Summary
Al3+ tolerance of barley was enhanced in transgenic plants by expression of the SbMATE gene from sorghum and the FRD3 gene from Arabidopsis, which increased citrate efflux in roots.
Key words: Acid soil, cereal, citrate, Hordeum vulgare, MATE transporters, resistance, root exudates, transgenic.
Abstract
Malate and citrate efflux from root apices is a mechanism of Al3+ tolerance in many plant species. Citrate efflux is facilitated by members of the MATE (multidrug and toxic compound exudation) family localized to the plasma membrane of root cells. Barley (Hordeum vulgare) is among the most Al3+-sensitive cereal species but the small genotypic variation in tolerance that is present is correlated with citrate efflux via a MATE transporter named HvAACT1. This study used a biotechnological approach to increase the Al3+ tolerance of barley by transforming it with two MATE genes that encode citrate transporters: SbMATE is the major Al3+-tolerance gene from sorghum whereas FRD3 is involved with Fe nutrition in Arabidopsis. Independent transgenic and null T3 lines were generated for both transgenes. Lines expressing SbMATE showed Al3+-activated citrate efflux from root apices and greater tolerance to Al3+ toxicity than nulls in hydroponic and short-term soil trials. Transgenic lines expressing FRD3 exhibited similar phenotypes except citrate release from roots occurred constitutively. The Al3+ tolerance of these lines was compared with previously generated transgenic barley lines overexpressing the endogenous HvAACT1 gene and the TaALMT1 gene from wheat. Barley lines expressing TaALMT1 showed significantly greater Al3+ tolerance than all lines expressing MATE genes. This study highlights the relative efficacy of different organic anion transport proteins for increasing the Al3+ tolerance of an important crop species.
Introduction
The prevalence of toxic aluminium cations (Al3+) in acid soils (pH <5.0) is a major limitation to crop production around the world (Kochian et al., 2004, 2005). Soluble Al3+ rapidly inhibits root growth by damaging interactions at the growing root apices (Ryan et al., 1993; Sivaguru and Horst, 1998). This reduces the ability of roots to penetrate the soil and absorb water and nutrients. Some plant species have evolved mechanisms to combat this stress which either exclude Al3+ from the growing root apices or safely accommodate Al3+ once it enters the cytosol and efficiently repair stress-induced damage. One exclusion mechanism that has been described in a wide range of species relies on the release of organic anions, such as citrate and malate, from roots (Miyasaka et al., 1991; Delhaize et al., 1993; Ma et al., 2001). These anions can form strong complexes with metal ions and it is hypothesized they protect cells at the growing root apex by chelating toxic Al3+ cations in the apoplasm and rhizosphere (Kinraide et al., 2005).
The first gene identified in plants that was able to explain genotypic variation in Al3+ tolerance was TaALMT1 (aluminium-activated anion transporter) from wheat (Triticum aestivum). TaALMT1 encodes an Al3+-activated anion channel that facilitates malate efflux in roots (Sasaki et al., 2004; Pineros et al., 2008a; Zhang et al., 2008). Other members of the ALMT family perform similar transport functions in Arabidopsis thaliana (Hoekenga et al., 2006), Brassica napus (Ligaba et al., 2006) and rye (Secale cereale) (Collins et al., 2008). The release of citrate from roots is mediated by different transporters from the MATE (multidrug and toxic compound extrusion) family. The first MATE genes involved in Al3+ tolerance were identified by mapped-based cloning in barley (Hordeum vulgare) (Furukawa et al., 2007) and sorghum (Sorghum bicolor) (Magalhaes et al., 2007). The aluminium-activated citrate transporter gene (HvAACT1) in barley is constitutively expressed in the root apices, whereas SbMATE expression in sorghum is induced by Al3+ treatment over several days. However, in both cases, relative tolerance to Al3+ toxicity among different genotypes of barley and sorghum is highly correlated with the level of expression of these genes. Furthermore, these plants need to be exposed to Al3+ for citrate efflux to occur indicating that an interaction, either direct or indirect, between Al3+ and the MATE proteins is required to activate their function. Heterologous expression of HvAACT1 in tobacco (Nicotiana tabacum) (Furukawa et al., 2007) and SbMATE in an Al3+-sensitive mutant Arabidopsis line (Magalhaes et al., 2007) increased the tolerance of these plants to Al3+ stress. Expression of SbMATE in wheat also increased the tolerance of transgenic T1 wheat lines but the phenotype proved unstable and was lost in subsequent generations (Magalhaes et al., 2007; L.V. Kochian, personal communication). MATE genes were later linked with Al3+ tolerance in other species including Arabidopsis, maize (Zea mays), wheat, rice (Oryza sativa), and rice bean (Vigna umbellata) (Liu et al., 2009; Ryan et al., 2009, 2011; Yang et al., 2011; Yokosho et al., 2011; Maron et al., 2013; Tovkach et al., 2013). Most of these MATE proteins also require soluble Al3+ to activate their function by mechanisms that remain unclear. Two exceptions to this pattern include TaMATE1 in wheat and VuMATE1 from rice bean because both these proteins release citrate in the absence of Al3+. Interestingly, VuMATE1 expression is still induced by Al3+ treatment (Yang et al., 2011) whereas TaMATE1 is expressed constitutively (Ryan et al., 2009, 2011).
FRD3 is a member of the MATE family of proteins in Arabidopsis which transports citrate but not for Al3+ tolerance. Instead, FRD3 is expressed in the xylem paremchyma of roots where it transports citrate into the xylem to facilitate iron movement to the shoots in the transpiration stream (Durrett et al., 2007). Nevertheless, when overexpressed in Arabidopsis with the 35SCaMV promoter, FRD3 confers citrate efflux in roots and enhanced Al3+ tolerance compared to control plants. The FRD3 protein does not require Al3+ to activate its function so efflux in the roots of the transgenic Arabidopsis lines is constitutive. This contrasts with the MATE transporters from barley and sorghum, which are both activated by Al3+.
Although variation in HvAACT1 expression in barley largely accounts for the genotypic variation in Al3+ tolerance, this species remains one of the most sensitive of agriculturally important grasses. Furthermore, there appears to be little potential for improvement in barley using conventional breeding methods beyond the levels currently provided by HvAACT1 (Minella and Sorrells, 1992). Biotechnology has provided alternative strategies for increasing the basal tolerance of barley. Indeed, Al3+ tolerance has been improved in transgenic plants by increasing the expression of endogenous genes such as HvAACT1 (Zhou et al., 2013) and HvALMT1 (Gruber et al., 2011), and by heterologous expression of TaALMT1 from wheat (Delhaize et al., 2004) and the thioredoxin gene (PTrx) from Phalaris coerulescensi (Li et al., 2010). Transgenic barley lines expressing TaALMT1 also show improved phosphate uptake and grain yield when grown on an acid soil with low levels of plant-available phosphorus, which could largely be attributed to improved root growth (Delhaize et al., 2009). There is no a priori reason to predict whether or not similar MATE genes from other species can confer even stronger phenotypes. Therefore, in the present study, barley was transformed with two MATE genes with contrasting characteristics to determine whether they could increase citrate release from roots and tolerance to Al3+ toxicity in hydroponics and in acid soil. The tolerance of these lines was compared with previously generated transgenic barely lines expressing the organic anion transporter proteins TaALMT1 and HvAACT1.
