Abstract
In the mammalian heart, the circadian protein Clock regulates glucose and fatty acid metabolism. In this study, we determined some of the factors that regulate Clock expression and subcellular distribution in myocytes. Using immunochemistry and biochemical subcellular fractionation, we have shown that Clock localizes to the Z-disk of the myofilaments. Increasing calcium and cross-bridge cycling with 10μM phenylephrine for 48 hours resulted in a 3 fold increase in Clock and a translocation of the protein to the nucleus. When myofilament cross-bridge cycling was inhibited with 10 μM verapamil or 7.5 mM butanedione monoxime for 48 hours, both significantly reduced the presence of Clock in the nucleus and cytoskeleton. These results suggest that the expression and subcellular distribution of Clock can be altered by changes in cross-bridge cycling, a major source of energy expenditure in myocytes. We suggest that the circadian Clock protein may help coordinate the sensing of energy expenditure with energy supply.
Keywords: circadian, myocytes, Clock, myofilaments, Z-disk, cross-bridge cycling, nuclear, nucleo-cytoplasmic shuttling
Introduction
The normal human heart displays diurnal variation in contractile activity in which both heart rate and blood pressure are reduced at night [1]. These variations are part of the heart's response to a circadian “internal clock” influenced by the stimulus of the daily light-dark cycle [2]. The heart responds to the daily variation in mechanical activity by altering its efficiency and activity accordingly. In rats that are nocturnal, contractile performance, carbohydrate oxidation, oxygen consumption and the expression of metabolic genes are greatest at night [3]. The myocardium expresses a number of circadian genes including BMAL1(brain and muscle ARNT-like protein1), Clock, cryptochrome (CRY) and the period genes (PER1, PER2, PER3) which are thought to regulate a number of cellular processes [4]. Clock protein exerts its effects by forming a heterodimer with BMAL1 which increases the transcription of target genes including PER and CRY genes. PER and CRY proteins then form a complex which enters the nucleus, repressing the activities of the Clock/BMAL1 complex [5]. Thus, these proteins form a feedback loop, regulating gene expression in a cyclic manner.
The Clock/Bmal1 complex has been shown to regulate glucose homeostasis [6] and fatty acid metabolism [7]. These findings indicate that Clock is likely to play an important role in the regulation of metabolism within the myocardium. Clock protein has recently been shown to be a histone acetyl transferase, a function likely to increase gene transcription through the remodelling of chromatin [8]. Alterations in the diurnal expression of Clock protein within the heart are often associated with external influences such as the sleep/wake cycle of an animal or serum-shock in cultured cells. The effect of intracellular events such as altered metabolic or mechanical activity on Clock protein has never been investigated in the heart.
In this study we show the subcellular distribution of Clock protein in cardiac myocytes for the first time, using biochemical subcellular fractionation. Clock strongly localises to the Z-disk of the myofilaments. Increases in contractile activity with phenylephrine treatment resulted in increased Clock protein expression and a translocation of the protein to the nucleus. A decrease in contractile activity with either verapamil or butanedione monoxime (BDM) treatment resulted in decreased nuclear cycling. The presence of Clock protein within the cytoskeleton was also dependent upon active cross-bridge cycling in these cells. This suggests that both the expression and subcellular distribution of Clock protein can be influenced by intracellular activity in cardiac myocytes. These results show for the first time that Clock may provide an important link between the regulation of energy expenditure and energy supply within myocytes by “sensing” myofilament cross-bridge activity and then influencing metabolic gene expression through its role as a transcription factor.
Material and methods
Cell culture
Myocytes were isolated from the cardiac ventricles of 1-2 day old Sprague Dawley rats by sequential collagenase digestion, as previously described [9]. Briefly, cells were preplated to reduce non-myocyte cell contamination and then plated (1 million cells/cm2) on fibronectin (25 μM/ml) coated petri dishes in PC1 medium (BioWhittaker, Walkersville, MD) for 24 hours and transferred to a DMEM:M199 serum free medium. Non-myocytes were taken from the preplated cells and cultured separately for other studies. For the studies comparing fibroblasts with myocytes, both cell types were cultured in 5% serum in DMEM from the same neonatal hearts for 5 days and harvested at the same time.
