Abstract
IMPORTANCE
Minimally invasive colectomies are increasingly popular options for colon resection.
OBJECTIVE
To compare the perioperative outcomes and costs of robot-assisted colectomy (RC), laparoscopic colectomy (LC), and open colectomy (OC).
DESIGN, SETTING, AND PARTICIPANTS
The US Nationwide Inpatient Sample database was used to examine outcomes and costs before and after propensity score matching across the 3 surgical approaches. This study involved a sample of US hospital discharges from 2008 to 2010 and all patients 21 years of age or older who underwent elective colectomy.
MAIN OUTCOMES AND MEASURES
In-hospital mortality, complications, ostomy rates, conversion to open procedure, length of stay, discharge disposition, and cost.
RESULTS
Of the 244 129 colectomies performed during the study period, 126 284 (51.7%) were OCs, 116 261 (47.6%) were LCs, and 1584 (0.6%) were RCs. In comparison with OC, LC was associated with a lower mortality rate (0.4%vs 2.0%), lower complication rate (19.8%vs 33.2%), lower ostomy rate (3.5 vs 13.0%), shorter median length of stay (4 vs 6 days), a higher routine discharge rate (86.1%vs 68.4%), and lower overall cost than OC ($11 742 vs $13 666) (all P < .05). Comparison between RC and LC showed no significant differences with respect to in-hospital mortality (0.0%vs 0.7%), complication rates (14.7%vs 18.5%), ostomy rates (3.0% vs 5.1%), conversions to open procedure (5.7%vs 9.9%), and routine discharge rates (88.7%vs 88.5%) (all P > .05). However, RC incurred a higher overall hospitalization cost than LC ($14 847 vs $11 966, P < .001).
CONCLUSIONS AND RELEVANCE
In this nationwide comparison of minimally invasive approaches for colon resection, LC demonstrated favorable clinical outcomes and lower cost than OC. Robot-assisted colectomy was equivalent in most clinical outcomes to LC but incurred a higher cost.
The application of minimally invasive procedures in colorectal surgery has been rapidly gaining acceptance.1 Laparoscopic colectomy (LC) has been shown by single-institution studies to be associated with equivalent or superior clinical outcomes in comparison with open colectomy (OC).2–5 Owing to the shortened length of stay (LOS) and decreased complication rate, LC was also associated with lower overall cost.2However, the introduction of the laparoscopic surgical approach also highlights drawbacks such as loss of 3-dimensional view, long instruments that amplify physiologic tremors, and loss of dexterity and ergonomic discomfort for the surgeon.6 These factors may contribute to technical difficulty with the laparoscopic procedure as well as a long learning curve.7
Robot-assisted surgery could be considered an advancement of laparoscopic surgery because it aims to minimize these technical challenges with the use of robotic arms and a separate operating console.8–10However, robot-assisted surgery has gained acceptance at a slower pace in colorectal surgery.11,12 Past research studies have focused mainly on robot-assisted total mesorectal excision for rectal cancers.12–14 To our knowledge, only limited published data exist on robot-assisted colectomies (RCs). They mainly consist of single-institution early outcome reports15–19 and retrospective comparative studies on RCs.20–22 These early results have demonstrated that RC, while being equivalent in safety and feasibility, usually incurs a higher cost, even beyond the initial purchase of the robot.
To our knowledge, this is the first comprehensive national study of minimally invasive approaches for colon resection. We reviewed the current use pattern of the 3 approaches for colectomies—open, laparoscopic, and robotic—and performed a comparative analysis of their outcomes and costs using propensity score matching.
Methods
Study Population
A sample of adult patients (aged ≥21 years) who underwent elective colectomies from October 1, 2008, to December 31, 2010, across the nation was identified using the US Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS). The NIS included a 20% stratified probability sample of inpatient discharge data from approximately 1040 hospitals in 44 states.
We extracted patients with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for OC or LC as shown in the eTable (Supplement). Patients with rectal resection were not included owing to lack of separate coding of laparoscopic and open procedures in ICD-9 classification. Patients with distant metastases were also excluded for preservation of cohort homogeneity. Beginning October 1, 2008, the robot-assisted modifier code (ICD-9-CM 17.42) was used to identify robot-assisted laparoscopic procedures. Minimally invasive procedures that were later converted to open procedures were identified with the ICD-9 diagnosis code V64.41 and categorized under their original procedure. Patients admitted for nonelective procedures were excluded.
Patient characteristics included age, sex, race/ethnicity, surgical indication, median household income, and primary health care payer. The Charlson Comorbidity Index,23 a prognostic measure calculated from the presence or absence of several comorbid conditions using a weighted formula, was used as a measure of comorbidity burden at the time of surgery. Patients with missing data on sex or race/ethnicity were excluded from the analysis. Surgical indications were inferred using ICD-9-CM diagnostic codes as shown in the eTable (Supplement).
Hospital characteristics included hospital size, teaching status, location, and case volume. Hospital size, defined by the NIS database using the number of inpatient beds, was categorized into small, medium, and large. Hospital case volume was calculated as the total number of colectomies performed per hospital per year and analyzed in tertiles. Hospitals with missing values for the described characteristics were excluded.
