Abstract
OBJECTIVES
We conducted a population-based analysis of time trends in length of stay (LOS), predictors of prolonged LOS and emergency readmission following resection for non-small-cell lung cancer (NSCLC).
METHODS
Incident lung cancers (ICDO2:C34), diagnosed between 2002 and 2008, were identified from the National Cancer Registry (NCR) of Ireland, and linked to hospital in-patient episodes (HIPE). For those with NSCLC who underwent lung resection, the associated hospital episode was identified. Factors predicting longer LOS (upper quartile, >20 days), and emergency readmission within 28 days of the index procedure (IP) were investigated using Poisson regression.
RESULTS
A total of 1284 patients underwent resection. Eighty-four (7%) subsequently died in hospital and 1200 (93%) were discharged. Hundred and nineteen of 1200 (10%) were readmitted as an emergency within 28 days of discharge. Median LOS after the IP was 13 days (inter-decile range: 7–35). Risk of prolonged LOS was significantly greater in patients >75 years, resident in an area of highest deprivation, with 2+ comorbidities, who had undergone surgery in a lower-volume hospital, and died in hospital subsequent to the IP. Emergency readmission was significantly more likely in patients who were resident in an area of highest deprivation, with 2+ comorbidities, and had Stage III disease or worse. The main reasons for emergency readmission were: pulmonary complications (29%), cardio/cerebrovascular events (21%) or infection (20%).
CONCLUSIONS
Half of the patients had a LOS in excess of 13 days, which was longer than any other country with published data. Patient and health-service factors were associated with prolonged LOS, while patient and tumour characteristics were associated with risk of emergency readmission. Deprivation was a conspicuous determinant of both LOS and readmission.
Keywords: Lobectomy, Pneumonectomy, Length of stay, Emergency readmission
INTRODUCTION
The number of new cases of lung cancer in the EU will increase by 27% between 2008 and 2025, in part due to population ageing, and in part due to long-term trends in tobacco use [1]. Surgery remains the mainstay of treatment, with curative intent for non-small-cell lung cancer (NSCLC) patients who are medically fit, with lobectomy as the treatment of first choice [2]. Length of stay (LOS) in hospital after surgery impacts on cost and hospital performance [3–5]. There is little definitive information on LOS following lung cancer resection; yet, the rate of lung cancer resection (as a proportion of NSCLC cases) is increasing in several European countries [6–8]. Internationally, the few available studies suggest that there is much variation in the reported median LOS after NSCLC resection [5, 9–12]. Postoperative complications are common after lung resection and can result in prolonged hospitalization and early readmission [9, 13]. We conducted a population-based analysis of time trends in LOS and predictors of prolonged LOS following resection for NSCLC in Ireland. We further investigated the factors predicting emergency readmission within 28 days of discharge after the index procedure (IP).
METHODS
The primary data sources for this study were the National Cancer Registry (NCR) and the Hospital In-Patient Enquiry (HIPE) database in Ireland [14, 15]. HIPE is a computer-based information system that records data on discharges from all acute public hospitals and a few private hospitals [15]. Lung cancer patients (ICD-O2: C34) newly diagnosed between 2002 and 2008 were identified from the NCR. Individuals who had another primary cancer prior to the lung cancer (other than non-melanoma skin) were excluded. The dataset was then limited to those who had lung cancer resection according to NCR records (ICD9-CM codes 32.2X, 32.3, 32.4, 32.5, 32.6, 32.9, 34.4X) [16]. Using probabilistic matching techniques, these patients were linked to HIPE episodes (Fig. 1). Coverage of private hospitals by HIPE is very incomplete and we limited our analysis to patients treated in public hospitals. Cases with tumour morphology codes of M8039-8046 (small cell carcinoma) were then excluded. HIPE hospitalization episodes were ordered by date of admission. The date of surgical resection (IP) recorded by the NCR was matched to the corresponding HIPE episode. The final analysis dataset included 1284 patients with NSCLC who underwent a resection (Fig. 1).
Figure 1:
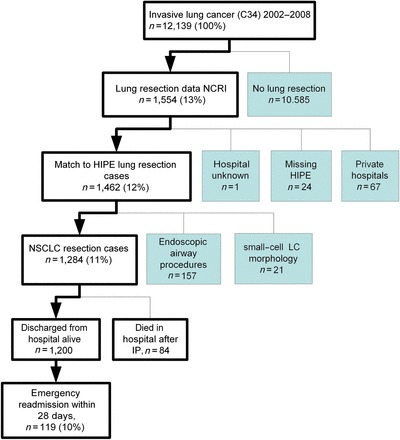
Study overview: patient selection.
