Summary
This study characterized ANU10, a novel chloroplast protein encoded by a nuclear gene in Arabidopsis, which influences leaf and chloroplast shape and is required for thylakoid stacking and grana formation.
Key words: Arabidopsis thaliana, chloroplast, grana, LHCII trimers, mesophyll development, thylakoid biogenesis, thylakoid stacking.
Abstract
The chloroplasts of land plants contain internal membrane systems, the thylakoids, which are arranged in stacks called grana. Because grana have not been found in Cyanobacteria, the evolutionary origin of genes controlling the structural and functional diversification of thylakoidal membranes in land plants remains unclear. The angulata10-1 (anu10-1) mutant, which exhibits pale-green rosettes, reduced growth, and deficient leaf lateral expansion, resulting in the presence of prominent marginal teeth, was isolated. Palisade cells in anu10-1 are larger and less packed than in the wild type, giving rise to large intercellular spaces. The ANU10 gene encodes a protein of unknown function that localizes to both chloroplasts and amyloplasts. In chloroplasts, ANU10 associates with thylakoidal membranes. Mutant anu10-1 chloroplasts accumulate H2O2, and have reduced levels of chlorophyll and carotenoids. Moreover, these chloroplasts are small and abnormally shaped, thylakoidal membranes are less abundant, and their grana are absent due to impaired thylakoid stacking in the anu10-1 mutant. Because the trimeric light-harvesting complex II (LHCII) has been reported to be required for thylakoid stacking, its levels were determined in anu10-1 thylakoids and they were found to be reduced. Together, the data point to a requirement for ANU10 for chloroplast and mesophyll development.
Introduction
In land plants, mutants with defective pigment content have allowed the identification of numerous nuclear genes that are crucial for organelle division and other plastid-specific processes (Lopez-Juez and Pyke, 2005; Hricová et al., 2006). Many of these genes encode plant-specific proteins whose closest homologues are found in Cyanobacteria, as expected if they have been acquired by the nuclear genome in a horizontal transfer event, originating in the genome of the cyanobacterial ancestor of plastids. As an example, the nuclear genomes of land plants encode homologues of Filamenting temperature-sensitive Z (FtsZ) (Osteryoung and Vierling, 1995; Osteryoung et al., 1998), Minicell D (MinD) (Colletti et al., 2000), MinE (Itoh et al., 2001), and many other components of the prokaryotic cell division apparatus.
The chloroplasts of land plants contain internal membrane systems, the thylakoids, which are arranged in stacks called grana. Grana thylakoids form cylindrical stacks that are connected to adjacent grana by non-stacked stroma thylakoids, which form right-handed helices around the grana (Austin and Staehelin, 2011). The thylakoidal membranes harbour the photosynthetic protein complexes, photosystems I and II (PSI and PSII), and other protein complexes, including ATP synthase, cytochrome b 6 f, and the light-harvesting complexes I and II (LHCI and LHCII). Grana underlie a differential specialization of thylakoid membranes in the lateral dimension: the PSII and LHCII complexes are more abundant in grana, and the PSI and ATP synthase are preferentially found in the adjacent stroma thylakoids (Albertsson, 2001; Dekker and Boekema, 2005). Because grana have not been found in Cyanobacteria, the evolutionary origin of genes controlling the structural and functional diversification of thylakoidal membranes in land plants remains unclear (Mullineaux, 2005). Mutants with abnormal thylakoid stacking and loss of typical grana have long been known in species such as maize (Bachmann et al., 1969), barley (Nielsen et al., 1979), tobacco (Archer and Bonnett, 1987), and Arabidopsis thaliana (hereafter, Arabidopsis) (Reiter et al., 1994). However, the molecular basis of the phenotypes observed in these species has only been determined in a few cases. Mutations in the Arabidopsis CHLORATA-42 (CH-42) gene (Koncz et al., 1990), which encodes one of the three subunits of the chloroplast magnesium chelatase complex, significantly reduce thylakoid stacking, suggesting a link between chlorophyll biosynthesis and grana formation (Apchelimov et al., 2007). Another Arabidopsis gene, GRANA-DEFICIENT CHLOROPLAST1 (GDC1), encodes an ankyrin-domain protein that is essential for grana formation. GDC1 is required for the assembly of the trimeric forms of the LHCII complex, which are barely detected in the gdc1-3 mutant (Cui et al., 2011). Current models emphasize the role of LHCII trimers in the formation of grana and the stacking of thylakoidal membranes (Mullet and Arntzen, 1980; Day et al., 1984; Garab and Mustardy, 1999; Allen and Forsberg, 2001), mainly through electrostatic interactions between the positively charged N-terminal domains of LHCII trimers (stroma-exposed) and the negatively charged surface of LHCII trimers in adjacent thylakoidal membranes (Steinback et al., 1979; Standfuss et al., 2005). In addition, members of the CURVATURE THYLAKOID1 (CURT1) family of proteins have recently been shown to be enriched at the margin of grana, where they are thought to promote the curvature of thylakoidal membranes in Arabidopsis. Interestingly, the Arabidopsis CURT1A protein can replace the function of a distant cyanobacterial orthologue, showing that at least some proteins with crucial roles in thylakoid architecture are evolutionarily conserved, even though Cyanobacteria lack grana (Armbruster et al., 2013).
Here it is shown that the ANGULATA10 (ANU10) gene of Arabidopsis encodes a novel plastid-localized protein that is conserved among land plants, and the phenotypic and molecular characterization of loss-of-function anu10 mutants is reported. It is demonstrated that the ANU10 protein localizes to plastids, including amyloplasts and chloroplasts, where it is associated with thylakoidal membranes. Thylakoid biogenesis is seriously impaired in anu10-1 chloroplasts. Supporting the implication of LHCII trimers in grana formation, the anu10-1 mutant contains reduced levels of LHCII trimers and shows defective thylakoid stacking, therefore lacking typical grana. A relationship between plastid integrity and leaf development can be inferred from the larger, sparsely packed cells observed in the palisade mesophyll of the anu10-1 mutant.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana (L.) Heynh. wild-type accessions Columbia-0 (Col-0) and Landsberg erecta (Ler), as well as the T-DNA insertion lines SAIL_708_F05 (N831342) and SAIL_659_F07 (N828696), were obtained from the Nottingham Arabidopsis Stock Centre (NASC). The anu10-1 mutant was isolated after ethylmethane sulphonate (EMS)-induced mutagenesis as previously described (Berná et al., 1999). All plants in this work were grown on Murashige and Skoog agar medium (2.15g l–1), at 20±1 °C and 60–70% relative humidity under continuous fluorescent light (~90 μmol m−2 s−1) as previously described (Ponce et al., 1998). For the reactive oxygen species (ROS) production assay, low intensity (~55 μmol m−2 s−1) and moderately high intensity (~180 μmol m−2 s−1) light conditions were additionally used. Crosses and allelism tests were performed as reported in Berná et al. (1999).
Positional cloning and molecular characterization of anu10 alleles
Low-resolution mapping of the anu10-1 mutation was performed as described in Ponce et al. (2006). For fine mapping, the nga392, SO392, and cer479911 insertion/deletion polymorphisms from Monsanto (http://www.arabidopsis.org/browse/Cereon) and the F3M18 and F1K23 single nucleotide polymorphisms (SNPs) from the 1001 genomes project database (Weigel and Mott, 2009) were used. To find the anu10-1 mutation, a 3918bp fragment encompassing the entire transcription unit of At1g28530 was PCR amplified from Ler and anu10-1 genomic DNA, and sequenced on an ABI PRISM 3130xl Genetic Analyser (Applied Biosystems). All the primers used for the cloning and sequencing of At1g28530 are listed in Supplementary Table S1 available at JXB online. The T-DNA insertions in N831342 and N828696 lines were confirmed by PCR using primers recommended by the ‘T-DNA Primer Design’ tool (http://signal.salk.edu/tdnaprimers.2.html; Supplementary Table S1).
Bioinformatic analyses
Four cDNA (BT005784.1, BT008607.1, BX816836.1, and AK228671.1) and nine expressed sequence tag (EST; AU228187.1, AU237163.1, BP599159.1, BP606540.1, BP662266.1, BP807381.1, BP846942.1, ES017536.1, and EL984216.1) sequences from GenBank were used to assemble the transcriptional unit of At1g28530 using CAP3 (http://pbil.univ-lyon1.fr/cap3.php) (Huang and Madan, 1999). Subcellular localization was predicted with TargetP (http://www.cbs.dtu.dk/services/TargetP/) (Emanuelsson et al., 2000) and Multiloc2 (http://abi.inf.uni-tuebingen.de/Services/MultiLoc2) (Blum et al., 2009). Chloroplast transit peptide sequences and transmembrane domains were predicted with ChloroP 1.1 (http://www.cbs.dtu.dk/services/ChloroP/) (Emanuelsson et al., 1999) and SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui/) (Hirokawa et al., 1998), respectively.
To identify ANU10 homologues, BLASTP searches (Altschul et al., 1997) were carried out at the NCBI server using a word size of 2 and default values for all other parameters. Full-length sequences were selected based on a BLAST E-value cut-off of 3×10–6, and were subsequently aligned using the consistency-based method implemented in T-Coffee (Notredame et al., 2000). Alignments were refined with MUSCLE (http://www.ebi.ac.uk/Tools/msa/muscle/) (Edgar, 2004) and shaded with BOXSHADE3.21 (http://www.ch.embnet.org/software/BOX_form.html). Identity percentages were calculated using the Sequence Manipulation Suite (http://www.bioinformatics.org/sms2/index.html) (Stothard, 2000). Phylogenetic trees were obtained using MEGA5 (Tamura et al., 2011). Searches for distant homologues of ANU10 were carried out with HMMER (http://hmmer.janelia.org/) (Finn et al., 2011).
RNA isolation, cDNA synthesis, and qRT-PCR
Total RNA from Col-0 rosettes [collected 21 d after stratification (das)] was extracted using TRI Reagent (Sigma), and DNA was removed using the TURBO DNA-free Kit (Invitrogen). First-strand cDNA was synthesized using random hexamers and the Maxima Reverse Transcriptase system (Fermentas). For quantification of the expression of nuclear and plastid genes, the primers listed on Supplementary Table S1 available at JXB online were used. The 18S rRNA gene was used as an internal control (Yamauchi et al., 2004). Three different biological replicates and triplicate reactions were used. Amplification reactions were prepared in a volume of 20 μl by adding 7.5 μl of Maxima SYBR Green/ROX qPCR Master Mix (Fermentas), 3 μl of the corresponding primer pair (2.5 μM each), and 1 μl of cDNA template. Relative quantification of gene expression data was performed using the comparative C T method (Schmittgen and Livak, 2008) on a Step One Plus System (Applied Biosystems).
