Abstract
Phox2b is a transcription factor expressed in the central and peripheral neurons that control cardiovascular, respiratory and digestive functions and essential for their development. Several populations known or suspected to regulate visceral functions express Phox2b in the developing hindbrain. Extensive cell migration and lack of suitable markers have greatly hampered studying their development. Reasoning that intersectional fate mapping may help to overcome these impediments, we have generated a BAC transgenic mouse line, P2b::FLPo, which expresses codon-optimized FLP recombinase in Phox2b expressing cells. By partnering the P2b::FLPo with the FLP-responsive RC::Fela allele, we show that FLP recombination switches on lineage tracers in the cells that express or have expressed Phox2b, permanently marking them for study across development. Taking advantage of the dualrecombinase feature of RC::Fela, we further show that the P2b::FLPo transgene can be partnered with Lbx1Cre as Cre driver to generate triple transgenics in which neurons having a history of both Phox2b and Lbx1 expression are specifically labelled. Hence, the P2b::FLPo line when partnered with a suitable Cre driver provides a tool for tracking and accessing genetically subsets of Phox2b-expressing neuronal populations, which has not been possible by Cremediated recombination alone.
Keywords: BAC transgenic mouse line, fate mapping, FLP recombinase, Phox2b, hindbrain
Phox2b is a transcription factor found in a limited set of central and peripheral neurons. It is expressed in and essential for the development of the reflex circuits that control bodily homeostasis by regulating cardio-vascular, respiratory and digestive functions (Brunet and Goridis, 2008; Brunet and Pattyn, 2002). In addition to the core components of the visceral reflex loops —the visceral sensory ganglia, their central target (the nucleus of the solitary tract (nTS)), the visceromotor neurons and the autonomic ganglia (Dauger et al., 2003; Pattyn et al., 1997)— Phox2b is expressed in only a few other places, some of which have functional or phylogenetic links with the visceral nervous system. These are (i) the branchiomotor neurons (Pattyn et al., 1997), whose visceral function is obscured in mammals but which control respiration in fish, (ii) the central (nor)adrenergic cell groups involved in modulating autonomic functions (Pattyn et al., 2000), (iii) a population of hindbrain interneurons whose function is mostly unknown except for one group involved in respiratory control (Goridis et al., 2010; Guyenet et al., 2008), and (iv) the oculomotor (nIII) and trochlear (nIV) motor neurons (Pattyn et al., 1997).
We recently generated a BAC transgenic mouse line that exploits a Phox2b-containing BAC to drive Cre recombinase expression in a pattern mirroring that of the endogenous Phox2b gene (D’Autreaux et al., 2011). We thus took the same approach to produce a mouse line (henceforth termed P2b::FLPo) expressing codon-optimized FLP recombinase (FLPo) (Raymond and Soriano, 2007). Using homologous recombination in bacteria, we replaced the Cre open reading frame in Phox2b::Cre with the FLPo-encoding sequence (Fig. 1a). When paired with an Lbx1Cre (Sieber et al., 2007) and a dual recombinase-responsive indicator allele (Fig. 1b) (Jensen et al., 2008), the P2b::FLPo line could be used to track the subset of neurons that have a history of both Phox2b and Lbx1 expression. Therefore, P2b::FLPo mice are suitable for intersectional genetics (Dymecki et al., 2010) and can be used to subdivide Phox2b lineage cells into subsets defined by the past or present expression of a second gene. In addition to interrogating cell lineage relationships, P2b::FLPo mice will provide a tool for genetically manipulating the cells that express or have expressed Phox2b and a second, independent gene.
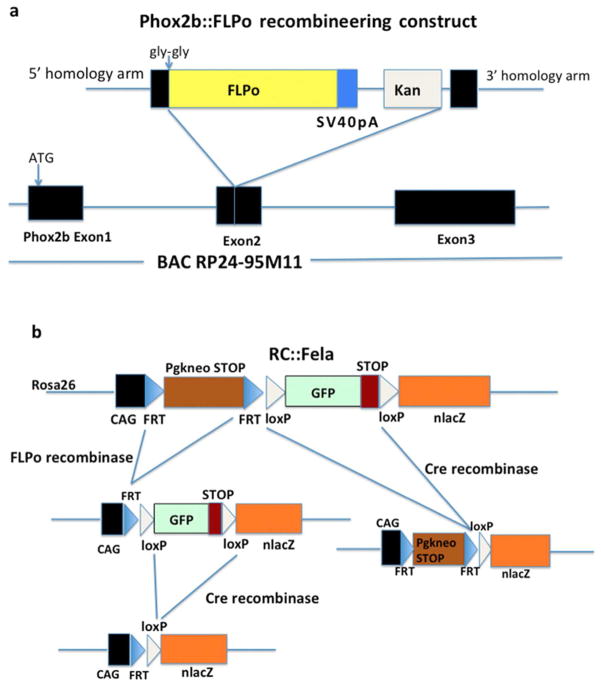
Fig. 1.
