Abstract
Seeking a remedy for the radiation fear in Japan, the author re-examined an article on radiation hormesis. It describes the background for this fear and evidence in the first UNSCEAR report of a reduction in leukemia of the Hiroshima survivors in the low dose zone. The data are plotted and dose-response models are drawn. While UNSCEAR suggested the extra leukemia incidence is proportional to radiation dose, the data are consistent with a hormetic J-shape and a threshold at about 100 rem (1 Sv). UNSCEAR data on lifespan reduction of mammals exposed continuously to gamma rays indicate a 2 gray/year threshold. This contradicts the conceptual basis for radiation protection and risk determination established in 1956–58. In this paper, beneficial effects and thresholds for harmful effects are discussed, and the biological mechanism is explained. The key point: the rate of DNA damage (double-strand breaks) caused by background radiation is 1000 times less than the endogenous (spontaneous) rate. It is the effect of radiation on an organism’s very powerful adaptive protection systems that determines the dose-response characteristic. Low radiation up-regulates the protection systems, while high radiation impairs these systems. The remedy for radiation fear is to expose and discard the politicized science.
The great tragedy of science—the slaying of a beautiful hypothesis by an ugly fact.
—Huxley TH. English biologist (1825–1895)
INTRODUCTION
Almost three years have passed since a major earthquake and devastating tsunami damaged the Fukushima-Daiichi nuclear power plant. An evacuation order forced 70,000 people to leave the area, while an additional 90,000 left voluntarily and subsequently returned. Many of those who left under the forced order have not gone back to their homes as removal of radioactivity continues. Approximately 1,600 people died, mainly due to psychological stress, in the evacuation process (Mainichi 2013)—about the same number of deaths in the Fukushima prefecture from the earthquake and tsunami combined (Japan National Police Agency 2013). The United Nations Committee on the Effects of Atomic Radiation reported that no health effects attributable to radiation were observed (UNSCEAR 2012). The World Health Organization’s health risk assessment (WHO 2013), employing invalid LNT methodology (there is no other method), estimates the increased lifetime risks of cancer and calculates the cumulative risks for the 15 years following the radioactive release. The radiation levels in the evacuated areas were within the range of naturally occurring radiation. No adverse effects of those higher doses have ever been observed (Jaworowski 1999). The precautions taken to avoid hypothetical health risks that are highly questionable have proved to be very harmful.
The tragedy is that the radiation dose-response characteristic for leukemia in humans had been determined in 1958, but it was disregarded because of the policy decision to adopt the linear no-threshold (LNT) dose-response model. The threshold model had been the “gold standard” for medicine and physiology since the 1930s; however, in 1956, the US National Academy of Sciences adopted the LNT model for evaluating genomic risks due to ionizing radiation. The Genetics Panel members believed there was no safe exposure for reproductive cells. They thought that the mutation risk increased with even a single ionization. In 1958, the National Committee for Radiation Protection and Measurement generalized the LNT concept to somatic cells and cancer risk assessment. Soon after, the other national and international organizations adopted this model for radiation-induced genetic and cancer risks (Calabrese 2013a, 2013b).
Why is the dose-response for leukemia so important? Fliedner et al. (2012) point out that the hemopoietic cell system in mammals is generally more radiosensitive than the gastrointestinal cell system or skin. Hemopoietic failure occurs at doses lower than the doses that cause the GI-tract failure or acute skin damage. Therefore, radiation-induced leukemia is expected to occur at lower doses and much sooner than other radiation-induced cancers. So, the dose-response behaviour for leukemia would be an indication of the likely dose-response for these other cancers. Less radiosensitive cells are affected to a greater degree by the non-radiogenic factors that contribute to their transformations into malignancy. With increased latency, the effects of these confounding factors are more difficult to correct for when estimating the effect of radiation. In the latest update to the atomic bomb survivor data (Ozasa et al. 2012), the authors have claimed that zero dose is the best estimate for a dose threshold for solid cancer mortality, apparently supporting the LNT model. However, their analysis restricted the possible functional forms of the dose-response relationship a priori. An analysis that used a more general functional form to fit the data demonstrates that a dose threshold cannot be excluded (Doss 2013). And a recent analysis of this data using artificial neural networks reveals the presence of thresholds and negative risk (Sasaki et al. 2014).
RADIATION HORMESIS - A REMEDY FOR FEAR
The enormous social fear and media frenzy surrounding the release of radioactivity from the damaged Fukushima NPP led the author to study again the facts presented in a remarkable paper by Jaworowski (2010) on radiation hormesis. He described the exaggerated fear of irradiating healthy tissues that arose during the Cold War period with its massive production and incessant testing of nuclear weapons. Radioactive materials from the atmospheric tests spread over the whole planet. People were quite rightly scared of the terrifying prospect of a global nuclear war and large doses of radiation from fallout. However, it was the leading physicists responsible for inventing nuclear weapons who instilled a fear of small doses in the general population. In their highly ethical endeavour to stop preparations for atomic war, they were soon joined by many scientists from other fields. Eventually, this developed politically into opposition against atomic power stations and all things nuclear.
