Abstract
BACKGROUND
The proportion of screening colonoscopic examinations performed by a physician that detect one or more adenomas (the adenoma detection rate) is a recommended quality measure. However, little is known about the association between this rate and patients’ risks of a subsequent colorectal cancer (interval cancer) and death.
METHODS
Using data from an integrated health care delivery organization, we evaluated the associations between the adenoma detection rate and the risks of colorectal cancer diagnosed 6 months to 10 years after colonoscopy and of cancer-related death. With the use of Cox regression, our estimates of attributable risk were adjusted for the demographic characteristics of the patients, indications for colonoscopy, and coexisting conditions.
RESULTS
We evaluated 314,872 colonoscopies performed by 136 gastroenterologists; the adenoma detection rates ranged from 7.4 to 52.5%. During the follow-up period, we identified 712 interval colorectal adenocarcinomas, including 255 advanced-stage cancers, and 147 deaths from interval colorectal cancer. The unadjusted risks of interval cancer according to quintiles of adenoma detection rates, from lowest to highest, were 9.8, 8.6, 8.0, 7.0, and 4.8 cases per 10,000 person-years of follow-up, respectively. Among patients of physicians with adenoma detection rates in the highest quintile, as compared with patients of physicians with detection rates in the lowest quintile, the adjusted hazard ratio for any interval cancer was 0.52 (95% confidence interval [CI], 0.39 to 0.69), for advanced-stage interval cancer, 0.43 (95% CI, 0.29 to 0.64), and for fatal interval cancer, 0.38 (95% CI, 0.22 to 0.65). Each 1.0% increase in the adenoma detection rate was associated with a 3.0% decrease in the risk of cancer (hazard ratio, 0.97; 95% CI, 0.96 to 0.98).
CONCLUSIONS
The adenoma detection rate was inversely associated with the risks of interval colorectal cancer, advanced-stage interval cancer, and fatal interval cancer. (Funded by the Kaiser Permanente Community Benefit program and the National Cancer Institute.)
Colonoscopy is a commonly used primary or follow-up screening test to detect colorectal cancer,1-3 the second leading cause of death from cancer in the United States.4,5 Colonoscopy can reduce the risk of death from colorectal cancer through detection of tumors at an earlier, more treatable stage and through removal of precancerous adenomas.3,6 Conversely, failure to detect adenomas during colonoscopy may increase the subsequent risk of cancer.
The adenoma detection rate, the proportion of screening colonoscopies performed by a physician that detect at least one histologically confirmed colorectal adenoma or adenocarcinoma, has been recommended as a quality benchmark by specialty societies7 and has been recently proposed by the Centers for Medicare and Medicaid Services as a reportable quality measure. Currently, professional societies recommend adenoma detection rates of 15% or higher for female patients and 25% or higher for male patients as indicators of adequate colonoscopy quality, although data are lacking to validate these thresholds.7 Adenoma detection rates vary widely among providers in both academic and community settings8-20; however, the adenoma detection rate has not been well validated with respect to its use in predicting the risk of important outcomes such as the development of colorectal cancer after colonoscopy (hereafter referred to as interval cancer), advanced-stage interval cancer, and fatal interval cancer. A study of 42 interval cancers identified over a period of 5 years showed an association between the colonoscopy-related adenoma detection rate (<20% vs. ≥20%) and the risk of interval cancer.21
We evaluated the relationship between the adenoma detection rates in a large group of endoscopists in a community-based setting and their patients’ risks of interval colorectal cancer, advanced-stage interval cancer, and fatal interval cancer.