Gene constructs and plant transformation
To make the 35S pro :ANU10 and 35S pro :ANU10:GFP (green fluorescent protein) transgenes, the full-length coding sequence of ANU10 was amplified from Col-0 cDNA using Phusion polymerase (Thermo Scientific) with the ANU10cds-F and ANU10cds-R primers (Supplementary Table S1 at JXB online). The amplification product was cloned into the pENTR/D-TOPO entry vector (Invitrogen) and transferred into the pMDC32 and pMDC83 destination vectors, which include a dual 35S promoter (Curtis and Grossniklaus, 2003). For the ANU10 pro :GUS (β-glucuronidase) transgene, a 1.5-kb fragment encompassing the intergenic region between At1g28530 and At1g28540, including the At1g28530 5′-untranslated region (UTR) and At1g28540 3′-UTR, was amplified using Col-0 genomic DNA as the template. The resulting product was cloned into the pGEM-T Easy221 vector (kindly provided by B. Scheres), and then transferred into the pMDC163 destination vector (Curtis and Grossniklaus, 2003). Alternatively, the ANU10 promoter region was fused with the ANU10 full-length cDNA in a two-template PCR of overlapping products, using the primers ANU10cds-pro and ANU10pro-cds (Supplementary Table S1). The resulting fusion product was cloned into the pGEM-T Easy221 vector and transferred into pMDC111 (Curtis and Grossniklaus, 2003) to obtain the ANU10 pro :ANU10:GFP construct. All the constructs were transformed into Agrobacterium tumefaciens LBA4404. Col-0, Ler, and anu10-1 plants were transformed by the floral dip method (Clough and Bent, 1998). T1 transgenic plants were selected on plates supplemented with 15 μg ml–1 hygromycin B (Invitrogen).
Microscopy, histology, and morphometry
For transmission electron microscopy, mutant, wild-type, and transgenic plants were harvested 16 das. Leaf tissue excluding the primary vein and leaf margin was excised and fixed in McDowell’s solution (McDowell and Trump, 1976), and prepared as previously described (Hricová et al., 2006). Samples were visualized at 80kV using a JEOL 1011 transmission electron microscope equipped with a Gatan 792 BioScan digital camera. Grana diameter and height measurements were obtained from transmission electron micrographs using the ImageJ software. Leaf tissues were imaged using a Leica TCS SPE confocal microscope. Root tissues were imaged using a Nikon C1 confocal microscope. Transverse sections of leaves were obtained as described by Serrano-Cartagena et al. (2000), embedding the tissue in Technovit7100 resin and obtaining 10 μm sections. Rosette area measurement and morphometric analysis of palisade cells and transverse sections of leaves were performed as described previously (Pérez-Pérez et al., 2011; Ferrández-Ayela et al., 2013).
Dry weight and pigment determination
For dry weight measurement, eight plants of each genotype were oven-dried for 48h at 55 ºC. For determination of chlorophylls and carotenoids, four independent samples of 100mg of fresh leaves from rosettes collected 16 das were pooled, frozen in liquid N2, and homogenized with 3.5ml of cold 80% acetone. The samples were centrifuged for 5min at 5000rpm and the pigment concentration in the supernatant was spectrophotometrically determined as previously described (Wellburn, 1994).
ROS, GUS, and Lugol staining
To determine ROS accumulation, a minimum of six first-node leaves from each genotype were excised and incubated overnight in 1mg ml–1 3,3′-diaminobenzidine (DAB; Sigma-Aldrich), under vacuum and in the dark. Leaves were cleared by boiling in acetic acid:glycerol:ethanol (1:1:3 v/v/v) for 5min, and then incubated in 96% (v/v) ethanol until chlorophyll was bleached. GUS staining of plant tissues and Lugol staining of root tips were performed as described in Robles et al. (2010) and Willemsen et al. (1998), respectively. Samples were visualized with a Nikon C1 microscope.
Western blot analysis of chloroplast proteins and blue native PAGE of thylakoid membranes
Chloroplasts from rosette leaves collected 16 das were isolated as described by Grabsztunowicz and Jackowski (2012), omitting the Percoll step gradient. Stroma and thylakoid membranes from isolated chloroplasts were separated as previously reported (Armbruster et al., 2010). A volume of isolated chloroplasts and thylakoids equivalent to 20 μg of chlorophyll, as well as 20 μg of proteins from the stroma fraction, including a lane with an EZ-Run Pre-stained Rec Protein Ladder (Fisher BioReagents), were resolved by 10% SDS–PAGE, blotted on nitrocellulose membranes (Amersham Hybond ECL, RPN203D; GE Healthcare), and subjected to immunoblot analysis with specific antibodies (G1544, Sigma; AS03 037-10 and AS05 092, Agrisera AB). Isolation of thylakoids from rosettes collected 16 das and blue native PAGE were carried out as reported in Pérez-Pérez et al. (2013).
Results
Leaf and whole-plant defects in anu10-1 plants
In a large-scale screen for EMS-induced mutants with abnormal leaf shape, 18 mutants with pale-green leaves and dentate margins were previously identified. The causative mutations were dubbed angulata (anu), and complementation tests showed that they damage 12 different genes (ANU1–ANU12) (Berná et al., 1999).
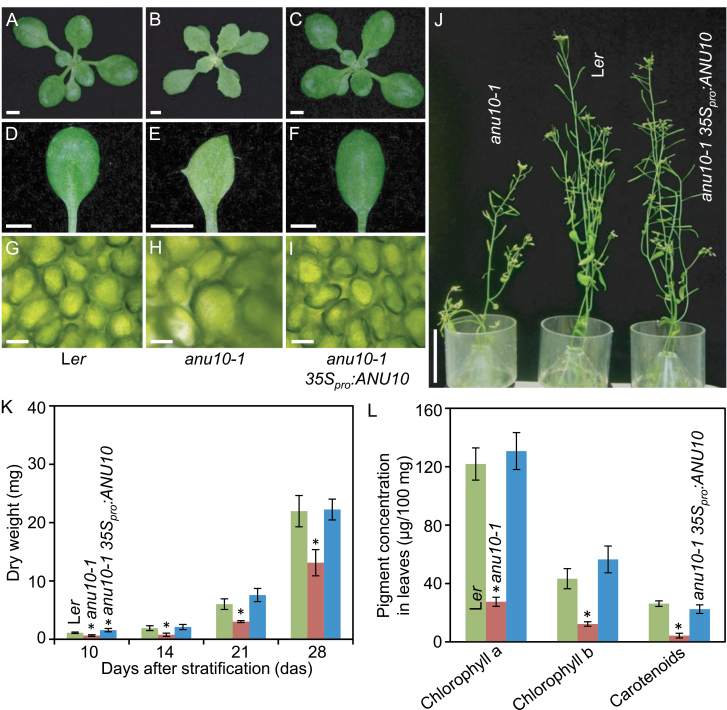
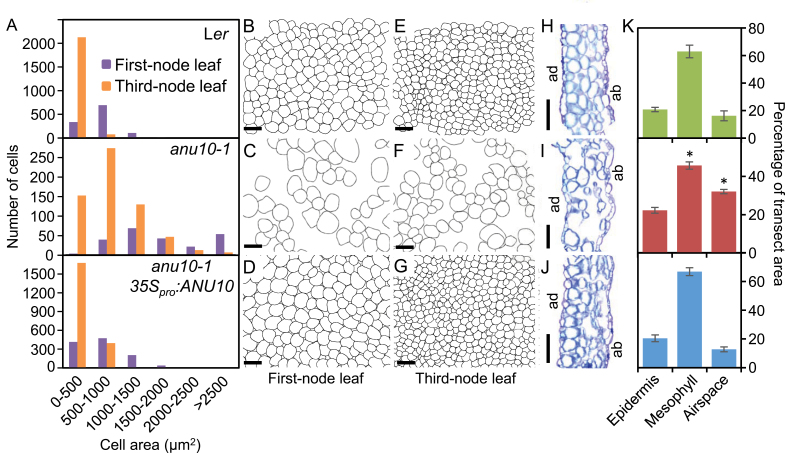
The only mutant allele of the ANU10 gene, anu10-1, causes reduced rosette and stem growth (Fig. 1A, B, J). The dry weight and projected area of anu10-1 rosettes were significantly lower than in wild-type Ler plants throughout the study (Fig. 1K; Supplementary Fig. S1 at JXB online). Rosettes of the anu10-1 mutant harvested 21 das showed a dry weight of 3.02±0.16mg and an area of 147.72±32.68mm2, while the corresponding values for Ler were 6.02±0.91mg and 701.48±56.88mm2, respectively. Adult anu10-1 plants also exhibited a significant reduction in shoot length: 28.47±2.69cm in Ler, but only 18.22±3.55cm in anu10-1 42 das (Fig. 1J; Supplementary Fig. S1). The number of secondary stems was lower in anu10-1 (4.67±0.52) than in Ler (9.25±0.71) determined 35 das, although this difference disappeared over time (Supplementary Fig. S1). The leaves of anu10-1 were pale green and their margins had prominent teeth (Fig. 1E). As seen in paradermal sections, the palisade mesophyll cells were irregular in size, with larger cells in anu10-1 than in the wild type (Fig. 1G, H). By measuring the size of individual cells, a shift in the distribution of palisade cell size towards greater values (Fig. 2A), as well as the presence of large intercellular spaces in the palisade mesophyll of anu10-1 leaves (Fig. 2B, C, E, F) was detected. Similar defects were observed in transverse sections of first-node leaves (Fig. 2H, I). In these sections, mesophyll cells filled a significantly smaller percentage of the section area, which was matched by a significant increase in the area occupied by air spaces in anu10-1 leaves (Fig. 2K).
Fig. 1.