As discussed later, the justifications of physicists and their followers were invalid, but they were effective; atmospheric tests were stopped in 1963. However, this was achieved at a price—a terrifying specter had emerged of small, near zero radiation doses endangering all future generations. Jaworowski explains in his paper that this became a long-lived and worldwide societal affliction nourished by the LNT assumption, according to which any dose, even that close to zero, would contribute to the disastrous effect. Radiation hormesis (Luckey 1991) is an excellent remedy for this affliction, and it is perhaps for this reason he believes that it has been ignored and dismissed over the past half century. What happened more than 50 years ago still influences the current thinking of both the decision makers and those who elect them (Jaworowski 2010).
He points out that the linearity assumption was not confirmed by early or later epidemiological studies of Hiroshima and Nagasaki survivors. No hereditary disorders were found in the children of highly irradiated parents (Sanders 2010). UNSCEAR was concerned mainly with the effects of nuclear tests, fulfilling a political task to stop weapons testing. The committee had mixed opinions regarding the LNT model, and its first report, UNSCEAR 1958, contains conflicting statements. Jaworowski states: “hormesis is clearly evident . . . in a table showing leukemia incidence in the Hiroshima population, which was lower by 66.3% in survivors exposed to 20 mSv, compared to the unexposed group (p.165). This evidence of radiation hormesis was not commented upon. Since then, the standard policy line of UNSCEAR and of international and national regulatory bodies over many decades has been to ignore any evidence of radiation hormesis and to promote LNT philosophy.”
The very important data in UNSCEAR 1958, Table VII were not presented in graphical form. In Figure 1, the tabulated data were plotted and two dose-response models were drawn—a hormetic J-shape line and a straight line (the LNT model) from 1300 to 0 rem.1 A line through 100 rem was added to take into account Footnote c, which states that the doses in Zone C “were greater than 50 rem.” UNSCEAR 1958, paragraph 31, states: “In zones A (1300 rem), B (500 rem), and C (50 rem), the values of PL were calculated2 to be . . . This finding was taken to support the suggestion that the extra leukemia incidence is directly proportional to radiation dose, and conversely to argue against the existence of a threshold for leukemia induction.” However, Figure 1 shows that the data are more consistent with a hormetic J-shape for dose response and a threshold dose at about 100 rem (1 Sv).
UNSCEAR 1958. TABLE VII.
Leukemia incidence for 1950–57 after exposure at Hiroshimaa
| Zone | Distance from hypocentre (metres) | Dose (rem) | Persons exposed | L (Cases of leukemia) | Nb (total cases per 104) | |
|---|---|---|---|---|---|---|
| A | under 1,000 | 1,300 | 1,241 | 15 | 3.9 | 12,087 ± 3,143 |
| B | 1,000–1,499 | 500 | 8,810 | 33 | 5.7 | 3,746 ± 647 |
| C | 1,500–1,999 | 50c | 20,113 | 8 | 2.8 | 398 ± 139 |
| D | 2,000–2,999 | 2 | 32,692 | 3 | 1.7 | 92 ± 52 |
| E | over 3,000 | 0 | 32,963 | 9 | 3.0 | 273 ± 91 |
Based on data in reference 13 (Wald N. Science 127:699–700. 1958). Prior to 1950 the number of cases may be understated rather seriously.
The standard error is taken as: N times ( ).
FIGURE 1.
Leukemia incidence in the Hiroshima survivors for 1950–57.
The discussion in paragraph 33 states “that a threshold for leukemia induction might occur. In fact, according to Table VII a dose of 2 rem is associated with a decreased leukemia rate.” But this observation was rejected because “the estimates of dose ... are much too uncertain ...” UNSCEAR should not have ignored its observation of a decreased leukemia incidence (based on one standard deviation of uncertainty) for the 32,692 survivors in Zone D, which was below the leukemia incidence of the 32,963 survivors in Zone E (the controls). The evidence of a threshold at about 100 rem or 1 Sv disproved the LNT dose-response model, and UNSCEAR should have rejected the LNT model in its report. There are no grounds for suggesting an increased risk of leukemia following an acute radiation dose below 50 rem or 500 mSv.