METHODS
STUDY POPULATION AND DATA SOURCES
The patients in the study were members of Kaiser Permanente Northern California, an integrated health services organization that serves approximately 3.3 million people annually at 17 medical centers. Demographic characteristics of the members closely approximate those of the region’s diverse population (when compared with the regional demographic characteristics estimated by the decennial census). The patients were enrolled in Medicare, Medicaid, or a commercial insurance plan.22,23 We obtained data from electronic records on the patients’ sex, age, race or ethnic group, family history of colorectal cancer, and Charlson comorbidity index score (calculated for the year before the index colonoscopy). The Charlson index predicts the influence of coexisting diseases on mortality; higher scores indicate the presence of more (or more severe) coexisting conditions.24
Patients were included in the study if they had undergone a colonoscopy between January 1, 1998, and December 31, 2010; were 50 years of age or older at the time of the colonoscopic examination; and had at least 6 months of subsequent follow-up. The gastroenterologists who performed the colonoscopies had to have completed 300 or more total colonoscopic examinations and 75 or more screening examinations during the study period.
Patients were followed from the date of the colonoscopy to the first of the following events: completion of 10 years of follow-up, diagnosis of a colorectal adenocarcinoma, discontinuation of membership in Kaiser Permanente Northern California, or the end of the follow-up period (December 31, 2010).
STUDY OVERSIGHT
The study was approved by the institutional review board of Kaiser Permanente Northern California, which waived the requirement for informed consent. The listed authors had sole responsibility for the study design, data collection, decision to submit the manuscript for publication, and drafting of the manuscript. This study was conducted within the National Cancer Institute (NCI) Cancer Research Network and as part of the NCI-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium. The PROSPR consortium conducts multisite, coordinated, transdisciplinary research to evaluate and improve cancer-screening processes.
COLONOSCOPIC EXAMINATIONS
Colonoscopies were identified from electronic records with the use of Current Procedural Terminology codes, and adenomas detected at colonoscopy were identified with the use of Systematized Nomenclature of Medicine codes.25 We used an algorithm that incorporated data from electronic consultation records, clinical diagnostic codes from the International Classification of Diseases, 9th revision, and laboratory, pathological, and radiologic tests to categorize the indication for colonoscopy as screening, surveillance, or diagnosis. A screening examination was defined as a colonoscopy for which there was no surveillance or diagnostic indication. A surveillance examination was defined as a colonoscopy for which there was no diagnostic indication and which was performed in a patient who had undergone a colonoscopy within the previous 10 years or a sigmoidoscopy within the previous 5 years or who had a history of polyps or colorectal cancer. A diagnostic indication was assigned if the patient had one or more of the following findings before the colonoscopy: a positive test for hemoglobin in stool within the past year; a gastrointestinal condition, such as abdominal pain, iron-deficiency anemia, gastrointestinal bleeding, unexplained weight loss, a change in bowel habits, an abnormal finding on abdominal imaging, a polyp detected by means of flexible sigmoidos-copy, or diverticulitis, within the past 6 months; or a diagnosis of inflammatory bowel disease within the past 10 years. Validation studies confirmed high levels of sensitivity and accuracy for capture of colonoscopic examinations, indications for colonoscopy, detection of cancers, and detection of adenomas.26
CANCER OUTCOMES
An interval cancer was defined as a colorectal cancer diagnosed between 6 months and 10 years after colonoscopy; cancers diagnosed within 6 months after colonoscopy were considered to be “detected” by the index examinations. Early interval cancers were those diagnosed between 6 and 35 months after the colonoscopy; delayed cancers were those diagnosed 3 or more years after the colonoscopy. The risk of interval cancer among patients who underwent more than one colonos-copy was assigned to the physician who performed the most recent colonoscopy.
Data on the diagnosis and stage of colorectal adenocarcinoma were obtained from the Kaiser Permanente Northern California and California cancer registries, which are part of the Surveillance, Epidemiology, and End Results (SEER) registry. Advanced-stage cancers were defined as stage III (regional disease with spread to the regional lymph nodes only) or stage IV (distant metastasis) according to the American Joint Committee on Cancer staging system; for patients who did not undergo such staging, advanced-stage cancers were defined as code 3 (disease in the regional lymph nodes), code 4 (regional disease with direct extension and spread to the regional lymph nodes), or code 7 (distant metastasis) according to the SEER Program Coding and Staging Manual 2013.27 Proximal cancers were those in the cecum, ascending colon, hepatic flexure, and transverse colon; distal cancers were those in the splenic flexure, descending colon, sigmoid colon, and rectum. The underlying causes of death were obtained from cancer-registry and state mortality files.