LOS was calculated as the number of days between the admission date for the IP and the discharge date, or death (if the patient died in hospital) (i.e. this was the total of preoperative and postoperative LOS). Duration of discharge was calculated as the time from discharge following the IP to the first emergency readmission to a public hospital (if any). Readmissions were recorded by HIPE as ‘emergency’ when the patient required immediate care and treatment as a result of a severe, life-threatening or potentially disabling condition [15]. In the UK, 28-day readmission rate is a key hospital performance indicator [http://www.nchod.nhs.uk]; therefore, we based our analysis on emergency readmissions where the duration of discharge was <29 days.
A range of patient, tumour and health-service-related variables were abstracted from the NCR and HIPE databases and investigated for associations with LOS and emergency readmission. Details on age at diagnosis, gender and marital status were available from the NCR. Socioeconomic status was measured in terms of the level of deprivation of each patient's local area of residence at diagnosis using a score created from 2002 census variables [17]. The score was derived for each of the 3409 electoral districts in Ireland; the districts were categorized into five levels of deprivation. In our dataset, the number of patients resident within a quintile 5 district (‘most deprived’) was double that of any other quintile in our study. We therefore pooled quintiles 1–4 (‘less deprived’) for comparison with quintile 5; this is also consistent with what was done in a large US study of LOS and lung resections among Medicare recipients [5]. Each case was classified according to smoking status at diagnosis, which was derived by the NCR from hospital charts, and defined as: (i) ‘never smoked’, (ii) ‘ex-smoker’ did not smoke more than once a month for the past year and (iii) ‘current smoker’ smoked at least once a month for the past year. Cases were classified by summary stage of disease. Where information on distant metastasis (MX) was not recorded, these cases were treated as M0 [18]. A comorbidity score for each patient, based on the Charlson index, was derived from all diagnoses recorded in HIPE for the IP episode; the lung cancer diagnosis was disregarded in this calculation [19]. Resection procedures were categorized as: lobectomy, pneumonectomy, segmental/wedge resections and ‘other procedures’. Patients were classified as ‘private’ or ‘public’ (this refers to whether the patient saw the surgeon as a private or public patient). The patient's destination at discharge after IP was classified as: home, other healthcare facility (i.e. another acute/step-down hospital, nursing home or hospice), or death in hospital after IP. Resections for NSCLC were performed in six public hospitals during 2002–8. The number of resections undertaken per hospital for each year was counted. The patients were then classified as ‘higher volume’ or ‘lower volume’ depending on whether they were operated on in a hospital above, or below, the median resection count/hospital/year respectively (40/hospital/year).
Statistical analysis
Analyses were conducted using Stata 11. Median LOS, and ranges (upper and lower decile of LOS) were computed at each level of the sociodemographic, clinical and health-service-related variables. Variations in LOS were examined using the Kruskal–Wallis equality-of-populations rank test and Cuzick's test for trend. In the absence of any published definition of prolonged LOS in Europe, the cut-point defining the upper quartile of patients (>20 days) was selected as a practical threshold for prolonged LOS; this also happened to be 1 week longer than the median LOS (13 days).
As the outcome was common, instead of using logistic regression to estimate odds ratios, we modelled prolonged LOS using Poisson regression with a log link and robust variance to estimate risk ratios and associated 95% confidence intervals [20]. Three types of variables were considered for inclusion in the model: sociodemographic (age, gender, marital status, deprivation, smoking status and public/private status); clinical (IP type, stage and comorbidity) and health-service related (hospital volume, destination at discharge). A backward stepwise variable selection approach for modelling was used, removing variables in order of least significance until all retained variables achieved P < 0.1 in likelihood ratio tests (LRTs). As a sensitivity analysis, we also modelled LOS as a continuous variable using linear regression after a log transformation to normalize the distribution of LOS.
Poisson regression with a log link and robust variance [20] was also used to estimate risk ratios for factors predicting emergency readmissions within 28 days after discharge. Patients who had not died in hospital were classified as: (a) emergency readmission within 28 days of discharge, or (b) not readmitted as an emergency within 28 days of discharge. The primary reason for the emergency readmission was derived from the HIPE diagnostic codes for that admission.
