Background: The cell adhesion molecule L1 plays important roles in the developing and adult nervous system.
Results: L1 is cleaved by myelin basic protein (MBP) yielding a L1 fragment that promotes L1-dependent functions.
Conclusion: L1 functions in the nervous system depend on MBP.
Significance: Study of the L1 and MBP functions may contribute to understanding the pathogenesis of demyelinating and neurodegenerative diseases.
Keywords: Carbohydrate, Carbohydrate-binding Protein, Cell Adhesion, Cell Death, Cell Surface, Myelin, Neurite Outgrowth, Neurobiology, Protease, Protein Processing
Abstract
The cell adhesion molecule L1 is a Lewisx-carrying glycoprotein that plays important roles in the developing and adult nervous system. Here we show that myelin basic protein (MBP) binds to L1 in a Lewisx-dependent manner. Furthermore, we demonstrate that MBP is released by murine cerebellar neurons as a sumoylated dynamin-containing protein upon L1 stimulation and that this MBP cleaves L1 as a serine protease in the L1 extracellular domain at Arg687 yielding a transmembrane fragment that promotes neurite outgrowth and neuronal survival in cell culture. L1-induced neurite outgrowth and neuronal survival are reduced in MBP-deficient cerebellar neurons and in wild-type cerebellar neurons in the presence of an MBP antibody or L1 peptide containing the MBP cleavage site. Genetic ablation of MBP in shiverer mice and mutagenesis of the proteolytically active site in MBP or of the MBP cleavage site within L1 as well as serine protease inhibitors and an L1 peptide containing the MBP cleavage site abolish generation of the L1 fragment. Our findings provide evidence for novel functions of MBP in the nervous system.
Introduction
Development of the nervous system and its correct functioning in the adult depend on many cellular features, such as cell proliferation and migration, neuronal survival and programmed cell death, neuritogenesis, synapse formation, and synaptic plasticity. The protein backbones and distinct carbohydrate structures of cell adhesion molecules participate in these important cellular events (1–3). In particular, recognition molecules at the cell surface and in the extracellular matrix contribute to these events, and among these the immunoglobulin superfamily member L1 has been implicated in all these functions. L1 is a transmembrane cell surface glycoprotein and is not only a glycan-carrying but also a glycan binding cell adhesion molecule (4–7) that plays a crucial role in neuronal cell migration and survival, neurite outgrowth, axon guidance and fasciculation, myelination, synaptic plasticity, and regeneration after injury (1, 2, 8–10). In addition, it is associated with neural disorders, such as the L1 syndrome, fetal alcohol syndrome, and schizophrenia as well as Alzheimer, Parkinson, Huntington, and Hirschsprung diseases (2, 9–15). Besides other functionally important glycan residues, L1 carries the Lewisx glycan epitope (Lex),3 which has been implicated in cell adhesion and migration as well as neurite outgrowth (6, 16–18).
In search of binding partners for the Lex glycan, we identified myelin basic protein (MBP) as a Lex-binding protein and show that it interacts with L1 in a Lex-dependent manner. MBP is a major structural component of mature myelin and is implicated in formation, compaction, and maintenance of myelin (19, 20). Different MBP isoforms originate from a gene complex called golli (gene in the oligodendrocyte lineage), which in mice comprises 11 exons and includes the 7 exons coding for the so-called classic MBP proteins (19, 21, 22). Golli and MBP proteins are generated from different transcription start sites and are encoded by different exons (19, 21, 22). The different MBP proteins are expressed in the central and peripheral nervous system, whereas the golli proteins are found in both the immune and nervous system but not in compact myelin. The importance of MBP proteins for myelination is well exemplified by the severe phenotype of the naturally occurring MBP-deficient shiverer mutant mouse, which lacks most of the compact myelin structures in the central nervous system (23, 24).
In trying to identify the molecular binding partners of L1 on myelin-forming cells, we found abnormalities in L1 proteolytic processing in the shiverer mouse mutant. We, therefore, undertook a more detailed investigation of the relationship between neuronal L1 and myelin forming glial cells, with a particular view on MBP. Using a phage display approach, we found that MBP binds to Lex and to Lex carrying L1 from mouse brain but not to recombinantly expressed L1 devoid of Lex. Binding of MBP to L1 leads to proteolytic cleavage of L1 at Arg687. This proteolytic cleavage induces L1-mediated functions in vitro, such as L1-dependent neurite outgrowth and neuronal survival. Our findings describing these novel functions of MBP point to more direct roles of this protein in the pathogenesis of demyelinating and neurodegenerative diseases.
EXPERIMENTAL PROCEDURES
Animals
L1-deficient mice (25) and MBP-deficient shiverer mice (23, 26) were kept as heterozygous breeding pairs. L1-deficient mice were maintained on a mixed genetic background (129SVJ × C57BL/6 × Black Swiss), and MBP-deficient shiverer mice were maintained on an inbred C57BL/6J background. In experiments using L1- or MBP-deficient mice, wild-type littermates were used. In all other experiments C57BL/6J mice were used. All experiments were conducted in accordance with the German and European Community laws on protection of experimental animals and were approved by the responsible committee of The State of Hamburg.
Antibodies and Reagents
L1 antibody 172-R was from HISS Diagnostics. Pan-MBP antibody (sc-13914) and dynamin I antibody (sc-12724) were purchased from Santa Cruz Biotechnology, and βIII-tubulin antibody (PRB-435P) was from Covance. The MBP antibody against the exon II-encoded domain (27) was kindly provided by David Colman (Montreal Neurological Institute and Hospital of McGill University, Montreal, Canada). Polyclonal L1 antibody, L1 antibodies 555 and 557, antibody L3 against oligomannoses, antibody L5 against Lex, and antibody HNK-1 against the HNK-1 glycan have been described (4, 7, 28, 29). Carbohydrates and glycoconjugates were from Dextra Laboratories, PNGase F was from Roche Diagnostics, and α-(1–3,4)-fucosidase from QA-Bio. Synthetic peptides were from Schafer-N, and MBP purified from bovine brain was from AbD Serotec (#6420-0100). Secondary antibodies were from Dianova, and streptavidin coupled to horseradish peroxidase (HRP) was obtained from Thermo Fisher Scientific.
Phage Display
Phage display screening of a Pre-Made T7 Select Library of normal human brain tissue (Novagen; catalogue number 70637-7) was performed as described in the manufacturer's protocol using BSA-Lex or BSA-Lea in PBS (100 μg/ml) as substrate-coated baits and 100 μg/ml antibody L5 in PBS for elution. After four rounds of panning, DNA was isolated from individual clones and sequenced.
Isolation of L1 and Myelin, Western Blot Analysis, and Cell Surface Biotinylation
Western blot analysis, cell surface biotinylation, immunopurification of L1 from early postnatal mouse brains, and isolation of crude myelin from adult mouse brains were described (7, 30, 31).
Purification and Treatments of Brain L1
L1 was immunopurified from early postnatal mouse brains (7). For N-deglycosylation, brain L1 was denatured at 95 °C in 1% SDS and 20 mm sodium phosphate (pH 7.2), diluted 1:10, and incubated overnight with 0.1 units of PNGase F and 0.5% CHAPS at 37 °C. For defucosylation, it was resuspended in 250 mm sodium phosphate (pH 5.0) and incubated with 2 milliunits of α-(1–3,4)-fucosidase overnight at 37 °C. For the protease assay, 1 μg of brain L1 and 2 μg of bovine MBP were incubated overnight at 37 °C in 30 μl of PBS containing 1 mm CaCl2 and 1 mm MgCl2 without or with 15 μg of aprotinin (5.4 trypsin inhibitory units/mg).
Binding Assays
ELISA using 5 μg/ml brain L1, L1-Fc, or MBP in TBS as substrate coats, different concentrations of MBP, brain L1, or L1-Fc in PBS as soluble binding partners, and L1 antibody 555 or pan-MBP antibody, HRP-coupled secondary antibodies (Dianova), and o-phenylenediamine dihydrochloride (Thermo Fisher Scientific) for detection of binding was described (7). Peak wavelength shift of reflected light was measured in a label-free binding assay using BIND Technology (SRU Biosystems), a 384-well plate with a TiO2 biosensor surface for substrate-coating, and soluble binding partners (32).
Immunocytochemistry
Dissociated murine cerebellar neurons were prepared from 6–8-day-old mice and maintained on poly-l-lysine for 24 h in serum-containing medium. For MBP surface immunostaining, pan-MBP antibody was added to live cells and incubated for 30 min at 4 °C. For double immunostaining of MBP and L1, cells were incubated with serum-free medium for 4 h, with L1 antibody 557 or 555 for 30 min, and with pan-MBP antibody for 20 min at 37 °C. After fixation with 4% paraformaldehyde for 30 min and blocking with 2% BSA in PBS for 1 h at room temperature, cells were incubated with Cy2- or Cy3-conjugated secondary antibodies in PBS for 2 h at room temperature. For double immunostaining of MBP and βIII-tubulin, fixed cells were incubated in 2% BSA, 0.3% Triton X-100 in PBS for 1 h at room temperature followed by incubation with pan-MBP and βIII-tubulin antibodies in PBS for 2 h at room temperature and with Cy2- or Cy3-conjugated secondary antibodies in PBS for 1 h at room temperature. Coverslips were embedded in Aqua-Poly/Mount (Polysciences) or Roti®-Mount FluorCare DAPI (Carl Roth), and confocal images were taken with an Olympus Fluoview FV1000 confocal laser scanning microscope.
Neurite Outgrowth and Neuronal Cell Survival
Preparation and analysis of cerebellar neurons from 6–8-day-old mice were described, and cells were cultured in chemically defined serum-free medium (33). Neurite outgrowth was quantified 24 h after seeding by measuring total neurite length of at least 100 neurons per condition using an AxioVision system 4.6 (Carl Zeiss). Glycans (10 μm), Lex peptide (16) and scrambled peptide (100 μg/ml), glycan-specific antibodies (10 μg/ml), and aprotinin (0.03 trypsin inhibitory units/ml) were added 1 h (neurite outgrowth) or 15 h (cell survival) after cell seeding. For induction of cell death, hydrogen peroxide (10 μm) or sodium glutamate (100 μm) was added 16 h after plating. After an additional 24 h, cells were treated with 1 μg/ml calcein AM (Molecular Probes) and 1 μg/ml propidium iodide (Sigma) for 1 h at 37 °C. Viability of cells was assessed by counting the numbers of calcein versus propidium iodide-positive cells. Twelve randomly chosen areas of a microscopic field (20× magnification) from three wells per treatment and experiment were counted.
Site-directed Mutagenesis of L1 and Transfection of HEK293 Cells
Site-directed mutagenesis and transfection of HEK293 cells were described (9). For mutation of R687A (L1R/A) or of F686L/R687A (L1FR/LA), the following primers were used: fwR/A (5′-CCC TAT GTC CAC TAC ACC TT T GCG GTC ACT GCC ATT AAC AAA TAT-3′) and revR/A (5′-GTT AAT GGC AGT GAC CGC AAA GGT GTA GTG GAC ATA GGG GGA CAG-3′); fwFR/LA (5′-CCC TAT GTC CAC TAC ACC CTT GCG GTC ACT GCC ATT AAC AAA TAT-3′) and revFR/LA (5′-GTT AAT GGC AGT GAC CGC AAG GGT GTA GTG GAC ATA GGG GGA CAG-3′).
Site-directed Mutagenesis of MBP and Transduction of Neurons with Red Fluorescent Protein (RFP)-MBP-encoding Adeno-associated Virus 1 (AAV1) Vectors
For site-directed mutagenesis of Ser139 and Ser176 in MBP to Ala139 and Ala176, the following primers and a vector coding for RFP N-terminally fused to the murine 21.5-kDa MBP (34) were used: fw139 (5′-GGC CTG TCC CTC AGC AGA TTT GCC TGG GGG GCC GAG GGG CAG AAG CCA G-3′) and rev139 (5′-CCC CTC GGC CCC CCA GGC AAA TCT GCT GAG GGA CAG GCC TCT CCC-3′); fw179 (5′-TAC GAC GCC CAG GCC ACG CTT GCC AAA ATC TTT AAG CTG GGA GGA-3′) and rev179 (5′-CAG CTT AAA GAT TTT GGC AAG CGT GCC CTG GGC GTC GTA GGC CCC-3′).
