Abstract
The AlamarBlue assay is based on fluorometric detection of metabolic mitochondrial activity of cells. In this study, we determined the methodology for application of the assay to radiation response experiments in 96-well plates. AlamarBlue was added and its reduction measured 7 hours later. Selection of the initial number of plated cells was important so that the number of proliferating cells remains lower than the critical number that produced full AlamarBlue reduction (plateau phase) at the time points of measurements. Culture medium was replaced twice a week to avoid suppression of viability due to nutrient competition and metabolic waste accumulation. There was no need to replace culture medium before adding AlamarBlue. Cell proliferation continued after irradiation and the suppression effect on cell viability was most evident on day 8. At this time point, by comparing measurements from irradiated vs. non-irradiated cells, for various dose levels, a viability dose response curve was plotted. Immediately after the 8th day (nadir), cells started to re-grow at a rate inversely related to the radiation dose. By comparing measurements at the time point of nadir vs. a convenient subsequent time point, re-growth dose response abilities were plotted, simulating clonogenic assays.
Keywords: AlamarBlue, radiation, dose response, 96-well plate
INTRODUCTION
Cell viability can be measured in 96-well plates, following exposure to drugs or physical agents, like hypoxia, hyperthermia or ionizing radiation. A variety of methods have been applied, each having advantages and disadvantages (Protocols and Applications Guide). Such methods include the measurement of the overall DNA content, LDH release from cells, incorporation of bromodeoxyuridine or of thymidine analogues during DNA synthesis, measurement of specific proteins involved in apoptosis, and measurement of the cell metabolic activity like oxidore-ductase activity or ATP levels.
The AlamarBlue assay is a fluorometric method for the detection of metabolic activity of cells (Page et al. 1993). The method is based on the reduction of resazurin (oxidized form; 7-hydroxy-3H-phenoxazin-3-1-10-oxide) to resorufin (reduced form), by mitochondrial enzymes that carry diaphorase activity, like NADPH dehydrogenase (O’Brien et al. 2000). Optically, the blue and poorly fluorescent resazurin is gradually transformed by cells into the red, highly fluorescent, resorufin. Fluorescence of both forms can be monitored at 530–560nm excitation wavelength and at 590nm emission wavelength and the oxidized form does not fluoresce much. Absorbance is monitored at 570nm and 600nm for the oxidized and the reduced forms, respectively.
Several advantages of the AlamarBlue assay have been reported (O’Brien et al. 2000, Nakayama et al. 1997, Nociari et al 1998). This assay is simple method based on a water soluble substance that works on both suspended and attached cells. Furthermore, the reagents seem to be non-toxic to cells and technicians. A disadvantage of the method is that it relies on a metabolic pathways that can be affected by the individual cell reducing ability and by agents affecting mitochondrial activity or having a direct reducing effect on resazurin.
In the current study, we evaluated the potential and limitations of the AlamarBlue assay to detect the effect of ionizing radiation on cell viability and cell re-growth as a function of time and radiation dose. In this way, time-response and dose-response curves could be plotted for further studies of radiosensitization or radioprotection of adherent cancer and normal cells, respectively.
MATERIALS AND METHODS
Irradiation details
96-well plates were used to assess multi-dose radiation survival curves at various time points. Irradiation of the plates was performed using the 6MV beam of a Linear Accelerator PRECISE (ELEKTA) supplied with a MultiLeaf Collimator. The 6MV photon energy produced has a maximum depth dose 16mm in water and TPR20,10 = 0.680. Whole plate irradiation was performed using a posterior field of 10x10cm placed in a box of plexiglass providing adequate space below (2cm) and above the 96-well plate to allow electron balance and accurate delivery of the desired dose to the cells in the wells.
For multidose irradiation of the same 96-well plate, a previously validated and reported technique was used (Abatzoglou et al. 2013), that allows the administration of 8 different desired doses based on specific equations created following a series of dosimetric experiments.