STATISTICAL ANALYSIS
We examined whether physicians’ adenoma detection rates, categorized either in quintiles or as a continuous variable, predicted patients’ risk of interval colorectal cancer. We used multilevel Cox proportional-hazards regression, adjusting for patient sex and age, Charlson comorbidity index score, and indication for colonoscopy; the race or ethnic group of the patients did not substantially alter physicians’ adenoma detection rates and was not included in the final model. We accounted for within-physician clustering and within-patient correlation using marginal modeling, with a robust sandwich estimate of the covariance matrix.28 Thus, for each physician, we used a standardized exposure (i.e., the adenoma detection rate for screening examinations) to predict their patients’ outcomes for all colonoscopies, irrespective of indications, after balancing for potential differences in their patient populations. We tested for non-proportionality of covariates using time–covariate interaction terms in the main model. There was heterogeneity in the effect over time for the age of the patient (≤85 years vs. >85 years) and the indication for diagnostic examination but not for the adenoma detection rate; inclusion of time–covariate interaction terms had no effect on hazard-ratio estimates. Thus, we included only main-effect terms in our final model.
The change in the potential number of interval cancers, from the lowest to the highest quintile of adenoma detection rates, was analogous to the number needed to treat and was calculated as 1 divided by the risk difference between quintiles 1 and 5. The risk difference was estimated with the use of the following equation: risk difference = incidence − (hazard ratio × incidence), where hazard ratio is the adjusted hazard ratio for quintile 5 relative to quintile 1, and incidence is the unadjusted incidence per 10 years of follow-up after colonoscopy in the lowest-performing quintile.
Analyses were performed with the use of SAS software, version 9.3 (SAS Institute), and Stata software, version 10.1 (StataCorp).
RESULTS
COLONOSCOPIES AND PHYSICIAN CHARACTERISTICS
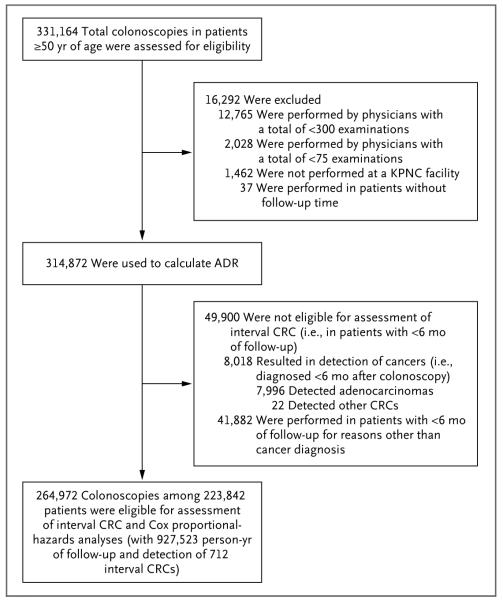
We identified 331,164 eligible colonoscopic examinations during the study period; exclusion of 12,765 colonoscopies by endoscopists who had performed fewer than 300 total examinations, 2028 by providers who had performed fewer than 75 screening examinations, 1462 that were not performed at Kaiser Permanente facilities, and 37 performed in patients with no follow-up time yielded 314,872 examinations, 8730 colorectal cancers, and 136 gastroenterologists for the calculation of adenoma detection rates. Exclusion of 8018 examinations in which a cancer was detected within 6 months after colonoscopy and an additional 41,882 examinations with less than 6 months of follow-up data resulted in 264,972 colonoscopies among 223,842 patients, with 712 interval colorectal adenocarcinomas (8.2% of all colorectal cancers) and 927,523 person-years of follow-up for the analyses of interval cancer (Table 1 and Fig. 1, and Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The median interval between the index examination and the diagnosis of an interval cancer was 39 months.
Table 1.
