Abstract
Background:
Leishmaniasis is a major worldwide public health problem with about two to three million humans threatened by this disease annually. Allium cepa (onion) is an important dietary vegetable and was used as a herbal medicine for centuries. The root of Ixora brachiata is medicinally important.
Objectives:
The aim of this study was to investigate the anti-Leishmania effect of the ethanolic and methanolic extracts of Ixora brachiata root and aqueous onion extracts on Leishmania major promastigotes.
Patients and Methods:
The parasites isolated from cutaneous leishmaniasis were exposed with different concentrations of selected plant extracts and their inhibitory effects on the promastigotes were evaluated after 24 and 48 hours.
Results:
Among tested plant extracts, Ixora brachiata root extracts revealed the best activity against Leishmania major promastigotes with IC100 value of 2.5 mg/mL and IC50 value of 0.078 mg/mL.
Conclusions:
This study showed that aqueous Allium cepa and Ixora brachiata root extracts as natural products could be used as alternative drugs in treatment of leishmaniasis.
Keywords: Leishmania major, Onions , Leishmaniasis
1. Background
Allium cepa (onion), belongs to family Gallium, is rich in sulfur. It has been traditionally used as carminative, diuretic, expectorant, stomachic as well as antispasmodic, anthelmintic, and anti-infective factor (1). In addition to, A. cepa and its extracts have been used as a poultice for alleviation of skin diseases and insect bites (2). Antibacterial, antifungal, and anthelmintic activities of this vegetable and its preparations have also been reported (3). Ixora brachiata belongs to Rubiaceae family; it is a gigantic shrub or small tree that grows in rain forests. The root of I. brachiata is medically important and although its medicinal properties are not mentioned in medical texts, tribes are using it for skin diseases. Since review of literature revealed that leishmanicidal activity of the root of I. brachiata has not been evaluated so far, the present study was conducted to investigate the in-vitro antileishmanial activity of this plant. Leishmania is intracellular parasitic hemoflagellates that infect skin macrophages and leads to visceral leishmaniasis in vertebrate hosts. Despite cardiac and renal toxicity of pentavalent antimonies such as glucantime, these drugs are still the first choice among drugs used for the treatment of leishmaniasis (4). Due to side effects of commercial drugs for treatment of these diseases, it is necessary to find effective medicinal plants, easily available, and cheap drugs for prevention of the Leishmania growth. Traditional medicines are often a good source of bioactive compounds useful against many diseases such as leishmaniasis. Hence, we evaluated the antileishmanial effect of aqueous onion and I. brachiata extracts on the Leishmania promastigotes. Moreover, antileishmanial activity of many plant extracts were reported in previous studies (5-8). Although literature on I. brachiata root extract has shown good antidermatophytic activity (9), the effect of this plant has not been reported on Leishmania parasites up to this date. In addition, previous studies showed that all tested strains of Leishmania were sensitive to the onion juice (10).
2. Objectives
The aim of this study was to investigate the antileishmanial effect of the ethanolic and methanolic extracts of I. brachiata root and aqueous onion extracts against Leishmania major promastigotes.
3. Patients and Methods
3.1. Plant Material and Extract Preparation
The roots of I. brachiata were collected from district Ratnagiri, Maharashtra State, India. Fresh A. cepa (onion) was prepared from local market. They were flayed, cut into small pieces, and crushed in a blender. The homogenate was filtered through cleansing cloth. The filtrate was centrifuged and the supernatant of onion extract was filtrated, then sterilized with the help of 0.22 um Millipore filter (10) and stored at -20˚C. Aqueous Allium cepa extract was used immediately after the extraction. The dried and powdered leaves (10 g) from plants were extracted with ethanol 80 % by maceration. The crude extracts were obtained after evaporation under room temperature. According to procedure of described in previous studies, an aliquot of 1 g dried plant extracts was dissolved in 5 mL dimethyl sulfoxide (DMSO 1% solvent) as stock to prepare serial double dilutions to working concentrations of 0.078-20 mg/mL (11). All extracts were kept at 4˚C until used in future experiments.