Materials and Methods
Plant materials and plasmid vectors
The Al3+-sensitive barley cv. Golden Promise was used in the transformation experiments and cv. Dayton was included as an Al3+-tolerant control. cDNAs for SbMATE and FRD3 were inserted into the pWBVec8 binary vector (Wang et al., 1998) where expression of the transgenes is driven by the maize ubiquitin promoter (Schunmann et al., 2003). The pWBVec8::SbMATE and pWBVec8::FRD3 vectors were introduced into Agrobacterium by triparental mating (Wise et al., 2006).
Barley transformation
Barley was transformed using the Agrobacterium method as described by Tingay et al. (1997). Primary transgenic plants (T0) transformed with SbMATE were analysed for the presence of the transgene using the following primers: forward 5′-GTCACCACGTCGTTCGTC-3′ and reverse 5′-GGGTGCAGATCTGGAAGG-3′. Four independent T1 transgenic lines (SbMATE:T1_9A, SbMATE:T1_22, SbMATE:T1_100E, and SbMATE:T1_133) exhibiting higher citrate efflux in root tips than wild-type plants were selected to generate T3 families, and from these putative homozygous and null lines were selected using PCR. Primary transgenic plants (T0) transformed with FRD3 were tested for the presence of the transgene with the following primers: forward 5′-GCCCATGTCATTTCTCAGTACTTCA-3′ and reverse 5′-TTCCAAACTGCAAATCCCCGAAG-3′. Eight T1 lines were tested for citrate efflux, and two with the highest fluxes (FRD3:T1_40 and FRD3:T1_55) were selected to generate T3 families. Putative homozygous and null lines were selected for each using PCR.
Quantitative reverse-transcription PCR
Three biological replicates each consisting of eight root apices (~5mm) were collected from seedlings and total RNA was extracted using a RNeasy Minikit (Qiagen) with DNAase treatment. First-strand cDNA was synthesized using 1 μg total RNA, 1× RT buffer, 10mM each dNTP, 500ng oligo(dT)15 primer, 0.2M dithiothreitol and 1 unit SuperScript II Reverse Transcriptase (Invitrogen). Reactions were incubated at 25 °C for 5min and then at 42 °C for 60min, followed by a RNaseH degradation step at 37 °C for 30min. Real-time PCR was performed in a Rotor-Gene 3000 Real Time Cycler (Corbett Research, Australia) using 10 μl reaction mixture containing 4.5 μl cDNA diluted to 1:20, 5 µl SYBR Green JumpStart Taq ReadyMix (Sigma), and 0.5 μl of 10 pmol μ l–1 each primer. The barley endogenous actin gene (forward: 5′-GACTCTGGTGATGGTGTCAGC-3′, reverse: 5′-GGCTGGAAGAGGACCTCAGG-3′) was used to normalize the transgene expression level. Primers used to measure SbMATE expression were 5′-ACCTGATAACGCTGATAATGCTGAG and 5′-CAGCAGAAGGAATCCGCATCC-3′ and for FRD3 were 5′-GCCCATGTCATTTCTCAGTACTTCA-3′ and 5′-TTCCAAACTGCAAATCCCCGAAG-3′.
Measurements of citrate and malate efflux
Citrate efflux from excised root apices was measured as described by Wang et al. (2007) and Zhou et al (2013). Seedlings were grown in nutrient solution without added AlCl3 for 4 d. Malate concentrations in samples were measured with an enzyme assay as described previously (Ryan et al., 1995; Pereira et al., 2010).
Relative root length in hydroponic culture
A nutrient solution (pH 4.3) containing 500 μM KNO3, 500 μM CaCl2, 500 μM NH4NO3, 150 μM MgSO4, 10 μM KH2PO4, 2 μM Fe:EDTA, 11 μM H3BO3, 2 μM MnCl2, 0.35 μM ZnCl2, 0.2 μM CuCl2, and AlCl3 concentrations of 0, 1, 2, and 4 μM was prepared. The seeds were germinated in the dark for 2 d at 4 °C and 2 d at 24 °C. After the length of the middle primary root was measured, the seedlings were placed on floats in tanks each containing 20 l aerated nutrient solution with 0, 1, 2, and 4 μM AlCl3. After 4 d, the seedlings were removed and net root growth was calculated.
Soil experiments
An acidic red ferrosol soil obtained from the Robertson region of New South Wales, Australia (34° 35′ S 150° 36′ E) was used in the soil experiment. The pH of half of the soil was raised from pH 4.33 to 5.18 (measured with 0.01M CaCl2) with addition of 5g CaCO3 kg–1 dry soil. This also reduced exchangeable Al3+ in the soil from approximately 30% of total exchangeable cations to below 1%. Each pot (diameter 9cm and height 22cm) contained 1.3kg soil. Field capacity of the soil was 36% and water was added to maintain moisture at 90% of the field capacity. No additional water was applied. Seeds from each line were germinated on Petri dishes and seedlings with similar root lengths were planted in three pots with acid soil and three pots with limed soil (two seedlings per pot). The pots were placed in a temperature-controlled glasshouse under a 16h/8h light/dark cycle (22 and 18 °C, respectively). After 6 d, the plants were harvested and shoot fresh weight obtained. Roots were washed using a gentle water spray and measurements made of the length of the longest two roots on each seedling. The whole root system was stored in 50% ethanol for later processing. Preserved roots were floated on a plastic tray and scanned using a flatbed scanner (Epson Expression 800) at a resolution of 400 dpi for total root length and diameter using WINRhizo Pro (version 2002). Roots were then dried at 70 °C for 48h and weighed.
Statistical analysis
This study commonly compared root length in an Al3+ treatment (hydroponics or an acid soil) with root length in a control treatment (e.g. zero Al3+ in hydroponics or limed soil) to account for inherent differences in growth between lines. The resulting value is called relative root length (RRL). Therefore RRL = x/y where x and y represent the mean net root length in the Al3+ treatment and control conditions, respectively. Since standard errors (SE) are associated with the measurements of root length in the controls and treatments, the ratio of the means requires a new accumulated standard error. The formula for this accumulated error and the procedure used for determining whether two RRL values are statistically different from one another was described previously (Zhou et al., 2013).