This study was deemed exempt from review by the Johns Hopkins Medicine institutional review board. Written informed consent from patients was waived as this was a secondary data analysis using deidentified data.
Outcomes of Interest
The primary out come was in-hospital mortality. Secondary out comes included complication rates, ostomy rates, LOS, discharge disposition, overall hospitalization cost, and cost per hospitalization day. Discharge disposition was categorized as (1) routine discharge; (2) transfer to other health care facility including short-term hospital, skilled nursing facility, intermediate care, or other type of facility; and (3) other including home health care or discharge against medical advice. Overall hospitalization cost24 was estimated by multiplying total hospital charges (adjusted for inflation to reflect 2010 US dollars) by hospital-specific cost-to-charge ratios and re-weighted to account for the hospitals where the cost-to-charge ratio was not available. To adjust for the influence of LOS on cost, cost per hospitalization day was calculated as over all cost divided by LOS. For robot-assisted and laparoscopic procedures, conversion to open procedure was examined as an additional outcome variable. Complications were identified using ICD-9-CM diagnostic codes for perioperative complications25 for each major organ system. Ostomy rates, conversion to open procedure, and transfusion were identified using ICD-9-CM procedure codes (eTable in Supplement).
Statistical Analysis
National estimates were calculated using stratification, clustering, and survey weights in accordance with the NIS sampling design. Subtracted data were stratified by procedure type: RC, LC, and OC. For continuous variables such as age, LOS, and cost, values were presented asmedians accompanied by inter-quartile ranges. For categorical variables, including sex, race/ethnicity, Charlson Comorbidity Index score, surgical indication, median household income, primary health care payer, hospital size, teaching status, hospital location, and national estimates of patients, number along with the corresponding percentage within each procedure were presented. The Pearson χ2 test was used to assess categorical variables, and the Mann-Whitney U test was used to assess continuous variables.
Outcome variables were examined by procedure type and included in-hospital mortality, complication rates, ostomy rate, LOS, discharge disposition, and hospitalization cost. Owing to differences between patient cohorts who underwent each respective procedure, we relied on propensity score matching to adjust for those differences.26 Two separate comparisons were performed: LC vs OC and RC vs LC. In each comparison, only patients from hospitals where both procedures were performed were included for analysis. Propensity scores were assigned for each patient based on multivariate logistic regression using both patient characteristics (ie, age, sex, race/ethnicity, Charlson Comorbidity Index score, surgical indication, median household income, primary health care payer, and resection type) and hospital characteristics (ie, size, teaching status, location, region, and case volume). The assignment was repeated twice for comparison between LC and OC and between RC and LC, and the balance of score distribution between groups was checked. We performed 1:1 fixed ratio nearest neighbor matching with replacement between LC and OC and between RC and LC. The 1:1 ratio was chosen to minimize bias without sacrificing too much power in accordance with recommendations from previous literature.27 As a measure of sensitivity analysis, exact matching between RC and LC using patient characteristics, including age (in bins of 10 years), sex, race/ethnicity, surgical indication, and Charlson Comorbidity Index score, was also performed to examine the aforementioned outcomes and cost variables. All tests were 2-sided, with the significance level set at α = .05. All data transformation and statistical analyses were performed using Stata version 12.1 (StataCorp).
Results
Study Population
Between October 2008 and December 2010, there were 48 237 elective colectomies in the NIS database that fit our inclusion criteria, representing 244 129 colectomies across the nation after incorporating NIS survey weights. Patient and hospital baseline characteristics are summarized in Table 1. The median patient age was 64 years. Patients who underwent colectomy were more likely to be female (n = 133 307 [54.6%]) and white (n = 201 089 [82.4%]). Most cases were right hemicolectomies (n = 90 838 [37.2%]) and sigmoidectomies (n = 90 171 [36.9%]).The remainder were left hemicolectomies (n = 24 423 [10.0%]), transverse colon resections (n = 11 001 [4.5%]), cecectomies (n = 8913 [3.7%]), and other types of resections (n = 17 291 [7.1%]) and were grouped together as others for subsequent analysis. The 3 most common indications for colectomy were colon cancer (n = 81 423 [33.5%]), diverticular disease (n = 77 900 [32.1%]), and inflammatory bowel disease (n = 7393 [3.0%]).
Table 1.