Generation of the Phox2b::FLPo BAC transgene and schematic of the RC::Fela indicator allele. (a) Cloning strategy. From 5′ to 3′, the recombineering construct contains a 5′ Phox2b gene homology arm, two glycine codons introduced to correct the reading frame and interrupt structured protein domains, the FLPo sequence followed by a SV40 polyadenylation signal, the kanamycin resistance gene and a 3′ Phox2b gene homology arm. It was placed in fusion with the Phox2b second exon contained in BAC RP24-95M11 by recombination in bacteria. This will create a fusion protein between the first 82 residues of Phox2b and the FLPo sequence. (b) Schematic of the RC::Fela dual recombinase-responsive indicator allele taken from Jensen et al. (2008) and of its derivatives generated by FLP and/or Cre recombinases. GFP expression will be activated in response to FLP-mediated recombination only and nβgal expression in response to both FLP- and Cre-mediated recombination. Germ line excision by Cre recombinase provided by Pgk::Cre will produce an indicator allele expressing nβgal in response to FLP.
Pronuclear injections of the P2b::FLPo BAC construct yielded two founders, 3276 and 3278. In both lines, FLPo-mediated recombination was limited to Phox2b lineage cells, but only the 3276 line activated indicator gene expression in CNS neuron progenitors while in 3278 mice FLPo activity was strictly postmitotic. We thus selected the former for detailed analysis. The hemizygous P2b::FLPo transgenics from the 3276 line were viable and fertile, had a normal life span and showed no obvious phenotype. However, by analyzing the zygosity of the offspring of crosses between P2b::FLPo mice using real-time quantitative PCR (Sakurai et al., 2008), we found that all progeny harbouring the transgene were hemizygotes. Among 26 pups examined, 6 were wild-type, and none homozygous. This result suggests that homozygosity of the transgene is lethal during embryonic development.
To evaluate FLPo activity and specificity, we used the dual recombinase-dependent RC::Fela indicator line that expresses GFP in response to FLP recombinase and nuclearly located β-galactosidase (nβgal) in response to both FLP and Cre recombinase (Jensen et al., 2008) (Fig. 1b). Germline excision of the loxP-flanked GFP cassette in RC::Fela mice by maternally expressed Pgk::Cre will thus result in lacZ expression in all FLPo-expressing cells. In P2b::FLPo;RC::Fela embryonic day 12.5 (E12.5) embryos, lacZ expression was activated by FLPo in all major sites of the central and peripheral nervous system in which Phox2b is known to be present (Fig. 2). No ectopic expression was observed in cells in which Phox2b is never found. In particular, staining was absent in the forebrain where Phox2b is not expressed.
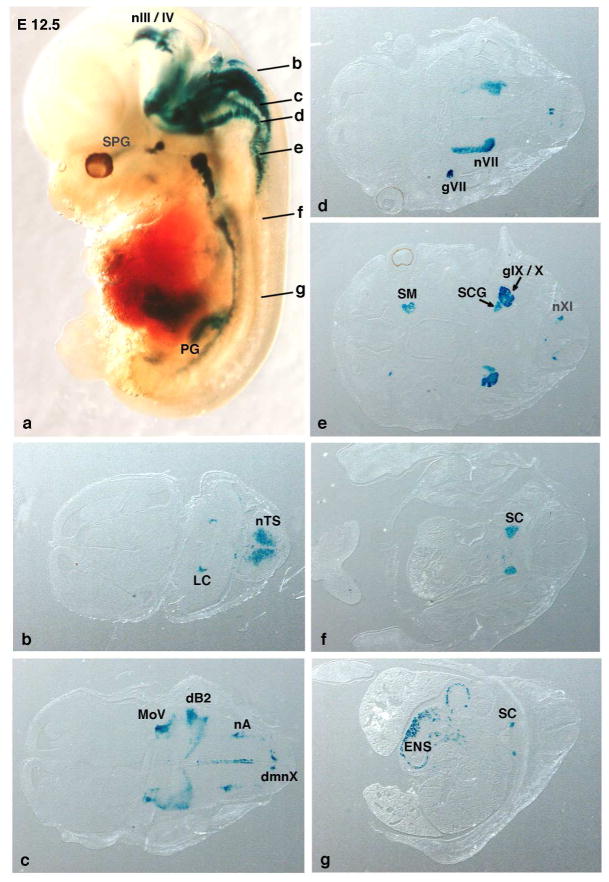
Fig. 2.
