Abstract
Psychotic symptoms, delusions and hallucinations, occur in approximately 50% of individuals with Alzheimer’s disease (AD) (AD with psychosis [AD + P]). Pharmacotherapies for AD + P have limited efficacy and can increase short-term mortality. These observations have motivated efforts to identify the underlying biology of AD + P. Psychosis in AD indicates a more severe phenotype, with more rapid cognitive decline beginning even before psychosis onset. Neuroimaging studies suggest that AD + P subjects demonstrate greater cortical synaptic impairments than AD subjects without psychosis, reflected in reduced gray matter volume, reduced regional blood flow, and reduced regional glucose metabolism. Neuroimaging and available postmortem evidence further indicate that the impairments in AD + P, relative to AD subjects without psychosis, are localized to neocortex rather than medial temporal lobe. Neuropathologic studies provide consistent evidence of accelerated accumulation of hyperphosphorylated microtubule associated protein tau in AD + P. Finally, studies of familial aggregation of AD + P have established that the risk for psychosis in AD is, in part, genetically mediated. Although no genes are established as associated with AD + P, the first genome-wide association study of AD + P has generated some promising leads. The study of the neurobiology of AD + P is rapidly accelerating and may be poised for translational discovery. This process can be enhanced by identifying points of convergence and divergence with the neurobiology of AD proper and of schizophrenia, by innovative extension of current approaches, and by development of relevant animal models.
Keywords: Alzheimer’s disease, genetics, heritability, neuroimaging, neuropathology, psychosis
Psychotic symptoms, delusions and hallucinations, are frequent in Alzheimer’s disease (AD) (AD with psychosis [AD + P]). Common delusions in AD patients include delusions of persecution, infidelity, abandonment, or that deceased individuals (e.g., parents) are still living (1). Other misidentification delusions are also frequent in AD patients: beliefs that one’s home is not one’s home; that a family member is someone else, has been reduplicated, or is an imposter; the presence of phantom boarders; and that images on the television are actually people present in the house (2). Unlike schizophrenia, delusions in AD are typically not bizarre or complex, and Schneiderian first-rank symptoms are rare (3). Hallucinations in AD can occur in any sensory modality, but visual hallucinations are most common in AD (1), again a contrast with schizophrenia.
In a comprehensive review of clinical studies, the median prevalence of AD + P was 41% (range = 12.2–74.1%), with a 3-year cumulative incidence approximating 50% (4,5). Epidemiologic studies have found a lower point prevalence of AD + P, closer to 25% (6). These differences may reflect that the rate of AD + P is dependent on AD stage, with low rates of psychosis in prodromal and early AD and higher rates in middle and later stages (7,8). Regardless, with current estimates of over 5 million Americans affected by AD and estimates of greater than 13 million affected by 2050 (9), AD + P would currently be the second most prevalent psychotic disorder (after schizophrenia) in the United States, and may soon be the most prevalent.
In addition to the individual distress that delusions and hallucinations may confer, when present, AD + P is a marker for a number of additional adverse outcomes in AD patients. Alzheimer’s disease with psychosis is associated with the co-occurrence of other behavioral disturbances, the most troublesome of which are agitation (10) and aggression (11,12). Depressive symptoms are also increased in AD + P (8,13). Alzheimer’s disease with psychosis is associated with greater distress for family and caregivers (14), greater functional impairment (15), greater rates of institutionalization (16–19), worse general health for the patient (20), and increased mortality (21). In addition, the other behavioral disturbances that co-occur with AD + P may themselves influence outcomes adversely (22,23).
Current pharmacotherapies for psychosis in AD have limited efficacy and high toxicity in this age group. Haloperidol is the most studied of the conventional antipsychotics. It has mild to moderate efficacy relative to placebo in AD patients with psychosis and/or agitated behaviors (24). However, it causes serious side effects, namely parkinsonism, tardive dyskinesia, and akathisia. More recent studies have examined atypical antipsychotics, such as risperidone, olanzapine, and aripiprazole. These medications have efficacy similar to conventional antipsychotics for AD + P, with lower rates of motor side effects (25). However, they have been associated with increased cerebrovascular adverse events and increased mortality after short-term treatment (25), a risk that appears to be shared with conventional antipsychotics (26). In contrast to antipsychotic medications, the evidence base for the efficacy of other medications, such as selective serotonin reuptake inhibitors, is much smaller (27).
The shortcomings of antipsychotic medications, which were developed for similar symptoms occurring in patients without dementia, may be due to a lack of biologic specificity. Evidence from clinical, genetic, brain imaging, and neuropathology studies, however, are now emerging to provide an initial understanding of the neurobiology of AD + P. In the following, we review that evidence as it relates to cognitive, genetic, neuroimaging, and postmortem correlates of psychosis in AD. Based on that review, we attempt to provide a critique and suggestions for how the field may move forward toward transformative discovery.
Cognition and Psychosis in Alzheimer’s Disease
The risk of psychosis in AD is inextricably linked to cognitive decline. Ropacki and Jeste (28) reviewed 55 studies of AD + P, comprised of 9749 subjects. In cross-sectional assessments, greater cognitive impairment in AD + P than in AD without psychosis (AD − P) was found in 20 of the 30 studies that assessed this association (28). Although most studies utilized measures of global cognition, several studies that have evaluated cognitive domains suggest a frontal localization of the greater cognitive deficits in AD + P, with working memory particularly affected (4,29–31). The association between AD + P and cognitive burden is not readily attributable to other factors, as age, age of onset of AD, duration of AD, sex, education, race, and family history of psychiatric illness show only modest or equivocal associations with AD + P as compared with AD − P (28).
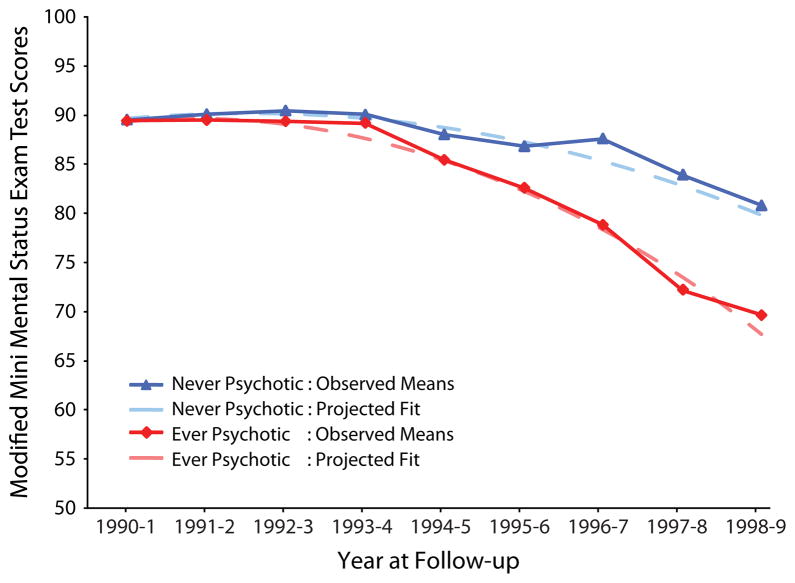
Alzheimer’s disease with psychosis was even more strongly associated with more rapid cognitive decline, which was the most consistent correlate of AD + P compared with AD − P (28). Nine of nine studies found a significant association between a greater rate of cognitive decline and the presence of AD + P (28). Recent studies have continued to support the relationship between both greater cognitive impairment and more rapid cognitive decline and AD + P (8,32–35). A number of studies now indicate that more rapid cognitive deterioration begins before onset of psychosis, during prodromal and early stages of AD, subsequently manifesting as frank psychotic symptoms. For example, greater cognitive dysfunction was already present in the earliest stages of AD, preceding the onset of psychosis by at least 1 to 2 years (32). Paulsen et al. (4) found that more rapid cognitive decline was present a year before psychosis in early AD. A more recent report evaluated individuals without dementia at their time of entry into the population-based Cardiovascular Health Study and who, by the end of the 10-year study period, developed dementia (33). Individuals who ultimately developed AD + P declined significantly more rapidly than individuals who developed AD − P, despite equivalent baseline scores (33) (Figure 1).
Figure 1.
Cognitive trajectory in individuals with incident Alzheimer’s disease with and without psychosis. Observed and quadratic fit lines of Modified Mini-Mental Status Exam test scores in elderly individuals characterized by the Cardiovascular Health Study as without cognitive disorder at study baseline. Groups did not differ at baseline, but the more rapid decline in the group who developed Alzheimer’s disease with psychosis is readily apparent. [Reproduced with permission from Emanuel et al. (33)].
