM and preterm labor.
The process of membrane rupture targets the predominant matrix component, collagen, and may involve local (e.g. intra-amniotic infection) or remote factors (e.g. procoagulants). Clinical evidence for a role of thrombin in preterm birth includes the following: (i) thrombin-antithrombin complexes, markers of in vivo generation of thrombin, are increased in plasma (2) and amniotic fluid (3) from women in preterm labor or preterm PROM; (ii) increased plasma thrombin-antithrombin complexes in the second trimester are associated with subsequent preterm PROM (4); (iii) retroplacental hematomas in the first trimester are associated with adverse pregnancy outcomes, including preterm birth (5); and (iv) vaginal bleeding in the first or second trimester is associated with preterm birth (6, 7). Several studies implicate decidual cells as the primary source and target of thrombin (8). Thrombin stimulates the production of matrix metalloproteinase-1 (MMP-1) (9) in endometrial stromal cells in culture, suggesting that matrix degradation occurs through collagenase activity. Abruption-induced thrombin generation has been associated with fetal membrane weakening and PPROM (4, 10), and treatment of intact amnion explants with thrombin results in increased levels of MMP-9 and mechanical weakening (11). The precise mechanisms by which thrombin leads to preterm labor are not known.
The primary load-bearing structure of the fetal membranes is the amnion which comprises a single layer of avascular epithelial cells and an underlying layer of mesenchymal cells (12). Mesenchymal cells are the primary source of collagen and matrix support of the amnion. Interstitial collagens (types I, III, and V) maintain the mechanical integrity of the amnion. Fetal membrane rupture is preceded by degradation of collagen that is mediated primarily by MMPs in amnion. Interstitial collagenase, MMP-1, cleaves the triple helix of fibrillar collagen, which is then further degraded by the gelatinases, MMP-2 and MMP-9. MMP-1 is increased in amniotic fluid obtained from women with PPROM (13). Moreover, MMP-9 is increased in amniotic membranes obtained from women in normal labor (14) and in those from women with PPROM (15, 16).
Prostaglandins (PGs) play pivotal roles in human parturition by stimulating cervical ripening, myometrial contraction, and fetal membrane rupture (17). In human pregnancy, the principle source of PGs is the amnion (18). In this study, we investigated the effects of thrombin on MMPs and PGE2 synthesis in amnion cells in vitro and in vivo. Collectively, the data reported herein suggest novel mechanisms of action and a pivotal role of thrombin in the pathogenesis of preterm labor and PPROM.
EXPERIMENTAL PROCEDURES
Reagents
DMEM/F12 (Ham, 11320), antibiotic-antimycotic solution (15240), and 10% gelatin zymogram gel (EC61755) were purchased from Invitrogen. Thrombin from human plasma (T7009) and hirudin were purchased from Sigma, and recombinant thrombin was from R&D Systems. One NIH unit/ml of human plasma thrombin activity was equivalent to 0.324 μg/ml (i.e. 9 nm). WEDE15 (Monoclonal Antibody Antithrombin receptor IM2085) was purchased from Beckman Coulter. PAR-1-activating peptide (TFLLRN, 61530), Sensolyte® Plus 520 MMP-1 Assay kit (72012), and Sensolyte 520 Thrombin Activity Assay kit (72129) were purchased from Anaspec (Fremont, CA). PAR-1 selective receptor antagonist, SCH79797 was obtained from Tocris (Ellisville, MO). Rabbit anti-human fibronectin polyclonal antibody (AB1945) was purchased from Millipore. Goat anti-rabbit IgG (H+L)-HRP conjugate (170-6515) and rabbit anti-sheep IgG (H+L)-HRP conjugate (172-1017) were purchased from Bio-Rad. Polyclonal goat IgG (AB108-C), Anti-human TLR4 antibody (AF1478), human collagen I α1 antibody (AF6220), and MMP-1 were purchased from R&D Systems. Human collagen type 1 was purchased from Millipore (CC050). Protease inhibitor mixture tablets (complete Mini, 04 693 124 001) were purchased from Roche Applied Science. Percoll (17-0891-01) and Amersham Biosciences ECL Plus Western Blotting Detection kit (RPN2132) were purchased from GE Healthcare. BCA (bicinchoninic acid) assay (23225) was purchased from Thermo Scientific.
Thrombin Activity Assay in Human Amnion
Human amnion tissues were obtained from pregnancies delivered preterm (n = 8) or term (n = 6). The preterm group included both PPROM and non-PPROM, and delivery mode was either vaginal or cesarean section. Term normal amnion was derived from spontaneous vaginal delivery. Tissues were rinsed with saline and, after thorough removal of blood, frozen in liquid nitrogen, stored at −80 °C. Pulverized human amnion (∼50 mg) was homogenized in 500 μl of PBS. Homogenates were centrifuged at 3000 × g for 5 min at 4 °C. Supernatant was collected and assayed using Sensolyte 520 Thrombin Activity Assay kit according to the manufacturer's instruction. The proteolytic activity of thrombin was quantified using fluorescence resonance energy transfer (FRET) peptide.
Isolation and Culture of Amnion Epithelial and Mesenchymal Cells
Preparation and isolation of amnion epithelial and mesenchymal cells were performed as described previously (19). Briefly, amnion tissue was separated by blunt dissection. The amnion tissue was minced, and cells were dispersed by enzymatic digestion. Isolated amnion cells were suspended in DMEM/F12 that contained fetal bovine serum (10%, v/v) and antibiotic-antimycotic solution (1%, v/v). Cells were plated in plastic culture dishes, maintained at 37 °C in a humidified atmosphere of 5% CO2 in air, and allowed to replicate in monolayer to confluence.
Isolation of Decidual Cells
Decidual cells were isolated from human decidual tissue by limited enzymatic digestion and separation on Percoll gradients according to previously described methods (20). Cells were plated in plastic plates and allowed to replicate to confluence in primary monolayer culture.
Quantitative Real-time PCR
Quantitative RT-PCR was used to determine the relative levels of gene expression as described previously (21). Primer sequences for amplifications are shown in Table 1. Taqman probe with FAM dye label was used for COX2, and SYBR Green was used for other genes. Gene expression was normalized to that of the housekeeping gene GAPDH for human amnion cells and cyclophilin A for mouse tissue.
TABLE 1.
Sequences of primers used for quantitative RT-PCR
| Gene | Primer sequences |
|---|---|
| Human | |
| GAPDH | Fwd 5′-GGA GTC AAC GGA TTT GGT CGT A-3′ |
| Rev 5′-CAA CAA TAT CCA CTT TAC CAG AGT TA-3′ | |
| MMP1 | Fwd 5′-AGA TGA AAG GTG GAC CAA CAA TTT-3′ |
| Rev 5′-CCA AGA GAA TGG CCG AGT TC-3′ | |
| MMP2 | Fwd 5′-TTG ATG GCA TCG CTC AGA TC-3′ |
| Rev 5′-TGT CAC GTG GCG TCA CAG T-3′ | |
| MMP9 | Fwd 5′-CCA CCA CAA CAT CAC CTA TTG G-3′ |
| Rev 5′-GCA AAG GCG TCG TCA ATC A-3′ | |
| COX2 | Fwd 5′-GAA TCA TTC ACC AGG CAA ATT G-3′ |
| Rev 5′-TCT GTA CTG CGG GTG GAA CA-3′ | |
| Probe 6-FAM TCC TAC CAC CAG CAA CCC TGC CA 6-TAMRA | |
| PAR1 | Fwd 5′-CCG CCT GCT TCA GTC TGT-3′ |
| Rev 5′-ATC TAA GGT GGC ATT TGT TGC T-3′ | |
| Mouse | |
| Cpha | Fwd 5′-GCT TTT CGC CGC TTG CT-3′ |
| Rev 5′-TCG TCA TCG GCC GTG AT-3′ | |
| Mmp2 | Fwd 5′-GGA CTA TGA CCG GGA TAA GAA ATA TG-3′ |
| Rev 5′-GGG CAC CTT CTG AAT TTC CA-3′ | |
| Mmp9 | Fwd 5′-AGA CCA AGG GTA CAG CCT GTT C-3′ |
| Rev 5′-GCA CGC TGG AAT GAT CTA AGC-3′ | |
| Mmp8 | Fwd 5′-CAC ACA CAG CTT GCC AAT GC-3′ |
| Rev 5′-TTT CGT AGG TAA TTC TCA GCA GTT TTT-3′ | |
| Mmp13 | Fwd 5′-GGC AAA AGC CAT TTC ATG CT-3′ |
| Rev 5′-TCT TCA TCG CCT GGA CCA TAA-3′ | |
| Cox1 | Fwd 5′-TGCCCTCACCAGTCAATCC-3′ |
| Rev 5′-GTAGCCCGTGCGAGTACAATC-3′ | |
| Cox2 | Fwd 5′-AGT TTG TTG AGT CAT TCA CCA GAC A-3′ |
| Rev 5′-CCA CTG CTT GTA CAG CAA TTG G-3′ | |
| Pgdh | Fwd 5′-TAG CAG GGC TCA TGC CTG TT-3′ |
| Rev 5′-TGG GCA AAT GAC ATT CAG TCT T-3′ | |
MMP-1 Activity Assay
MMP-1 activity of the conditioned media was assayed by The Sensolyte Plus 520 MMP-1 Assay kit according to the manufacturer's instruction. Proteolytic activity of MMP-1 was quantified using FRET peptide.