Patient and Hospital Characteristics of Elective Colectomiesa
| No. (%) | |||||
|---|---|---|---|---|---|
| Characteristic | Total (N = 244 129 [100%]) |
Robot-Assisted Colectomy (n = 1584 [0.6%]) |
Laparoscopic Colectomy (n = 116 261 [47.6%]) |
Open Colectomy (n = 126 284 [51.7%]) |
P Valueb |
| Patient characteristics | |||||
| Resection type | |||||
| Right hemicolectomy | 90838 (37.2) | 536 (33.9) | 47532 (40.9) | 42769 (33.9) | <.001c |
| Sigmoidectomy | 90 171 (36.9) | 874 (55.2) | 45 965 (39.5) | 43 332 (34.3) | |
| Others | 63 120 (25.9) | 174 (10.9) | 22 765 (19.6) | 40 181 (31.8) | |
| Age, median (IQR), y | 64 (21) | 61.5 (18) | 62 (20) | 65 (21) | <.001c |
| Race/ethnicity | |||||
| White | 20 1089 (82.4) | 1219 (76.9) | 96 878 (83.3) | 10 2992 (81.6) | .002c |
| Black | 21 175 (8.7) | 86 (5.5) | 9053 (7.8) | 12 036 (9.5) | |
| Hispanic | 12 659 (5.2) | 185 (11.7) | 6176 (5.3) | 6484 (4.9) | |
| Others | 9206 (3.8) | 93 (5.9) | 4154 (3.6) | 5051 (3.9) | |
| Sex | |||||
| Male | 110822 (45.4) | 640 (40.4) | 54609 (47.0) | 55573 (44.0) | <.001c |
| Female | 133 307 (54.6) | 944 (59.6) | 61 652 (53.0) | 70 710 (56.0) | |
| Charlson Comorbidity Index score | |||||
| 0 | 95 644 (39.2) | 767 (48.4) | 52 883 (45.5) | 41 993 (33.3) | <.001c |
| 1–2 | 80 857 (33.1) | 533 (33.6) | 39 099 (33.7) | 41 224 (32.6) | |
| ≥3 | 67 628 (27.7) | 284 (17.9) | 24 279 (20.9) | 43 066 (34.1) | |
| Surgical indication | |||||
| Colon cancer | 81 423 (33.4) | 456 (28.8) | 37 203 (32.0) | 43 764 (34.7) | <.001c |
| Diverticular disease | 77 900 (31.9) | 642 (40.5) | 42 590 (36.6) | 34 669 (27.5) | |
| Others | 84 805 (34.7) | 487 (30.7) | 35 967 (31.3) | 47 851 (37.8) | |
| Primary payer | |||||
| Private | 115 325 (47.2) | 918 (58.0) | 61 387 (52.8) | 53 020 (42.0) | <.001c |
| Medicare | 113 614 (46.5) | 603 (38.1) | 49 055 (42.2) | 63 956 (50.6) | |
| Medicaid/others | 15190 (6.2) | 63 (4.0) | 5819 (5.0) | 9308 (7.4) | |
| Median household income, $ | |||||
| <38 999 | 54 659 (22.4) | 214 (13.5) | 21 496 (18.5) | 32 949 (26.1) | <.001c |
| 39 000–47 999 | 63 516 (26.0) | 352 (22.2) | 27 508 (23.7) | 35 656 (28.2) | |
| 48 000–62 999 | 62 081 (25.4) | 494 (31.2) | 31 107 (26.8) | 30 480 (24.1) | |
| >63 000 | 63 874 (26.2) | 524 (33.1) | 36 151 (31.1) | 27 198 (21.5) | |
| Hospital characteristics | |||||
| Size | |||||
| Small | 27 303 (11.2) | 157 (9.9) | 11 723 (10.1) | 15 423 (12.2) | .14 |
| Medium | 58 638 (24.0) | 379 (23.9) | 29 305 (25.2) | 28 954 (22.9) | |
| Large | 158 188 (64.8) | 1048 (66.1) | 75 233 (64.7) | 81 907 (64.9) | |
| Type | |||||
| Nonteaching | 123 706 (50.7) | 418 (26.4) | 57 543 (49.5) | 65 745 (52.1) | .001c |
| Teaching | 120 424 (49.3) | 1166 (73.6) | 58 718 (50.5) | 60 539 (47.9) | |
| Location | |||||
| Rural | 26 882 (11.0) | <11 (0.3) | 8652 (7.4) | 18 226 (14.4) | <.001c |
| Urban | 217 248 (89.0) | 1580 (99.7) | 107 610 (92.6) | 108 058 (85.6) | |
| Volume tertile | |||||
| Small | 81 671 (33.5) | 297 (18.8) | 33 186 (28.5) | 48 188 (38.2) | <.001c |
| Middle | 80 955 (33.2) | 612 (38.6) | 39 275 (33.8) | 41 068 (32.5) | |
| Large | 81 503 (33.3) | 675 (42.6) | 43 801 (37.7) | 37 028 (29.3) | |
Abbreviation: IQR, interquartile range.
Weighted counts using Nationwide Inpatient Sample complex survey weights; numbers may not sum to group totals or percentages may not add to 100 owing to the need for rounding. Numbers are rounded to nearest integral number and percentages are based on rounded numbers.
The P values address comparison across all 3 procedures.
Statistically significant, with P < .05.
Of all the colectomies, 126 284 (51.7%) were OCs, 116 261 (47.6%) were LCs, and 1584 (0.6%) were RCs. While the proportion of colectomies performed laparoscopically has experienced modest growth over the study period (45.5%in 2008 vs 47.4% in 2009 vs 48.3% in 2010), RC cases appeared to be growing exponentially (0.1%in 2008 vs 0.5%in 2009 vs 0.9% in 2010; eFigure in Supplement), although overall numbers were still small.