Genetics of Psychosis in Alzheimer’s Disease
Heritability and Familial Aggregation
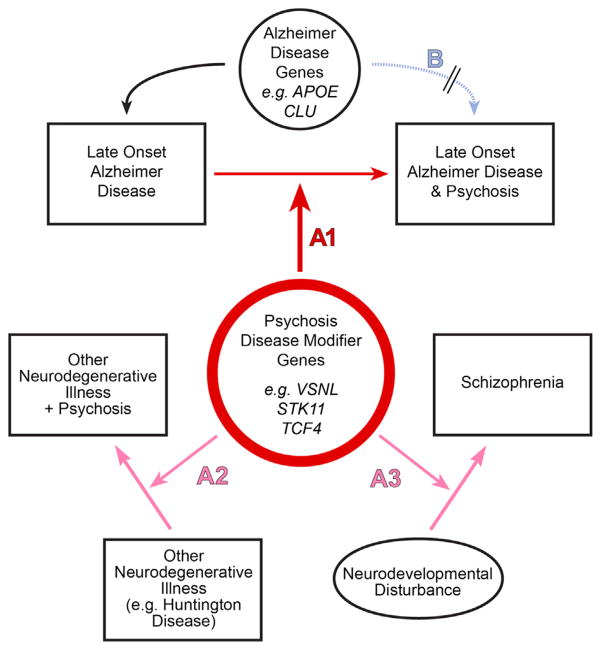
Perhaps the most compelling evidence that AD + P has a biology that is distinct from that of AD − P is the finding that the risk for psychosis in AD is transmitted in families. An initial report found that AD + P is familial, with an odds ratio for psychosis of 3.2 in siblings of AD + P subjects who themselves were affected with AD (36). The finding of familial aggregation of AD + P has since been replicated in two additional cohorts (8,37). The estimated heritability of psychosis in AD is 61% when psychosis is defined by the presence of multiple or recurrent psychotic symptoms and is 30% for any single occurrence of a symptom (38). These findings provide strong support for efforts to identify genetic variants causally related to AD + P, through one of several models (Figure 2). We summarize below studies to date.
Figure 2.
Genetic model of Alzheimer’s disease (AD) with psychosis (AD + P). In a heterogeneity model (B), genetic variants would increase the risk for a type of AD with more rapid cognitive decline and psychosis. This might occur, for example, by altering clearance of beta-amyloid directly affecting the early neurodegenerative process. Current genetic data do not support this model in AD + P (see text). In the alternative disease modifying model (A1), genetic variants that increase risk for AD + P do not themselves cause AD but lead to vulnerability, e.g., by accelerating the deleterious effects of beta-amyloid or microtubule associated protein tau on its downstream synaptic targets. Some of these variants may be shared with other psychoses, e.g., conferring synaptic vulnerability during other neurodegenerative diseases or during adolescent development in schizophrenia (A2, A3).
Linkage Studies
Linkage studies aimed at identifying chromosomal loci involved with AD + P found significant linkage to loci on chromosomes 2, 7, 8, and 15 (37,39–41). Suggestive linkage has also been found on chromosomes 6 and 21 (40), but significance was lost with follow-up analysis (37). One study found that chromosome 14, at a locus near to but independent of Presenilin 1, is linked to the absence of hallucinations in AD + P (39).
Candidate Gene Studies
Apolipoprotein E
More than 20 studies have evaluated whether carrying one or more epsilon 4 alleles of the apolipo-protein E (APOE) gene, the best established genetic risk factor for late-onset AD (42), may increase risk for AD + P. Initial findings were mixed (Table S1 in Supplement 1), likely due to differences in sample sizes (with small sample sizes having both false positive and negative findings), variability across patient populations, and varying diagnostic criteria of psychosis across studies. To avoid some of these problems, a recent report analyzed a large cohort with uniform and standardized criteria for diagnosing both AD and psychosis available through the National Alzheimer’s Disease Coordinating Center uniform data set, finding no association of APOE epsilon 4 alleles with AD + P (43). More recently, it has been suggested that a poly-T repeat sequence polymorphism in translocase of outer mitochondrial membrane 40 homolog, TOMM40, which is in linkage disequilibrium with APOE, may explain some of the association of APOE with AD risk. However, no association of poly-T repeat length with AD + P was found (44).
Other Candidate Genes
Association studies of candidate genes have predominantly, but not exclusively, focused on monoamine neurotransmitter systems. As a group, these studies experienced many of the same limitations as described for the early APOE studies. In addition, unlike APOE, most have studied only one or a few genetic variants without established biologic effect and/or known genetic mechanisms of the studied variant. Not surprisingly, then, these studies have likewise yielded conflicting results [reviewed in (45); Table S2 in Supplement 1]. Overall, the substantial limitations associated with the candidate gene approach mandates interpreting findings as conservatively as possible.
Genome-Wide Association
The first genome-wide association study of AD + P was recently reported (46). This study meta-analytically combined three AD genome-wide association datasets (47–49). In total, there were 1299 cases with AD + P, 735 cases with AD − P, and 5659 control subjects unaffected by AD. The AD + P versus AD − P analysis included 1,882,172 single nuclear polymorphisms (SNPs); the AD + P versus control analyses included 1,847,262 SNPs.
The results for the AD + P versus AD − P and AD + P versus control analyses are shown in Table 1. Among the most significant SNPs in the AD + P versus AD − P analysis was rs3764640 in serine/threonine kinase 11 (STK11). Although STK11 deletions are present in Peutz-Jeghers syndrome, one case with an unusually large STK11 deletion has been described in which Peutz-Jeghers syndrome, mental retardation, and schizophrenia co-occurred (50). Similarly, a genome-wide screen in siblings co-affected by schizophrenia found reduced copy numbers of STK11 in 3 of 18 individuals, significantly more often than in control subjects (51). Of interest, STK11, also known as liver kinase B1, triggers phosphorylation of tau (52), and amyloid precursor protein overexpression promotes tau phosphorylation in an liver kinase B1-dependent manner (53).
Table 1.
Genome-wide Association Study of AD + P (46)
| SNP | Chr | MB | MAF | Closest RefSeq Gene | GWAS p | OR |
|---|---|---|---|---|---|---|
| AD + P vs. AD − P | ||||||
| rs753129 | 4 | 56.4 | .24 | AC110611.1 | 2.85E-07 | .66 |
| rs2969775 | 2 | 47.7 | .37 | AC079250.1 | 2.11E-06 | .68 |
| rs257016 | 5 | 123.2 | .36 | AC008541.1 | 4.06E-06 | .70 |
| rs6509701 | 19 | 58.1 | .30 | ZNF320 | 5.41E-06 | .71 |
| rs16922670a | 9 | 105.1 | .14 | RP11-341A22.2a | 7.22E-06 | 1.63 |
| rs17716202 | 5 | 55.9 | .06 | AC022431.2 | 7.70E-06 | .45 |
| rs3764640a | 19 | 1.2 | .21 | STK11a | 7.88E-06 | .68 |
| rs11252926a | 10 | 0.6 | .36 | DIP2Ca | 8.08E-06 | .72 |
| AD + P vs. Control Subjects | ||||||
| rs6834555 | 4 | 9.7 | .21 | SLC2A9 | 3.06E-07 | 1.39 |
| rs4038131a | 2 | 17.6 | .07 | VSNL1a | 5.90E-07 | .64 |
| rs16970672 | 17 | 73.5 | .29 | AC015804.1 | 1.67E-06 | 1.29 |
| rs9811423a | 3 | 114.3 | .47 | RP11-572M11.4a | 4.18E-06 | 1.28 |
| rs733175a | 4 | 9.7 | .18 | SLC2A9a | 4.97E-06 | 1.36 |
| rs4360367 | 9 | 31.6 | .09 | RP11-402B2.1 | 5.90E-06 | .68 |
| rs4746003 | 10 | 71.2 | .25 | RP11-242G20.2 | 5.95E-06 | 1.29 |
| rs9789748a | 2 | 17.7 | .07 | VSNL1a | 7.39E-06 | 1.50 |
| rs1464108 | 12 | 129.6 | .32 | RIMBP2 | 8.19E-06 | 1.27 |
Results for all loci at p < 1 × 10−5 are shown. For AD + P versus AD − P, 1740 loci had p < 1 × 10−4. For AD + P versus control subjects, 1628 loci had p < 1 × 10−4.
AD + P, Alzheimer’s disease with psychosis; AD − P: Alzheimer’s disease without psychosis; Chr, chromosome; GWAS, genome-wide association study; MAF, minor allele frequency; MB, megabase; OR, odds ratio; RefSeq, reference sequence; SNP, single nucleotide polymorphism.