Gelatin Zymography
Gelatin zymography was performed as described (22). Briefly, conditioned medium was concentrated 10-fold with Amicon Ultra-4 Centrifugal Filter Units (Millipore) at 3200 × g for 1 h at 4 °C. Mouse fetal membranes were homogenized with buffer (10 mm Tris, 150 mm NaCl, 10 mm CaCl2, and 0.1% Triton X-100, pH 7.4), centrifuged at 16,000 × g for 15 min at 4 °C, and the supernatant was used for assay. Concentrated media (4 μg) or homogenized mouse fetal membranes (12 μg) were applied to 10% gelatin polyacrylamide minigels in standard sodium dodecyl sulfate loading buffer containing 0.1% sodium dodecyl sulfate. After electrophoresis, gels were soaked in renaturing buffer (2.7% Triton X-100 in distilled water) in a shaker for 30 min with one change after 30 min to remove sodium dodecyl sulfate. Gels were soaked in assay buffer (50 mm Tris, 200 mm NaCl, 10 mm CaCl2, 0.05% Brij 35, pH 7.5) for 16 h at 34 °C and stained with Coomassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid followed by destaining with 25% methanol and 7% acetic acid. Areas of lysis were analyzed using a Fuji LAS 3000 image analysis system (Fujifilm Life Science, Tokyo, Japan).
PGE2 ELISA
PGE2 concentration in the condition media was assayed by Parameter PGE2 immunoassay (KGE004B; R&D Systems) according to the manufacturer's instructions.
Immunohistochemistry
Immunohistochemistry was performed as described previously (22). Formalin-fixed, paraffin-embedded human fetal membranes from term noncomplicated deliveries were sectioned at 5 μm. Slides were deparaffinized in xylene and rehydrated in graded alcohols to distilled water. Endogenous peroxidase activity was quenched for 10 min at room temperature, using 0.3% H2O2 with 0.1% sodium azide. Slides were subjected to steam heat epitope retrieval in EDTA buffer (1 mm, pH 8.0) for 30 min. After rinsing in PBS, slides were incubated in primary antibody (WEDE15) for 30 min at 25 °C. Negative control specimens were processed simultaneously in an identical manner, with the exception that control mouse IgG was used in place of primary antibody. After another rinse in PBS, slides were incubated with appropriate horseradish peroxidase-conjugated polymer for 30 min at 25 °C. Finally, the slides were immersed for 5 min in 25 °C diaminobenzidine, enhanced with 0.5% copper sulfate in PBS for 5 min at 25 °C, counterstained in hematoxylin, dehydrated in graded alcohols, cleared in xylene, and covered with a coverslip.
Immunoblotting
Protein extraction from cells with urea buffer was performed as described previously (21). Briefly, after washing with PBS, cells were extracted overnight at 4 °C with 6 m urea buffer (6 m urea, 16 mm potassium phosphate, pH 7.8, and 0.12 m NaCl containing a protease inhibitor mixture), centrifuged at 10,000 × g for 30 min, and supernatants were used for immunoblot analysis. Conditioned media from treated amnion cells were filter-concentrated with Amicon Ultra-4 Centrifugal Filter Units at 3200 × g for 50 min at 4 °C. Concentrated samples were supplemented with a protease inhibitor mixture for immunoblotting. Total protein (30 μg/lane) was applied to 6% polyacrylamide gels, separated by electrophoresis, and transferred to nitrocellulose membranes overnight at 4 °C. Membranes were blocked with TBST containing 5% nonfat powdered milk for 1 h at 37 °C. Membranes were incubated with primary antibodies of rabbit anti-human fibronectin polyclonal antibody (AB1945) or human collagen I α1 antibody (AF6220) overnight at 4 °C. Thereafter, blots were incubated with a second antibody (goat anti-rabbit IgG-HRP conjugate or rabbit anti-sheep IgG-HRP conjugate, 1:10,000) at room temperature for 1 h. Signals were detected by chemiluminescence using a Amersham Biosciences ECL Plus Western blotting Detection kit.
Thrombin-induced Model of Preterm Labor
C57BL/6 female mice were crossed with male mice for 5 h (from 10:00 to 15:00), and pregnancy was determined by the presence of a vaginal plug. On 17 days postcoitum at 19:00, pregnant mice were anesthetized with tribromoethanol (250 μg/g body weight, intraperitoneally). A ventral incision was made to visualize the uterus and thrombin (4 μl, 1 unit/μl), or PBS with 1% methylene blue was injected in the interface between fetal membranes and uterine lining of each fetus opposite the placental site (see Fig. 8A). Injection was limited to 6 pups. Thereafter, the maternal abdomen was closed with 5-0 silk. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Southwestern Medical Center.
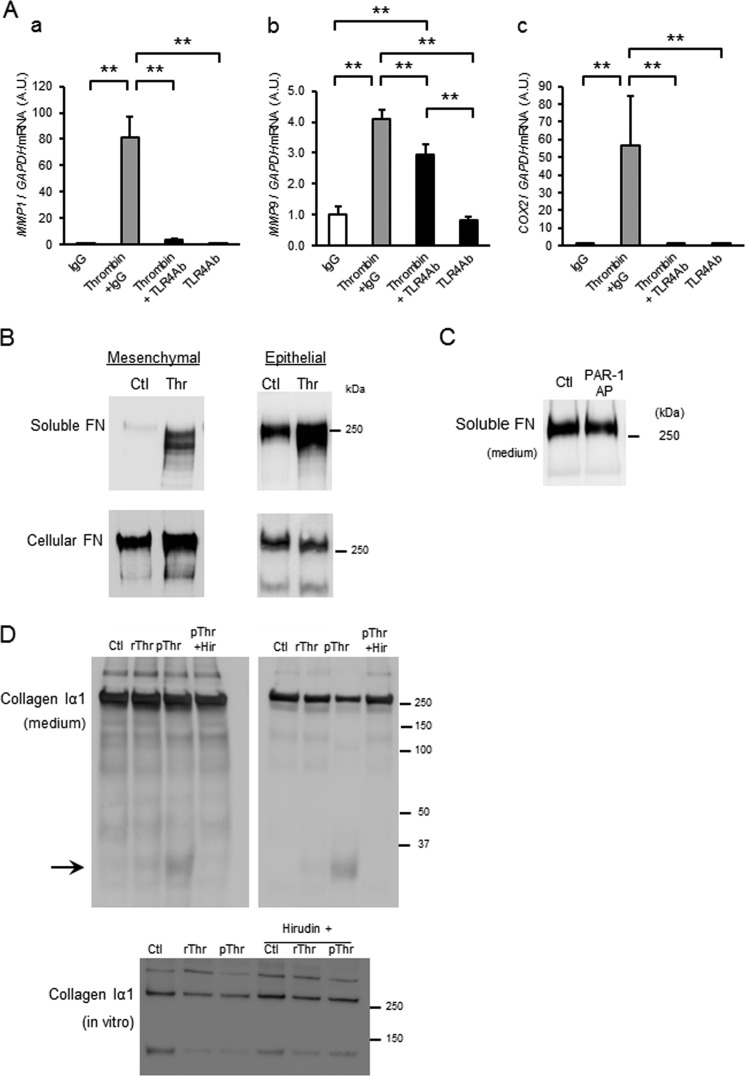
FIGURE 8.
Thrombin-induced up-regulation of MMP-1 and COX2 is mediated via Toll-like receptor-4. A, amnion mesenchymal cells were pretreated with control IgG or anti-human TLR4 neutralizing antibody for 30 min prior to treatment with control (medium only) or thrombin (2 units/ml). After 48 h, relative mRNA levels of MMP1 (a), MMP9 (b), and COX2 (c) were analyzed using quantitative PCR. Data represent mean ± S.D. (error bars), n = 3 in each group. **, p < 0.01. A.U., arbitrary units. B, immunoblot analysis of soluble (medium) and cellular fibronectin (FN) in mesenchymal (TLR4-responsive) and epithelial (TLR4-nonresponsive) cells treated with control (Ctl) or thrombin (Thr, 4 units/ml, 36 nm). 30 μg of protein from conditioned media (soluble) or 6 m urea extract (cellular) was applied in each lane. C, immunoblot of soluble fibronectin in media of primary amnion mesenchymal cells treated with control (Ctl) or PAR-1 AP × 48 h. D, immunoblot analysis of collagen type I α1 in media from mesenchymal cells treated with recombinant (4 units/ml) or plasma (4 units/ml) Thr and hirudin (6.5 units/ml). Immunoblot of purified collagen type I treated with thrombin ± hirudin in vitro is also shown.
