Summary
Grafting together two different genotypes results in the upregulation of stress responses at the graft interface during graft union formation in comparison to the wound-like responses of autografts.
Key words: Gene expression, graft interface, grafting, grapevine, rootstock, stress response.
Abstract
Although grafting is widely used in the agriculture of fruit-bearing crops, little is known about graft union formation in particular when two different species are grafted together. It is fascinating that two different plant species brought together can develop harmoniously as one organism for many decades. The objective of this study was to determine whether grafting two different grapevine genotypes alters gene expression at the graft interface in comparison to the presumably wound-like gene expression changes induced in autografts. Gene expression at the graft interface was studied 3, 7, 14, and 28 d after grafting in hetero- and autografts of grapevine (Vitis spp.). Genes differentially expressed between the hetero- and autografts during graft union formation were identified. These genes were clustered according to their expression profile over the time course. MapMan and Gene Ontology enrichment analysis revealed the coordinated upregulation of genes from numerous functional categories related to stress responses in the hetero- compared to the autografts. This indicates that heterografting with nonself rootstocks upregulates stress responses at the graft interface, potentially suggesting that the cells of the graft interface can detect the presence of a nonself grafting partner.
Introduction
Grafting has been used in horticulture in China since before 2000 bc (Liu, 2006; Mudge et al., 2009). Grafting is still widely used today, for example in the cultivation of grapevine, apples, Prunus spp., and vegetables (e.g. Lee et al., 2010; Gregory et al., 2013). In viticulture, grafting began in Europe to facilitate grapevine growth in soils infected with phylloxera, a soil-dwelling insect pest introduced to Europe from America (May, 1994). The role of rootstocks in defence responses may go beyond resistance to soil-borne pests and diseases: for example, in apple, certain rootstocks can confer resistance to fireblight to the scion (Jensen et al., 2012). It has been suggested recently that grafting per se could increase plant defence responses by activating systemic defence mechanisms (Guan et al., 2012). Despite the old and wise use of grafting in agriculture, the early stages of the grafting process and the molecular mechanisms involved in the communication between two different genotypes at the graft interface are still poorly understood.
Successful grafting is a complex process that begins with adhesion of the two grafted partners, followed by callus formation and the establishment of a functional vascular system (as reviewed by Pina and Errea, 2005). When callus cells come into contact, the cell walls undergo dissolution, holes in the cell walls appear, plasma membranes contact, and plasmodesmata appear (Jeffree and Yeoman, 1983).
The changes in graft union morphology and vascular formation have been studied using classical histology and various imaging techniques (e.g. Weatherhead and Barnett, 1986; Soumelidou et al., 1994; Olmstead et al., 2006; Leszczynski et al., 2000; Bahar et al., 2010; Milien et al., 2012). At the molecular level, graft union formation presumably requires considerable reprogramming of gene expression, protein translation, and metabolism. Global changes in gene expression during the process of graft formation have been analysed in hickory (Carya tomentosa) with cDNA amplified fragment length polymorphism 0, 3, 7, and 14 d after grafting (Zheng et al., 2010). This has revealed that some genes related to indole-3-acetic acid, cell cycle, metabolism, and signal transduction are differentially expressed (Zheng et al., 2010). Graft union development in Arabidopsis thaliana hypocotyl grafts has been studied at the histological and transcriptional level (Yin et al., 2012) and graft union development was shown to involve wound and hormone signalling and the clearing of cellular debris. However, hypocotyl grafting appears to be quite different from grafting in woody perennial species because the graft union formation is not accompanied by the development of callus tissue in the graft zone. In grapevine, grafting is traditionally performed on overwintering stems in the spring so that graft union formation and the reactivation of metabolism and growth of the cambium in the spring occur in parallel. In a previous study, this study group has shown that graft union development in grapevine involves the upregulation of many genes involved in cell-wall synthesis, wound responses, secondary metabolism, and signalling (Cookson et al., 2013). These previous gene expression studies have identified genes involved in autografting of the same species (e.g. Zheng et al., 2010; Yin et al., 2012; Cookson et al., 2013) but have not examined gene expression induced by grafting different species together.
To date, it is not known whether any degree of nonself recognition occurs between two different genotypes or species at a graft interface. Empirically it is known that not all species or genotypes graft together successfully and graft incompatibility has been widely reported (as reviewed by Pina and Errea, 2005). It has long been known that incompatible heterografts develop less mechanical strength and fewer trans-union xylem connections than compatible grafts (Yeoman and Brown, 1976; Yeoman et al., 1978). However, the underlying causes of graft incompatibility are unknown and incompatible responses can arise many years after grafting has taken place. Incompatibility between different genotypes at the graft interface has been associated with the accumulation of phenolic compounds (Pina and Errea, 2008b ), reactive oxygen species (Nocito et al., 2010), and UDP-glucose pyrophosphorylase (Pina and Errea, 2008a ), and modifications of cell-wall composition (Pina et al., 2012).
In this work, gene expression was studied at the graft interface in hetero- and autografts of grapevine during a time course (3, 7, 14, and 28 d) after grafting using whole-genome microarrays. Many genes were differentially expressed over time, from 3 to 28 d after grafting, but they will not be discussed here as they have been described previously (Cookson et al., 2013). The scion/rootstock graft interfaces studied were: the autograft Vitis vinifera cv. ‘Cabernet-Sauvignon N’ (CS; CS/CS) and the heterografts CS/V. riparia cv. ‘Riparia Gloire de Montpellier’ (RG; CS/RG) and CS/V. berlandieri x V. rupestris hybrid cv. ‘1103 Paulsen’ (1103P; CS/1103P). Many genes were differentially expressed between the different graft interface zones (i.e. between the graft interfaces made of only CS cells and those made up of cells of two different genotypes; data not shown). However, the present paper will focus on the identification of genes differentially regulated during the time course between the hetero- and autografted plants.