Whole-mount staining of of nβgal expression from the RC::Fela indicator allele in E12.5 P2b::FLPo embryos after germ line excision of the GFP-STOP cassette. (a) Whole-mount X-gal staining of Phox2b::FLPo;RC::Fela embryos after removal of the loxP-flanked cassette in the germ line by maternal Pgk::Cre. The nβgal expression is readily detected at the known Phox2b+ sites in the midbrain and hindbrain and in the autonomic ganglia but is absent from Phox2b-negative tissues (forebrain and thoracic and lumbar spinal cord). Letters b-g in (a) indicate the position and approximate plane of the sections shown in b-g of a whole-mount-labelled embryo. dB2, dB2 interneuron progenitor region; dmnX, dorsal motor nucleus of the vagus nerve; ENS, enteric nervous system; gVII, geniculate ganglion; gIX/X, petrosal-nodose ganglionic complex; LC, locus coeruleus; MoV, motor nucleus of the trigeminal nerve; nIII/IV, oculomotor and trochlear nuclei; nVII, facial nucleus; nXI, neurons of the XIth (accessory) cranial nerve; nA, nucleus ambiguus; nTS, solitary tract nucleus; PG, pelvic ganglia; SC, sympathetic chain; SCG, superior cervical ganglion; SM, submandibular ganglion; SPG, sphenopalatine ganglion.
We further investigated the extent and specificity of FLPo-mediated recombination on mid- and hindbrain sections of E14.5 P2b::FLPo;RC::Fela embryos that express GFP as lineage tracer after FLPo-mediated recombination only (Fig. 3a–i). Virtually all Phox2b+ cells in nIII and nIV (Fig. 3a and b) and the branchio-visceromotor neurons of the facial nucleus (nVII) (Fig. 3d), the nucleus ambiguus (nA) (Fig. 3e and i) and the dorsal motor nucleus of the vagus nerve (dmnx) (Fig. 3h and i) also expressed GFP. The same was true for the second order sensory neurons of the nTS (Fig. 3h and i). GFP expression was also strongly activated in the trigeminal motor nucleus (MoV) in which Phox2b expression is fading out at this stage (Fig. 3c). Co-expression of GFP and Phox2b in the dB2 progenitor domain (see below) shows that FLPo is already active in ventricular zone progenitors (Fig. 3f and g). GFP was also prominently expressed in the midline raphe probably because of early Phox2b expression in the floorplate from which the midline cells are derived (Fig. 3d and i).
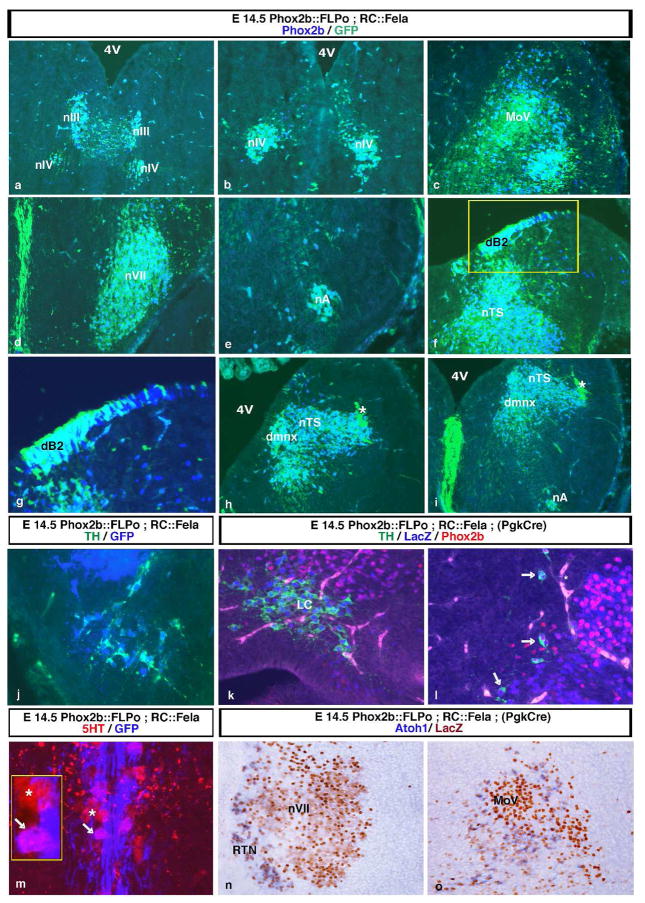
Fig. 3.