Characteristics of the Patients and Physicians.
| Characteristic | All Colonoscopies (N = 314,872) |
Interval Colorectal Cancer (N = 712)* |
|---|---|---|
| Patients | ||
| Sex — no. (%) | ||
| Female | 164,711 (52.3) | 344 (48.3) |
| Male | 150,161 (47.7) | 368 (51.7) |
| Race or ethnic group — no. (%)† | ||
| Non-Hispanic white | 205,827 (65.4) | 541 (76.0) |
| Hispanic | 28,209 (9.0) | 55 (7.7) |
| Black | 20,608 (6.5) | 46 (6.5) |
| Asian or Pacific Islander | 35,531 (11.3) | 41 (5.8) |
| Native American | 1,365 (0.4) | 2 (0.3) |
| Mixed or other | 10,293 (3.3) | 24 (3.4) |
| Unknown | 13,039 (4.1) | 3 (0.4) |
| Age — yr | ||
| Median | 64 | 69 |
| Interquartile range | 57–72 | 63–77 |
| Charlson comorbidity index score‡ | ||
| Median | 0 | 0 |
| Interquartile range | 0–1 | 0–1 |
| Indication for colonoscopy — no. (%)§ | ||
| Screening | 57,588 (18.3) | 90 (12.6) |
| Surveillance | 76,418 (24.3) | 232 (32.6) |
| Diagnosis | 180,866 (57.4) | 390 (54.8) |
| Location of interval colorectal cancer — no. (%) | ||
| Distal colon | 267 (37.5) | |
| Proximal colon | 427 (60.0) | |
| Unknown | 18 (2.5) | |
| Follow-up time between last colonoscopy and data censoring — mo¶ | ||
| Median | 35 | 39 |
| Interquartile range | 19–59 | 21–64 |
| Physicians | ||
| Total — no. (%) | 136 (100) | |
| Year of graduation from medical school | ||
| Median | 1989 | |
| Interquartile range | 1980–1997 | |
| Adenoma detection rate quintile — no. (%)∥ | ||
| Quintile 1: 16.56% (7.35–19.05%) | 27 (19.9) | |
| Quintile 2: 21.50% (19.06–23.85%) | 27 (19.9) | |
| Quintile 3: 25.70% (23.86–28.40%) | 28 (20.6) | |
| Quintile 4: 30.96% (28.41–33.50%) | 27 (19.9) | |
| Quintile 5: 38.86% (33.51–52.51%) | 27 (19.9) |
Interval cancers were defined as colorectal adenocarcinomas diagnosed between 6 months and 10 years after the index colonoscopy.
Information about race or ethnic group was derived from numerous sources, including medical records (physician report), patient self-report on questionnaires, and cancer registries.
Scores on the Charlson comorbidity index, a validated method of classifying prognosis according to coexisting conditions in longitudinal studies, range from 0 to 33, with higher scores indicating a greater burden of coexisting conditions.
For patients with interval cancers, the indication for colonoscopy was the same as the indication for the colonoscopy that preceded the diagnosis of the interval cancer.
Follow-up time is shown for the 264,972 colonoscopic examinations included in Cox analyses. Patients were followed from the date of the colonoscopy to the first of the following events: completion of 10 years of follow-up, diagnosis of a colorectal adenocarcinoma, discontinuation of membership in Kaiser Permanente Northern California, or the end of follow-up (December 31, 2010).
Quintiles are expressed as medians and ranges.
Figure 1. Colonoscopic Examinations Performed by 136 Gastroenterologists.
ADR denotes adenoma detection rate, CRC colorectal cancer, and KPNC Kaiser Permanente Northern California.
Characteristics of the physicians are shown in Table 1, and in Table S1 in the Supplementary Appendix. The average number of colonoscopies performed by an individual physician was 2150 (range, 355 to 6005); adenoma detection rates ranged from 7.4 to 52.5% (9.7 to 60.5% for male patients and 3.9 to 45.9% for female patients). There was a strong correlation between the adenoma detection rates based on screening examinations alone and those based only on diagnostic examinations (r = 0.75, P<0.001) or only on surveillance examinations (r = 0.72, P<0.001).