RESULTS
Of 12 139 incident cases of invasive lung cancer diagnosed in Ireland between 2002 and 2008, 1284 had NSCLC and underwent lung resection in public hospitals (Fig. 1). Of these, 72% underwent lobectomy, 17% underwent pneumonectomy, 4% had segmental/wedge resection and 7% had another excision procedure. There was a significant increase in lung cancer resections performed per annum (expressed as a proportion of NSCLC cases) between 2002 and 2008 (142/1415 (10%) in 2002, rising to 228/1613 (14%) in 2008; P-trend = 0.000012). The median total LOS was 13 days (inter-decile range (IDR): 7–35 days, mean = 18 days and range: 1–343 days). The median LOS decreased significantly from 15 days in 2002 to 12 days in 2008 (P-trend = 0.037) (Table 1). The median preoperative LOS for the period 2002–8 was 1 day (IDR: 0–6 days). This decreased significantly over time, from 3 days [IDR: 1–16] in 2002, to 1 day [IDR: 0–3] in 2008 (P-trend = 0.0000000000021).
Table 1:
Patients with NSCLC undergoing resection, 2002–8: numbers (n), percentages (%) and median and IDR LOS and P-values
| Variables |
Categories | n | % | Median LOS | IDR | P-value |
|---|---|---|---|---|---|---|
| Total | 1284 | 100% | 13 | [7–35] | ||
| Age at diagnosis | <55 year | 204 | 16% | 10 | [5–25] | 0.000000017 |
| 55–64 year | 423 | 33% | 12 | [7–30] | ||
| 65–74 year | 479 | 37% | 13 | [7–37] | ||
| >75 year | 178 | 14% | 15 | [7–50] | ||
| Gender | Female | 541 | 42% | 12 | [7–32] | 0.22 |
| Male | 743 | 58% | 13 | [7–36] | ||
| Marital status | Married | 810 | 63% | 12 | [7–31] | 0.019 |
| Other | 474 | 37% | 13 | [7–40] | ||
| Deprivationa | 1 least | 230 | 18% | 12 | [6–35] | 0.016 |
| 2 | 166 | 13% | 12 | [5–27] | ||
| 3 | 144 | 11% | 12.5 | [7–30] | ||
| 4 | 187 | 15% | 12 | [6–28] | ||
| 5 most | 480 | 37% | 13 | [7–39] | ||
| Unknown | 77 | 6% | 13 | [7–31] | ||
| Smoking status | Never smoker | 124 | 10% | 12.5 | [6–34] | 0.67 |
| Ex-smoker | 434 | 34% | 12 | [7–33] | ||
| Current smoker | 604 | 47% | 13 | [7–38] | ||
| Unknown | 122 | 10% | 10 | [2–23] | ||
| Health insuranceb | Public | 884 | 69% | 13 | [7–36] | 0.017 |
| Private (held private health insurance) | 400 | 31% | 12 | [6–31] | ||
| Stage | Stage I/II | 854 | 67% | 13 | [7–36] | 0.023 |
| Stage III+ | 309 | 24% | 13 | [7–33] | ||
| Unstaged | 121 | 9% | 10 | [4–22] | ||
| Comorbidity | 0 | 931 | 73% | 12 | [6–32] | 0.000000026 |
| 1 | 264 | 21% | 13 | [8–36] | ||
| 2+ | 89 | 7% | 18 | [8–59] | ||
| Procedure | Lobectomy | 909 | 71% | 12 | [7–35] | 0.14 |
| Pneumonectomy | 234 | 18% | 13 | [7–33] | ||
| Segmental/wedge excision | 49 | 4% | 13 | [6–42] | ||
| Other excision | 92 | 7% | 15 | [4–37] | ||
| Discharge status | To home | 970 | 76% | 12 | [7–29] | 0.00000059 |
| To care (other acute hospital/nursing home/hospice) | 230 | 18% | 12 | [2–45] | ||
| Died in hospital post-IP | 84 | 7% | 21.5 | [10–91] | ||
| Hospital volumec | Higher volume: ≥40/year | 661 | 51% | 12 | [6–31] | 0.000076 |
| Lower volume: <40/year | 623 | 49% | 13 | [7–37] | ||
| Year of incidence | 2002 | 142 | 11% | 15 | [8–36] | 0.037 |
| 2003 | 174 | 14% | 12 | [7–34] | ||
| 2004 | 153 | 12% | 13 | [8–32] | ||
| 2005 | 187 | 15% | 12 | [4–34] | ||
| 2006 | 176 | 14% | 13 | [7–29] | ||
| 2007 | 224 | 17% | 13 | [7–33] | ||
| 2008 | 228 | 18% | 12 | [6–40] |
P-value: Kruskal–Wallis test for binary and categorical variables and test for trend with ordinal categorical variables.
aSmall area (electoral district) based quintile of deprivation.
bPatient had private health insurance or settled own account (‘private’), or did not have health insurance (‘public’) at discharge.
cThe patients were classified as ‘high volume’ or ‘low volume’ depending on whether they were operated in a hospital above or below the median resection count/hospital/year respectively (40/hospital/year).