Efficient FLP-mediated recombination in neurons that express or have expressed Phox2b. (a–i) Coronal sections through the midbrain (a and b) and hindbrain (c–i) from E14.5 doubly transgenic Phox2b::FLPo;RC::Fela embryos. Shown are images stained with anti-GFP (green) and anti-Phox2b (blue) antibodies. Double-labelled cells are turquoise; cells that have lost Phox2b expression are light green. There are no blue cells that express Phox2b alone. Dorsal is at the top in all panels, the midline at the left in panels c to i. Except for panel g, panels a to i are a rostral to caudal sequence. Virtually all Phox2b+ cells in the nIV, nVII, nA, dmnX, the nTS and the dB2 progenitor domain also express GFP. The MoV, in which Phox2b expression is fading out at this stage, is essentially singly GFP+ (c). The indicator allele is also activated in the Phox2b-negative midline raphe, a derivative of the floor plate that expresses Phox2b at early stages of development (d and i). A higher magnification of the boxed region in panel f demonstrates the complete overlap between GFP and Phox2b expression in the dB2 progenitors (g). The asterisks in h and i mark incoming GFP+ fibers of the solitary tract. In panel h, the choroid plexus is labelled because of blood vessel autofluorescence. 4V, 4th ventricle. (j–l) Activation of RC::Fela expression in all (nor)adrenergic cells identified by TH expression (green). (j) GFP expression (blue) in the neurons of the C1 adrenergic cell group from an E14.5 Phox2b::FLPo;RC::Fela embryo. (k and l) E14.5 Phox2b::FLPo;RC::Fela embryos with germ line excision of the loxP-flanked cassette by maternal expression of Pgk::Cre stained by anti-βgal (blue) and anti-Phox2b antibodies (red) in addition to anti-TH. The locus coeruleus (LC) has lost Phox2b expression at this stage, but shows strong labelling for nβgal (k). The C2 adrenergic cells at the lateral border of the nTS (arrows) that have mostly lost Phox2b expression are nβgal+. (m) Section through the ventral raphe of the caudal hindbrain from an E14.5 Phox2b::FLPo;RC::Fela embryo labelled with anti-5HT (red) and anti-GFP (blue) antibodies. Pink cells, in which GFP expression is activated, are intermingled with red cells that have not recombined the RC::Fela allele. The arrow and the asterisk point at examples of GFP-positive and GFP-negative cells, respectively. A higher magnification of them is shown by the inset. There is strong GFP expression in the midline as in panels d and i. (n) and (o) In situ hybridization with an Atoh1 probe (blue) combined with immunodetection of nβgal (brown) on sections through nVII or MoV from E14.5 Phox2b::FLPo;RC::Fela embryos in which the loxP-flanked cassette has been excised by maternal expression of Pgk::Cre. Virtually all perifacial and peritrigeminal Atoh1-expressing neurons have activated the LacZ gene. RTN, retrotrapezoid nucleus.
RC::Fela expression was also activated in the tyrosine hydroxylase (TH)-positive (nor)adrenergic neurons, all of which express or have expressed Phox2b (Pattyn et al., 2000): the C1 adrenergic cell group, the locus coeruleus, which at this stage has lost Phox2b expression, and the C2 cell group, in which Phox2b was also mostly absent at this stage (Fig. 3j–l). The Phox2b-negative serotonergic (5HT) neurons in the medulla have a history of Phox2b expression since they originate from the Phox2b+ branchio-visceromotor progenitor domain (Pattyn et al., 2003). Accordingly, we observed FLPo-mediated recombination of the indicator allele in medullary 5HT neurons (Fig. 3m), but never in the pons (not shown) where the majority of 5HT neurons originate from progenitors in rhombomere 1 that do not express Phox2b (Jensen et al., 2008; Pattyn et al., 2004). However, activation of GFP expression was incomplete and observed in only about half of the 5HT neurons. Mosaic activation of RC::Fela expression in the visceromotor progenitor domain may be the cause. Alternatively, the time of FLPo expression in visceromotor progenitors may be too short to achieve recombination in early born 5HT neurons.
The retrotrapezoid nucleus (RTN) is a loose collection of neurons that reside close to the medullary surface ventral to nVII and have been found to be crucial for respiratory CO2 chemosensitivity and normal breathing (Goridis et al., 2010; Guyenet et al., 2008). The RTN neurons originate from the dB2 dorsal interneuron progenitors and can be identified by co-expression of Phox2b and Atoh1 (Dubreuil et al., 2009). The only other Atoh1+ cells in the Phox2b lineage are the peritrigeminal neurons of still unknown function that surround MoV (Rose et al., 2009). LacZ expression was strongly activated in both cell groups in P2b::FLPo;RC::Fela mice that have expressed Pgk::Cre in the germline (Fig. 3n and o), showing that the P2b::FLPo allele partnered with a suitable Cre driver such as Atoh1 may be used to track these cells specifically.