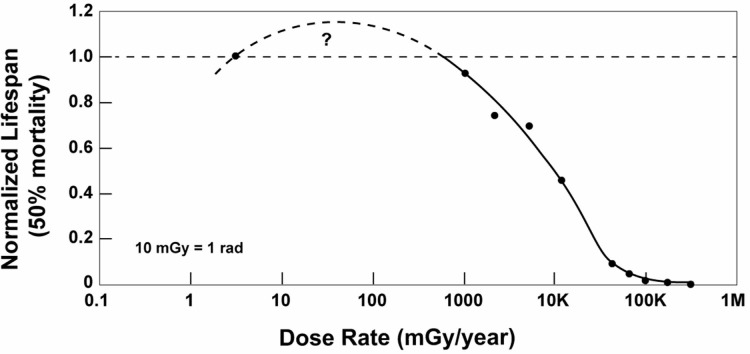
The discussion above concerns evidence of a threshold for an acute exposure. There is also evidence of a threshold for chronic irradiations, which is relevant for the residents of the Fukushima prefecture. Fliedner et al. (2012) pointed out that bone marrow stem cells, which produce the blood cell components, are very sensitive to radiation, yet they are remarkably resistant to chronic low-dose exposure regarding function and maintenance of blood supply. Their results of the lifetime irradiations of the beagle dogs at various dose rates demonstrate that lifespan increases as dose rate decreases, as expected. Mortality due to failures of the hematopoietic system decreased and mortality due to cancer increased, as the dose rate decreased. It is very surprising that the 92 dogs in the group exposed to 3 mGy/day (1100 mGy/year) had life spans that were almost as long as those in the control group, and the causes of death in these dogs were similar to those in the control group, dominated by fatal tumor. Figure 2, a graph of normalized lifespan versus dose rate, indicates a threshold at 700 mGy/year for the onset of lifespan reduction. There was no evidence that any of these chronic irradiations increased the risk of fatal tumors. This evidence—a threshold at 700 mGy per year and the absence of increased lifetime cancer risk with chronic irradiation—adds to many other data of this kind (Muckerheide 2000, Sanders 2010) and should cause UNSCEAR, the NAS and all radiation protection organizations to revoke the generalized link they created in 1958 between low radiation and a risk of cancer. This link is the basis for the fear we see today.
FIGURE 2.
Lifespan versus radiation level, (Cuttler 2013).
Regarding the present concern about radiation-induced “health effects” on the residents around the Fukushima NPP, UNSCEAR states that that none were observed (UNSCEAR 2012, Chapter IIB, Section 9(a)) and discusses in Chapter III, Section 1 the “difficulties in attributing health effects to radiation exposure and inferring risks.” Section 2 points out that failure to properly address uncertainties can cause anxiety and undermine confidence among the public, decision-makers and professionals. If it wished, UNSCEAR could have attributed beneficial health effects to the low radiation, based on the extensive evidence in Annex B of its UNSCEAR 1994 report. This report contains summaries of 192 studies on adaptive responses. There have also been hundreds of additional scientific studies published during the subsequent 20 years.
BENEFICIAL EFFECTS
Positive health effects from low dose radiation were identified by medical scientists and practitioners soon after x-rays and radioactivity were discovered in 1895–96. High, short-term exposures were harmful, but low acute doses or low dose-rate long-term exposures were beneficial. Often this was found inadvertently, while diagnosing bone fractures or other medical conditions. Recent review papers describe accepted medical applications, such as accelerated healing of wounds and infections, cancer cures, and treatments of inflammations and arthritis, before the introduction of the low dose radiation cancer scare in the late 1950s (Cuttler 2013). A new review discusses the historical use of low radiation to cure pneumonia (Calabrese 2013c), a very common occurrence in hospitals.
Beneficial effects of low dose radiation have been known and studied for well over a century. The mechanism is explained in a medical textbook, in a chapter by Feinendegen et al. (2013). The key point is the discovery more than 25 years ago that spontaneous (endogenous) DNA damage, by the attack of reactive oxygen species (ROS), occurs at a relatively very high rate compared to the damage rate caused by natural background radiation. The natural rate of single-strand breaks from ROS attacks per average cell is many millions of times greater than the rate induced by ∼ 1 mGy per year. Single-strand breaks are readily repaired, but double-strand breaks (DSBs) are relevant to induction of cancer and other genetic changes. Non-irradiated cells contain from about 0.1 to numerous DSBs at steady state. This agrees with the calculated probability of 0.1 for a DSB to occur per average cell in the human body per day from endogenous, mainly ROS sources (Pollycove and Feinendegen 2003). The probability of a radiogenic DSB to occur per day in background radiation is on average only about 1 in 10,000 cells. So the ratio of spontaneous to radiogenetic DSBs produced per day is about 1,000; i.e., the natural damage rate is a thousand times greater than the damage rate due to background radiation.
The critical factor is the effect of radiation on an organism’s very powerful biological defences and protection systems, which involve the actions of more than 150 genes. They act on all of the damage that is occurring (and its consequences) due to both internal causes and the effects of external agents. Although a low radiation dose or low level radiation causes cell damage, it up-regulates adaptive protection systems in cells, tissues, animals and humans that produce beneficial effects far exceeding the harm caused by the radiation (Feinendegen et al. 2013). The net beneficial effects are very significant in restoring and improving health. The detailed behaviours of the defences are very complex, but the evidence is very clear. They range from prevention/cure of cancers to the very important medical applications of enhanced adaptive protections in the responses to stresses and enhanced healing of wounds, curing of infections, and reduction of inflammation, as mentioned earlier. In contrast, high level irradiation impairs these systems.