Results
Generation of T3 transgenic and null lines
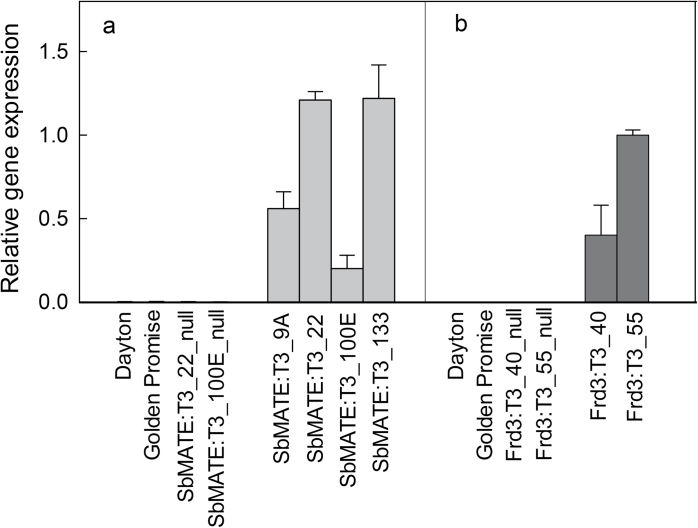
Barley (cv. Golden Promise) was transformed with the SbMATE gene from sorghum and the FRD3 gene from Arabidopsis. Seedlings from independent T1 lines expressing these genes were grown hydroponically and citrate efflux measured in excised root apices in the presence of 50 μM AlCl3 (Supplementary Table S1 available at JXB online). Five of the seven SbMATE T1 lines tested showed significantly greater citrate efflux than the untransformed controls and four of these (SbMATE:T1_9A, SbMATE:T1_22, SbMATE:T1_100E, and SbMATE:T1_133) were used to generate T3 families. Among the T3 families, likely homozygous lines were identified for each transgenic event using PCR (Supplementary Table S2). Two null lines (untransformed segregants) were also identified from the SbMATE:T3_22 and SbMATE:T3_100E families and included as controls in later experiments along with the Al3+-sensitive parent cv. Golden Promise. Expression level in the root apices of the SbMATE T3 lines was measured with quantitative reverse-transcription PCR. All four transgenic lines had SbMATE expression in the roots, with SbMATE:T3_22 and SbMATE:T3_133 showing greater expression than the other two transgenic lines (Fig. 1A). No SbMATE expression was detected in the null lines, the parental cv. Golden Promise, and the Al3+-tolerant cv. Dayton.
Fig. 1.
Relative expression of SbMATE (A) and FRD3 (B) in transgenic and control barley lines. Quantitative reverse-transcription PCR was measured in T3 transgenic lines, null lines, and two wild-type control cultivars: Dayton (Al3+-resistant) and Golden Promise (Al3+-sensitive parental line). cDNA was prepared from root apices of barley lines and expression measured relative to the reference gene actin. Data are mean and standard error (n=3, biological replicates).
Eight FRD3 T1 lines were tested for citrate efflux and six showed greater efflux than controls (Supplementary Table S1). Two of these lines with the highest citrate efflux, FRD3:T1_40 and FRD3:T1_55, were used to generate T3 families, and likely T3 homozygous lines and null lines were identified from each transgenic event with PCR (Supplementary Table S3). The null lines FRD3:T3_40_null and FRD3:T3_55_null were included as controls in subsequent experiments. Expression of FRD3 in FRD3:T3_55 was about 2.5-fold greater than in FRD3:T3_40. No FRD3 expression was detected in the null lines or in the nontransgenic controls, as expected (Fig. 1B).
Organic acid efflux in T3 lines
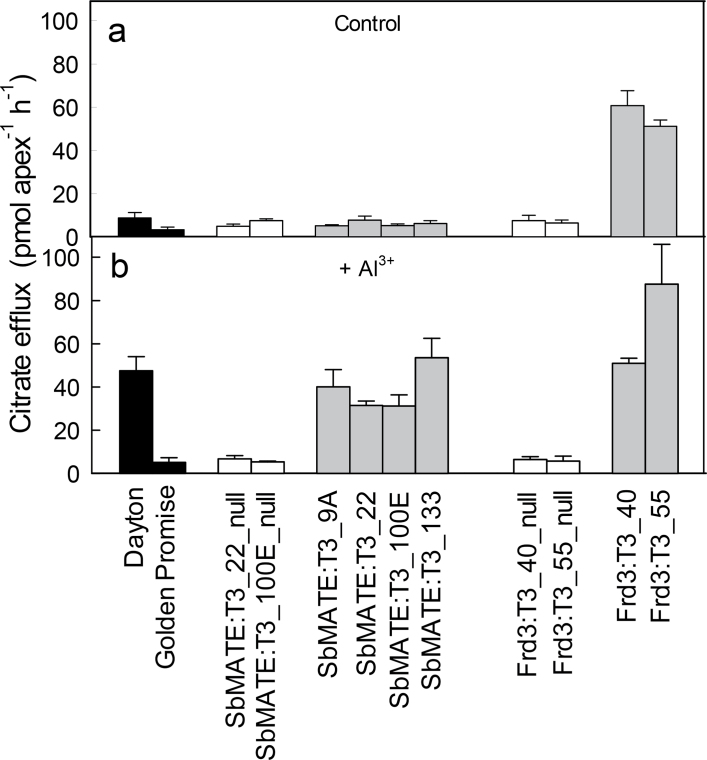
Citrate efflux in the root apices of SbMATE and FRD3 T3 lines were measured in the presence and absence of 50 μM AlCl3. In the absence of Al3+, citrate efflux from the SbMATE transgenic and null lines were low and similar to the untransformed controls which included the parental cv. Golden Promise and the Al3+-tolerant cv. Dayton (Fig. 2). By contrast, citrate efflux was detected in both FRD3 transgenic lines in the absence of Al3+. In the presence of Al3+, citrate efflux was 40–80 pmol apex–1 h–1 from the transgenic lines expressing SbMATE and FRD3 and in cv. Dayton. Only background efflux was measured in the null lines and cv. Golden Promise. Citrate efflux in cv. Dayton is controlled by the endogenous MATE gene HvAACT1. Wheat cv. Carazinho was also included as a positive control (data not shown) because it displays a high constitutive citrate efflux (Ryan et al., 2009). Citrate efflux from Carazinho was about 2-fold greater than the barley lines, reaching 113±32 pmol apex–1 h–1 in the absence of Al3+ and 136±21 pmol apex–1 h–1 in the presence of 50 μM Al3+.
Fig. 2.
Citrate efflux from root apices. Citrate efflux from excised root apices of control lines and transgenic barley lines expressing SbMATE or FRD3 in the absence of Al3+ (A) and in the presence of 50 μM AlCl3 (B). Black indicates the two wild-type controls, cv. Dayton (Al3+-resistant) and Golden Promise (Al3+-sensitive parental line); white indicates the two independent null segregant lines (T3) for the SbMATE and FRD3 transformation events; grey indicates the independent T3 transgenic lines expressing the transgenes. Data are mean and standard error (n= 3 or 4),
Malate efflux was also measured from root apices in the presence of 50 μM Al3+ but only low background fluxes were detected in the transgenic lines and control lines (data not shown). Malate efflux was detected in the wheat cv. Carazinho, which was included as a positive control. Efflux in Carazinho was 0.35 nmol apex–1 h–1 the presence of Al3+. This response is controlled by the TaALMT1 anion channel in wheat (Sasaki et al., 2004).