Cell line and measurement
The T98G glioblastoma cell line (Karim et al. 2005) was used for experiments. Cells were cultured in Minimum Essential Medium (Gibco® MEM, Grand Island, New York 10370-070), in CO2 incubators at 37°C and 21% oxygen / 5% CO2 ambient. MEM was supplemented with 10% Fetal Bovine Serum, 1mM Sodium Pyruvate and 2mM L-Glutamine. Before plating, the cells were first washed with DPBS (Gibco, Grand Island, New York 15400-054) and then trypsinized with 1X trypsin diluted in DPBS. The appropriate volume of culture medium was then placed in a flask to stop trypsinization and cells were counted using a hematocytometer (Marienfeld, Germany) after being stained with trypan Blue (Gibco, Grand Island, New York, 15250-061). The appropriate volume of the cell solution was placed in a new falcon tube containing culture medium to give a desired final concentration, e.g.. 1250 cells/ml. 200μl of this solution was placed in each well, e.g. 250 cells/200μl.
For cancer cell susceptibility testing, flat clear-bottomed tissue culture treated 96-well plates were used and bottom reading was utilized. Outer perimeter wells were filled with culture medium to prevent dehydration in inner wells. A microplate reader (FLUOstar® Omega; BMG LABTECH GmbH, Ortenberg, Germany) was used to assess fluorescence.
The AlamarBlue method
The AlamarBlue® assay was used to assess cell viability (Serotec, Oxon, UK) (Page et al. 1993, Nakayama et al. 1997, Nociari et al 1998). Following incubation of cells in wells (200μl of culture medium), 10% v/v AlamarBlue (20μl) was added and fluorescence was measured (excitation 530nm, emission 590nm). Wells containing culture medium without cells, 10% v/v alamar blue, and vitamin C (ascorbic acid 0.75 mg (5μl)/well; Pascorbin® 750mg/5ml, PASCOE, Germany) that results in rapid full reduction of the AlamarBlue were used as positive controls. Wells with culture medium without cells containing 10% v/v AlamarBlue were assays as negative controls.
Gain adjustment of fluorescence for every well was performed against the well of the maximum fluorescence (wells with fully reduced AlamarBlue; see below). The cell concentration was a subject of the current investigation.
Analysis was based on :
the relative fluorescence units (RFU) recorded.
the ratio of RFU recorded from a well divided by the RFU recorded from the reference well (RFU-ratio).
- the calculation of the ratio of RFU compared to non-irradiated cells (i/niRFU-ratio): the ratio of mean RFU obtained from the irradiated well minus the mean signal obtained from three negative control wells, divided by the mean signal recorded from non-irradiated wells (or irradiated at an earlier time point) minus the mean signal from three negative control wells.
- the calculation of the absolute % AlamarBlue reduction (%ABr): the ratio of mean RFU obtained from the irradiated wells minus the mean signal obtained from three negative control wells, divided by the mean signal recorded from 3 fully reduced (positive control) wells minus the signal from negative control wells.
Statistical analysis
Statistical analysis and curve plotting (linear regression and polynomial analysis) were performed using standard equations included in the GraphPad Prism 5.01 package (GraphPad Software Inc., USA).
RESULTS AND DISCUSSION
Time point to measure fluorescence after AlamarBlue injection
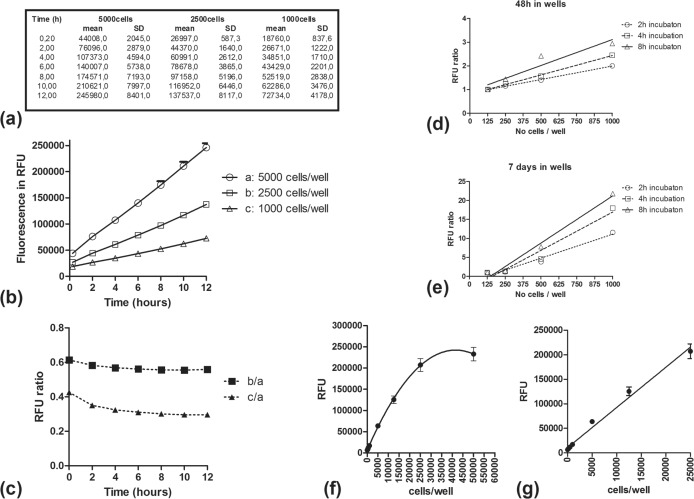
In order to assess the optimal time to measure fluorescence following addition of AlamarBlue in wells, three different cell concentrations were applied in one 96-well plate; (a) 5000, (b) 2500 and (c) 1000 cells per 200μl total culture medium volume (two columns of 6 wells each per concentration). 20μl of AlamarBlue was added and the 96-well plate was placed into the reader for consecutive measurements every 2h for 12 hours. Figure 1a shows the mean ± SD RFU values which are plotted in Figure 1b. A clear difference among the three cell concentrations was evident even at the first time point (12min following AlamarBlue addition). By plotting the RFU ratio of b/a and c/a cell concentration against time (Figure 1c), we noted that this declined within the first 3h, reaching a plateau at 6 to 8 hours. It was, therefore, concluded that at least 6h should be allowed between of addition of AlamarBlue and the measurement of fluorescence.