Materials and methods
Two independent grafting experiments were done in the spring of 2011 and 2012. The samples from 2011 were used for microarray and quantitative real-time PCR (qPCR) analysis whereas the samples from 2012 were used only for qPCR analysis.
Plant material and grafting procedure
Hardwood from CS (clone 15), RG (clone 1030), and 1103P (clone 198) was collected from a vineyard in France in January and stored as 1-m-long stems in a cold chamber (4 °C) until grafting in March. The scion CS was grafted onto RG (CS/RG) and 1103P (CS/1103P) as well as autografted onto CS rootstocks (CS/CS) (as described by Cookson et al., 2013).
RNA extraction
Three pools of 15 graft interface zones (approximately 5mm in length including both scion and rootstock tissues) were harvested 3, 7, 14, and 28 d after grafting for CS/RG, CS/1103P, and CS/CS and were immediately snap frozen in liquid nitrogen. Total RNA was extracted using the protocol described by Cookson et al. (2013).
Microarray analysis
The microarray hybridizations were done for 36 samples (three pools of graft interface zones of CS/RG, CS/1103P, and CS/CS harvested 3, 7, 14, and 28 d after grafting) by the Plateforme Biopuces, Institut National des Sciences Appliquées, Toulouse, France according to the manufacturer’s instructions. The microarrays used were the grape whole-genome microarrays from Nimblegen, Roche (design name 090918 Vitus exp HX12) and were background corrected, quantile-normalized, and summarized as described by Cookson et al. (2013). The raw and normalized microarray data is available at http://www.ebi.ac.uk/arrayexpress (last accessed 20 March 2014) (accession number E-MTAB-1610).
The genes differentially expressed between the scion/rootstock combinations at 3–7, 7–14, and 14–28 d after grafting were identified using limma (Smyth, 2005; log2 fold-change >1, P<0.05, adjusted with Holm). This method identified genes that were differentially expressed between the hetero- and autografts between each time point of the time course: these genes were interesting because they behaved differently during graft union formation, depending of the scion/rootstock combination under consideration. The genes differentially expressed between CS/RG and CS/CS were further analysed using K-means clustering; the number of clusters chosen was selected from visual inspection of a hierarchical clustering dendrogram of the data (Supplementary Fig. S1 available at JXB online).
Differences in gene expression were visualized using MapMan (Thimm et al., 2004; Usadel et al., 2005). The MapMan mapping file was obtained from http://www.gomapman.org/ (last accessed 20 March 2014); 27 837 of the 29 549 genes on the microarray are present in the mapping file (Rotter et al., 2009). Enrichments of functional categories of the MapMan annotation in the significantly differentially expressed genes were tested for significance by applying Fisher exact tests with a Bonferroni correction for multiple tests using Mefisto version 0.23beta (http://www.usadellab.org, last accessed 20 March 2014). Enrichment of Gene Ontology (GO) terms in significantly differentially expressed genes was evaluated using analysis tool from AgriGO (http://bioinfo.cau.edu.cn/agriGO, last accessed 20 March 2014; Du et al., 2010) with Fisher tests and Bonferroni multiple testing correction (P<0.05).
qPCR analysis
For qPCR experiments, genomic DNA contamination was removed from the RNA using a Turbo DNA-free kit (Ambion) and reverse transcription was done using the Superscript III kit (Invitrogen) using oligo dT primers and 1.5 μg RNA, both according to the manufacturer’s instructions. Gene expression was analysed on a Biorad CFX96 machine using iQ Sybr Green Supermix with a primer concentration of 250nM, according to the manufacturer’s instructions. The quality and quantity of cDNA synthesized was tested using two sets of primers that amplified the 3′ and 5′ regions of the same reference gene (a SAND protein, VIT_06s0004g02820, which was not differentially expressed between the samples) and genomic DNA contamination was checked by qPCR using intron-specific primers (Supplementary Table S1). The expression of genes of interest was normalized using VIT_02s0025g01050 (an additional reference gene which was also not differentially expressed between the samples); this gene was also used to confirm the stability of expression of VIT_06s0004g02820 (quantified using the 3′ primers, Supplementary Table S1). PCR efficiency for each primer pair was calculated using LinRegPCR (Ramakers et al., 2003). Data are expressed as deltadeltaCq, with a CS/CS sample used as the reference.
Results
Heterografting with a nonself rootstock alters the expression of many genes at the graft interface in comparison to autografting
The gene expression differences between the graft interface of the heterograft CS/RG and the autograft were analysed at 3–7, 7–14, and 14–28 d after grafting: a total of 1105 differentially expressed genes were identified (Fig. 1, Supplementary Table S2). These 1105 genes behaved differently during graft union formation between the auto- and heterografts. A similar result was obtained when the graft interfaces of CS/CS and CS/1103P were compared (Supplementary Fig. S2A) as there were very few genes differentially expressed at the graft interface between the two heterografted plants (Supplementary Fig. S2B). Because of the similarity between the transcriptional response of CS/RG and CS/1103P to grafting, only the results of the comparison between CS/RG and CS/CS are described in this manuscript; some results for the comparison between CS/1103P and CS/CS are given in Supplementary Fig. S2.