Significant differences with respect to the patient characteristics were observed across the 3 surgical approaches. The median age of patients receiving OC was 65 years, significantly older than those receiving LC (62 years) or RC (61.5 years). There were more women than men receiving colectomies in all 3 surgical approaches (OC: 56.0%, LC: 53.0%, and RC: 59.6%). Most patients who underwent colectomy in all 3 approaches were white (OC: 81.6%, LC: 83.3%, and RC: 76.9%). There was a significantly higher proportion of Hispanics in the RC group (OC: 4.9%, LC: 5.3%, and RC: 11.7%). The patients receiving OC had a significantly higher comorbidity burden than either LC or RC patients, as evidenced by the higher proportion of patients with Charlson Comorbidity Index scores of 3 or greater (OC: 34.1%, LC: 20.9%, and RC: 17.9%). Open colectomy patients were also more likely to have undergone surgery for colorectal malignancy (OC: 34.7%, LC: 32.0%, and RC: 28.8%). A higher proportion of the RC patients had private payer insurance (RC: 58.0%, LC: 52.8%, and OC: 42.0%), whereas OC patients were more likely to have their medical expenses covered by Medicare (OC: 50.6%, LC: 42.2%, and RC: 38.1%). Robot-assisted colectomy patients were also more likely to be wealthier, as evidenced by the higher proportion of these patients with a median household income of more than $63 000 (RC: 33.1%, LC: 31.1%, and OC: 21.5%).
No significant difference was observed in the distribution of the hospitals’ size in which the 3 surgical procedures were performed. However, a significantly higher proportion of RC and LC was performed at teaching hospitals (RC: 73.6%, LC: 50.5%, and OC: 47.9%).Robot-assisted colectomy was performed almost exclusively in urban regions (RC: 99.7%, LC: 92.6%, and OC: 85.6%), at higher-volume hospitals (large hospital volume, as measured in tertiles = RC: 42.6%, LC: 37.7%, and OC: 29.3%), and at teaching hospitals (RC: 99.7%, LC: 92.6%, and OC: 85.6%).
Unmatched Outcomes
Overall, patients who under went colectomies during the study experienced an in-hospital mortality rate of approximately 1.3% (n = 3062) and a complication rate of 26.7% (n = 65 125). While there was no obvious temporal trend of in-hospital mortality, LOS, or cost, the overall complication rate decreased from year to year (28.2% in 2008 vs 27.1%in 2009 vs 25.9% in 2010). The median LOS was 5 days with an interquartile range of 4 days. About 77.0% of the patients (n = 185 579) had a routine discharge after hospitalization, 8.7% (n = 20 890) were transferred to other health care facilities, and 14.4% (n = 34 594) were discharged to home health care.
The in-hospital mortality rate was highest among OC patients and lowest among RC patients (OC: 2620 [2.1%], LC: 442 [0.4%], and RC: 0 [0.0%]; P < .001). The complication rate was highest among OC patients and lowest among RC patients (OC: 41 888 [33.2%], LC: 23 005 [19.8%], and RC: 232 [14.7%]; P < .001). The ostomy rate was highest among OCs, followed by RCs, and then LCs (OC: 166 595 [13.1%], LC: 4102 [3.5%], and RC: 80 [5.1%]). Conversion to open procedure occurred more frequently among LC than among RC patients (LC: 14.953 [12.4%] and RC: 95 [5.7%]).Median LOS was significantly longer with OC than with RC or LC (OC: 6 days, LC: 4 days, and RC: 4 days). As opposed to transfer to another health care facility, routine discharge occurred less commonly with OC than with LC or RC (OC: 84 472 [68.3%], LC: 99 702 [86.1%], and RC: 1405 [88.7%]).Median overall hospitalization cost was the highest among RC, followed by OC, and then LC (OC: $13 911, LC: $10 782, and RC: $14 847).
Propensity Score–Matched Comparisons
Owing to differences in patient and hospital characteristics between the 3 procedures, separate propensity score– matched comparisons between LC and OC and between RC and LC were performed to evaluate the outcomes with minimized bias.
Laparoscopic vs Open Colectomies
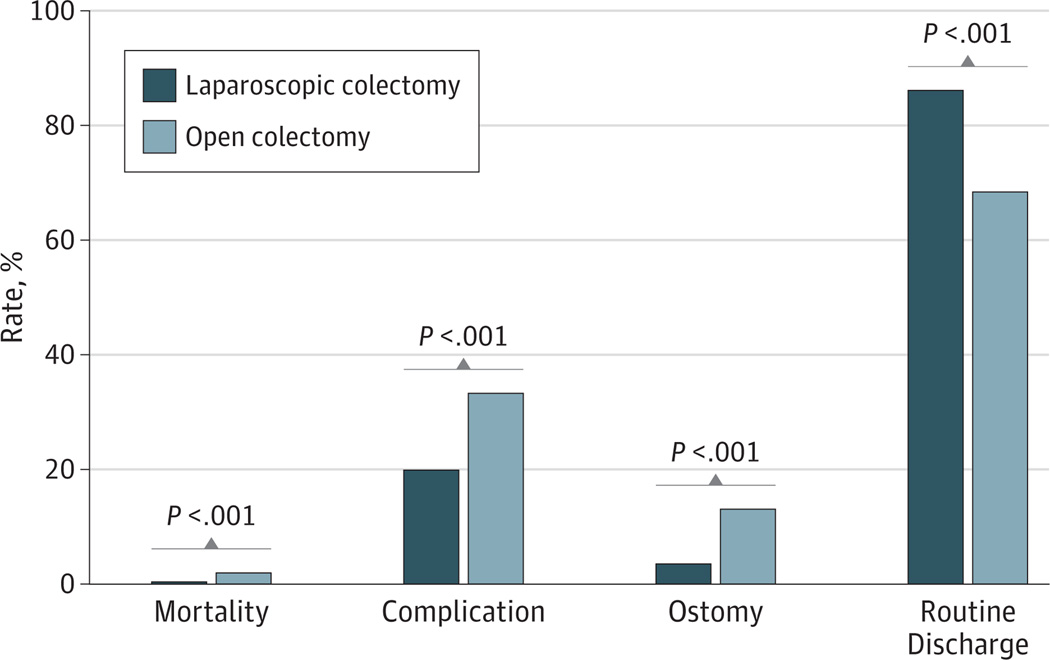
After 1:1 fixed ratio propensity score matching, 115 694 LCs and 116 261 OCs were retained for comparison. The in-hospital mortality rate for LC patients (0.4%) was significantly lower than for OC patients (2.0%). Moreover, in comparison with OC, LC was associated with a significantly lower complication rate (LC: 19.8% and OC: 33.2%), lower ostomy rate (LC: 3.5% and OC: 13.0%), shorter median LOS (LC: 4 days and OC: 6 days), and a higher routine discharge rate (LC: 99 702 [86.1%] and OC: 77 584 [68.4%]) (Figure).