For subcloning of RFP-tagged wild-type and mutated MBP into pAAV-MCS vector (Cellbiolabs; CMV promoter) via an SalI restriction site using the InFusion Cloning kit (Clontech), PCR amplification with Phusion Polymerase (New England Biolabs) and the following primers was performed: fw (5′-ATCCTCTAGAGTCGAC ATG GTG AGC AAG GGC GAG GAG-3′) and rev (5′-GCTTCTGCAGGTCGAC TCA GCG TCT CGC CAT GGG-3). AAV1 pseudotyped vectors were generated by co-transfection of HEK293-AAV cells (Cellbiolabs) with the particular pAAV transfer plasmid (Cellbiolabs) and the AAV packaging plasmid pDP1rs (a kind gift from Jürgen Kleinschmidt, DKFZ Heidelberg, Germany), which provides the AAV2 rep and AAV1 cap genes and adenoviral helper functions (35). Generation of recombinant AAV1 particles was carried out as described previously (36) with some modifications. Briefly, 1 × 107 HEK293-AAV cells were transfected with 17.5 μg of pDP1rs and 4.5 μg of pAAV plasmid per plate complexed with Max-polyethyleneimine (PEI, Polysciences) at a PEI:DNA ratio of 3:1 (37). After 72 h cells were harvested, washed 3 times with PBS, and resuspended in 5 ml of lysis buffer (50 mm Tris base, 150 m NaCl, 5 mm MgCl2 (pH 8.5)). After three freeze-thaw cycles, lysates were incubated with 250 units/ml Benzonase (Merck) for 1 h at 37 °C. Cell debris was pelleted, and vector containing lysates were purified using iodixanol step gradients and ultrafiltration (Amicon: Ultra Cartridges, 50 Mr cutoff). The genomic titers of DNase-resistant recombinant AAV particles were determined after alkaline treatment and subsequent neutralization by quantitative PCR (50 °C for 2 min and 95 °C for 10 min followed by 35 cycles of 95 °C for 15 s and 60 °C for 60 s) using the SYBR Green qPCR Master MIX 2 (ThermoScientific), CMV promoter sequences (5′-GGCGGAGTTGTTACGACAT-3′ and 5′-GGGACTTTCCTACTTGGCA-3′), and an ABI PRISM® 7900HT cycler (Applied Biosystems). pAAV-MCS plasmid was used as a copy number standard, and calculations were done using the SDS 2.4 software (Applied Biosystems).
For viral transduction, AAV1 carrying RFP-tagged wild-type and mutant MBP were incubated at a 1000-fold multiplicity of infection with MBP-deficient cerebellar neurons for 24 h in culture medium (Neurobasal A supplemented with 10% fetal calf serum, 1 mm sodium pyruvate, 2 mm l-glutamine, 4 nm l-tyrosine, 1× B27 supplement, 10 μg/ml BSA, 100 μg/ml transferrin, 10 μg/ml insulin, 10 ng/ml selenium, 50 units/ml penicillin, and 50 units/ml streptomycin).
In Situ Hybridization
The templates for the synthesis of cRNA hybridization probes were amplified by PCR using the vector coding for murine 21.5-kDa MBP (34), the forward primers 5′-TAATACGACTCACTATAGGGAGA atg gca tca cag aag aga ccc tc-3′ or 5′-TAATACGACTCACTATAGGGAGA gtg aca cct cga aca cca cct cc-3′, both containing the T7 promoter (capital letters), and the reverse primers 5′-tca gcg tct cgc cat ggg aga tc-3′ or 5′-cag ctt aaa gat ttt gga aag cgt gcc-3′. For the preparation of the fluorescently labeled probes the FISH TagTM RNA kit (Invitrogen) and Alexa Fluor® 488 dye were used, and hybridization was done as suggested in the manufacturer's manual.
In Utero Injection of Viral Particles and Explant Culture
After exposure of the uterine horns, AAV1 carrying wild-type MBP or mutated MBP (1 μl; 4.5 × 105 virus particles) were in utero-injected into the brain ventricle system of 13.5-day-old shiverer embryos. On postnatal day 5.5, cerebellar explant cultures were prepared and analyzed as described (10). Briefly, explants were maintained on Matrigel (BD Biosciences) diluted 1:3 in serum-free medium for 1 h followed by incubation in serum-free medium, fixation, and staining. Total neurite lengths per explant and the number of migrating cells for 10 explants per group and animal were determined using the OLYMPUS FV1000 imaging system (Olympus, Hamburg, Germany) and AxioVision 4.6 software (Carl Zeiss, Oberkochen, Germany).
RESULTS
Identification of MBP as a Lex-binding Protein
To identify novel neural Lex binding partners, we screened a phage display library of human brain proteins using BSA-conjugated Lex glycan as bait, BSA-conjugated Lewisa glycan (Lea) for exclusion of non-specifically bound phages, and Lex-specific antibody L5 for phage elution. After panning, we identified among the clones that bound to Lex, but not to Lea, a clone that contained a cDNA coding for amino acids 121–304 of the MBP-golli isoform 1 (also known as HOG7, homologous to J37 in the mouse) (38). This result suggests that MBP binds to Lex-carrying proteins.
Binding of MBP to L1 Depends on Lex-carrying N-Glycans
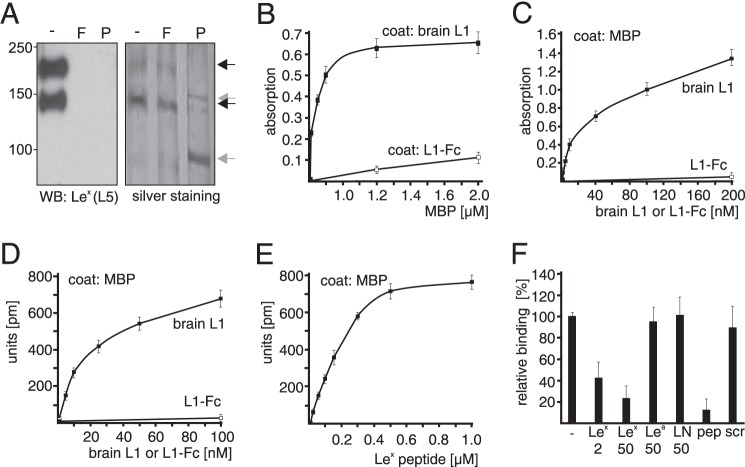
Because L1 carries Lex and is involved in Lex-dependent neurite outgrowth (4–6), we investigated whether MBP binds to L1 in a Lex-dependent manner. To this aim, L1 immunopurified from early postnatal mouse brains, recombinant L1 consisting of the extracellular L1 domain and human Fc (L1-Fc), and MBP from bovine brain were used in binding assays. L1-Fc does not carry Lex glycans as it was expressed in Lex-negative CHO cells (39). Western blot analysis of brain L1 with the Lex-specific antibody revealed that the 200-kDa full-length L1 (L1–200) and the 140-kDa L1 fragment (L1–140) (40) carry Lex, whereas no Lex-positive bands were seen after removal of N-linked glycans or terminal fucose residues by PNGase F or α1–3,4-fucosidase treatment (Fig. 1A), showing that mouse brain L1 carries Lex glycans in N-glycosidic linkage. Silver staining showed that similar amounts of L1–200 and L1–140 were detectable in the untreated sample and after α1–3,4-fucosidase treatment (Fig. 1A). After PNGase F treatment, L1–200 and L1–140 bands were shifted to 150 and 95 kDa, but the amounts of these bands correlated with those of the L1–200 and L1–140 bands in the untreated sample.
FIGURE 1.
Lex-dependent interaction between L1 and MBP. A, L1 from mouse brain was treated without (-) or with α1–3,4-fucosidase (F) or PNGase F (P) and subjected to Western blot (WB) analysis using Lex-specific antibody L5 or to silver staining to control loading. For ELISA (B and C) and label-free binding assay (D–F), substrate-coated brain L1 (B), L1-Fc (B), or MBP (C–F) were incubated with soluble MBP (B), L1-Fc (C and D), or brain L1 in the absence (C, D, and F) or presence of Lex peptide (E) or a 2- or 50-fold molar excess of Lex, Lea, N-acetyllactosamine (LN), Lex peptide (pep), or scrambled Lex peptide (scr) (F). Mean values ± S.D. from four representative experiments with triplicates are shown for absorption or reflected wavelength shifts relative to values obtained without additions (set to 100%).
By ELISA, MBP showed a concentration-dependent binding to substrate-coated Lex-carrying brain L1 but not to Lex-lacking L1-Fc (Fig. 1B). Moreover, concentration-dependent binding of brain L1, but not L1-Fc, to substrate-coated MBP was observed both in ELISA and a label-free binding assay (Fig. 1, C and D). In the label-free assay, the synthetic Lex-mimicking peptide (Lex peptide) showed a concentration-dependent binding to substrate-coated MBP (Fig. 1E), and binding of brain L1 to substrate-coated MBP was inhibited by the Lex glycan and Lex peptide but not by N-acetyllactosamine, the Lea glycan, or a scrambled version of the Lex peptide (scrambled Lex peptide) (Fig. 1F). These results show that MBP binds to brain L1 via Lex.
The MBP-induced and L1-mediated Neurite Outgrowth and Neuronal Survival Are Lex-dependent
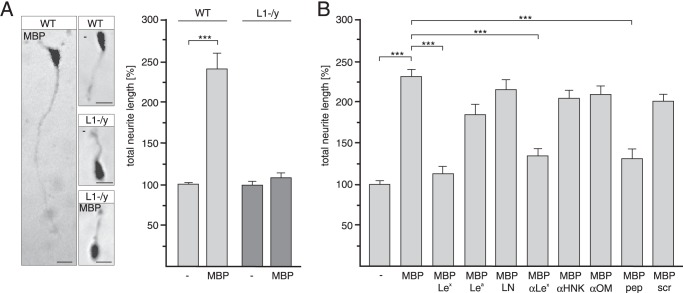
To determine the functional relevance of the interaction between L1 and MPB in the brain, neurite outgrowth experiments with cerebellar neurons from wild-type or L1-deficient mice were performed in the absence or presence of MBP. The total neurite lengths of wild-type neurons were increased in the presence of MBP when compared with neurite lengths observed in its absence. Neurite outgrowth of L1-deficient neurons was not promoted by MBP (Fig. 2A), indicating that L1 is required for MBP-triggered neurite outgrowth.
FIGURE 2.
Promotion of neurite outgrowth by MBP depends on L1 and Lex. Wild-type (WT) (A and B) and L1-deficient (L1-/y) cerebellar neurons (A) were incubated with MBP in the absence (A and B) or presence of Lex, Lea, N-acetyllactosamine (LN), Lex antibody (αLex), Lex peptide (pep), scrambled Lex peptide (scr), HNK1 antibody (αHNK), or oligomannose antibody (αOM) (B). Representative images of untreated and MBP-stimulated wild-type and L1-deficient neurons are shown, and the bars represent 10 μm (A). Mean values ± S.E. (***, p < 0.001; two-tailed Student's t test) from four independent experiments with duplicates counting at least 100 neurons per sample are shown for neurite lengths relative neurons on poly-l-lysine without additives (set to 100%) (A and B).
Next, we examined whether the L1-dependent effect of MBP on neurite outgrowth is Lex-dependent. To competitively block binding of MBP to Lex-carrying L1 at the cell surface and, thus, to inhibit MBP-induced neuritogenesis, Lex antibody, glycan, and peptide were used. As controls, we used the glycans Lea and N-acetyllactosamine, scrambled Lex peptide, and antibodies directed against oligomannoses and the HNK-1 glycan, which are also carried by brain L1. MBP-enhanced neurite outgrowth was not observed in the presence of Lex-specific antibody, peptide, or glycan when compared with neurite outgrowth in their absence or in the presence of Lea, N-acetyllactosamine, scrambled Lex peptide, and antibodies against the glycans HNK1 or oligomannose (Fig. 2B). These results indicate that the effects of MBP on neurite outgrowth are mediated by a Lex-dependent interaction between MBP and L1.