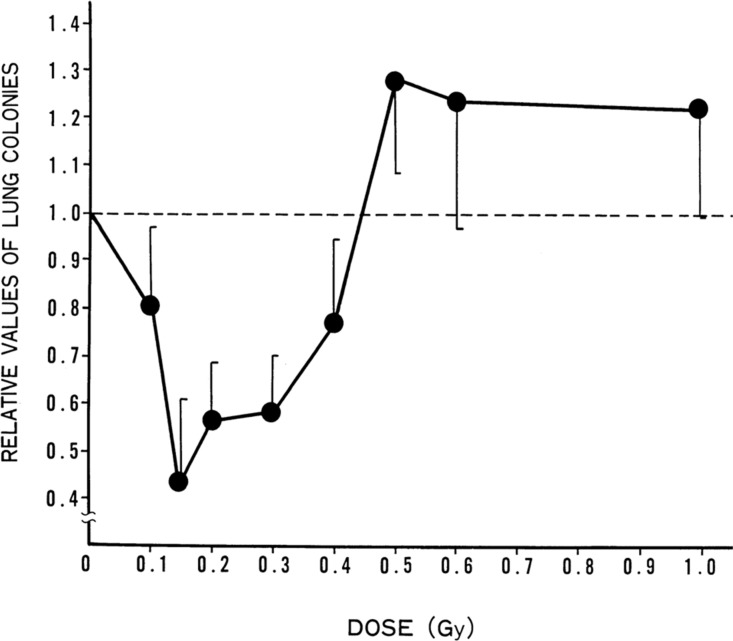
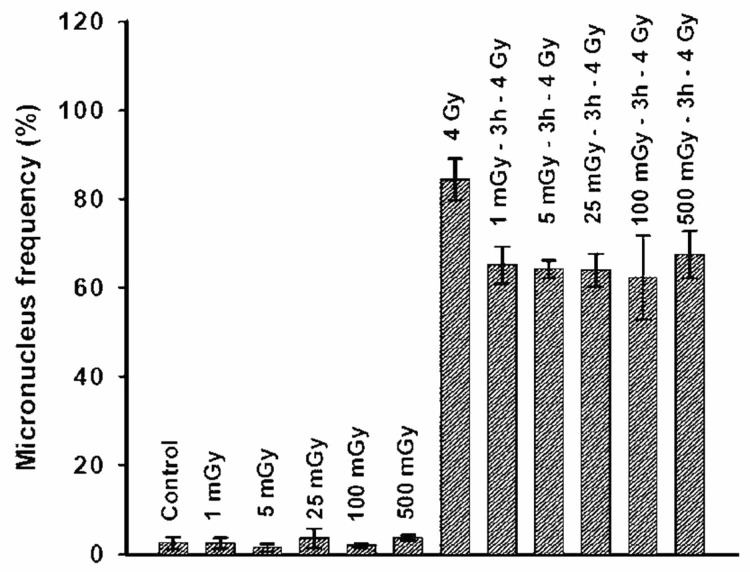
This mechanism was demonstrated in fundamental studies by Kiyohiko Sakamoto, starting in 1975, on mice and later on human cancer patients (Sakamoto 2004). Figure 3 demonstrates the effect of a single whole-body dose of x-rays. The up-regulated immune system destroys significantly more spontaneous lung cancer metastases. The data suggest that that effectiveness for suppression of lung-colony formation peaks at about 150 mGy. Experimental results from Mitchel (2007a) demonstrate the adaptive response in cells that are given a conditioning dose of gamma radiation at a low dose rate (3 mGy/min) in the range from 1 to 500 mGy, three hours before receiving a high dose (4 Gy) at a high dose rate (1.8 Gy/min). The 4 Gy dose often results in a break in one or more chromosomes (DNA double-strand breaks). If cells divide before repairing those breaks, the remaining pieces are packaged into micronuclei. Figure 4 shows the frequency of micronuclei in cells that have been allowed to repair, an indication of cell repair competence. The low radiation dose up-regulates the cell repair process, which results in fewer broken chromosomes from the 4Gy dose. This enhanced repair capability occurs after a dose of just 1 mGy, an average of a single ionization track per cell.
FIGURE 3.
Effect of TBI dose on spontaneous lung metastasis: TBI given 12 days after tumor-cell transplantation into groin, (Sakamoto 2004) Figure 12.
FIGURE 4.
Low doses enhance the repair of broken chromosomes in human cells, (Mitchel 2007a) Figure 2.