Figure. Propensity Score–Matched Comparison Between Laparoscopic Colectomy and Open Colectomy.
Statistically significant differences were observed in mortality, complication, ostomy, and routine discharge rates between laparoscopic colectomy and open colectomy.
While the median cost per hospitalization day for LC was significantly higher than OC (LC: $2666 and OC: $2120), the overall hospitalization cost was lower for LC than OC (LC: $11 742 and OC: $13 666) (both P < .001). Outcomes and costs for LC and OC patients are shown in Table 2.
Table 2.
Propensity Score–Matched Perioperative Outcomes Between LC and OC
| Outcome | LC (n = 115 694) |
OC (n = 116 261) |
P Value |
|---|---|---|---|
| Mortality, No. (%) | 442 (0.4) | 2258 (2.0) | <.001a |
| Complications, No. (%) | 23 005 (19.8) | 38 454 (33.2) | <.001a |
| Ostomy, No. (%) | 4102 (3.5) | 15 056 (13.0) | <.001a |
| LOS, median (IQR), d | 4 (3) | 6 (4) | <.001a |
| Discharge disposition, No. (%) | |||
| Routine discharge | 99 702 (86.1) | 77 584 (68.4) | <.001a |
| Transfer to other health care facilities | 5335 (4.6) | 13 700 (12.1) | |
| Others | 10 782 (9.3) | 22 147 (19.5) | |
| Overall cost, median (IQR), USD, $ | 11 742 (6792) | 13 666 (11 196) | <.001a |
| Cost per day, median (IQR), USD, $ | 2666 (1482) | 2120 (1128) | <.001a |
Abbreviations: IQR, interquartile range; LC, laparoscopic colectomy; LOS, length of stay; OC, open colectomy.
Statistically significant, with P < .05.
Robot-Assisted vs Laparoscopic Colectomies
After 1:1 fixed ratio propensity score matching, 1584 RCs and 1500 LCs were retained for comparison. No significant difference of in-hospital mortality was observed between RC and LC (RC: 0% and LC: 0.7%). There was no significant difference in overall complication rate (RC: 14.7% and LC: 18.5%), ostomy rate (RC: 3.0%and LC: 5.1%), conversion to open rate (RC: 5.7% and LC: 9.9%), or routine discharge rate (RC: 88.7% and LC: 88.50%). Patients who underwent RC had a marginally shorter LOS than LC patients. How ever, both the median cost per hospitalization day (RC: $3407 and LC: $2617) and median overall hospitalization cost (RC: $14 847 and LC: $11 966) were higher for RC than LC (both P < .001). Outcomes and costs for RC and LC patients are shown in Table 3.
Table 3.
Propensity Score–Matched Perioperative Outcomes Between RC and LC
| Outcome | LC (n = 1584) |
OC (n = 1500) |
P Value |
|---|---|---|---|
| Mortality, No. (%) | 0 (0.0) | <11 (0.7) | .12 |
| Complications, No. (%) | 232 (14.7) | 277 (18.2) | .26 |
| Ostomy, No. (%) | 45 (3.0) | 80 (5.1) | .18 |
| Conversion to open, No. (%) | 90 (5.7) | 149 (9.9) | .05 |
| LOS, median (IQR), d | 4 (2) | 4 (3) | .008a |
| Discharge disposition, No. (%) | |||
| Routine discharge | 1405 (88.7) | 1319 (88.5) | .98a |
| Transfer to other health care facilities | 58 (3.6) | 52 (3.5) | |
| Others | 122 (7.7) | 120 (8.0) | |
| Overall cost, median (IQR), USD, $ | 14 847 (8620) | 11 966 (6582) | <.001a |
| Cost per day, median (IQR), USD, $ | 3407 (2353) | 2617 (1344) | <.001a |
Abbreviations: IQR, interquartile range; LC, laparoscopic colectomy; LOS, length of stay; RC, robot-assisted colectomy.