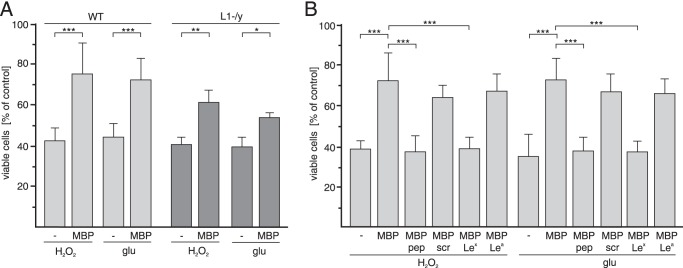
Because L1 has been shown to be neuroprotective (33, 41), we tested whether this L1-dependent function is affected by MBP. To this aim, survival of cultured cerebellar neurons after oxidative or glutamate stress was analyzed. The number of viable wild-type cerebellar neurons after hydrogen peroxide or glutamate treatment was increased in the presence of MBP relative to the number determined in its absence (Fig. 3A and B). The protective effect of MBP on L1-deficient neurons was less pronounced when compared with the effect on wild-type neurons (Fig. 3A), indicating that neuroprotection by MBP is mainly, but not completely, mediated by L1. However, MBP-promoted neuronal survival was completely blocked by the Lex peptide and glycan, but not by the Lea glycan or scrambled Lex peptide (Fig. 3B), indicating that the neuroprotective effect of MBP depends on Lex. The combined results indicate that MBP not only promotes L1-dependent neurite outgrowth of cerebellar neurons, but also protects cerebellar neurons from hydrogen peroxide-induced oxidative stress and glutamate excitotoxicity.
FIGURE 3.
MBP enhances survival of cerebellar neurons exposed to oxidative or excitotoxic stress in an L1- and Lex-dependent manner. WT (A and B) and L1-deficient (L1-/y) (A) cerebellar neurons were treated with H2O2 or glutamate (glu) in the absence or presence of MBP (A and B) and Lex, Lea, Lex peptide (pep), or scrambled Lex peptide (scr) (B). Mean values ± S.E. (*, p < 0.05; **, p < 0.01; ***, p < 0.001; two-tailed Student's t test) from four independent experiments with triplicates are shown for the numbers of viable cells relative to those determined without additives (set to 100%).
MBP Acts as a Serine Protease That Cleaves L1 and Modulates L1-dependent Functions
It has been shown that L1 is cleaved by different proteases and that proteolytic processing of L1 is involved in regulation of L1-dependent neurite outgrowth (9, 40, 42). Because MBP modulates L1-induced neurite outgrowth and neuronal survival and has an autocatalytic serine protease activity (43) as well as proteolytically degrades amyloid-β (44), we hypothesized that MBP may cleave L1 to modulate L1-dependent functions. To test this possibility, we investigated whether ablation of MBP affects proteolytic processing of L1 by determining the levels of full-length L1 and L1 fragments in crude axolemma-containing myelin fractions from brains of wild-type mice and MBP-deficient shiverer mice. Western blot analysis using antibody 555 directed against an extracellular epitope between the second and third fibronectin type III (FnIII) domains of L1 showed that the levels of full-length L1–200 and its proteolytic 140- and 180-kDa fragments (L1–140 and L1–180) were similarly present in myelin fractions from wild-type and MBP-deficient mice (Fig. 4A). This observation indicates that MBP neither affects L1 expression nor the proteolytic processing by plasmin, PC5a, metalloproteases, or neuropsin, which generate L1–140 or L1–180 (40, 45, 46). Interestingly, a 70-kDa L1 fragment (L1–70) was detectable in the myelin fraction from wild-type mice but not in the crude myelin fraction from shiverer mice (Fig. 4A), implying that MBP is involved in the regulation of proteolytic processing of L1 resulting in generation of L1–70. Also, the L1 antibody 172-R directed against the intracellular domain of L1 recognized L1–70 in the wild-type but not in the MBP-deficient myelin fraction (Fig. 4A).
FIGURE 4.
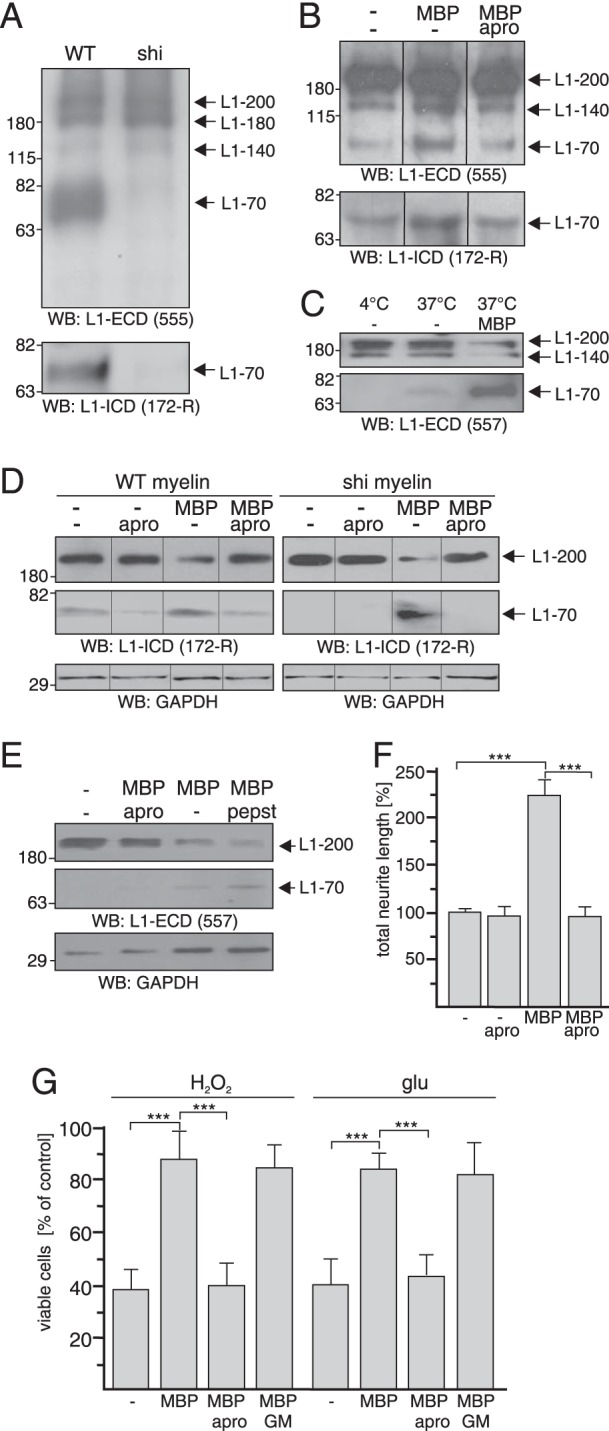
Cleavage of L1 by MBP results in generation of L1–70. Myelin fractions isolated from WT and shiverer (shi) brains (A), brain L1 treated with MBP in the absence or presence of aprotinin (B), myelin fraction from wild-type or shiverer mouse brains treated with MBP and aprotinin (apro) at 4 °C or 37 °C (C and D), wild-type cerebellar neurons treated with MBP and aprotinin or pepstatin (pepst) (E) were subjected to Western blot analysis with the L1 antibodies 555, 557, and 172-R and GAPDH antibody to control loading. Representative Western blots (WB) from three experiments with identical results are shown. Full-length L1 and L1 fragments are indicated by arrows. Upper and lower panel were derived from the same blot after different exposure times (C–E). Lanes that were not adjacent to each other but derived from the same blot are indicated by dividing lines (B and D). F and G, cerebellar neurons from wild-type mice were treated with H2O2 or glutamate (glu) in the absence or presence of MBP and aprotinin or GM6001 (GM). Mean values ± S.E. (***, p < 0.001; two-tailed Student's t test) from four independent experiments with duplicates counting at least 100 neurons per sample are shown for neurite lengths, and mean values ± S.E. from four independent experiments with triplicates are shown for the numbers of viable relative to those determined without additives (set to 100%).
L1–70 is generated in cerebellar neurons by a serine protease that is unidentified so far (9). To analyze whether MBP acts as a serine protease that cleaves L1 to generate L1–70, purified MBP was incubated with brain L1 in the absence or presence of the serine protease inhibitor aprotinin. L1–70 was detected by monoclonal L1 antibodies reacting with its intracellular and extracellular domains (Fig. 4B). The addition of MBP increased the levels of L1–70, whereas this increase was not observed in the presence of aprotinin (Fig. 4B). These results indicate that MBP acts as a serine protease that cleaves brain L1.
To further substantiate this notion, we treated crude myelin fractions from wild-type or MBP-deficient brains with MBP and aprotinin. Western blot analysis revealed that L1–70 was not detectable in the fraction from wild-type mice at the proteolysis inhibiting temperature 4 °C (Fig. 4C). Small amounts of the fragment were observed upon incubation at 37 °C, whereas L1–70 levels were drastically enhanced by incubation at 37 °C in the presence of MBP, and the corresponding level of full-length L1–200 decreased (Fig. 4, C and D). L1–70 was not detectable in crude myelin of MBP-deficient mice upon incubation at 37 °C in the absence of MBP (Fig. 4D), whereas it was seen upon the addition of MBP (Fig. 4D). Enhancement of L1–70 levels by MBP in the wild-type myelin fraction was reduced by aprotinin, and the MBP-mediated generation of L1–70 in the MBP-deficient fraction was completely abolished by aprotinin (Fig. 4D). These results show that MBP and its serine protease activity are required for the generation of L1–70.
We further analyzed whether MBP promotes generation of L1–70 in cultured cerebellar neurons and observed that the addition of MBP triggered the generation of L1–70 and decrease of L1–200 levels (Fig. 4E), whereas aprotinin abolished the MBP-mediated generation of L1–70 from L1–200 (Fig. 4E). Interestingly, in the presence of the aspartyl protease inhibitor pepstatin, an increase in the L1–70 levels was observed (Fig. 4E), suggesting that pepstatin may inhibit an aspartyl protease that consecutively cleaves L1–70. The combined results indicate that MBP has serine protease activity and that it cleaves L1 to generate the recently described transmembrane 70-kDa L1 fragment (9).
To address the question of whether the serine protease activity of MBP is necessary for L1-dependent functions, cellular responses were determined in the absence and presence of MBP and aprotinin. MBP-enhanced neurite outgrowth of cerebellar neurons was abolished in the presence of aprotinin, whereas aprotinin did not alter neurite outgrowth in the absence of MBP (Fig. 4F). The MBP-enhanced neuroprotection against stress through hydrogen peroxide and glutamate was also diminished by aprotinin, whereas the metalloprotease inhibitor GM6001 had no effect (Fig. 4G). These results imply that the promotion of cellular responses by MBP depends on MBP serine protease activity.
L1-induced Cellular Functions Depend on MBP
We showed that exogenously applied MBP promotes L1-dependent neurite outgrowth and protects cerebellar neurons from insult by hydrogen peroxide and glutamate excitotoxicity. To investigate whether these L1-mediated functions depend on endogenous MBP, we determined whether L1-induced neurite outgrowth and survival of cerebellar neurons are affected by blocking of endogenous MBP functions using the pan-MBP antibody or by ablation of MBP. Stimulation of L1 signaling in wild-type cerebellar neurons by the addition of brain L1 or function-triggering L1 antibody 557 (28) led to an increase in neurite lengths when compared with values observed in their absence, whereas neurite outgrowth from MBP-deficient neurons was only slightly increased by brain L1 and L1 antibody (Fig. 5A). Promotion of neurite outgrowth from wild-type neurons by brain L1 or antibody 557 was less pronounced in the presence of a pan-MBP antibody in comparison to that determined in its absence and was similar to that of MBP-deficient neurons observed in the presence of brain L1 or antibody 557 (Fig. 5A). Interestingly, exogenous MBP enhanced neurite outgrowth of wild-type and MBP-deficient neurons to the same extent (Fig. 5A).
FIGURE 5.