Fig. 1.
Transcriptomic analysis of the graft interface of the grapevine heterograft CS/RG and autograft CS/CS at 3, 7, 14, and 28 d after grafting, showing the number of genes differentially expressed after grafting (log2 fold-change >1, P<0.05, adjusted with Holm). Upper values indicate upregulation; lower values indicate downregulation.
The MapMan categories (BINs; Thimm et al., 2004; Usadel et al., 2005) enriched in all the 1105 genes differentially expressed between CS/CS and CS/RG were associated with cell walls, development (storage proteins), jasmonate signalling (lipoxygenase), various miscellaneous enzymes, and not assigned genes (Table 1). Among these genes, the GO compartments membrane, external encapsulating structure, and extracellular region/space/part were enriched, along with the functions catalytic and oxidoreductase activity (Table 2).
Table 1.
Enrichment of MapMan functional categories (BINs) in the 1105 genes differentially expressed in the callus 3, 7, 14, and 28 d after grafting between the heterograft CS/RG and the autograftContingency gives the number of genes (i) from the BIN in the input list, (ii) in the background (microarray), (iii) not in the BIN in input list, and (iv) not in the background. P-values adjusted with Bonferroni. NA, not assigned.
| BIN code | BIN name | Contingency | Adjusted P-value |
|---|---|---|---|
| 10 | Cell wall | 41–515–988–26 293 | 9.80E-03 |
| 33.1 | Development, storage proteins | 9–42–1020–26 766 | 2.84E-02 |
| 17 | Hormone metabolism | 43–540–986–26 268 | 5.77E-03 |
| 17.7 | Hormone metabolism, jasmonate | 11–29–1018–26 779 | 4.38E-05 |
| 17.7.1 | Hormone metabolism, jasmonate, synthesis/degradation | 11–28–1018–26 780 | 3.29E-05 |
| 17.7.1.2 | Hormone metabolism, jasmonate, synthesis/degradation, lipoxygenase | 7–9–1022–26 799 | 2.40E-04 |
| 26 | Miscellaneous enzymes | 131–1596–898–25 212 | 1.21E-12 |
| 26.4 | Miscellaneous enzymes, β-1,3 glucan hydrolases | 10–38–1019–26 770 | 2.52E-03 |
| 26.1 | Miscellaneous enzymes, cytochrome P450 | 32–305–997–26 503 | 3.72E-04 |
| 26.3 | Miscellaneous enzymes, gluco-, galacto-, and mannosidases | 12–68–1017–26 740 | 1.10E-02 |
| 26.8 | Miscellaneous enzymes, nitrilases, nitrile lyases, berberine bridge enzymes, reticuline oxidases, troponine reductases | 13–79–1016–26 729 | 1.01E-02 |
| 35 | NA | 324–11 861–705–14 947 | 7.07E-14 |
| 35.3 | NA, new | 67–3837–962–22 971 | 4.06E-12 |
| 35.1.5 | NA, no ontology, pentatricopeptide repeat-containing protein | 5–558–1024–26 250 | 1.83E-02 |
Table 2.
Enrichment of GO terms in the 1105 genes differentially expressed in the callus 3, 7, 14, and 28 d after grafting between the heterograft CS/RG and the autograftContingency gives the number of genes (i) in the BIN in the input list, (ii) in the background (microarray), (iii) not in the BIN in input list, and (iv) not in the background. P-values adjusted with Bonferroni.
| GO accession number | Term type | Term | Contingency | Adjusted P-value |
|---|---|---|---|---|
| GO:0005576 | C | Extracellular region | 106–1139–672–17 499 | 2E-10 |
| GO:0005615 | C | Extracellular space | 37–255–741–18 452 | 2E-07 |
| GO:0044421 | C | Extracellular region part | 37–271–741–18 436 | 7E-07 |
| GO:0030312 | C | External encapsulating structure | 43–468–735–18 233 | 4E-04 |
| GO:0016020 | C | Membrane | 305–6073–473–12 366 | 0.026 |
| GO:0003824 | F | Catalytic activity | 491–9610–287–8643 | 6E-06 |
| GO:0016491 | F | Oxidoreductase activity | 130–1836–648–16 758 | 8E-06 |
Key: F, molecular function and C, cellular compartment.