IDR: inter-decile range (10th–90th centile).
The characteristics of the 1284 cases, together with median and IDR LOS, are shown in Table 1. Table 2 presents crude and adjusted risk ratios (RR) for factors that were significantly associated with prolonged LOS in multivariate analyses (greater than the upper quartile of LOS, i.e. >20 days). In the adjusted analysis, the risk of prolonged LOS was significantly higher in patients who were older than 75 years; lived in the most deprived areas; had two or more comorbid conditions; and had undergone surgery in a lower-volume hospital. Patients who died in hospital subsequent to the IP were also significantly more likely to have prolonged LOS. Although the percentage of patients who had prolonged LOS varied slightly according to the procedure received (lobectomy 25%, pneumonectomy 23%, segmental 31% and other excision 31%), this was not statistically significant in adjusted analysis. Similarly, stage of disease was not significantly associated with prolonged LOS after adjusting for other variables (Stage I/II 25%, Stage III+ 25% and unstaged 16%). In the sensitivity analysis, using linear regression, the same variables predicted LOS as predicted prolonged LOS.
Table 2:
Factors significantly associated with prolonged LOS in patients having resection for NSCLC, 2002–8: number (n) of total (N) (%) who had prolonged LOS (>20 days), univariate and adjusted RR, with 95% CI and LRTs
| Prolonged LOS (>20 days) |
Univariate |
Adjusteda |
LRT | |||||
|---|---|---|---|---|---|---|---|---|
| n | N | % | RR | 95% CI | RR | 95% CI | P-value | |
| Total | 312 | 1284 | 24% | |||||
| Age | ||||||||
| <55 year | 37 | 204 | 18% | 1 | 1 | 0.064 | ||
| 55–64 year | 85 | 423 | 20% | 1.11 | [0.78, 1.57] | 1.03 | [0.73, 1.45] | |
| 65–74 year | 128 | 479 | 27% | 1.47 | [1.06, 2.04] | 1.32 | [0.95, 1.82] | |
| >75 year | 62 | 178 | 35% | 1.92 | [1.35, 2.74] | 1.55 | [1.08, 2.23] | |
| Sex | ||||||||
| Female | 132 | 541 | 24% | 1 | ||||
| Male | 180 | 743 | 24% | 0.99 | [0.82, 1.21] | |||
| Deprivation | ||||||||
| Less deprived (q1–4) | 158 | 727 | 22% | 1 | 1 | 0.060 | ||
| Most deprived (q5) | 138 | 480 | 29% | 1.32 | [1.09, 1.61] | 1.30 | [1.07, 1.58] | |
| Unknown | 16 | 77 | 21% | 0.96 | [0.61, 1.51] | 1.03 | [0.66, 1.62] | |
| Comorbidity | ||||||||
| None | 206 | 931 | 22% | 1 | 1 | 0.073 | ||
| 1 | 70 | 264 | 27% | 1.20 | [0.95, 1.51] | 1.11 | [0.88, 1.40] | |
| 2+ | 36 | 89 | 40% | 1.83 | [1.38, 2.42] | 1.60 | [1.20, 2.13] | |
| Hospital volume | ||||||||
| Higher: ≥40/year | 141 | 661 | 21% | 1 | 1 | 0.086 | ||
| Lower: <40/year | 171 | 623 | 27% | 1.29 | [1.03, 1.61] | 1.24 | [0.99, 1.56] | |
| Discharge status | ||||||||
| Alive at discharge | 266 | 1200 | 22% | 1 | 1 | 0.000074 | ||
| Died in hospital post IP | 46 | 84 | 55% | 2.47 | [1.98, 3.08] | 2.03 | [1.60, 2.57] | |
aMutually adjusted for age, deprivation, comorbidity, hospital volume and discharge status (and year of incidence, not shown).
RR: risk ratio; LRT: likelihood ratio test for exclusion of that variable from multivariable model.