RISK OF INTERVAL COLORECTAL CANCER
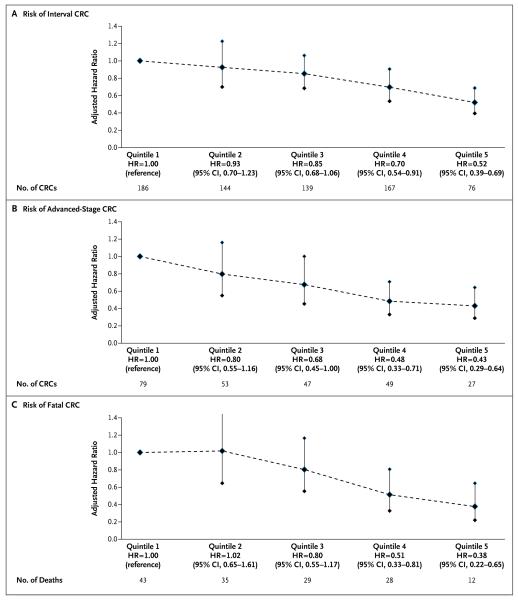
The unadjusted risks of interval colorectal cancer according to quintiles of adenoma detection rates, from lowest to highest, were 9.8, 8.6, 8.0, 7.0, and 4.8 cases per 10,000 person-years of follow-up, respectively. For patients of physicians with adenoma detection rates in the highest quintile, as compared with patients of physicians with rates in the lowest quintile, the risk of receiving a diagnosis of an interval cancer was 0.52 (95% confidence interval [CI], 0.39 to 0.69), after adjustment for covariates (Table 2 and Fig. 2A). The risk of interval cancer decreased approximately linearly with increasing adenoma detection rates, without evidence of a threshold effect within the observed range of rates. With the adenoma detection rate modeled as a continuous variable, each 1.0% increase in the rate predicted a 3.0% decrease in the risk of interval cancer (hazard ratio, 0.97; 95% CI, 0.96 to 0.98).
Table 2.
Adenoma Detection Rate and Risk of an Interval Colorectal Cancer among All Patients.
| Adenoma Detection Rate |
Interval
Cancer |
Hazard Ratio
(95% CI) * |
Unadjusted
Risk |
|---|---|---|---|
|
no. of
cases |
no. of cases/
10,000 person-yr |
||
| Continuous rate | 712 | 0.97 (0.96–0.98) | 7.7 |
| Rate quintile | |||
| Quintile 1: 7.35–19.05% | 186 | 1.00 (reference) | 9.8 |
| Quintile 2: 19.06–23.85% | 144 | 0.93 (0.70–1.23) | 8.6 |
| Quintile 3: 23.86–28.40% | 139 | 0.85 (0.68–1.06) | 8.0 |
| Quintile 4: 28.41–33.50% | 167 | 0.70 (0.54–0.91) | 7.0 |
| Quintile 5: 33.51–52.51% | 76 | 0.52 (0.39–0.69) | 4.8 |
Hazard ratios were adjusted for age, Charlson comorbidity score, sex (in the model including both men and women), and indication for colonoscopy, with clustering according to physician.
Figure 2. Hazard Ratios for Colorectal Cancer, According to Quintile of Adenoma Detection Rates.
Data were adjusted for sex, age, Charlson comorbidity index score, and indication for colonoscopy, with clustering according to physician. Vertical lines indicate 95% confidence intervals. HR denotes hazard ratio.
The results were similar in an analysis restricted to examinations involving persons with at least 2 years of follow-up data (hazard ratio for an interval cancer, 0.97; 95% CI, 0.96 to 0.99; P<0.001) and separate analyses of screening colonoscopies (hazard ratio, 0.97; 95% CI, 0.95 to 1.00; P = 0.05), diagnostic colonoscopies (hazard ratio, 0.97; 95% CI, 0.96 to 0.98; P<0.001), and surveillance colonoscopies (hazard ratio, 0.98; 95% CI, 0.97 to 0.99; P = 0.02). The unadjusted risks according to the most recent indication for colonoscopy were 5.0 cases of interval cancer per 10,000 person-years of follow-up for screening, 8.9 cases per 10,000 person-years for surveillance, and 8.0 cases per 10,000 person-years for diagnostic examinations. The adjusted hazard ratio of 0.52 suggests that physicians who increase their adenoma detection rate from less than 19% (the lowest quintile) to 34 to 53% (the highest quintile) might prevent 1 additional interval cancer over the next 10 years for every 213 colonos-copies performed.