Clustering reveals the divergent profiles of the genes differentially expressed during the time course
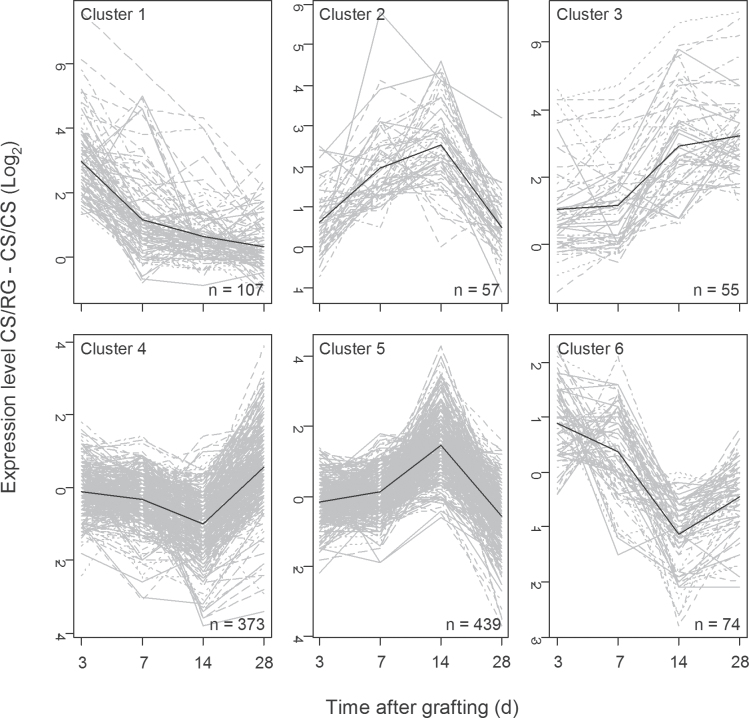
In order to simplify the interpretation of the genes differentially expressed between CS/CS and CS/RG, the profiles of gene expression were clustered into six clusters (Fig. 2, Supplementary Table S3). The expression of six of these genes was confirmed by qPCR (Supplementary Fig. S3).
Fig. 2.
Clustering of gene expression profiles of genes differentially expressed between the heterograft CS/RG and the autograft control at the graft interface in the time course after grafting.
Cluster 1 contained genes that were particularly upregulated in the heterograft CS/RG in comparison to the autograft control 3 d after grafting (Fig. 2). The functional categories were associated with cell wall, jasmonate signalling (lipoxygenase), miscellaneous enzymes, not assigned genes, and pathogenesis-related (PR) proteins were enriched in these early responding differentially expressed genes (Supplementary Table S4). Examination of the genes in cluster 1 identified the early upregulation in the heterograft of two dormancy-associated proteins (VIT14s0083g00250 and VIT_14s0083g00290), some genes involved in oxidative stress (e.g. a peroxiredoxin VIT_05s0020g00600, a peroxidase VIT_08s0058g00990, l-ascorbate oxidase VIT_06s0009g01320), and PR protein 10 (VIT_07s0005g00930) (Supplementary Table S3).
Cluster 2 contained genes particularly upregulated 7 and 14 d after grafting in the heterograft CS/RG in comparison to the autograft control (Fig. 2). The functional category polyamine oxidase was enriched in this cluster (Supplementary Table S4 available at JXB online) along with the GO term catalytic activity (Supplementary Table S5). This cluster included two ankyrin repeat family proteins (VIT_00s0256g00010 and VIT_00s0256g00020) which may be involved in protein–protein interactions, two genes from the category DNA (VIT_08s0058g00090 and VIT_16s0050g02320), and two peroxidases (VIT_12s0055g01010 and VIT_12s0055g01030) (Supplementary Table S3).
Cluster 3 contained genes that were particularly upregulated in the heterograft CS/RG towards the end of the time course, 14–28 d after grafting (Fig. 2). In this cluster, genes associated with secondary metabolism (particularly phenylpropanoid) were enriched (Supplementary Table S4). Cluster 3 contained four genes involved cell organization (VIT_12s0059g00050, VIT_14s0081g00370, VIT_14s0081g00400, and VIT_14s0081g00420), SENESCENCE ASSOCIATED GENE 101 (SAG101 VIT_05s0077g01730), a lateral organ boundaries gene (VIT_00s0340g00090), and five receptor kinases (VIT_04s0008g00920, VIT_17s0000g02360, VIT_12s0028g01430, VIT_15s0021g00840, VIT_15s0045g 00680, and VIT_18s0041g01770) (Supplementary Table S3).
Cluster 4 contained genes upregulated 28 d after grafting in the heterograft CS/RG compared to autograft control (Fig. 2); the categories cell wall (particularly cellulose synthesis), miscellaneous enzymes, not assigned genes, secondary metabolism (flavonols), S-locus glycoprotein-like receptor kinases, and peptide/oligopeptide transport were enriched in this cluster (Supplementary Table S4). The GO compartment membrane and the functions kinase, molecular transducer, oxidoreductase, receptor, signal transducer, and transferase activities were enriched in this cluster (Supplementary Table S5). Cluster 4 also included a number of receptor kinases, two genes from the category DNA (VIT_19s0014g05320 and VIT_07s0130g00370), and a number of transcription factors, including a SET domain-containing protein (VIT_08s0056g01660) (Supplementary Table S3).
Cluster 5 contained genes that were upregulated 14 d after grafting and then downregulated 28 d after grafting (Fig. 2); the functional categories β-1,3 glucan hydrolyases and trehalose-6-phosphate phosphatase were enriched in this cluster (Supplementary Table S4 available at JXB online) along with the GO terms catalytic activity and the extracellular compartment (Supplementary Table S5). In addition, cluster 4 contained two SAG101 genes (VIT_05s0077g01720 and VIT_14s0066g01830), one SAG6 gene (VIT_00s0301g00100) (Supplementary Table S5), four PR proteins (VIT_18s0001g03570, VIT_09s0002g06880, VIT_10s0003g03690, and VIT_12s0034g01230), and many transcription factors, receptor kinases, and genes involved in calcium signalling (Supplementary Table S3).