Statistical Analysis
Values were expressed as means ± S.D. (in vitro experiments) or means ± S.E. (in vivo experiments). Data were analyzed by unpaired Student's t test in human amnion tissues. In PAR-1 or TLR4 blocking experiments, one-way analysis of variance followed by the Student-Newman-Keuls test was used. Mann-Whitney U test was used in analyzing data from the mice preterm delivery model. p values < 0.05 were regarded as statistically significant.
RESULTS
Thrombin Activity in Human Amnion from Pregnancies Delivered Preterm and Term
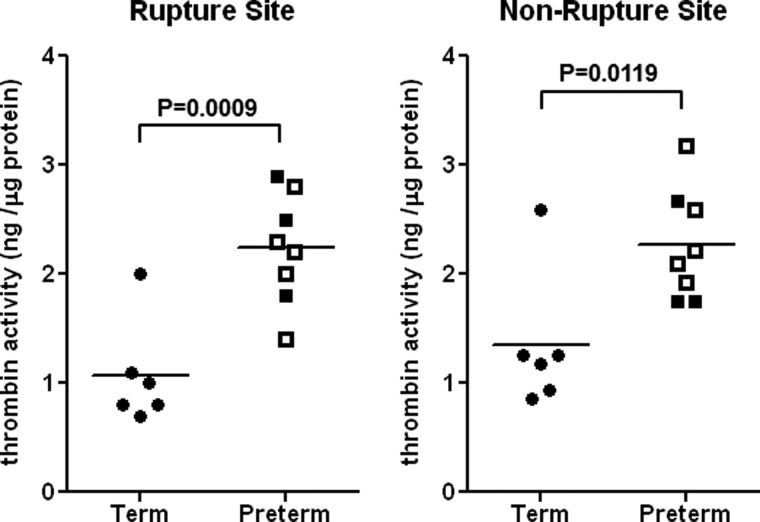
To determine whether thrombin activity was increased at the site of membrane rupture, thrombin activity was analyzed in human amnion from pregnancies delivered preterm (33.0 ± 1.3 weeks) or term (38.3 ± 0.4 weeks) (Fig. 1). Thrombin activity was increased significantly in amniotic membranes from women delivered preterm regardless of sampling site. Within the group delivered preterm, differences in thrombin activity were not significant between PPROM and non-PPROM or between vaginal or cesarean delivery (data not shown).
FIGURE 1.
Thrombin activity in human amnion from pregnancies delivered preterm or term. Thrombin activity was quantified using the FRET system after complete removal of blood from human amnion. Preterm amnion (n = 8) includes both PPROM (open symbols) and non-PPROM (filled symbols), and the delivery mode was either vaginal or cesarean section. Term normal amnion (n = 6) was obtained from spontaneous vaginal delivery.
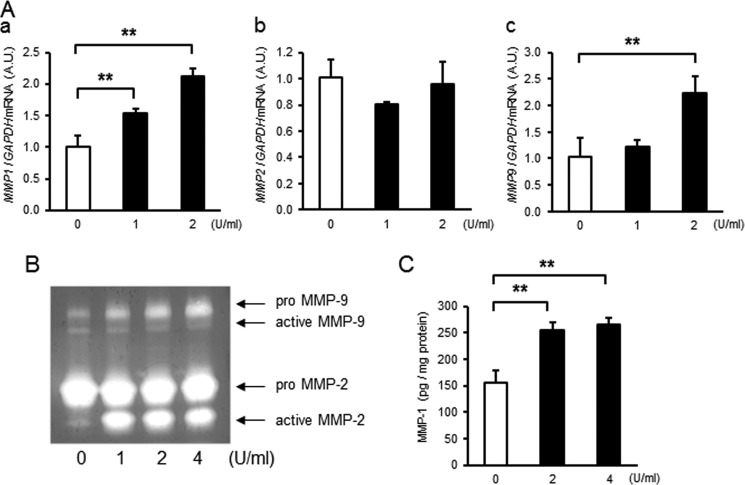
Thrombin Dose- and Time-dependently Increased MMP Activity in Primary Amnion Mesenchymal but Not Epithelial Cells
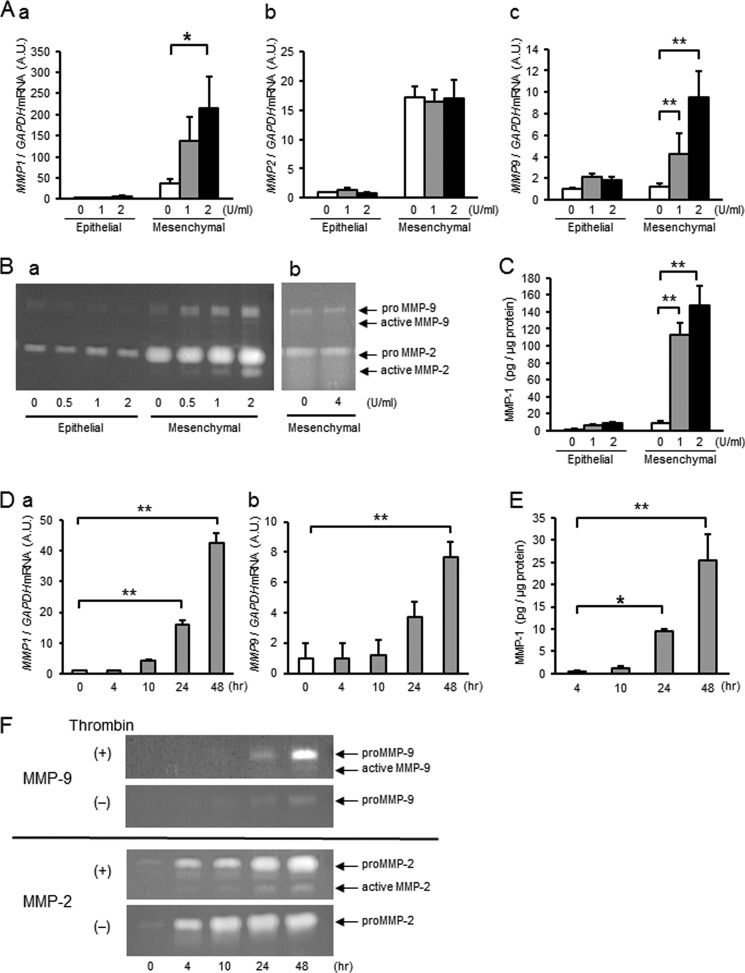
To assess the effect of thrombin on MMP-1, MMP-2, or MMP-9, primary amnion cells were treated with thrombin (0–2 units/ml) for 4–48 h, and mRNA and activity levels were quantified using quantitative PCR, FRET, or quantitative zymography (Fig. 2). Whereas MMP1 mRNA levels were low and unchanged by thrombin treatment in epithelial cells, MMP1 mRNA increased dose- and time-dependently in mesenchymal cells (Fig. 2, Aa and Da). Although not regulated in epithelial cells, thrombin induced significant increases in MMP-1 activity in a dose- and time-dependent manner in mesenchymal cells (Fig. 2, C and E). Like MMP1, basal expression of MMP2 was low in epithelial compared with mesenchymal cells. Thrombin, however, did not regulate MMP-2 gene expression in either cell type (Fig. 2Ab). Thrombin treatment resulted in increased MMP9 mRNA in mesenchymal, but not epithelial, cells (Fig. 2, Ac and Db). In agreement with mRNA levels, gelatin zymography of the conditioned media revealed that proMMP-2 was not regulated by thrombin in either cell type (Fig. 2Ba). Interestingly, however, thrombin resulted in dose- and time-dependent increases in active MMP-2 in mesenchymal cells (Fig. 2, Ba and F). Incubation of conditioned media from mesenchymal cells with thrombin (4 units/ml × 48 h) under cell-free conditions did not result in conversion of proMMP-2 to its active form, suggesting that the protease activity of thrombin was not directly involved in activation of MMP-2 (Fig. 2Bb). Although low and not changed in epithelial cells, proMMP9 was increased significantly in mesenchymal cells (Fig. 2, Ba and F). Thus, thrombin treatment results in increased expression of MMP-1 and MMP-9 mRNA and enzymatic activity in amnion mesenchymal, but not epithelial, cells. Further, although thrombin did not regulate MMP2 mRNA, treatment of mesenchymal cells with thrombin resulted in conversion of MMP-2 to its active form possibly through cleavage of the pro form by MMP-1 or MMP-9.