Statistically significant, with P < .05.
To address the concern that significant differences might be concealed owing to the 1:1 propensity matching reducing the sample size of LC patients, exact matching between RC and LC on key patient characteristics was performed for sensitivity analysis. There were 1043 RC patients and 5536 LC patients retained after exact matching for comparison. Again, no significant difference was found in the mortality rate (RC: 0 [0.0%] and LC: 0 [0.0%]), complication rate (RC: 155 [14.8%] and LC: 826 [14.9%]), ostomy rate (RC: 52 [5.0%] and LC: 114 [2.1%]), conversion to open rate (RC: 61 [5.8%] and LC: 543 [9.8%]), median LOS (RC: 4 days and LC: 4 days), or routine discharge rate (RC: 944 [90.5%] and LC: 5176 [93.5%]) (all P > .05). Robot-assisted colectomy still incurred significantly higher median cost per hospitalization day (RC: $3531 and LC: $2708) and median overall hospitalization cost (RC: $14 384 and LC: $10 600) (both P < .001).
Discussion
Since the first laparoscopic right hemicolectomy was described28 in 1990, LC has appeared to be equivalent to OC in both short-term quality of life4 and long-term oncologic outcomes.5 However, studies so far have been either high-volume single-institution series or those performed in the setting of clinical trials.29 Therefore, it was unclear whether similar outcomes would be observed in a nationwide population data set. Our study is important because it found that LC, in comparison with OC, was associated with a lower in-hospital mortality rate, lower complication rate, lower transfusion rate, lower ostomy rate, shorter LOS, and a higher likelihood of routine discharge. While the cost per hospitalization day was higher among LC patients than OC patients, the overall cost of LC was lower than OC, most likely owing to the shorter LOS and lower complication rate. We confirmed the study result of Alkhamesi et al2 and earlier national outcome studies of LC in patients with colon cancer and diverticulitis.30,31
Our data also show that the hospital use of LCs was evenly distributed between teaching and nonteaching hospitals and between low-, middle-, and high-volume hospitals. This implies that LC is transitioning from its early phase, when it was performed by highly trained specialists, to more widespread use.
Despite its clear benefits, laparoscopic surgery was found to be used at lower frequencies than would be expected.1 As of 2010,more than half of all colectomies (51.7%) in the United States are still performed via an open approach. This might be attributed to the higher technical difficulty and learning curve associated with the laparoscopic procedure.32–34
Robot-assisted surgery, with its relative ease of use, was introduced as a way to mitigate difficulties associated with LC. To our knowledge, to date, most of the published studies have been small, single-institution case series lacking a laparoscopic control group. They consistently demonstrated the safety and feasibility of RC.16–19,22 So far, 3 retrospective comparative studies20,22,35 and 1 randomized clinical trial36 have been published. None of them found significant differences in LOS or complication rate between RC and LC. Available literature suggests that RC, despite being a safe and feasible procedure, is not associated with a significant improvement in clinical outcomes as compared with LC.
To our knowledge, our report is the first national study comparing outcomes between RC and LC with a propensity score–matching approach. While most robot-assisted surgery patients were white, similar to findings from previous studies,37 there was a significantly higher proportion of Hispanic patients who underwent RC. This might be explained by geographical location: hospitals performing RC were concentrated in urban areas where a higher proportion of Hispanics reside.38,39 Matched comparisons between RC and LC yielded no significant differences in in-hospital mortality, complication, transfusion, ostomy, conversion to open, or routine discharge rates. While the difference in most outcomes was not statistically significant, there are some trends that appear to favor RC especially conversion to open procedures and ostomy rates. The lack of statistical significance could be partially attributable to the relative small sample sizes. Although no significant temporal trends were found in either the complication rate or the cost associated with RC during the short time span of our study, results of previous studies have been optimistic with respect to improved outcomes after the learning curve for RC is overcome.10,22 Robot-assisted colectomy is in its infancy, and it remains to be seen whether RC produces improved outcomes as technology and techniques gradually mature.
When LC was first introduced, it faced similar skepticism of its clinical benefits as RC does today.40–43 In the case of LCs, the additional complications specific to laparoscopic procedures were mitigated with increased experience, while the additional operating room cost was compensated for by a decrease in postoperative complications and LOS.2,5 Limitations of our study included the lack of standardization of the robot-assisted approach. Variability in size of incision, port placement and robot docking location,44 use of intra-corporeal anastomosis,36 and use of an entirely robot-assisted approach or a hybrid approach45 could not be ascertained owing to the nature of documentation in the NIS. Another limitation was our inability to account for potential confounders that were not available in the NIS such as severity of disease and expertise level of individual surgeons. The third limitation was the short postoperative follow-up period: complications occurring after discharge could not be captured in our study.
Conclusions
Our study confirmed the benefits of LC over OC on a national level by demonstrating its lower complication profile and lower costs. Also, our data show that RC is at least equivalent in clinical outcomes to LC. We look forward to seeing the higher cost associated with RC decrease in the future with more prevalent use of the technology.
Footnotes
Author Contributions: Dr Ahuja had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Analysis and interpretation of data: Juo, Hyder, Haider, Lidor, Ahuja.