Finally, cluster 6 contained genes that were strongly downregulated in the heterograft CS/RG compared to autograft control 14 d after grafting (Fig. 2). The categories development (storage proteins), jasmonate signalling, miscellaneous enzymes, and both abiotic and biotic (proteinase inhibitors) stress were enriched in this cluster (Supplementary Table S4) along with the GO term extracellular region (Supplementary Table S5). This cluster included six PR proteins including two tumour-related proteins (VIT_17s0119g00150 and VIT_17s0119g00230) (Supplementary Table S5) and a number of transcription factors, including GROWTH REGULTING FACTOR 3 (VIT_09s0002g01350) (Supplementary Table S3).
Differential expression of genes associated with biotic stress
One of the common themes emerging from the genes differentially expressed between CS/CS and CS/RG during the time course is the differential expression of many genes involved in defence and/or stress responses. A summary of the gene expression differences of genes from the MapMan BIN biotic stress responses between the heterograft CS/RG and the autograft is given in a MapMan visualization (Supplementary Fig. S4 available at JXB online). The strong upregulation of genes from the functional categories jasmonate, cell wall, PR proteins, and secondary metabolites 3 d after grafting was evident (Supplementary Fig. S4A). Gradually, the expression of most genes associated with biotic stress decreased over time except the resistance genes (i.e. biotic stress receptors), which remained upregulated throughout the time course (Supplementary Fig. S4).
A conceptual model of the gene expression changes at the graft interface during heterograft union formation
In grapevine, graft union formation in both auto- and heterografts began (within a few hours) with the formation of a brown necrotic layer and the first callus cells developing 14 d after grafting. By 28 d after grafting, considerable callus tissue had developed and the graft union was functional (Fig. 3). Morphological changes are accompanied by many gene expression changes in autografted plants (Cookson et al., 2013). Compared to autografting, heterografting resulted in additional gene expression changes: for example, the rapid upregulation of genes involved in jasmonate signalling, PR protein expression, and biotic stress and the sustained upregulation of many genes involved in secondary metabolism. Some polyamine oxidase genes were upregulated 7–14 d after grafting and S-locus receptor kinases were generally upregulated and protease inhibitors generally downregulated towards the end of graft union formation (14–28 d after grafting) (Fig. 3).
Fig. 3.
Conceptual model of the mechanisms involved in grafting together different plant genotypes.
Discussion
Genes differentially expressed in a heterograft and autograft of grapevine
The objective of this work was to determine whether the transcriptome of grapevine is altered in response to grafting with a nonself genotype during the first month after grafting. As early as 1930, Kostoff suggested that the two different genotypes at the graft interface communicate with each other and induce an immune response (Kostoff, 1930). The presence of cell-wall projections on the cells of the graft interface suggested that cellular recognition processes are involved (Yeoman et al., 1978; Jeffree and Yeoman, 1983). In addition, a difference in protein profile between in vitro callus auto- and heterografts of Prunus spp. has been reported (Pina and Errea, 2008a ).
The gene expression differences at the graft interface between auto- and heterografts could be difficult to interpret because they may be related to a number of factors. In this study, the formation of the graft union concurred with the spring-time reactivation of metabolism in both auto- and heterografts; therefore, differences in gene expression between the different genotypes could be due to their different responses to warming in the spring. However, many genes were differentially expressed between the auto- and heterografts but not differentially 3–28 d after grafting (data not shown). Additionally, there could be differences in the timings of wound-healing processes in the different scion/rootstock combinations; however, in all combinations, the callus tissue was visible from 14 d after grafting and was well developed by 28 d after grafting (data not shown).
Grafting with nonself rootstock triggers the differential expression of a large number of genes involved in plant defence and/or stress responses
Plant defence and/or stress responses are designed to facilitate plant survival under abiotic stresses and/or to hinder pathogen development and progression of the disease upon pathogen infection. There are many similarities in the recognition and signalling pathways of abiotic and biotic stress responses (e.g. as reviewed by Tippmann et al., 2006); therefore, it is difficult to determine whether biotic or abiotic pathways were differentially regulated at the graft interface of hetero- and autografts.
Local pathogen attack can induce a form of programmed cell death, the hypersensitive response (as reviewed by Van Breusegem and Dat, 2006). Typically, the cells involved in the hypersensitive response generate an oxidative burst by producing reactive oxygen species, superoxide anions, hydrogen peroxide, hydroxyl radicals, and nitrous oxide. These responses are not unique to biotic stress responses and can also be induced in response to numerous abiotic stress conditions (as reviewed by Tippmann et al., 2006). The differential expression of genes involved in oxidative stress in this work (e.g. glutathione S-transferases, ascorbate oxidase, polyphenol oxidases, and peroxidases) could be associated with the induction of an oxidative burst at the graft interface. The enrichment of polyamine metabolism in cluster 2 was due to the presence of polyamine oxidase in this cluster: polyamine oxidase has been shown to be a key element of oxidative burst to induce programmed cell death (Yoda et al., 2006). Grafting in plants is presumably perceived as a considerable stress by the plant involved, and the grafting of two different genotypes described in this work further upregulates oxidative stress responses at the transcriptional level. Oxidative stress has also been implicated in the grafting incompatibility response of in vitro heterocallus grafts (Nocito et al., 2010): the activity of five antioxidant enzymes was increased in incompatible pear/quince heterografts in comparison to the compatible pear/pear autografts.