As proof-of-principle for the use of the P2b::FLPo line for intersectional genetics, we attempted at labeling a subset of Phox2b-expressing neurons. The progenitor region of the dorsal hindbrain that constitutes the ventricular zone can be subdivided into domains, which give rise to different populations of interneurons, termed dA1-4 and dB1-4 (Sieber et al., 2007). Lbx1 is expressed postmitotically in a major class of dorsal hindbrain interneurons, the class B neurons, which arise from the dB1–dB4 progenitor domains, while Phox2b is expressed postmitotically in the dA3-derived neurons and in the dB2 progenitors and their postmitotic progeny (Sieber et al., 2007). Already in E12.5 P2b::FLPo;RC::Fela embryos, the indicator allele is strongly activated in the Phox2b+ dB2 progenitors and their postmitotic descendants that have left the ventricular zone (Fig. 4a–c). By contrast, the great majority of B class neurons that originate in dB1, dB3 and dB4 do not express Phox2b (Fig. 4d and e) and neither do their progenitors. Since Lbx1 is not expressed in dA-derived neurons, the only neurons having a history of both, Lbx1 and Phox2b expression are those derived from dB2 progenitors. We thus generated triple transgenics that express Lbx1Cre (Sieber et al., 2007) in addition to P2b::FLPo and RC::Fela, in order to track the dB2-derived interneurons by nβgal expression.
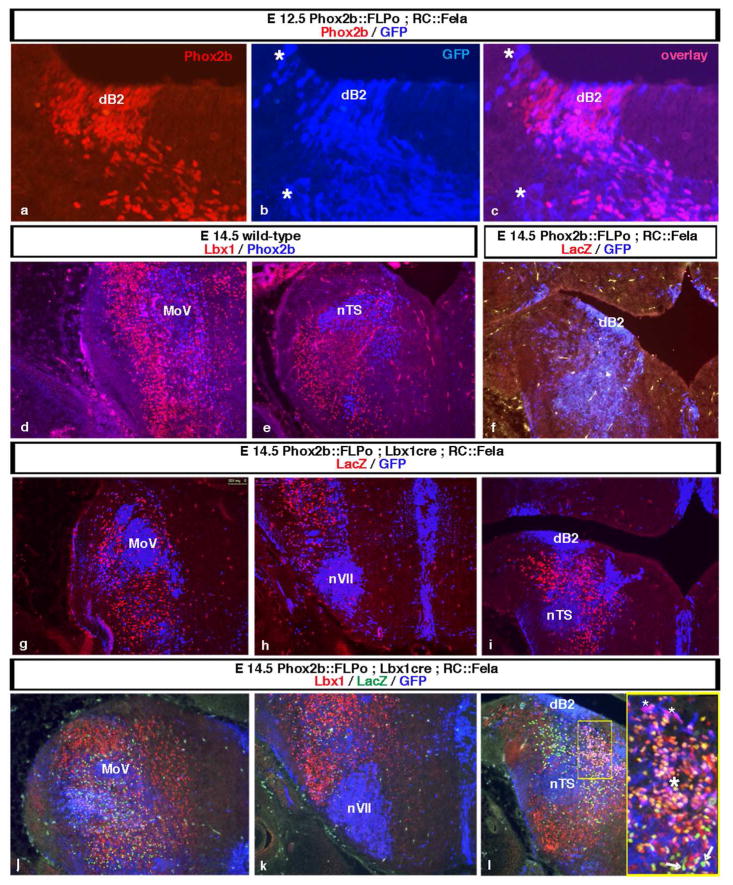
Fig. 4.
Identification of dB2-derived interneurons by intersectional labelling of coronal hindbrain sections from E12.5 (a–c) or E14.5 (d–l) embryos. The midline is at the right and dorsal at the top in all panels. (a–c) In the dB2 progenitor domain from E12.5 Phox2b::FLPo;RC::Fela embryos, the indicator allele (GFP expression in blue) is already strongly activated. Its expression accurately mirrors that of Phox2b (red). The asterisk marks incoming fibers. (d and e) Lbx1-expressing neurons (red) occupy a vast domain of the dorsal hindbrain. The great majority of them are Phox2b (blue)-negative except for cells surrounding and ventral to MoV (pink). (f) In the absence of Cre, GFP (blue), but not LacZ (red) expression is activated in Phox2b::FLPo;RC::Fela control littermates showing that βgal is not produced from RC::Fela that underwent only FLP- but not Cre-mediated recombination. (g–i) Mapping of the dB2-derived interneurons that have a history of both Lbx1 and Phox2b expression in Phox2b::FLPo;Lbx1Cre;RC::Fela triple transgenics. Within the Phox2b lineage, the LacZ gene is activated in the dB2-derived subset detected by anti-βgal antibodies (red) while GFP expression (blue) is activated in cells that have never expressed Lbx1. Remark that the dB2 progenitors in the ventricular zone themselves do not express βgal since Lbx1 is switched on only postmitotically in their descendants. Panels g to i are a rostral to caudal sequence. (j–l) Triple immunostaining for Lbx1 (red), nβgal (green) and GFP (blue). Panels j to l are a rostral to caudal sequence. Most nβgal-expressing cells with a history of both Lbx1 and Phox2b expression that are born in the dB2 domain have lost Lbx1 expression at this stage. The inset in panel i shows a higher magnification of the boxed area. The LacZ gene is activated and GFP expression lost in the dB2 cells as they become post-mitotic, leave the ventricular zone and acquire Lbx1 and thus Cre expression. Pink cells (small asterisks) in the transitional zone have switched on Lbx1 but express still GFP, orange-pink appearing cells (large asterisk) express Lbx1 and have now switched on nβgal expression. During their further outward migration, they become Lbx1-negative and express solely nβgal (green cells, arrows).
