Abstract
The presence of circulating plasma 17β-estradiol (E2) is beneficial in women against abnormal vascular tone development, such as coronary arterial vasospasms. Several vascular diseases have demonstrated that an increased expression of the sarcoplasmic reticulum Ca2+-ATPase pump (SERCA2b) serves to limit the excessive accumulation of intracellular Ca2+. Therefore, the hypothesis of this study was that E2 would increase SERCA2b expression in the coronary vasculature. Coronary arteries were dissected from hearts obtained from mature female pigs. Artery segments were cultured for 24 hrs in E2 (1 pM or 1 nM) and homogenized for Western blot analysis. E2 (1 nM) induced a ~ 50% increase in the immunoreactivity for SERCA2b. E2 also increased the protein expression of the known SERCA regulatory proteins, protein kinase A (PKA) and protein kinase G (PKG). The E2-induced increase in SERCA2b was attenuated when the culture media was supplemented with the α/β estrogen receptor antagonist, ICI 182,780, and the PKG antagonist, KT5823. The PKA antagonist (KT5720) had no effect on SERCA2b expression. Removal of the endothelium (using a wooden toothpick) decreased the E2-mediated increase in SERCA2b and PKG expression by 45% and 47%, respectively. Overall, these findings suggest that one of the potential cardiovascular benefits of E2 in women is the upregulation of SERCA2b, via the activation of the classical α and β estrogen receptor pathway.
Keywords: sarcoplasmic reticulum, Ca2+-ATPase pump, artery, estrogen, protein kinase G, endothelium
INTRODUCTION
Circulating plasma 17β-estradiol (estrogen; E2) in women reduces abnormal vascular tone development (such as coronary vasospasms) by regulating the mechanisms responsible for controlling the intracellular smooth muscle cell Ca2+ concentration (1, 2). For example, E2 decreases Ca2+ entry into the smooth muscle cells by inhibiting voltage-gated Ca2+ channels (3, 4) and activating Ca2+-activated K+ channels (5, 6). Prakash et al. (7) has suggested that E2 may enhance Ca2+ removal from the cells by stimulating the plasma membrane Ca2+ pump. The sarcoplasmic reticulum Ca2+-ATPase pump (SERCA) also reduces the intracellular Ca2+ concentration by pumping Ca2+ back into the sarcoplasmic reticulum (8, 9); however, it is not known if E2 affects SERCA2 in the coronary vasculature. Therefore, the purpose of this study was to determine if E2 affects the protein expression of SERCA2b in coronary arteries. Several studies have previously found that hearts from OVX animal models have reduced SERCA2a expression and function (10–12). The predominate SERCA isoforms in the myocardium and vasculature are SERCA2a and SERCA2b, respectively (13, 14).
Using cardiomyocytes from OVX rats, Bupha-Intr and Wattanapermpool (10) were the first to demonstrate that estrogen deficiency reduced SERCA2a expression independent of its regulatory protein, phospholamban. This decrease in SERCA2a was reversed with E2 supplementation. Bupha-Intr and Wattanapermpool (10) speculated that the reduced expression of SERCA2a was due to SERCA2a phosphorylation, which would target the protein for degradation. Later, Bupha-Intr et al. (11) showed that moderate exercise could also reverse the OVX-induced decrease in SERCA2a expression in the rat heart. Based upon these studies we hypothesized that E2 would increase SERCA2b expression in coronary arteries from female pigs. We have previously found that SERCA2b activity and expression increased in coronary arteries from diabetic dyslipidemic male pigs to attenuate the elevation in intracellular Ca2+ concentration in vascular smooth muscle cells (8). Subsequently, Witczak et al. (9) showed that when these diabetic dyslipidemic pigs were exercise trained both SERCA2b expression and the basal intracellular Ca2+ levels decreased.
As previously mentioned, in pig coronary arteries an increase in SERCA2b expression is accompanied by an increase in the ability of SERCA2b to sequester Ca2+ into the sarcoplasmic reticulum (SR) (8, 9). Several studies using cardiomyocytes have demonstrated that protein kinase A (15), protein kinase G (16), and/or CaMKII (17) can phosphorylate the SERCA regulatory protein, phospholamban, to increase pump activity. Also, using pig coronary arteries, Grover et al. (18) previously found that CaMKII could directly phosphorylate SERCA2b (independent of phospholamban) to increase its activity. Therefore, in this study we sought to determine if these protein kinases could also influence the expression of SERCA2b.
RESULTS
Effect of estrogen on SERCA2b protein expression
Western blot analysis of a 24 hr exposure to 1 nM of E2 increased (p=0.03) SERCA2b expression (Fig. 1a). In contrast, the vehicle solvent for E2 (ethanol; EtOH; 0.01%), and an E2 concentration of 1 pM had no effect. The densitometric data is expressed as a ratio of the band intensity of artery segments cultured without any EtOH or E2 supplementation (i.e. “untreated” group) for each Western blot. The representative raw Western blot inset in Fig. 1a demonstrates the specificity of the antibody for SERCA2b. As shown in Fig. 1b, the increase in SERCA2b expression by E2 (1 nM) was inhibited (p=0.001) by a 24 hr incubation with the estrogen receptor α/β antagonist, ICI 182,780 (10 μM). ICI 182,780 is also a partial agonist for the G-protein coupled estrogen receptor (GPER) (21, 22). Under our experimental conditions, ICI 182,780 had no effect on SERCA2b expression; there was no difference between arteries incubated in ICI 182,780 in the presence or absence of E2.