Cellular composition and subcellular fractionation
For subcellular fractionation of myocytes, the ProteoExtract Subcellular Proteome Kit from Calbiochem was used. This method uses a detergent-based protocol[10] and has been previously described [11]. Cellular proteins were sequentially extracted into four compartments: cytosolic, membrane/organelles, nuclei and cytoskeleton. Digitonin/EDTA is used to remove the cytosol. Triton/EDTA is used to remove the membrane/organelle fraction. Tween/deoxycholate/benzonase removes the nuclei. Finally SDS is used to remove the cytoskeleton. Cells were briefly washed 3 times in PBS between each extraction fraction to prevent cross-contamination. After each fraction, cells were observed by microscopy to ensure that they were still attached to the dish. Cell integrity is maintained throughout the fractionation process. The accuracy of the fractionation method was verified with antibodies to well documented subcellular distributions markers.
Western blotting for analysis of protein expression
Neonatal rat ventricular myocytes were rinsed with warm PBS and then scraped from the silicone membranes or dishes in lysis buffer containing 1% SDS and protease inhibitor cocktail (Sigma). The Bradford method was used to determine total protein using crystalline bovine serum albumin as standard. For whole heart protein analysis, tissue was ground in liquid nitrogen and added to lysis buffer containing 1% SDS, 50 mM NaF and protease inhibitor cocktail (Sigma). Samples were treated with β-mecaptoethanol and heated to 100°C for 5 minutes. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Hybond C, Amersham). Blots containing either whole cell lysates or fractionated cells were probed for anti-Clock (Abcam), alpha actinin (Abcam) and vimentin (Santa Cruz Biotechnology Inc). Horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Research Diagnostics Inc) were used to visualize proteins by enhanced chemiluminescnce (ECL, Amersham). The bands corresponding to the various proteins were quantified by laser densitometry. Protein bands were further standardized to total protein loading by using the amido black-stained nitrocellulose membrane as described previously[12].
Immuno-chemistry and image analysis
After the various experimental protocols, cells for immuno-cytochemical staining were fixed in 4% paraformaldehyde for 3 minutes and then 100% methanol at -20°C for 1 minute. Fixed cells were immunostained with antibodies as described previously [13]. Rhodamine and Alexa Fluor-conjugated secondary antibodies (Molecular Probes) were used to visualize the specific proteins. Fluorescently-labeled cells were then viewed using a Zeiss Model LSM 510 laser scanning confocal microscope.
Statistics
For the experiments described here, at least three separate primary cultures were averaged. Each culture used about 30 neonatal hearts. All values are means ± SEM. All values of significance were calculated using the appropriate comparisons: one way analysis of variance or the Students unpaired t-test. Differences among means were considered significant at p< 0.05. Data were analyzed using GraphPad and SigmaStat statistical software.
Results
Clock protein expression and distribution in the heart
To determine the importance of the circadian protein Clock in the heart, its expression was compared in several tissues from adult Sprague Dawley female rats. Samples were collected from heart, lung, liver and kidney from the same animals at Zeitgeber 9.00 hours when the animals were well into their resting period. This time was chosen to ensure that there would be minimal influences from physical activity and feeding habits. Figure 1A shows the results from Western blotting of Clock protein in heart, lung liver and kidney. Clock expression in the heart was about 5 fold higher than the other tissues. The other three tissues had a similar expression of protein though. Clock protein expression was not different between different chambers of the adult rat heart (data not shown). Next we determined whether the expression of Clock was altered from the neonatal period to adulthood in these animals. The neonatal hearts were collected from 1-2 day old pups from the mothers used in Figure 1A. Clock protein expression was not significantly different between neonatal hearts and adult left ventricles, suggesting that this level of Clock expression is required throughout the life of these animals.
Figure 1.