Phenotypic characterization and rescue of the anu10-1 mutant. (A–C) Rosettes, (D–F) first-node leaves, and (G–I) bright-field micrographs of the subepidermal layer of palisade mesophyll cells from (A, D, G) the Ler wild type, (B, E, H) the anu10-1 mutant, and (C, F, I) a transgenic anu10-1 35Spro:ANU10 plant. (J) Adult plants. Pictures were taken (A–I) 16 and (J) 42 das. Scale bars indicate (A–F) 2mm, (G–I) 30 μm, and (J) 5cm. (K) Dry weight and (L) pigment content in Ler, anu10-1, and transgenic anu10-1 35Spro:ANU10 plants. Error bars indicate standard deviations. Asterisks indicate values significantly different from Ler in a Mann–Whitney U-test [(K) P<0.01, n=8 and (L) P<0.05, n=4].
Fig. 2.
Morphometry of anu10-1 mesophyll cells. (A) Distribution of palisade mesophyll cell area in first- and third-node leaves from Ler, anu10-1, and anu10-1 35S pro :ANU10 plants (n=8). (B–G) Representative diagrams of the subepidermal layer of palisade mesophyll cells from (B–D) first- and (E–G) third-node leaves. Diagrams were drawn from differential interference contrast pictures taken from cleared leaves. (H–J) First-node leaf transverse sections from (H) Ler, (I) anu10-1, and (J) anu10-1 35S pro :ANU10 plants. ad, adaxial surface, ab, abaxial surface. Scale bars indicate (B–G) 50 μm and (H–J) 500 μm. (K) Percentage of leaf transect area occupied by epidermis, mesophyll (including palisade and spongy mesophyll cells and bundle sheath cells), or air spaces in Ler (green), anu10-1 (red), and anu10-1 35S pro :ANU10 (blue) first-node leaves. Error bars indicate standard deviations. Asterisks indicate values significantly different from Ler in a Mann–Whitney U-test (P<0.01, n=6).
To gain insight into the physiological basis of the pale-green phenotype of anu10-1, pigment levels were measured in the mutant. In line with the observed pale-green phenotype (Fig. 1B, E), a reduction in the levels of chlorophyll a, chlorophyll b, and carotenoids was detected (Fig. 1L). Because carotenoids play an important role in photoprotection, the presence of ROS, visualized by staining with DAB, was also tested. Darker staining was observed in anu10-1 leaves relative to Ler (Supplementary Fig. S2 available at JXB online), which indicates the presence of increased levels of H2O2. In the mutant, the accumulation of H2O2 was positively correlated with light intensity (Supplementary Fig. S2).
Positional cloning of ANU10
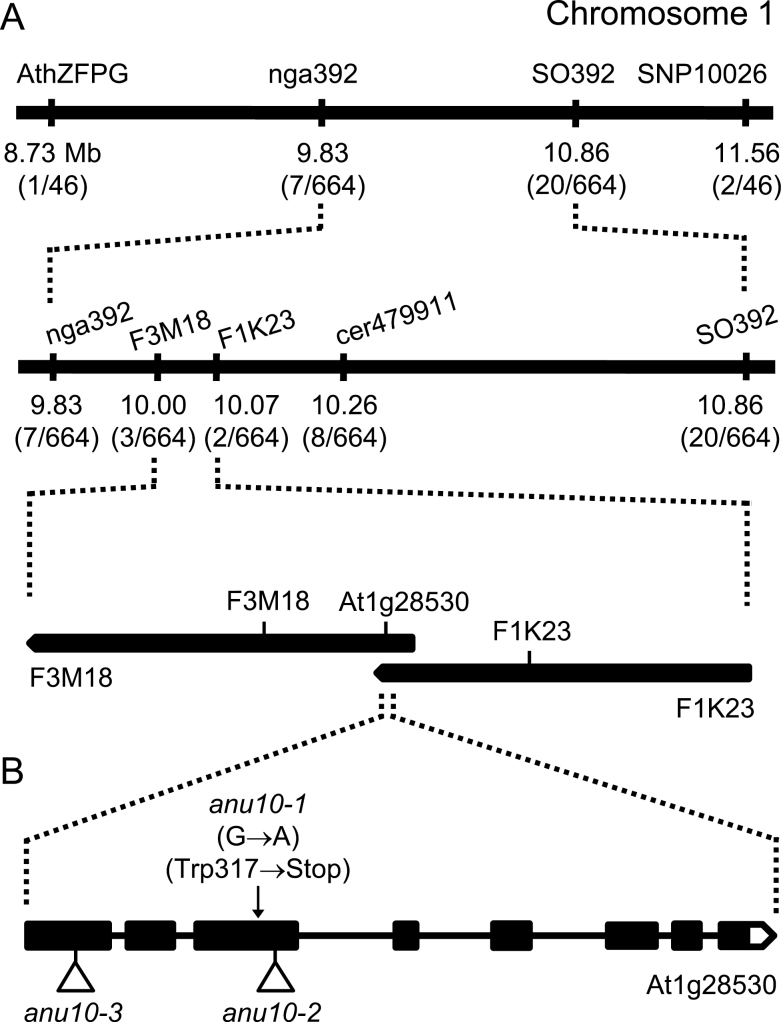
The anu10-1 mutation was previously mapped to chromosome 1 (Robles and Micol, 2001). To understand further the molecular basis of the phenotype of anu10-1, a positional cloning approach was undertaken to identify the causal gene. First a 72kb candidate interval (Fig. 3A) flanked by two SNP markers, F3M18 and F1K23 (Supplementary Table S1 available at JXB online), was defined. This interval encompassed 24 candidate genes. Because the phenotype of anu10-1 suggests a defect in a chloroplast-related function (Fig. 1B, E), the At1g28530 gene, which is the only gene in this interval predicted to encode a chloroplast-localized protein, was the focus of further study. The At1g28530 gene was sequenced in mutant and wild-type plants, and a G→A transition was found in its coding region only in anu10-1 mutant plants (Fig. 3B). Expression of the At1g28530 gene is supported by four cDNA and nine EST sequences deposited in GenBank (see the Materials and methods). In order to determine the intron–exon structure of the At1g28530 gene, these sequences were assembled using CAP3 and the consensus mRNA sequence was aligned to the sequence of Arabidopsis chromosome 1 (NC_003070). The consensus mRNA sequence has eight exons and its longest open reading frame encodes a protein with 614 amino acids and a molecular mass of 68.46kDa. The anu10-1 mutation introduces a stop codon (Trp317→Stop) in the third exon of the gene (Fig. 3B), and is predicted to truncate the protein prematurely (from 614 amino acids in Ler to only 316 in anu10-1).
Fig. 3.
Positional cloning of ANU10. (A) A mapping population of 332 F2 plants derived from an anu10-1×Col-0 cross allowed a candidate region of 72kb to be defined in chromosome 1. Names and physical map positions of the molecular markers used for linkage analysis are shown. The number of recombinant chromosomes found and the total number of chromosomes analysed are indicated in parentheses. (B) Structure of the ANU10 gene with indication of the nature and position of the anu10 mutations. Boxes and lines indicate exons and introns, respectively. A white box represents the 3′-UTR. Triangles indicate T-DNA insertions.
To identify additional alleles of the ANU10 gene, two publicly available lines, SAIL_708_F05 and SAIL_659_F07, which carry T-DNA insertions in the coding sequence of At1g28530 were characterized. When homozygous for the T-DNA insertion, these two lines displayed phenotypes similar to those of anu10-1, including leaves with prominent marginal teeth and paler rosettes, when compared with their wild-type Col-0 (Supplementary Fig. S3A–D at JXB online). In general, these phenotypes were less severe than those of anu10-1 and were more apparent in younger rosettes, becoming indistinguishable from Col-0 14–15 das. The mutant phenotype of the F1 progeny from the crosses of anu10-1 to SAIL_708_F05 and SAIL_659_F07 indicated that both T-DNA lines carry anu10 alleles (Supplementary Fig. S3E, F), which were named anu10-2 and anu10-3 (Fig. 3B). Because the anu10-2 and anu10-3 mutants carry T-DNA insertions in exons 3 and 1, respectively, which are expected to disrupt the coding potential of the mRNA, it is speculated that the milder effects of these mutant alleles might reflect differences between the Ler and Col-0 genetic backgrounds.
To confirm further the identity of the ANU10 gene, a construct was made to express the coding region of At1g28530 constitutively. When transformed into anu10-1 plants, the 35S pro :ANU10 transgene fully complemented the defects in leaf shape (Fig. 1C, F), growth (Fig. 1C, J; Supplementary Fig. S1 at JXB online), mesophyll development (Figs 1I, 2A, D, G, J, K), pigment levels (Fig. 1L), and H2O2 accumulation (Supplementary Fig. S2) in each of six independent transformants. Therefore, the correct identification of ANU10 as the At1g28530 gene is supported both by the transgenic complementation studies and by the independent isolation of three mutant alleles carrying lesions in the coding region of At1g28530.
Analysis of ANU10 expression
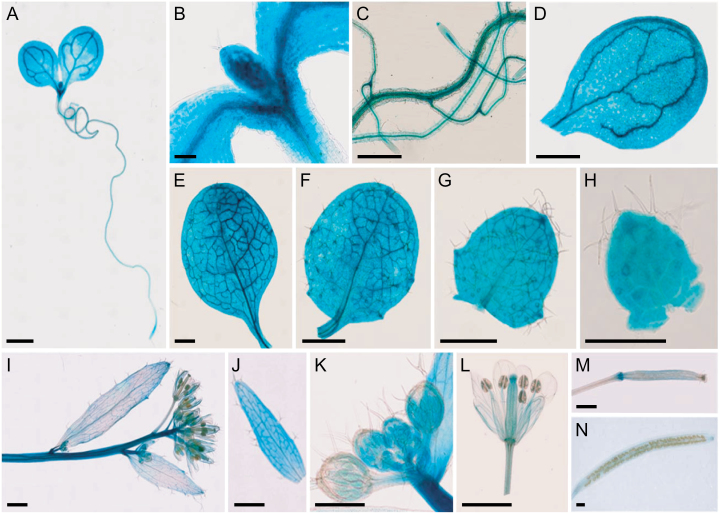
To determine the expression pattern of ANU10 in wild-type plants, an ANU10 pro :GUS reporter transgene was constructed. Four independent transgenic plants stably expressing the transgene and showing the same staining pattern were studied. The GUS signal was broad in seedlings collected 3 das (Fig. 4A), being particularly intense in incipient leaves (Fig. 4B). In plants collected 13 das, GUS activity was detected in roots, cotyledons, and leaves (Fig. 4C–H). In older plants, GUS activity was observed in stems, cauline leaves, flowers, and siliques (Fig. 4I–N). Remarkably, the GUS signal was more intense in young cauline leaves (Fig. 4J), flowers (Fig. 4K), and siliques (Fig. 4M) than in the corresponding mature organs (Fig. 4I, L, N). The spatio-temporal expression analysis suggests that ANU10 is expressed in all plant organs and that its expression is particularly important in developing organs.