FIGURE 1.
Fluorescence recorded in RFU for three different cell concentrations at various time points following injection of AlamarBlue (1a). Fluorescence in RFU (1b) and of RFU-ratio (1c) using as a baseline the highest cell concentration, plotted against time (hours) following AlamarBlue addition. RFU ratio (mean RFU from wells of different concentrations divided by the mean RFU from wells of 125cell/well concentration), at 48h (1d) and at 7 days (1e) after placement of cells in wells. Measurements were performed at 2h, 4h and 8h following AlamarBlue addition. Correlation of cell concentration in wells with RFU, 7 hours after AlamarBlue addition to the wells (1f and 1g).
An additional experiment was set up using a series of lower cell concentrations of 125, 250, 500 and 1000 cells per well. The fluorescence was measured at 0, 48, 72 hours and 7 days after placement of cells in the wells. At these specific time points, AlamarBlue was added and measurements were performed 2h, 4h and 8h after incubation. Gain adjustment was performed against the well producing the maximum fluorescence. Figure 1d,e shows the ratio of RFU recorded from any well divided by the RFU from wells of the lowest (125 cell) concentration. It was noted that measurements performed after 8h incubation with AlamarBlue resulted in the largest difference between different cell concentrations. This suggested that an 8h incubation time is optimal compared to shorter incubation times. A 7h interval was chosen as a convenient time point that allows the whole procedure to be accomplished within the 8 working-hours of the day.
Creation of a RFU/cell-number line
After placing different numbers of cells per well (100, 250, 500, 1000, 5000, 12500, 25000, 50000) in triplicates, AlamarBlue (AB) was added and RFUs were measured at 7h. Additional wells containing AB in culture medium alone (0 cells) and fully reduced AB after adding vitamin C (corresponding to the concentration of cells that would result in full AB reduction at 7h) were also included. Gain adjustment for fluorescence measurement was performed on this latter well. Considering the RFU of 0-cell wells as the minimum value (0 cells), the RFUs were plotted against the cell/well concentrations (Figure 1f,g). It was noted that up to a concentration of 25000 cells/well, there was an overall linear correlation, with a line slope of 8200±0.35 and an r2=0.98). Thereafter the curve started to flatten due to saturation of the method (full AlamarBlue reduction at 7 hours estimated to be achieved by a concentration of 35000 cells/well).
This suggests that any experiment on cell proliferation should include an initial number of cells that would reach a final cell population of less than 25000 cells/well at the desired time point of analysis. However, this may vary upon cell type as AlamarBlue reduction is mitochondrial mediated and depends on individual cell metabolic activity. Thus, an RFU/cell-number line should be always performed before any experiment with a new cell line.