There was no βgal expression in control littermates that had not inherited the Lbx1Cre allele (Fig. 4f). By contrast, the intersectional marker nβgal was clearly detected in triple transgenic P2b::FLPo; Lbx1Cre;RC::Fela embryos. The dB2-derived cells in which the lacZ gene has been switched on by the combined action of both FLPo and Cre recombinase could be clearly distinguished from the GFP-expressing cells that had undergone FLPo-mediated recombination only (Fig. 4g–i). Among the Lbx1- or Phox2b- expressing hindbrain neurons, the nβgal+ intersectional population represented a small subpopulation (Fig. 4j–l). The dB2 progenitors themselves also expressed GFP since Lbx1 expression is switched on only postmitotically (Fig. 4i and l). Upon exit from the ventricular zone, the newly born neurons start expressing both, nβgal and Lbx1, but they lose Lbx1 expression as they migrate further away (Fig. 4l). At this stage, Lbx1 has already disappeared from a more laterally located β-gal+ population. In fact, at E14.5, most βgal+ cells have lost Lbx1 expression and could thus not have been identified by double-labelling for Phox2b and Lbx1 (Fig. 4j–l).
The exploration of development and fate of hindbrain interneurons has been hampered by the lack of specific markers, in particular of those that persist at later stages of development. Recombinase-based genetic fate mapping offers a solution by switching on permanent lineage tracers in cell populations that express recombinases in a highly specific manner as defined by transgene regulatory elements. The P2b::FLPo line presented here can be used for intersectional genetic fate mapping when paired with Cre driver lines and a dual-recombinase responsive indicator allele. As shown here at the example of the dB2-derived interneurons, the P2b::FLPo line, if partnered with a suitable Cre driver line, constitutes a powerful tool to define subsets of Phox2b-expressing interneurons.
Defining cell identities based on the expression, past or present, of two separate genes greatly refines cell lineage relationships previously defined only by Cre-based lineage analysis (Dymecki et al., 2010). It also provides a genetic tool to express any gene of interest solely at the intersection of two expression domains thus greatly improving selectivity. In the past, obstacles to the widespread use of FLP driver lines have been the lower efficiency of FLP-mediated compared to Cre-mediated recombination (Raymond and Soriano, 2007) and the paucity of dual recombinase-responsive reporter lines. These obstacles have now been overcome by the development of enhanced (Rodriguez et al., 2000) and then of enhanced and codon-optimized (Raymond and Soriano, 2007) FLP and of indicator lines such as RC::Fela (Jensen et al., 2008). In Phox2b::FLPo;RC::Fela embryos, the indicator gene was activated in all cells that express or have expressed Phox2b or descend from Phox2b+ progenitors. Recombination was essentially complete, except for 5HT neurons, and appeared as efficient as that previously shown for the Phox2b::Cre allele (D’Autreaux et al., 2011). Hence, the Phox2b::FLPo line may serve as a novel tool for studying development and function of neurons that express or have expressed Phox2b.