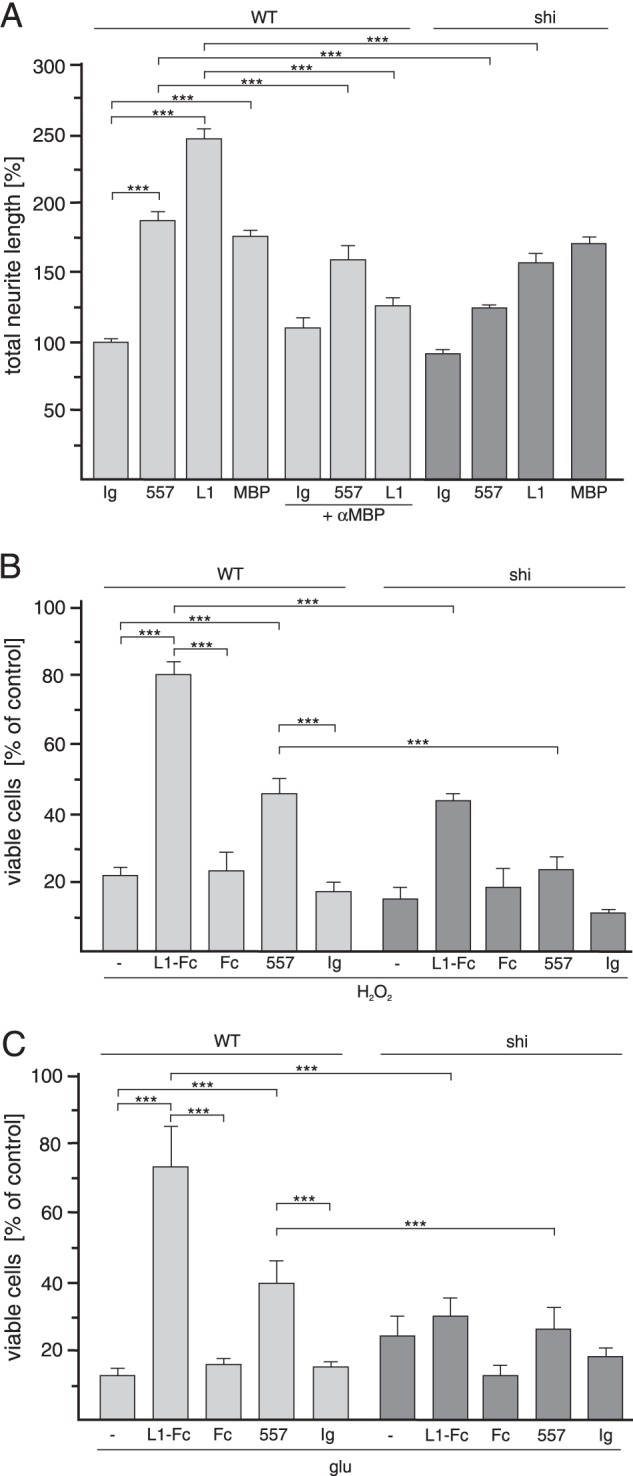
L1-induced promotion of neurite outgrowth and survival of cerebellar neurons depend on MBP. Cerebellar neurons from WT and MBP-deficient shiverer (shi) mice were treated with the L1 antibody 557 (A–C), brain L1 (A), L1-Fc (B and C), Fc (B and C), MBP (A), or control antibody (Ig) (A–C) in the absence or presence of the pan-MBP antibody (αMBP) (A), H2O2 (B), or glutamate (glu) (C). Mean values ± S.E. (***, p < 0.001; two-tailed Student's t test) from four independent experiments with duplicates counting at least 100 neurons per sample are shown for neurite lengths, and mean values ± S.E. from four independent experiments with triplicates are shown for the numbers of viable cells relative to those determined without additives (set to 100%).
Upon stimulation of L1 signaling by function-triggering L1-Fc or antibody 557, wild-type cerebellar neurons were more resistant to hydrogen peroxide or glutamate toxicity than non-treated neurons or neurons treated with Fc or non-immune control antibody (Fig. 5, B and C). In contrast, L1-Fc had only a slight effect, and the L1 antibody had no effect on survival of MBP-deficient cerebellar neurons (Fig. 5, B and C). The combined results show that L1-induced neurite outgrowth and survival of cerebellar neurons depend on endogenous MBP and that ablation or blocking of endogenous MBP function(s) impairs L1-induced cellular functions.
MBP Is Expressed by Cerebellar Neurons and Associates with L1 at the Cell Surface
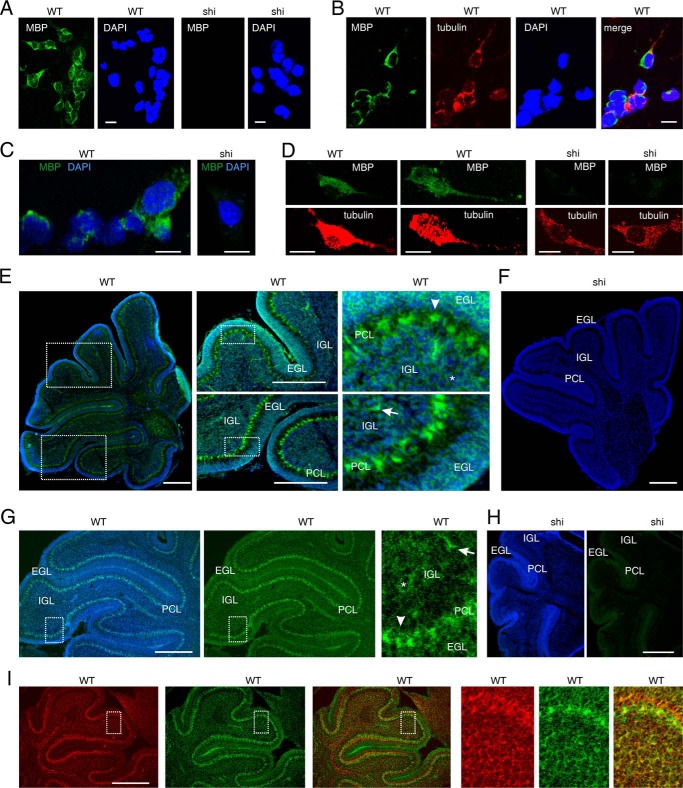
Because L1-triggered neurite outgrowth and survival of cerebellar neurons depends on endogenous MBP, we concluded that MBP is expressed by cultured cerebellar neurons and released into the extracellular space, where MBP cleaves L1 to regulate L1-induced cellular functions. To test this assumption we performed immunostaining of live cerebellar neurons. First, the specificity of the pan-MBP antibody was validated by Western blot analysis of brain homogenates from wild-type and MBP-deficient mice. The pan-MBP antibody recognized three major bands of ∼14, 18, and 21 kDa in the homogenate of wild-type brains, whereas no bands were detectable in the homogenate of MBP-deficient brains from shiverer mice (Fig. 6A), demonstrating that the antibody is MBP-specific. A pronounced punctate MBP staining at the neuronal cell surface was observed when this antibody was used for immunocytochemistry of live cerebellar cells in culture (Fig. 6B), which consist of well more than 95% neurons and contain less than 0.5% immature oligodendrocytes that do not express MBP. These results indicate that MBP is present extracellularly and suggests that MBP is released from cerebellar neurons.
FIGURE 6.
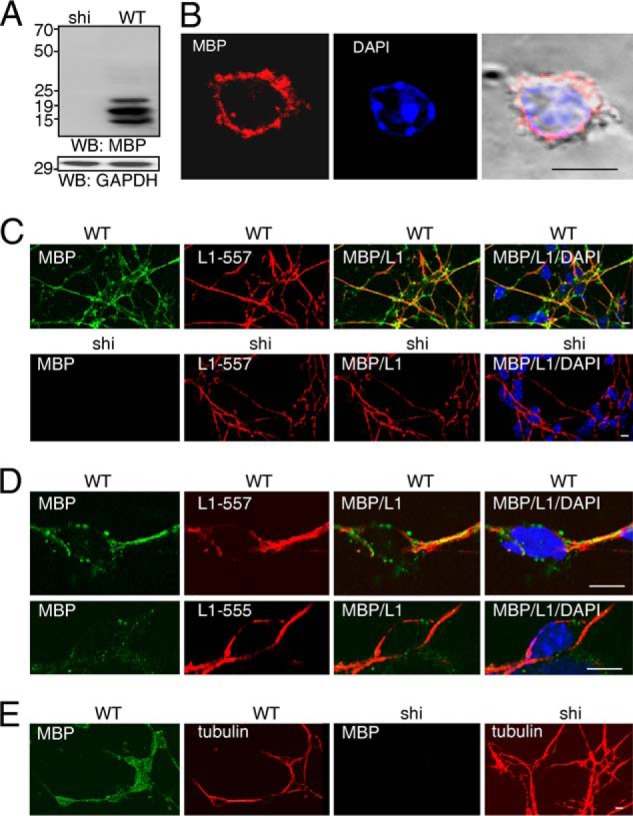
MBP is expressed by cerebellar neurons and associates with L1 at the surface of cerebellar neurons. A, Western blot analysis of brain homogenates from WT and shiverer (shi) mice with pan-MBP antibody or GAPDH antibody is shown. A representative Western blot (WB) from three experiments with identical results is shown. B, live wild-type cerebellar neurons were stained with pan-MBP antibody followed by staining with Cy3-labeled secondary antibody and DAPI. C and D, wild-type and MBP-deficient (shi) cerebellar neurons were treated with L1 antibody 557 (C and D) or 555 (D) followed by staining with pan-MBP antibody, fixation, and staining with Cy2- and Cy3-labeled secondary antibodies and DAPI. E, wild-type and MBP-deficient (shi) cerebellar neurons were stained with βIII-tubulin and pan-MBP antibody (E) after fixation and permeabilization followed by staining with Cy2- and Cy3-labeled secondary antibodies and DAPI. Representative images are shown, and the bars represent 10 μm.
To test whether MBP is released from cerebellar neurons upon L1 stimulation, wild-type and MBP-deficient cerebellar cell cultures were treated with function-triggering L1 antibody 557 or with L1 antibody 555, which does not trigger L1 functions (28). Pronounced MBP surface immunostaining and partial co-localization of L1 and MBP at the cell surface of wild-type, but not MBP-deficient cells, was found after treatment with antibody 557 (Fig. 6, C and D). In contrast, no co-localization of MBP and L1 and weak MBP surface immunostaining were observed in wild-type cells after treatment with antibody 555 (Fig. 6D), indicating that MBP is released from cerebellar neurons upon L1 stimulation. To verify that the MBP-expressing cells represent neurons, cerebellar cell cultures were subjected to immunostaining with pan-MBP antibody and an antibody against the neuron-specific marker βIII-tubulin. In cultures of wild-type cells, the vast majority of the cells (>90%) were MBP- and βIII-tubulin-positive, whereas in cultures of MBP-deficient cells, all βIII-tubulin-positive were MBP-negative (Fig. 6E). This result shows that cultured cerebellar neurons express MBP.
To confirm the expression of MBP in cerebellar neurons in vitro and in vivo, we performed in situ hybridization using brain tissue or cultured cerebellar neurons from wild-type and MBP-deficient mice. The MBP probe comprising the entire cDNA sequence coding for the 21.5-kDa MBP isoform detected MBP mRNA in cultured wild-type granule neurons, whereas no MBP mRNA was detectable in MBP-deficient neurons (Fig. 7, A–E). Staining with βIII-tubulin antibody confirmed that the MBP-expressing cells represent neurons (Fig. 7B). MBP mRNA was also detected in βIII-tubulin positive wild-type neurons when using a MBP probe comprising exon IV-VI of the MBP gene (21), whereas no MBP mRNA was detected in βIII-tubulin positive MBP-deficient neurons (Fig. 7, C and D). This result demonstrates that MBP is expressed in cerebellar neurons.
FIGURE 7.
MBP is expressed by cerebellar neurons in vitro and in vivo. Cultured cerebellar neurons (A–D) and cerebellar tissue sections (E–I) from WT and MBP-deficient shiverer (shi) mice were analyzed by in situ hybridization using a probe for the 21.5-kDa MBP isoform (green) (A, B, E, and F) or exon IV-VI-containing MBP isoforms (green) (C, D, and G–I). DAPI (A–D) or bisbenzimide (E–I) was used for nuclear staining (blue) (A–I), and an antibody against the neuronal marker βIII-tubulin (red) was used to identify neurons (B, D, and I). A–I, representative images are shown, and the bars represent 10 μm (A–D) and 300 μm (E–I). EGL, external granular layer; PCL, Purkinje cell layer; IGL, internal granular layer. Granule neurons, interneuron, and Purkinje cells are indicated by asterisks, arrows, and arrowheads, respectively.
Using the MBP probes on brain tissue cyrosections from 6-day-old wild-type and MBP-deficient mice, MBP mRNA expression was observed in wild-type cerebella but not in MBP-deficient cerebella (Fig. 7, E–I). MBP mRNA was not only seen in granule precursor cells in the external granular layer and in granule neurons in the internal granular layer but also in other neurons such as Purkinje cells and interneurons (Fig. 7, E and G). The expression of MBP mRNA in wild-type cerebella coincided with the expression of βIII-tubulin (Fig. 7I). These results demonstrate that MBP is not only expressed in cerebellar neurons in vitro but also in vivo.