FIGURE 2.
Thrombin dose- and time-dependently increases MMP-1 and MMP-9 mRNA and enzyme activity in amnion mesenchymal but not epithelial cells. Primary human amnion epithelial or mesenchymal cells were treated with thrombin for the indicated dose and time. A, amnion cells were treated with different doses of thrombin for 48 h and analyzed for MMP1 (a), MMP2 (b), or MMP9 (c) mRNA. A.U., arbitrary units. B, gelatin zymography of conditioned media from cells treated with various concentrations of thrombin for 48 h (a) or cell-free incubation of conditioned media with thrombin (4 units/ml, 36 nm) for 48 h (b). C, MMP-1 enzymatic activity in conditioned media from cells treated with different doses of thrombin for 48 h. D, thrombin (2 units/ml, 18 nm)-induced increases in MMP1 (a) and MMP9 (b) mRNA as a function of time. E, MMP-1 enzymatic activity in conditioned media from amnion mesenchymal cells treated with 2 units/ml (18 nm) of thrombin as a function of time. F, gelatin zymography of conditioned media (5 μg of protein) from amnion mesenchymal cells. Upper zymogram represents MMP-9; lower, MMP-2. Cells were stimulated with 2 units/ml thrombin (+) or medium only (−) for different time periods. In A and C–E error bars represent mean ± S.D., n = 3 in each group. B and F represent results from at least three experiments. *, p < 0.05; **, p < 0.01 compared with controls (0 units/ml vehicle, A and C), 0 h (D), or 4 h thrombin treatment (E).
Thrombin Increases COX2 mRNA and PGE2 in Primary Amnion Mesenchymal Cells
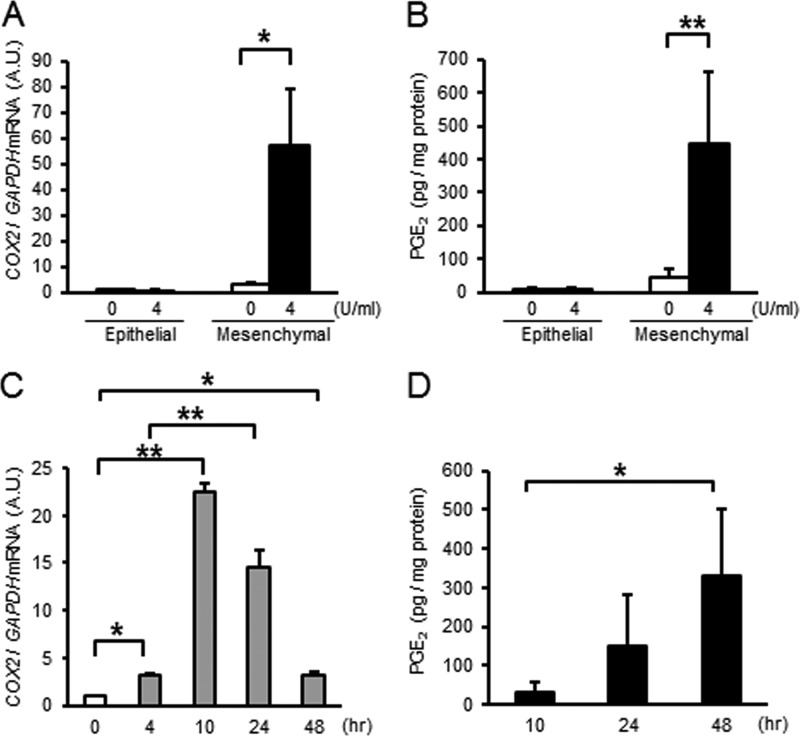
Prostaglandins are believed to be involved in PPROM, initiation of myometrial contractions of labor, and cervical ripening during parturition. Thus, we quantified the effect of thrombin on PG synthesis in primary amnion cells. In epithelial cells, COX2 mRNA was unchanged, whereas thrombin increased COX2 mRNA 10-fold in mesenchymal cells (Fig. 3, A and C). Although unchanged in the conditioned media of epithelial cells, PGE2 was increased 10-fold in media from mesenchymal cells (Fig. 3, B and D).
FIGURE 3.
Thrombin increases COX2 mRNA and PGE2 synthesis in primary amnion mesenchymal cells. Primary amnion epithelial or mesenchymal cells were treated for the indicated dose or time. A, COX2 mRNA levels in amnion cells treated with 4 units/ml (36 nm) of thrombin × 24 h. A.U., arbitrary units. B, PGE2 levels in conditioned media. Amnion cells were treated with 4 units/ml (36 nm) thrombin ×48 h. C, COX2 mRNA in amnion mesenchymal cells treated with control (medium only) or thrombin (4 units/ml, 18 nm) as a function of time. D, PGE2 in conditioned media from amnion mesenchymal cells treated with thrombin (4 units/ml, 36 nm) for the indicated times. n = 3 in each group. *, p < 0.05; **, p < 0.01 compared with control (0 units/ml, A and B), 0 h (C), or 10 h thrombin treatment (D).
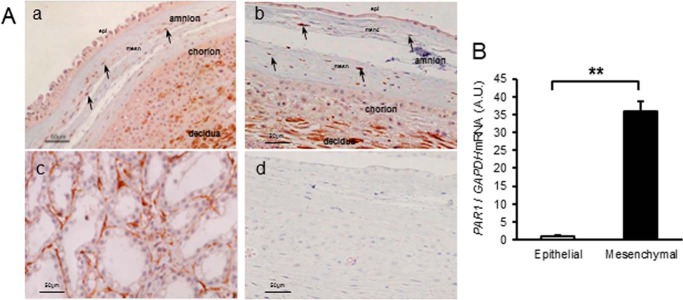
Thrombin Receptor, PAR-1, Is Expressed in Human Amnion Mesenchymal and Decidual Cells
Imunohistochemistry was used to localize expression of the major thrombin receptor, PAR-1, in human fetal membranes (Fig. 4A). PAR-1 was strongly expressed in amnion mesenchymal cells of both rupture and nonrupture sites. PAR-1 was not expressed in amnion epithelial cells. PAR-1 was also expressed in chorion fibroblasts and interestingly, highly expressed in decidua (Fig. 4A, a and b). In primary culture, PAR-1 mRNA levels were increased 30-fold in mesenchymal cells compared with very low expression in epithelial cells (Fig. 4B), compatible with the expression pattern observed using immunohistochemistry. Because PAR-1 was highly expressed in decidua, we determined the effect of thrombin on primary decidual cells (Fig. 5). Thrombin treatment of human decidua cells in culture resulted in increased MMP1 and MMP9 mRNA levels (Fig. 5A, a and c). The magnitude of stimulation, however, was much less relative to that in amnion mesenchymal cells (Fig. 2). Similar to our findings in mesenchymal cells, proMMP-2 and MMP2 mRNA were not regulated by thrombin in decidual cells (Fig. 5, Ab and B). By gelatin zymography, however, active MMP-2 was not only more abundant in decidual cells but also increased significantly by thrombin treatment (Fig. 5B). ProMMP-9 increased dose-dependently whereas active MMP-9 was not regulated in decidual cells (Fig. 5B). Thrombin also increased MMP-1 activity in decidual cells (Fig. 5C), but the effect was modest compared with its dramatic effect on amnion mesenchymal cells. Together, these results suggest that thrombin regulates MMPs mRNA and enzymatic activity in PAR-1-expressing decidual and amnion mesenchymal cells, but the magnitude of stimulation is more dramatic in mesenchymal cells.
FIGURE 4.
Thrombin receptor, PAR-1, is expressed in human amnion mesenchymal cells. A, immunostaining of PAR-1 in human fetal membranes from normal vaginal deliveries. a, nonrupture site. b, rupture site. Arrows indicate immunoreactive mesenchymal cells in amnion. c, prostate cancer as positive control. d, negative control in which mouse control IgG was used as a first antibody. Results are consistent among three fetal membranes in each group. epi, epithelial cells; mesn, mesenchymal layer. B, PAR1 mRNA levels in primary amnion cells. Results represent mean ± S.D. (error bars), n = 3 in each group. A.U., arbitrary units. **, p < 0.01.
FIGURE 5.
Effect of thrombin on primary decidual cells. A, primary decidual cells were treated with various doses of thrombin for 48 h and analyzed for relative levels of MMP1 (a), MMP2 (b), or MMP9 (c) mRNA. A.U., arbitrary units. B, gelatin zymography of conditioned media (2 μg of protein) from decidual cells treated with different doses of thrombin for 48 h. C, MMP-1 enzymatic activity in conditioned media from thrombin-treated decidual cells. Results represent mean ± S.D. (error bars), n = 3 in each group. **, p < 0.01 compared with control (0 units/ml).