Of 1200 patients who were discharged alive after surgery (none of whom died within 28 days of discharge), 119 (10%) were readmitted as emergencies within 28 days. Four of these 119 died in hospital after their emergency readmission. Table 3 presents crude and adjusted risk ratios for factors significantly associated with risk of emergency readmission. In the adjusted analysis, risk of readmission was increased in patients from the most deprived areas, with two or more comorbid conditions, and with Stage III+ disease (Table 3). Patients >75 years (14% of whom were readmitted, compared with 7, 10 and 9% in the <55 year, 55–64 year and 65–74 year age groups, respectively), and those who underwent segmental procedures (17% readmitted, compared with 9% for lobectomy, 12% for pneumonectomy and 9% for other procedures) were also more prone to emergency readmission in univariate analyses; however, age and procedure type were not significantly associated with readmission after adjustment for the factors in the model. The main reasons for emergency readmission were: pulmonary complications (29%), cardiovascular/vascular events (21%) and infections (20%) (Table 4).
Table 3:
Factors significantly associated with emergency readmission in patients having resection for NSCLC, 2002–8: number (n) of total (N) (%) who were readmitted within 28 days), univariate and adjusted RR with 95% CI and LRT
| Readmitted within 28 days |
Crude |
Adjusteda |
LRT | |||||
|---|---|---|---|---|---|---|---|---|
| n | N | % | RR | [95% CI] | RR | [95% CI] | P-value | |
| Total | 119 | 1200 | 10% | |||||
| Age | ||||||||
| <55 year | 15 | 201 | 7% | 1 | ||||
| 55–64 year | 42 | 406 | 10% | 1.39 | [0.79, 2.44] | |||
| 65–74 year | 41 | 441 | 9% | 1.25 | [0.71, 2.20] | |||
| >75 year | 21 | 152 | 14% | 1.85 | [0.99, 3.47] | |||
| Sex | ||||||||
| Female | 50 | 521 | 10% | 1 | ||||
| Male | 69 | 679 | 10% | 1.06 | [0.75, 1.50] | |||
| Deprivation | ||||||||
| Less deprived (q1–4) | 57 | 685 | 8% | 1 | 1 | 0.0095 | ||
| Most deprived (q5) | 59 | 441 | 13% | 1.61 | [1.14, 2.27] | 1.56 | [1.11, 2.20] | |
| Unknown | 3 | 74 | 4% | 0.49 | [0.16, 1.52] | 0.48 | [0.16, 1.45] | |
| Comorbidity | ||||||||
| None | 74 | 879 | 8% | 1 | 1 | 0.011 | ||
| 1 | 30 | 244 | 12% | 1.46 | [0.98, 2.18] | 1.43 | [0.96, 2.12] | |
| 2+ | 15 | 77 | 19% | 2.31 | [1.40, 3.83] | 2.38 | [1.43, 3.96] | |
| Stage | ||||||||
| Stage I/II | 72 | 801 | 9% | 1 | 1 | 0.039 | ||
| Stage III+ | 39 | 281 | 14% | 1.54 | [1.07, 2.23] | 1.62 | [1.13, 2.34] | |
| Unstaged | 8 | 118 | 7% | 0.75 | [0.37, 1.53] | 0.83 | [0.41, 1.69] | |
aMutually adjusted for deprivation, comorbidity and stage.
RR: risk ratio; LRT: P-value of likelihood ratio test for exclusion of that variable in multivariable model.