Cerebellar Neurons Release a 60-kDa MBP Form That Cleaves L1
To further analyze the expression of MBP by cerebellar neurons, cultured wild-type and MBP-deficient cerebellar neurons were subjected to Western blot analysis using the pan-MBP antibody. Several major MBP-positive bands ranging from ∼14 to 21 kDa were detectable in cell lysates of wild-type cerebellar neurons, whereas no bands were detected in the lysates of cerebellar neurons from MBP-deficient shiverer mice (Fig. 8A). This result confirms that cerebellar neurons express MBP.
FIGURE 8.
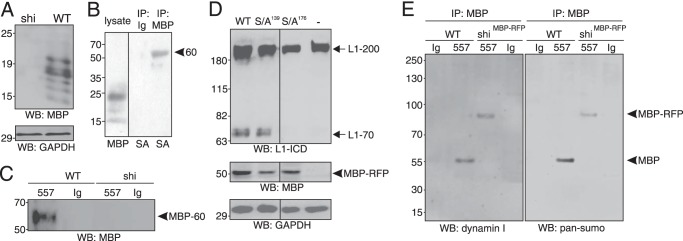
MBP is released from cerebellar neurons upon L1 stimulation as 60-kDa protein containing exon II-encoded sequences, sumo, and part of dynamin. A, Western blot (WB) analysis of cerebellar neurons from WT and shiverer (shi) mice using pan-MBP or GAPDH antibodies is shown. B, immunoprecipitates (IP) obtained by pan-MBP or control (Ig) antibodies after cell surface biotinylation of cerebellar neurons from WT and shiverer (shi) mice were analyzed by Western blot analysis with pan-MBP antibody or streptavidin (SA). C, cell culture supernatants of cerebellar neurons from WT and shiverer (shi) mice after treatment with L1 antibody 557 or control antibody (Ig) were subjected to immunoprecipitation with pan-MBP antibody and Western blot analysis with a MBP antibody recognizing the exon II-encoded domain. The 60-kDa MBP form is depicted by an arrowhead. D, cerebellar neurons from shiverer mice were incubated without (−) or with AAV1 coding for RFP-tagged wild-type MBP or MBP S139A or S176A mutants and treated with the L1 antibody 557. Western blot analysis of cell lysates with L1 antibody 172-R, pan-MBP, or GAPDH antibodies is shown. RFP-tagged MBP is depicted by an arrowhead. E, cell culture supernatants from MBP-deficient cerebellar neurons from shiverer mice transduced with AAV1 coding for RFP-tagged MBP (shiRFB-MBP) and from wild-type cerebellar neurons were collected after treatment with the L1 antibody 557 or control antibody and subjected to immunoprecipitation with MBP antibody specific for exon II-encoded sequences. The immunoprecipitates were probed by Western blot analysis with dynamin I and pan-sumo antibodies. The 60-kDa MBP form and the 90-kDa MBP-RFP form are depicted by an arrowhead. A–E, representative Western blots from three experiments with identical results are shown.
To investigate whether MBP is released from cerebellar neurons, wild-type cerebellar neurons were subjected to cell surface biotinylation and immunoprecipitation with the pan-MBP antibody. Western blot analysis of the MBP immunoprecipitates using HRP-conjugated streptavidin revealed that only one protein band of ∼60 kDa was detected as biotinylated protein (Fig. 8B), indicating that MBP is present at the cell surface of cerebellar neurons with an apparent molecular mass of ∼60 kDa. To characterize this 60-kDa MBP form and its release, cell culture supernatants from wild-type and MBP-deficient cerebellar neurons after treatment with the function-triggering L1 antibody 557 or with non-immune control antibody were subjected to immunoprecipitation with a pan-MBP antibody. Western blot analysis of the MBP immunoprecipitates using an MBP antibody directed against exon II-encoded sequences showed only the 60-kDa MBP form in the cell culture supernatant of wild-type neurons after stimulation with the L1 antibody, whereas no band was seen when the control antibody was applied (Fig. 8C). No immunoreactive band was seen in immunoprecipitates isolated from MBP-deficient neurons upon treatment with L1 or control antibody (Fig. 8C).
In the human 18.5-kDa MBP isoform, the serine at position 152 has been proposed to be the active site for the serine protease activity (43). In the murine 21.5-kDa isoform, which also contains the exon II-encoded sequences, the putative active site serine is at position 176. To test whether this MBP isoform is an extracellular L1-cleaving serine protease, MBP-deficient cerebellar neurons were treated with L1 antibody 557 after transduction with AAV1 coding for a fusion protein of RFP and the murine 21.5-kDa MBP isoform (34) or coding for a RFP-tagged mutant of 21.5-kDa MBP carrying a serine to alanine exchange at position 176 (S176A). For the control, AAV1 coding for a RFP-tagged mutant with a serine to alanine replacement at position 139 (S139A) was used. Western blot analysis of cell lysates with the pan-MBP antibody showed that the wild-type and mutated MBP forms were expressed in MBP-deficient neurons, with the cellular levels of the wild-type MBP being higher than those of the MBP mutants (Fig. 8D). Using L1 antibody 172-R, L1–70 was not detectable in non-transduced MBP-deficient neurons after L1 stimulation, whereas significant levels of L1–70 were seen in lysates of L1-stimulated MBP-deficient neurons transduced with AAV1 coding for wild-type MBP or the S139A mutant MBP. However, no L1–70 was detectable after transduction of the S176A mutant MBP (Fig. 8D). This result shows that MBP with exon II-encoded sequences functions as the serine protease that cleaves L1 and generates L1–70 and that the serine at position 176 is the active center of this protease.
Because a 60-kDa MBP form has been detected at the cell surface of a non-myelinating cell line and sequence analysis suggests that this form is a fusion between MBP and the C-terminal part of a dynamin-like protein (47), we analyzed if the 60-kDa MBP form is a fusion of MBP and a dynamin-like protein. To test whether the MBP form contains a dynamin portion after its L1-triggered release, cell culture supernatants were collected from wild-type cerebellar neurons after treatment with L1 antibody 557 or non-immune control antibody and from MBP-deficient cerebellar neurons after transduction with AAV1 coding for RFP-tagged wild-type MBP (RFP-MBP) and treatment with L1 or control antibodies and subjected to immunoprecipitation with pan-MBP antibody followed by Western blot analysis of immunoprecipitates with dynamin I antibody. In the immunoprecipitate from the cell culture supernatant of L1-stimulated wild-type neurons one band of ∼60 kDa was detectable with the dynamin I antibody, and one band of approximately ∼90 kDa was observed in the immunoprecipitate of the cell culture supernatant from L1-stimulated MBP-deficient neurons transduced with AAV coding for RFP-MBP (Fig. 8E). No dynamin-immunopositive bands were seen in immunoprecipitates from cell culture supernatants of wild-type or RFP-MBP-expressing neurons after treatment with control antibody (Fig. 8E). These results indicate that the 60-kDa MBP form contains part of dynamin I or a dynamin I-related protein and that the ∼30-kDa RFP at the N terminus of 21.5-kDa MBP shifts the apparent molecular mass of the dynamin-containing 60-kDa MBP form to ∼90 kDa.
Based on the observations that stimulation of cerebellar neurons with L1 antibody 557 results in sumoylation of L1 (9), sumo is secreted (48), and amyloid precursor protein is sumoylated in its extracellular domain (49), we hypothesized that the 60-kDa MBP form may be sumoylated. We, therefore, probed the MBP immunoprecipitates from cell culture supernatants of wild-type and MBP-deficient neurons transduced with AAV1 coding for RFP-MBP by Western blot analysis with a pan-sumo antibody. As seen with the dynamin antibody, a ∼60-kDa band was detected by the sumo antibody in the immunoprecipitate from cell culture supernatants of L1-stimulated wild-type cerebellar neurons, whereas an ∼90-kDa band was detected in the immunoprecipitates from cell culture supernatants of L1-stimulated MBP-deficient neurons transduced with AAV1 coding for RFP-MBP (Fig. 8E). No bands were seen in the immunoprecipitates of neurons that had been treated with a non-immune control antibody (Fig. 8E). These results indicate that the 60-kDa MBP form represents a sumoylated MBP form containing exon II-encoded MBP sequences and part of dynamin I or a dynamin I-related protein.
MBP Cleaves Murine L1 within Its First FnIII Domain at Arg687
In a previous study we showed that L1–70 is monosumoylated (9). Here, we show that cleavage of L1 by MBP generates this transmembrane fragment, which contains the epitope recognized by antibody 557 at the N terminus of the third FnIII domain. Based on these findings and taking the molecular weight of sumo into account, this fragment could be generated by MBP cleavage within the first or second FnIII domain. Because MBP autocatalytically cleaves itself at Gln-Lys75 and/or Phe-Lys91 (43), and amyloid-β42 at several sites including Phe-Arg5 (44), we scanned the first and second FnIII domains of murine and human L1 for potential MBP cleavage sites, e.g. for Gln-Lys, Phe-Lys, or Phe-Arg bonds, and identified Phe-Arg687 in the first FnIII domain as a potential MBP cleavage site. Importantly, this potential cleavage site is located within a highly conserved sialic acid binding site on L1 (7).
To verify that L1 is cleaved by MBP within this motif at Phe-Arg687, we first analyzed whether L1-stimulated neurite outgrowth, which depends on L1 cleavage by MBP is affected by a previously described mouse L1 synthetic peptide (“siabind”; amino acids 672–705), which contains the potential MBP cleavage site and the sialic acid binding motif and inhibits L1-induced neurite outgrowth (6). As a control, a scrambled peptide was used. The stimulation of neurite outgrowth with L1 antibody 557 was abolished by siabind, whereas the scrambled peptide had no effect (Fig. 9A). This result indicates that siabind, but not the scrambled peptide, competitively binds to MBP to impede the interaction of MBP with the putative cleavage site in L1 and thereby to block cleavage of L1 by MBP.
FIGURE 9.
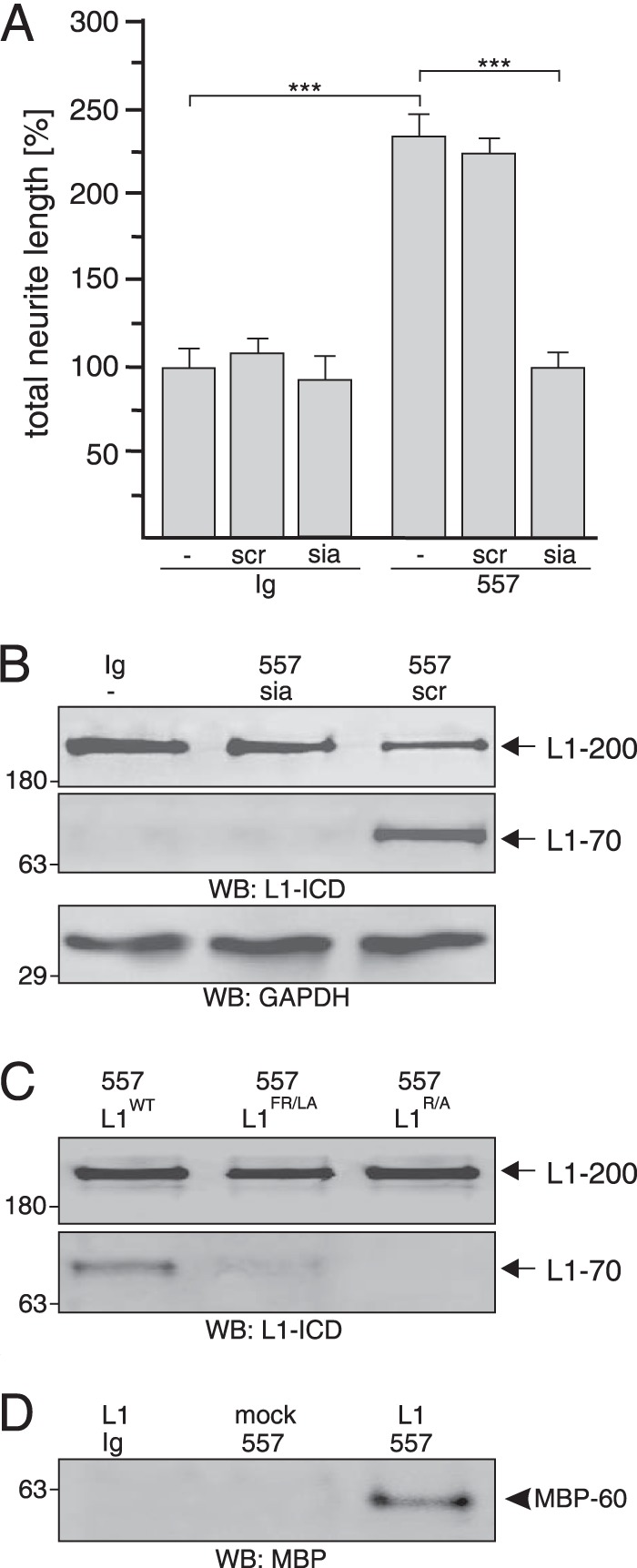
Extracellular MBP cleaves L1 at Arg687. A and B, cerebellar neurons were treated with control antibody (Ig) or L1 antibody 557 in the absence (−) or presence of the L1-derived peptide siabind (sia) or a scrambled version of siabind (scr). Mean values ± S.E. (***, p < 0.001; two-tailed Student's t test) for neurite lengths from three independent experiments (A) and Western blot (WB) analysis of cell lysates with L1 antibody 172-R (L1-ICD) and GAPDH antibody (B) are shown. C, lysates of HEK293 cells transfected with wild-type L1 (L1WT), L1R/A mutant, or L1FR/LA mutant and treated with L1 antibody 557 were subjected to Western blot analysis with L1 antibody 172-R. D, immunoprecipitates with pan-MBP antibody from cell culture supernatants of mock- or L1-transfected HEK293 cells treated with L1 antibody 557 or control antibody (Ig) were subjected Western blot analysis with a MBP antibody specific for the exon II-encoded domain. B–D, representative Western blots of three experiments with identical results are shown. L1–200 and L1–70 are indicated by arrows, and the 60-kDa MBP form is depicted by an arrowhead.