Intragenic SNPs.
The most significant intragenic SNP in the AD + P versus control analysis was rs4038131, an intronic SNP in visinin-like 1 (VSNL1). This SNP also showed evidence of association with AD + P versus AD − P (odds ratio: .72, p = 1.84 × 10−2). VSNL1 encodes visinin-like protein-1 (VILIP-1), a neuronal calcium sensor (54). It has recently been reported that cerebrospinal fluid and plasma concentrations of VILIP-1 are elevated in AD subjects relative to normal control subjects (55,56) and to non-AD dementia subjects (56). Elevated cerebrospinal fluid VILIP-1 levels predict more rapid cognitive decline in early AD (57). Of interest, expression of VSNL1 messenger RNA and VILIP-1 protein are also reported to be altered in schizophrenia (58,59).
Finally, loci recently identified as associated with risk of AD, including clusterin, phosphatidylinositol binding clathrin assembly protein, complement receptor 1, bridging integrator 1, adenosine triphosphate binding cassette transporter 7, membrane-spanning 4-domains subfamily A, CD2-associated protein, CD33, and ephrin type-A receptor 1 did not show evidence of association with AD + P when compared with AD − P cases and there was no association of AD + P with these loci as a group. Similarly, APOE/TOMM40 SNPs were not associated with AD + P when compared with AD − P.
Neuroimaging Studies of Psychosis in Alzheimer’s Disease
It should be noted that many imaging studies of psychosis in AD test associations with delusions separately from associations with hallucinations in AD subjects. This contrasts with the majority of clinical and genetic studies summarized above, which analyze associations with psychosis defined by delusions and/or hallucinations. The difference results from the hypothesis in imaging studies that delusions and hallucinations arise from pathology in distinct neural circuits. However, clinical studies indicate that delusions and hallucinations in AD are highly comorbid, and the latter rarely occur in the absence of delusions (60). We have therefore referred to all groups below as AD + P, unless subjects were restricted to those with delusions only or hallucinations only.
Computed Tomography and Magnetic Resonance Imaging Studies
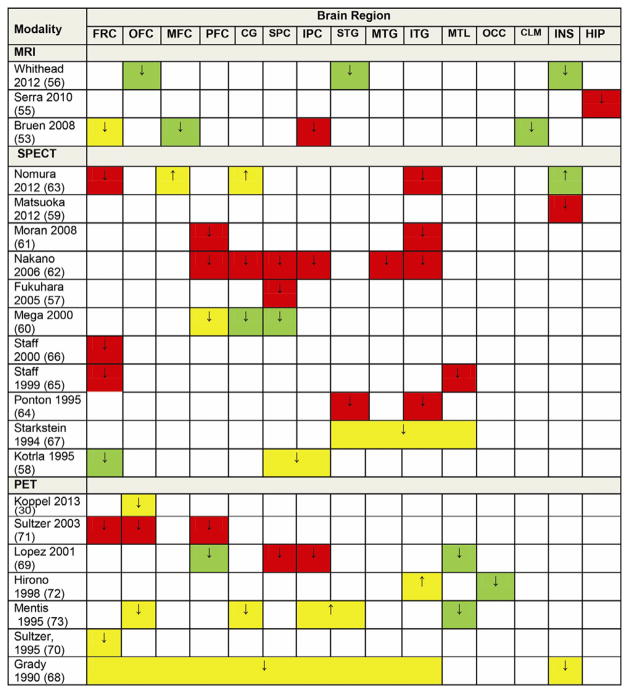
A few studies using computed tomography scan have found associations of AD + P with right frontal lobe atrophy and with changes in brain asymmetry (Table S3 in Supplement 1). A number of magnetic resonance imaging studies of gray matter volume have been reported in AD + P, using both region of interest and voxel-based comparisons (61–64). Studies were consistent in demonstrating an association of AD + P with decreased gray matter volumes (Figure 3). Reductions in frontal cortex gray matter were most consistently reported across studies, although parietal cortex was also affected. One study also reported sex differences in AD + P with only female subjects showing reduced cortical thickness in left orbitofrontal, superior temporal, and insular regions (64). Magnetic resonance imaging has also been used to evaluate whether white matter hyperintensities associate with AD + P, with inconsistent results (Table S3 in Supplement 1).
Figure 3.
Summary of findings from neuroimaging studies of Alzheimer’s disease with psychosis. ↓, decreased volume or activity; ↑, increased volume or activity; green, left; red, right; yellow, bilateral; CG, cingulate gyrus; CLM, claustrum; FRC, frontal cortex; HIP, hippocampus; INS, insula; IPC, inferior parietal cortex; ITG, inferior temporal gyrus; MFC, medial frontal cortex; MTG, middle temporal gyrus; MTL, medial temporal cortex; MRI, magnetic resonance imaging; OCC, occipital cortex; OFC, orbitofrontal cortex; PET, positron emission tomography; PFC, prefrontal cortex; SPC, superior parietal cortex; SPECT, single-photon emission computed tomography; STG, superior temporal gyrus.
Single-Photon Emission Computed Tomography Studies
Single-photon emission computed tomography studies have findings most consistent with reduced cerebral perfusion across cortical regions in AD + P subjects in comparison with AD − P (Figure 3). While there is a slight predominance of findings for frontal cortical regions, parietal and temporal cortex are also frequently affected (65–75). Some of the findings need to be interpreted with caution, as the analyses were not corrected for multiple comparisons and also the groups were different in terms of severity and other characteristics across studies. In some studies, differences were noted to depend on sex. For example, one study found male subjects with AD + P to have hyperperfusion in right striatum (69). Female AD + P subjects were found to have right insular hypoperfusion (67).
[18F]-Fluorodeoxyglucose Positron Emission Tomography Studies
Positron emission tomography studies of AD + P have consistently revealed greater hypometabolism in neocortex, particularly in bilateral frontal and prefrontal cortex, than in AD − P subjects (Figure 3) (76–79). Although uncommon, two studies reported at least one region of increased metabolism in AD + P, including in bilateral sensory association areas, raising the possibility of sensory disinhibition in some cases (80,81). The presence of hallucinations in AD has been investigated separately in only one small study and was correlated with lower regional cerebral blood flow in the right parietal cortex (77). Only one positron emission tomography study has used receptor probes to evaluate AD + P. In this study measuring dopamine D2/D3 receptor availability using [11C]raclopride, significantly higher striatal D2/D3 receptor availability was found in AD + P compared with AD − P subjects (82).
Neuropathologic Studies of Alzheimer’s Disease with Psychosis
Amyloid-Beta Pathology
Several studies have investigated whether AD + P associates with more severe fibrillar amyloid-beta (Aβ) pathology, in the form of neuritic plaques, compared with AD − P patients (Table 2). Results in earlier studies varied (83–85), possibly due to factors in the design, such as not accounting for the presence of comorbid Lewy body pathology or not correcting for multiple comparisons. In contrast, Sweet et al. (86) examined ratings of area densities of neuritic plaques across several brain regions in a well-matched cohort of AD + P and AD − P subjects, with matching criteria that included Lewy body pathology. No significant associations of AD + P with neuritic plaque severity were found.
Table 2.