Fig. 1.
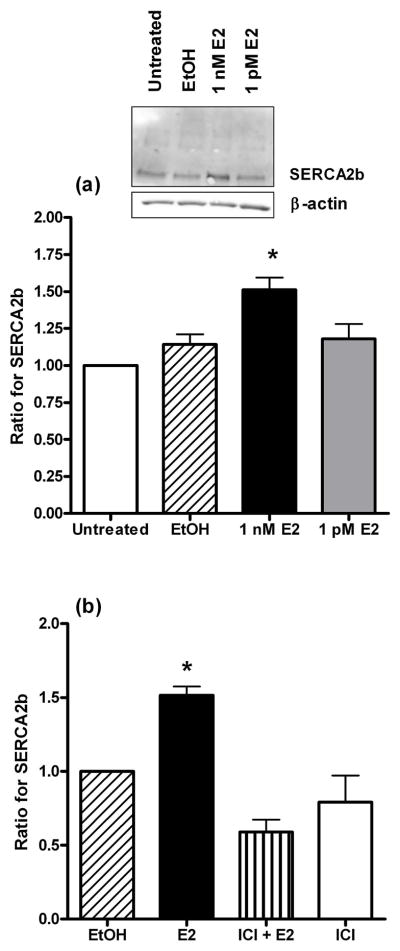
(a) The effect of estrogen on the protein expression of SERCA2b. The data represent the ratio of the band intensity for the EtOH and estrogen (E2) groups compared to the untreated group for each Western blot. The insert shows a representative raw Western blot for SERCA2b and the loading control, β-actin. * indicates P < 0.05, n = 6. (b) SERCA2b expression in the presence of estrogen and the estrogen receptor α/β antagonist, ICI 182,780. The data represent the ratio of the band intensity for the experimental groups compared to the EtOH group for each Western blot. * indicates P < 0.05, n = 4. In both (a) and (b) the endothelium was intact.
The effect of protein kinase A, G, and CaMKII on SERCA2b protein expression
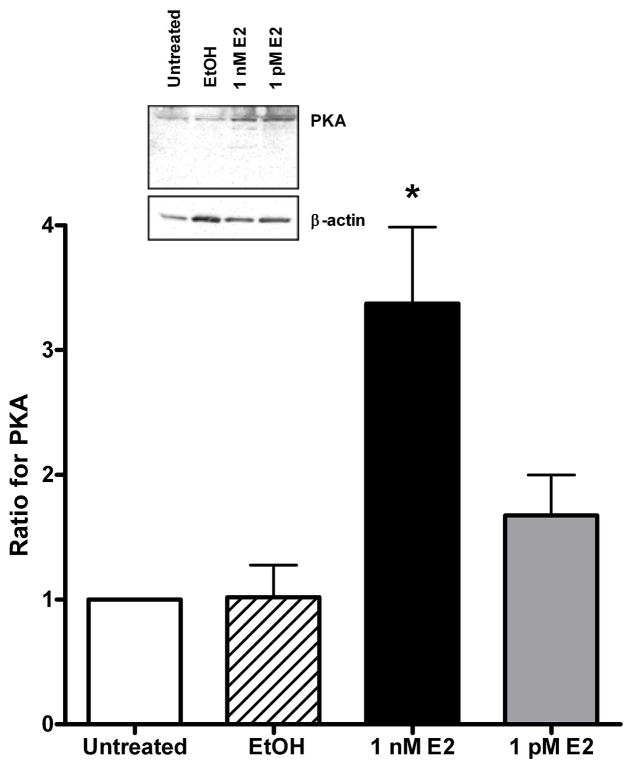
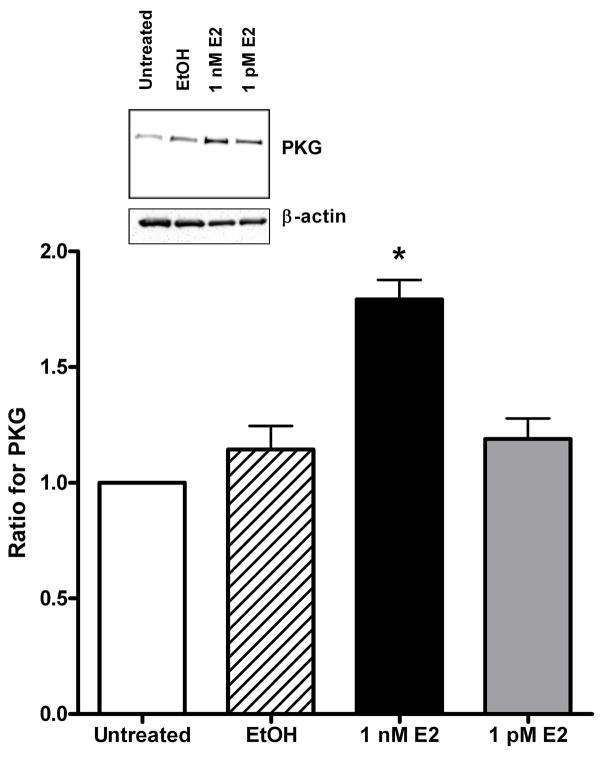
A 24 hr exposure to 1 nM E2 significantly increased the expression of protein kinase A (PKA, Fig. 2) by 330% (p=0.001) compared to the EtOH group. Likewise, E2 increased (p=0.002) PKG expression by 63% (Fig. 3) compared to EtOH treated arteries. Similar to Fig. 1a for SERCA2b, EtOH and 1 pM E2 had no effect on the expression of these protein kinases. There was a slight increase in the expression of Ca2+/calmodulin-dependent protein kinase II (CaMKII), but the results were not significantly different from the EtOH group (p=0.28, n=5, data not shown).