3.2. Parasites
The L. major promastigotes were maintained in blood agar medium supplemented with RPMI-1640 medium (Sigma chemical Co.) and 5% fetal bovine serum (FBS) at 26˚C and were suspended in RPMI-1640 medium to adjust to a final concentration of 10 × 106 parasites/mL.
3.3. Determination of the 50% Effective Concentration
The leishmanicidal activity of the plant extracts of aqueous A. cepa and I. brachiata root on parasites in the promastigote stage were assessed by using 96-well microplate. For determination of the 100% and 50% inhibition concentration (IC100 and IC50, respectively), each well was filled with 100 µL of the parasites suspension (1 × 106 parasites/mL). Subsequently, 100 µL serial dilutions of the selected plant extracts were added to the same wells of microplate and the plate was incubated at 26˚C for 48 hours. A negative control (DMSO 1% solvent without any plant extract) and positive control (85 mg/mL of Glucantime) were used on the same plate. This is worthy of mention that neither the ethanol nor DMSO (up to 1%) used in tests had an effect on promastigotes (12). At the end of the incubation time, the plate was shaken over a plate shaker and the number of promastigotes in each concentration was calculated using a hemocytometer slide. Each assay was repeated three times. The surviving parasites were enumerated.
4. Results
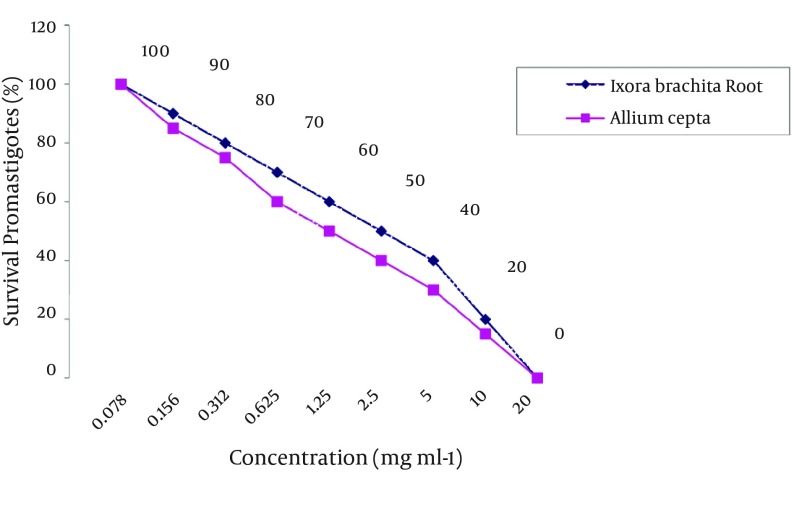
In the present investigation, extracts from selected plants were assayed for their in vitro leishmanicidal activity against L. major promastigotes cultures. The viability of L. major promastigotes in the concentration of 0.312 mg/mL ethanolic and methanolic extracts of I. brachiata root was 20% while over 80% of promastigotes in this concentration were passive. In addition, at the concentration of 2.5 mg/mL ethanolic and methanolic extracts of I. brachiata root, 100% of the L. major promastigotes were unmovable and the viability of the L. major promastigotes in the same concentration was 0%. The viability of the L. major promastigotes in the concentration of 0.312 mg/mL aqueous onion extracts was 80% and in the same concentration, 20% of the L. major promastigotes were unmovable. At the concentration of 2.5 mg/mL aqueous A. cepa extracts, 70% of the L. major promastigotes were unmovable and the viability of promastigotes was 30%. Moreover, in the concentration of 5.0 mg/mL of aqueous A. cepa extracts, 100% of the L. major promastigotes were unmovable and the viability of parasites in this concentration was 0% (Table 1 and Figure 1).