Neuropathologic Studies of Alzheimer’s Disease with Psychosis
| Author, Date | Focus | n, Mean Age at Death (Years) | Psychosis Defined As | Method | Regions Examined | Findings |
|---|---|---|---|---|---|---|
| Zubenko et al. 1991 (85) | Senile plaques, NFTs, NA, 5-HT | 13 AD + P, 68.8 ± 7.6 14 AD − P, 75.1 ± 7.2 |
Presence of delusions or hallucinations | Bielschowsky, Congo red, and Masson trichrome stain | Middle frontal, superior temporal, and entorhinal cortices, presubiculum | Higher senile plaque density in prosubiculum of AD + Pa Higher NFT density in middle frontal cortex of AD + Pa,b Higher NE in substantia nigra of AD + Pa Lower 5-HT in Prosubiculum of AD + Pa |
| Förstl et al. 1993 (84) | Senile plaques | 3 AD + P, 69.3 ± 6.7 5 AD − P, 75.6 ± 4.8 |
Presence of delusions or hallucinations | Glees and Marsland silver impregnation | Frontal and temporal lobes, hippocampus, basal nucleus of Meynert, midbrain, pons | Nonec |
| Förstl et al. 1994 (98) | 5-HT | 22 AD + P 34 AD − P Age not differentiated by diagnosis, 75.4 ± 7.4 overall |
Auditory hallucinations, paranoid delusions, misidentification delusions | Luxol-fast blue/cresyl violet | Basal nucleus of Meynert, parahippocampal gyrus, hippocampus, substantia nigra, locus coeruleus, dorsal raphe nucleus | Higher cell counts in the parahippocampal gyrus, lower cell counts in dorsal raphe in AD + Pc |
| Mukaetova- Ladinska et al. 1995 (83) | Neuritic plaques, PHF tau | 18 AD (ages not specified) | Not specified | ELISA | Frontal, temporal, parietal, occipital, hippocampal, entorhinal cortices | Higher area densities of neuritic plaques associated with AD + Pc Higher mean PHF tau concentration across regions in AD + Pc |
| Farber et al. 2000 (89) | NFT, Senile plaques | 69 AD + P, 80.9 ± 8.2 40 AD − P, 84.6 ± 8.2 |
Presence of delusions or hallucinations | Bielschowsky | Middle frontal and superior temporal gyri, inferior parietal lobule, hippocampus, entorhinal cortex | Higher NFT area density in neocortex, but not medial temporal lobe, in AD + P No difference in plaque area density |
| Sweet et al. 2000 (86) | Neuritic plaques, NFTs | 24 AD + P, 77.0 ± 9.2 25 AD − P, 80.5 ± 7.8 |
Presence of delusions or hallucinations | Bielschowsky, anti- βA4, Congo red | Cingulate gyrus, caudate nucleus, basal ganglia, thalamus, hippocampus, cerebellum, dentate nucleus, midbrain, pons, medulla, spinal cord, inferior parietal lobule, superior and middle temporal cortex, primary visual cortex, nucleus basalis, amygdala, and transentorhinal cortex | None |
| Lai et al. 2001 (99) | ACh | 7 AD + P 19 AD − P Age not differentiated by diagnosis, 81 ± 1 overall |
Anxiety, persecutory ideation, visual/auditory hallucinations | Radioligand binding | Orbitofrontal gyrus (BA 11) and midtemporal gyrus (BA 21) | Higher M2 receptor density in BA 11 and BA 21 |
| Sweet et al. 2001 (95) | DA | 5 AD + P, 74.4 ± 11.9 8 AD − P, 85.6 ± 4.5 |
Presence of delusions or hallucinations | Radioligand binding | Nucleus accumbens, head of caudate nucleus | Higher striatal D3 receptor density in AD + P |
| Sweet et al. 2002 (91) | Synapses, GABA | 12 AD + P, 77.2 ± 8.0 16 AD − P, 80.5 ± 7.2 |
Presence of delusions or hallucinations | MRS | Amygdala, DLPFC, superior temporal, inferior parietal, occipital, cerebellum | Higher GPE in the DLPFC, inferior parietal, occipital; higher GPC in the DLPFC, superior temporal, occipital; lower NAA in the DLPFC, superior temporal, inferior parietal. Lower NAA, increased GPE in cortex (not medial temporal). Lower mean GABA concentration across all regions examined in AD + P. |
| Garcia-Alloza et al. 2005 (96) | 5-HT | 22 AD, Age not differentiated by diagnosis, 81.06 ± 1.6 overall | Hallucinations, persecutory ideas, inappropriate anxiety | HPLC | Frontal lobe (BA 10) and temporal lobe (BA 20) | Lower 5-HT in BA 20 of AD + Pa |
| Marcos et al. 2008 (97) | 5-HT | 22 AD, Age not differentiated by diagnosis, 81.06 ± 1.6 years overall | Hallucinations, persecutory ideas, inappropriate anxiety | HPLC | Temporal lobe (BA 20) | Lower 5-HT in BA 20, reduced adenylate cyclase activity after 5-HT6 stimulationa |
| Tsang et al. 2008 (100) | ACh | 7 AD + P 14 AD − P Age not differentiated by diagnosis, 83 ± 2 overall |
Anxiety, persecutory ideation, visual/auditory hallucinations | Radioligand binding | Orbitofrontal cortex (BA 11) | Reduced non-M2 binding in BA 11 |
| Murray et al. 2012 (88) | Soluble Aβ | 30 AD + P, 80.7 ± 7.9 22 AD − P, 80.6 ± 8.7 |
Presence of delusions or hallucinations | Aβ1–42 and Aβ1–40 ELISA Kalirin Western blot |
Superior frontal gyrus, inferior parietal cortex, superior temporal gyrus, occipital cortex | Lower soluble cortical Aβ1–40, but not Aβ1–42, in AD + P Kal-7, -9, and -12 are lower in AD + P DLPFC, but not Kal-5 |
| Murray et al. 2013 (90) | PhosphoMAPT | 26 AD + P, 81.4 ± 8.1 19 AD − P, 80.4 ± 8.9 |
Presence of delusions or hallucinations | IHC | Middle frontal gyrus | Elevated cortical phosphoMAPT concentration |
Aβ, amyloid beta; Aβ1–40, amyloid beta 1–40; Aβ1–42, amyloid beta 1–42; Ach, acetylcholine; AD, Alzheimer’s disease; AD + P, Alzheimer’s disease with psychosis; AD − P, Alzheimer’s disease without psychosis; anti-βA4, anti-beta amyloid A4 peptide antibody; BA, Brodmann area; DA, dopamine; D3, dopamine subtype 3; DLFPC, dorsolateral prefrontal cortex; ELISA, enzyme-linked immunosorbent assay; 5-HT, serotonin; 5-HT6, serotonin receptor subtype 6; GABA, gamma-aminobutyric acid; GPC, glycerophosphocholine; GPE, glycerophosphoethanolamine; HPLC, high pressure liquid chromatography; IHC, immunohistochemistry; Kal-5, -7, -9, -12, kalirin isoforms; M2, muscarinic subtype 2; MRS, magnetic resonance spectroscopy; NA, noradrenalin; NAA, N-acetyl-L-aspartate; NE, norepinephrine; NFT, neurofibrillary tangle; PHF, paired helical filament; phosphoMAPT, phosphorylated microtubule-associated protein tau.
Did not control for or specify exclusion of Lewy body pathology.
Does not persist after correction for multiple comparison.
Specified Lewy body cases, but did not stratify in analyses.
Increasingly, studies indicate that soluble forms of Aβ are the primary contributors to synapse impairment in AD (87). Soluble Aβ was also recently assessed in AD + P. Concentrations of soluble Aβ1–40 and Aβ1–42 were evaluated in cortical gray matter from multiple brain regions, in a well-matched cohort of AD + P and AD − P subjects. Soluble Aβ1–40 levels were significantly lower in the AD + P group, without any associated change in concentrations of Aβ1–42. This selective reduction in Aβ1–40 supported a significant increase in the Aβ1–42:Aβ1–40 ratio in dorsolateral prefrontal cortex (88). The increase in Aβ1–42:Aβ1–40 ratio highlights its importance as an indicator of Aβ toxicity, especially in AD + P where fibrillar Aβ neuropathology findings have been inconsistent.
Microtubule Associated Protein Tau Pathology
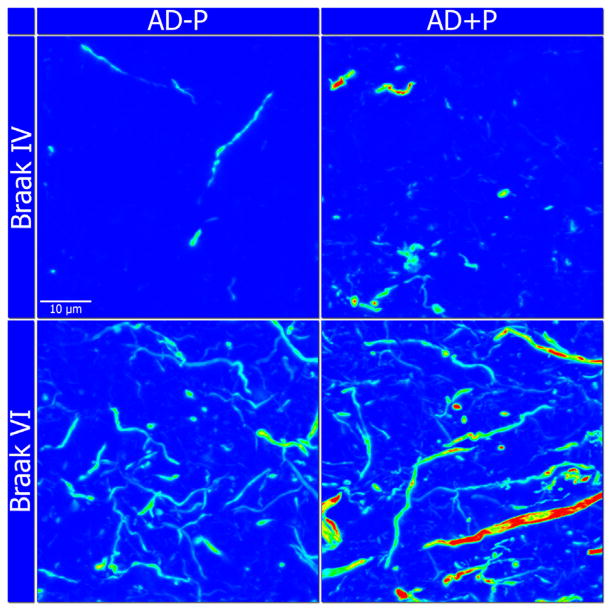
In contrast to studies of fibrillar Aβ, all but one of the studies to evaluate microtubule associated protein tau (MAPT) pathology have found some evidence of increased indices of pathologic MAPT aggregation in AD + P (Table 2) (84–86,89). In the largest and most comprehensive assessment of neurofibrillary tangles, Farber et al. (89) found a significant association between AD + P and increased neurofibrillary tangle area density across neo-cortical regions but not in medial temporal lobe structures. This association persisted even after accounting for comorbid Lewy body pathology. Similar findings resulted when measuring MAPT concentration in formic acid extracts of cortical gray matter, detected with an antibody directed against paired helical filament epitopes (83). Thus, available evidence supports increased aggregation of MAPT in AD + P. The contribution of MAPT to AD + P was further highlighted in a recent study that found no increased spread of phosphorylated microtubule-associated protein tau but increased concentrations of phosphoMAPT aggregates in dorsolateral prefrontal cortex of AD + P subjects (Figure 4) (90).