Al3+ tolerance: root growth in hydroponic experiments
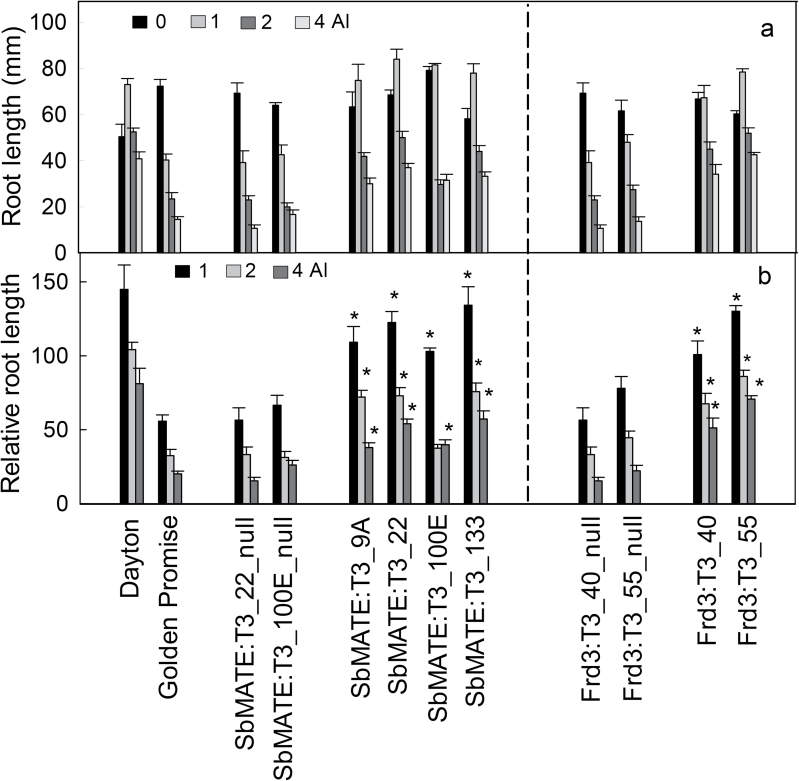
After 4 d in hydroponic solution without Al3+, roots of all the lines grew 50–80mm (Fig. 3A). In the presence of 1 μM Al3+, growth of the null lines and cv. Golden Promise was inhibited by ~30%, whereas growth of the SbMATE and FRD3 transgenic lines was either unaffected or stimulated. Increasing the Al3+ concentration to 2 or 4 μM inhibited root growth of all lines but the null lines and cv. Golden Promise were inhibited to a greater degree than either set of transgenic lines.
Fig. 3.
Al3+ tolerance of transgenic and control lines in hydroponic experiments. (A) Net root growth of transgenic and control seedlings was measured after 4 d in nutrient solution containing 0, 1, 2 or 4 μM AlCl3. Data are mean and standard error (n=7). (B) Relative root growth of lines was calculated as net root growth in Al3+ compared to zero Al3+ control. Data are means and standard error. Asterisks indicate significant differences from the null lines at the same Al3+ concentration (P<0.05).
Relative root length compares net growth at each Al3+ concentration with the zero Al3+ treatment. As the Al3+ concentrations increased, RRL for the SbMATE transgenic lines and the FRD3 transgenic lines remained 2–3-fold greater than their respective nulls. These differences were statistically significant (P<0.05) except for SbMATE:T3_100E, which was not different from its null lines at 2 μM Al3+ (Fig. 3B).
Al3+ tolerance: root growth in soil experiments
Soil experiments were performed over 6 d with an acidic ferrosol and the same soil amended with lime to increase the pH and decrease Al3+ toxicity. Root and shoot measurements were expressed as relative values from the acid soil compared to the limed soil. Representative plants immediately after harvest are shown in Fig. 4. Root fresh weight was similar or slightly greater in the acid soil compared to the limed soil for all lines except SbMATE:T3_100E_null, which was significantly lower in the acid soil (Supplementary Table S4). However, there were no consistent trends in the relative fresh weight of roots or shoots (data not shown) between the transgenic lines and their null controls for either of the SbMATE and FRD3 lines.
Fig. 4.
Barley plants at the end of the soil experiment. Representative plants of SbMATE:T3_100E_null (A), SbMATE:T3_100E (B), FRD3:T3_40_null (C), and FRD3:T3_40 (D) grown in limed and acid soil. Note that the roots on the transgenic plants and null plants are similar in the limed soil but longer on the transgenic plants than the null controls but in acid soil. Shoots do not show strong phenotypes between the acid and limed soils (this figure is available in colour at JXB online).
Length of the longest root on seedlings in limed soil ranged from 160 to 210mm (Supplementary Fig. S1A). Roots were shorter in acid soil for both sets of null lines, with RRL approximately 60–70% (Fig. 5A). By contrast, RRL for the SbMATE transgenic lines was 85–115% and for the two FRD3 transgenic lines RRL was 90 and 100%. Root growth of cv. Dayton was the same in the acid and limed soil. RRL for cv. Golden Promise was greater than both SbMATE null lines and one of the FRD3 null lines in this experiment.
Fig. 5.
Al3+ tolerance in soil experiment: relative root lengths. (A) Relative root length based on the length of the longest root from seedlings grown in acid soil compared to the limed soil; data are mean and standard error; asterisks indicate significant differences from the null lines (P<0.05). (B) Relative total root growth of lines based on root length in acid soil compared to the limed soil; data are mean and standard error; asterisks above the SbMATE transgenic lines indicate significant differences from both null lines (P<0.05). Asterisks above the FRD3 transgenic lines indicate significant differences from their respective null lines (P<0.05). See Supplementary Fig. S1 for raw data used to calculate these results.
Total root length in both sets of null lines was ~60% less in acid soil compared to the limed soil (Supplementary Fig. S1B). Relative total root length in three of the four SbMATE transgenic lines and both FRD3 transgenic lines was 2-fold greater than their respective nulls (Fig. 5B). The single transgenic line not fitting this trend was SbMATE:T3_22, which showed a larger-than-expected decrease in root growth in acid soil. Relative total root length in cv. Dayton was similar in acid and limed soils.