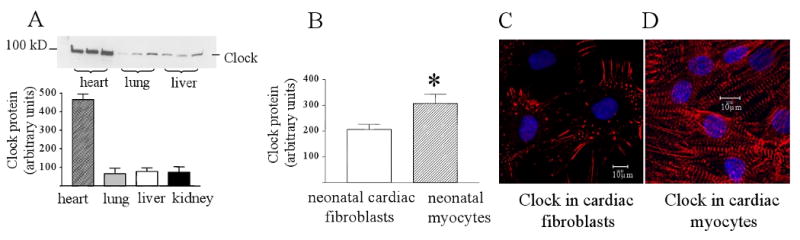
(A) Western blot of Clock protein in the adult rat heart, lung, liver and kidney. Samples were collected at Zeitgeber 9.00 hours during the resting period for rats. Clock protein expression was 5-fold higher than the three other tissues. (B) Clock protein expression in neonatal rat cardiac myocytes and cardiac fibroblasts cultured in 5% serum for 5 days. Myocytes had significantly more Clock protein than fibroblasts. P<0.05, n=4. (C) Immunostaining of Clock protein in cardiac fibroblasts. (D) Immunostaining of Clock protein in cardiac myocytes.
The distribution of Clock protein between different cardiac cell types has not been previous described, so we determined this by measuring Clock expression in cultured neonatal myocytes and non-myocytes, which are mostly fibroblasts. Figure 1B shows that Clock protein is expressed in both myocytes and non-myocytes, with myocytes having a significantly higher expression of the protein. Both cells types were cultured from the same neonatal rat hearts at the same time. To determine the distribution of Clock protein within these cell types, myocytes and non-myocytes were immunostained for the protein and examined using confocal microscopy. Figure 1C shows that in fibroblasts, Clock localizes to the focal adhesions and actin stress cables. Figure 1D shows that in myocytes, Clock has a striated appearance suggesting that it mostly associates with the myofilaments.
Clock distribution in the myofilaments
To determine the location of Clock within the myofilaments, neonatal cardiac myocytes were immunostained for Clock and the Z-disk protein alpha actinin. Figure 2 A-C shows that the two proteins colocalize in the same region, demonstrating that Clock strongly localizes to the Z-disk of the myofilaments. A confocal Z-stack of these cells also showed that Clock is located from the focal adhesion to the top of the myofilaments. Lesser amounts of the protein also extend into the A-band of the myofilaments.
Figure 2.
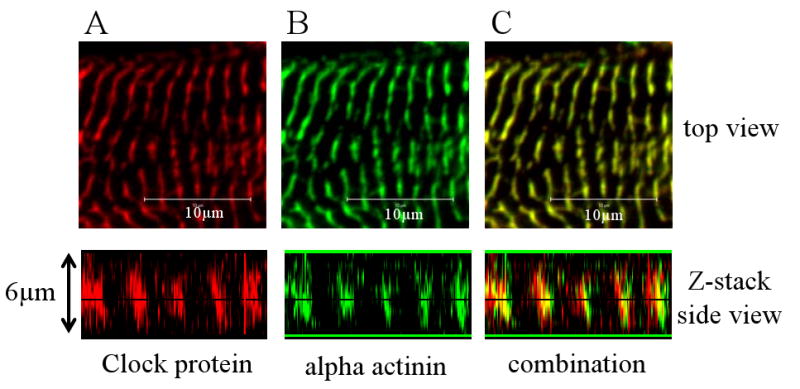
Clock localizes to the myofilaments of cardiac myocytes. (A) High resolution confocal image of Clock protein (in red) in the myofilaments of a neonatal cardiac myocyte, along with a confocal z-stack image showing that the protein is localized from the focal adhesions to the top of the cell. (B) Immunostaining for the Z-disk protein alpha actinin (in green) in the same myocyte. Panel C shows the combination of the double staining. The two proteins colocalize in the Z-disk.