Changing culture medium in wells
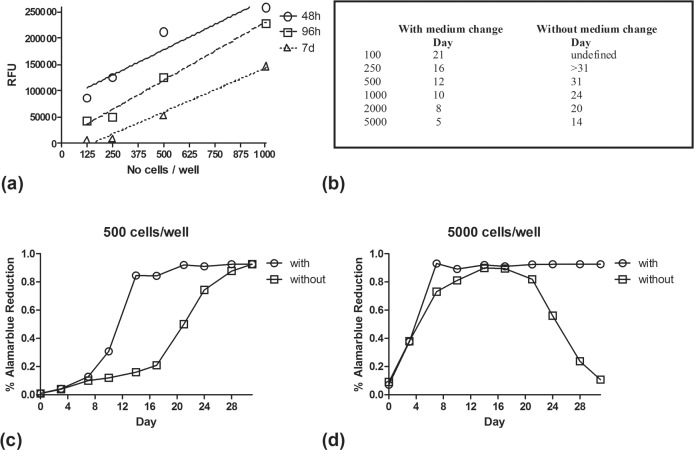
In order to assess how the number of cells change in the wells through time, measurements of fluorescence was performed at 48h, 96h, and 7 days after placement in the wells. Culture medium was not changed. AlamarBlue was added at the selected time points and fluorescence was recorded 7 hours later. At all cell concentrations (125, 250, 500 and 1000 cells/well) the RFU obtained gradually decreased over the number of days cell were kept in wells of non-renewed culture medium (Figure 2a). This could show that cell death due to nutrient deprivation results in a reduction of cell number over time. This phenomenon seemed to proportionally affect all cell concentrations as the RFU/cell lines in the three time points are parallel.
FIGURE 2.
RFU for different cell concentrations recorded at 48h, 96h and 7 days following their placement in the 96-well plate. Recording was performed 7 hours after AlamarBlue injection (2a). Days needed to reach AlamarBlue plateau (cell concentration >30.000/well), with and without culture medium renewal (2b). The % AlamarBlue reduction calculated for 500 cells/well and 5000cells/well, plotted against days, is shown in figure 2c and 2d respectively.
In order to further investigate the phenomenon, a new experiment was performed directly comparing the cell concentration when changing culture medium every 3 days vs. when culture medium was left unchanged. In this experiment the non-reduced and fully reduced forms of AlamarBlue were used to obtain the “ % reduction of AlamarBlue” (%ABr) according to the formula reported in the methods.
The increase of cell number (proliferation) was monitored twice-a-week for 5 weeks, for various cell concentrations (100, 250, 500, 1000100, 250, 500, 2000, 5000 cells/well). In cells with regular culture medium replacement, the %ABr increased with time reaching a plateau (cell concentration >25000/well) at specific time points shown in Figure 2b. Higher cell concentrations reached the plateau earlier, as expected. For cell cultures where no medium change was performed, the plateau phase was reached with a significant delay (Figure 2b). Figure 2c shows comparatively the %ABr of cells with vs. without medium change for a cell concentration of 500 cells/well. After reaching plateau, cells maintained a maximum %ABr when culture medium was regularly renewed, as nutrients were available and the only restriction parameter was the available space in the well. On the contrary, cells left in non-renewed culture medium showed a gradual reduction reflecting cell death due to nutrient deprivation (Figure 2d).
Culture medium replacement at a rate of twice-a-week prevented death from starvation and allowed cells to survive above the plateau phase even at initial cell concentration as high as 5000 cells/well. It is stressed that the plateau reached does not reflect the maximum cell concentration that can be achieved in the well, as this simply reflects saturation of the method, meaning that at 7 hours all AB available was reduced. Plateau means that the number of cells are above 25000/well. The number of cells may, therefore, further increase. Thus experiments should avoid comparing irradiated (or treated) cells with control cells at time points where the latter have reached the plateau/saturation phase. According to Figure 2b and for the specific cell line we investigated, any experiment should have been accomplished within less than 16 days for a cell concentration of 250 cells/well and within less than 8 days for a cell concentration of 2000 cells/well.
It is obvious from the curves of the 500cells/well group that changing of culture medium resulted in proliferation enhancements, presumably by providing the necessary nutrients and removing metabolic waste products that negatively affect proliferation and survival. After several days, depending upon initial cell concentration, the %ABr reached a plateau (due to saturation of the method) that is sustained for wells with regular replacement of the culture medium, but rapidly declines for wells without medium replacement. This reflected the cell death and proliferation inhibition as a result of nutrient deprivation and metabolic waste accumulation. This time point where the effect of adverse medium conditions become evident was estimated to be 14 days for a cell concentration of 5000 cells/well, and seemed to exceed 30 days for concentrations as low as 500 cells/well.