Fig. 4.
Visualization of ANU10 pro :GUS activity in a wild-type background. (A) Seedling, (B) detail of the shoot apex, (C) roots, and (D) cotyledon. (E–H) Vegetative leaves from the (E) first, (F) third, (G) fifth, and (H) seventh nodes. (I) Inflorescence, (J) young cauline leaf, (K) immature flowers, (L) mature flower, and (M) immature and (N) mature siliques. Pictures were taken (A) 3, (B) 6, (C–H) 13, and (I–N) 42 das. Scale bars indicate (A, D–G, I, J, L–N) 1mm, (C, H, K) 500 μm, and (B) 100 μm.
The ANU10 gene encodes a protein of unknown function
ANU10 is a single-copy gene in the nuclear genome of Arabidopsis. Because the gene is predicted to encode a protein of unknown function with no conserved domains, BLAST searches were carried out to identify similar protein sequences in public databases. Significant hits were found in the genomes of other higher plants and the moss Physcomitrella patens, but not in those of animals or other eukaryotes, including algae, suggesting that ANU10 belongs to a family of embryophyte-specific proteins (Supplementary Fig. S4 available at JXB online). The chloroplast localization of ANU10 was consistently predicted by several computational tools (see the Materials and methods), including TargetP (score=0.929) and Multiloc2 (score=0.57). ChloroP 1.1 predicted a chloroplast transit peptide in ANU10 and in most of its orthologues from other land plants (Supplementary Fig. S4A, Supplementary Table S2). In addition to the transit peptide, ANU10 and most of its orthologues were also predicted to have a transmembrane domain (Supplementary Fig. S4A, Supplementary Table S2), suggesting that these proteins are anchored to chloroplast membranes. As an example, SOSUI predicted a transmembrane domain spanning residues 421–443 in ANU10.
To gain insight into the evolutionary origin of this protein family, HMMER searches were carried out using a profile made with the sequences of several ANU10 homologues from land plants. HMMER allowed the identification of some distantly related sequences in Cyanobacteria. In line with these results, a search for known domains in the Pfam database (Punta et al., 2012) yielded a low significance hit to a domain of unknown function (DUF4335) present in some cyanobacterial proteins. Together, these data indicate that ANU10 is conserved among land plants. However, unlike proteins such as CURT1A (Armbruster et al., 2013), which is functionally conserved in Cyanobacteria, the search for cyanobacterial orthologues of ANU10 did not yield obvious candidates.
ANU10 localizes to plastids
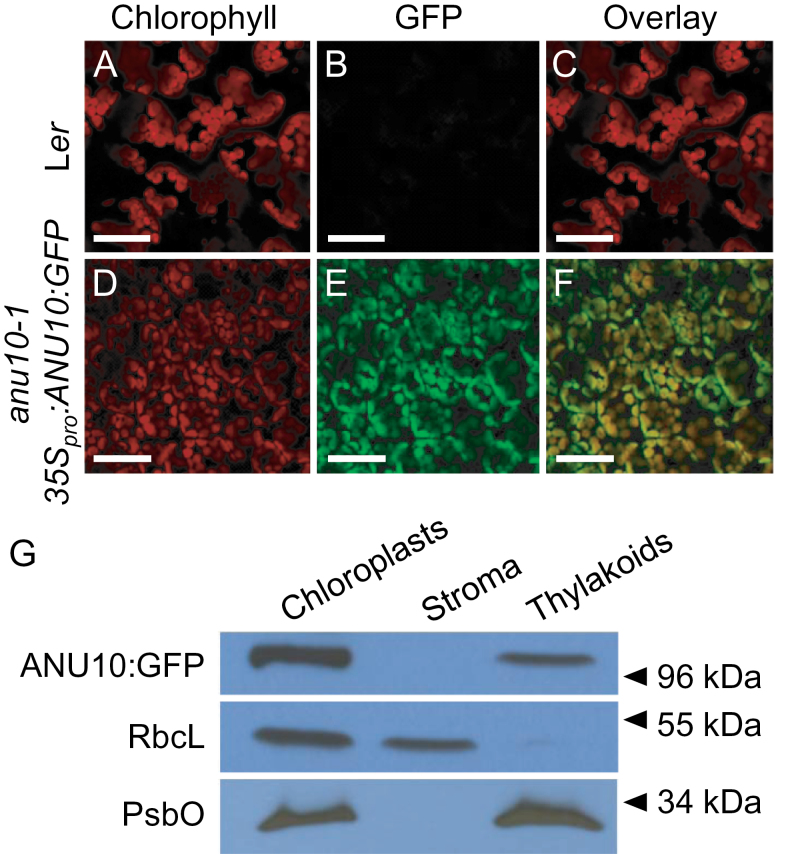
To determine experimentally the subcellular localization of the ANU10 protein, an in-frame translational fusion of GFP to the C-terminal end of ANU10 was made. Transgenic anu10-1 plants expressing 35S pro :ANU10:GFP were used to visualize the GFP signal by confocal laser scanning microscopy. The GFP signal was specifically detected in chloroplasts from four independent lines carrying the 35S pro :ANU10:GFP transgene (Fig. 5A–F). To exclude that the observed localization pattern represents an artefact due to overexpression of the ANU10:GFP fusion protein, the same translational fusion was also placed under the control of the endogenous ANU10 promoter (ANU10 pro :ANU10:GFP). An identical distribution of GFP signal was observed in anu10-1 plants expressing the ANU10 pro :ANU10:GFP transgene (Supplementary Fig. S5 at JXB online), demonstrating that the promoter chosen does not affect the subcellular localization of the fusion protein. Interestingly, both transgenes were able partially to rescue the anu10-1 phenotype in young rosettes (Supplementary Fig. S6). The rescue was complete 21 das regardless of the promoter used, indicating that the ANU10:GFP fusion protein retains sufficient activity to complement the mutant phenotype at this stage. To determine the suborganellar localization of the ANU10 protein, stromal and thylakoidal fractions were isolated from the chloroplasts of anu10-1 35S pro :ANU10:GFP transgenic plants. The presence of the ANU10:GFP fusion protein in each fraction was tested by western blotting using an anti-GFP antibody. The fusion protein was specifically detected in thylakoids, but not in the soluble stromal fraction (Fig. 5G), indicating that ANU10 is associated with thylakoidal membranes.
Fig. 5.
Subcellular and suborganellar localization of ANU10. (A–F) Confocal micrographs of the subepidermal layer of palisade mesophyll cells from (A–C) Ler and (D–F) anu10-1 35S pro :ANU10:GFP transgenic plants. Micrographs show (A, D) the chlorophyll autofluorescence of the chloroplasts, (B, E) the GFP fluorescence, and (C, F) an overlay of the chlorophyll and GFP signals, showing their co-localization in (F). Pictures were taken from first-node leaves collected 16 das. Scale bars indicate 50 μm. (G) Western blot analysis of the proteins in chloroplast, stroma, and thylakoid fractions isolated from anu10-1 35S pro :ANU10:GFP transgenic plants collected 16 das. Primary antibodies against GFP, the large Rubisco subunit (RbcL), and the PsbO subunit of PSII were used. Molecular mass markers are indicated on the right.
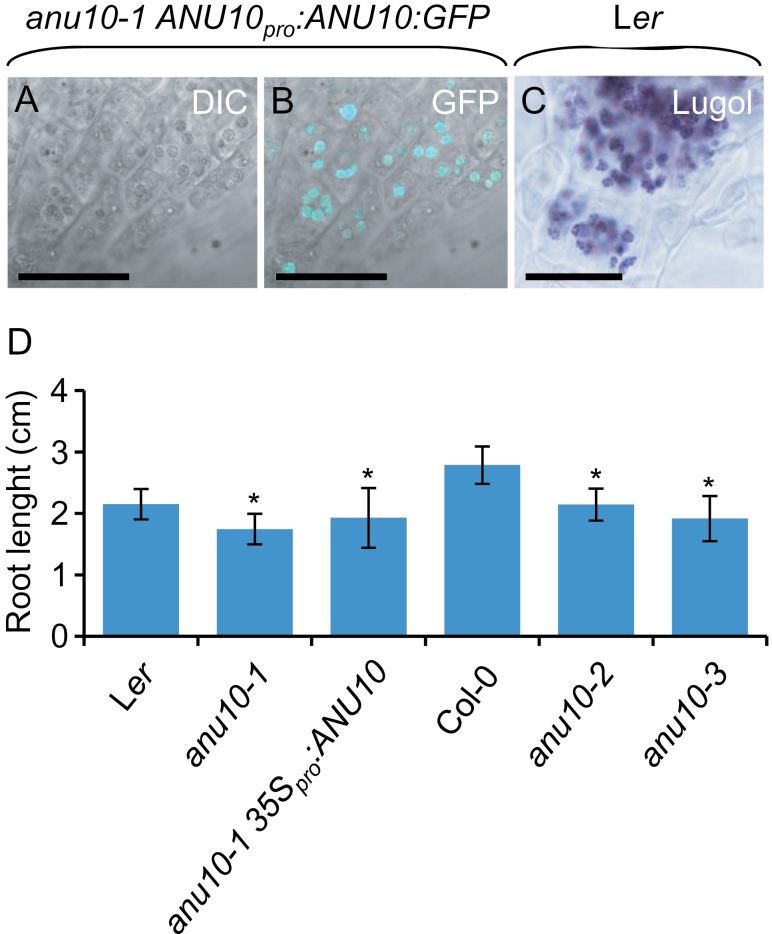
Because ANU10 was found to be expressed in roots (Fig. 4C), root tissues were also examined under a confocal microscope. A strong GFP signal was detected in root tips from four independent lines expressing the ANU10 pro :ANU10:GFP transgene (Fig. 6A, B). The pattern of GFP signal paralleled the distribution of amyloplasts in root tips stained with lugol (Fig. 6C). Although amyloplasts play a central role in root graviperception, no obvious defects were detected in the gravitropic response of anu10 roots (Supplementary Fig. S7A at JXB online). Starch content was similar between anu10 mutants and the wild type, as visualized after lugol staining (Supplementary Fig. S7B). The primary root length was reduced in anu10-1 (1.75±0.25cm) compared with Ler (2.15±0.25cm) (Fig. 6D). Roots of anu10-2 and anu10-3 were also shorter (2.14±0.26 and 1.92±0.37cm, respectively) than those of Col-0 (2.79±0.30cm). However, this phenotype, unlike the leaf phenotypes, was not restored in anu10-1 35S pro :ANU10 transgenic plants, in which the mean length of the main root was 1.93±0.49cm (Fig. 6D).