Plant genomes contain large numbers of receptor kinases with very divergent extracellular domains and functions. The receptor kinases that were differentially expressed in response to grafting with nonself genotypes included genes from the functional categories LRR, kinase receptor-like cytoplasmic kinase VII, S-locus glycoprotein-like, DUF 26, and wheat LRK10-like. LRR proteins are the largest group of receptor kinases in plants and the motif is thought to be involved in signal transduction and to mediate protein–protein interactions. LLR domain-containing proteins have been implicated in many developmental pathways and defence responses (as reviewed by Haffani et al., 2004). S-locus glycoprotein-like receptor kinases were first identified as being important in self-incompatibility responses in Brassica flowers and have since been shown to be involved in plant defence responses (as reviewed by Sanabria et al., 2008). LRK10 receptor kinases were first identified as leaf rust resistance genes in wheat and are similar in structure to S-locus motifs. The biological functions of receptor-like cytoplasmic kinases are much less understood; it has been suggested that their lack of an apparent extracellular domain implies that receptor-like cytoplasmic kinases more likely function in signal transduction rather than ligand perception (Lu et al., 2010). The differential expression of receptor kinases in hetero- compared to autografts could suggest that there is some degree of nonself recognition at the graft interface in heterografted plants.
Genes associated with jasmonate signalling also particularly enriched in the genes upregulated in the heterograft CS/RG compared to the autograft plants at early time points after grafting. Jasmonate and its derivates are involved in wound and defence signalling (as reviewed by Browse, 2009); therefore, the upregulation of genes involved in jasmonate signalling could also be associated with defence and wound responses during the early stages of heterografting.
Towards the end of the time course, 28 d after grafting, cell-wall precursor and lignin synthesis were enriched in the genes upregulated in the heterograft CS/RG compared to the autograft control: this could also be due to differences in plant wounding and defence responses. The upregulation of phenylalanine ammonia-lyase (PAL) gene expression has been reported during the formation of in vitro callus grafts of apricot and plum (Pina and Errea, 2008b ). PAL expression was further increased by the callus grafting of incompatible heterograft (apricot/plum and plum/apricot) combinations in comparison to both autograft control samples (apricot/apricot and plum/plum) (Pina and Errea, 2008b ). The upregulation of PAL expression was accompanied by the presence of soluble and wall-bound phenolic compounds but not by the production of lignin during the first 3 weeks after callus grafting (Pina and Errea, 2008b ).
PR proteins form part of the general mechanism of plant responses to unfavourable conditions and are considered the executioners of plant immune responses as they generally have antifungal or bacterial properties (such as β-1–3 glucanase, thaumatin, chitinase, and defensins). PR proteins were particularly enriched in the genes upregulated between the autograft and the heterograft at early time points after grafting. This could suggest that the wound response to grafting induces PR proteins but that the induction is not maintained at later time points due to absence of pathogens. Upregulation of PR1 expression has also been observed in sterile in vitro callus grafts of pear/quince heterografts in comparison to pear/pear autograft control (Nocito et al., 2010). However, upregulation of PR1 in heterografts could also be related to its function in hormone signalling (Naseem et al., 2012).
It has recently been proposed that grafting with nonself genotypes confers an increase in plant defence responses by activating systemic defence mechanisms (Guan et al., 2012); however, this may be difficult to demonstrate because of the indirect effects of rootstock on plant development: e.g. mineral nutrition, vigour). The work presented here shows a possible means by which grafting with nonself rootstocks could activate defence mechanisms, by the differential regulation of numerous defence-related genes at the graft interface of heterografted plants. Interestingly, gene expression is altered in the shoot apex of the autograft control compared with the heterograft CS/RG, but there are no gene expression differences between the heterografts CS/RG and CS/1103P (Cookson and Ollat, 2013). Furthermore, a number of defence-related genes are differentially expressed between CS/CS and CS/RG in the shoot apex (Cookson and Ollat, 2013), specifically the downregulation of secondary metabolism and PR protein expression and the differential regulation of a number of receptor kinases.
Hybrid necrosis in grapevine suggests that CS and RG have some degree of immune incompatibility
The observation that hybrids between V. vinifera and V. riparia show high levels of hybrid necrosis (Filler et al., 1994a,b ) could suggest that autoimmune-induced hybrid necrosis is involved. It has been suggested that hybrid necrosis is due to genetic incompatibilities that involve an immune response (Bomblies and Weigel, 2007). Wheat plants undergoing hybrid necrosis were found to have increased levels of superoxide (a molecule involved in oxidative burst and hypersensitive response) (Khanna-Chopra et al., 1998). Elevated levels of PR proteins and defence response genes have also been associated with hybrid necrosis (Bomblies et al., 2007). The genes that have been identified as responsible for hybrid necrosis to date have been involved in receptor kinase-mediated defence responses (as reviewed by Bomblies, 2009). The idea that immune responses are involved in grafting two different genotypes together and hybrid necrosis was proposed in 1930 (Kostoff, 1930) and has only relatively recently resurfaced in the literature (e.g. Wulff et al., 2004; Bomblies and Weigel, 2007). The gene expression differences observed at the graft interface of heterografted grapevines known to induce hybrid necrosis (CS is V. vinifera and RG is V. riparia) adds further support to this hypothesis.
Conclusion
Grafting with nonself genotypes triggers the differential expression of numerous genes at the graft interface during the first month after grafting; this begins with the upregulation of oxidative stress responses and PR proteins and is followed by the upregulation of many other genes involved in plant stress responses. These findings suggest that the cells at the graft interface are capable of detecting the presence of the nonself grafting partner, which may induce an immune-type response.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Sequences and PCR efficiency of primers used for qPCR analysis.