To test whether siabind comprising the putative MBP cleavage site inhibits cleavage of L1 by MBP upon L1 stimulation, we determined the intracellular levels of the MBP-generated L1–70 after stimulation of cerebellar neurons with L1 antibody in the absence or presence of siabind or its scrambled version. Lysates of neurons after L1 stimulation in the presence of the scrambled peptide were positive for L1–70 in Western blot analysis with L1 antibody 172-R, whereas the fragment was not detectable upon L1 stimulation in the presence of siabind (Fig. 9B). These results indicate that the L1 peptide binds to MBP and interferes with the MBP-mediated cleavage of L1.
To further substantiate that MBP cleaves L1 at Arg687, this putative cleavage site was disrupted by exchanging the arginine residue to an alanine residue or by exchanging both the phenylalanine and arginine residues to leucine and alanine residues. L1-deficient HEK293 cells transfected with vectors encoding wild-type L1 or L1 mutants were treated with non-immune control antibody or with L1 antibody 557, which triggers generation of L1–70 in L1-transfected HEK293 cells (9). Western blot analysis of cell lysates using L1 antibody 172-R showed similar expression levels of wild-type and mutated L1–200 (Fig. 8C). After L1 stimulation, L1–70 was seen in lysates of cells expressing wild-type L1 and hardly or not detectable in lysates of cells expressing the L1 mutants (Fig. 9C).
Generation of L1–70 in L1-transfected HEK293 cells implied that these cells express and release MBP into the extracellular space after L1-stimulation. To confirm this notion, cell-free culture supernatants were isolated from mock-transfected or L1-transfected HEK293 cells upon treatment with antibody 557 or control antibody and subjected to immunoprecipitation with pan-MBP antibody. In Western blot analysis with MBP antibody recognizing exon II-encoded sequences, only the 60-kDa MBP form was detected in the immunoprecipitate from L1-stimulated cells expressing L1, whereas no immunopositive MBP band was detectable in the supernatants from mock-transfected cells treated with L1 antibody or from L1-expressing cells treated with control antibody (Fig. 9D), indicating an L1-dependent and L1-induced release of the 60-kDa MBP form from HEK293 cells. In summary, our results show that the released 60-kDa MBP form with the exon II-encoded domain cleaves mouse L1 at the Phe-Arg687 bond in its first FnIII domain to generate L1–70.
Serine Protease Activity of MBP Is Required for Neurite Outgrowth and Cell Migration in Vivo
To investigate whether the serine protease activity of MBP is important for promotion of neurite outgrowth and cell migration in vivo, we injected AAV1 coding for the wild-type 21.5-kDa MBP or the proteolytically inactive S176A mutant into the ventricle system of MBP-deficient shiverer embryos in utero at embryonic day 13.5, isolated cerebellar explants at postnatal day 5.5, cultured these explants for 2 days and analyzed the explants for neurite outgrowth and cell migration (Fig. 10A). After transduction with wild-type MBP, values for total neurite lengths and migrating cells reached values observed for explants from untreated wild-type mice and were much higher than those observed for explants after transduction with proteolytically inactive MBP (Fig. 10, B and C). After in utero transduction with proteolytically inactive MBP, postnatal cerebellar explants showed impaired neurite outgrowth and cell migration similarly to explants from untreated MBP-deficient shiverer mice (Fig. 10C). Western blot analysis showed that both the wild-type and mutated MBP were expressed in the cerebellum after transduction of MBP-deficient embryos, whereas L1–70 was only generated after transduction with wild-type MBP (Fig. 10D). These results show that the serine protease activity is required for MBP-triggered neurite outgrowth and neuronal migration in the cerebellum in vivo and suggest that promotion of neurite outgrowth and cell migration in vivo depends on the generation of L1–70 by the serine protease activity of MBP.
FIGURE 10.
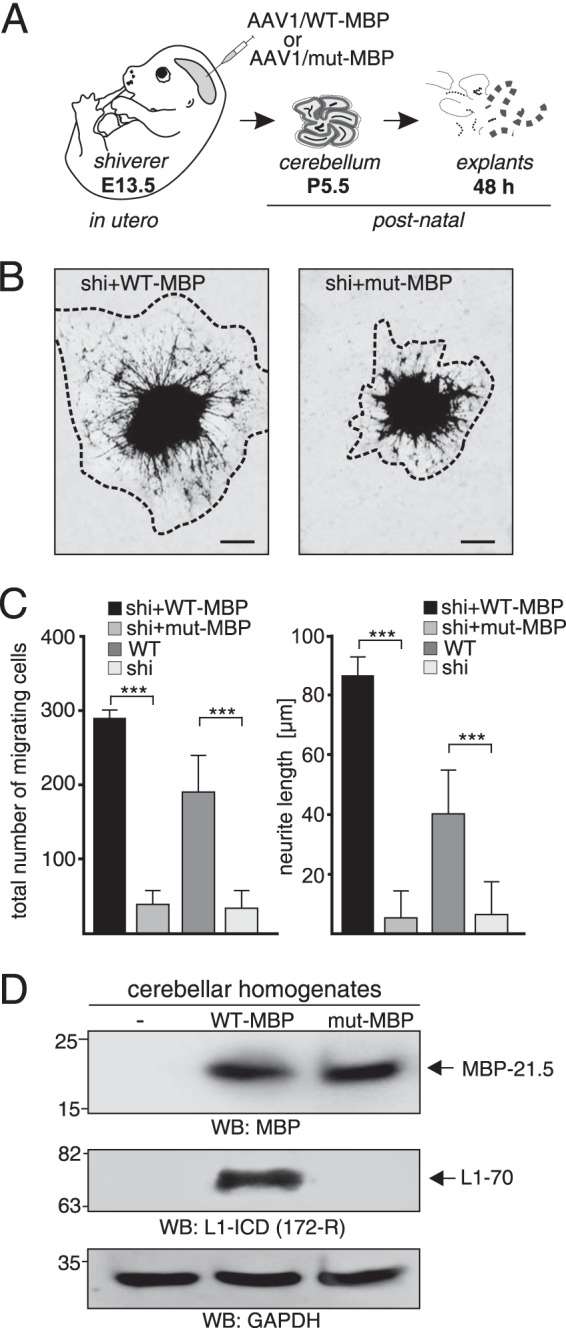
Neurite outgrowth and neuronal cell migration depend on serine protease activity of MBP in vivo. A, schematic representation of the experimental design. AAV1 coding for wild-type MBP (AAV1 WT-MBP) or for a proteolytically inactive MBP mutant (AAV1 mut-MBP) was injected into MBP-deficient shiverer embryos in utero at embryonic day 13.5 (E13.5). At postnatal day 5.5 (P5.5) explants were prepared from the cerebella of virus-transduced MBP-deficient mice and maintained for 2 days in culture. B, representative images of explants from cerebella of MBP-deficient mice transduced with wild-type or mutated MBP are shown. C, explants from treated animals and untreated wild-type and shiverer mice were analyzed for neurite outgrowth and cell migration by counting total neurite lengths and number of migrating cells of 10 explants from 3 animals per group. Mean values ± S.E. (***, p < 0.001; two-tailed Student's t test) for neurite lengths and number of migrating cells are shown. D, homogenates from 5.5-day-old untreated and transduced mice were subjected to Western blot (WB) analysis with MBP, L1, and GAPDH antibodies. A representative blot with homogenates from one animal of three animals per group is shown.
DISCUSSION
Here, we show that extracellular MBP acts as a serine protease that cleaves L1 in its extracellular domain at Arg687. The notion that MBP functions as a serine protease has been underscored by the observation that autocatalytic cleavage activity of purified or recombinant MBP and the cleavage of amyloid-β by purified or recombinant MBP are abolished in the presence of serine protease inhibitors (43, 44). In the present study we provide further evidence that MBP is a serine protease: proteolytic cleavage of L1 by MBP yields a transmembrane L1–70 fragment that is not generated in MBP-deficient mice or after mutation of the serine at the enzymatically active site within MBP.
Application of MBP to cultured cerebellar neurons generates L1–70 and induces L1-dependent cellular responses such as neurite outgrowth and neuroprotection against oxidative and glutamate stress, indicating that generation of L1–70 by MBP regulates L1 functions. Functional ablation of extracellular MBP as a serine protease inhibits cleavage of L1 in its extracellular domain and generation of the transmembrane L1–70 and, in consequence, L1-triggered cellular responses in vitro and in vivo, such as neurite outgrowth and neuronal cell migration.
Intriguing and novel findings of our study are the expression of MBP isoforms by cerebellar neurons, the L1-induced release of a 60-kDa MBP form, and the localization of MBP at the surface of cerebellar neurons. We find it highly unlikely that MBP observed in cultures of cerebellar neurons originates as contamination from myelin during culture preparation, because the dissociated cells derive from early postnatal cerebellum containing negligible amounts of myelin, and by far, remnant MBP released from myelin is completely digested by trypsin used for cell dissociation. We also exclude the possibility that MBP derives from oligodendrocytes in the culture, because >95% of all cells in the culture are neurons, and <0.5% are oligodendrocytes. Indeed, in situ hybridization experiments confirm that MBP is expressed by cerebellar neurons in vitro and in vivo. It has been shown that MBP isoforms are not only expressed by myelinating oligodendrocytes and Schwann cells but also by non-myelinating cells (47) and by non-neural cells (50) including peripheral T cells, which secrete several MBP isoforms (51). It is noteworthy in this context that L1 stimulation of the human neuroblastoma cell line SH-SY5Y triggers cleavage of L1 and generation of L1–70 (9), indicating that MBP is also expressed and released by these cells and that cleavage of L1 by MBP at the cell surface yields the L1–70 fragment. Here, we show that MBP is also expressed by L1-deficient HEK293 cells and that L1 stimulation of HEK293 cells transfected to express L1 results in release of a 60-kDa MBP form and MBP-mediated cleavage of L1 at Arg687. MBP forms of ∼60 kDa have been identified in studies using cell lines or myelin-containing fractions (47, 52). Moreover, the 60-kDa MBP form contains dynamin sequences and citrulline residues and has been detected at the cell surface of a non-myelinating cell line, suggesting that this MBP-dynamin fusion protein is released from the cells into the extracellular space (47). Interestingly, the 60-kDa MBP form released from cultured cerebellar neurons after L1 stimulation is recognized by a dynamin I antibody and, in addition, by a sumo antibody, suggesting that either the MBP or the dynamin part of the fusion protein is sumoylated. The 60-kDa MBP form was also identified in the bovine MBP preparation, which cleaves brain L1,4 indicating that this MBP form is able to cleave L1.
Our results indicate that the extracellular 60-kDa MBP form released by cerebellar neurons contains murine MBP isoforms that comprise exon II-encoded sequences, such as the 21.5-kDa isoform (19, 24). In fact, generation of L1–70 after transduction of MBP-deficient cerebellar neurons with AAV1 coding for the RFP-tagged 21.5-kDa MBP form suggests that the 21.5-kDa MBP isoform cleaves L1.