THRESHOLDS FOR HARMFUL EFFECTS
The evidence of net beneficial effects requires the determination of the threshold for harmful effects. This was known through more than thirty years of human experience when the first radiation protection tolerance dose, 0.2 roentgen per day or ∼ 700 mGy per year, was established for radiologists in the early 1930s. Figure 2 is the result of a recent assessment of lifespan data for dogs exposed to cobalt-60 gamma radiation (Cuttler 2013). The threshold for net harm is also ∼ 700 mGy per year. Similar data are found in UNSCEAR 1958, Annex G, p. 162. The threshold for lifespan reduction of mice and Guinea pigs exposed to radium gamma rays is 4 roentgen per week or ∼ 2000 mGy per year. Their mean survival time is 7% longer than the controls at a dose rate of 0.5 roentgen per week, which is about 240 mGy per year.
The accepted threshold for recognizing harmful late effects after a short-term exposure, according to a large set of experimental and epidemiological data, is an absorbed dose of about 100 mGy. However, the UNSCEAR data for leukemia incidence among the Hiroshima survivors, shown in Figure 1, suggest a threshold of about 500 mGy for leukemia.
INVALID BASIS FOR THE LNT MODEL
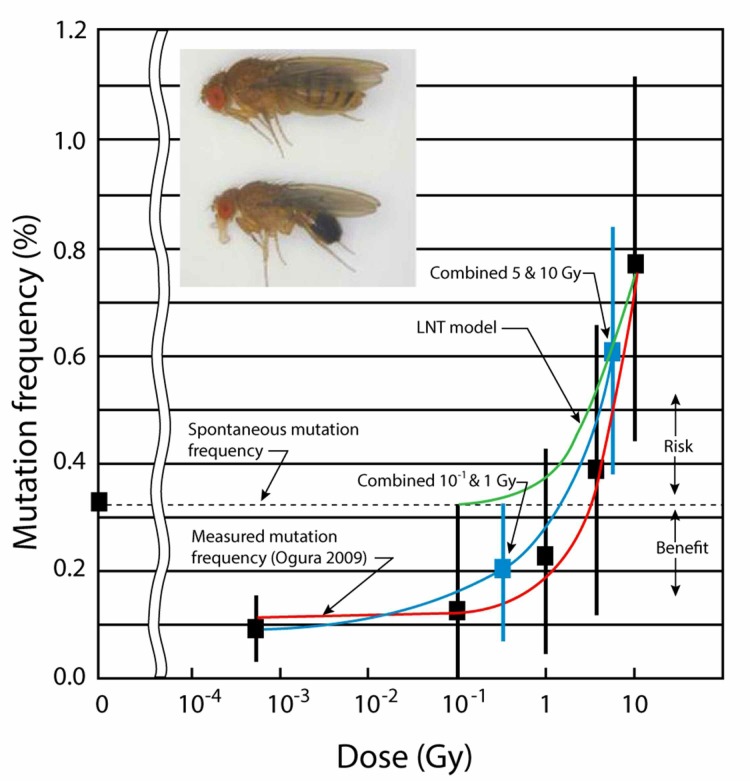
Calabrese reviewed the evolution of radiation protection from the tolerance dose (threshold) concept to the LNT concept. It began when early geneticists discovered that large numbers of mutations could be induced in germ cells of fruit flies by ionizing radiation. This would enable eugenicists to modify organisms for utilitarian purposes (Muller 1927). A high dose, at a high rate, produced a mutation rate that was 150 times greater than the spontaneous rate. This and other high-dose studies indicated that the mutation rate was proportional to the dose. A radiation target theory was developed by physicists to model the process of radiation-induced mutation, with mathematical calculations related to quantum mechanics (Calabrese 2013a). They established a conceptual framework for gene structure, target theory for the induction of mutations by ionizing radiation, the single-hit mechanism hypothesis to account for the shape of the LNT dose response and the application of this dose-response model for what was to become modern cancer risk assessment. However, organisms do not behave according to this model. The Caspari and Stern (1948) study that irradiated 50,000 fruit flies to a dose of ∼ 50 roentgen at a low rate, revealed a mutation rate that was the same as the 50,000 controls. This study was ignored. Recent studies on fruit flies at very low dose rate indicate a mutation frequency far below the spontaneous rate—genetic benefit instead of risk—below an absorbed dose of about 1 Gray, Figure 5 (Cuttler 2013). This evidence clearly falsifies the LNT model.
FIGURE 5.
Fruit fly mutation frequency versus radiation dose (Cuttler 2013). A binomial distribution is assumed for the occurrence of the mutations. Each error bar is two standard deviations from the mean frequency. The data points at 0.3 Gy (0.19%) and at 7 Gy (0.61%) are obtained by “pooling” the Ogura et al. (2009) data at 10−1 and 1 Gy, and at 5 and 10 Gy, respectively. Note that the mean mutation frequency is below the spontaneous level (0.32%) when the dose is below 1 Gy.