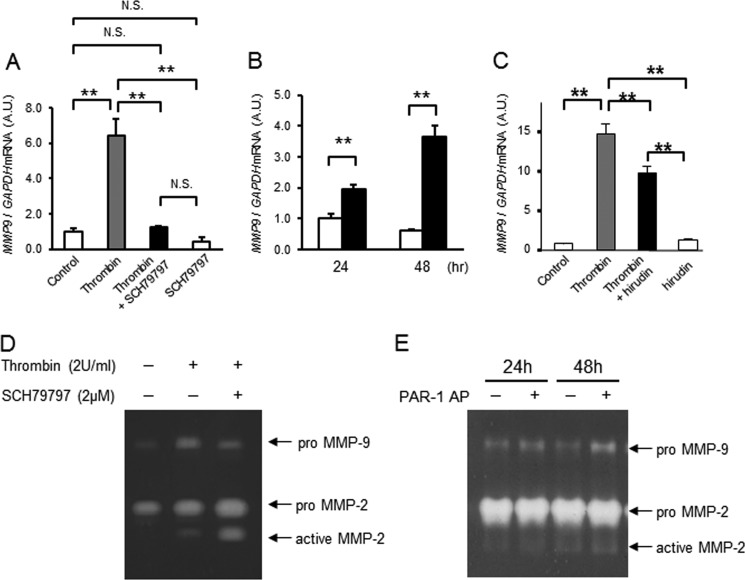
Up-regulation of MMP9 mRNA Is Mediated via PAR-1
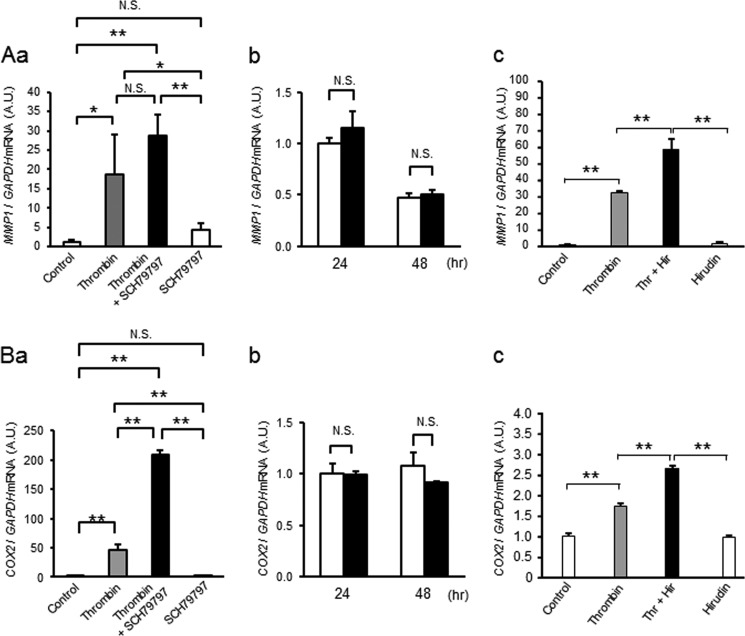
To clarify whether thrombin-induced up-regulation of MMPs and COX-2 in mesenchymal cells is mediated through PAR-1, two approaches were taken. First, we tested whether pretreatment with a PAR-1-specific inhibitor, SCH79797, blocked thrombin-induced activation of MMP gene expression. Second, we determined whether a PAR-1-activating peptide (PAR-1 AP, TFLLRN) mimicked thrombin-induced effects on these cells. The PAR-1 inhibitor completely blocked thrombin-induced increases in MMP9 mRNA, and PAR-1 AP increased MMP9 mRNA levels significantly (Fig. 6, A and B). The thrombin protease inhibitor, hirudin, also partially blocked thrombin-induced up-regulation of MMP9 (Fig. 6C). Further, by gelatin zymography, thrombin-induced increases of proMMP-9 were partially blocked by the PAR-1 inhibitor (Fig. 6D), and PAR-1 AP increased proMMP9 (Fig. 6E). Zymography also indicated that increased activation of MMP-2 was not suppressed by the PAR-1 inhibitor (Fig. 6D) nor mediated by the PAR-1-activating peptide (Fig. 6E). These data indicate that activation of PAR-1 is important for thrombin-induced increases in MMP-9. In contrast, thrombin-induced increases in MMP1 and COX2 were not suppressed by the PAR-1 inhibitor nor increased by the PAR-1 AP (Fig. 7). Rather, COX2 mRNA was increased by the addition of SCH79797 (Fig. 7Aa). Inhibition of thrombin protease activity with hirudin amplified, rather than suppressed, the effect of thrombin on MMP1 and COX2 gene expression (Fig. 7Ac). We considered the possibility that thrombin may mediate COX2 or MMP1 gene expression through PAR-2–4. MMP1, MMP9, and COX2 mRNA, however, were not increased by PAR-2- (SLIGRL-NH2 (200 μm), Anaspec), PAR-3- (TFRGAP-NH2 (200 μm), BACHEM), nor PAR-4- (AYPGKF-NH2 (200 μm), Tocris) activating peptides (data not shown).
FIGURE 6.
Thrombin-induced up-regulation of MMP-9 is mediated by PAR-1. A, effect of a PAR-1-selective receptor antagonist (SCH79797, 2 μm) and thrombin (2 units/ml, 18 nm) on MMP9 mRNA levels in amnion mesenchymal cells. Cells were pretreated with SCH79797 30 min prior to thrombin treatment. Data represent mean ± S.D. (error bars), n = 3 in each group. **, p < 0.01; N.S., not significant. A.U., arbitrary units. B, effect of control (open bars) or PAR-1 AP (TFLLRN, 200 μm, filled bars) on MMP9 mRNA in amnion mesenchymal cells treated for 24 or 48 h. Data represent mean ± S.D. (error bars, n = 3 in each group). **, p < 0.01; N.S., not significant. C, effect of control (open bars), thrombin (2 units/ml, 18 nm, gray bars), hirudin (4 units/ml, white bars), or thrombin + hirudin (filled bars) on MMP9 mRNA in amnion mesenchymal cells treated for 48 h. Data represent mean ± S.D., n = 3 in each group. **, p < 0.01. D, gelatin zymography of conditioned media of mesenchymal cells treated with thrombin (2 units/ml, 18 nm), with or without the PAR-1 antagonist SCH79797 (5 μm) for 48 h. E, gelatin zymography of conditioned media of mesenchymal cells treated with control or PAR-1 AP (TFLLRN, 200 μm) × 24 or 48 h. N.S., not significant.
FIGURE 7.
Thrombin-induced up-regulation of MMP-1 (A) and COX2 (B) is not mediated by PAR-1. Aa and Ba, effect of a PAR-1-selective receptor antagonist (SCH79797, 2 μm) and thrombin (2 units/ml, 18 nm) on MMP1 (Aa) and COX2 (Ba) mRNA levels in amnion mesenchymal cells. Cells were pretreated with SCH79797 30 min prior to thrombin treatment. Data represent mean ± S.D. (error bars), n = 3 in each group. *, p < 0.05; **, p < 0.01; N.S., not significant. A.U., arbitrary units. Ab and Bb, effect of control (open bars) or PAR-1 AP (TFLLRN, 200 μm, filled bars) on MMP1 (Ab) and COX2 (Bb) mRNA in amnion mesenchymal cells treated for 24 or 48 h. Data represent mean ± S.D. (error bars), n = 3 in each group. *, p < 0.05; **, p < 0.01; N.S., not significant. Ac and Bc, effect of control (open bars), thrombin (2 units/ml, 18 nm, gray bars), hirudin (4 units/ml, white bars), or thrombin + hirudin (filled bars) on MMP1 (Ac) and COX2 (Bc) mRNA in amnion mesenchymal cells treated for 48 h. Data represent mean ± S.D., n = 3 in each group. **, p < 0.01.
In addition to thrombin, MMPs may activate PAR-1. To determine whether MMP-1-induced activation of PAR-1 contributed to increased MMP-9 or MMP-1 mRNA in amnion mesenchymal cells, cells were treated with recombinant MMP-1 in the presence or absence of the PAR-1 inhibitor, SCH79797. MMP-1 increased MMP-9 mRNA 14-fold which was inhibited 35% by SCH79797. In contrast, MMP-1 had little effect on its own gene expression (from 1.0 ± 0.06 to 2.84 ± 0.2 relative units/GAPDH) which was not blocked by SCH79797. Together, these data suggest that up-regulation of MMP-9 was mediated by PAR-1 both directly through its own hirudin-sensitive protease activity and indirectly through MMP1. In contrast, thrombin-induced increases in MMP1 and COX2 were mediated by mechanisms other than its protease activity or activation of PARs.