Fig. 6.
Localization of the ANU10 protein and effect of anu10 mutations on root growth. (A, B) Confocal micrographs of the root apex from an anu10-1 ANU10 pro :ANU10:GFP transgenic plant: (A) differential interference contrast (DIC) image and (B) an overlay of the GFP fluorescence and the DIC image. (C) Root apex from a Ler plant stained with lugol. Scale bars indicate 30 μm. (D) Main root length of anu10-1, anu10-2, and anu10-3 mutants, their respective wild types, and anu10-1 35S pro :ANU10 transgenic plants. Error bars indicate standard deviations. Asterisks indicate values significantly different from the wild type in a t-test (P<0.05, n=30).
Thylakoid membranes and LHCII trimers are less abundant in anu10-1
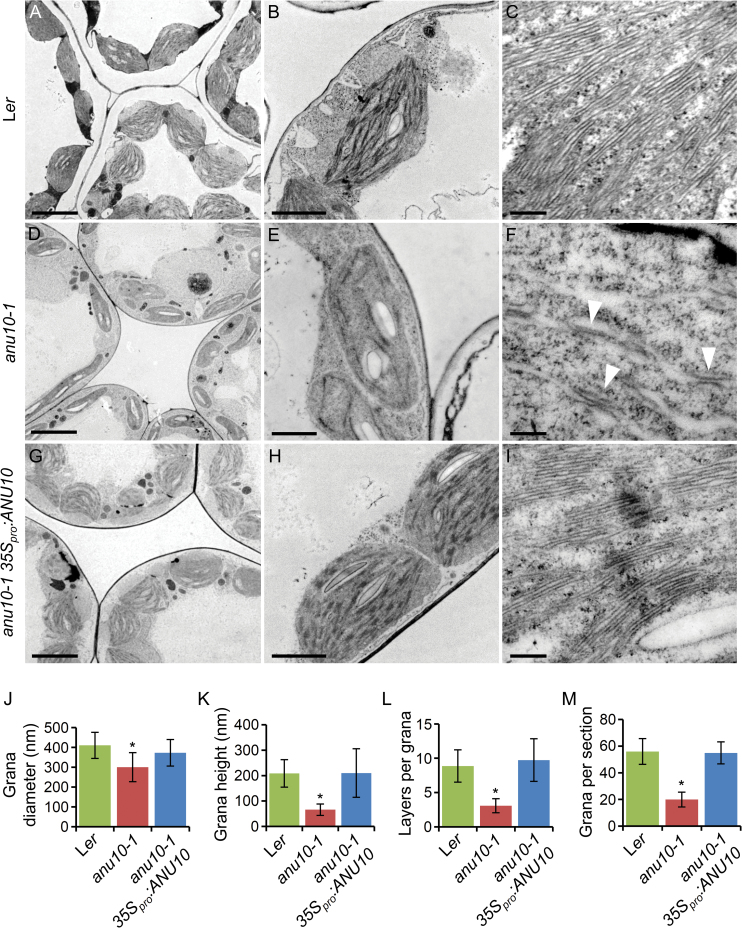
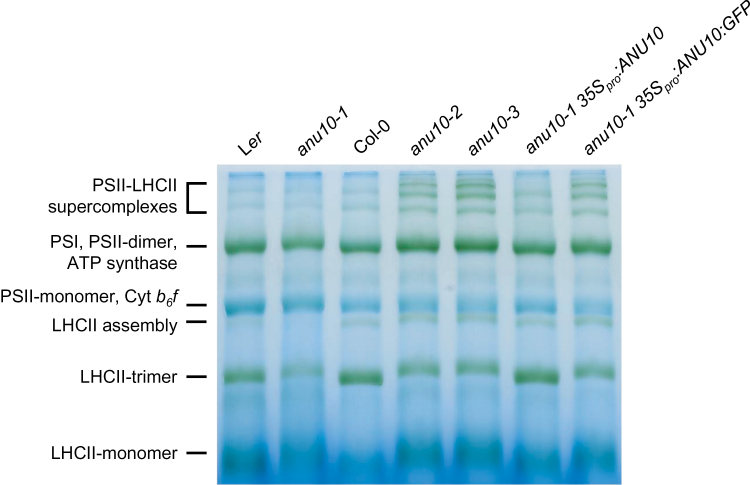
To investigate whether ANU10 deficiency affects the suborganellar organization of chloroplasts, the ultrastructure of chloroplasts from Ler, anu10-1, and anu10-1 35S pro :ANU10 mesophyll cells was studied using transmission electron microscopy. Chloroplasts were smaller in anu10-1 plants than in the wild type. While Ler chloroplasts are typically lens shaped (Fig. 7A, B), anu10-1 chloroplasts were smaller and abnormally shaped (Fig. 7D, E). Fewer thylakoidal membranes were observed in anu10-1 chloroplasts (Fig. 7B, E). When observed at high magnification, it was found that anu10-1 thylakoids failed to stack and form typical grana. Under the conditions used here, wild-type grana were composed of 8.37±2.36 layers of thylakoidal membranes, while the number of layers per grana was only 3.08±1.02 for anu10-1 (Fig. 7F, L), as also reflected by the decreased height of anu10-1 grana (Fig. 7K). Moreover, the diameter of the grana and the number of grana per chloroplast section were found to be reduced in anu10-1 (Fig. 7J, M). Chloroplast size, morphology, thylakoid abundance, and grana morphology and stacking were totally restored to those of the wild type in anu10-1 plants expressing the 35S pro :ANU10 transgene (Fig. 7G–I, J–M). Because LHCII trimers are thought to participate in thylakoid stacking, blue native PAGE was used to study the levels of trimeric LHCII complexes in isolated thylakoids from 16 das plants. LHCII trimers migrated slightly more slowly and their levels were reduced in thylakoids of anu10-1 compared with those of Ler (Fig. 8A, lanes 1 and 2). The anu10-2 and anu10-3 mutants showed a similar reduction in the amount and mobility of LHCII trimers relative to the Col-0 wild type (Fig. 8A, lanes 3–5). The mobility and levels of LHCII trimers were fully restored in thylakoids of anu10-1 plants carrying the 35S pro :ANU10 transgene (Fig. 8A, lane 6). The levels of LHCII trimers were partially restored to wild-type levels in anu10-1 35S pro :ANU10:GFP plants (Fig. 8A, lane 7), in line with the partial phenotypic rescue seen in anu10-1 35S pro :ANU10:GFP plants collected 16 das (Supplementary Fig. S6B at JXB online). Taken together, these results suggest that ANU10 is important for thylakoid biogenesis and grana formation, and do not allow the exclusion that a defect in the levels or composition of the LHCII trimers might be responsible for the reduced thylakoid stacking seen in the anu10-1 mutant.
Fig. 7.
Ultrastructure of anu10-1 chloroplasts. Transmission electron micrographs of palisade mesophyll cell chloroplasts from (A–C) Ler, (D–F) anu10-1, and (G–I) anu10-1 35S pro :ANU10. Arrowheads in (F) indicate unstacked thylakoid membranes in the anu10-1 mutant. Pictures were taken from first-node leaves collected 16 das. Scale bars indicate (A, D, G) 5 μm, (B, H) 2 μm, (E) 1 μm, and (C, F, I) 200nm. (J–M) Comparison of (J) the diameter (x-axis) and (K) height (y-axis) of granal stacks, (L) the number of membrane layers in granal stacks, and (M) the number of stacks in chloroplast sections from Ler, anu10-1, and anu10-1 35S pro :ANU10. The number of grana per chloroplast section was determined from images of individual chroroplasts similar to those shown in B, E, and H. Asterisks indicate values significantly different from the wild type in a t-test (P<0.05, n=10–20).
Fig. 8.
Thylakoidal protein complexes in the anu10 mutants. Blue native PAGE of photosynthetic protein complexes from anu10-1, anu10-2, and anu10-3 mutants, their respective wild types, and anu10-1 35S pro :ANU10 and anu10-1 35S pro :ANU10:GFP transgenic plants. PSII, photosystem II; PSI, photosystem I; Cyt b 6 f, cytochrome b 6 f complex; LHCII, light-harvesting chlorophyll a/b-binding protein complex II.