Figure 4.
Representative heat map images highlighting the difference in immunoreactivity of phosphorylated microtubule-associated protein tau (AT8 antibody) in the dorsolateral prefrontal cortex, between Alzheimer‘s disease with psychosis (AD + P) and Alzheimer‘s disease without psychosis (AD − P) subjects at lower (IV) and higher (VI) Braak stages. Blue and red colors represent lower and higher immunofluorescence intensities, respectively. Images captured at 60× magnification.
Synaptic Pathology
As synapse loss is the strongest correlate of cognitive decline in AD and the trajectory of decline is more severe in AD + P, synapse loss may be greater in AD + P. Findings from a magnetic resonance spectroscopy study of postmortem AD + P tissue support greater synaptic loss in AD + P (Table 2). In cortex, N-acetyl-L-aspartate (a marker of neuronal integrity) concentrations were significantly lower in AD + P, and concentrations of the phosphodiester membrane breakdown product, glycerophosphoethanolamine, were significantly elevated in AD + P. Superior temporal gyrus, dorsolateral prefrontal cortex, and inferior parietal cortex were most affected. However, there were no differences in these markers between AD + P and AD − P in the medial temporal lobe (amygdala) and cerebellum (91). This excess synaptic disruption could result either from the increased accumulation of pathology in AD + P (e.g., as described for soluble Aβ and MAPT) or from an enhanced synaptic vulnerability to these pathologic factors due to other molecular changes in AD + P. Indeed, kalirin, a dendritic spine-enriched protein essential for maintenance of dendritic spines in the cortex (92–94), is reduced in AD + P cortex (88).
Neurotransmitter Systems
A number of neurotransmitter system alterations have been reported in AD + P compared with AD − P. Nucleus accumbens dopamine D3 receptor density is significantly higher in AD + P with no difference in receptor affinity, findings that were independent of neuroleptic use or Lewy body pathology (95). Reduced serotonin (5-HT) in the ventral temporal cortex and prosubiculum (85,96,97), as well as lower adenylate cyclase activity after stimulation of 5-HT6 receptors (97), have been reported in AD + P. The lower 5-HT levels could be related to lower cell counts in dorsal raphe nucleus in AD + P (98). Alzheimer’s disease with psychosis has been associated with an increased ratio of acetylcholinesterase/5-HT. Other cholinergic alterations include higher muscarinic M2 receptor density in orbitofrontal gyrus of AD patients with delusions and in middle temporal gyrus of AD patients with hallucinations (99), while non-M2 binding is reduced in orbitofrontal gyrus (100). Finally, one report identified increased norepinephrine in the substantia nigra in AD + P (85).
Comorbid Pathology—Lewy Bodies and Vascular Lesions
The presence of well-formed visual hallucinations is among the criteria for the clinical diagnosis of dementia with Lewy bodies (DLB) and thus may contribute to differentiating between DLB and AD. Recent neuropathologic data using antibodies against alpha-synuclein to detect Lewy bodies (including screening for pathology in amygdala and entorhinal cortex) have found alpha-synuclein aggregation to be present in up to ~50% of cases with neuropathologically confirmed AD (101). In the majority of such cases, i.e., those with limited Lewy body pathology in the presence of both amyloid plaques and moderate to severe neurofibrillary tangles (e.g., Braak stage IV–VI), the clinical syndrome and neuropathologic diagnosis would be conceptualized as primarily due to AD with comorbid Lewy body pathology and not as primarily due to DLB (102).
Current evidence indicates that the presence of comorbid Lewy body pathology in AD may contribute to psychosis, although by no means can the occurrence of psychosis in AD be attributed principally to Lewy body pathology. Visual hallucinations are more frequent in individuals with primary AD plus comorbid Lewy body pathology, although other hallucinations and delusions may not be more frequent (103,104). In fact, psychosis (including delusions and/or auditory and visual hallucinations) is present in 40% to 60% of AD subjects without any Lewy body pathology detectable by stringent screening (104). Nevertheless, Lewy body pathology may contribute in some cases, especially in individuals with neocortical stage Lewy body pathology (103).
As vascular lesions have been implicated in occurrence of late-onset psychosis in the absence of any other known neurodegenerative disease (105), vascular disease could influence the clinical manifestation of illness by supporting a lower threshold for the development of psychotic symptoms. However, a recent examination of clinical and neuroimaging correlates of AD + P did not find increased rates of vascular risk factors or vascular lesions (43). Other pathways such as inflammation or altered cholesterol transport may also contribute to the neurobiology of AD (106) but have not been examined for an association with AD + P.
Discussion
This review reveals a number of areas of convergent findings.
The Presence of Psychosis in AD Clearly Demarcates a More Severe Phenotype of AD
Subjects who eventually manifest with psychotic symptoms undergo a sustained, more rapid cognitive decline that precedes their psychosis onset. Alzheimer’s disease with psychosis subjects demonstrate greater evidence of cortical synapse loss and impairment than subjects with AD − P, reflected in measures of gray matter volume, regional blood flow, regional glucose metabolism, and postmortem membrane breakdown products.
Psychosis in AD Is Most Closely Associated with Exaggerated Reductions of Gray Matter Volume, Blood Flow, and Glucose Metabolism in Neocortex Rather than in Medial Temporal Lobe Structures
This is not to say that individuals with AD + P do not have short-term memory impairment and pathologic changes in the entorhinal cortex and hippocampus. Indeed, they share these changes with AD − P subjects. What differentiates AD + P from AD − P, however, is greater impairment across neocortical regions. While no single brain region is solely affected, heteromodal association regions predominate, with few studies implicating sensory cortex. In particular, frontal cortical regions, including dorsolateral prefrontal cortex, appear commonly affected in AD + P.
Accumulation of Pathologic MAPT in Neocortical Regions Is Increased in AD + P
Findings across multiple neocortical regions of increased phosphoMAPT, or increased fibrillar MAPT in tangles, has been identified using a variety of technical approaches, indicating a robustness of the association. Whether the increase is causally related to onset of psychosis or is a correlated outcome of an accelerated pathologic process, of course, cannot be distinguished in these studies.
The Risk for AD + P Is, in Part, Genetically Mediated
Three independent cohorts have demonstrated evidence for familial aggregation of psychosis in AD, strongly suggesting that at least a portion of the risk is genetic in origin. Although no single genetic variant has yet to be unequivocally demonstrated as contributing to this risk, there is an emerging picture of the genetic architecture. Psychosis in AD does not appear to arise in conjunction with genetic variants that increase the risk for AD itself. Instead, the most likely genetic (and neurobiological) model is one in which genetic variants for AD + P independently modify the progression of the cascade of pathology induced by AD genes (Figure 2A1).
Moving Forward
These points of consistency in this emerging field of study suggest that the neurobiology of psychosis in AD is poised for translational discovery. Importantly, there is the opportunity to inform and accelerate this process by leveraging the findings emerging from the study of the neurobiology of AD itself and of idiopathic psychosis (schizophrenia). Achieving this goal will require extension and innovation within the current approaches of genetic, imaging, and postmortem studies. Additional development of model systems and biomarkers will also be needed.
There is a need to further characterize intermediate phenotypes, which will provide a key link for bridging from animal models to human brain pathology and from pathology to the pathophysiologic changes manifesting as symptoms. Additional structural and functional brain imaging are clearly among these options but could be extended by the conduct of multimodal imaging within subjects and by the incorporation of additional measures such as amyloid (or when available MAPT) imaging. Such studies should also give consideration to inclusion of cerebrospinal fluid biomarkers, in particular measures of phosphoMAPT and of VILIP-1. Importantly, there is a current lack of longitudinal imaging studies that could shed more light on the temporal relationship between functional and structural changes and psychosis onset.