The association between the adenoma detection rate and the risk of interval cancer was observed both among women (hazard ratio with quintile 5 vs. quintile 1, 0.43; 95% CI, 0.28 to 0.66) and among men (hazard ratio, 0.60; 95% CI, 0.42 to 0.88). There was no significant interaction according to sex (P = 0.23) (Table S2 in the Supplementary Appendix).
There was an inverse association between the adenoma detection rate and the subsequent risks of cancer in the proximal colon (hazard ratio with quintile 5 vs. quintile 1, 0.49; 95% CI, 0.35 to 0.69), cancer in the distal colon (hazard ratio, 0.55; 95% CI, 0.39 to 0.79), early cancer (hazard ratio, 0.40; 95% CI, 0.23 to 0.68), and delayed cancer (hazard ratio, 0.61; 95% CI, 0.39 to 0.96) (Table S2 in the Supplementary Appendix).
RISK OF ADVANCED-STAGE AND FATAL INTERVAL CANCERS
For patients of physicians with rates in the highest quintile of adenoma detection rates, as compared with patients of physicians with rates in the lowest quintile, the risk of receiving a diagnosis of advanced-stage interval colorectal cancer was reduced by 57% (hazard ratio, 0.43; 95% CI, 0.29 to 0.64) (Fig. 2B) and the risk of a fatal interval colorectal cancer was reduced by 62% (hazard ratio, 0.38; 95% CI, 0.22 to 0.65) (Fig. 2C). Each 1% increase in the adenoma detection rate was associated with a 5% decrease in the risk of a fatal interval colorectal cancer (hazard ratio, 0.95; 95% CI, 0.94 to 0.97) within the observed range of adenoma detection rates.
DISCUSSION
A physician’s adenoma detection rate, derived from screening examinations, is recommended as a measure of the quality of colonoscopic examination, but the relationship of the adenoma detection rate to the subsequent risks of colorectal cancer and death has been unclear. Our study, which involved 136 gastroenterologists, more than 250,000 colonoscopies, and 712 cases of interval cancer diagnosed during a 10-year follow-up period, showed a strong inverse association between the adenoma detection rate and the risk of interval cancer in a dose-dependent fashion, within the observed range of adenoma detection rates. Higher detection rates also predicted decreased risks of advanced-stage disease, fatal interval cancer, both early and delayed interval cancers, and cancers in both the proximal and distal colon. In separate analyses according to the indication for colonoscopy (i.e., screening, surveillance, or diagnosis), higher adenoma detection rates also predicted decreased risks of interval cancers.
The current study adds to our knowledge of how variation in the performance of colonoscopy may influence its effectiveness with respect to cancer detection and prevention. A prior study of 42 interval cancers in a Polish screening program also evaluated the relationship between the adenoma detection rate for colonoscopy and the risk of interval cancer; that study showed that adenoma detection rates of less than 20% and rates of 20% or higher differed in predicting the risk of interval cancer.21 Although the study represented an important advance, it was limited by a relatively small number of cancers, with no data on deaths, a maximum follow-up of 5 years, and a low percentage of physicians with an adenoma detection rate of 20% or higher (14%, vs. >80% for physicians in the current study).21 Two additional studies have evaluated the relationship between the rate of colonoscopic polypectomy (without pathological classification of polyps) and the risk of interval cancers over a 3-year period: one study showed an inverse association29; the other study showed an inverse association only for cancer in the proximal colon.30 The polypectomy rate alone, however, is difficult to use as a quality metric because of the potential subjectivity of assigning polyp status in the absence of histo-logic findings.30 Finally, a recent study showed an inverse relationship between the adenoma detection rate with flexible sigmoidoscopy and the risk of an interval cancer in the distal colon.31
The association between the adenoma detection rate and the risk of interval cancer is presumed to be due to the enhanced detection of precancerous adenomas. However, other factors such as the completeness of polyp removal may be involved.32 If physicians with lower adenoma detection rates are also less likely to completely resect polyps, the increase in the subsequent risk of cancer among their patients may be due in part to incomplete resection rather than decreased detection alone. Higher adenoma detection rates may also lead to more frequent surveillance. However, increased detection of asymptomatic cancers (and, therefore, total interval cancers) might be expected in patients undergoing enhanced surveillance. In contrast, we found fewer interval cancers in patients of physicians with higher adenoma detection rates.