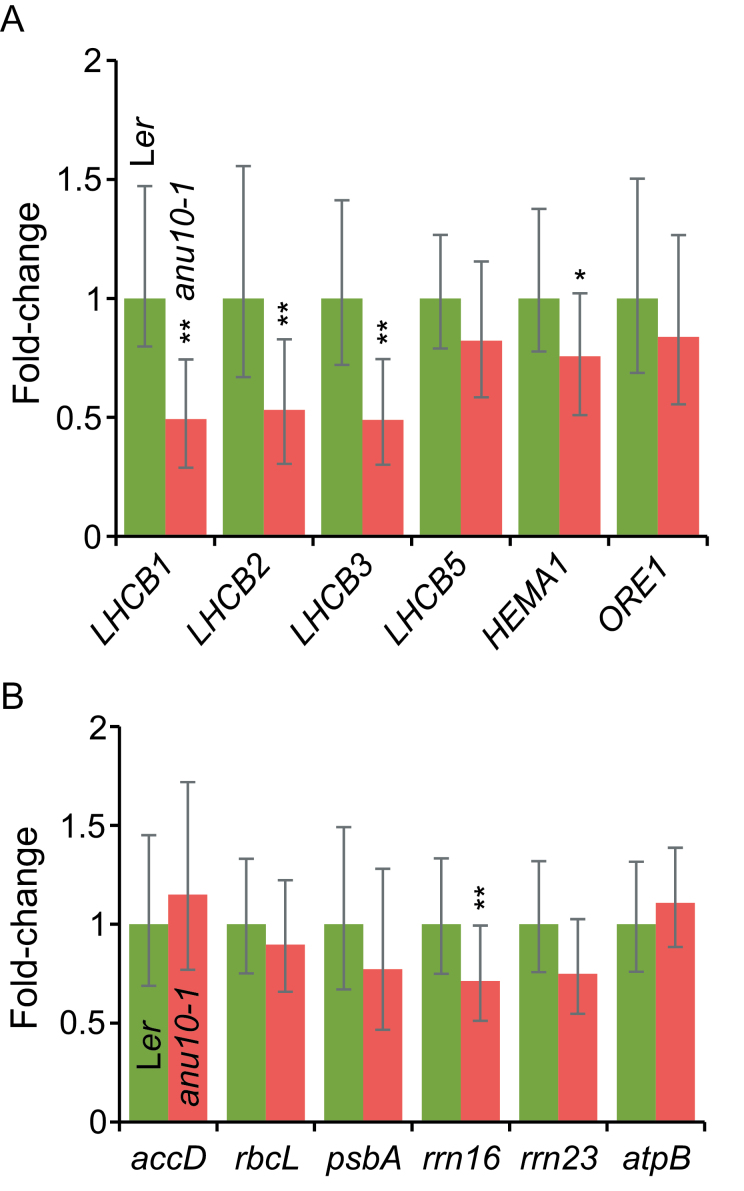
Expression of nuclear and chloroplast genes in anu10-1
Quantitative RT-PCR (qRT-PCR) was used to explore whether ANU10 deficiency affects the expression of nuclear and plastid genes. The relative expression of nuclear genes encoding subunits of LHCII trimers (LHCB1, LHCB2 and LHCB3) was found to be ~0.5-fold decreased in anu10-1 compared with Ler (Fig. 9A). Because the LHCB5 gene is known to be overexpressed in plants that do not express the LHCB1 and LHCB2 proteins (Ruban et al., 2003), the expression of LHCB5 was also analysed. However, the expression levels of LHCB5 were not significantly different in anu10-1 and Ler (Fig. 9A). Because the impact of the anu10-1 mutation on the expression of LHCB genes might be an indirect consequence of a more general problem that triggers retrograde signals from the chloroplast to the nucleus, the expression of the HEMA1 gene, which is involved in chlorophyll biosynthesis and is particularly tightly regulated by chloroplast-to-nucleus signals (Mochizuki et al., 2008), was also examined. The expression of HEMA1 was significantly although mildly reduced in anu10-1 (Fig. 9A), suggesting that the reduced expression of LHCB genes is at least in part an indirect effect of the mutation on these signalling pathways.
Fig. 9.
Expression of nuclear and plastid genes in the anu10-1 mutant. (A, B) qRT-PCR analysis of the expression of (A) LHCB1, LHCB2, LHCB3, LHCB5, HEMA1, and ORE1 nuclear genes, and (B) accD, psbA, rbcL, rrn16, rrn23, and atpB plastid genes in Ler and anu10-1 rosettes collected 16 das. Bars indicate the relative expression levels, determined by the comparative CT method, and normalized with the expression of the 18S rRNA housekeeping gene. Error bars indicate the interval delimited by 2–(ΔΔCT± SD). Asterisks indicate ΔCT values significantly different from those of Ler in a Mann–Whitney U-test (P<0.01; n=9).
Because the phenotype of anu10-1 mutants might also be interpreted as a consequence of premature senescence, which might lead to grana disassembly and thylakoid swelling (Evans et al., 2010; Krupinska et al., 2012), the expression level of the nucleus-encoded ORESARA1 (ORE1) transcription factor, a senescence marker that has recently been reported to be a key regulator of the senescence response (Kim et al., 2009; Balazadeh et al., 2010), was also monitored. ORE1 was expressed at similar levels in anu10-1 mutants and wild-type rosettes (Fig. 9A), suggesting that premature senescence is not the cause of the observed phenotype.
To survey the effects of the anu10-1 mutation on the expression of plastid genes, the transcript levels of the plastid-encoded accD, rbcL, psbA, rrn16, rrn23, and atpB genes were also quantified. A reduction only of rrn16 expression in anu10-1 mutants was observed, while the expression levels of the other genes were not significantly different from those of Ler (Fig. 9B), indicating that the defects of anu10-1 chloroplasts do not result from a general deregulation of organelle transcription.
Discussion
ANU10 encodes a novel plastid protein conserved throughout land plants
The chloroplast proteome comprises ~1300 different proteins unambiguously identified in proteomic studies (Zybailov et al., 2008; Ferro et al., 2010), ~92% of which are encoded in the nuclear genome. However, the function of a significant fraction (~30%) of these proteins remains unknown (Kleffmann et al., 2004), indicating that additional research is needed to understand fully the processes that take place in the chloroplast. In the present work, the ANU10 gene has been identified using a map-based cloning strategy. ANU10 is the founding member of a plant-specific family of proteins that contain a chloroplast transit peptide and a transmembrane domain. Using translational fusions to GFP, it has been shown that the ANU10 protein localizes to chloroplasts. A transmembrane domain is also predicted to occur in most ANU10 homologues from other species. In line with this prediction, the ANU10 protein was detected using immunoblotting in the fraction corresponding to thylakoidal membranes, but not in the soluble (stromal) fraction derived from the chloroplasts. Study of the expression pattern and subcellular localization of the ANU10 protein shows that the gene is expressed in a variety of tissues, including cotyledons, leaves, flowers, and roots, and is consistent with microarray data available from the Arabidopsis eFP Browser (http://bar.utoronto.ca/efp/cgi-bin/efpWeb.cgi). In close agreement with this expression pattern, GFP signal corresponding to the ANU10:GFP fusion protein was detected not only in the leaf chloroplasts, but also in the root amyloplasts. The data point to a differential requirement for ANU10 protein in roots and leaves. Although the expression of the full-length coding sequence of ANU10 restored the wild-type phenotype in anu10-1 mutant leaves, the root length was not restored to wild-type values, suggesting that roots are sensitive to an excess of ANU10 protein.
ANU10 is required for thylakoid biogenesis and grana stacking
Very little is known as yet about the mechanisms that lead to normal thylakoid biogenesis and grana stacking. The present finding that anu10-1 mutants exhibit aberrant thylakoid stacking and lack the typical grana seen in normal chloroplasts is expected to help in furthering understanding of these processes. Although a mechanistic link between the molecular function and the observed phenotypes is still lacking, ANU10 might contribute to the characteristic internal organization of chloroplasts in several different ways.
First, previous authors have proposed that LHCII trimers play a crucial role in thylakoid stacking and grana formation (Day et al., 1984; Allen and Forsberg, 2001; Cui et al., 2011). A lower amount of LHCII trimers in anu10-1 thylakoids was detected here, in line with the proposed role of trimeric LHCII complexes in thylakoid stacking. Reduced levels of LHCII trimers in the gdc1-3 mutant have been linked to impaired grana stacking in Arabidopsis (Cui et al., 2011), and other authors have shown that normal thylakoid stacking can still occur in plants with reduced levels of LHCB1 and LHCB2 (Andersson et al., 2003), which are compensated by higher levels of LHCB5 (Ruban et al., 2003). Stacks of thylakoidal membranes have been found in other mutants with reduced levels of LHCB transcripts, such as genomes uncoupled1 (gun1) (Susek et al., 1993), immutans (Wetzel et al., 1994), and CAB underexpressed4 (cue4) and cue9 (Lopez-Juez et al., 1998). Therefore, it is presently unclear if the moderate reduction in LHCB transcription and LHCII trimers detected in anu10 mutants can account for the thylakoid phenotype.
Secondly, the lack of grana stacks in anu10 mutants might be a consequence of a more general deficiency in thylakoid biogenesis. Thylakoidal membranes, which are less abundant in anu10-1 chloroplasts, are thought to originate from vesicles that form at the inner envelope of chloroplasts and subsequently incorporate into developing thylakoids (Eggink et al., 2001; Tanz et al., 2012). Three proteins known to participate in vesicle formation and transport during thylakoid biogenesis are VESICLE-INDUCING PROTEIN IN PLASTIDS1 (VIPP1), CHLOROPLAST SECRETION-ASSOCIATED RAS1 (CPSAR1), and SNOWY COTYLEDON2/SHI-YO-U1 (SCO2/CYO1). The VIPP1 protein localizes to the thylakoidal and chloroplast inner membranes, and the high-chlorophyll fluorescence155 (hcf155) mutant shows that VIPP1 is required for the formation of the vesicles (Kroll et al., 2001). CPSAR1 dually localizes to the chloroplast stroma and the inner envelope, and has been proposed to participate in the initiation of vesicles from the inner envelope (Garcia et al., 2010). SCO2/CYO1 is required for the trafficking of vesicles from the inner envelope to developing thylakoids (Tanz et al., 2012). Interestingly, the anu10-1 phenotype resembles the phenotype of loss-of-function hcf155, cpsar1, and sco2-1 mutants as regards the low abundance of thylakoids (Kroll et al., 2001; Garcia et al., 2010; Tanz et al., 2012). This raises the possibility that ANU10 participates in thylakoid biogenesis by modulating vesicle integration into developing thylakoids. Under this scenario, the reduced levels of LHCII trimeric forms detected in anu10-1 thylakoids would simply be an indirect consequence of inefficient thylakoid biogenesis, as LHCB proteins have been proposed to be targeted to thylakoids, at least in part, through their incorporation into developing vesicles in the inner membranes of chloroplasts (Eggink et al., 2001; Tanz et al., 2012).
Because some proteins involved in plastid gene expression, such as pTAC14 (Gao et al., 2011) or the nucleus-encoded RPOTmp polymerase (Azevedo et al., 2008), are associated with the thylakoidal membranes, and ptac14 mutants have been reported to lack grana and thylakoidal membranes (Gao et al., 2011), it was also considered here that the defects in thylakoid abundance and stacking might result from a more general problem in plastid gene expression in anu10-1. However, the results of the present study indicate that the expression of plastid genes is not generally affected.
Leaf senescence has also been associated with thylakoid defects in higher plants (Evans et al., 2010; Krupinska et al., 2012). In the anu10-1 mutant, however, the senescence marker ORE1 was expressed at normal levels, suggesting that senescence is not the primary cause of the observed plastid phenotypes.
