Abstract
The neurovascular unit (NVU) comprises brain endothelial cells, pericytes or vascular smooth muscle cells, glia and neurons. The NVU controls blood–brain barrier (BBB) permeability and cerebral blood flow, and maintains the chemical composition of the neuronal ‘milieu’, which is required for proper functioning of neuronal circuits. Recent evidence indicates that BBB dysfunction is associated with the accumulation of several vasculotoxic and neurotoxic molecules within brain parenchyma, a reduction in cerebral blood flow, and hypoxia. Together, these vascular-derived insults might initiate and/or contribute to neuronal degeneration. This article examines mechanisms of BBB dysfunction in neurodegenerative disorders, notably Alzheimer’s disease, and highlights therapeutic opportunities relating to these neurovascular deficits.
Neurons depend on blood vessels for their oxygen and nutrient supplies, and for the removal of carbon dioxide and other potentially toxic metabolites from the brain’s interstitial fluid (ISF). The importance of the circulatory system to the human brain is highlighted by the fact that although the brain comprises ~2% of total body mass, it receives up to 20% of cardiac output and is responsible for ~20% and ~25% of the body’s oxygen consumption and glucose consumption, respectively1. To underline this point, when cerebral blood flow (CBF) stops, brain functions end within seconds and damage to neurons occurs within minutes2.
Neurodegenerative disorders such as Alzheimer’s disease and amyotrophic lateral sclerosis (ALS) are associated with microvascular dysfunction and/or degeneration in the brain, neurovascular disintegration, defective blood–brain barrier (BBB) function and/or vascular factors1,3–12. Microvascular deficits diminish CBF and, consequently, the brain’s supply of oxygen, energy substrates and nutrients. Moreover, such deficits impair the clearance of neurotoxic molecules that accumulate and/or are deposited in the ISF, non-neuronal cells and neurons. Recent evidence suggests that vascular dysfunction leads to neuronal dysfunction and neurodegeneration, and that it might contribute to the development of proteinaceous brain and cerebrovascular ‘storage’ disorders. Such disorders include cerebral β-amyloidosis and cerebral amyloid angiopathy (CAA), which are caused by accumulation of the peptide amyloid-β in the brain and the vessel wall, respectively, and are features of Alzheimer’s disease1.
In this Review, I will discuss neurovascular pathways to neurodegeneration, placing a focus on Alzheimer’s disease because more is known about neurovascular dysfunction in this disease than in other neurodegenerative disorders. The article first examines transport mechanisms for molecules to cross the BBB, before exploring the processes that are involved in BBB breakdown at the molecular and cellular levels, and the consequences of BBB breakdown, hypoperfusion, and hypoxia and endothelial metabolic dysfunction for neuronal function. Next, the article reviews evidence for neurovascular changes during normal ageing and neurovascular BBB dysfunction in various neurodegenerative diseases, including evidence suggesting that vascular defects precede neuronal changes. Finally, the article considers specific mechanisms that are associated with BBB dysfunction in Alzheimer’s disease and ALS, and therapeutic opportunities relating to these neurovascular deficits.
The neurovascular unit
The neurovascular unit (NVU) comprises vascular cells (that is, endothelium, pericytes and vascular smooth muscle cells (VSMCs)), glial cells (that is, astrocytes, microglia and oliogodendroglia) and neurons1,2,13 (FIG. 1). In the NVU, the endothelial cells together form a highly specialized membrane around blood vessels. This membrane underlies the BBB and limits the entry of plasma components, red blood cells (RBCs) and leukocytes into the brain. The BBB also regulates the delivery into the CNS of circulating energy metabolites and essential nutrients that are required for proper neuronal and synaptic function. Non-neuronal cells and neurons act in concert to control BBB permeability and CBF. Vascular cells and glia are primarily responsible for maintenance of the constant ‘chemical’ composition of the ISF, and the BBB and the blood–spinal cord barrier (BSCB) work together with pericytes to prevent various potentially neurotoxic and vasculotoxic macromolecules in the blood from entering the CNS, and to promote clearance of these substances from the CNS1.