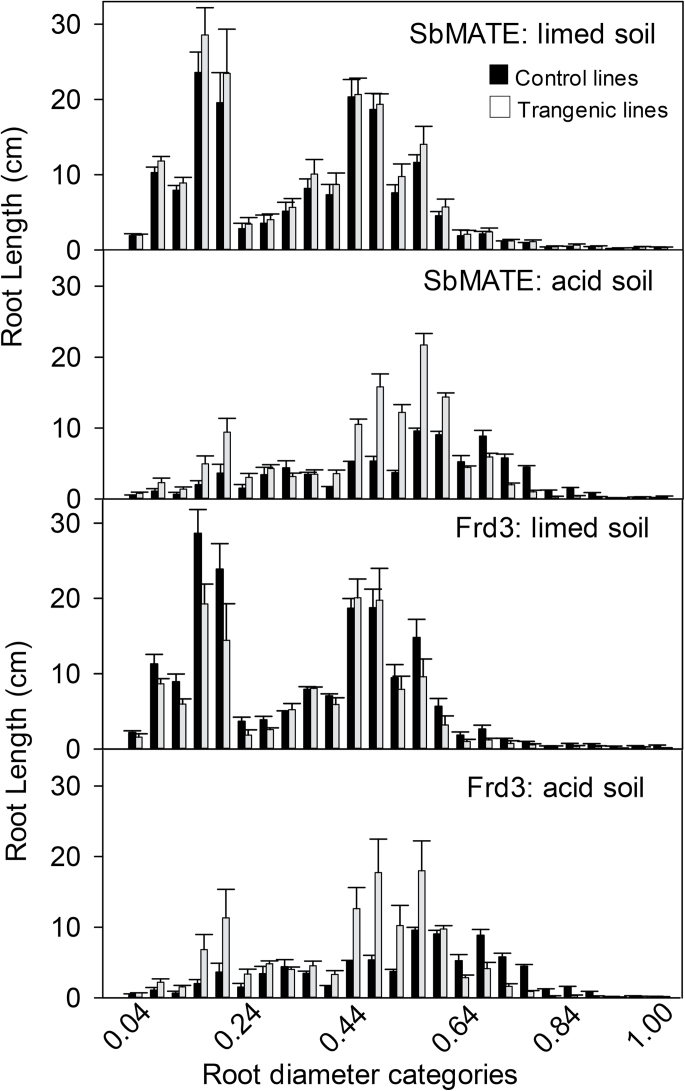
The combined root length within each category of root diameter was plotted for the transgenic and null lines (Fig. 6). For clarity, data from the independent null lines were combined and compared with the combined data from the transgenic lines. In limed soil, the distribution of root diameters was qualitatively similar for all the null and transgenic lines with two prominent peaks emerging at about 0.2 and 0.5mm (Fig. 6A, C). In acid soil, the distribution of diameters from Golden Promise and null lines of SbMATE (Fig. 6B) and FRD3 (Fig. 6D) was significantly flatter than the limed soil with no peak close to 0.2mm, indicating proportionally more roots had diameters 0.6mm and greater. Transgenic lines expressing SbMATE and FRD3 also showed a flatter profile and a shift towards thicker roots; however, the changes were not as large and the two distinct peaks close to 0.2 and 0.5mm remained. These results indicate that expression of single gene in barley enables roots to grow longer in an acid soil and maintains a greater proportion of thinner roots.
Fig. 6.
Distribution of root diameters on soil-grown plants. Transgenic plants expressing SbMATE or FRD3 and control plants were grown in a limed soil or acid soil for 6 d. The roots were washed and scanned with WinRHIZO and the total root length falling within each diameter class was estimated. Black indicates control lines not expressing a transgene and these include the two nulls and Golden Promise (mean and standard error, n=3); grey indicates transgenic lines expressing a transgene; data are mean and standard error for the SbMATE lines (n=4) and mean and range for FRD3 lines (n=2). The diameter categories increase in 40-μm increments from 0 to 1.0mm.
Comparing transgenic barley lines transformed with MATE and ALMT genes
Previous studies have improved the Al3+ tolerance of barley by transforming the sensitive cv. Golden Promise with MATE genes and with ALMT genes. The present work directly compared the transgenic lines generated here with transgenic lines generated previously. The first experiment compared lines expressing FRD3 and SbMATE with lines overexpressing the endogenous HvAACT1 gene from barley (Zhou et al., 2013). Plants were grown in hydroponics with 0, 1, 2, and 4 μM AlCl3 and relative root growth was estimated after 4 d. The tolerance conferred by the three MATE genes was similar for 1 and 2 μM AlCl3 but the FRD3 line was significantly more tolerant than the other two lines at 4 μM (Supplementary Fig. S2).
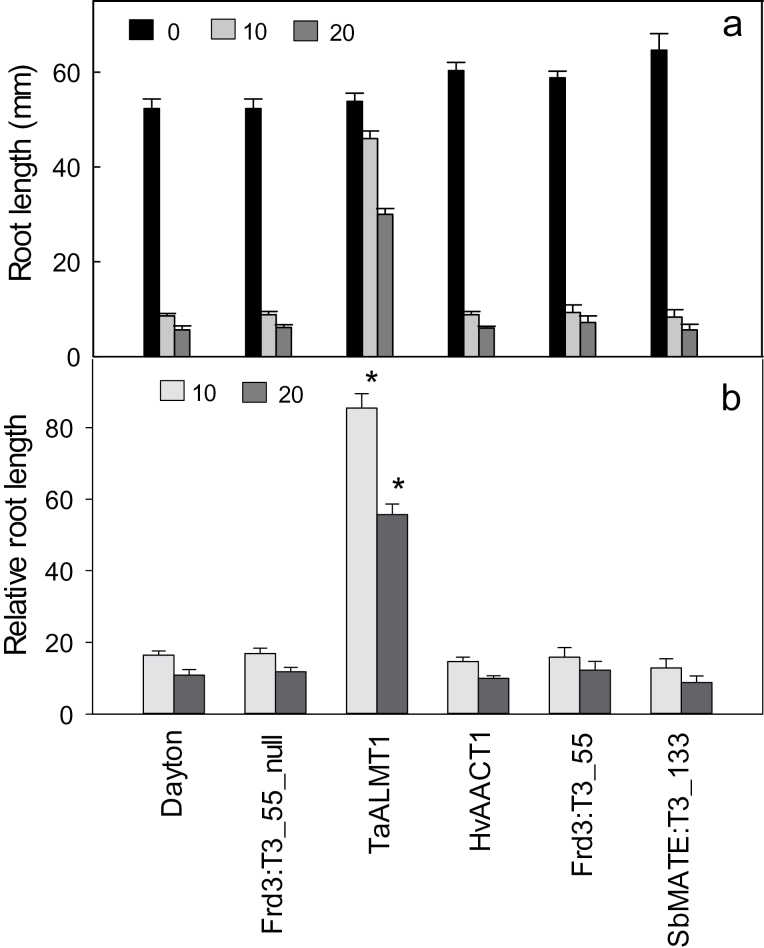
In separate experiments, barley lines expressing the same three MATE genes were compared with barley expressing TaALMT1 from wheat (Delhaize et al., 2004). These experiments used higher concentrations of AlCl3 because preliminary experiments indicated they differentiated these lines more clearly. The results indicate that expression of the wheat gene TaALMT1 conferred significantly greater tolerance at 10 and 20 μM Al3+ than any of the three MATE genes tested (Fig. 7).
Fig. 7.
Direct comparison of Al3+ tolerance in transgenic barley lines expressing MATE genes and TaALMT1. T3 barley lines expressing SbMATE and FRD3 (generated here) and HvAACT1 (generated previously; Zhou et al., 2013) were compared with a transgenic line expressing TaALMT1 from wheat (Delhaize et al., 2004). (A) Net root length was measured after plants were grown for four d in hydroponics with 0, 10 and 20 μM AlCl3. (B) Relative root length of lines was calculated as net root growth in Al3+ compared to zero Al3+ control. Data are mean and standard error (n= 5 or 6). Asterisks indicate significant differences from all other lines in the same condition (P<0.05).