Drafting of the manuscript: Juo, Hyder.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Juo, Hyder, Haider, Lidor.
Obtained funding: Haider.
Administrative, technical, or material support: Haider, Ahuja.
Study supervision: Haider, Ahuja.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Robinson CN, Chen GJ, Balentine CJ, et al. Minimally invasive surgery is underutilized for colon cancer. Ann Surg Oncol. 2011;18(5):1412–1418. doi: 10.1245/s10434-010-1479-0. [DOI] [PubMed] [Google Scholar]
- 2.Alkhamesi NA, Martin J, Schlachta CM. Cost-efficiency of laparoscopic versus open colon surgery in a tertiary care center. Surg Endosc. 2011;25(11):3597–3604. doi: 10.1007/s00464-011-1765-3. [DOI] [PubMed] [Google Scholar]
- 3.Lai JH, Law WL. Laparoscopic surgery for colorectal cancer. Br Med Bull. 2012;104:61–89. doi: 10.1093/bmb/lds026. [DOI] [PubMed] [Google Scholar]
- 4.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Clinical Outcomes of Surgical Therapy (COST) Study Group. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287(3):321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer: abstracted from. Nelson H, Sargent D, Wieand HS. for the Clinical Outcomes of Surgical Therapy Study Group: a comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004; 350:2050–2059. Cancer Treat Rev. 2004;30(8):707–709. doi: 10.1016/j.ctrv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Delaney CP, Lynch AC, Senagore AJ, Fazio VW. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum. 2003;46(12):1633–1639. doi: 10.1007/BF02660768. [DOI] [PubMed] [Google Scholar]
- 7.Braga M, Vignali A, Zuliani W, et al. Training period in laparoscopic colorectal surgery. Surg Endosc. 2002;16(1):31–35. doi: 10.1007/s00464-001-9035-4. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne GH. Robotic surgery, telerobotic surgery, telepresence, and telementoring. Review of early clinical results. Surg Endosc. 2002;16(10):1389–1402. doi: 10.1007/s00464-001-8283-7. [DOI] [PubMed] [Google Scholar]
- 9.Weber PA, Merola S, Wasielewski A, Ballantyne GH. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum. 2002;45(12):1689–1694. doi: 10.1007/s10350-004-7261-2. discussion 1695–1696. [DOI] [PubMed] [Google Scholar]
- 10.Huettner F, Dynda D, Ryan M, Doubet J, Crawford DL. Robotic-assisted minimally invasive surgery: a useful tool in resident training: the Peoria experience, 2002–2009. Int J Med Robot. 2010;6(4):386–393. doi: 10.1002/rcs.342. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JE, Chang DC, Parsons JK, Talamini MA. The first national examination of outcomes and trends in robotic surgery in the United States. J Am Coll Surg. 2012;215(1):107–114. doi: 10.1016/j.jamcollsurg.2012.02.005. discussion 114–116. [DOI] [PubMed] [Google Scholar]
- 12.Pigazzi A, Garcia-Aguilar J. Robotic colorectal surgery: for whom and for what? Dis Colon Rectum. 2010;53(7):969–970. doi: 10.1007/DCR.0b013e3181db8055. [DOI] [PubMed] [Google Scholar]
- 13.Pigazzi A, Luca F, Patriti A, et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17(6):1614–1620. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 14.Baik SH, Kwon HY, Kim JS, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16(6):1480–1487. doi: 10.1245/s10434-009-0435-3. [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, Jiang ZW, Xie LF, Liu FT, Li JS. Robotic-assisted laparoscopic colectomy for colon cancer: a report of 13 cases [in Chinese] Zhonghua Wei ChangWai Ke Za Zhi. 2011;14(5):327–329. [PubMed] [Google Scholar]
- 16.Zimmern A, Prasad L, Desouza A, Marecik S, Park J, Abcarian H. Robotic colon and rectal surgery: a series of 131 cases. World J Surg. 2010;34(8):1954–1958. doi: 10.1007/s00268-010-0591-4. [DOI] [PubMed] [Google Scholar]
- 17.Buchs NC, Pugin F, Bucher P, Morel P. Totally robotic right colectomy: a preliminary case series and an overview of the literature. Int J Med Robot. doi: 10.1002/rcs.404. [published online June 15, 2011] [DOI] [PubMed] [Google Scholar]
- 18.Huettner F, Pacheco PE, Doubet JL, Ryan MJ, Dynda DI, Crawford DL. One hundred and two consecutive robotic-assisted minimally invasive colectomies: an outcome and technical update. J Gastrointest Surg. 2011;15(7):1195–1204. doi: 10.1007/s11605-011-1549-z. [DOI] [PubMed] [Google Scholar]
- 19.D’Annibale A, Pernazza G, Morpurgo E, et al. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol. 2010;17(11):2856–2862. doi: 10.1245/s10434-010-1175-0. [DOI] [PubMed] [Google Scholar]
- 20.Deutsch GB, Sathyanarayana SA, Gunabushanam V, et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc. 2012;26(4):956–963. doi: 10.1007/s00464-011-1977-6. [DOI] [PubMed] [Google Scholar]