METHODS
Transgene construction
The FLPo coding sequence was introduced into BAC RP24-95M11 (obtained from the CHORI BACPAC Distribution Center), which contains the Phox2b gene together with 150kb upstream and 50kb downstream sequences, fused to the Phox2b second exon at the same residue as in the Phox2b::Cre construct (D’Autreaux et al., 2011). The Cre coding sequence from the Phox2b::cre transgene was replaced with the FLPo sequence using a bacterial recombineering approach. The FLPo recombineering construct (Fig. 1a) with the added 5′ and 3′ homology arms was assembled by overlap extension PCR (Horton et al., 1993). The sequences to be assembled were amplified from the following templates: the 5′ and 3′ homology arms from RP24-95M11, FLPo from plasmid 13792 (Addgene), SV40polyA from plasmid PRK5 (Addgene) and the kanamycin resistance cassette from pCR4-TOPO (Invitrogen). The primer sequences used are available upon request. The forward primer used to amplify FLPo introduced two glycine residues at the junction with the Phox2b exon to correct the reading frame and interrupt structured domains. After sequence verification, the assembled sequence was electroporated into SW102 bacteria that harboured the Phox2b::Cre construct (D’Autreaux et al., 2011) within BAC RP24-95M11 from which the loxP sites had been removed, providing ampicillin but not kanamycin resistance. The cells that contained the recombined BAC were selected on chloramphenicol/ kanamycin plates and tested for correct recombination by PCR. We verified the integrity of the BAC by restriction enzyme digestion and PCR across multiple sites, followed by sequencing the amplicons. The P2b::FLPo BAC construct was then purified using the Large-Construct Kit from Qiagen.
Mice
The BAC DNA was injected into C57BL/6 X DBA/2 F1 blastocysts by SEAT (CNRS UPS44, Villejuif, France). Two founders that transmitted into the germ line were obtained, one of which (founder 3276) was analysed in this study. The founder lines were maintained by crossing with C57BL/6 X DBA/2 F1 mice. Offspring harbouring the FLPo gene were identified by PCR using the following primers:
Forward primer GAGCTTCGACATCGTGAACA
Reverse primer ACAGGGTCTTGGTCTTG.
The P2b::FLPo mouse line will be available to the academic research community upon acceptance of the manuscript.
Transgenic P2b::FLPo mice were crossed with mice harbouring the RC::Fela indicator allele that expresses GFP after FLP-mediated recombination and nβgal after both, FLP- and Cre-mediated recombination (Fig. 1b). Its efficiency as a dual recombinase responsive indicator has been documented (Jensen et al., 2008). They were then maintained as P2b::FLPo;RC::Fela double heterozygotes. To achieve germline deletion of the loxP-flanked GFP cassette and thus nβgal expression in all cells with a history of Phox2b expression, P2b::FLPo;RC::Fela males were crossed with Pgk::Cre females that will provide Cre activity in all offspring through Cre expression in the female germ line (Lallemand et al., 1998). Lbx1 was used as Cre driver for intersectional labelling of a subset of the Phox2b lineage. The generation and use of Lbx1Cre mice has been described (Sieber et al., 2007). All animal studies were done in accordance with the guidelines issued by the European Community and the French Ministry of Agriculture and have been approved by Direction départementale de la protection des populations de Paris (Service Protection & Santé Animales-Environnement).
Determination of zygosity by real-time quantitative PCR
Pieces of ear obtained using an ear punch were digested overnight at 55°C in lysis buffer (0.1 M Tris HCl, pH 8, 0.5% SDS, 5 mM EDTA, 0.2 mg/ml proteinase K). After inactivation of proteinase K at 85°C for 30 min, the extracts were spun down (5 min at 15,000 g) and the DNA collected from the supernatants by isopropanol precipitation. After a wash in 70% ethanol, the pellet was resuspended in 0.1 M Tris HCl, pH 8 and its concentration adjusted to 40 μg/ml (ND-1000 spectrophotometer, NanoDrop). SYBR Green real-time genomic PCR was done using SYBR Green I Master mixture (Roche) and Light Cycler 480 (Roche). The PCR program was initiated by 95°C for 30 sec, followed by 40 cycles consisting of 95°C for 15 sec, 60°C for 30 sec and 72°C for 20 sec. The generation of specific PCR products was confirmed by melting curve analysis. The following primers were used: for the reference gene β-actin 5′GGCTGTATTCCCCTCCATCG and 5′CCAGTTGGTAACAATGCCATGT, for FLPo 5′CAGCCTGAAGAAGCTGATCC and 5′GACACGATGTCGGTGATGTC. The zygosity of P2b::FLPo transgenics was determined using the 2−ddCt method as described (Sakurai et al., 2008) on 32 μg DNA. The 2−ddCt values were calculated from the dCt of each sample (FLPo-β-actin) using as calibrator DNA from the parents known to be hemizygous. Analysis of 64 μg of parent DNA served to imitate the homozygous state. The values for 64 μg of parent DNA (n=6) ranged from 2.1 to 2.4 as expected, the values for the offspring harbouring the transgene (n=20) from 0.86 to 1.4 (triplicate determinations from 3 separate PCR reactions).