Supplementary Table S2. Gene expression differences between the graft interface zones of the heterograft CS/RG and the autograft 3, 7, 14, and 28 d after grafting.
Supplementary Table S3. Clustering of genes differentially expressed between the heterograft CS/RG and the autograft in the time course after grafting.
Supplementary Table S4. Enrichment of MapMan BINs in the six clusters shown in Fig. 2.
Supplementary Table S5. Enrichment of GO terms in the six clusters shown on Fig. 2.
Supplementary Fig. S1. Cluster dendrogram of the expression profiles of genes differentially expressed between the heterograft CS/RG and the autograft in the time course after grafting.
Supplementary Fig. S2. Transcriptomic analysis of the graft interface 3, 7, 14, and 28 d after grafting in different scion/rootstock combinations of grapevine heterografts CS/RG and CS/1103P and the autograft.
Supplementary Fig. S3. Validation of microarray data by qPCR in the graft interface.
Supplementary Fig. S4. MapMan visualization of genes assigned to the functional category biotic stress differentially expressed between the heterograft CS/RG and the autograft control at 3, 7, 14, and 28 d after grafting.
Acknowledgements
The authors thank the Bernard Douens, Guillaume Pacreau, Jean-Pierre Petit and Jean-Paul Robert for their technical help. This work was supported by a grant from the Institut National de la Recherche Agronomique department Génétique et Amélioration des Plantes.
References
- Bahar E, Korkutal I, Carbonneau A, Akcay G. 2010. Using magnetic resonance imaging technique (MRI) to investigate graft connection and its relation to reddening discoloration in grape leaves. Journal of Food Agriculture and Environment 8, 293–297 [Google Scholar]
- Bomblies K. 2009. Too much of a good thing? Hybrid necrosis as a by-product of plant immune system diversification. Botany 87, 1013–1022 [Google Scholar]
- Bomblies K, Lempe J, Epple P, Warthmann N, Lanz C, Dangl JL, Weigel D. 2007. Autoimmune response as a mechanism for a Dobzhansky-Muller-type incompatibility syndrome in plants. PLoS Biology 5, 1962–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K, Weigel D. 2007. Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nature Reviews Genetics 8, 382–393 [DOI] [PubMed] [Google Scholar]
- Browse J. 2009. Jasmonate passes muster: a receptor and targets for the defense hormone. Annual Review of Plant Biology 60, 183–205 [DOI] [PubMed] [Google Scholar]
- Cookson SJ, Moreno MJC, Hevin C, Mendome LZN, Delrot S, Trossat-Magnin C, Ollat N. 2013. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. Journal of Experimental Botany 64, 2997–3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson SJ, Ollat N. 2013. Grafting with rootstocks induces extensive transcriptional re-programming in the shoot apical meristem of grapevine. BMC Plant Biology 13, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang ZH, Su Z. 2010. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Research 38, W64-–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler DM, Luby JJ, Ascher PD. 1994a. Incongruity in the interspecific crosses of Vitis L reproductive expression in the F1 progeny. Euphytica 78, 155–164 [Google Scholar]
- Filler DM, Luby JJ, Ascher PD. 1994b. Incongruity in the interspecific crosses of Vitis L morphological abnormalities in the F-2 progeny. Euphytica 78, 227–237 [Google Scholar]
- Gregory PJ, Atkinson CJ, Bengough AG, Else MA, Fernandez-Fernandez F, Harrison RJ, Schmidt S. 2013. Contributions of roots and rootstocks to sustainable, intensified crop production. Journal of Experimental Botany 64, 1209–1222 [DOI] [PubMed] [Google Scholar]
- Guan WJ, Zhao X, Hassell R, Thies J. 2012. Defense mechanisms involved in disease resistance of grafted vegetables. Hortscience 47, 164–170 [Google Scholar]
- Haffani YZ, Silva NF, Goring DR. 2004. Receptor kinase signalling in plants. Canadian Journal of Botany 82, 1–15 [Google Scholar]
- Jeffree CE, Yeoman MM. 1983. Development of intercellular connections between opposing cells in a graft union. New Phytologist 93, 491–509 [Google Scholar]
- Jensen PJ, Halbrendt N, Fazio G, et al. 2012. Rootstock-regulated gene expression patterns associated with fire blight resistance in apple. BMC Genomics 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna-Chopra R, Dalal M, Kumar GP, Laloraya M. 1998. A genetic system involving superoxide causes F1 necrosis in wheat (T-aestivum L.). Biochemical and Biophysical Research Communications 248, 712–715 [DOI] [PubMed] [Google Scholar]
- Kostoff D. 1930. Ontogeny, genetics and cytology of Nicotiana hybrids. Genetica 12, 33–118 [Google Scholar]
- Lee JM, Kubota C, Tsao SJ, Bie Z, Echevarria PH, Morra L, Oda M. 2010. Current status of vegetable grafting: diffusion, grafting techniques, automation. Scientia Horticulturae 127, 93–105 [Google Scholar]
- Leszczynski R, Byczkowski B, Jurga S, Korszun S. 2000. NMR microimaging studies of the union between stock and scion. Applied Magnetic Resonance 18, 147–153 [Google Scholar]
- Liu Y. 2006. Historical and modern genetics of plant graft hybridization. Advances in Genetics 56, 101–129 [DOI] [PubMed] [Google Scholar]