Evidence That Up-regulation of MMP-1 and COX2 Is Mediated via Toll-like Receptor-4
Previously, we found that fetal fibronectin up-regulated MMP-1 and COX-2 mRNA and enzymatic activity in mesenchymal cells through extra domain A-mediated activation of TLR4 (23). To determine whether thrombin signals through TLR4, the effect of a TLR4 neutralizing antibody on thrombin-induced increases in MMP1 and COX2 mRNA was determined. Surprisingly, TLR4 neutralizing antibody completely blocked thrombin-induced increases in MMP1 and COX2 mRNA and partially blocked MMP9 mRNA (Fig. 8A, a–c). Furthermore, thrombin treatment (4 units/ml (36 nm) × 48 h) dramatically increased soluble fibronectin in conditioned media from both amnion mesenchymal cells (TLR4-responsive) and epithelial cells which do not express functional TLR4 (Fig. 8B) (23). Further, in contrast with mesenchymal cells, thrombin-induced release of fibronectin was not accompanied by increased expression of COX2 and MMP1 in epithelial cells (data not shown). PAR-1 AP did not increase release of fibronectin (Fig. 7C). Thrombin treatment also resulted in increased degradation of collagen type I α1 (Fig. 8B). Specifically, thrombin purified from human plasma resulted in reduced amounts of collagen I α1 monomers and the appearance of a 30-kDa collagen degradation product in the media, an effect reversed by hirudin. Using purified collagen type I in vitro, thrombin resulted in 50% loss of collagen type I dimers and trimers, which was rescued by hirudin. Collagen type I α1 monomers were most susceptible to thrombin with 85% degradation partially rescued to 63% by hirudin. Thus, collagen degradation is mediated by thrombin protease activity, not PAR-1 nor TLR4-induced up-regulation of MMP-1. Together, these data suggest that thrombin (i) activates PAR-1 both directly and indirectly to increase MMP-9 in amnion mesenchymal cells, (ii) activates TLR4 to increase COX-2 and MMP-1 in these cells, (iii) acts to release free fetal fibronectin from both epithelial and mesenchymal cell matrix, and (iv) converts collagen type I to its degradative products through its protease activity.
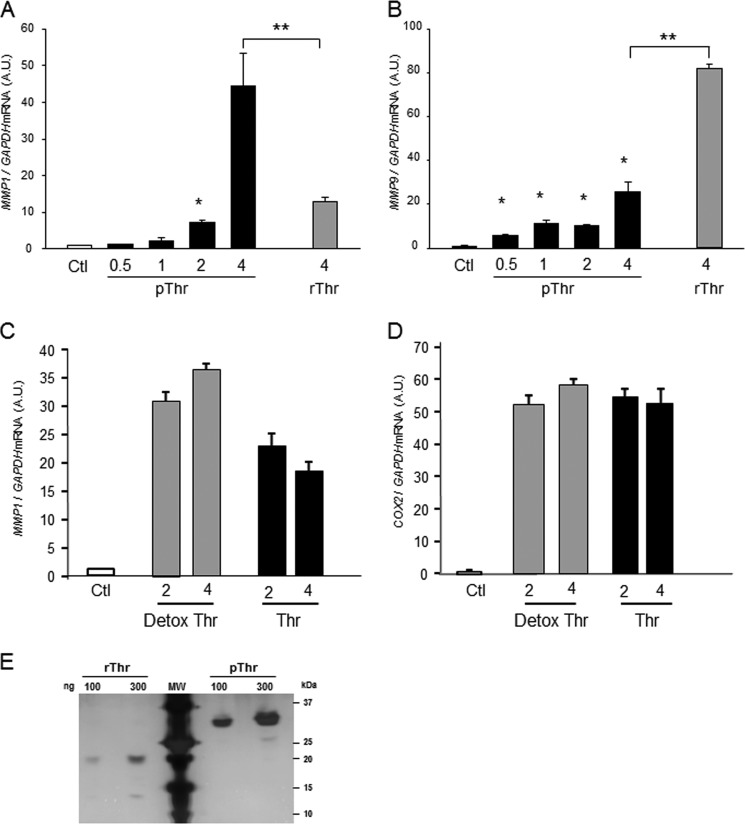
To ensure that thrombin-induced activation of TLR4 was not due to contamination with either collagenases or endotoxin, the effects of recombinant thrombin (rThr) were compared with those of plasma thrombin (pThr). Thrombin activity assays revealed that plasma thrombin was 28% greater than that of rThr. rThr was much less active in terms of collagenase activity (Fig. 8D), and induction of MMP-1 gene expression (Fig. 9A) rThr was more potent that pThr in increasing MMP9 gene expression (Fig. 9B). Although a complete dose-response analysis of rThr was not completed primarily due to its costs, concentrations of 6 units/ml rThr (54 nm) did not increase MMP1 gene expression more than 4 units/ml (36 nm). To ensure that pThr effects on TLR4 were not due to endotoxin contamination, endotoxin was removed from plasma thrombin using a Detoxi-Gel Endotoxin Removing column (24, 25). Endotoxin removal did not affect pThr-induced up-regulation of MMP1 or COX2 (Fig. 9, C and D). Taken together, therefore, pThr-induced increases in TLR4 targets were not mediated through LPS contamination; rather, rThr was less active than pThr in terms of collagenase activity, and activation of TLR4 was likely to due to changes in glycosylation. Silver-stained gels using 100 and 300 ng of purified pThr and rThr demonstrated remarkable changes in molecular mass due to changes in glycosylation (Fig. 9E).
FIGURE 9.
Comparison of pThr and rThr and endotoxin removal on MMP9, MMP1, and COX2 gene expression in amnion mesenchymal cells. A and B, cells were treated with various doses of pThr (0.5 (4.5 nm) to 4 (36 nm) units/ml or rThr (4 units/ml), and MMP1 (A) or MMP9 (B) mRNA levels were quantified after 48 h. *, p < 0.01 compared with control. A.U., arbitrary units. C and D, effect of endotoxin removal on pThr-induced MMP1 (C) and COX2 (D) mRNA. E, silver-stained gel of purified rThr and pThr. Thrombin was applied at 100 and 300 ng/lane as indicated. **, p < 0.01.
Thrombin-induced Up-regulation of MMPs, PGE2 Synthesis, and Preterm Labor in Mice
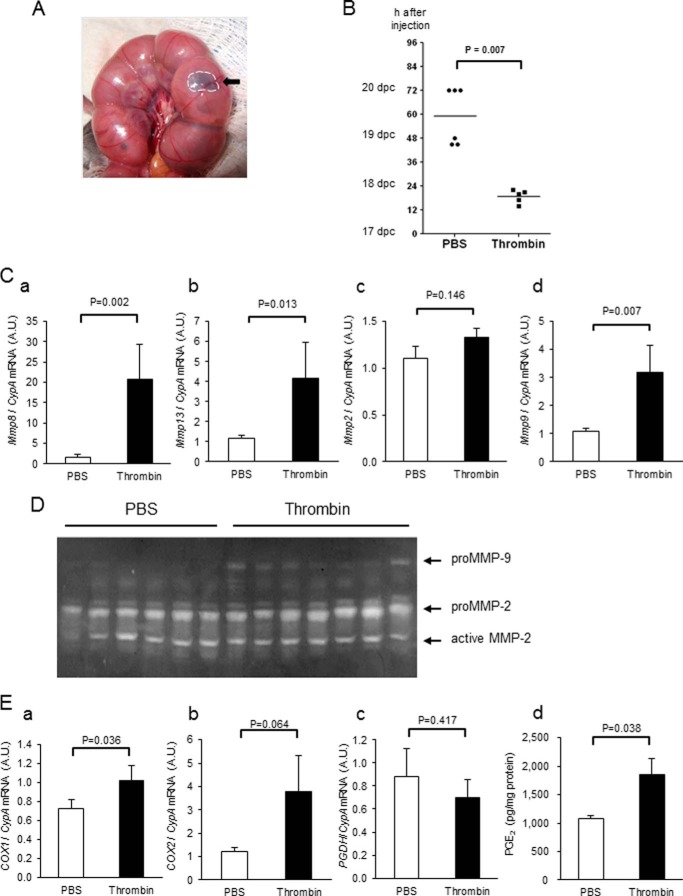
To determine the effects of thrombin in vivo, precisely timed pregnant mice were injected in the interface between fetal membranes and the uterine wall with either 4 units/fetus thrombin or PBS at 17 days postcoitum. In this model, thrombin activity increased 1.6-fold in thrombin-injected fetal membranes compared with PBS-injected controls (1.26 ± 0.08 and 1.86 ± 0.13 ng/μg protein, respectively, n = 3 in each group, p = 0.013). This dose and increase in activity were comparable with that in human preterm relative to term amnion (Fig. 1) and were thereby considered physiologically relevant. All thrombin-injected mice delivered preterm (within 24 h after injection, average 18.8 ± 1.5 h, Fig. 10B), and all pups either died or were cannibalized shortly after birth. All pups delivered, even in mice that were injected in only one horn. In contrast, PBS-injected mice delivered at term (59.0 ± 5.8 h after injection, p < 0.01, Fig. 10B) and survived. To determine whether thrombin-induced preterm labor was accompanied by activation of MMPs and COX-2 in mouse fetal membranes, membranes were collected 14 h after control or thrombin injection. Consistent with previous reports (26), MMP1 mRNA levels were almost undetectable in murine membranes (data not shown). In mice, MMP-1 is functionally substituted by other collagenases (e.g. Mmp-8 and Mmp-13) (26–29). Local injection of thrombin at the maternal-fetal interface resulted in increased mRNA levels of both collagenase-2 (Mmp8) and collagenase-3 (Mmp13) (Fig. 10C, a and d). Mmp9, but not Mmp2, mRNA also increased significantly after thrombin injection (Fig. 10C, c and b), and proMMP-9 was increased in thrombin-injected fetal membranes (Fig. 10D). COX1 and COX2 mRNA tended to increase (Fig. 10E, a and b), whereas mRNA level of 15-hydroxyprostaglandin dehydrogenase, which inactivates prostaglandins, was not changed (Fig 10Ec). PGE2 synthesis was significantly increased by thrombin (Fig. 10Ed). Thus, we confirmed that injection of thrombin at the maternal-fetal interface resulted in increased collagenase gene expression (Mmp8 and Mmp13), Mmp9 mRNA, and enzymatic activity, and PGE2 synthesis in vivo.