More consideration of longitudinal course also is needed in future postmortem studies of AD + P. The vast majority of individuals studied to date have had end stage AD neuropathology, with extensive neurofibrillary tangles throughout the neo-cortex (i.e., largely Braak stage VI). However, clinical studies clearly indicate that the most rapid increase in rates of psychosis occurs during the transition from mild cognitive symptoms to early and middle stages of cognitive impairment (28,60), corresponding roughly to Braak stages III to V (107,108). Studies of tissue from individuals in earlier pathologic stages are clearly needed before findings can be interpreted as having a potential causal role in psychosis. In addition, greater emphasis must be given to identifying postmortem findings that are selective for psychosis in AD and not just exaggerated in AD + P relative to AD − P. That is, relevant discoveries for mechanisms of psychosis in AD should involve molecular or structural pathologies that are present in AD + P relative to AD − P and to normal control subjects and not present in AD − P when compared with control subjects.
Molecular discovery could be enhanced by expanding the cohorts of families and unrelated individuals available for further genetic discovery in AD + P by incorporating rigorous behavioral phenotyping into the many such collections of AD subjects. Evaluating AD + P using innovative genomic approaches including genome-wide association, assessment of copy number variations (which has been fruitful in schizophrenia), detection of rare alleles, and integrated genomic/transcriptomic strategies should be pursued. It will be essential to complement any molecular discovery from genomics with examination of the same molecules in postmortem samples of AD + P subjects and with examination of genetic animal models. For example, a potentially useful strategy would be to cross a mouse model of a knockout for a gene implicated in AD + P with one of several current animal models of AD and then assess modification of the AD pathology by the psychosis-associated gene. This could facilitate discovery of intermediate phenotypes that could then be forward translated for testing in humans.
Supplementary Material
Acknowledgments
This work was supported by Veterans Health Administration Grant BX000452 and National Institutes of Health Grants MH071533, AG05133, and AG027224. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Footnotes
Dr. Sweet serves as a consultant for Lilly, USA. Drs. Murray, Kumar, and DeMichele-Sweet report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.08.020.
References
- 1.Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, et al. The Behavior Rating Scale for Dementia of the Consortium to Establish a Registry for Alzheimer’s Disease. The Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer’s Disease. Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 2.Rubin EH, Drevets WC, Burke WJ. Nature of psychotic symptoms in senile dementia of the Alzheimer type. J Geriatr Psychiatry Neurol. 1988;1:17–20. doi: 10.1177/089198878800100104. [DOI] [PubMed] [Google Scholar]
- 3.Jeste DV, Finkel SI. Psychosis of Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Paulsen JS, Salmon DP, Thal L, Romero R, Weisstein-Jenkins C, Galasko D, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable Alzheimer’s disease. Neurology. 2000;54:1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- 5.Wilkosz PA, Miyahara S, Lopez OL, DeKosky ST, Sweet RA. Prediction of psychosis onset in Alzheimer disease: The role of cognitive impairment, depressive symptoms, and further evidence for psychosis subtypes. Am J Geriatr Psychiatry. 2006;14:352–360. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]
- 6.Leroi I, Voulgari A, Breitner JC, Lyketsos CG. The epidemiology of psychosis in dementia. Am J Geriatr Psychiatry. 2003;11:83–91. [PubMed] [Google Scholar]
- 7.Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry. 1989;25:39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- 8.Sweet RA, Bennett DA, Graff-Radford NR, Mayeux R. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain. 2010;133:1155–1162. doi: 10.1093/brain/awq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thies W, Bleiler L Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Gilley DW, Whalen ME, Wilson RS, Bennett DA. Hallucinations and associated factors in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1991;3:371–376. doi: 10.1176/jnp.3.4.371. [DOI] [PubMed] [Google Scholar]
- 11.Gilley DW, Wilson RS, Beckett LA, Evans DA. Psychotic symptoms and physically aggressive behavior in Alzheimer’s disease. J Am Geriatr Soc. 1997;45:1074–1079. doi: 10.1111/j.1532-5415.1997.tb05969.x. [DOI] [PubMed] [Google Scholar]
- 12.Sweet RA, Pollock BG, Sukonick DL, Mulsant BH, Rosen J, Klunk WE, et al. The 5-HTTPR polymorphism confers liability to a combined phenotype of psychotic and aggressive behavior in Alzheimer’s disease. Int Psychogeriatr. 2001;13:401–409. doi: 10.1017/s1041610201007827. [DOI] [PubMed] [Google Scholar]
- 13.Lyketsos CG, Sheppard JM, Steinberg M, Tschanz JA, Norton MC, Steffens DC, Breitner JC. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: The Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 14.Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 15.Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabins PV, Mace NL, Lucas MJ. The impact of dementia on the family. JAMA. 1982;248:333–335. [PubMed] [Google Scholar]
- 17.Lopez OL, Wisniewski SR, Becker JT, Boller F, DeKosky ST. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Arch Neurol. 1999;56:1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- 18.Magni E, Binetti G, Bianchetti A, Trabucchi M. Risk of mortality and institutionalization in demented patients with delusions. J Geriatr Psychiatry Neurol. 1996;9:123–126. doi: 10.1177/089198879600900303. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JL, Diaz C, Levy M, Binetti G, Litvan II. Neuropsychiatric syndromes in neurodegenerative disease: Frequency and signficance. Semin Clin Neuropsychiatry. 1996;1:241–247. doi: 10.1053/SCNP00100241. [DOI] [PubMed] [Google Scholar]
- 20.Bassiony MM, Steinberg M, Rosenblatt A, Baker A, Lyketsos CG. Delusions and hallucinations in Alzheimer’s disease: Prevalence and clinical correlates. Int J Geriatr Psychiatry. 2000;15:99–107. doi: 10.1002/(sici)1099-1166(200002)15:2<99::aid-gps82>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RS, Tang Y, Aggarwal NT, Gilley DW, Mccann JJ, Bienias JL, Evans DA. Hallucinations, cognitive decline, and death in Alzheimer’s disease. Neuroepidemiology. 2006;26:68–75. doi: 10.1159/000090251. [DOI] [PubMed] [Google Scholar]
- 22.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geda YE, Schneider LS, Gitlin LN, Miller DS, Smith GS, Bell J, et al. Neuropsychiatric symptoms in Alzheimer’s disease: Past progress and anticipation of the future. Alzheimers Dement. 2013;9:602–608. doi: 10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38:553–563. doi: 10.1111/j.1532-5415.1990.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 25.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: Meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 26.Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: Population based cohort study. BMJ. 2012;344:e977. doi: 10.1136/bmj.e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock BG, Mulsant BH, Rosen J, Mazumdar S, Blakesley RE, Houck PR, Huber KA. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. Am J Geriatr Psychiatry. 2007;15:942–952. doi: 10.1097/JGP.0b013e3180cc1ff5. [DOI] [PubMed] [Google Scholar]
- 28.Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–2030. doi: 10.1176/appi.ajp.162.11.2022. [DOI] [PubMed] [Google Scholar]
- 29.Jeste DV, Wragg RE, Salmon DP, Harris MJ, Thal LJ. Cognitive deficits of patients with Alzheimer’s disease with and without delusions. Am J Psychiatry. 1992;149:184–189. doi: 10.1176/ajp.149.2.184. [DOI] [PubMed] [Google Scholar]
- 30.Koppel J, Sunday S, Goldberg TE, Davies P, Christen E, Greenwald BS. Psychosis in Alzheimer’s disease is associated with frontal metabolic impairment and accelerated decline in working memory: Findings from the Alzheimer’s disease neuroimaging initiative. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2012.10.028. published online ahead of print March 15. [DOI] [PubMed] [Google Scholar]