- Lu DP, Wu SJ, Gao XQ, Zhang YL, Shan LB, He P. 2010. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proceedings of the National Academy of Sciences, USA 107, 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. 1994. Using grapevine rootstocks—the Australian perspective. Adelaide, Australia: Winetitles [Google Scholar]
- Milien M, Renault-Spilmont AS, Cookson SJ, Sarrazin A, Verdeil JL. 2012. Visualization of the 3D structure of the graft union of grapevine using X-ray tomography. Scientia Horticulturae 144, 130–140 [Google Scholar]
- Mudge K, Janick J, Scofield S, Goldschmidt EE. 2009. A history of grafting. In: Janick J, ed, Horticultural reviews, vol. 35 Hoboken, NJ: John Wiley; pp 437–493 [Google Scholar]
- Naseem M, Philippi N, Hussain A, Wangorsch G, Ahmed N, Dandekar T. 2012. Integrated systems view on networking by hormones in Arabidopsis immunity reveals multiple crosstalk for cytokinin. The Plant Cell 24, 1793–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocito FF, Espen L, Fedeli C, Lancilli C, Musacchi S, Serra S, Sansavini S, Cocucci M, Sacchi GA. 2010. Oxidative stress and senescence-like status of pear calli co-cultured on suspensions of incompatible quince microcalli. Tree Physiology 30, 450–458 [DOI] [PubMed] [Google Scholar]
- Olmstead MA, Lang NS, Ewers FW, Owens SA. 2006. Xylem vessel anatomy of sweet cherries grafted onto dwarfing and nondwarfing rootstocks. Journal of the American Society for Horticultural Science 131, 577–585 [Google Scholar]
- Pina A, Errea P. 2005. A review of new advances in mechanism of graft compatibility–incompatibility. Scientia Horticulturae 106, 1–11 [Google Scholar]
- Pina A, Errea P. 2008a. Influence of graft incompatibility on gene expression and enzymatic activity of UDP-glucose pyrophosphorylase. Plant Science 174, 502–509 [Google Scholar]
- Pina A, Errea P. 2008b. Differential induction of phenylalanine ammonia-lyase gene expression in response to in vitro callus unions of Prunus spp. Journal of Plant Physiology 165, 705–714 [DOI] [PubMed] [Google Scholar]
- Pina A, Zhebentyayeva T, Errea P, Abbott A. 2012. Isolation and molecular characterization of cinnamate 4-hydroxylase from apricot and plum. Biologia Plantarum 56, 441–450 [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters 339, 62–66 [DOI] [PubMed] [Google Scholar]
- Rotter A, Camps C, Lohse M, et al. 2009. Gene expression profiling in susceptible interaction of grapevine with its fungal pathogen Eutypa lata: extending MapMan ontology for grapevine. BMC Plant Biology 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria N, Goring D, Nurnberger T, Dubery I. 2008. Self/nonself perception and recognition mechanisms in plants: a comparison of self-incompatibility and innate immunity. New Phytologist 178, 503–513 [DOI] [PubMed] [Google Scholar]
- Smyth GK. 2005. Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, eds, Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer; pp 397–420 [Google Scholar]
- Soumelidou K, Battey NH, John P, Barnett JR. 1994. The anatomy of the developing bud union and its relationship to dwarfing apple. Annals of Botany 74, 605–611 [Google Scholar]
- Thimm O, Blasing O, Gibon Y, et al. 2004. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal 37, 914–939 [DOI] [PubMed] [Google Scholar]
- Tippmann HF, Schlüter U, Collinge DB. 2006. Common themes in biotic and abiotic stress signalling in plants. In: Teixeira da Silva JA, ed, Floriculture, ornamental and plant biotechnology, vol. 3 Ikenobe, Japan: Global Science Books; pp 52–67 [Google Scholar]
- Usadel B, Nagel A, Thimm O, et al. 2005. Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of coresponding genes, and comparison with known responses. Plant Physiology 138, 1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Dat JF. 2006. Reactive oxygen species in plant cell death. Plant Physiology 141, 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherhead I, Barnett JR. 1986. Development and structure of unusual xylem elements during graft union formation in Picea sitchensis L. Annals of Botany 57, 593–598 [Google Scholar]
- Wulff BBH, Kruijt M, Collins PL, Thomas CM, Ludwig AA, De Wit P, Jones JDG. 2004. Gene shuffling-generated and natural variants of the tomato resistance gene Cf-9 exhibit different auto-necrosis-inducing activities in Nicotiana species. The Plant Journal 40, 942–956 [DOI] [PubMed] [Google Scholar]
- Yeoman MM, Brown R. 1976. Implications of formation of graft union for organization in intact plant. Annals of Botany 40, 1265–1276 [Google Scholar]
- Yeoman MM, Kilpatrick DC, Miedzybrodzha MB, Gould AR. 1978. Cellular interactions during graft formation in plants, are they cognition phenomenon? Symposia of the Society for Experimental Biology 32, 139–160 [PubMed] [Google Scholar]
- Yin H, Yan B, Sun J, et al. 2012. Graft-union development: a delicate process that involves cell-cell communication between scion and stock for local auxin accumulation. Journal of Experimental Botany 63, 4219–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Hiroi Y, Sano H. 2006. Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiology 142, 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng BS, Chu HL, Jin SH, Huang YJ, Wang ZJ, Chen M, Huang JQ. 2010. cDNA-AFLP analysis of gene expression in hickory (Carya cathayensis) during graft process. Tree Physiology 30, 297–303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.