The current analyses also add to our knowledge about the timing of interval cancers: two thirds of such cancers in our study were diagnosed more than 3 years after the index colonos-copy, and we would expect this proportion to be even larger if all the patients had undergone 10 years of follow-up. Previous studies have focused primarily on cancers occurring within 3 to 5 years after the colonoscopic examination; rapidly progressing adenomas or cancers that were present but not detected at the time of colonoscopy would presumably account for these interval cancers.15,21,29,30,33-41 The number of cancers detected in this study is similar to or lower than the numbers observed in other studies (which often evaluated cancers over a shorter period).29,30 Interval cancers accounted for only 8.2% of all colorectal cancers detected in our study cohort, and the absolute risk of an interval cancer was relatively low (9.8 interval cancers per 10,000 person-years of follow-up in quintile 1 of adenoma detection rates and 4.8 interval cancers per 10,000 person-years of follow-up in quintile 5). A preliminary modeling study has suggested that these differences in detection may influence the effectiveness of colonoscopic screening programs.42
Possible limitations of our study include an inability to adjust for other risk factors (e.g., bowel preparation and the extent of the colonoscopic examination) and an inability to compare procedure-related complications among quintiles of adenoma detection rates. A prior study based on a manual (not electronic) record review of data from Kaiser Permanente Northern California showed perforation rates of 1.1 per 1000 colonoscopies that included biopsy or polypectomy and 0.6 per 1000 colonoscopies that did not include biopsy or polypectomy. There were also 4.8 occurrences of postprocedure bleeding per 1000 colonoscopies that included biopsy or polypectomy.43
In conclusion, in a large community-based U.S. population across multiple medical centers, physicians’ adenoma detection rates were inversely related to the risk of interval colorectal cancer, including advanced-stage cancer and fatal interval colorectal cancer, among patients with up to 10 years of follow-up. This association was approximately linear across quintiles of adenoma detection rates in our population and was observed for male and female patients, cancers in the proximal and distal colon, and early and delayed interval cancers. These findings support the validity of the adenoma detection rate as a quality measure of physicians’ performance of colonoscopy in community practice.44 Studies to determine whether improving the adenoma detection rate leads to improved outcomes are warranted.20
Supplementary Material
Acknowledgments
Supported by grants from the Kaiser Permanente Community Benefit program and the National Cancer Institute (U54 CA163262 [PROSPR] and U24 CA171524).