DISCUSSION
Many researchers use the LNT model to predict the lifetime risk of excess cancer from a small dose of radiation. They calculate the expected excess cancer incidence from a very low dose by connecting a straight line between the zero-dose, zero-incidence point and the high-dose cancer incidence data of the atomic bomb survivors. This procedure can only yield a positive risk of cancer. Most epidemiological studies are designed to measure radiation-induced cancer incidence, so they do not report any observations of beneficial effects. The data are fitted to the LNT model, presuming it is valid. Scott et al. (2008) list seven approaches that make it difficult to recognize bio-positive effects and thresholds, concluding that there is no credible evidence to support the contention that CT scans will cause future cancers. Scott (2008) points out three epidemiological “tricks” that are commonly employed to obtain a LNT curve. Relative risk and odds ratio values are often shown instead of cancer incidence data. Jaworowski’s (1999, 2010) discussion, of the political and vested interests behind the activities of many scientists to sustain the radiation scare, explains the misrepresentations of data and deceptions that have been carried out to fit the LNT model since the 1950s.
Mitchel (2007b) has reviewed the radiation protection methodology employed to calculate radiation risk estimates for humans. He pointed out many contradictions with the biological evidence. Radiation protection practice has been based on the LNT concept, extrapolating high dose epidemiological data to predict risk at low dose, many auxiliary concepts and assumptions, and radiation physics models. The radiation protection system uses dose as a surrogate for risk. The biological information indicates this is incorrect.
Regulations for radiation protection have a large impact on health, the environment and the economy; they should be based on science. This requires strict adherence to The Scientific Method (Seiler and Alvarez 1994, 1998). When the term risks of health effects is used, two aspects are involved—the analysis of risks and the valuation and management of these risks. The latter are social issues. Risk analysis uses scientific models to predict consequences of events by calculating the probabilities for their occurrences. Although the uncertainties may be considerable, that does not change the character of the discipline; the scientific method is applied as rigorously as it can be. The basic tools are scientific concepts and models, which need to be subjected to rigorous tests to be useful and credible. The method has six basic requirements;
Sufficiency of information: A sufficient amount of data is needed to support the formulation of a hypothesis or a model. In some cases, a lack of data is replaced by convenient assumptions without subjecting these assumptions to a reality test.
Replicability of critical experiments: Confirmation by repeated experiments by the same experimenter and by others is one of the key requirements of the scientific method.
Comprehensiveness of data evaluation: All data available must be addressed in evaluating the model or hypothesis. Contradictory data sets must not be ignored or set aside without a valid reason.
Logical approach: Models and hypotheses should follow in a logical manner from the data available and be free from internal inconsistencies.
Scientific honesty: This is one of the most important but often also one of the most difficult requirements. An author and the supporters of a model or a hypothesis must avoid self-deception in evaluating the requirements listed above. Vigorous peer review is an indispensable ingredient.
Falsifiability of hypotheses and models: Theories must be subjected to tests that may prove them wrong. This requirement is the cornerstone of the scientific method. If it is known that a low dose-rate exposure to a toxicant exists in a population, it is imperative to perform a test as to whether our knowledge allows the statement that the probability of health effects due to this exposure is different from zero. Once that fact has been established, then the question arises as to how large the risk is and how well we know it.
The application of the scientific method is a way to impose accountability and ethics on the practitioners of a science. Trying to evade the requirements of the scientific method could be interpreted as an attempt to avoid accountability and scientific honesty, and substitute another agenda (Seiler and Alvarez 1994).
CONCLUSIONS AND RECOMMENDATIONS
Social concerns about the safety of all nuclear technologies is caused by ideological linkage of any (human-made) radiation exposure to an excess risk of health effects, namely cancer and genetic harm, using the LNT model to calculate excess health risks. This link, created in the 1950s to stop the development and production of nuclear weapons, is maintained in spite of the extensive biological evidence of beneficial effects from low dose or low dose rate exposures. Ignoring biological facts and refusing to revert to the threshold model concept for radiation protection has created an enormous barrier against social acceptance of nuclear energy and the use of radiation-based medical diagnostics. The remedy is to discard this politicized science.
This enormous Fukushima-Daiichi radiation scare is a very serious crisis. It should be viewed as an opportunity to make changes in attitudes and concepts that would not otherwise be possible.
The following three fundamental messages should be communicated to everyone in order to explain the real effect of radiation on health and to eliminate the irrational fear.
Spontaneous DNA damage, mainly from reactive oxygen species, occurs at very high rate; the rate of double-strand breaks (DSBs) is more than 1000 times the rate of DSBs induced by a background radiation level of 1 mGy per year.
Biological organisms have very powerful adaptive protection systems against harm to their cells, tissues and the entire organism, regardless of whether the harm is caused by natural internal processes or by external agents.