Discussion
In this study, barley was transformed with two MATE genes that encode citrate transporters with distinct properties. SbMATE is an Al3+-activated transport protein that confers Al3+ tolerance to sorghum (Magalhaes et al., 2007) and FRD3 is involved in the long-distance transport of Fe from roots to shoots in Arabidopsis (Durrett et al., 2007). Independent transgenic lines expressing these genes were generated and their Al3+ tolerance were compared with null lines. Transgenic lines expressing SbMATE displayed an Al3+-dependent citrate release from roots not detected in null segregant lines. These lines also showed greater tolerance to Al3+ and maintained a higher proportion of thinner roots, which is important for soil exploration and nutrient uptake. Barley lines expressing FRD3 showed similar phenotypes except that citrate release occurred in the presence and absence of Al3+. These phenotypes are consistent with previous results from transgenic Arabidopsis expressing FRD3 with the 35SCaMV promoter (Durrett et al., 2007) and they confirm that, when expressed ectopically, FRD3 can facilitate citrate efflux from monocotyledons in the absence of Al3+. The Al3+-tolerant barley cv. Dayton also showed citrate efflux but only in the presence of Al3+, which is consistent with previous findings (Furukawa et al., 2007; Wang et al., 2007).
Al3+ tolerance was evaluated in hydroponic experiments and in acid soil. Fresh shoot weight and fresh root weight were not strongly correlated with Al3+ tolerance which is similar to previous reports for young seedlings (Muhling et al., 1988; Carr and Ritchie, 1993). Relative root length in the short-term hydroponics and soil trials proved to be a convenient screening method for comparing lines. At low Al3+ concentrations, both sets of transgenic lines and cv. Dayton had longer roots than in zero Al3+ solution. This stimulation of growth in acid conditions by low levels of Al3+ has been observed previously and is interpreted as Al3+ alleviating H+ toxicity (Kinraide et al., 1992). Moroni et al. (2010) screened a range of cereal genotypes for Al3+ tolerance and concluded that the rankings differed between hydroponic and field trials because barley appeared more tolerant in soil than the hydroponic screens. While the present study found broad agreement between the results in hydroponics and short soil experiments, longer field trials will be necessary to assess how effective these transgenes are in improving grain yield on acid soils. In soil experiments, cv. Golden Promise did not always perform the same as the null lines even though the lines should have been genetically identical (Fig. 5). This is not unexpected since, unlike cv. Golden Promise, the nulls were regenerated from callus in tissue culture, which might have resulted in somaclonal variation. This highlights the importance of including null-segregant lines as controls when evaluating transgenic material.
Barley lines expressing SbMATE and FRD3 showed similar levels of tolerance and none were more tolerant than the wild-type cv. Dayton. Interestingly citrate efflux in independent transgenic lines expressing SbMATE was similar despite differences in expression level (Figs 1 and 2). This result suggests that beyond a certain level of expression, citrate efflux, and consequently Al3+ tolerance, does not increase further. It is unclear why this saturation in tolerance occurs. Perhaps once the capacity to release citrate from root cells reaches a certain threshold, other metabolic processes begin to limit efflux, or the citrate efflux in nontargeted tissues becomes counterproductive to growth. Organic anion synthesis might limit citrate efflux when expression of the transporters is sufficiently high. Consistent with this idea are the findings that overexpression of genes involved in organic anion synthesis can also enhance Al3+ tolerance by increasing organic anion efflux in roots (Fuente et al., 1997; Koyama et al., 2000; Tesfaye et al., 2001; Anoop et al., 2003; Barone et al., 2008; Trejo-Tellez et al., 2010; Wang et al., 2010). The present study found that a barley line transformed with TaALMT1 from wheat (conferring malate efflux) was more tolerant than any of the lines expressing MATE genes. The efflux of citrate in lines expressing the MATE genes (~50 pmol apex–1 h–1) was approximately 20-fold smaller than malate efflux in lines expressing TaALMT1 (~1 nmol apex–1 h–1). The stability constants for aluminium citrate compounds are many orders of magnitude greater than for aluminium malate compounds (Jones, 1998) suggesting that citrate should provide much greater tolerance than malate, even if less is released. This is partly supported by predictions of the chemical speciation program GEOCHEM (Shaff et al., 2010). In a test solution of 1mM CaCl2, 100 μM AlCl3, and either 100 μM citrate or malate (fixed pH 4.5), the free Al concentration was 9.9 μM for malate and 0.07 μM for citrate. However, when 10-fold less malate and citrate are tested (ie. 10 μM citrate), the free concentration of Al was ~70 μM for both. Therefore the difference between these anions appears to depend on the ratio of their concentrations to Al. The relative toxicity of Al solutions to plants are best predicted by modelling the activities of the Al3+ species at the surface of the root cell membranes but this is a more complex calculation requiring some knowledge of the zeta potential or surface charge density of the roots (Kinraide et al., 1992, 2005). The present finding that citrate efflux was not as effective as expected (Zhao et al., 2003) might also be explained, in part, by inappropriate stability constants due to the experimental conditions used to derive these values. High concentrations of reagents are commonly used in high-ionic-strength background solutions which contrasts with the low ionic strength of the hydroponic growth solution. Furthermore, the theoretical stability constants derived for Al3+ and citrate might be difficult to interpret: at pH 4.3, the molar fraction of the trivalent citrate is relatively small. Nevertheless, the results appear to indicate that the stability constants for Al3+:citrate and Al3+:malate are less important than the magnitudes of fluxes. As the organic anions are released from the root-cell cytosol into the more acidic apoplastic environment, they will bind with H+ and potentially raise the pH slightly near the membrane surface; therefore, it is possible that differences in the capacity of these anions to influence local pH could also contribute to the tolerance they confer.
The finding that Al3+ tolerance was increased by FRD3 expression, a gene not naturally involved with this phenotype, is consistent with a hypothesis for the evolution of Al3+ tolerance in plants (Magalhaes, 2010; Ryan and Delhaize, 2010). It proposes that Al3+ tolerance in some species is a relatively recent trait acquired from mutations to genes encoding organic anion transporters that perform other functions. These mutations affect the level or distribution of protein expression which extends their function to include organic anion release from root apices. This hypothesis is supported by reports describing how different mutations upstream of organic anion transporter genes change expression and alter responses to Al3+ stress. For example, multiple, perfect, tandem repeats of sequence in the promoter of TaALMT1 in wheat (Sasaki et al., 2006, Ryan et al., 2009) and cis-acting elements in the promoter of HlALMT1 in Holcus lantanus drive higher expression of these genes. Similarly, higher expression of SbMATE in different genotypes of sorghum is associated with a greater numbers of Tourist-like miniature inverted-repeat transposable elements (MITE) several kilobases upstream from the coding region (Magalhaes et al., 2007). Other mutations that increase gene expression include transposon-like insertions in the 5′-untranscribed region of HvAACT1 in barley (Fujii et al., 2012) and in TaMATE1B in wheat (Tovkach et al., 2013).