Cell proliferation after irradiation
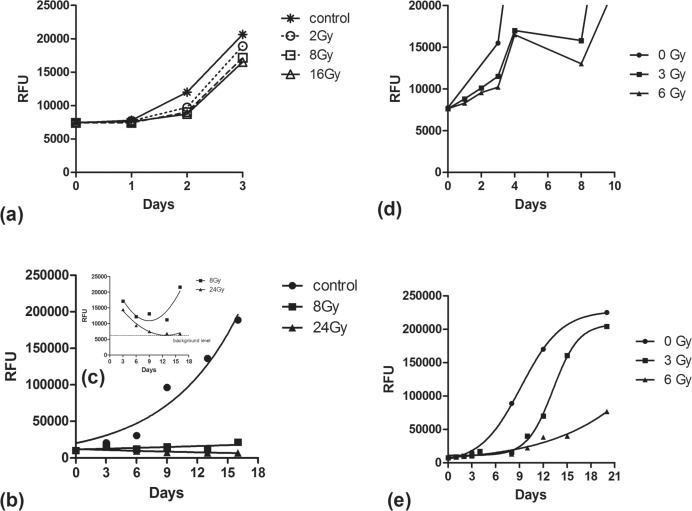
T98 cells at a concentration of 250 cells/well were plated in 96-well plates. Wells in triplicates received 0Gy (control), 8Gy and 16Gy or 24Gy at day 0. Culture medium was replaced twice a week. AlamarBlue was added on days 3, 6, 9, 13 and 16 and measured 7 hours thereafter.
Analysis of RFU during the first post-irradiation days showed that even highly irradiated cells maintain initially an almost equal ability to proliferate compared to non-irradiated cells. This is in contrast to a cytotoxic agent exposure, like chloroquine, that resulted in a sharp reduction of RFU hours following cell exposure (data not shown). Figures 3a and 3b show the proliferation during the first 3 days of non-irradiated cells and cells receiving 2, 8 and 16 or 24Gy. Proliferation on day 1 and 2 was clearly lower than that on day 3 for all groups. After the 3rd day, irradiated cells showed a reduction of the RFUs and a trend for re-growth after the 8th day for the 8Gy dose level (Figure 3c). A more profound reduction down to the background levels (after the 9th day) was noted for the 24Gy. In both dose levels the 8th–12th day seemed to be the nadir of the RFU.
FIGURE 3.
RFU obtained from T98 cell cultures in non-irradiated and irradiated cells: (3a) during the first 3 days; (3b and 3c) during 16 consecutive days; (3d) during 10 consecutive days; (3e) during 21 days.
To further investigate the post-irradiation proliferation ability of cells at lower dose levels an additional experiment was performed, using doses of 3Gy and 6Gy and follow-up of measurements on days 0, 1, 2, 3, 4, 8, 10, 12, 15 and 20. The RFU were counted and plotted (Figure 3d,e). During the first 4 days there was a proliferation of cells despite irradiation, although this was slightly lower than non-irradiated cells. After day 5, a drop of the RFU (thus of number of cells reached) began, which lasted till day 8. The drop was more pronounced at 6Gy compared to 3Gy. Thereafter, cell proliferation occurred in the 3Gy irradiated cells approaching saturation after day 16, while the 6Gy irradiated cells exhibited a far more restricted proliferation capacity.
Thus, cells are proliferating in both non-irradiated and irradiated wells. In irradiated wells, however, cells seem to enter a three phase response due to radiation induced cell death or to radiation induced proliferation delay, as recorded by the AlamarBlue assay:
inert phase The initial proliferation rate is slightly reduced compared to non-irradiated cells and this reduction is more intense at higher dose levels. This phase lasts 4 days for the cell line under evaluation.
the death phase As cell death is gradually intensified over time, reaching the highest intensity around the 8th day, the estimated proliferation rate reaches negative values, as surviving cells after the 4th day are reduced in numbers.
the re-growth phase After the 8th day, the cell death phase has weakened and proliferation of the surviving cells starts to prevail. The regrowth rate is inversely related to the radiation dose level.
The AlamarBlue assay gave an estimate of the combined result of cell proliferation and death, thereon reported as ‘suppression effect’. Moreover, following cells for longer intervals, the ‘re-growth ability’ was also estimated.