- 21.Shin JY. Comparison of short-term surgical outcomes between a robotic colectomy and a laparoscopic colectomy during early experience. J Korean Soc Coloproctol. 2012;28(1):19–26. doi: 10.3393/jksc.2012.28.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum. 2010;53(7):1000–1006. doi: 10.1007/DCR.0b013e3181d32096. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Finkler SA. The distinction between cost and charges. Ann Intern Med. 1982;96(1):102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- 25.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32(7):700–715. doi: 10.1097/00005650-199407000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(supp 2):69–80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172(9):1092–1097. doi: 10.1093/aje/kwq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1(3):144–150. [PubMed] [Google Scholar]
- 29.Stucky CC, Pockaj BA, Novotny PJ, et al. Long-term follow-up and individual item analysis of quality of life assessments related to laparoscopic-assisted colectomy in the COST trial 93-46-53 (INT 0146) Ann Surg Oncol. 2011;18(9):2422–2431. doi: 10.1245/s10434-011-1650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masoomi H, Buchberg B, Nguyen B, Tung V, Stamos MJ, Mills S. Outcomes of laparoscopic versus open colectomy in elective surgery for diverticulitis. World J Surg. 2011;35(9):2143–2148. doi: 10.1007/s00268-011-1117-4. [DOI] [PubMed] [Google Scholar]
- 31.Vaid S, Tucker J, Bell T, Grim R, Ahuja V. Cost analysis of laparoscopic versus open colectomy in patients with colon cancer: results from a large nationwide population database. Am Surg. 2012;78(6):635–641. [PubMed] [Google Scholar]
- 32.Reissman P, Cohen S, Weiss EG, Wexner SD. Laparoscopic colorectal surgery: ascending the learning curve. World J Surg. 1996;20(3):277–281. doi: 10.1007/s002689900044. discussion 282. [DOI] [PubMed] [Google Scholar]
- 33.Schlachta CM, Mamazza J, Seshadri PA, Cadeddu M, Gregoire R, Poulin EC. Defining a learning curve for laparoscopic colorectal resections. Dis Colon Rectum. 2001;44(2):217–222. doi: 10.1007/BF02234296. [DOI] [PubMed] [Google Scholar]
- 34.Miskovic D, Ni M, Wyles SM, Tekkis P, Hanna GB. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum. 2012;55(12):1300–1310. doi: 10.1097/DCR.0b013e31826ab4dd. [DOI] [PubMed] [Google Scholar]
- 35.Rawlings AL, Woodland JH, Vegunta RK, Crawford DL. Robotic versus laparoscopic colectomy. Surg Endosc. 2007;21(10):1701–1708. doi: 10.1007/s00464-007-9231-y. [DOI] [PubMed] [Google Scholar]
- 36.Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg. 2012;99(9):1219–1226. doi: 10.1002/bjs.8841. [DOI] [PubMed] [Google Scholar]
- 37.Sukumar S, Sun M, Karakiewicz PI, et al. National trends and disparities in the use of minimally invasive adult pyeloplasty. J Urol. 2012;188(3):913–918. doi: 10.1016/j.juro.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Ellin JC. Areas of Hispanic concentration: a differentiation of urban subareas in forty US counties. Res Contemp Appl Geography. 2000;24(4):1–23. [Google Scholar]
- 39.Hofer MD, Meeks JJ, Cashy J, Kundu S, Zhao LC. Impact of increasing prevalence of minimally invasive prostatectomy on open prostatectomy observed in the national inpatient sample and national surgical quality improvement program. J Endourol. 2013;27(1):102–107. doi: 10.1089/end.2012.0315. [DOI] [PubMed] [Google Scholar]
- 40.Holzman MD, Eubanks S. Laparoscopic colectomy: prospects and problems. Gastrointest Endosc Clin N Am. 1997;7(3):525–539. [PubMed] [Google Scholar]
- 41.Hohenberger W, Schneider C, Reymond MA, Scheidbach H, Köckerling F. Laparoscopic resection of colorectal malignancy: an oncological risk? [in German] Zentralbl Chir. 1997;122(12):1127–1133. [PubMed] [Google Scholar]
- 42.Berman IR. Laparoscopic resection for colon cancer: cause for pause. Important Adv Oncol. 1996:231–243. [PubMed] [Google Scholar]
- 43.Bertagnolli MM, DeCosse JJ. Laparoscopic colon resection for cancer: an unfavorable view. Adv Surg. 1996;29:155–164. [PubMed] [Google Scholar]
- 44.Park SY, Choi GS, Park JS, Kim HJ, Choi WH, Ryuk JP. Robot-assisted right colectomy with lymphadenectomy and intracorporeal anastomosis for colon cancer: technical considerations. Surg Laparosc Endosc Percutan Tech. 2012;22(5):e271–e276. doi: 10.1097/SLE.0b013e31826581bd. [DOI] [PubMed] [Google Scholar]
- 45.Koh DC, Tsang CB, Kim SH. A new application of the four-arm standard da Vinci surgical system: totally robotic-assisted left-sided colon or rectal resection. Surg Endosc. 2011;25(6):1945–1952. doi: 10.1007/s00464-010-1492-1. [DOI] [PubMed] [Google Scholar]