Table 4:
Primary reason for first emergency readmission within 28 days of IP
| Readmission cause | Description | n (%) | Subtotal number (%) |
|---|---|---|---|
| Pulmonary complication | Atelectasis | 11 (9%) | |
| Pneumonia | 8 (7%) | ||
| Pulmonary air leak | 6 (5%) | ||
| Pleural effusion | 7 (6%) | ||
| Empyema | 2 (2%) | 34 (29%) | |
| Cardiovascular/vascular | Dysrhythmia | 18 (15%) | |
| Myocardial infarction | 4 (3%) | ||
| Cerebrovascular event | 3 (3%) | 25 (21%) | |
| Infection | Wound infection | 6 (5%) | |
| Fever | 14 (12%) | ||
| Urinary tract infection | 3 (3%) | 23 (20%) | |
| Others | Renal insufficiency | 2 (2%) | |
| Others | 35 (29%) | 37 (31%) | |
| Total | 119 (100%) |
DISCUSSION
Strengths and limitations
This study is based on high-quality population-based cancer registration data. Our dataset of >1200 lung cancer resections conducted in patients incident during 2002–8 is relatively large by European standards and provides for the first time, as far as we are aware, detailed information on factors predicting prolonged LOS, and emergency readmission from a European population-based series. To our knowledge, apart from one study in Japan [11], it is the only population-based study to investigate LOS in patients of all ages; the other large population-based studies of LOS—from the USA—were restricted to patients aged 65 and older [4, 5]. Only 24 (<2%) cases recorded by the NCR as having a resection in a public hospital had no corresponding HIPE record. Failure to find a match can occur for several reasons including: typographical errors in fields used for matching, missing data on either system or no mention of cancer on the HIPE record, in which case the record would not be made available to NCR. The small numbers of missing episodes were distributed across hospitals and years. Compared with other cancers, surgery with curative intent for NSCLC is relatively uncommon, typically being undertaken in a minority of patients (e.g. 10–11% in England [6] and 16–19% in Norway [8]). Selection for surgery is partly determined by preoperative lung function tests. [21] We did not have access to data on lung function and derived a comorbidity score instead to provide some measure of likely health status.
International comparisons in length of stay
In our study, the median LOS (13 days) was considerably greater than that observed in Canada (6 days, based on 360 patients) [10] and Spain (7 days, n = 727) [9]. However, these data derived from case series from single centres may not be directly comparable to other hospitals or countries. Several large population-based studies have presented median LOS after lung cancer resection: US thoracic surgery database audit (6 days) [3], US Medicare database audit (10 days) [4], US SEER subset of Medicare recipients (6 days) [5], Japan (national audit of lobectomy, mean 13.7 days) [11], and a UK multicentre cohort study (9 days for pneumonectomy, n = 312, 2005) [12]. LOS in Ireland was greater than that reported for each of these studies. There is no single obvious reason why Ireland should have such prolonged LOS for lung resection compared with other countries. In published papers, it is not always entirely clear how LOS has been computed and whether the authors included (as we have done) preoperative LOS. When we discounted preoperative LOS, postoperative LOS in Ireland (median = 12 days, data not shown) was still much greater than that of any other country with published information. LOS in Ireland following colorectal cancer surgery [22] was also relatively high compared with international norms; this suggests that system-level or hospital-level practices account for the findings to some degree.
The largest studies on LOS were undertaken within the US healthcare system where every cost item (including LOS) is rigorously controlled by health insurers, which probably explains why the LOS in these studies is markedly lower than that of Ireland. The (postoperative mean) estimate of LOS in Ireland was not much greater than that of Japan [11]. Otake et al. [11] note that unlike in the USA, immediate postoperative care and subsequent nursing care tend to be combined within the same hospital episode, which is why LOS is much greater in Japan than in the USA. It is possible that a portion of the ‘excess’ days LOS in Irish hospitals may be devoted to subsequent nursing care rather than immediate postoperative care, as happens in Japan, but we are unable to determine this from the data available.
Our study was undertaken within a mixed public–private healthcare system. We observed a modest trend for reduced LOS over the period 2002–8. The Dutch National Medical Registration system has proposed that all hospitals should aspire to achieve an average LOS for each specialty, equal to that of a benchmark model hospital for that specialty [23]. Our findings suggest that a similar systematic approach is required in Ireland. Shorter hospital stay as a result of improved discharge efficiency could reduce cost per patient and increase patient throughput.
Factors associated with length of stay
The observed associations between older age and more comorbidities and increased risk of prolonged LOS in our study are probably unsurprising. However, they do suggest that LOS following lung resection is not easily reduced, short of restricting surgery to the youngest and healthiest patients. Our finding of reduced risk of prolonged LOS in higher-volume hospitals is consistent with a large national audit in Japan [11], but not with a large national audit of the US Medicare population (>65 years) in the USA [4]. Apart from the much smaller number of hospitals performing lung cancer surgery in Ireland, the number of NSCLC resections/hospital carried out within Irish hospitals was within the same order of magnitude as that of the USA and Japan. Although the ‘excess’ LOS for patients in lower-volume hospitals in our study was modest (only 2 days on average), this has the potential to impact significantly on hospital budgets in Ireland (and elsewhere); this should be a particular concern for service providers in light of the increasing incidence of lung cancer projected in many populations and the increasing resort to surgical interventions [6–8]. One potential implication of our findings is that further centralization of surgical expertise towards higher-volume hospitals in a small county like Ireland could provide a way to constrain LOS and achieve better economy of scale.