Fig. 2.
The effect of estrogen on protein kinase A (PKA) expression in arteries with an intact endothelium. The data represent the ratio of the band intensity for the EtOH and estrogen (E2) groups compared to the untreated group for each Western blot. The insert shows a representative raw Western blot for PKA and the loading control, β-actin. * indicates P < 0.05, n = 6.
Fig. 3.
Protein kinase G (PKG) expression in the presence of physiological concentrations of estrogen. The data represent the ratio of the band intensity for the EtOH and estrogen (E2) groups compared to the untreated group for each Western blot. The insert shows a representative raw Western blot for PKG and the loading control, β-actin. The data are from endothelial intact arteries. * indicates P < 0.05, n = 5.
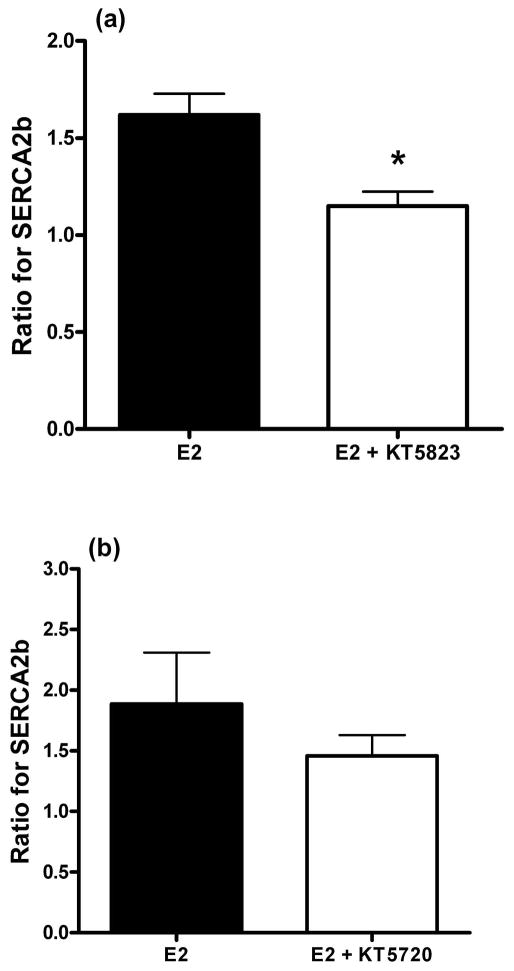
As demonstrated in Fig. 4a, the increased expression of SERCA2b by E2 was attenuated (p=0.007) by the presence of the protein kinase G inhibitor, KT5823 (10 μM). The vehicle solvents for E2 and KT5823 (0.01% EtOH and DMSO, respectively) had no effect on SERCA2b expression (p=0.58, n=6). In contrast, arteries incubated with E2 and the protein kinase A inhibitor, KT5720 (10 μM), did not significantly (p=0.38, n=4) attenuate the E2-induced increase in SERCA2b expression (Fig. 4b). Even though E2 did not significantly increase CaMKII protein expression, the effect of the CaMKII inhibitor, KN93 (10 μM), was still evaluated on SERCA2b expression in the presence of E2. The results indicated there was no effect of KN93 on the increased expression of SERCA2b by E2 (n=5, data not shown). Both KT5720 and KN93 have previously been demonstrated to selectively inhibit kinase activity in arterial smooth muscle (23, 24).
Fig. 4.
(a) Inhibition of protein kinase G (PKG) attenuates the estrogen (E2)-induced increase in SERCA2b. The data represent the ratio of the band intensity for the E2 and E2 + KT5823 groups. (b) The protein kinase A inhibitor, KT5720, did not significantly inhibit the E2-mediated increase in SERCA2b expression. The data represent the ratio of the band intensity for the E2 and E2 + KT5720 groups. For both (a) and (b) there was no difference (P > 0.05) between the vehicle solvents (EtOH and DMSO) for E2 and the kinase inhibitors. Both sets of data are from endothelial intact arteries. * indicates P < 0.05, n = 6.