FIGURE 10.
Effect of thrombin on gestational length, MMPs, and PGE2 in fetal membranes. Thrombin (1 unit/μl, 4 unit/fetus) or PBS (4 μl) with 1% methylene blue was injected in the interface between fetal membranes and uterus in pregnant mice on 17 days postcoitum (dpc). A, mouse uterus after injection. Injected-reagent (thrombin or PBS) was localized in the interface between fetal membranes and the uterine wall. B, time to delivery after thrombin injection. Black bar indicates the mean. Mice were injected with either PBS (n = 6) or thrombin (n = 5), and time of delivery after injection was recorded. C, Mmp8 (a), Mmp13 (b), Mmp2 (c), and Mmp9 (d) mRNA levels in fetal membranes collected 14 h after injection of PBS or thrombin. Data represent mean ± S.E. (error bars), n = 10 in each group. A.U., arbitrary units. D, gelatin zymography of protein (14 μg/lane) from fetal membranes collected 14 h after injection of PBS or thrombin. E, COX1 mRNA (a), COX2 mRNA (b), 15-hydroxyprostaglandin dehydrogenase mRNA (c), and PGE2 content (d) in fetal membranes 14 h after injection of pregnant mice with PBS or thrombin. n = 10 in each group.
DISCUSSION
In this investigation, we confirmed the effects of thrombin on MMP-1 in decidual cells and extended these findings to show that MMP-2 and MMP-9 were also activated. Relative to decidual cells, however, the effects of thrombin were more pronounced in mesenchymal cells of the amnion. We suggest, therefore, that thrombin-induced activation of MMPs in both cell types may synergistically contribute to degradation of collagen in the fetal membranes.
Thrombin activity was increased in amnion from pregnancies complicated by preterm delivery compared with term delivery. In normal uncomplicated pregnancy, amniotic fluid concentrations of thrombin-antithrombin III complexes are decreased in the mid-trimester than at term (3). Thus, the increased thrombin activity in amnions from women with preterm labor is likely the result of pathological change and suggests that thrombin may be involved in the pathophysiology of preterm labor.
Thrombin up-regulates MMPs in fetal membrane organ culture (11, 30, 31). In this study, we used primary amnion cells to clarify that mesenchymal, not epithelial, cells are involved in thrombin-induced up-regulation of collagenase, MMP-1, as well as the gelatinases MMP-2 and MMP-9. Thrombin-induced increases in MMP-1 and MMP-9 were associated with increased mRNA transcripts for MMP1 and MMP9, whereas MMP-2 was activated at the post-transcriptional level. Thrombin also increased COX2 mRNA as well as PGE2 synthesis in amnion mesenchymal cells. Consistent with previous reports (32), amnion mesenchymal cells were the major source of PGE2 in amnion. Thrombin further up-regulated PGE2 synthesis in amnion mesenchymal cells. These findings suggest that thrombin-mediated increases in PGE2 synthesis may lead to uterine contractions and cervical ripening of preterm labor. Prostaglandin-inactivating enzymes in chorion or cervix (i.e. 15-hydroxyprostaglandin dehydrogenase) may prevent delivery of bioactive prostaglandins to these sites. Nonetheless, thrombin is a direct activator of uterine contractility (33), and our findings of thrombin-induced preterm birth in mice suggest that thrombin induces preterm birth either directly or indirectly through prostaglandin biosynthesis.
Use of a PAR-1-selective inhibitor and activating peptide indicated that thrombin-induced up-regulation of MMP-9 in mesenchymal, but not epithelial, cells was mediated by PAR-1. The results are consistent with our findings that PAR-1 receptors were localized to mesenchymal cells. Activation of PAR-1 was mediated both directly through its hirudin-sensitive protease activity and indirectly through MMP-1-induced activation of PAR-1. In contrast with MMP-9, thrombin-induced increases in MMP1 and COX2 in amnion mesenchymal cells were not mediated by activation of PAR-1. Our previous results indicated that the extra domain A of fetal fibronectin up-regulated MMP-1 and COX-2 through activation of TLR4 in amnion mesenchymal cells (23). Hence, we suspected that thrombin also sent signals via TLR4. TLR4 neutralizing antibody completely blocked thrombin-induced increases in MMP1 and COX2 mRNA. Further, thrombin-induced increases in these TLR4 target genes were not mitigated by endotoxin removal, and endotoxin-free recombinant thrombin also increased MMP1 and COX2. Interestingly, we found that thrombin also stimulated release of fibronectin from its cell-bound fibrillar form to its soluble form, which is known to activate TLR4 (23). Thrombin-induced release of fibronectin occurred even in epithelial cells with nonfunctioning TLR4 complexes, indicating that thrombin-induced activation of MMP1 was not required. Although multiple mechanisms may be involved, these findings suggest that solubilization of fetal fibronectin plays an important role in thrombin-induced activation of TLR4 and thereby up-regulation of MMP-1 and COX-2 in amnion mesenchymal cells. Our results indicate that glycosylated mature pThr is more active that nonglycoslyated rThr in terms of TLR4 activation and collagenase activity. Further, pThr is a more potent collagenase that rThr. The experiments, therefore, indicate that several mechanisms are involved in thrombin-induced effects on human mesenchymal cells, including activation of PAR-1 to increase MMP-9, release of fibronectin from epithelial and mesenchymal matrix, and TLR4-mediated activation of MMP1 and COX-2 in mesenchymal cells. The results are compatible with those of Puthiyachirakkal et al. in which thrombin directly weakened devitalized fetal membranes through its protease activity (34).
Thrombin caused preterm labor in pregnant mice, up-regulating MMP expression and PGE2 synthesis in murine fetal membranes. Thus, thrombin-induced up-regulation of collagenases, gelatinase, and PGE2 in vivo supports our data obtained in human amnion cells in vitro.
In summary, these studies indicate that thrombin, a multifunctional serine protease, plays a pathologic role in preterm labor and PPROM, particularly in amnion mesenchymal cells. We suggest that development of drugs or strategies to block the actions of thrombin or inhibit serine (i.e. thrombin) and MMP protease activity in tissues at the maternal-fetal interface may contribute to successful prevention and treatment of preterm rupture of the fetal membranes and preterm birth.
Acknowledgments
We thank the physicians and staff of Parkland Memorial Hospital, and Valencia Hoffman for valuable assistance in tissue procurement and Dr. John J. Moore (Case Western Reserve University School of Medicine) for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant HD11149. This work was also supported by a fellowship from SUMITOMO Life Social Welfare Service Foundation and the Human Tissue and Biologic Fluid Core Laboratory.
- PPROM
- preterm premature rupture of membrane
- PAR-1
- protease-activated receptor-1
- PAR-1 AP
- PAR-1-activating peptide
- PGE2
- prostaglandin E2
- PROM
- premature rupture of membranes
- pThr
- plasma thrombin
- rThr
- recombinant thrombin
- TLR4
- Toll-like receptor 4.