The contribution of dead cells to the AlamarBlue reduction
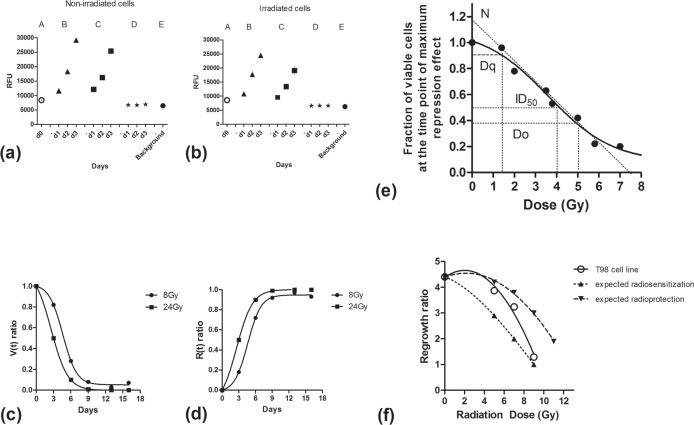
In an attempt was made to clarify whether AlamarBlue can be added in the culture medium of wells or if culture medium should be replaced before AlamarBlue addition. The question raised was whether dead or dying cells detached from the well bottom and floating in the culture medium could have an effect on AlamarBlue reduction. Two 96-well plates were prepared, one irradiated with 6Gy and one without irradiation. RFU was measured on day 0, and on days 1, 2 and 3, with and without culture medium replacement before addition of AlamarBlue. The discarded culture medium was placed in wells and measured separately. Figure 4a,b shows the results obtained, confirming that the discarded culture medium, up to 3 days following seeding of cells in the wells, had RFU levels equal to the background (culture medium without cells), whether cultures were irradiated or not. This shows, that the dead or dying cells in the overlying culture medium did not affect the overall fluorescence of the reduced AlamarBlue and that fluorescence was entirely due to the living cells attached to the well bottom.
FIGURE 4.
Analysis of RFU from non-irradiated (4a) and irradiated by 6Gy (4b) T98 cells, at days 0, 1, 2 and 3. ‘A’ (circles) represents RFU 7hours after placement in 96-well plates. ‘B’ (triangles) shows the RFU in wells where the culture medium is not replaced before adding AlamarBlue. ‘C’ (squares) shows the RFU in wells where the culture medium is replaced before adding AlamarBlue. ‘D’ (asterisks) shows the RFU in the supernatant culture medium removed by the wells and ‘E’ (black circle) shows the RFU recorded from well containing culture medium without cells (background fluorescence). Plots of the viability (4c) and the repression (4d) ratio vs. post-irradiation days for 8 and 24Gy. Figure 4e shows a typical dose response survival curve of the T98 glioblastoma cells, also indicating the key radiobiological parameters. Regrowth dose response curve for the T98 cell line, considering the 8th day as the nadir of cell viability and measurements recorded on day 12 of the regrowth phase (Figure 4f). Dotted lines represent theoretical curves of combination of radiotherapy with radiosensitizing and radioprotective agents.
Estimation of the ‘viability’ and of the ‘repression’ radiation effect
By comparing the RFU of irradiated cells at a time point ‘t’ with the RFU of non-irradiated cells at the same time point, we have an estimate of the fraction of cells that have not contributed to the proliferation (due to death and/or cell cycle arrest) because of irradiation. The i/niRFU-ratio gives the viable cell ratio at a specific time point (Viability-ratio; V(t)-ratio).
The non-surviving cells (that would have been accumulated if cells were left unirradiated) are ‘1-i/niRFU-ratio’ (1- V(t)). We can, therefore, calculate the repression effect R at a time point ‘t’ by the following Repression-ratio, ‘R(t) ratio’.
In our case, the V(t) ratio showed a dose dependent drop, which was more profound at the 24Gy dose level compared to 8Gy. In this later case the V-ratio approached 0 showing no viable cells after the 9th day. On the contrary, 3% of cells survived at day 13 and increased to 7% at day 16 for the 8Gy dose level. This ‘V(t) ratio’ gave an estimate of the percentage of viable cells that may maintain proliferation capacity at the specific time point. This declined over time and, if dose allowed, cells recovered (Figure 4c). On the contrary, the repression (R(t) ratio) effect increased over time (Figure 4d) and then declined after a maximum effect had been reached.