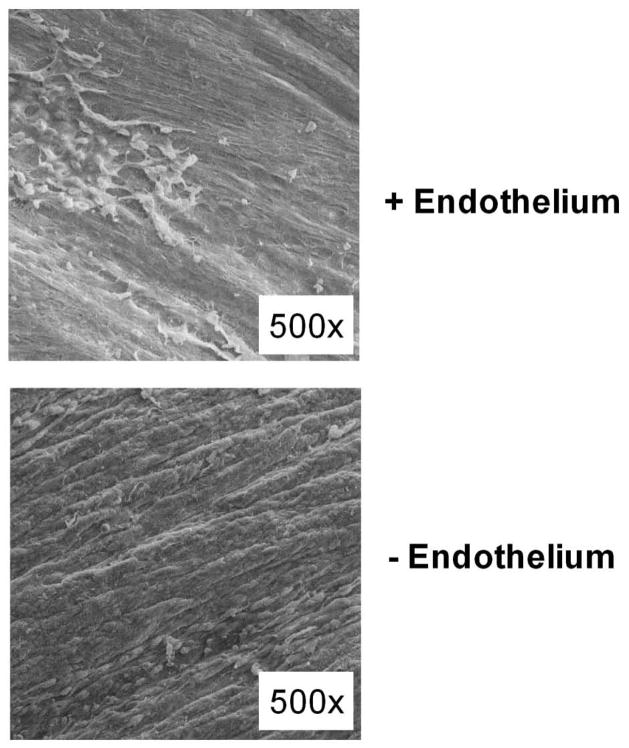
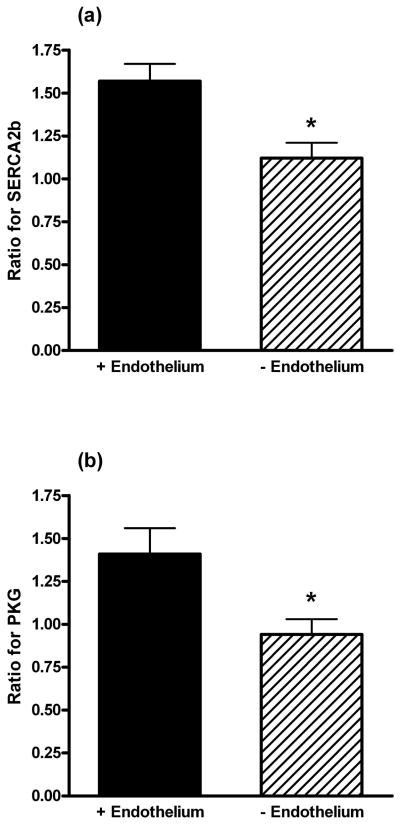
Using scanning electron microscopy, Fig. 5 shows the integrity of the endothelium after rubbing the arterial lumen with a wooden toothpick (n=4). Although not done in this study, we have previously confirmed that rubbing the lumen with a toothpick will prevent the nitric oxide mediated relaxation response induced by bradykinin in pig coronary arteries (19). This type of endothelial damage elicited a decrease in SERCA2b (Fig. 6a) and PKG (Fig. 6b) expression by 45% (p=0.03) and 47% (p=0.04), respectively, compared to an arterial segment with an intact endothelium. The data in Fig. 6 are expressed as a ratio of SERCA2b and PKG expression, respectively, of EtOH treated arteries. The vehicle solvent for E2, 0.01% EtOH, did not have an effect on SERCA2b (p=0.24) and PKG (p=0.92) expression in the presence and absence of an endothelium.
Fig. 5.
Scanning electron micrograph image of the luminal surface of a coronary artery. (A) The arterial endothelium was left intact and several red blood cells are visible. (B) The endothelium was rubbed off using a wooden toothpick and the elongated smooth muscle cells are clearly present.
Fig. 6.
The effect of the endothelium on SERCA2b and protein kinase G (PKG) expression in the presence of estrogen (E2). The data for SERCA2b (a) and PKG (b) represent the ratio of the band intensity for E2 compared to EtOH in the presence and absence of the endothelium. * indicates P < 0.05, n=7 (a) and n=6 (b).
DISCUSSION
Our study suggests that E2 elicits an endothelium-dependent increase in SERCA2b expression in coronary arteries. This SERCA2b increase appears to be mediated through the activation of the classical α and β estrogen receptors. E2 significantly increased the expression of protein kinase A (PKA) and protein kinase G (PKG); however, only the selective inhibition of PKG attenuated the E2-induced increase in SERCA2b expression. These results corroborate previous findings, which suggest that the increase in SERCA2b expression serves to limit the excessive accumulation of intracellular Ca2+ in vascular smooth muscle cells (25). Furthermore, this may contribute to the observation that women have less coronary arterial tone and reactivity than men (26).
We have previously used organ cultured porcine coronary arteries for four days to induce smooth muscle cells into a synthetic phenotype to investigate endothelin-1 and purinergic nucleotide receptor expression (19, 27, 28). However, we cultured coronary arteries for just 24 hours to investigate the effect of E2 exposure on SERCA2b expression. There is a possibility that the 24 hr culture period may have induced some phenotypic change in the smooth muscle cells; however, Throne and Paul (29) demonstrated that the porcine coronary arteries still maintain their normal contractile phenotype after 24 hours in organ culture.