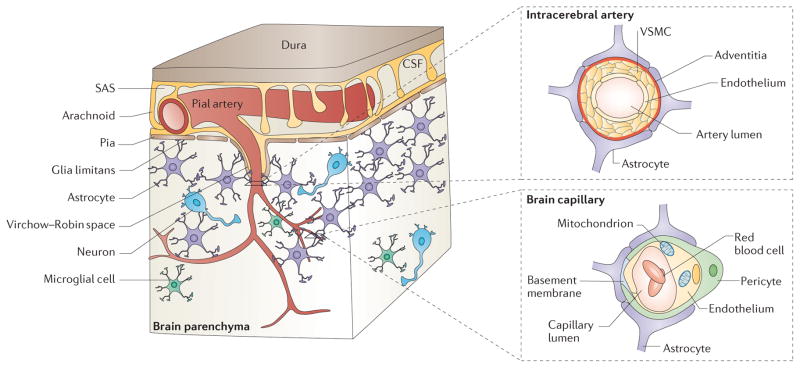
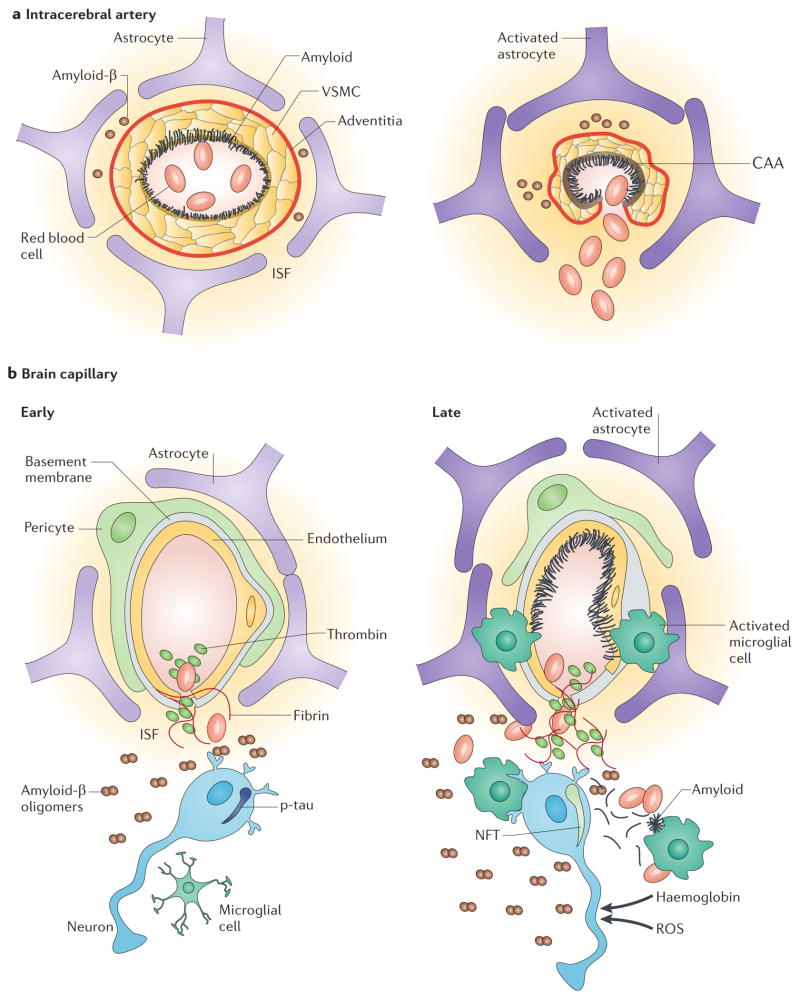
Figure 1. Cerebral microcirculation and the neurovascular unit.
In the brain, pial arteries run through the subarachnoid space (SAS), which contains the cerebrospinal fluid (CSF). These vessels give rise to intracerebral arteries, which penetrate into brain parenchyma. Intracerebral arteries are separated from brain parenchyma by a single, interrupted layer of elongated fibroblast-like cells of the pia and the astrocyte-derived glia limitans membrane that forms the outer wall of the perivascular Virchow–Robin space. These arteries branch into smaller arteries and subsequently arterioles, which lose support from the glia limitans and give rise to pre-capillary arterioles and brain capillaries. In an intracerebral artery, the vascular smooth muscle cell (VSMC) layer occupies most of the vessel wall. At the brain capillary level, vascular endothelial cells and pericytes are attached to the basement membrane. Pericyte processes encase most of the capillary wall, and they communicate with endothelial cells directly through synapse-like contacts containing connexins and N-cadherin. Astrocyte end-foot processes encase the capillary wall, which is composed of endothelium and pericytes. Resting microglia have a ‘ramified’ shape and can sense neuronal injury.
Transport across the blood–brain barrier
The endothelial cells that form the BBB are connected by tight and adherens junctions, and it is the tight junctions that confer the low paracellular permeability of the BBB1. Small lipophilic molecules, oxygen and carbon dioxide diffuse freely across the endothelial cells, and hence the BBB, but normal brain endothelium lacks fenestrae and has limited vesicular transport.
The high number of mitochondria in endothelial cells reflects a high energy demand for active ATP-dependent transport, conferred by transporters such as the sodium pump ((Na++K+) ATPase) and the ATP-binding cassette (ABC) efflux transporters. Sodium influx and potassium efflux across the abluminal side of the BBB is controlled by (Na++K+) ATPase (FIG. 2). Changes in sodium and potassium levels in the ISF influence the generation of action potentials in neurons and thus directly affect neuronal and synaptic functions1,12.