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 3.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 8.Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst. 1990;82:1769–72. doi: 10.1093/jnci/82.22.1769. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 10.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126:1247–56. doi: 10.1053/j.gastro.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 12.Bretthauer M, Skovlund E, Grotmol T, et al. Inter-endoscopist variation in polyp and neoplasia pick-up rates in flexible sigmoidoscopy screening for colorectal cancer. Scand J Gastroenterol. 2003;38:1268–74. doi: 10.1080/00365520310006513. [DOI] [PubMed] [Google Scholar]
- 13.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonos-copy. Am J Gastroenterol. 2007;102:856–61. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez W, Harewood GC, Petersen BT. Evaluation of polyp detection in relation to procedure time of screening or surveillance colonoscopy. Am J Gastroenterol. 2004;99:1941–5. doi: 10.1111/j.1572-0241.2004.40569.x. [DOI] [PubMed] [Google Scholar]
- 15.Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right-sided colon cancer: a population-based analysis. Gastroenterology. 2004;127:452–6. doi: 10.1053/j.gastro.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Hosokawa O, Shirasaki S, Kaizaki Y, Hayashi H, Douden K, Hattori M. Invasive colorectal cancer detected up to 3 years after a colonoscopy negative for cancer. Endoscopy. 2003;35:506–10. doi: 10.1055/s-2003-39665. [DOI] [PubMed] [Google Scholar]
- 17.Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36:499–503. doi: 10.1055/s-2004-814399. [DOI] [PubMed] [Google Scholar]
- 18.Schoen RE, Pinsky PF, Weissfeld JL, et al. Results of repeat sigmoidoscopy 3 years after a negative examination. JAMA. 2003;290:41–8. doi: 10.1001/jama.290.16.2123-b. [DOI] [PubMed] [Google Scholar]
- 19.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656–65. doi: 10.1016/j.gie.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon NP. How does the adult Kaiser Permanente membership in northern California Compare with the larger community? Kaiser Permanente Division of Research; Oakland, CA: 2006. [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.CPT (current procedural terminology) 2011: professional edition. American Medical Association Press; Chicago: 2010. [Google Scholar]
- 26.Corley DA, Jensen CD, Marks AR, et al. Variation of adenoma prevalence by age, sex, race, and colon location in a large population: implications for screening and quality programs. Clin Gastroenterol Hepatol. 2013;11:172–80. doi: 10.1016/j.cgh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamo MB, Johnson CH, Ruhl JL, Dickie LA, editors. 2013 SEER program coding and staging manual. National Cancer Institute; Bethesda, MD: 2013. http://seer.cancer.gov/manuals/2013/SPCSM_2013_maindoc.pdf. [Google Scholar]
- 28.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–73. [Google Scholar]
- 29.Cooper GS, Xu F, Barnholtz Sloan JS, Schluchter MD, Koroukian SM. Prevalence and predictors of interval colorectal cancers in Medicare beneficiaries. Cancer. 2012;118:3044–52. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with post-colonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Rogal SS, Pinsky PF, Schoen RE. Relationship between detection of adenomas by flexible sigmoidoscopy and interval distal colorectal cancer. Clin Gastroenterol Hepatol. 2013;11:73–8. doi: 10.1016/j.cgh.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy — results of the Complete Adenoma Resection (CARE) study. Gastroenterology. 2013;144(1):74.e1–80.e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Wang YR, Cangemi JR, Loftus EV, Jr, Picco MF. Rate of early/missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. Am J Gastroenterol. 2013;108:444–9. doi: 10.1038/ajg.2012.429. [DOI] [PubMed] [Google Scholar]
- 35.Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology. 1997;112:17–23. doi: 10.1016/s0016-5085(97)70213-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/ missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588–96. doi: 10.1038/ajg.2010.390. [DOI] [PubMed] [Google Scholar]
- 37.Bressler B, Paszat LF, Chen Z, Roth-well DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259–64. doi: 10.1016/j.cgh.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med. 2008;359:1218–24. doi: 10.1056/NEJMoa0803597. [DOI] [PubMed] [Google Scholar]; N Engl J Med. 2009;361:2004. Erratum. [Google Scholar]
- 41.Nakao SK, Fassler S, Sucandy I, Kim S, Zebley DM. Colorectal cancer following negative colonoscopy: is 5-year screening the correct interval to recommend? Surg Endosc. 2013;27:768–73. doi: 10.1007/s00464-012-2543-6. [DOI] [PubMed] [Google Scholar]
- 42.Meester RG, Corley DA, Doubeni CA, et al. Impact of variation in screening colonos-copy quality on the prevention of colorectal cancer deaths: a modelling study. Gastroenterology. 2013;144(Suppl):S190. abstract. [Google Scholar]
- 43.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 44.Hewett DG, Kahi CJ, Rex DK. Efficacy and effectiveness of colonoscopy: how do we bridge the gap? Gastrointest Endosc Clin N Am. 2010;20:673–84. doi: 10.1016/j.giec.2010.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.