- 31.Koppel J, Goldberg TE, Gordon ML, Huey E, Davies P, Keehlisen L, et al. Relationships between behavioral syndromes and cognitive domains in Alzheimer disease: The impact of mood and psychosis. Am J Geriatr Psychiatry. 2012;20:994–1000. doi: 10.1097/JGP.0b013e3182358921. [DOI] [PubMed] [Google Scholar]
- 32.Weamer EA, Emanuel JE, Varon D, Miyahara S, Wilkosz PA, Lopez OL, et al. The relationship of excess cognitive impairment in MCI and early Alzheimer’s disease to the subsequent emergence of psychosis. Int Psychogeriatr. 2009;21:78–85. doi: 10.1017/S1041610208007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emanuel JE, Lopez OL, Houck PR, Becker JT, Weamer EA, DeMichele-Sweet MA, et al. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am J Geriatr Psychiatry. 2011;19:160–168. doi: 10.1097/JGP.0b013e3181e446c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkosz PA, Seltman HJ, Devlin B, Weamer EA, Lopez OL, DeKosky ST, Sweet RA. Trajectories of cognitive decline in Alzheimer’s disease. Int Psychogeriatr. 2010;22:281–290. doi: 10.1017/S1041610209991001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweet RA, Seltman H, Emanuel JE, Lopez OL, Becker JT, Bis JC, et al. Effect of Alzheimer’s disease risk genes on trajectories of cognitive function in the Cardiovascular Health Study. Am J Psychiatry. 2012;169:954–962. doi: 10.1176/appi.ajp.2012.11121815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002;58:907–911. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- 37.Hollingworth P, Hamshere ML, Holmans PA, O’Donovan MC, Sims R, Powell J, et al. Increased familial risk and genomewide significant linkage for Alzheimer’s disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:841–848. doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- 38.Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:624–627. doi: 10.1176/appi.ajgp.13.7.624. [DOI] [PubMed] [Google Scholar]
- 39.Avramopoulos D, Fallin MD, Bassett SS. Linkage to chromosome 14q in Alzheimer’s disease (AD) patients without psychotic symptoms. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:9–13. doi: 10.1002/ajmg.b.30074. [DOI] [PubMed] [Google Scholar]
- 40.Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Linkage analysis of Alzheimer disease with psychosis. Neurology. 2002;59:118–120. doi: 10.1212/wnl.59.1.118. [DOI] [PubMed] [Google Scholar]
- 41.Go RC, Perry RT, Wiener H, Bassett SS, Blacker D, Devlin B, Sweet RA. Neuregulin-1 polymorphism in late onset Alzheimer’s disease families with psychoses. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:28–32. doi: 10.1002/ajmg.b.30219. [DOI] [PubMed] [Google Scholar]
- 42.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 43.DeMichele-Sweet MA, Lopez OL, Sweet RA. Psychosis in Alzheimer’s disease in the national Alzheimer’s disease coordinating center uniform data set: Clinical correlates and association with apolipoprotein e. Int J Alzheimers Dis. 2011;2011:926597. doi: 10.4061/2011/926597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu SH, Roeder K, Ferrell RE, Devlin B, DeMichele-Sweet MA, Kamboh MI, et al. TOMM40 poly-T repeat lengths, age of onset and psychosis risk in Alzheimer disease. Neurobiol Aging. 2011;32:2328–2329. doi: 10.1016/j.neurobiolaging.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer’s disease: A review. J Alzheimers Dis. 2010;19:761–780. doi: 10.3233/JAD-2010-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hollingworth P, Sweet R, Sims R, Harold D, Russo G, Abraham R, et al. Genome-wide association study of Alzheimer’s disease with psychotic symptoms. Mol Psychiatry. 2012;17:1316–1327. doi: 10.1038/mp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wijsman EM, Pankratz ND, Choi Y, Rothstein JH, Faber KM, Cheng R, et al. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamboh MI, Demirci FY, Wang X, Minster RL, Carrasquillo MM, Pankratz VS, et al. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry. 2012;2:e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kam M, Massare J, Gallinger S, Kinzie J, Weaver D, Dingell JD, et al. Peutz-Jeghers syndrome diagnosed in a schizophrenic patient with a large deletion in the STK11 gene. Dig Dis Sci. 2006;51:1567–1570. doi: 10.1007/s10620-006-9102-8. [DOI] [PubMed] [Google Scholar]
- 51.Lee CH, Liu CM, Wen CC, Chang SM, Hwu HG. Genetic copy number variants in sib pairs both affected with schizophrenia. J Biomed Sci. 2010;17:2. doi: 10.1186/1423-0127-17-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kojima Y, Miyoshi H, Clevers HC, Oshima M, Aoki M, Taketo MM. Suppression of tubulin polymerization by the LKB1-micro-tubule-associated protein/microtubule affinity-regulating kinase signaling. J Biol Chem. 2007;282:23532–23540. doi: 10.1074/jbc.M700590200. [DOI] [PubMed] [Google Scholar]
- 53.Wang JW, Imai Y, Lu B. Activation of PAR-1 kinase and stimulation of tau phosphorylation by diverse signals require the tumor suppressor protein LKB1. J Neurosci. 2007;27:574–581. doi: 10.1523/JNEUROSCI.5094-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amici M, Doherty A, Jo J, Jane D, Cho K, Collingridge G, Dargan S. Neuronal calcium sensors and synaptic plasticity. Biochem Soc Trans. 2009;37:1359–1363. doi: 10.1042/BST0371359. [DOI] [PubMed] [Google Scholar]
- 55.Lee JM, Blennow K, Andreasen N, Laterza O, Modur V, Olander J, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–1623. doi: 10.1373/clinchem.2008.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarawneh R, D’Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, et al. Visinin-like protein-1: Diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70:274–285. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarawneh R, Lee JM, Ladenson JH, Morris JC, Holtzman DM. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78:709–719. doi: 10.1212/WNL.0b013e318248e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein HG, Braunewell KH, Spilker C, Danos P, Baumann B, Funke S, et al. Hippocampal expression of the calcium sensor protein visinin-like protein-1 in schizophrenia. Neuroreport. 2002;13:393–396. doi: 10.1097/00001756-200203250-00006. [DOI] [PubMed] [Google Scholar]
- 60.Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 61.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuro-anatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- 62.Lee DY, Choo IH, Kim KW, Jhoo JH, Youn JC, Lee UY, Woo JI. White matter changes associated with psychotic symptoms in Alzheimer’s disease patients. J Neuropsychiatry Clin Neurosci. 2006;18:191–198. doi: 10.1176/jnp.2006.18.2.191. [DOI] [PubMed] [Google Scholar]
- 63.Serra L, Perri R, Cercignani M, Spano B, Fadda L, Marra C, et al. Are the behavioral symptoms of Alzheimer’s disease directly associated with neurodegeneration? J Alzheimers Dis. 2010;21:627–639. doi: 10.3233/JAD-2010-100048. [DOI] [PubMed] [Google Scholar]
- 64.Whitehead D, Tunnard C, Hurt C, Wahlund LO, Mecocci P, Tsolaki M, et al. Frontotemporal atrophy associated with paranoid delusions in women with Alzheimer’s disease. Int Psychogeriatr. 2012;24:99–107. doi: 10.1017/S1041610211000974. [DOI] [PubMed] [Google Scholar]
- 65.Fukuhara R, Ikeda M, Nebu A, Kikuchi T, Maki N, Hokoishi K, et al. Alteration of rCBF in Alzheimer’s disease patients with delusions of theft. Neuroreport. 2001;12:2473–2476. doi: 10.1097/00001756-200108080-00037. [DOI] [PubMed] [Google Scholar]
- 66.Kotrla KJ, Chacko RC, Harper RG, Jhingran S, Doody R. SPECT findings on psychosis in Alzheimer’s disease. Am J Psychiatry. 1995;152:1470–1475. doi: 10.1176/ajp.152.10.1470. [DOI] [PubMed] [Google Scholar]
- 67.Matsuoka T, Narumoto J, Shibata K, Okamura A, Nakamura K, Okuyama C, et al. Insular hypoperfusion correlates with the severity of delusions in individuals with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2010;29:287–293. doi: 10.1159/000295115. [DOI] [PubMed] [Google Scholar]
- 68.Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL. Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69:167–171. doi: 10.1136/jnnp.69.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moran EK, Becker JA, Satlin A, Lyoo IK, Fischman AJ, Johnson KA. Psychosis of Alzheimer’s disease: Gender differences in regional perfusion. Neurobiol Aging. 2008;29:1218–1225. doi: 10.1016/j.neurobiolaging.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 70.Nakano S, Yamashita F, Matsuda H, Kodama C, Yamada T. Relationship between delusions and regional cerebral blood flow in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:16–21. doi: 10.1159/000089215. [DOI] [PubMed] [Google Scholar]