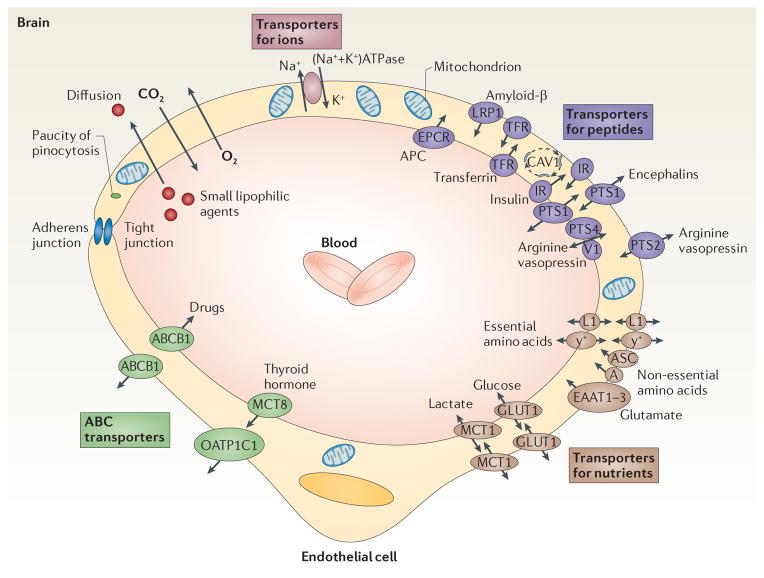
Figure 2. Blood–brain barrier transport mechanisms.
Small lipophilic drugs, oxygen and carbon dioxide diffuse across the blood–brain barrier (BBB), whereas ions require ATP-dependent transporters such as the (Na++K+) ATPase. Transporters for nutrients include the glucose transporter 1 (GLUT1; also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1)), the lactate transporter monocarboxylate transporter 1 (MCT1) and the L1 and y+ transporters for large neutral and cationic essential amino acids, respectively. These four transporters are expressed at both the luminal and albuminal membranes. Non-essential amino acid transporters (the alanine, serine and cysteine preferring system (ASC), and the alanine preferring system (A)) and excitatory amino acid transporter 1 (EAAT1), EAAT2 and EAAT3 are located at the abluminal side. The ATP-binding cassette (ABC) efflux transporters that are found in the endothelial cells include multidrug resistance protein 1 (ABCB1; also known as ATP-binding cassette subfamily B member 1) and solute carrier organic anion transporter family member 1C1 (OATP1C1). Finally, transporters for peptides or proteins include the endothelial protein C receptor (EPCR) for activated protein C (APC); the insulin receptors (IRs) and the transferrin receptors (TFRs), which are associated with caveolin 1 (CAV1); low-density lipoprotein receptor-related protein 1 (LRP1) for amyloid-β, peptide transport system 1 (PTS1) for encephalins; and the PTS2 and PTS4–vasopressin V1a receptor (V1AR) for arginine vasopressin.
Brain endothelial cells express transporters that facilitate the transport of nutrients down their concentration gradients, as described in detail elsewhere (FIG. 2). Glucose transporter 1 (GLUT1; also known as solute carrier family 2, facilitated glucose transporter member 1 (SLC2A1)) — the BBB-specific glucose transporter — is of special importance because glucose is a key energy source for the brain. Monocarboxylate transporter 1 (MCT1), which transports lactate, and the L1 and y+ amino acid trans- porters are expressed at the luminal and abluminal membranes. Sodium-dependent excitatory amino acid transporter 1 (EAAT1), EAAT2 and EAAT3 are expressed at the abluminal side of the BBB15 and enable removal of glutamate, an excitatory neurotransmitter, from the brain (FIG. 2). Glutamate clearance at the BBB is essential for protecting neurons from overstimulation of glutaminergic receptors, which is neurotoxic16. ABC transporters limit the penetration of many drugs into the brain17. For example, multidrug resistance protein 1 (ABCB1; also known as ATP-binding cassette subfamily B member 1) controls the rapid removal of ingested toxic lipophilic metabolites17 (FIG. 2). Some ABC transporters also mediate the efflux of nutrients from the endothelium into the ISF. For example, solute carrier organic anion transporter family member 1C1 (OATP1C1) transports thyroid hormones into the brain. MCT8 mediates influx of thyroid hormones from blood into the endothelium18 (FIG. 2).
The transport of circulating peptides across the BBB into the brain is restricted or slow compared with the transport of nutrients19. Carrier-mediated transport of neuroactive peptides controls their low levels in the ISF20–24 (FIG. 2). Some proteins, including transferrin, insulin, insulin-like growth factor 1 (IGF1), leptin25–27 and activated protein C (APC)28, cross the BBB by receptor-mediated transcytosis (FIG. 2).