Plotting a ‘viability’ (survival) dose response curve
Using the viability (V) at the time point where the maximum repression value was noted for a series of dose levels, we plotted a survival/viability dose-response curve of the specific cell line. Using a multi-dose irradiation method in a single 96-plate (Abatzoglou et al. 2013), we calculated the V at the time of maximum repression, the 8th post-irradiation day. The dose/V values were put on two axis and a polynomial fit curve was plotted by the statistical package. On this curve (Figure 4e) we calculated the traditional radiobiology parameters: i. Extrapolation number ‘N’ = 1.18, ii. ‘Dq’ shoulder width (quasi threshold dose) = 1.4Gy, iii. Do (37% viable cells) = 5.1Gy and, iv. ID50 (half maximum inhibitory dose) = 4Gy.
Assessment of the re-growth or ‘clonogenic’ ability
After the radiation induced death effect, the remaining cells restarted their re-growth. Radiation can have induced an effect that may stimulate or suppress growth of the surviving cells as compared to the non-irradiated cells. This post-irradiation growth ability was traditionally estimated by seeding irradiated cells in petri-dishes and counting the number of colonies that are created after a period of 1–3 weeks (Franken et al. 2006). Only a fraction of cells maintain the ability for ‘unlimited’ division and these were postulated to form the colonies. As the dose increased,, the number of clonogenic cells decreased and this was paralleled by a decrease in the number of colonies.
By using the AlamarBlue assay and recording the rate of cell re-growth and proliferation after the nadir time point of the V, we provided an estimate of this clonogenic activity after exposing cells to a range of radiation doses.
We counted the RFU increase at defined time points after the day of maximum repression effect (day 8), and calculated the ‘Regrowth or Clonogenic ratio’ (‘C(t)-ratio’).
Experiments were conducted to assess the regrowth ratio. Multidose irradiated 96-well plates were left to grow for 8 and 12 days before assessment with the AlamarBlue assay. At this time point, plateau had been reached for all dose levels except 7Gy and 9Gy. At these dose levels the C(t)-ratio was 3.24 and 1.29, respectively, while for 5Gy, assuming the plateau was reached on day 12, C(t)-ratio was 3.87. Non-irradiated cells (0Gy), according to previously reported experiments, showed a growth rate of 4.40 from day 8 to day 12. Taking into account these data we created a regrowth dose response curve shown in Figure 4f. It is stressed that regrowth ratio can be only estimated if the regrowing cells have not reached plateau levels at the time point of measurement (t), otherwise it is impossible to estimate the cell density reached and subsequently calculate the C(t)-ratio. The fact that regrowth started abruptly after the nadir noted at 8 days showed that the effect of a single radiotherapy fraction resolved within this time frame. Suppression of the regrowth ability thereafter seemed to be directly related to the radiation dose delivered. The biological mechanisms behind these two radiation induced phenomena may be different and demand thorough investigation.
Acknowledgments
The study has been financially supported by the Tumour and Angiogenesis Research Group.
REFERENCES
- Abatzoglou I, Zois CE, Pouliliou S, Koukourakis MI. Establishment and validation of a method for multi-dose irradiation of cells in 96-well microplates. Biochem Biophys Res Commun. 2013;431:456–459. doi: 10.1016/j.bbrc.2012.12.146. [DOI] [PubMed] [Google Scholar]
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Karim A, McCarthy K, Jawahar A, Smith D, Willis B, Nanda A. Differential cyclooxygenase-2 enzyme expression in radiosensitive versus radioresistant glioblastoma multiforme cell lines. Anticancer Res. 2005;25:675–679. [PubMed] [Google Scholar]
- Nakayama GR, Caton MC, Nova MP, Parandoosh Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J Immunol Methods. 1997;204:205–208. doi: 10.1016/s0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Nociari MM, Shalev A, Benias P, Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J Immunol Methods. 1998;213:157–167. doi: 10.1016/s0022-1759(98)00028-3. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Protocols & Applications Guide. www.promega.com. rev. 3/11.
- Page B, Page M, Noel C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int J Oncol. 1993;3:473–476. [PubMed] [Google Scholar]