The sows used in this study were retired breeders that weighed ~ 600 lbs and were still cycling (19). Sexually mature sows typically have a circulating E2 concentration around 20 pM (30). In women, the plasma E2 concentration before ovulation is ~ 0.2 nM and at ovulation is ~ 1 nM (31). Therefore, the 1 nM E2 used in this study to induce SERCA2b expression is comparable to a normal ovulatory plasma E2 concentration in women. In our study, incubating the arteries with 1 pM E2 served as an experimental control because it was a lower than normal E2 concentration in both women and sows. As shown in Fig. 1a, SERCA2b expression was not different between 1 pM E2 and the E2 vehicle, EtOH. Many in vitro studies that have investigated the effect of E2 on arteries have used pharmacological concentrations of E2 (32–35). In contrast, this study effectively utilizes an E2 concentration that is closer to physiological levels to induce changes in protein expression.
To our knowledge this is the first study to demonstrate that E2 can selectively increase SERCA2b in the vasculature. Several studies have used ovariectomized rats and found that the gonadal depletion of E2 decreases SERCA2a expression in the heart (10–12). Similar to our findings for SERCA2b, the attenuation of SERCA2a expression in the heart could be reversed to control levels by E2 supplementation (36) or moderate exercise (11, 37). The mechanistic regulation of the SERCA protein has not been clearly established (10), however, this study provides evidence that it is mediated by the α and β estrogen receptors. Besides being an antagonist for the α and β estrogen receptors, ICI 182,780 is also a partial agonist for the G-protein coupled estrogen receptor (GPER). However, ICI 182,780 alone did not affect SERCA2b expression, thus suggesting that GPER does not have a role in the E2-mediated increase in SERCA2b expression.
Because protein kinases may activate cellular transcription factors (such as the cAMP response-element binding protein, CREB) for the gene (i.e. SERCA2) that encodes SERCA2b (38) we desired to determine if the expression of PKA, PKG, and/or CaMKII could be linked to the upregulation of SERCA2b. Even though PKA and PKG were significantly increased by E2, PKG was the primary kinase coupled to SERCA expression. Coincidentally, removal of the endothelium similarly (based upon percent decrease) reduced the expression of SERCA2b and PKG, thus suggesting that the upregulation of SERCA is endothelium-dependent. Cohen et al. (39) initially showed the functional relationship between SERCA and the endothelium by demonstrating that endothelial-derived nitric oxide could induce arterial relaxation by stimulating SERCA2b activity. Several studies have subsequently demonstrated that E2 can enhance the arterial relaxation response by stimulating endothelial nitric oxide synthase (eNOS) activity and the release of nitric oxide (40, 41). Moreover, nitric oxide is known to stimulate cGMP and PKG formation (16). Although endothelium removal decreased SERCA2b expression to control levels, additional studies will be needed to determine exactly how the endothelium regulates SERCA2b expression.
Although there was an increase in PKA expression, the kinase did not appear to have a significant effect on SERCA2b protein expression. However, this does not discount that the increased expression of this kinase by E2 can have beneficial effects on the coronary vasculature. The goal of this study was not to determine the overall cadioprotective benefits of PKA and CaMKII; however, other investigators have found that the expression and activation of these two kinases can serve as a compensatory response to hyperpolarize the plasma membrane and decrease vascular tone. For example, Valero et al. (42) demonstrated that the β estrogen receptor induces arterial relaxation through the PKA activation of potassium channels. In the coronary vasculature, PKA can decrease vascular tone via the stimulation of Ca2+-activated and ATP-sensitive K+ channels (43). CaMKII has been implicated in the activation of voltage-activated and Ca2+-activated Cl− channels (44). In ventricular myocytes, CaMKII has even been shown to increase SERCA2a activity under conditions that induce cellular acidosis (45).
When used at high concentrations, the PKG and PKA inhibitors (KT5823 and KT5720, respectively) may lose some of their selectivity and inhibit additional protein kinases. However, the potential loss of specificity for KT5823 and KT5720 at 10 μM has not been found to inhibit those additional kinases involved with SERCA2b function (46, 47). Additionally, Taylor et al. (48) demonstrated that 10 μM KT5823 was needed to maximally dilate cGMP-simulated PKG activity in cerebral arteries. In this study we did not assess the potential effect of KT5823 alone on SERCA2b expression; instead we determined the inhibitory effect of KT5823 against the E2-mediated increase in SERCA2b expression. However, previous reports have suggested that KT5823 alone has similar effects as the controls when evaluating the cGMP/PKG pathway in arterial endothelial and smooth muscle cells (48–50).
In conclusion, the extrapolation of our in vitro results using porcine coronary arteries to human females suggest that the elevated expression of SERCA2b is likely to occur in those premenopausal women with a “healthy” endothelium, and thus, free of vascular disease (e.g. hypertension, atherosclerosis). This could potentially protect the coronary vasculature from any excessive accumulation of intracellular Ca2+ and the resultant development of abnormal coronary arterial tone.