Because the exon II-encoded domain is only present in MBP proteins, but not in golli proteins, and because MBP proteins are not expressed in shiverer mice (19, 24, 53), one has to conclude that the extracellular 60-kDa MBP form released from cerebellar neurons contains an MBP isoform with the exon II-encoded domain, most likely the 21.5-kDa isoform.
Based on the findings obtained by us and others, we assume that the 60-kDa MBP form represents a sumoylated fusion protein of the 21.5-kDa MBP isoform and a C-terminal portion of dynamin I or a related protein. It remains to be determined by which mechanisms the 60-kDa MBP form is formed and released from neurons.
The 60-kDa MBP form secreted from cerebellar neurons acts as an L1 cleaving protease. A number of observations (19) indicate that different MBP isoforms are localized in sundry cellular compartments as well as in myelin, suggesting miscellaneous functions of the isoforms. For example, when expressed individually in MBP-lacking cells, isoforms containing the exon II-encoding domain are detectable in the cytosol and the nucleus but not at the plasma membrane, whereas the isoforms lacking this domain have been found exclusively at the plasma membrane (54). Isoforms containing the exon II-encoded domain are highly expressed during fetal development and early in myelination, whereas the forms lacking this domain are expressed later in myelination and represent the primary MBP forms in adult myelin (55, 56).
An intriguing observation of the present study is that MBP binds to L1 via Lex-carrying N-glycans and that this Lex-dependent interaction regulates several L1-mediated functions. In a previous study we obtained indications that Lex in conjunction with L1 plays a role in neurite outgrowth and myelination (6). L1 is one of the key players in myelination and maintenance of the myelin sheaths in the central and peripheral nervous systems, and it enhances remyelination of re-grown axons after nerve injury (8, 25, 57–61). It is noteworthy that L1 delivery to the lesioned nervous system by different means promotes locomotor recovery, axonal regrowth, and remyelination (57–63). Interestingly, levels of the 21.5-kDa MBP isoform, but not other MBP isoforms, are increased in the injured spinal cords of mice transduced by an L1-encoding AAV (58). This observation may indicate that an elevated L1 level in vivo may trigger the release of distinct MBP isoforms, such as the 21.5-kDa isoform, and that the enhanced release of MBP results in cleavage of L1 and generation of L1–70, the level of which is increased 1 week after spinal cord injury (9).
Expression of MBP isoforms, particularly the 21.5-kDa isoform, is associated with the pathogenesis of multiple sclerosis (64–67), which is characterized by demyelination and remyelination. Interestingly, MBP isolated from multiple sclerosis patients shows higher rates of autocatalytic cleavage than MBP isolated from normal individuals, suggesting that the protease activity of MBP is crucial for its putative role in degenerative diseases associated with demyelination, such as multiple sclerosis (43).
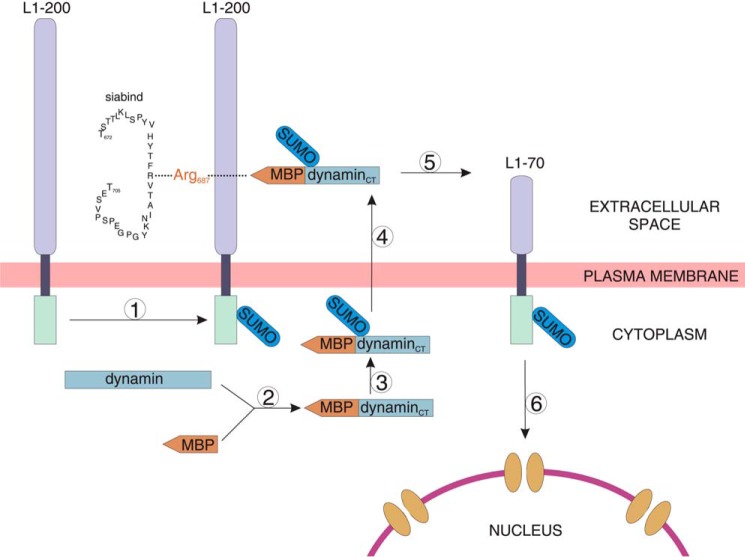
Based on our findings, we propose that L1 stimulation leads to formation of a MBP-dynamin fusion protein, sumoylation of L1 and the MBP-dynamin fusion protein, and release of the MBP-dynamin fusion protein (Fig. 11). The extracellular MBP-dynamin cleaves sumoylated L1 at Arg687, resulting in the generation of sumoylated transmembrane L1–70, which is imported into the nucleus (9) (Fig. 11). It is conceivable that the nuclear L1–70 may regulate nuclear signaling and/or gene transcription to induce L1-dependent cellular functions. Thus, our findings imply that L1-regulated release of a 60-kDa MBP form from cerebellar neurons, the Lex-dependent binding of extracellular MBP to L1, the proteolytic cleavage of L1 by extracellular MBP, and the MBP-mediated generation and nuclear import of L1–70 may play pivotal roles in L1-dependent events during development of the nervous system, such as neurito- and myelinogenesis. These MBP-mediated L1 functions may be relevant in many of the cognate functions of L1, such as neurite outgrowth, neuronal survival, and migration, formation, and maintenance of myelin, and synaptic activity and plasticity. The unexpected link between the functions of a major myelin component and a multifunctional neural adhesion molecule of the central nervous system in connection with an important glycan remains to be further investigated in demyelinating and neurodegenerative diseases.
FIGURE 11.
Working model for the proteolytic processing of L1 by MBP. Stimulation of L1 signaling triggers attachment of one sumo molecule to full-length L1–200 (1), the formation of a fusion protein between a MBP molecule containing the exon II-encoded domain and the C-terminal (CT) part of dynamin or a related protein (2), sumoylation (3), and secretion of the sumoylated MBP-dynamin (4). The extracellular MBP-dynamin fusion protein cleaves sumoylated L1 in its extracellular domain at Arg687 and generates the transmembrane L1–70 fragment containing the intracellular domain and part of the extracellular moiety (5). The sequence of the synthetic L1-derived peptide siabind comprising amino acids 672–705 and containing the sialic binding site and the MBP cleavage site is depicted. Sumoylated L1–70 is translocated to the nucleus (6), where it may interact with nuclear proteins or with DNA to regulate nuclear signaling and/or transcription and to induce L1-dependent cellular responses and functions.
Acknowledgments
We are very grateful to Ute Bork and Emanuela Szpotowicz for excellent technical assistance, Achim Dahlmann for genotyping, Eva Kronberg for breeding and maintenance of mice, David Colman and coworkers for MBP antibodies, and Jürgen Kleinschmidt for the plasmid pDP1rs.
This work was supported by the Deutsche Forschungsgemeinschaft (KL1378/2-2 and SFB470/C3).
D. Lutz, unpublished observation.
- Lex
- Lewisx
- Lea
- Lewisa
- MBP
- myelin basic protein
- PNGase F
- peptide N-glycosidase F
- RFP
- red fluorescent protein
- FnIII
- fibronectin type III
- AAV1
- adeno-associated virus 1.
REFERENCES
- 1. Kleene R., Schachner M. (2004) Glycans and neural cell interactions. Nat. Rev. Neurosci. 5, 195–208 [DOI] [PubMed] [Google Scholar]
- 2. Maness P. F., Schachner M. (2007) Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 10, 19–26 [DOI] [PubMed] [Google Scholar]
- 3. Schmid R. S., Maness P. F. (2008) L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr. Opin. Neurobiol. 18, 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Streit A., Yuen C. T., Loveless R. W., Lawson A. M., Finne J., Schmitz B., Feizi T., Stern C. D. (1996) The Le(x) carbohydrate sequence is recognized by antibody to L5, a functional antigen in early neural development. J. Neurochem. 66, 834–844 [DOI] [PubMed] [Google Scholar]
- 5. Wing D. R., Rademacher T. W., Schmitz B., Schachner M., Dwek R. A. (1992) Comparative glycosylation in neural adhesion molecules. Biochem. Soc. Trans. 20, 386–390 [DOI] [PubMed] [Google Scholar]
- 6. Lieberoth A., Splittstoesser F., Katagihallimath N., Jakovcevski I., Loers G., Ranscht B., Karagogeos D., Schachner M., Kleene R. (2009) Lewis(x) and α2,3-sialyl glycans and their receptors TAG-1, Contactin, and L1 mediate CD24-dependent neurite outgrowth. J. Neurosci. 29, 6677–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kleene R., Yang H., Kutsche M., Schachner M. (2001) The neural recognition molecule L1 is a sialic acid-binding lectin for CD24, which induces promotion and inhibition of neurite outgrowth. J. Biol. Chem. 276, 21656–21663 [DOI] [PubMed] [Google Scholar]
- 8. Guseva D., Zerwas M., Xiao M. F., Jakovcevski I., Irintchev A., Schachner M. (2011) Adhesion molecule L1 overexpressed under the control of the neuronal Thy-1 promoter improves myelination after peripheral nerve injury in adult mice. Exp. Neurol. 229, 339–352 [DOI] [PubMed] [Google Scholar]
- 9. Lutz D., Wolters-Eisfeld G., Joshi G., Djogo N., Jakovcevski I., Schachner M., Kleene R. (2012) Generation and nuclear translocation of a sumoylated transmembrane fragment of the cell adhesion molecule L1. J. Biol. Chem. 287, 17161–17175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutz D., Wolters-Eisfeld G., Schachner M., Kleene R. (2014) Cathepsin E generates a sumoylated intracellular fragment of the cell adhesion molecule L1 to promote neuronal and Schwann cell migration as well as myelination. J. Neurochem. 128, 713–724 [DOI] [PubMed] [Google Scholar]
- 11. Strekalova H., Buhmann C., Kleene R., Eggers C., Saffell J., Hemperly J., Weiller C., Müller-Thomsen T., Schachner M. (2006) Elevated levels of neural recognition molecule L1 in the cerebrospinal fluid of patients with Alzheimer disease and other dementia syndromes. Neurobiol. Aging 27, 1–9 [DOI] [PubMed] [Google Scholar]
- 12. Wakabayashi Y., Uchida S., Funato H., Matsubara T., Watanuki T., Otsuki K., Fujimoto M., Nishida A., Watanabe Y. (2008) State-dependent changes in the expression levels of NCAM-140 and L1 in the peripheral blood cells of bipolar disorders, but not in the major depressive disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 13. Schäfer M. K., Altevogt P. (2010) L1CAM malfunction in the nervous system and human carcinomas. Cell. Mol. Life Sci. 67, 2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poltorak M., Khoja I., Hemperly J. J., Williams J. R., el-Mallakh R., Freed W. J. (1995) Disturbances in cell recognition molecules (N-CAM and L1 antigen) in the CSF of patients with schizophrenia. Exp. Neurol. 131, 266–272 [DOI] [PubMed] [Google Scholar]
- 15. Kurumaji A., Nomoto H., Okano T., Toru M. (2001) An association study between polymorphism of L1CAM gene and schizophrenia in a Japanese sample. Am. J. Med. Genet. 105, 99–104 [PubMed] [Google Scholar]
- 16. Katagihallimath N., Mehanna A., Guseva D., Kleene R., Schachner M. (2010) Identification and validation of a Lewis x glycomimetic peptide. Eur. J. Cell Biol. 89, 77–86 [DOI] [PubMed] [Google Scholar]
- 17. Sajdel-Sulkowska E. M. (1998) Immunofluorescent detection of CD15-fucosylated glycoconjugates in primary cerebellar cultures and their function in glial-neuronal adhesion in the central nervous system. Acta Biochim. Pol. 45, 781–790 [PubMed] [Google Scholar]
- 18. Brito C., Escrevente C., Reis C. A., Lee V. M., Trojanowski J. Q., Costa J. (2007) Increased levels of fucosyltransferase IX and carbohydrate Lewis(x) adhesion determinant in human NT2N neurons. J. Neurosci. Res. 85, 1260–1270 [DOI] [PubMed] [Google Scholar]
- 19. Boggs J. M. (2006) Myelin basic protein: a multifunctional protein. Cell. Mol. Life Sci. 63, 1945–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Readhead C., Takasashi N., Shine H. D., Saavedra R., Sidman R., Hood L. (1990) Role of myelin basic protein in the formation of central nervous system myelin. Ann. N.Y. Acad. Sci. 605, 280–285 [DOI] [PubMed] [Google Scholar]
- 21. Feng J. M. (2007) Minireview: expression and function of golli protein in immune system. Neurochem. Res. 32, 273–278 [DOI] [PubMed] [Google Scholar]