- 71.Nomura K, Kazui H, Wada T, Sugiyama H, Yamamoto D, Yoshiyama K, et al. Classification of delusions in Alzheimer’s disease and their neural correlates. Psychogeriatrics. 2012;12:200–210. doi: 10.1111/j.1479-8301.2012.00427.x. [DOI] [PubMed] [Google Scholar]
- 72.Ponton MO, Darcourt J, Miller BL, Cummings JL, Schumann SW, Maen I. Psychometric and SPECT studies in Alzheimer disease with and without delusions. Neuropsychiatry Neuropsychol Behav Neurol. 1995;8:264–270. [Google Scholar]
- 73.Staff RT, Shanks MF, Macintosh L, Pestell SJ, Gemmell HG, Venneri A. Delusions in Alzheimer’s disease: Spet evidence of right hemispheric dysfunction. Cortex. 1999;35:549–560. doi: 10.1016/s0010-9452(08)70818-9. [DOI] [PubMed] [Google Scholar]
- 74.Staff RT, Venneri A, Gemmell HG, Shanks MF, Pestell SJ, Murray AD. HMPAO SPECT imaging of Alzheimer’s disease patients with similar content-specific autobiographic delusion: Comparison using statistical parametric mapping. J Nucl Med. 2000;41:1451–1455. [PubMed] [Google Scholar]
- 75.Starkstein SE, Vazquez S, Petracca G, Sabe L, Migliorelli R, Teson A, Leiguarda R. A SPECT study of delusions in Alzheimer’s disease. Neurology. 1994;44:2055–2059. doi: 10.1212/wnl.44.11.2055. [DOI] [PubMed] [Google Scholar]
- 76.Grady CL, Haxby JV, Schapiro MB, Gonzalez-Aviles A, Kumar A, Ball MJ, et al. Subgroups in dementia of the Alzheimer type identified using positron emission tomography. J Neuropsychiatry Clin Neurosci. 1990;2:373–384. doi: 10.1176/jnp.2.4.373. [DOI] [PubMed] [Google Scholar]
- 77.Lopez OL, Smith G, Becker JT, Cidis Meltzer C, DeKosky ST. The psychotic phenomenon in probable Alzheimer’s disease: A positron emission tomography study. J Neuropsychiatry Clin Neurosci. 2001;13:50–55. doi: 10.1176/jnp.13.1.50. [DOI] [PubMed] [Google Scholar]
- 78.Sultzer DL, Mahler ME, Mandelkern MA, Cummings JL, Van Gorp WG, Hinkin CH, Berisford MA. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1995;7:476–484. doi: 10.1176/jnp.7.4.476. [DOI] [PubMed] [Google Scholar]
- 79.Sultzer DL, Brown CV, Mandelkern MA, Mahler ME, Mendez MF, Chen ST, Cummings JL. Delusional thoughts and regional frontal/temporal cortex metabolism in Alzheimer’s disease. Am J Psychiatry. 2003;160:341–349. doi: 10.1176/appi.ajp.160.2.341. [DOI] [PubMed] [Google Scholar]
- 80.Hirono N, Mori E, Ishii K, Kitagaki H, Sasaki M, Ikejiri Y, et al. Alteration of regional cerebral glucose utilization with delusions in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1998;10:433–439. doi: 10.1176/jnp.10.4.433. [DOI] [PubMed] [Google Scholar]
- 81.Mentis MJ, Weinstein EA, Horwitz B, McIntosh AR, Pietrini P, Alexander GE, et al. Abnormal brain glucose metabolism in the delusional misidentification syndromes: A positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995;38:438–449. doi: 10.1016/0006-3223(94)00326-x. [DOI] [PubMed] [Google Scholar]
- 82.Reeves S, Brown R, Howard R, Grasby P. Increased striatal dopamine (D2/D3) receptor availability and delusions in Alzheimer disease. Neurology. 2009;72:528–534. doi: 10.1212/01.wnl.0000341932.21961.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mukaetova-Ladinska EB, Harrington CR, Xuereb J, Roth M, Wischik CM. Treating Alzheimer’s and Other Dementias. New York: Springer; 1995. Biochemical, Neuropathological, and Clinical Correlations of Neurofibrillary Degeneration in Alzheimer’s Disease; pp. 57–80. [Google Scholar]
- 84.Forstl H, Burns A, Luthert P, Cairns N, Levy R. The Lewy-body variant of Alzheimer’s disease clinical and pathological findings. Br J Psychiatry. 1993;162:385–392. doi: 10.1192/bjp.162.3.385. [DOI] [PubMed] [Google Scholar]
- 85.Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, Kopp U. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619–624. doi: 10.1001/archneur.1991.00530180075020. [DOI] [PubMed] [Google Scholar]
- 86.Sweet RA, Hamilton RL, Lopez OL, Klunk WE, Wisniewski SR, Kaufer DI, et al. Psychotic symptoms in Alzheimer’s disease are not associated with more severe neuropathologic features. Int Psychogeriatr. 2000;12:547–558. doi: 10.1017/s1041610200006657. [DOI] [PubMed] [Google Scholar]
- 87.Walsh DM, Selkoe DJ. A beta oligomers-a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 88.Murray PS, Kirkwood CM, Gray MC, Ikonomovic MD, Paljug WR, Abrahamson EE, et al. β-Amyloid 42/40 ratio and kalirin expression in Alzheimer disease with psychosis. Neurobiol Aging. 2012;33:2807–2816. doi: 10.1016/j.neurobiolaging.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farber NB, Rubin EH, Newhouse PA, Kinscherf DA, Miller JP, Morris JC, et al. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer’s disease. Arch Gen Psychiatry. 2000;57:1165–1173. doi: 10.1001/archpsyc.57.12.1165. [DOI] [PubMed] [Google Scholar]
- 90.Murray PS, Kirkwood CM, Ikonomovic MD, Fish KN, Sweet RA. Tau phosphorylation is exaggerated in Alzheimer disease with psychosis. Am J Geriatr Psychiatry. 2013;21:S80–S81. [Google Scholar]
- 91.Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, et al. Psychosis in Alzheimer disease: Postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002;23:547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 92.Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, et al. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie Z, Cahill ME, Penzes P. Kalirin loss results in cortical morphological alterations. Mol Cell Neurosci. 2010;43:81–89. doi: 10.1016/j.mcn.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sweet RA, Hamilton RL, Healy MT, Wisniewski SR, Henteleff R, Pollock BG, et al. Alterations of striatal dopamine receptor binding in Alzheimer disease are associated with Lewy body pathology and antemortem psychosis. Arch Neurol. 2001;58:466–472. doi: 10.1001/archneur.58.3.466. [DOI] [PubMed] [Google Scholar]
- 96.Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ. Cholinergic-serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia. 2005;43:442–449. doi: 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 97.Marcos B, Garcia-Alloza M, Gil-Bea FJ, Chuang TT, Francis PT, Chen CP, et al. Involvement of an altered 5-HT -{6} receptor function in behavioral symptoms of Alzheimer’s disease. J Alzheimers Dis. 2008;14:43–50. doi: 10.3233/jad-2008-14104. [DOI] [PubMed] [Google Scholar]
- 98.Forstl H, Burns A, Levy R, Cairns N. Neuropathological correlates of psychotic phenomena in confirmed Alzheimer’s disease. Br J Psychiatry. 1994;165:53–59. doi: 10.1192/bjp.165.1.53. [DOI] [PubMed] [Google Scholar]
- 99.Lai MK, Lai OF, Keene J, Esiri MM, Francis PT, Hope T, Chen CP. Psychosis of Alzheimer’s disease is associated with elevated muscarinic M2 binding in the cortex. Neurology. 2001;57:805–811. doi: 10.1212/wnl.57.5.805. [DOI] [PubMed] [Google Scholar]
- 100.Tsang SW, Francis PT, Esiri MM, Wong PT, Chen CP, Lai MK. Loss of [3H]4-DAMP binding to muscarinic receptors in the orbito-frontal cortex of Alzheimer’s disease patients with psychosis. Psychopharmacology (Berl) 2008;198:251–259. doi: 10.1007/s00213-008-1124-9. [DOI] [PubMed] [Google Scholar]
- 101.Hamilton RL. Lewy bodies in Alzheimer’s disease: A neuropathological review of 145 cases using a-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 103.Ballard CG, Jacoby R, Del Ser T, Khan MN, Munoz DG, Holmes C, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with lewy bodies. Am J Psychiatry. 2004;161:843–849. doi: 10.1176/appi.ajp.161.5.843. [DOI] [PubMed] [Google Scholar]
- 104.Tsuang D, Simpson K, Larson EB, Peskind E, Kukull W, Bowen JB, et al. Predicting lewy body pathology in a community-based sample with clinical diagnosis of Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2006;19:195–201. doi: 10.1177/0891988706292755. [DOI] [PubMed] [Google Scholar]
- 105.Breitner JC, Husain MM, Figiel GS, Krishnan KR, Boyko OB. Cerebral white matter disease in late-onset paranoid psychosis. Biol Psychiatry. 1990;28:266–274. doi: 10.1016/0006-3223(90)90582-m. [DOI] [PubMed] [Google Scholar]
- 106.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, Gertz HJ, Xuereb JH, Hills R, et al. Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer’s disease. Am J Pathol. 2000;157:623–636. doi: 10.1016/s0002-9440(10)64573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.