Low dose radiation generally up-regulates adaptive protection systems resulting in a net health benefit to the organism in terms of response to stress. High dose radiation generally impairs protection systems and results in more net harm than benefit. The effect of radiation on the protective systems is what determines the health benefit or risk.
Other recommendations are:
Scientific societies should organize meetings to discuss the health benefits and risks of radiation.
Regulatory bodies and health organizations should examine the scientific evidence.
Radiation protection regulations should be changed. They should be based on using The Scientific Method instead of politicized science.
The basis for radiation protection should be restored to the tolerance dose (threshold) concept, in light of more than a century of medical evidence.
Calculation of excess cancer risk using unscientific concepts, such as the LNT model, should be stopped.
Regulation of harmless radiation sources, such as radon in homes, should be stopped.
Based on biological evidence, the threshold for evacuations from low dose rate radiation should be raised from 20 to no more than 700 mGy per year, i.e., from 2 to ≤ 70 rad per year.
APPENDIX 1
Radiation dose is the amount of energy deposited per unit mass in an irradiated object. Many different units have been used during more than 115 years of work with ionizing radiation (Henriksen et al. 2013, Chapter 5).
Radiation dose is measured in units of gray (Gy), the System International (SI) unit. When one kilogram absorbs a joule of radiation energy, its radiation dose is one gray. So 1 Gy = 1 joule/kg, and 1 milligray (mGy) is a thousandth of a gray.
The roentgen unit R is a measure of radiation exposure, i.e., the ionization of air molecules. If soft tissue is exposed to gamma radiation of 1 R, the radiation dose will be approximately 9.3 mGy.
The radiation absorbed dose (rad) was developed in 1953. One rad is 100 erg per gram or 10-2 joule/kg. Therefore, 1 gray = 100 rad.
When biological organisms are irradiated with different types of radiation (x-rays, gamma rays, sub-atomic particles) the biological end result for the same dose given in Gy may vary. A relative biological effectiveness (RBE) factor is calculated for humans, and the dose is multiplied by the RBE weight factor to obtain “the effective dose.” The unit is called rem in the old system and sievert (Sv) in the SI system. For x-rays and gamma rays, the RBE = 1. For these types of radiation, rem = rad and sievert = gray. One Sv = 100 rem.
Footnotes
The different radiation units are discussed in Appendix 1.
PL is the extra probability of leukemia occurring in an exposed person per rem and per year elapsed after exposure.
REFERENCES
- Calabrese EJ. Origin of the linear no threshold (LNT) dose-response concept. Arch Toxicol. 2013a doi: 10.1007/s00204-013-1104-7. Available at: http://link.springer.com/article/10.1007%2Fs00204-013-1104-7. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. How the US National Academy of Sciences misled the world community on cancer risk assessment: new findings challenge historical foundations of the linear dose response. Arch Toxicol. 2013b doi: 10.1007/s00204-013-1105-6. Available at: http://link.springer.com/article/10.1007/s00204-013-1105-6. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. How radiotherapy was historically used to treat pneumonia: Could it be useful today? Yale Journal of Biology and Medicine. 2013c;86:555–570. [PMC free article] [PubMed] [Google Scholar]
- Caspari E, Stern C. The influence of chronic irradiation with gamma rays at low doses on the mutation rate in Drosophila Melanogaster. Genetics. 1948;33:75–95. doi: 10.1093/genetics/33.1.75. Available at: http://www.genetics.org/content/33/1/75.full.pdf+html?sid=cb861a39-fb63-48c4-bcbe-2433bb5c8d6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttler JM. Commentary on Fukushima and Beneficial Effects of Low Radiation. Dose-Response. 2013;11(4):432–443. doi: 10.2203/dose-response.13-008.Cuttler. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3834738/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss M. Linear No-Threshold Model vs. Radiation Hormesis. Dose Response. 2013;11(4):480–497. doi: 10.2203/dose-response.13-005.Doss. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3834742/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinendegen LE, Pollycove M, Neumann RD. Hormesis by low dose radiation effects: low-dose cancer risk modeling must recognize up-regulation of protection. In: Baum RP, editor. Therapeutic Nuclear Medicine. Springer; 2013. ISBN 973-3-540-36718-5. Available at: http://db.tt/UyrhlBpW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliedner TM, Graessle DH, Meineke V, Feinendegen LE. Hemopoietic response to low dose-rates of ionizing radiation shows stem cell tolerance and adaptation. Dose-Response. 2012;10:644–663. doi: 10.2203/dose-response.12-014.Feinendegen. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3526333/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T, Biophysics and Medical Physics Group at UiO . Radiation and Health. Taylor & Francis; 2013. ISBN 0-415-27162-2 (2003 updated to 2013). University of Oslo. Available at: http://www.mn.uio.no/fysikk/tjenester/kunnskap/straling/radiation-and-health-2013.pdf. [Google Scholar]
- Japan National Police Agency Damage situation and police countermeasures associated with 2011 Tohoku district - off the Pacific Ocean earthquake, November 8, 2013. 2013. Available at: http://www.npa.go.jp/archive/keibi/biki/higaijokyo_e.pdf.