METHODS
Tissue Preparation and Culture
Hearts from female Yorkshire pigs (3–4 years of age) were obtained from a local packing plant. The use of this animal model in our lab has been previously described (19). The right coronary artery was immediately dissected from the hearts in a cold, modified low Ca2+ Krebs buffer (in mM): 138 NaCl, 5 KCl, 0.2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose, with a pH of 7.4. The distal end (3 cm in length) of the artery was cut into four longitudinal strips (it is difficult to longitudinally cut more than four sections from the distal end of an artery). Each strip was placed in a separate petri dish and incubated (5% CO2 at 37°C) for 24 hrs in RPMI 1640 phenol-free culture media (Sigma, St. Louis, MO) containing an antibiotic/antimycotic solution (Sigma). When desired, the culture media was supplemented with 17β-estradiol (E2, Sigma) and protein kinase inhibitors against PKA (KT5720; EMD Millipore, Billerica, MA), PKG (KT5823; EMD Millipore), and CaMKII (KN93; Tocris Bioscience, Minneapolis, MN). The vehicle solvents used for E2 and the kinase inhibitors, respectively, were ethanol (EtOH, 0.01%) and dimethyl sulfoxide (DMSO, 0.01%). Similarly, the E2 antagonist used was ICI 182,780 (Tocris Cookson, Ellsville, MO) and its solvent was EtOH (0.01%).
To evaluate the effect of the endothelium, the lumen of the distal end of the artery was opened and longitudinally sectioned into two strips. The luminal side of one piece was rubbed using a wooden toothpick, while the endothelium was left intact on the other arterial piece. Each piece (without or with an endothelium) was further sectioned into two strips, whereby each strip was cultured for 24 hrs in E2 or EtOH.
Immunoblots
After 24 hrs, the arterial strips were rinsed in a Krebs low Ca2+ buffer solution and homogenized in a lysis buffer having the following composition: 50 mM HEPES, 150 mM NaCl, 1 mM EGTA, 1.5 mM MgCl2, 1% Triton-X, 10% Glycerol, 0.1 mM phenylmethylsulfonylfluoride (Sigma), 5 mg/mL leupeptin (Sigma), 5 mg/mL antipapin (Sigma), and 5 mg/mL aprotinin (Sigma). The whole cell homogenate protein for each treatment group was quantitated using the Bradford Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Equivalent amounts of protein for each sample were run on 4–15% polyacrylamide gradient gels and electroblotted to a PVDF membrane. The membrane was blocked for at least 60 min in 5% non-fat dry milk dissolved in a Tris-buffered saline containing 0.1% Tween 20 (TBST). The membrane was then incubated in the appropriate primary antibody solution for 60 minutes: anti-SECRA2b, 1:500 (Affinity Bioreagents, Golden, CO); anti-PKG, 1:200 (Stressgen Bioreagents, Ann Arbor, MI); anti-PKA, 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-CaMKII 1:100 (Abcam, Cambridge, MA). An anti-β-actin antibody (1:1000, Abcam) was used as the internal standard. The membrane was washed three times (10 min each) using TBST and incubated with the appropriate horseradish peroxidase (HRP) secondary antibody for 60 min. The respective secondary IgG antibodies used against the primary for SERCA2b, PKG, PKA, and CaMKII were: goat anti-mouse, 1:1000 (Santa Cruz); goat anti-rabbit, 1:1000 (Santa Cruz); donkey anti-goat. 1:1000; goat anti-rabbit, 1:1000 (Santa Cruz). The membrane was subsequently washed three times (10 min each) using TBST. Chemiluminescence was used to detect each HRP labeled antibody using the FluorChem (FC2) AlphaImager Documentation and Analysis System (Alpha Innotech Corp., San Leandro, CA). Band densitometry was analyzed using the AlphaImager and normalized to β-actin.
Scanning Electron Microscopy
The distal end (3 cm in length) of each artery was opened longitudinally and fixed with 5% glutaraldehyde for 60 min. It was then post-fixed using 4% osmium tetroxide (OsO4) for an additional 60 min. The specimens were dehydrated using ascending ratios of acetone/ethanol (1:1, 2:1, 4:1) followed by descending ratios of acetone/hexamethyldisilazane (4:1, 2:1, 1:1). They were then dried using a critical point dryer and sputter coated with gold particles. The luminal surface was observed using a PSEM II 2000 (ASPEX Corp., Delmont, PA) scanning electron microscope as previously described (20).
Data Analysis
The resulting bands on the immunoblots were visualized using the FluorChem (FC2) digital imager. Bands were analyzed using the spot densitometry program associated with the AlphaImager Analysis System. The intensity of each band is expressed as a ratio of the control group for each blot. Statistical analysis was conducted using SigmaStat 3.5 (SyStat Software, Point Richmond, CA) and significance was defined at P≤0.05. When evaluating two groups, a t-test was used. To assess the significance between more than two groups the data was analyzed using a one-way ANOVA followed by Bonferroni’s post-hoc analysis. Data are expressed as the mean±S.E.M. for the number (n) of pigs used.
Acknowledgments
This study was supported by grants from the National Center for Research Resources (5P20RR016460-11) and the National Institute of General Medical Sciences (8 P20 GM103429-11) from the National Institutes of Health. Undergraduate students at the University of Central Arkansas involved in this study were Cristian Bratu-Ene, Andreya Reed, Stuart Sherwood, Lauren Sideroff, and Tiffany Mattingly. We appreciate Odom’s Tennessee Pride Sausage, Inc. (Little Rock, Arkansas) and the United States Department of Agriculture for their donation of the pig hearts.
Footnotes
The authors have no conflict of interest with this study.