Table 1. In Vitro Antileishmanial Activity of Ixora brachiata Root and Aqueous Allium cepa Extracts a.
| Plant Material | Promastigotes | |
|---|---|---|
| IC100 Value, mg/mL | IC50 Value, mg/mL | |
| Ethanolic extract of Ixorabrachiata root | 2.5 | 0.078 |
| Aqueous onion | 5.0 | 1.25 |
| Control drug | ||
| Glucantime | 8.5 | 21.25 |
a IC50 and IC100 are the sample concentrations that inhibited 50% and 100% the growth of parasites.
Figure 1. The Viability of Leishmania major Promastigotes in the Various Concentration of Aqueous Allium cepa and I. brachita Extracts.
5. Discussion
Saleheen et al. (10), have reported aqueous A. cepa extracts (AOE) gave an IC100 and average IC50 values of 1.25 mg/mL and 0. 376 mg/mL, respectively, against tested L. major promastigotes (10). These figures are close to our finding in the present study. According to the mentioned reports above, the L. major promastigotes inhibited by the ethanolic and methanolic extracts of I. brachiata root had low viability (20%) while the L. major promastigotes inhibited with aqueous A. cepa extracts had high viability (80%). It was interesting to note that the ethanolic as well as methanolic extracts of I. brachiata root and aqueous onion revealed potential leishmanicidal activity, as shown in Tabe1, with the IC50 values of 0.078 and 1.25 mg/mL, respectively. These were comparable to the effects of glucantime (IC50 = 21.25 mg/mL) used as positive control. The IC100 values of tested plant extracts were 2.5-5.0 mg/mL, which were comparable to the IC100 of 85 mg/mL glucantime. These results revealed that the the tested plant extracts had high potential antileishmanial activity in comparison to glucantime (IC50 of 21.25 mg/mL and IC100 of 85 mg/mL). However, according to the previous studies, some other plant extracts such as Allium sativum, Plagiochila disticha, and Casearia sylvestris also have exhibited inhibitory activities against Leishmania (13). Moreover, the presence of compounds such as tannins, flavonoids, saponin carbohydrates, coumarins, and triterpenes reported by previous studies (9), may be responsible for leishmanicidal activity in this plant. These results are supported with the observations of Marine et al. and Firdous et al. (14, 15), who have shown the leishmanicidal activity of the flavonoids isolated from Consolida oliveriana and the efficacy of the carbohydrates in treatment of leishmaniasis. In addition, tannins isolated from Anogeissus leiocarpus was shown to possess a good activity against Leishmania (16). Other compound such as terpenoids (sesquiterpene lactones) have been reported to be active against Leishmania (17).
This study revealed that selected plants in this investigation contained potent compound with high potential leishmanicidal activity. Although glucantime is very toxic, it is still used because other drugs have been shown to be of variable effect or ineffective against the parasites (18). Since commercial drugs for treatment of leishmaniasis have many side effects, it would be timely to find effective medicinal plants in herbal medicine for the investigation and isolation of their active compound and the study of their toxicity.
Acknowledgments
There were no acknowledgments.
Footnotes
Implication for health policy/practice/research/medical education:This work was conducted for the first time because antileishmanial Effect of Ixora brachiata Roxb. Root extract on Leishmania major promastigotes had not been reported to the date of study.
Authors’ Contribution:All authors were in the same.
Financial Disclosure:There was no conflict of interest.
Funding/Support:There was no financial support.