In conclusion, these experiments demonstrate that heterologous expression of SbMATE and FRD3 can stably increase the Al3+ tolerance of an important cereal species by enhancing citrate efflux in root apices. Future studies will introgress these transgenes into Al3+-tolerant barley cultivars such as Dayton and perhaps pyramid them with other genes to assess whether the effects of the endogenous and transgenes are additive. Pyramiding MATE genes with ALMT genes is likely to be a more successful strategy than pyramiding multiple MATE genes. Since MATEs release citrate and ALMTs release malate, their combination could avoid the saturation of tolerance observed here when MATE genes were overexpressed. However overexpression of MATE or ALMT genes could have pleiotropic effects on plants through interactions with cell signalling or through chemical changes in the rhizosphere. For instance, some ALMTs are involved in mineral nutrition and ion homeostasis and so these processes could be perturbed in transgenic plants (Pineros et al., 2008b; Gruber et al., 2011). Malate release from Arabidopsis roots via AtALMT1 can induce the colonization of microorganisms in the rhizosphere and on the root surface (Rudrappa et al., 2008). Malate exudation was shown to recruit Bacillus subtilis biofilm formation on tomato roots in a similar way (Chen et al., 2012). Therefore, the root microbiome could be altered in transgenic plants with higher expression of MATE and ALMT transporters. It was recently shown that AtALMT1 expression in Arabidopsis was increased by indole acetic acid, abscisic acid, and the bacterial elicitor flagellin 22 as well as to low pH and Al3+, which indicates this gene is potentially involved in a wide array of biological functions in addition to Al3+ tolerance (Kobayashi et al., 2013). Therefore cell signalling, growth, or responses to stress could also be affected in plants overexpressing these transporters. The transgenic lines generated here and previously provide useful material for investigating these processes in more detail.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Al3+ tolerance in soil experiments: total root length.
Supplementary Fig. S2. Comparing the Al3+ tolerance of barley lines expressing three different MATE genes.
Supplementary Table S1. Citrate efflux in the excised root apices of T1 transgenic lines.
Supplementary Table S2. Screening T2 barley plants transformed with SbMATE.
Supplementary Table S3. Screening T2 barley plants transformed with FRD3.
Supplementary Table S4. Root fresh weight of transgenic and control barley lines grown in acid and limed soil.
Acknowledgements
The authors thank Elizabeth E. Rogers (University of Missouri, USA) for providing the FRD3 cDNA and Leon V. Kochian for providing the SbMATE cDNA. They also acknowledge Terese Richardson and Michael Ayliffe (CSIRO, Australia) for advice with barley transformation. J.F.P. was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (process number 4151-07-0) and Empresa Brasileira de Pesquisa Agropecuária. The authors thank Jiangfeng You for technical assistance and Jon Shaff (USDA) for an updated source file for GEOCHEM-EZ.
References
- Anoop VM, Basu U, McCammon MT, McAlister-Henn L, Taylor GJ. 2003. Modulation of citrate metabolism alters aluminum tolerance in yeast and transgenic canola overexpressing a mitochondrial citrate synthase. Plant Physiology 132, 2205–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Rosellini D, LaFayette P, Bouton J, Veronesi F, Parrott W. 2008. Bacterial citrate synthase expression and soil aluminum tolerance in transgenic alfalfa. Plant Cell Reports 27, 893–901 [DOI] [PubMed] [Google Scholar]
- Carr SJ, Ritchie GSP. 1993. Al toxicity of wheat grown in acidic subsoils in relation to soil solution properties and exchangeable cations. Australian Journal of Soil Research 31, 583–596 [Google Scholar]
- Chen Y, Cao SG, Chai YR, Clardy J, Kolter R, Guo JH, Losick R. 2012. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Molecular Microbiology 85, 418–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Shirley NJ, Saeed M, Pallotta M, Gustafson JP. 2008. An ALMT1 gene cluster controlling aluminum tolerance at the Alt4 locus of rye (Secale cereale L.). Genetics 179, 669–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente JM, Ramirez-Rodriguez V, Cabrera-Ponce JL, Herrera-Estrella L. 1997. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science 276, 5466–1568 [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. 2004. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proceedings of the National Academy of Sciences, USA 101, 15249–15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. 1993. Aluminum tolerance in wheat (Triticum-aestivum L.).2. Aluminum-stimulated excretion of malic-acid from root apices. Plant Physiology 103, 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Taylor P, Hocking PJ, Simpson RJ, Ryan PR, Richardson AE. 2009. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnology Journal 7, 391–400 [DOI] [PubMed] [Google Scholar]
- Durrett TP, Gassmann W, Rogers EE. 2007. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology 144, 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M, Yokosho K, Yamaji N, Saisho D, Yamane M, Takahashi H, Sato K, Nakazono M, Ma JF. 2012. Acquisition of aluminium tolerance by modification of a single gene in barley. Nature Communications 3, 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF. 2007. An aluminum-activated citrate transporter in barley. Plant and Cell Physiology 48, 1081–1091 [DOI] [PubMed] [Google Scholar]
- Gruber BD, Delhaize E, Richardson AE, Roessner U, James RA, Howitt SM, Ryan PR. 2011. Characterisation of HvALMT1 function in transgenic barley plants. Functional Plant Biology 38, 163–175 [DOI] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Pineros MA, et al. 2006. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis . Proceedings of the National Academy of Sciences, USA 103, 9738–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL. 1998. Organic acids in the rhizosphere—a critical review. Plant and Soil 205, 25–44 [Google Scholar]
- Kinraide TB, Parker DR, Zobel RW. 2005. Organic acid secretion as a mechanism of aluminium resistance: a model incorporating the root cortex, epidermis, and the external unstirred layer. Journal of Experimental Botany 56, 1853–1865 [DOI] [PubMed] [Google Scholar]
- Kinraide TB, Ryan PR, Kochian LV. 1992. Interactive effects of Al3+, H+ and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiology 99, 1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Sugimoto M, Lakshmanan V, Iuchi S, Kobayashi M, Bais HP, Koyama H. 2013. Characterization of the complex regulation of AtALMT1 expression in response to phytohormones and other inducers. Plant Physiology 162, 732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Pineros MA. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology 55, 459–493 [DOI] [PubMed] [Google Scholar]
- Kochian LV, Pineros MA, Hoekenga OA. 2005. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant and Soil 274, 175–195 [Google Scholar]
- Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata D. 2000. Overexpression of mitochondrial citrate synthase in Arabidopsis thaliana improved growth on a phosphorus-limited soil. Plant and Cell Physiology 41, 1030–1037 [DOI] [PubMed] [Google Scholar]