- Jaworowski Z. Radiation risk and ethics. Physics Today. 1999;59(9):24–29. Available at: http://dl.dropbox.com/u/71478013/Jaworowski-1999_Radiation-Risk-Ethics_PhysToday_copyright.pdf. [Google Scholar]
- Jaworowski Z. Radiation hormesis - A remedy for fear. Human Exper Toxicol. 2010;29(4):263–270. doi: 10.1177/0960327110363974. Available at: http://www.belleonline.com/newsletters/volume15/vol15-2.pdf. [DOI] [PubMed] [Google Scholar]
- Luckey TD. Radiation Hormesis. CRC Press; 1991. [Google Scholar]
- Mainichi Stress-induced deaths in Fukushima top those from 2011 natural disasters. The Mainichi. September. 2013;9:2013. Available at: http://news.ca.msn.com/top-stories/fukushima-evacuation-has-killed-more-than-earthquake-and-tsunami-survey-says. [Google Scholar]
- Mitchel REJ. Low Doses of Radiation Reduce Risk in Vivo. Dose-Response. 2007a;5:1–10. doi: 10.2203/dose-response.06-109.Mitchel. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2477704/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel REJ. Cancer and Low Dose Responses in Vivo: Implications for Radiation Protection. Dose-Response. 2007b;5(4):284–291. doi: 10.2203/dose-response.07-014.Mitchel. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2477713/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckerheide J. Apply radiation health effects data to contradict and overturn radiation protection policies and rules. Proc ICONE 8; April 2–6; Baltimore. 2000. Available at: http://www.ddpon-line.org/muckerheide.doc. [Google Scholar]
- Muller HJ. Artificial transmutation of the gene. Science. 1927;66(1699):84–87. doi: 10.1126/science.66.1699.84. [DOI] [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- Pollycove M, Feinendegen LE. Radiation-induced versus endogenous DNA damage: possible effect of inducible protective responses in mitigating endogenous damage. Human Exper Toxicol. 2003;22:290–306. doi: 10.1191/0960327103ht365oa. Available at: http://www.belleonline.com/newsletters/volume11/vol11-2.pdf. [DOI] [PubMed] [Google Scholar]
- Sakamoto K. Radiobiological Basis for Cancer Therapy by Total or Half-body Irradiation. Nonlinearity in Biology, Toxicology, and Medicine. 2004;2:293–316. doi: 10.1080/15401420490900254. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657505/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders CL. Radiation Hormesis and the Linear-No-Threshold Assumption. Springer; 2010. [Google Scholar]
- Sasaki MS, Tachibana A, Takeda S. Cancer risk at low doses of ionizing radiation: artificial neural networks inference from atomic bomb survivors. J Radiat Res. 2014. (in press) [DOI] [PMC free article] [PubMed]
- Scott BR. It’s time for a new low-dose-radiation risk assessment paradigm—one that acknowledges hormesis. Dose-Response. 2008;6:333–351. doi: 10.2203/dose-response.07-005.Scott. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2592992/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BR, Sanders CL, Mitchel REJ, Boreham DR. CT Scans May Reduce Rather than Increase the Risk of Cancer. J Am Phys Surg. 2008;13(1):8–11. Available at: http://www.jpands.org/vol13no1/scott.pdf. [Google Scholar]
- Seiler FA, Alvarez JL. The Scientific Method in Risk Assessment. Technology: Journal of the Franklin Institute. 1994;331A:53–58. Available at: http://www.researchgate.net/publication/259527706_The_Scientific_Method_in_Risk_Assessment. [Google Scholar]
- Seiler FA, Alvarez JL. The Scientific Method and Risk Management. Proc Waste Management Symposium; March 3; 1998. Available at: http://www.wmsym.org/archives/1998/html/sess07/07-01/07-01.htm. [Google Scholar]
- UNSCEAR 1958. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation. United Nations General Assembly Official Records Thirteenth Session Supplement 17 (A/3838). New York. Available at: http://www.unscear.org/unscear/en/publications/1958.html.
- UNSCEAR 1994. Adaptive Responses to Radiation in Cells and Organisms Sources and Effects of Ionizing Radiation Report to the United Nations General Assembly, with Scientific Annexes. Annex B. Available at: http://www.unscear.org/unscear/publications/1994.html.
- UNSCEAR 2012. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation. Fifty-ninth session (21–25 May 2012). Available at: http://www.unscear.org/
- WHO . Health risk assessment from the nuclear accident after the 2011 Great East Japan Earthquake and Tsunami, based on a preliminary dose estimation. World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/78218/1/9789241505130_eng.pdf. [Google Scholar]