- 22. Harauz G., Boggs J. M. (2013) Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. J. Neurochem. 125, 334–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolf M. K., Billings-Gagliardi S. (1984) CNS hypomyelinated mutant mice (jimpy, shiverer, quaking): in vitro evidence for primary oligodendrocyte defects. Adv. Exp. Med. Biol. 181, 115–133 [DOI] [PubMed] [Google Scholar]
- 24. Readhead C., Hood L. (1990) The dysmyelinating mouse mutations shiverer (shi) and myelin deficient (shimld). Behav. Genet. 20, 213–234 [DOI] [PubMed] [Google Scholar]
- 25. Dahme M., Bartsch U., Martini R., Anliker B., Schachner M., Mantei N. (1997) Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 17, 346–349 [DOI] [PubMed] [Google Scholar]
- 26. Mikoshiba K., Takamatsu K., Tsukada Y. (1983) Peripheral nervous system of shiverer mutant mice: developmental change of myelin components and immunohistochemical demonstration of the absence of MBP and presence of P2 protein. Brain Res. 283, 71–79 [DOI] [PubMed] [Google Scholar]
- 27. Pedraza L., Fidler L., Staugaitis S. M., Colman D. R. (1997) The active transport of myelin basic protein into the nucleus suggests a regulatory role in myelination. Neuron 18, 579–589 [DOI] [PubMed] [Google Scholar]
- 28. Appel F., Holm J., Conscience J. F., von Bohlen und Halbach F., Faissner A., James P., Schachner M. (1995) Identification of the border between fibronectin type III homologous repeats 2 and 3 of the neural cell adhesion molecule L1 as a neurite outgrowth promoting and signal transducing domain. J. Neurobiol. 28, 297–312 [DOI] [PubMed] [Google Scholar]
- 29. Heller M., von der Ohe M., Kleene R., Mohajeri M. H., Schachner M. (2003) The immunoglobulin-superfamily molecule basigin is a binding protein for oligomannosidic carbohydrates: an anti-idiotypic approach. J. Neurochem. 84, 557–565 [DOI] [PubMed] [Google Scholar]
- 30. Makhina T., Loers G., Schulze C., Ueberle B., Schachner M., Kleene R. (2009) Extracellular GAPDH binds to L1 and enhances neurite outgrowth. Mol. Cell. Neurosci. 41, 206–218 [DOI] [PubMed] [Google Scholar]
- 31. Norton W. T., Poduslo S. E. (1973) Myelination in rat brain: method of myelin isolation. J. Neurochem. 21, 749–757 [DOI] [PubMed] [Google Scholar]
- 32. Loers G., Makhina T., Bork U., Dörner A., Schachner M., Kleene R. (2012) The interaction between cell adhesion molecule L1, matrix metalloproteinase 14, and adenine nucleotide translocator at the plasma membrane regulates L1-mediated neurite outgrowth of murine cerebellar neurons. J. Neurosci. 32, 3917–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loers G., Chen S., Grumet M., Schachner M. (2005) Signal transduction pathways implicated in neural recognition molecule L1 triggered neuroprotection and neuritogenesis. J. Neurochem. 92, 1463–1476 [DOI] [PubMed] [Google Scholar]
- 34. Smith G. S., Paez P. M., Spreuer V., Campagnoni C. W., Boggs J. M., Campagnoni A. T., Harauz G. (2011) Classical 18.5-and 21.5-kDa isoforms of myelin basic protein inhibit calcium influx into oligodendroglial cells, in contrast to golli isoforms. J. Neurosci. Res. 89, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grimm D., Kay M. A., Kleinschmidt J. A. (2003) Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol. Ther. 7, 839–850 [DOI] [PubMed] [Google Scholar]
- 36. Grieger J. C., Choi V. W., Samulski R. J. (2006) Production and characterization of adeno-associated viral vectors. Nat. Protoc. 1, 1412–1428 [DOI] [PubMed] [Google Scholar]
- 37. Fukumoto Y., Obata Y., Ishibashi K., Tamura N., Kikuchi I., Aoyama K., Hattori Y., Tsuda K., Nakayama Y., Yamaguchi N. (2010) Cost-effective gene transfection by DNA compaction at pH 4.0 using acidified, long shelf-life polyethylenimine. Cytotechnology 62, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tosic M., Rakic S., Matthieu J. M., Zecevic N. (2002) Identification of Golli and myelin basic proteins in human brain during early development. Glia 37, 219–228 [DOI] [PubMed] [Google Scholar]
- 39. Zhang A., Potvin B., Zaiman A., Chen W., Kumar R., Phillips L., Stanley P. (1999) The gain-of-function Chinese hamster ovary mutant LEC11B expresses one of two Chinese hamster FUT6 genes due to the loss of a negative regulatory factor. J. Biol. Chem. 274, 10439–10450 [DOI] [PubMed] [Google Scholar]
- 40. Kalus I., Schnegelsberg B., Seidah N. G., Kleene R., Schachner M. (2003) The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J. Biol. Chem. 278, 10381–10388 [DOI] [PubMed] [Google Scholar]
- 41. Chen S., Mantei N., Dong L., Schachner M. (1999) Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. J. Neurobiol. 38, 428–439 [DOI] [PubMed] [Google Scholar]
- 42. Maretzky T., Schulte M., Ludwig A., Rose-John S., Blobel C., Hartmann D., Altevogt P., Saftig P., Reiss K. (2005) L1 is sequentially processed by two differently activated metalloproteases and presenilin/γ-secretase and regulates neural cell adhesion, cell migration, and neurite outgrowth. Mol. Cell. Biol. 25, 9040–9053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. D'Souza C. A., Wood D. D., She Y. M., Moscarello M. A. (2005) Autocatalytic cleavage of myelin basic protein: an alternative to molecular mimicry. Biochemistry 44, 12905–12913 [DOI] [PubMed] [Google Scholar]
- 44. Liao M. C., Ahmed M., Smith S.O., Van Nostrand W. E. (2009) Degradation of amyloid β protein by purified myelin basic protein. J. Biol. Chem. 284, 28917–28925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsumoto-Miyai K., Ninomiya A., Yamasaki H., Tamura H., Nakamura Y., Shiosaka S. (2003) NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J. Neurosci. 23, 7727–7736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Silletti S., Mei F., Sheppard D., Montgomery A. M. (2000) Plasmin-sensitive dibasic sequences in the third fibronectin-like domain of L1-cell adhesion molecule (CAM) facilitate homomultimerization and concomitant integrin recruitment. J. Cell Biol. 149, 1485–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ursell M. R., McLaurin J., Wood D. D., Ackerley C. A., Moscarello M. A. (1995) Localization and partial characterization of a 60-kDa citrulline-containing transport form of myelin basic protein from MO3–13 cells and human white matter. J. Neurosci. Res. 42, 41–53 [DOI] [PubMed] [Google Scholar]
- 48. Hosono H., Yokosawa H. (2008) Small ubiquitin-related modifier is secreted and shows cytokine-like activity. Biol. Pharm. Bull. 31, 834–837 [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y. Q., Sarge K. D. (2008) Sumoylation of amyloid precursor protein negatively regulates Aβ aggregate levels. Biochem. Biophys. Res. Commun. 374, 673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chignola R., Cestari T., Guerriero C., Riviera A. P., Ferrari S., Brendolan A., Gobbo M., Amato S., Sartoris S., Fracasso G., Liuzzi M. G., Riccio P., Tridente G., Andrighetto G. (2000) Expression of myelin basic protein (MBP) epitopes in human non-neural cells revealed by two anti-MBP IgM monoclonal antibodies. Clin. Exp. Immunol. 122, 429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guerriero C., Zoccatelli G., Stefani E., Sartoris S., Cestari T., Riviera A. P., Tridente G., Andrighetto G., Chignola R. (2003) Myelin basic protein epitopes secreted by human T cells encounter natural autoantibodies in the serum. J. Neuroimmunol. 141, 83–89 [DOI] [PubMed] [Google Scholar]
- 52. Miller S. L., Otvös-Papp E., Prichett W., Meyer R. D. (1989) Detection and partial biochemical characterization of a novel 57,500-dalton protein in rat brain myelin. J. Neurosci. Res. 22, 262–268 [DOI] [PubMed] [Google Scholar]
- 53. Landry C. F., Ellison J. A., Pribyl T. M., Campagnoni C., Kampf K., Campagnoni A. T. (1996) Myelin basic protein gene expression in neurons: developmental and regional changes in protein targeting within neuronal nuclei, cell bodies, and processes. J. Neurosci. 16, 2452–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Staugaitis S. M., Smith P. R., Colman D. R. (1990) Expression of myelin basic protein isoforms in nonglial cells. J. Cell Biol. 110, 1719–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nagasato K., Farris R. W., 2nd, Dubois-Dalcq M., Voskuhl R. R. (1997) Exon 2 containing myelin basic protein (MBP) transcripts are expressed in lesions of experimental allergic encephalomyelitis (EAE). J. Neuroimmunol. 72, 21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Woodruff R. H., Franklin R. J. (1998) The expression of myelin basic protein exon 1 and exon 2 containing transcripts during myelination of the neonatal rat spinal cord-an in situ hybridization study. J. Neurocytol. 27, 683–693 [DOI] [PubMed] [Google Scholar]
- 57. Barbin G., Aigrot M. S., Charles P., Foucher A., Grumet M., Schachner M., Zalc B., Lubetzki C. (2004) Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol. 1, 65–72 [DOI] [PubMed] [Google Scholar]
- 58. Chen J., Wu J., Apostolova I., Skup M., Irintchev A., Kügler S., Schachner M. (2007) Adeno-associated virus-mediated L1 expression promotes functional recovery after spinal cord injury. Brain 130, 954–969 [DOI] [PubMed] [Google Scholar]
- 59. Lavdas A. A., Chen J., Papastefanaki F., Chen S., Schachner M., Matsas R., Thomaidou D. (2010) Schwann cells engineered to express the cell adhesion molecule L1 accelerate myelination and motor recovery after spinal cord injury. Exp. Neurol. 221, 206–216 [DOI] [PubMed] [Google Scholar]
- 60. Zhang Y., Bo X., Schoepfer R., Holtmaat A. J., Verhaagen J., Emson P. C., Lieberman A. R., Anderson P. N. (2005) Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc. Natl. Acad. Sci. U.S.A. 102, 14883–14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roonprapunt C., Huang W., Grill R., Friedlander D., Grumet M., Chen S., Schachner M., Young W. (2003) Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J. Neurotrauma 20, 871–882 [DOI] [PubMed] [Google Scholar]
- 62. Xu J. C., Bernreuther C., Cui Y. F., Jakovcevski I., Hargus G., Xiao M. F., Schachner M. (2011) Transplanted L1 expressing radial glia and astrocytes enhance recovery after spinal cord injury. J. Neurotrauma 28, 1921–1937 [DOI] [PubMed] [Google Scholar]
- 63. Cui Y. F., Xu J. C., Hargus G., Jakovcevski I., Schachner M., Bernreuther C. (2011) Embryonic stem cell-derived L1 overexpressing neural aggregates enhance recovery after spinal cord injury in mice. PLoS ONE 6, e17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Capello E., Voskuhl R. R., McFarland H. F., Raine C. S. (1997) Multiple sclerosis: re-expression of a developmental gene in chronic lesions correlates with remyelination. Ann. Neurol. 41, 797–805 [DOI] [PubMed] [Google Scholar]
- 65. DeBruin L. S., Haines J. D., Bienzle D., Harauz G. (2006) Partitioning of myelin basic protein into membrane microdomains in a spontaneously demyelinating mouse model for multiple sclerosis. Biochem. Cell Biol. 84, 993–1005 [DOI] [PubMed] [Google Scholar]
- 66. Derfuss T., Meinl E. (2012) Identifying autoantigens in demyelinating diseases: valuable clues to diagnosis and treatment? Curr. Opin. Neurol. 25, 231–238 [DOI] [PubMed] [Google Scholar]
- 67. Musse A. A., Harauz G. (2007) Molecular “negativity” may underlie multiple sclerosis: role of the myelin basic protein family in the pathogenesis of MS. Int. Rev. Neurobiol. 79, 149–172 [DOI] [PubMed] [Google Scholar]