Histology
X-gal staining of whole-mount embryos was done as described (Knittel et al., 1995). After clearing in benzyl alcohol: benzyl benzoate (1:2), 16 μm transverse cryosections were prepared from stained embryos. The methods for immunofluorescence and combined in situ hybridisation and immunohistochemistry on coronal 16 μm cryosections have been described (Hirsch et al., 2007; Tiveron et al., 1996). Riboprobes for Atoh1 were synthesized using a DIG RNA labelling kit (Roche) as specified by the manufacturer. The primary antibodies used were: chicken anti-GFP (Aves), chicken (Aves) or rabbit (Cappel) anti-βgal, rabbit (Pattyn et al., 1997) or guinea pig (Dubreuil et al., 2009) anti-Phox2b, rabbit anti-TH (Millipore) and rabbit anti-5HT (Sigma). They were revealed for fluorescent staining by Alexa 488- (Invitrogen) or by Cy3- or Cy5-labelled (Jackson Immunoresearch) secondary antibodies of the appropriate specificity, for bright field observation by biotin-labelled secondary antibodies and Vectastain ABC kit (Vector) revealed with 3,3′-diaminobenzamide. Pictures were captured with either a Hamamatsu ORCA-ER or a Leica DFC420C camera mounted on a Leica DM5500B microscope for observation through fluorescence or bright field optics, respectively. They were contrast-adjusted and assembled in Adobe Photoshop.
Acknowledgments
Contract grant sponsors: Agence Nationale de la Recherche (to J.-F. B.) and institutional grants from CNRS, INSERM and Ecole normale supérieure.
We thank Carmen Birchmeier for the Lbx1Cre mouse line, Thomas Mueller and Carmen Birchmeier for the anti-Lbx1 antibody and Carmen Le Moal for animal husbandry.
LITERATURE CITED
- Brunet J-F, Goridis C. Phox2b and the homeostatic brain. In: Gaultier C, editor. Genetic basis for respiratory control diseases. New York: Springer; 2008. pp. 25–38. [Google Scholar]
- Brunet J-F, Pattyn A. Phox2 genes - from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- D’Autreaux F, Coppola E, Hirsch MR, Birchmeier C, Brunet J-F. Homeoprotein Phox2b commands a somatic-to-visceral switch in cranial sensory pathways. Proc Natl Acad Sci U S A. 2011;108:20018–20023. doi: 10.1073/pnas.1110416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, Gallego J, Brunet J-F. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet J-F, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymecki SM, Ray RS, Kim JC. Mapping cell fate and function using recombinase-based intersectional strategies. Methods Enzymol. 2010;477:183–213. doi: 10.1016/S0076-6879(10)77011-7. [DOI] [PubMed] [Google Scholar]
- Goridis C, Dubreuil V, Thoby-Brisson M, Fortin G, Brunet JF. Phox2b, congenital central hypoventilation syndrome and the control of respiration. Sem Cell Dev Biol. 2010;21:814–822. doi: 10.1016/j.semcdb.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Retrotrapezoid nucleus and central chemoreception. J Physiol. 2008;586:2043–2048. doi: 10.1113/jphysiol.2008.150870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch MR, Glover JC, Dufour HD, Brunet J-F, Goridis C. Forced expression of Phox2 homeodomain transcription factors induces a branchiovisceromotor axonal phenotype. Dev Biol. 2007;303:687–702. doi: 10.1016/j.ydbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knittel T, Kessel M, Kim MH, Gruss P. A conserved enhancer of the human and murine Hoxa-7 gene specifies the anterior boundary of expression during embryonal development. Development. 1995;121:1077–1088. doi: 10.1242/dev.121.4.1077. [DOI] [PubMed] [Google Scholar]
- Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet J-F. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci. 2000;15:235–243. doi: 10.1006/mcne.1999.0826. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, Brunet J-F. Mash1/Ascl1 is required for the development of central serotonergic neurons. Nat Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet J-F, Ericson J. Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 2003;17:729–737. doi: 10.1101/gad.255803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CS, Soriano P. High-efficiency FLP and PhiC31 site-specific recombination in mammalian cells. PLoS One. 2007;2:e162. doi: 10.1371/journal.pone.0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Rose MF, Ren J, Ahmad KA, Chao HT, Klisch TJ, Flora A, Greer JJ, Zoghbi HY. Math1 is essential for the development of hindbrain neurons critical for perinatal breathing. Neuron. 2009;64:341–354. doi: 10.1016/j.neuron.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Kamiyoshi A, Watanabe S, Sato M, Shindo T. Rapid zygosity determination in mice by SYBR Green real-time genomic PCR of a crude DNA solution. Transgenic Res. 2008;17:149–155. doi: 10.1007/s11248-007-9134-7. [DOI] [PubMed] [Google Scholar]
- Sieber MA, Storm R, Martinez-de-la-Torre M, Muller T, Wende H, Reuter K, Vasyutina E, Birchmeier C. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–4909. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron M-C, Hirsch M-R, Brunet J-F. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]