We also observed for the first time as far as we are aware that patients resident in the most deprived areas were more likely to have a prolonged LOS. Another study reported that patients with longer LOS following lung cancer surgery have higher mortality rates 2.5 years post-surgery [5]. This suggests that the association between deprivation and LOS explains, at least in part, the poorer survival found in socioeconomically disadvantaged lung cancer patients [24]. In terms of potential reasons why more deprived patients might have longer LOS, it is possible that they are not as prepared for discharge as less deprived patients, for reasons other than clinical factors. For example, in some instances, LOS could simply depend on access to transport on the proposed day of discharge (e.g. money for a taxi or availability of a car); such access may be more limited in patients resident in the most deprived areas. Also, some characteristics of the most deprived patients were different from other patients. For example, those resident in the most deprived areas were less likely to be married than those resident in other areas (41 vs 35%). Clinicians may be less likely to discharge a patient quickly if they lack a spouse who can provide support and care at home. Fewer patients in the most deprived areas were treated privately by the managing consultant (22 vs 36%), which effectively means that they did not have private health insurance. We have shown, for colorectal cancer, that private patients have shorter LOS post-surgery [22], most likely due to pressure from the insurer (or the patient) to constrain costs. Finally, current smoking was more common among patients resident in the most deprived areas (51 vs 45%). Smokers often have significant co-existing conditions (which may not have been fully captured by our measure of comorbidity) and may have poorer general health status, or be in poorer physical condition, thus resulting in longer LOS. Moreover, it is possible that they have poorer lung function, which has been previously reported to be associated with LOS [3, 10].
Emergency readmissions
The main reasons for emergency readmission within 28 days of discharge—pulmonary complications, cardio/cerebrovascular events and infection—were similar to those described in an audit of a US specialist centre [13]. In common with research in the USA [5], we found that multiple comorbidities and advanced stage predisposed to readmission, and these associations are unsurprising. To our knowledge, the association between deprivation and readmission has not been observed previously. The differentials between the ‘most’ and ‘least’ deprived for various characteristics were detailed above. Smoking, which was more common in the deprived category, may have been a factor driving readmissions, as was lack of social support in the unmarried. It is also possible that patients in the most deprived areas were inherently more prone to adverse events due to greater comorbidity, and perhaps were slower to recognize and act upon the development of an adverse event before it became serious enough for emergency readmission, but we are not aware of any data to support this hypothesis. Similar to the SEER subset study [5], prolonged LOS did not predict emergency readmission. By implication then, any efforts to reduce LOS in Ireland (or elsewhere) may not necessarily result in increased readmissions.
CONCLUSIONS
Half of the patients undergoing resection for NSCLC stay in hospital for >13 days. LOS is longer in Ireland when compared with other countries with published data. Deprivation, greater age, comorbidity and treatment at a lower-volume hospital were identified as risk factors for prolonged LOS. Deprivation also predicted emergency readmission with 28 days, as did comorbidity and more advanced stage. Since socioeconomic disadvantage is related to poorer survival from lung cancer, in the interests of equity, the reasons for the observed associations between deprivation and LOS and readmission require elucidation.