REFERENCES
- 1. Goldenberg R. L., Culhane J. F., Iams J. D., Romero R. (2008) Epidemiology and causes of preterm birth. Lancet 371, 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaiworapongsa T., Espinoza J., Yoshimatsu J., Kim Y. M., Bujold E., Edwin S., Yoon B. H., Romero R. (2002) Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J. Matern. Fetal Neonatal Med. 11, 368–373 [DOI] [PubMed] [Google Scholar]
- 3. Erez O., Romer R., Vaisbuch E., Chaiworapongsa T., Kusanovic J. P., Mazaki-Tovi S., Gotsch F., Gomez R., Maymon E., Pacora P., Edwin S. S., Kim C. J., Than N. G., Mittal P., Yeo L., Dong Z., Yoon B. H., Hassan S. S., Mazor M. (2009) Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J. Matern. Fetal Neonatal Med. 22, 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen T., Kuczynski E., O'Neill L. M., Funai E. F., Lockwood C. J. (2001) Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J. Matern Fetal Med. 10, 297–300 [DOI] [PubMed] [Google Scholar]
- 5. Nagy S., Bush M., Stone J., Lapinski R. H., Gardó S. (2003) Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet. Gynecol. 102, 94–100 [DOI] [PubMed] [Google Scholar]
- 6. Funderburk S. J., Guthrie D., Meldrum D. (1980) Outcome of pregnancies complicated by early vaginal bleeding. Br. J. Obstet. Gynaecol. 87, 100–105 [DOI] [PubMed] [Google Scholar]
- 7. Signore C. C., Sood A. K., Richards D. S. (1998) Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am. J. Obstet. Gynecol. 178, 336–340 [DOI] [PubMed] [Google Scholar]
- 8. Lockwood C. J., Krikun G., Papp C., Toth-Pal E., Markiewicz L., Wang E. Y., Kerenyi T., Zhou X., Hausknecht V., Papp Z. (1994) The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann. N.Y. Acad. Sci. 734, 57–79 [DOI] [PubMed] [Google Scholar]
- 9. Rosen T., Schatz F., Kuczynski E., Lam H., Koo A. B., Lockwood C. J. (2002) Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J. Matern. Fetal Neonatal Med. 11, 11–17 [DOI] [PubMed] [Google Scholar]
- 10. Mackenzie A. P., Schatz F., Krikun G., Funai E. F., Kadner S., Lockwood C. J. (2004) Mechanisms of abruption-induced premature rupture of the fetal membranes: thrombin enhanced decidual matrix metalloproteinase-3 (stromelysin-1) expression. Am. J. Obstet. Gynecol. 191, 1996–2001 [DOI] [PubMed] [Google Scholar]
- 11. Kumar D., Schatz F., Moore R. M., Mercer B. M., Rangaswamy N., Mansour J. M., Lockwood C. J., Moore J. J. (2011) The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro. Placenta 32, 206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parry S., Strauss J. F., 3rd (1998) Premature rupture of the fetal membranes. N. Engl. J. Med. 338, 663–670 [DOI] [PubMed] [Google Scholar]
- 13. Maymon E., Romero R., Pacora P., Gervasi M. T., Bianco K., Ghezzi F., Yoon B. H. (2000) Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 183, 914–920 [DOI] [PubMed] [Google Scholar]
- 14. Vadillo-Ortega F., González-Avila G., Furth E. E., Lei H., Muschel R. J., Stetler-Stevenson W. G., Strauss J. F., 3rd (1995) 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am. J. Pathol. 146, 148–156 [PMC free article] [PubMed] [Google Scholar]
- 15. Athayde N., Edwin S. S., Romero R., Gomez R., Maymon E., Pacora P., Menon R. (1998) A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am. J. Obstet. Gynecol. 179, 1248–1253 [DOI] [PubMed] [Google Scholar]
- 16. Draper D., McGregor J., Hall J., Jones W., Beutz M., Heine R. P., Porreco R. (1995) Elevated protease activities in human amnion and chorion correlate with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 173, 1506–1512 [DOI] [PubMed] [Google Scholar]
- 17. Challis J. R., Sloboda D. M., Alfaidy N., Lye S. J., Gibb W., Patel F. A., Whittle W. L., Newnham J. P. (2002) Prostaglandins and mechanisms of preterm birth. Reproduction 124, 1–17 [DOI] [PubMed] [Google Scholar]
- 18. Duchesne M. J., Thaler-Dao H., de Paulet A. C. (1978) Prostaglandin synthesis in human placenta and fetal membranes. Prostaglandins 15, 19–42 [DOI] [PubMed] [Google Scholar]
- 19. Casey M. L., MacDonald P. C. (1996) Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol. Reprod. 55, 1253–1260 [DOI] [PubMed] [Google Scholar]
- 20. Braverman M. B., Bagni A., de Ziegler D., Den T., Gurpide E. (1984) Isolation of prolactin-producing cells from first and second trimester decidua. J. Clin. Endocrinol. Metab. 58, 521–525 [DOI] [PubMed] [Google Scholar]
- 21. Wieslander C. K., Marinis S. I., Drewes P. G., Keller P. W., Acevedo J. F., Word R. A. (2008) Regulation of elastolytic proteases in the mouse vagina during pregnancy, parturition, and puerperium. Biol. Reprod. 78, 521–528 [DOI] [PubMed] [Google Scholar]
- 22. Rahn D. D., Acevedo J. F., Roshanravan S., Keller P. W., Davis E. C., Marmorstein L. Y., Word R. A. (2009) Failure of pelvic organ support in mice deficient in fibulin-3. Am. J. Pathol. 174, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mogami H., Kishore A. H., Shi H., Keller P. W., Akgul Y., Word R. A. (2013) Fetal fibronectin signaling induces matrix metalloproteases and cyclooxygenase-2 (COX-2) in amnion cells and preterm birth in mice. J. Biol. Chem. 288, 1953–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Issekutz A. C. (1983) Removal of Gram-negative endotoxin from solutions by affinity chromatography. J. Immunol. Methods 61, 275–281 [DOI] [PubMed] [Google Scholar]
- 25. Adam O., Vercellone A., Paul F., Monsan P. F., Puzo G. (1995) A nondegradative route for the removal of endotoxin from exopolysaccharides. Anal. Biochem. 225, 321–327 [DOI] [PubMed] [Google Scholar]
- 26. Balbín M., Fueyo A., Knäuper V., López J. M., Alvarez J., Sánchez L. M., Quesada V., Bordallo J., Murphy G., López-Otín C. (2001) Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J. Biol. Chem. 276, 10253–10262 [DOI] [PubMed] [Google Scholar]
- 27. Balbín M., Fueyo A., Knäuper V., Pendás A. M., López J. M., Jiménez M. G., Murphy G., López-Otin C. (1998) Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes: analysis of its potential role in postpartum involution of the uterus. J. Biol. Chem. 273, 23959–23968 [DOI] [PubMed] [Google Scholar]
- 28. Hartenstein B., Dittrich B. T., Stickens D., Heyer B., Vu T. H., Teurich S., Schorpp-Kistner M., Werb Z., Angel P. (2006) Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice. J. Invest. Dermatol. 126, 486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu N., Jansen E. D., Davidson J. M. (2003) Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J. Invest. Dermatol. 121, 926–932 [DOI] [PubMed] [Google Scholar]
- 30. Moore R. M., Schatz F., Kumar D., Mercer B. M., Abdelrahim A., Rangaswamy N., Bartel C., Mansour J. M., Lockwood C. J., Moore J. J. (2010) α-Lipoic acid inhibits thrombin-induced fetal membrane weakening in vitro. Placenta 31, 886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stephenson C. D., Lockwood C. J., Ma Y., Guller S. (2005) Thrombin-dependent regulation of matrix metalloproteinase (MMP)-9 levels in human fetal membranes. J. Matern. Fetal Neonatal Med. 18, 17–22 [DOI] [PubMed] [Google Scholar]
- 32. Economopoulos P., Sun M., Purgina B., Gibb W. (1996) Glucocorticoids stimulate prostaglandin H synthase type-2 (PGHS-2) in the fibroblast cells in human amnion cultures. Mol. Cell. Endocrinol. 117, 141–147 [DOI] [PubMed] [Google Scholar]
- 33. Elovitz M. A., Saunders T., Ascher-Landsberg J., Phillippe M. (2000) Effects of thrombin on myometrial contractions in vitro and in vivo. Am. J. Obstet. Gynecol. 183, 799–804 [DOI] [PubMed] [Google Scholar]
- 34. Puthiyachirakkal M., Lemerand K., Kumar D., Moore R., Philipson E., Mercer B. M., Mansour J. M., Hauguel-de Mouzon S., Moore J. J. (2013) Thrombin weakens the amnion extracellular matrix (ECM) directly rather than through protease-activated receptors. Placenta 34, 924–931 [DOI] [PubMed] [Google Scholar]