Loss of ANU10 function compromises leaf development
Altered leaf anatomy is a trait common to many mutants carrying lesions in nuclear genes that encode plastid proteins. Some examples are the immutans (Wetzel et al., 1994; Carol et al., 1999; Aluru et al., 2001), cue1 (Li et al., 1995; Streatfield et al., 1999), pale cress1 (pac1) (Reiter et al., 1994), and rugosa2 (rug2) (Quesada et al., 2011) mutants of Arabidopsis, the differentiation and greening (dag) mutant of Antirrhinum majus (Chatterjee et al., 1996), and the defective chloroplasts and leaves (dcl) mutant of tomato (Keddie et al., 1996). These mutants display variegated or pale-green phenotypes and have altered plastid and mesophyll development. In some variegated mutants, such as rug2, the pale sectors exhibit severe developmental defects in plastids and mesophyll cells, while the cells and chloroplasts within green sectors are usually less affected (Quesada et al., 2011).
Because some mutations damaging plastid-localized proteins affect both plastid development and mesophyll cell differentiation, plastid development and leaf morphogenesis have been hypothesized to be tightly coordinated processes via retrograde plastid-to-nucleus signalling (Rodermel, 2001). The signal is assumed to originate from chloroplasts with arrested differentiation or altered photosynthetic metabolism, and modulates the expression of nuclear genes encoding plastidial and other proteins (Susek et al., 1993; Koussevitzky et al., 2007). Pyke et al. (2000) proposed that a cell-autonomous signal originating in the plastids is required for normal development of palisade mesophyll cells. Like the above-mentioned mutants, the anu10-1 mutant exhibits altered mesophyll cell development, with larger and less densely packed palisade cells. The present morphometric analysis shows that anu10-1 leaves have fewer, larger mesophyll cells per area unit, suggesting either a premature transition from proliferation to cell expansion, or a defective cell proliferation. The increased extent of expansion observed in anu10-1 is in contrast to previous studies (Andriankaja et al., 2012), which have shown a delay in mesophyll cell differentiation when chloroplasts are prevented from differentiating. Different chloroplast-to-nucleus signals acting in differentiating and mature chloroplasts might explain the different effect on mesophyll cell expansion.
LHCB genes, among many other nuclear genes related to plastid photosynthetic machinery and other diverse functions, are known to be differentially expressed in response to chloroplast retrograde signalling (Susek et al., 1993; Koussevitzky et al., 2007). Furthermore, recent experimental evidence suggests that retrograde signalling also modulates the expression and activation of cyclin-dependent kinases, which are key regulators of the cell cycle (Kobayashi et al., 2009; Andriankaja et al., 2012). The defects of anu10-1 chloroplasts might trigger a retrograde signal leading to changes in nuclear gene expression, as exemplified by the reduced expression of HEMA1 and the LHCB1, LHCB2, and LHCB3 genes.
Concluding remarks
A nuclear gene, ANU10, has been identified, whose loss-of-function mutations lead to pleiotropic defects in leaf development and plastid internal organization, including a dramatic reduction in thylakoidal membranes and grana stacking. The results indicate that these phenotypes are not merely a consequence of a premature senescence response or of a general defect in the transcription of plastid-encoded genes. The expression of several nuclear genes, including HEMA1 and several LHCB genes, was found to be altered in the anu10-1 mutant, suggesting that the plastid defects trigger a retrograde (chloroplast-to-nucleus) signal that might account for the mesophyll phenotype.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Growth of the anu10-1 mutant.
Figure S2. ROS in anu10-1 leaves.
Figure S3. Rosette phenotype of homozygotes and heterozygotes for the anu10 alleles.
Figure S4. Phylogenetic analysis of ANU10 and its putative orthologues.
Figure S5. Subcellular localization of the ANU10:GFP fusion protein in the anu10-1 ANU10pro:ANU10:GFP transgenic line.
Figure S6. Phenotypic rescue of anu10-1 by the ANU10:GFP fusion protein.
Figure S7. Growth and starch content of anu10 roots.
Table S1. Primer sets used in this work.
Table S2. Transmembrane domains and chloroplast transit peptides in the ANU10 protein and its putative orthologues.
Acknowledgements
We wish to thank V. Quesada and P. Robles for their helpful comments on the manuscript, and J.M. Serrano, F.M. Lozano, T. Trujillo, L. Serna, and J.M. Sánchez-Larrosa for their excellent technical assistance. This work was supported by grants from the Ministerio de Economía y Competitividad of Spain [BFU2011-22825 and CSD2007-00057 (TRANSPLANTA)], the Generalitat Valenciana (PROMETEO/2009/112), and the European Commission [LSHG-CT-2006–037704 (AGRON-OMICS)] to JLM. HC is a recipient of a Marie Curie International Reintegration Grant (PIRG03-GA-2008–231073). RCS holds a fellowship from the Ministerio de Economía y Competitividad of Spain (BES-2009–014106). SK was supported by Academy of Finland projects 218157, 259888, and 130595.
References
- Albertsson P. 2001. A quantitative model of the domain structure of the photosynthetic membrane. Trends in Plant Science 6, 349–358 [DOI] [PubMed] [Google Scholar]
- Allen JF, Forsberg J. 2001. Molecular recognition in thylakoid structure and function. Trends in Plant Science 6, 317–326 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru MR, Bae H, Wu D, Rodermel SR. 2001. The Arabidopsis immutans mutation affects plastid differentiation and the morphogenesis of white and green sectors in variegated plants. Plant Physiology 127, 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Wentworth M, Walters RG, Howard CA, Ruban AV, Horton P, Jansson S. 2003. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II—effects on photosynthesis, grana stacking and fitness. The Plant Journal 35, 350–361 [DOI] [PubMed] [Google Scholar]
- Andriankaja M, Dhondt S, De Bodt S, et al. 2012. Exit from proliferation during leaf development in Arabidopsis thaliana: a not-so-gradual process. Developmental Cell 22, 64–78 [DOI] [PubMed] [Google Scholar]
- Apchelimov AA, Soldatova OP, Ezhova TA, Grimm B, Shestakov SV. 2007. The analysis of the ChlI 1 and ChlI 2 genes using acifluorfen-resistant mutant of Arabidopsis thaliana . Planta 225, 935–943 [DOI] [PubMed] [Google Scholar]
- Archer EK, Bonnett HT. 1987. Characterization of a virescent chloroplast mutant of tobacco. Plant Physiology 83, 920–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster U, Labs M, Pribil M, et al. 2013. Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature. The Plant Cell 25, 2661–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster U, Zuhlke J, Rengstl B, et al. 2010. The Arabidopsis thylakoid protein PAM68 is required for efficient D1 biogenesis and photosystem II assembly. The Plant Cell 22, 3439–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JR, 2nd, Staehelin LA. 2011. Three-dimensional architecture of grana and stroma thylakoids of higher plants as determined by electron tomography. Plant Physiology 155, 1601–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo J, Courtois F, Hakimi MA, Demarsy E, Lagrange T, Alcaraz JP, Jaiswal P, Marechal-Drouard L, Lerbs-Mache S. 2008. Intraplastidial trafficking of a phage-type RNA polymerase is mediated by a thylakoid RING-H2 protein. Proceedings of the National Academy of Sciences, USA 105, 9123–9128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MD, Robertson DS, Bowen CC. 1969. Thylakoid anomalies in relation to grana structure in pigment-deficiency mutants of Zea mays . Journal of Ultrastructure Research 28, 435–451 [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Siddiqui H, Allu AD, Matallana-Ramirez LP, Caldana C, Mehrnia M, Zanor MI, Kohler B, Mueller-Roeber B. 2010. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence. The Plant Journal 62, 250–264 [DOI] [PubMed] [Google Scholar]
- Berná G, Robles P, Micol JL. 1999. A mutational analysis of leaf morphogenesis in Arabidopsis thaliana . Genetics 152, 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum T, Briesemeister S, Kohlbacher O. 2009. MultiLoc2: integrating phylogeny and Gene Ontology terms improves subcellular protein localization prediction. BMC Bioinformatics 10, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M. 1999. Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. The Plant Cell 11, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743 [DOI] [PubMed] [Google Scholar]
- Colletti KS, Tattersall EA, Pyke KA, Froelich JE, Stokes KD, Osteryoung KW. 2000. A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Current Biology 10, 507–516 [DOI] [PubMed] [Google Scholar]
- Cui YL, Jia QS, Yin QQ, Lin GN, Kong MM, Yang ZN. 2011. The GDC1 gene encodes a novel ankyrin domain-containing protein that is essential for grana formation in Arabidopsis. Plant Physiology 155, 130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Sparvoli S, Edmunds C, Garosi P, Findlay K, Martin C. 1996. DAG, a gene required for chloroplast differentiation and palisade development in Antirrhinum majus. EMBO Journal 15, 4194–4207 [PMC free article] [PubMed] [Google Scholar]
- Day DA, Ryrie IJ, Fuad N. 1984. Investigations of the role of the main light-harvesting chlorophyll-protein complex in thylakoid membranes. Reconstitution of depleted membranes from intermittent-light-grown plants with the isolated complex. Journal of Cell Biology 98, 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker JP, Boekema EJ. 2005. Supramolecular organization of thylakoid membrane proteins in green plants. Biochimica et Biophysica Acta 1706, 12–39 [DOI] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggink LL, Park H, Hoober JK. 2001. The role of chlorophyll b in photosynthesis: hypothesis. BMC Plant Biology 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology 300, 1005–1016 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, von Heijne G. 1999. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Science 8, 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans IM, Rus AM, Belanger EM, Kimoto M, Brusslan JA. 2010. Dismantling of Arabidopsis thaliana mesophyll cell chloroplasts during natural leaf senescence. Plant Biology 12, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrández-Ayela A, Alonso-Peral MM, Sánchez-García AB, Micol-Ponce R, Pérez-Pérez JM, Micol JL, Ponce MR. 2013. Arabidopsis TRANSCURVATA1 encodes NUP58, a component of the nucleopore central channel. PLoS One 8, e67661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro M, Brugiere S, Salvi D, et al. 2010. AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Molecular and Cellular Proteomics 9, 1063–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. 2011. HMMER web server: interactive sequence similarity searching. Nucleic Acids Research 39, W29–W37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZP, Yu QB, Zhao TT, Ma Q, Chen GX, Yang ZN. 2011. A functional component of the transcriptionally active chromosome complex, Arabidopsis pTAC14, interacts with pTAC12/HEMERA and regulates plastid gene expression. Plant Physiology 157, 1733–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garab G, Mustardy L. 1999. Role of LHCII-containing macrodomains in the structure, function and dynamics of grana. Australian Journal of Plant Physiology 26, 649–658 [Google Scholar]