Subcellular distribution of Clock and contractile activity in cardiac myocytes
Since Clock strongly localizes to the myofilaments, we investigated whether the protein could be influenced by changes in myocyte contractile activity. To increase calcium cycling and myofilament cross-bridge cycling, myocytes were treated with 10 μM phenylephrine for 48 hours, a stimulus that also induces cellular hypertrophy. Following this period, cells were either fixed for immunostaining for Clock or fractionated into cytoplasmic, membrane, nuclear and cytoskeletal portions for Western blotting. Figure 3A and 3B show that following 48 hours of phenylephrine treatment, there was an increased translocation of Clock protein to the nucleus compared with controls. Figure 3C shows the results from Western blotting of subcellular fractions from control myocytes and those treated with 10 μM phenylephrine. There was an increase of Clock protein within the nucleus and a concomitant decrease in the proportion of the protein remaining in the membrane fraction. This finding is consistent with the immunostaining results. Further analysis showed that the total amount of Clock protein increased by 3 fold following phenylephrine treatment (Figure D). This demonstrates that increased contractile activity and calcium cycling alters both the subcellular distribution and total expression of Clock protein within cardiac myocytes.
Figure 3.
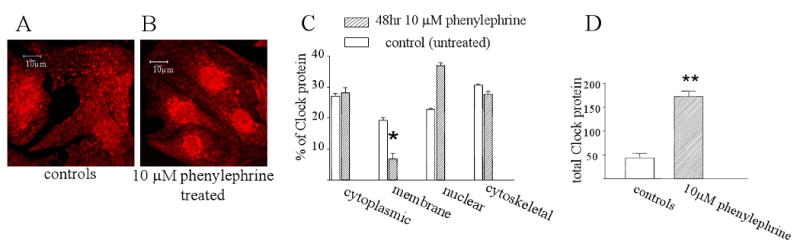
Clock subcellular localization and phenylephrine treatment. Panels A and B show immunostaining for Clock protein in untreated myocytes and those treated with 10 μM phenylephrine for 48 hours respectively. Panel C shows quantitation of Western blotting results of Clock protein distribution in subcellular fractions with and without 10μM phenylephrine treatment for 48 hours. Phenylephrine treatment results in a significant translocation of Clock from the membrane to the nuclear fraction. P<0.01, n=3 cultures. Panel D shows a Western blot of total Clock protein in untreated myocytes and following 48 hours of phenylephrine treatment. Clock protein increased about 3 fold following the drug treatment. P<0.01, n=3 cultures.
Clock protein and cross-bridge cycling in cardiac myocytes
Since increased contractile activity in myocytes increased the expression of Clock protein, we wanted to investigate whether decreasing activity would have the opposite effect. To inhibit the spontaneous contraction of these cultured myocytes, the cells were treated with 10 μM of the L-type calcium channel blocker verapmil. This drug inhibits both calcium cycling and cross-bridge cycling. In addition, we treated some cells with 7.5 mM BDM which inhibits cross-bridge cycling without influencing calcium cycling. Figure 4A shows that with verapmil or BDM treatment, the amount of Clock in the nucleus was significantly reduced. This suggests that inhibiting cross-bridge cycling alone is sufficient to reduce nuclear cycling of Clock protein. We also measured total Clock protein in control myocytes and those treated with verapmil or BDM. Total Clock protein was not significantly altered following inhibition of cross-bridge cycling with either drug.
Figure 4.
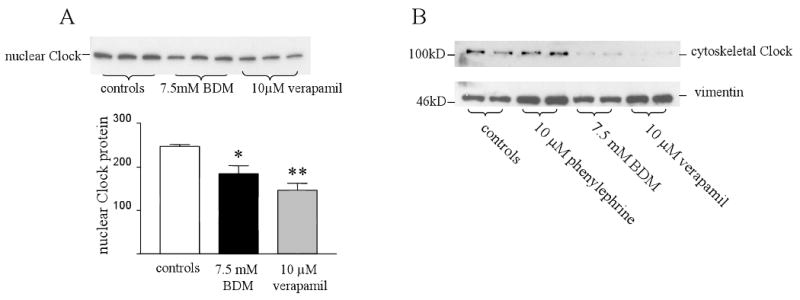
Subcellular distribution of Clock protein and myocyte cross-bridge cycling. Panel A shows a Western blot of Clock protein from nuclear fractions of untreated myocytes and those treated with either 7.5 mM BDM or 10 μM verapamil for 48 hours. There was a significant decrease in nuclear Clock in both groups. * controls vs BDM p<0.05 n=3. ** controls vs verapamil p<0.01 n=3. Panel B shows Clock protein from cytoskeletal fractions of myocytes treated with 10 μM phenylephrine, 7.5 mM BDM or 10 μM verapamil. Clock protein is present in the cytoskeleton when the myofilaments are actively contracting, whilst BDM and verapamil treatment significantly decrease its presence there.