References
- 1.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R49. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 2.Gilligan DM, Quyyumi AA, Cannon RO., III Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–51. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich ND, Koschak A, MacLeod KT. Oestrogen directly inhibits the cardiovascular L-type Ca2+ channel Cav1. 2. Biochemical and Biophysical Research Communications. 2007;361:522–7. doi: 10.1016/j.bbrc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 4.Crews JK, Murphy JG, Khalil RA. Gender differences in Ca(2+) entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34:931–6. doi: 10.1161/01.hyp.34.4.931. [DOI] [PubMed] [Google Scholar]
- 5.White RE, Han G, Maunz M, et al. Endothelium-independent effect of estrogen on Ca(2+)-activated K(+) channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–61. doi: 10.1016/s0008-6363(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 6.Yu X, Ma H, Barman SA, et al. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am J Physiol Endocrinol Metab. 2011;301:E882–8. doi: 10.1152/ajpendo.00037.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash YS, Togaibayeva AA, Kannan MS, Miller VM, Fitzpatrick LA, Sieck GC. Estrogen increases Ca2+ efflux from female porcine coronary arterial smooth muscle. Am J Physiol. 1999;276:H926–H34. doi: 10.1152/ajpheart.1999.276.3.H926. [DOI] [PubMed] [Google Scholar]
- 8.Hill BJ, Price EM, Dixon JL, Sturek M. Increased calcium buffering in coronary smooth muscle cells from diabetic dyslipidemic pigs. Atherosclerosis. 2003;167:15–23. doi: 10.1016/s0021-9150(02)00381-7. [DOI] [PubMed] [Google Scholar]
- 9.Witczak CA, Wamhoff BR, Sturek M. Exercise training prevents Ca2+ dysregulation in coronary smooth muscle from diabetic dyslipidemic yucatan swine. J Appl Physiol. 2006;101:752–62. doi: 10.1152/japplphysiol.00235.2006. [DOI] [PubMed] [Google Scholar]
- 10.Bupha-Intr T, Wattanapermpool J. Regulatory role of ovarian sex hormones in calcium uptake activity of cardiac sarcoplasmic reticulum. Am J Physiol Heart Circ Physiol. 2006;291:H1101–8. doi: 10.1152/ajpheart.00660.2005. [DOI] [PubMed] [Google Scholar]
- 11.Bupha-Intr T, Laosiripisan J, Wattanapermpool J. Moderate intensity of regular exercise improves cardiac SR Ca2+ uptake activity in ovariectomized rats. J Appl Physiol. 2009;107:1105–12. doi: 10.1152/japplphysiol.00407.2009. [DOI] [PubMed] [Google Scholar]
- 12.Paigel AS, Ribeiro RF, Jr, Fernandes AA, Targueta GP, Vassallo DV, Stefanon I. Myocardial contractility is preserved early but reduced late after ovariectomy in young female rats. Reprod Biol Endocrinol. 2011;9:54. doi: 10.1186/1477-7827-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggermont JA, Wuytack F, Verbist J, Casteels R. Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J. 1990;271:649–53. doi: 10.1042/bj2710649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–73. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 15.Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989;264:11468–74. [PubMed] [Google Scholar]
- 16.Lincoln TM, Dey N, Sellak H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol. 2001;91:1421–30. doi: 10.1152/jappl.2001.91.3.1421. [DOI] [PubMed] [Google Scholar]
- 17.Jiang M, Xu A, Narayanan N. Thyroid hormone downregulates the expression and function of sarcoplasmic reticulum-associated CaM kinase II in the rabbit heart. Am J Physiol Heart Circ Physiol. 2006;291:H1384–94. doi: 10.1152/ajpheart.00875.2005. [DOI] [PubMed] [Google Scholar]
- 18.Grover AK, Xu A, Samson SE, Narayanan N. Sarcoplasmic reticulum Ca 2+ pump in pig coronary artery smooth muscle is regulated by a novel pathway. Am J Physiol Cell Physiol. 1996;271:C181–C7. doi: 10.1152/ajpcell.1996.271.1.C181. [DOI] [PubMed] [Google Scholar]
- 19.Tummala S, Hill BJ. The enhanced endothelin-1-induced contraction in cultured coronary arteries from mature female pigs is not antagonized by 17beta-estradiol. Vascul Pharmacol. 2007;46:346–52. doi: 10.1016/j.vph.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta R, Chowdhury P, Ali N. Scanning electron microscope studies of bone samples: Influence of simulated microgravity. Nucl Instrum Meth B. 2007;261:908–12. [Google Scholar]
- 21.Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol. 2000;278:C537–45. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- 24.Keung W, Vanhoutte PM, Man RY. Acute impairment of contractile responses by 17beta-estradiol is cAMP and protein kinase G dependent in vascular smooth muscle cells of the porcine coronary arteries. Br J Pharmacol. 2005;144:71–9. doi: 10.1038/sj.bjp.0706018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturek M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J Appl Physiol. 2011;111:573–86. doi: 10.1152/japplphysiol.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momen A, Gao Z, Cohen A, Khan T, Leuenberger UA, Sinoway LI. Coronary vasoconstrictor responses are attenuated in young women as compared with age-matched men. J Physiol. 2010;588:4007–16. doi: 10.1113/jphysiol.2010.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill BJF, Wamhoff BR, Sturek M. Functional nucleotide receptor and sarcoplasmic reticulum morphology of dedifferentiated porcine coronary smooth muscle cells. Journal of Vascular Research. 2001;38:432–43. doi: 10.1159/000051076. [DOI] [PubMed] [Google Scholar]