- Li QY, Niu HB, Yin J, Shao HB, Niu JS, Ren JP, Li YC, Wang X. 2010. Transgenic barley with overexpressed PTrx increases aluminum resistance in roots during germination. Journal of Zhejiang University—Science B 11, 862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligaba A, Katsuhara M, Ryan PR, Shibasaka M, Matsumoto H. 2006. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiology 142, 1294–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Magalhaes JV, Shaff J, Kochian LV. 2009. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. The Plant Journal 57, 389–399 [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. 2001. Aluminium tolerance in plants and the complexing role of organic acids. Trends in Plant Science 6, 273–278 [DOI] [PubMed] [Google Scholar]
- Magalhaes JV. 2010. How a microbial drug transporter became essential for crop cultivation on acid soils: aluminium tolerance conferred by the multidrug and toxic compound extrusion (MATE) family. Annals of Botany 106, 199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimaraes CT, et al. 2007. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genetics 39, 1156–1161 [DOI] [PubMed] [Google Scholar]
- Maron LG, Guimaraes CT, Kirst M, et al. 2013. Aluminum tolerance in maize is associated with higher MATE1 gene copy number. Proceedings of the National Academy of Sciences, USA 110, 5241–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minella E, Sorrells ME. 1992. Aluminum tolerance in barley: genetic relationships among genotypes of diverse origin. Crop Science 32, 593–598 [Google Scholar]
- Miyasaka SC, Buta JG, Howell RK, Foy CD. 1991. Mechanism of aluminum tolerance in snapbeans: root exudation of citric acid. Plant Physiology 96, 737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni JS, Sato K, Scott BJ, Conyers M, Read BJ, Fisher R, Poile G. 2010. Novel barley (Hordeum vulgare L.) germplasm resistant to acidic soil. Crop and Pasture Science 61, 540–553 [Google Scholar]
- Muhling KH, Steffens D, Mengel K. 1988. Determination of phytotoxic soil aluminum by electroultrafiltration. Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 151, 267–271 [Google Scholar]
- Pereira JF, Zhou GF, Delhaize E, Richardson T, Zhou MX, Ryan PR. 2010. Engineering greater aluminium resistance in wheat by over-expressing TaALMT1 . Annals of Botany 106, 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros MA, Cancado GMA, Kochian LV. 2008a. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: functional and structural implications. Plant Physiology 147, 2131–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros MA, Cancado GMA, Maron LG, Lyi SM, Menossi M, Kochian LV. 2008b. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: the case of ZmALMT1—an anion-selective transporter. The Plant Journal 53, 352–367 [DOI] [PubMed] [Google Scholar]
- Rudrappa T, Czymmek KJ, Pare PW, Bais HP. 2008. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiology 148, 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E. 2010. The convergent evolution of aluminium resistance in plants exploits a convenient currency. Functional Plant Biology 37, 275–284 [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. 1995. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta 196, 103–110 [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV. 1993. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. Journal of Experimental Botany 44, 437–446 [Google Scholar]
- Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E. 2009. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiology 149, 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Tyerman SD, Sasaki T, Furuichi T, Yamamoto Y, Zhang WH, Delhaize E. 2011. The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. Journal of Experimental Botany 62, 9–20 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ryan PR, Delhaize E, Hebb DM, Ogihara Y, Noda K, Matsumoto H, Yamamoto Y. 2006. Analysis of the sequence upstream of the wheat (Triticum aestivum L.) ALMT1 gene and its relationship to aluminium tolerance. Plant Cell and Physiology 47, 1343–1354 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. 2004. A wheat gene encoding an aluminum-activated malate transporter. The Plant Journal 37, 645–653 [DOI] [PubMed] [Google Scholar]
- Schunmann PHD, Llewellyn DJ, Surin B, Boevink P, De Feyter RC, Waterhouse PM. 2003. A suite of novel promoters and terminators for plant biotechnology. Functional Plant Biology 30, 443–452 [DOI] [PubMed] [Google Scholar]
- Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV. 2010. GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant and Soil 330, 207–214 [Google Scholar]
- Sivaguru M, Horst WJ. 1998. The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiology 116, 155–163 [Google Scholar]
- Tesfaye M, Temple SJ, Allan DL, Vance CP, Samac DA. 2001. Overexpression of malate dehydrogenase in transgenic alfalfa enhances organic acid synthesis and confers tolerance to aluminum. Plant Physiology 127, 1836–1844 [PMC free article] [PubMed] [Google Scholar]
- Tingay S, McElroy D, Kalla R, Fieg S, Wang MB, Thornton S, Brettell R. 1997. Agrobacterium tumefaciens-mediated barley transformation. The Plant Journal 11, 1369–1376 [Google Scholar]
- Tovkach A, Ryan PR, Richardson AE, Lewis DC, Rathjen TM, Ramesh S, Tyerman SD, Delhaize E. 2013. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiology 161, 880–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo-Tellez LI, Stenzel R, Gomez-Merino FC, Schmitt JM. 2010. Transgenic tobacco plants overexpressing pyruvate phosphate dikinase increase exudation of organic acids and decrease accumulation of aluminum in the roots. Plant and Soil 326, 187–198 [Google Scholar]
- Wang JP, Raman H, Zhou MX, Ryan PR, Delhaize E, Hebb DM, Coombes N, Mendham N. 2007. High-resolution mapping of the Alp locus and identification of a candidate gene HvMATE controlling aluminium tolerance in barley (Hordeum vulgare L.). Theoretical and Applied Genetics 115, 265–276 [DOI] [PubMed] [Google Scholar]
- Wang MB, Li ZY, Matthews PR, Upadhyaya NM. 1998. Improved vectors for Agrobacterium tumefaciens-mediated transformation of monocot plants. Acta Horticulturae 461, 401–408 [Google Scholar]
- Wang Q-F, Zhao Y, Yi Q, Li K-Z, Yu Y-X, Chen L-M. 2010. Overexpression of malate dehydrogenase in transgenic tobacco leaves: enhanced malate synthesis and augmented Al-resistance. Acta Physiologiae Plantarum 32, 1209–1220 [Google Scholar]
- Wise AA, Liu Z, Binns AN. 2006. Three methods for the introduction of foreign DNA into Agrobacterium . Methods in Molecular Biology 343, 43–53 [DOI] [PubMed] [Google Scholar]
- Yang XY, Yang JL, Zhou Y, Pineros MA, Kochian LV, Li GX, Zheng SJ. 2011. A de novo synthesis citrate transporter, Vigna umbellata multidrug and toxic compound extrusion, implicates in Al-activated citrate efflux in rice bean (Vigna umbellata) root apex. Plant, Cell and Environment 34, 2138–2148 [DOI] [PubMed] [Google Scholar]
- Yokosho K, Yamaji N, Ma JF. 2011. An Al-inducible MATE gene is involved in external detoxification of Al in rice. The Plant Journal 68, 1061–1069 [DOI] [PubMed] [Google Scholar]
- Zhang WH, Ryan PR, Sasaki T, Yamamoto Y, Sullivan W, Tyerman SD. 2008. Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant and Cell Physiology 49, 1316–1330 [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Ma JF, Sato K, Takeda K. 2003. Differential Al resistance and citrate secretion in barley (Hordeum vulgare L.). Planta 217, 794–800 [DOI] [PubMed] [Google Scholar]
- Zhou GF, Delhaize E, Zhou MX, Ryan PR. 2013. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Annals of Botany 112, 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.