Finally, we determined the distribution of Clock protein in the myocyte cyctoskeleton following phenylephrine, verapamil and BDM treatment. Figure 4B shows that Clock was only present in the cytoskeleton of untreated myocytes and those treated with phenylephrine. The protein was absent from the cytoskeleton when contractile activity was inhibited with either verapamil or BDM treatment. These results show that the presence of Clock in the myocyte cytoskeleton is dependent on active cross-bridge cycling.
Discussion
The circadian proteins have created a great deal of interest in biological systems in recent years and they have been implicated in health, disease and aging [4, 14, 15]. Clock protein has been shown to be an important regulator of glucose and fatty acid metabolism [6, 7]. However, the effect of intracellular events on the expression and subcellular distribution of Clock have not been investigated in the mammalian heart. In this study, we show for the first time that Clock is expressed in the cytoplasm, membrane, nucleus and cytoskeleton of cardiac myocytes. Clock strongly localizes to the myofilaments and colocalizes with alpha actinin in the Z-disk from the focal adhesion to the top of the myofilaments. The Z-disk in cardiac myocytes is a complex structure containing many structural proteins and signaling molecules, including mechanosensors [11, 16]. Our study shows that the circadian Clock protein can be added to the growing list of proteins within the myocyte costamere.
Phenylephrine is a hypertrophic agent and increases myocyte contractility in culture [17]. Increasing contractile activity with phenylephrine resulted in an increase in nuclear Clock, with a concomitant decrease in the membrane fraction. This may suggest that most of the protein translocates from the focal adhesion membrane which is known to remodel in response to phenylephrine treatment in myocytes [18]. Recently, it has been shown that in oestoblasts, mechanical cyclic stretch decreased Clock mRNA expression [19]. However, in our present study, the changes in contractile activity would be associated with changes in the energy expenditure of the myocytes. It is not yet clear whether the observed changes were associated with alterations in energy usage/reserve or simply a response cross-bridge cycling alone. The presence of Clock within the myofilaments could enable the protein to “sense” cross-bridge cycling in myocytes, a major source of energy expenditure. Some have theorized that the circadian system in cells could operate through mechanotransduction in association with the cytoskeleton [20]. In myocytes, mechanosensing may play an important role in the regulation of the internal circadian clock. The translocation of Clock protein to the nucleus in response to increased cross-bridge cycling would be expected to result in increased metabolic gene expression through its role as a. histone acetyltransferase.
It is plausible that Clock is part of a system that coordinates energy usage with energy supply. Our data are also consistent with the finding that in the intact heart, Clock protein levels increase during the periods of high metabolic activity and decrease during those of reduced activity [3]. Therefore in addition to extracellular cues, Clock may also respond to intracellular events in order to regulate the energy pool of myocytes, thus ensuring that the supply matches the demand. The energy supply of the heart cannot be compromised even for short periods, so our finding that Clock expression is much higher in the heart seems appropriate. Our results also showed that the presence of Clock in the cytoskeleton was dependent upon active cross-bridge cycling. Both verapamil and BDM decrease the amount of myofilaments present in myocytes but do not significantly reduce non-myofilament proteins like vimentin [21]. This further suggests that the presence of Clock in the cytoskeleton is dependent upon an active contractile apparatus rather than the filamentous cytoskeleton alone.
In summary, we have shown for the first time that the circadian protein Clock associates with the contractile filaments by localizing to the Z-disk of cardiac myocytes. Increasing myocyte contractile activity with phenylephrine increased Clock protein and its translocation to the nucleus. Inhibition of cross-bridge cycling with either verapamil or BDM reduced its presence in the nucleus and cytoskeleton. These findings strongly suggest that Clock expression and subcellular distribution can be influenced by alterations in cross-bridge cycling, a major source of energy expenditure. These findings raise the possibility that Clock is part of a mechanism that coordinates energy usage with energy supply in cardiac myocytes.