- 28.Hill BJF, Sturek M. Pharmological characterization of a UTP-sensitive P2Y nucleotide receptor in organ cultured coronary arteries. Vascul Pharmacol. 2001;39:83–8. doi: 10.1016/s1537-1891(02)00306-3. [DOI] [PubMed] [Google Scholar]
- 29.Thorne GD, Paul RJ. Effects of organ culture on arterial gene expression and hypoxic relaxation: role of the ryanodine receptor. Am J Physiol Cell Physiol. 2003;284:C999–C1005. doi: 10.1152/ajpcell.00158.2002. [DOI] [PubMed] [Google Scholar]
- 30.Chatrath R, Ronningen KL, LaBreche P, et al. Effect of puberty on coronary arteries from female pigs. J Appl Physiol. 2003;95:1672–80. doi: 10.1152/japplphysiol.00099.2003. [DOI] [PubMed] [Google Scholar]
- 31.Marsh EE, Shaw ND, Klingman KM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 2011;96:3199–206. doi: 10.1210/jc.2011-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han SZ, Karaki H, Ouchi Y, Akishita M, Orimo H. 17 beta-Estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995;91:2619–26. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- 33.Salom JB, Burguete MC, Perez-Asensio FJ, Centeno JM, Torregrosa G, Alborch E. Acute relaxant effects of 17-beta-estradiol through non-genomic mechanisms in rabbit carotid artery. Steroids. 2002;67:339–46. doi: 10.1016/s0039-128x(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Mohamed G, Elmarakby A, Carrier GO, Catravas JD, Caldwell RW, White RE. Estradiol relaxes rat aorta via endothelium-dependent and -independent mechanisms. Pharmacology. 2003;69:20–6. doi: 10.1159/000071268. [DOI] [PubMed] [Google Scholar]
- 35.Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 -estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293:H3713–9. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- 36.Chu SH, Goldspink P, Kowalski J, Beck J, Schwertz DW. Effect of estrogen on calcium-handling proteins, beta-adrenergic receptors, and function in rat heart. Life Sci. 2006;79:1257–67. doi: 10.1016/j.lfs.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 37.Anwar A, Schluter KD, Heger J, Piper HM, Euler G. Enhanced SERCA2A expression improves contractile performance of ventricular cardiomyocytes of rat under adrenergic stimulation. Pflugers Arch. 2008;457:485–91. doi: 10.1007/s00424-008-0520-7. [DOI] [PubMed] [Google Scholar]
- 38.Eyster KM, Mark CJ, Gayle R, Martin DS. The effects of estrogen and testosterone on gene expression in the rat mesenteric arteries. Vascul Pharmacol. 2007;47:238–47. doi: 10.1016/j.vph.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res. 1999;84:210–9. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- 40.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circ Res. 1996;79:1024–30. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 41.Levy AS, Chung JC, Kroetsch JT, Rush JW. Nitric oxide and coronary vascular endothelium adaptations in hypertension. Vasc Health Risk Manag. 2009;5:1075–87. doi: 10.2147/vhrm.s7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valero MS, Pereboom D, Barcelo-Batllory S, Brines L, Garay RP, Alda JO. Protein kinase A signalling is involved in the relaxant responses to the selective beta-oestrogen receptor agonist diarylpropionitrile in rat aortic smooth muscle in vitro. J Pharm Pharmacol. 2011;63:222–9. doi: 10.1111/j.2042-7158.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- 43.Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507 (Pt 1):117–29. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenwood IA, Ledoux J, Leblanc N. Differential regulation of Ca(2+)-activated Cl(−) currents in rabbit arterial and portal vein smooth muscle cells by Ca(2+)-calmodulin-dependent kinase. J Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomura N, Satoh H, Terada H, Matsunaga M, Watanabe H, Hayashi H. CaMKII-dependent reactivation of SR Ca(2+) uptake and contractile recovery during intracellular acidosis. Am J Physiol Heart Circ Physiol. 2002;283:H193–203. doi: 10.1152/ajpheart.00026.2001. [DOI] [PubMed] [Google Scholar]
- 46.Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–97. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- 47.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor MS, Okwuchukwuasanya C, Nickl CK, Tegge W, Brayden JE, Dostmann WR. Inhibition of cGMP-dependent protein kinase by the cell-permeable peptide DT-2 reveals a novel mechanism of vasoregulation. Mol Pharmacol. 2004;65:1111–9. doi: 10.1124/mol.65.5.1111. [DOI] [PubMed] [Google Scholar]
- 49.Wu BN, Chen CF, Hong YR, Howng SL, Lin YL, Chen IJ. Activation of BKCa channels via cyclic AMP- and cyclic GMP-dependent protein kinases by eugenosedin-A in rat basilar artery myocytes. Br J Pharmacol. 2007;152:374–85. doi: 10.1038/sj.bjp.0707406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasseckert SA, Schafer C, Kluger A, et al. Stimulation of cGMP signalling protects coronary endothelium against reperfusion-induced intercellular gap formation. Cardiovasc Res. 2009;83:381–7. doi: 10.1093/cvr/cvp065. [DOI] [PubMed] [Google Scholar]