- Garcia C, Khan NZ, Nannmark U, Aronsson H. 2010. The chloroplast protein CPSAR1, dually localized in the stroma and the inner envelope membrane, is involved in thylakoid biogenesis. The Plant Journal 63, 73–85 [DOI] [PubMed] [Google Scholar]
- Grabsztunowicz M, Jackowski G. 2012. Isolation of intact and pure chloroplasts from leaves of Arabidopsis thaliana plants acclimated to low irradiance for studies on Rubisco regulation. Acta Societatis Botanicorum Poloniae 82, 91–95 [Google Scholar]
- Hirokawa T, Boon-Chieng S, Mitaku S. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14, 378–379 [DOI] [PubMed] [Google Scholar]
- Hricová A, Quesada V, Micol JL. 2006. The SCABRA3 nuclear gene encodes the plastid RpoTp RNA polymerase, which is required for chloroplast biogenesis and mesophyll cell proliferation in Arabidopsis. Plant Physiology 141, 942–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Madan A. 1999. CAP3: a DNA sequence assembly program. Genome Research 9, 868–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R, Fujiwara M, Nagata N, Yoshida S. 2001. A chloroplast protein homologous to the eubacterial topological specificity factor minE plays a role in chloroplast division. Plant Physiology 127, 1644–1655 [PMC free article] [PubMed] [Google Scholar]
- Keddie JS, Carroll B, Jones JD, Gruissem W. 1996. The DCL gene of tomato is required for chloroplast development and palisade cell morphogenesis in leaves. EMBO Journal 15, 4208–4217 [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. 2009. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057 [DOI] [PubMed] [Google Scholar]
- Kleffmann T, Russenberger D, von Zychlinski A, Christopher W, Sjolander K, Gruissem W, Baginsky S. 2004. The Arabidopsis thaliana chloroplast proteome reveals pathway abundance and novel protein functions. Current Biology 14, 354–362 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kanesaki Y, Tanaka A, Kuroiwa H, Kuroiwa T, Tanaka K. 2009. Tetrapyrrole signal as a cell-cycle coordinator from organelle to nuclear DNA replication in plant cells. Proceedings of the National Academy of Sciences, USA 106, 803–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Mayerhofer R, Koncz-Kalman Z, Nawrath C, Reiss B, Redei GP, Schell J. 1990. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana . EMBO Journal 9, 1337–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J. 2007. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719 [PubMed] [Google Scholar]
- Kroll D, Meierhoff K, Bechtold N, Kinoshita M, Westphal S, Vothknecht UC, Soll J, Westhoff P. 2001. VIPP1, a nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proceedings of the National Academy of Sciences, USA 98, 4238–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupinska K, Mulisch M, Hollmann J, Tokarz K, Zschiesche W, Kage H, Humbeck K, Bilger W. 2012. An alternative strategy of dismantling of the chloroplasts during leaf senescence observed in a high-yield variety of barley. Physiologia Plantarum 144, 189–200 [DOI] [PubMed] [Google Scholar]
- Li H, Culligan K, Dixon RA, Chory J. 1995. CUE1: a mesophyll cell-specific positive regulator of light-controlled gene expression in Arabidopsis. The Plant Cell 7, 1599–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juez E, Jarvis RP, Takeuchi A, Page AM, Chory J. 1998. New Arabidopsis cue mutants suggest a close connection between plastid- and phytochrome regulation of nuclear gene expression. Plant Physiology 118, 803–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juez E, Pyke KA. 2005. Plastids unleashed: their development and their integration in plant development. International Journal of Developmental Biology 49, 557–577 [DOI] [PubMed] [Google Scholar]
- McDowell EM, Trump BF. 1976. Histologic fixatives suitable for diagnostic light and electron microscopy. Archives of Pathology and Laboratory Medicine 100, 405–414 [PubMed] [Google Scholar]
- Mochizuki N, Tanaka R, Tanaka A, Masuda T, Nagatani A. 2008. The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA 105, 15184–15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet JE, Arntzen CJ. 1980. Simulation of grana stacking in a model membrane system. Mediation by a purified light-harvesting pigment–protein complex from chloroplasts. Biochimica et Biophysica Acta 589, 100–117 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW. 2005. Function and evolution of grana. Trends in Plant Sciences 10, 521–525 [DOI] [PubMed] [Google Scholar]
- Nielsen NC, Smillie RM, Henningsen KW, Von Wettstein D. 1979. Composition and function of thylakoid membranes from grana-rich and grana-deficient chloroplast mutants of barley. Plant Physiology 63, 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. Journal of Molecular Biology 302, 205–217 [DOI] [PubMed] [Google Scholar]
- Osteryoung KW, Stokes KD, Rutherford SM, Percival AL, Lee WY. 1998. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial ftsZ . The Plant Cell 10, 1991–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Vierling E. 1995. Conserved cell and organelle division. Nature 376, 473–474 [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez JM, Esteve-Bruna D, González-Bayón R, Kangasjärvi S, Caldana C, Hannah MA, Willmitzer L, Ponce MR, Micol JL. 2013. Functional redundancy and divergence within the Arabidopsis RETICULATA-RELATED gene family. Plant Physiology 162, 589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez JM, Rubio-Díaz S, Dhondt S, Hernández-Romero D, Sánchez-Soriano J, Beemster GT, Ponce MR, Micol JL. 2011. Whole organ, venation and epidermal cell morphological variations are correlated in the leaves of Arabidopsis mutants. Plant, Cell and Environment 34, 2200–2211 [DOI] [PubMed] [Google Scholar]
- Ponce MR, Quesada V, Micol JL. 1998. Rapid discrimination of sequences flanking and within T-DNA insertions in the Arabidopsis genome. The Plant Journal 14, 497–501 [DOI] [PubMed] [Google Scholar]
- Ponce MR, Robles P, Lozano FM, Brotons MA, Micol JL. 2006. Low-resolution mapping of untagged mutations. Methods in Molecular Biology 323, 105–113 [DOI] [PubMed] [Google Scholar]
- Punta M, Coggill PC, Eberhardt RY, et al. 2012. The Pfam protein families database. Nucleic Acids Research 40, D290–D301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke K, Zubko MK, Day A. 2000. Marking cell layers with spectinomycin provides a new tool for monitoring cell fate during leaf development. Journal of Experimental Botany 51, 1713–1720 [DOI] [PubMed] [Google Scholar]
- Quesada V, Sarmiento-Mañús R, González-Bayón R, Hricová A, Pérez-Marcos R, Gracía-Martínez E, Medina-Ruiz L, Leyva-Díaz E, Ponce MR, Micol JL. 2011. Arabidopsis RUGOSA2 encodes an mTERF family member required for mitochondrion, chloroplast and leaf development. The Plant Journal 68, 738–753 [DOI] [PubMed] [Google Scholar]
- Reiter RS, Coomber SA, Bourett TM, Bartley GE, Scolnik PA. 1994. Control of leaf and chloroplast development by the Arabidopsis gene pale cress . The Plant Cell 6, 1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles P, Fleury D, Candela H, et al. 2010. The RON1/FRY1/SAL1 gene is required for leaf morphogenesis and venation patterning in Arabidopsis. Plant Physiology 152, 1357–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles P, Micol JL. 2001. Genome-wide linkage analysis of Arabidopsis genes required for leaf development. Molecular Genetics and Genomics 266, 12–19 [DOI] [PubMed] [Google Scholar]
- Rodermel S. 2001. Pathways of plastid-to-nucleus signaling. Trends in Plant Science 6, 471–478 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Wentworth M, Yakushevska AE, Andersson J, Lee PJ, Keegstra W, Dekker JP, Boekema EJ, Jansson S, Horton P. 2003. Plants lacking the main light-harvesting complex retain photosystem II macro-organization. Nature 421, 648–652 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C T method. Nature Protocols 3, 1101–1108 [DOI] [PubMed] [Google Scholar]
- Serrano-Cartagena J, Candela H, Robles P, Ponce MR, Perez-Perez JM, Piqueras P, Micol JL. 2000. Genetic analysis of incurvata mutants reveals three independent genetic operations at work in Arabidopsis leaf morphogenesis. Genetics 156, 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kuhlbrandt W. 2005. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO Journal 24, 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback KE, Burke JJ, Arntzen CJ. 1979. Evidence for the role of surface-exposed segments of the light-harvesting complex in cation-mediated control of chloroplast structure and function. Archives of Biochemistry and Biophysics 195, 546–557 [DOI] [PubMed] [Google Scholar]
- Stothard P. 2000. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques 28, 1102, 1104. [DOI] [PubMed] [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Hausler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flugge UI, Chory J. 1999. The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. The Plant Cell 11, 1609–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek RE, Ausubel FM, Chory J. 1993. Signal transduction mutants of Arabidopsis uncouple nuclear CAB and RBCS gene expression from chloroplast development. Cell 74, 787–799 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanz SK, Kilian J, Johnsson C, Apel K, Small I, Harter K, Wanke D, Pogson B, Albrecht V. 2012. The SCO2 protein disulphide isomerase is required for thylakoid biogenesis and interacts with LHCB1 chlorophyll a/b binding proteins which affects chlorophyll biosynthesis in Arabidopsis seedlings. The Plant Journal 69, 743–754 [DOI] [PubMed] [Google Scholar]
- Weigel D, Mott R. 2009. The 1001 genomes project for Arabidopsis thaliana . Genome Biology 10, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology 144, 307–313 [Google Scholar]
- Wetzel CM, Jiang CZ, Meehan LJ, Voytas DF, Rodermel SR. 1994. Nuclear–organelle interactions: the immutans variegation mutant of Arabidopsis is plastid autonomous and impaired in carotenoid biosynthesis. The Plant Journal 6, 161–175 [DOI] [PubMed] [Google Scholar]
- Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B. 1998. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development 125, 521–531 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. 2004. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. The Plant Cell 16, 367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ. 2008. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3, e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.