Acknowledgments
This research is supported by the National Institutes of Health AG 17022, HL-64956 and HL-62426 and AHA 0630307N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murali NS, Svatikova A, Somers VK. Cardiovascular physiology and sleep. Frontiers in Bioscience. 2003;8:s636–652. doi: 10.2741/1105. [DOI] [PubMed] [Google Scholar]
- 2.Portman MA. Molecular clock mechanisms and circadian rhythms intrinsic to the heart,(Editorial) Circ Res. 2001;89:1084. [PubMed] [Google Scholar]
- 3.Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circulation. 2001;104:2923–2931. doi: 10.1161/hh2401.100741. [DOI] [PubMed] [Google Scholar]
- 4.Young ME. The circadian clock within the heart: potential influence on myocardial gene expression, metabolism, and function, American Journal of Physiology - Heart &. Circulatory Physiology. 2006;290:H1–16. doi: 10.1152/ajpheart.00582.2005. [DOI] [PubMed] [Google Scholar]
- 5.Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biological Chemistry. 2003;384:711–719. doi: 10.1515/BC.2003.079. [DOI] [PubMed] [Google Scholar]
- 6.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biology. 2004;2:1893–1899. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 8.Hardin PE, Yu W. Circadian transcription: passing the HAT to CLOCK. Cell. 2006;125:424–426. doi: 10.1016/j.cell.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. American Journal of Physiology - Cell Physiology. 2003;285:C171–182. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 10.Ramsby ML, Makowski GS, Khairallah EA. Differential detergent fractionation of isolated hepatocytes: biochemical, immunochemical and two-dimensional gel electrophoresis characterization of cytoskeletal and noncytoskeletal compartments. Electrophoresis. 1994;15:265–277. doi: 10.1002/elps.1150150146. [DOI] [PubMed] [Google Scholar]
- 11.Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, de Tombe PP, Russell B. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. Am J Physiol Heart Circ Physiol. 2006 Sep 8; doi: 10.1152/ajpheart.00766.2006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Boateng SY, Seymour AM, Bhutta NS, Dunn MJ, Yacoub MH, Boheler KR. Sub-antihypertensive doses of ramipril normalize sarcoplasmic reticulum calcium ATPase expression and function following cardiac hypertrophy in rats. J Mol Cell Cardiol. 1998;30:2683–2694. doi: 10.1006/jmcc.1998.0830. [DOI] [PubMed] [Google Scholar]
- 13.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288:C30–38. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 14.Cutolo M, Masi AT. Circadian rhythms and arthritis. Rheumatic Diseases Clinics of North America. 2005;31:115–129. doi: 10.1016/j.rdc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Hofman MA. The human circadian clock and aging. Chronobiology International. 2000;17:245–259. doi: 10.1081/cbi-100101047. [DOI] [PubMed] [Google Scholar]
- 16.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–1325. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circulation Research. 1985;56:884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- 18.Kim DJ, Park SH, Lim CS, Chun JS, Kim JK, Song WK. Cellular localization of integrin isoforms in phenylephrine-induced hypertrophic cardiac myocytes. Cell Biochemistry & Function. 2003;21:41–48. doi: 10.1002/cbf.988. [DOI] [PubMed] [Google Scholar]
- 19.Kanbe K, Inoue K, Xiang C, Chen Q. Identification of clock as a mechanosensitive gene by large-scale DNA microarray analysis: downregulation in osteoarthritic cartilage. Mod Rheumatol. 2006;16:131–136. doi: 10.1007/s10165-006-0469-3. [DOI] [PubMed] [Google Scholar]
- 20.Shweiki D. The physical imperative in circadian rhythm: a cytoskeleton-related physically resettable clock mechanism hypothesis. Medical Hypotheses. 1999;53:413–420. doi: 10.1054/mehy.1998.0785. [DOI] [PubMed] [Google Scholar]
- 21.Byron KL, Puglisi JL, Holda JR, Eble D, Samarel AM. Myosin heavy chain turnover in cultured neonatal rat heart cells: effects of [Ca2+]i and contractile activity. American Journal of Physiology. 1996;271:C01447–01456. doi: 10.1152/ajpcell.1996.271.5.C01447. [DOI] [PubMed] [Google Scholar]