Funding
The National Cancer Registry is funded by the Department of Health in Ireland.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The authors are grateful to the tumour registration officers and data managers at the National Cancer Registry Ireland who abstracted the case information, and to the analysts at the ESRI who created and administered the HIPE database.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. http://globocan.iarc.fr. 17 May 2013, date last accessed. [Google Scholar]
- 2.NICE. Lung cancer: The diagnosis and treatment of lung cancer. 2011. NICE clinical guideline 121 http://guidance.nice.org.uk. (17 May 2013, date last accessed)
- 3.Wright CD, Gaissert HA, Grab JD, O'Brien SM, Peterson ED, Allen MS. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database Risk-Adjustment Model. Ann Thorac Surg. 2008;85:1857–65. doi: 10.1016/j.athoracsur.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg. 2003;238:161–7. doi: 10.1097/01.SLA.0000081094.66659.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farjah F, Wood DE, Varghese TK, Massarweh NN, Symons RG, Flum DR. Health care utilization among surgically treated Medicare beneficiaries with lung cancer. Ann Thorac Surg. 2009;88:1749–56. doi: 10.1016/j.athoracsur.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Riaz SP, Linklater KM, Page R, Peake MD, Møller H, Lüchtenborg M. Recent trends in resection rates among non-small cell lung cancer patients in England. Thorax. 2012;67:811–4. doi: 10.1136/thoraxjnl-2012-201768. [DOI] [PubMed] [Google Scholar]
- 7.Beattie G, Bannon F, McGuigan J. Lung cancer resection rates have increased significantly in females during a 15-year period. Eur J Cardiothorac Surg. 2010;38:484–90. doi: 10.1016/j.ejcts.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Strand TE, Bartnes K, Rostad H. National trends in lung cancer surgery. Eur J Cardiothorac Surg. 2012;42:355–8. doi: 10.1093/ejcts/ezs002. [DOI] [PubMed] [Google Scholar]
- 9.Varela G, Aranda JL, Jiménez MF, Novoa N. Emergency hospital readmission after major lung resection: prevalence and related variables. Eur J Cardiothorac Surg. 2004;26:494–7. doi: 10.1016/j.ejcts.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Gagarine A, Urschel JD, Miller JD, Bennett WF, Young JE. Preoperative and intraoperative factors predictive of length of hospital stay after pulmonary lobectomy. Ann Thorac Cardiovasc Surg. 2003;9:222–5. [PubMed] [Google Scholar]
- 11.Otake H, Yasunaga H, Horiguchi H, Matsutani N, Matsuda S, Ohe K. Impact of hospital volume on chest tube duration, length of stay, and mortality after lobectomy. Ann Thorac Surg. 2011;92:1069–74. doi: 10.1016/j.athoracsur.2011.04.087. [DOI] [PubMed] [Google Scholar]
- 12.Powell ES, Pearce AC, Cook D, Davies P, Bishay E, Bowler GM, et al. and Co-ordinators. UKPOS. UK pneumonectomy outcome study (UKPOS): a prospective observational study of pneumonectomy outcome. J Cardiothorac Surg. 2009;4:41–50. doi: 10.1186/1749-8090-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handy JR, Jr, Child AI, Grunkemeier GL, Fowler P, Asaph JW, Douville EC, et al. Hospital readmission after pulmonary resection: prevalence, patterns, and predisposing characteristics. Ann Thorac Surg. 2001;72:1855–9. doi: 10.1016/s0003-4975(01)03247-7. [DOI] [PubMed] [Google Scholar]
- 14.Data Quality and Completeness at the Irish National Cancer Registry. National Cancer Registry, Cork, Ireland. http://www.ncri.ie/pubs/pubfiles/CompletenessQuality.pdf. 17 May 2013, date last accessed.
- 15.Wiley M. Using HIPE data as research and planning tool: limitations and opportunities: a response. Ir J Med Sci. 2005;174:52–7. doi: 10.1007/BF03169128. [DOI] [PubMed] [Google Scholar]
- 16.Karaffa MC. ICD-9-CM: The International Classification of Diseases, 9th Edition, Clinical Modification. Los Angeles Ca. USA: Practice Management Information Corporation; 1992. [Google Scholar]
- 17.Kelly A, Teljeur C. A New National Deprivation Index for Health and Health Services Research, Small Area Health Research Unit. Trinity College Dublin, Ireland; 2004. http://www.sahru.tcd.ie/services/deprivation/DeprivationFiles/DeprivationReport2013.pdf. (01 June 2013, date last accessed). [Google Scholar]
- 18.Sobin LH, Wittekind Ch. TNM Classification of Malignant Tumours. 5th edn. Geneva, Switzerland: UICC; 1997. [Google Scholar]
- 19.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for non small cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–62. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur J Cardiothorac Surg. 2009;36:181–4. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 22.Kelly M, Sharp L, Dwane F, Kelleher T, Comber H. Factors predicting hospital length-of-stay and readmission after colorectal resection: a population-based study of elective and emergency admissions. BMC Health Serv Res. 2012;12:77. doi: 10.1186/1472-6963-12-77. doi:10.1186/1472-6963-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borghans I, Heijink R, Kool T, Lagoe R J, Westert GP. Benchmarking and reducing length of stay in Dutch hospitals. BMC Health Serv Res. 2008;8:220. doi: 10.1186/1472-6963-8-220. doi:10.1186/1472–6963-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH., Jr Neighborhood-level socioeconomic determinants impact outcomes in non small cell lung cancer patients in the Southeastern United States. Cancer. 2012;118:5117–23. doi: 10.1002/cncr.26185. [DOI] [PubMed] [Google Scholar]