References
- 1.Leung A. Encyclopedia of common natural ingredients used in food, drugs and cosmetics. New York, NY: John Wiley; 1980. [Google Scholar]
- 2.Vohora SB, Rizwan M, Khan JA. Medicinal uses of common Indian vegetables. Planta Med. 1973;23(4):381–93. doi: 10.1055/s-0028-1099460. [DOI] [PubMed] [Google Scholar]
- 3.Elnima EI, Ahmed SA, Mekkawi AG, Mossa JS. The antimicrobial activity of garlic and onion extracts. Pharmazie. 1983;38(11):747–8. [PubMed] [Google Scholar]
- 4.Mendonca-Filho RR, Rodrigues IA, Alviano DS, Santos AL, Soares RM, Alviano CS, et al. Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocos nucifera Linn. (Palmae). Res Microbiol. 2004;155(3):136–43. doi: 10.1016/j.resmic.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Mirzaie M, Nosratabadi SJ, Derakhshanfar A, Sharif I. Antileishmanial activity of Peganum harmala extract on the in vitro growth of Leishmania major promastigotes in comparison to a trivalent antimony drug. Vet Arh. 2007;77(4):365. [Google Scholar]
- 6.Raynaud-Le Grandic S, Fourneau C, Laurens A, Bories C, Hocquemiller R, Loiseau PM. In vitro antileishmanial activity of acetogenins from Annonaceae. Biomed Pharmacother. 2004;58(6-7):388–92. doi: 10.1016/j.biopha.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Hoet S, Opperdoes F, Brun R, Adjakidje V, Quetin-Leclercq J. In vitro antitrypanosomal activity of ethnopharmacologically selected Beninese plants. J Ethnopharmacol. 2004;91(1):37–42. doi: 10.1016/j.jep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Feily A, Saki J, Maraghi S, Moosavi Z, Khademvatan S, Siahpoosh A. In vitro activity of green tea extract against Leishmania major promastigotes. Int J Clin Pharmacol Ther. 2012;50(3):233–6. doi: 10.5414/cp201571. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi-Nejad B, Deokule SS. Antidermatophytic activities of Ixora brachiata Roxb. Afr J Biochem Re. 2009;3(10):344–8. [Google Scholar]
- 10.Saleheen D, Ali SA, Yasinzai MM. Antileishmanial activity of aqueous onion extract in vitro. Fitoterapia. 2004;75(1):9–13. doi: 10.1016/j.fitote.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghi-Nejad B, Saki J, Khademvatan S, Nanaei S. In vitro antileishmanial activity of the medicinal plant—Satureja khuzestanica Jamzad. J Med Plant Res. 2011;5(24):5912–5. [Google Scholar]
- 12.Braga FG, Bouzada ML, Fabri RL, de OM, Moreira FO, Scio E, et al. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J Ethnopharmacol. 2007;111(2):396–402. doi: 10.1016/j.jep.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Sharma U, Velpandian T, Sharma P, Singh S. Evaluation of anti-leishmanial activity of selected Indian plants known to have antimicrobial properties. Parasitol Res. 2009;105(5):1287–93. doi: 10.1007/s00436-009-1554-2. [DOI] [PubMed] [Google Scholar]
- 14.Marin C, Boutaleb-Charki S, Diaz JG, Huertas O, Rosales MJ, Perez-Cordon G, et al. Antileishmaniasis activity of flavonoids from Consolida oliveriana. J Nat Prod. 2009;72(6):1069–74. doi: 10.1021/np8008122. [DOI] [PubMed] [Google Scholar]
- 15.Firdous R, Yasinzai M, Ranja K. Efficacy of glucantime in the treatment of Old World cutaneous leishmaniasis. Int J Dermatol. 2009;48(7):758–62. doi: 10.1111/j.1365-4632.2009.04072.x. [DOI] [PubMed] [Google Scholar]
- 16.Shuaibu MN, Wuyep PT, Yanagi T, Hirayama K, Ichinose A, Tanaka T, et al. Trypanocidal activity of extracts and compounds from the stem bark of Anogeissus leiocarpus and Terminalia avicennoides. Parasitol Res. 2008;102(4):697–703. doi: 10.1007/s00436-007-0815-1. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt TJ. Structure activity relationships of Sesquite lactones. Stud Nat Prod Chem. 2002;3:309–92. doi: 10.1016/S1572-5995(06)80030-X. [DOI] [Google Scholar]
- 18.Lira R, Sundar S, Makharia A, Kenney R, Gam A, Saraiva E, et al. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J Infect Dis. 1999;180(2):564–7. doi: 10.1086/314896. [DOI] [PubMed] [Google Scholar]