Circumventricular organs
Several small neuronal structures that surround brain ventricles lack the BBB and sense chemical changes in blood or the cerebrospinal fluid (CSF) directly. These brain areas are known as circumventricular organs (CVOs). CVOs have important roles in multiple endocrine and autonomic functions, including the control of feeding behaviour as well as regulation of water and salt metabolism29. For example, the subfornical organ is one of the CVOs that are capable of sensing extracellular sodium using astrocyte derived lactate as a signal for local neurons to initiate neural, hormonal and behavioural responses underlying sodium homeostasis30. Excessive sodium accumulation is detrimental, and increases in plasma sodium above a narrow range are incompatible with life, leading to cerebral oedema (swelling), seizures and death29.
Vascular-mediated pathophysiology
The key pathways of vascular dysfunction that are linked to neurodegenerative diseases include BBB breakdown, hypoperfusion–hypoxia and endothelial metabolic dysfunction (FIG. 3). This section examines processes that are involved in BBB breakdown at the molecular and cellular levels, and explores the consequences of all three pathways for neuronal function and viability.
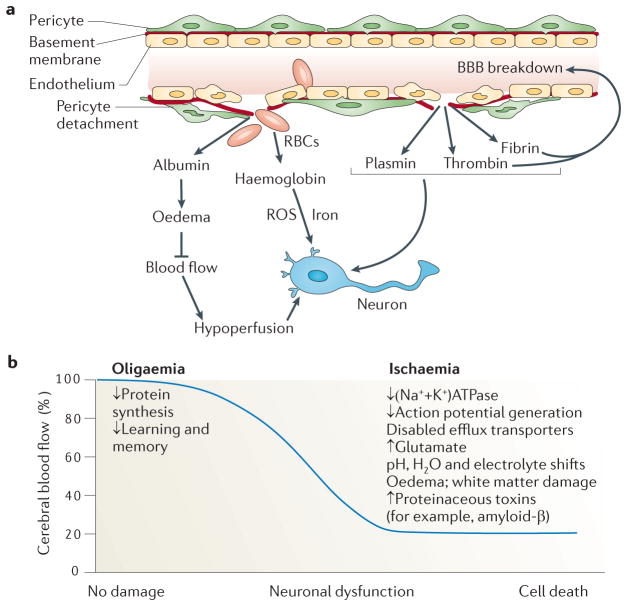
Figure 3. Vascular-mediated neuronal damage and neurodegeneration.
a, Blood–brain barrier (BBB) breakdown that is caused by pericyte detachment leads to leakage of serum proteins and focal microhaemorrhages, with extravasation of red blood cells (RBCs). RBCs release haemoglobin, which is a source of iron. In turn, this metal catalyses the formation of toxic reactive oxygen species (ROS) that mediate neuronal injury. Albumin promotes the development of vasogenic oedema, contributing to hypoperfusion and hypoxia of the nervous tissue, which aggravates neuronal injury. A defective BBB allows several potentially vasculotoxic and neurotoxic proteins (for example, thrombin, fibrin and plasmin) to enter the brain. b, Progressive reductions in cerebral blood flow (CBF) lead to increasing neuronal dysfunction. Mild hypoperfusion, oligaemia, leads to a decrease in protein synthesis, whereas more-severe reductions in CBF, leading to hypoxia, cause an array of detrimental effects.
Blood–brain barrier breakdown
Disruption to tight and adherens junctions, an increase in bulk-flow fluid transcytosis, and/or enzymatic degradation of the capillary basement membrane cause physical breakdown of the BBB. The levels of many tight junction proteins, their adaptor molecules and adherens junction proteins decrease in Alzheimer’s disease and other diseases that cause dementia1,9, ALS31, multiple sclerosis32 and various animal models of neurological disease8,33. These decreases might be partly explained by the fact that vascular-associated matrix metalloproteinase (MMP) activity rises in many neurodegenerative disorders and after ischaemic CNS injury34,35; tight junction proteins and basement membrane extracellular matrix proteins are substrates for these enzymes34. Lowered expression of messenger RNAs that encode several key tight junction proteins, however, has also been reported in some neurodegenerative disorders, such as ALS31.
Endothelial cell–pericyte interactions are crucial for the formation36,37 and maintenance of the BBB33,38. Pericyte deficiency can lead to a reduction in expression of certain tight junction proteins, including occludin, claudin 5 and ZO133, and to an increase in bulk- flow transcytosis across the BBB, causing BBB breakdown38. Both processes can lead to extravasation of multiple small and large circulating macromolecules (up to 500 kDa) into the brain parenchyma33,38. Moreover, in mice, an age-dependent progressive loss of pericytes can lead to BBB disruption and microvasular degeneration and, subsequently, neuronal dysfunction, cognitive decline and neurodegenerative changes33. In their lysosomes, pericytes concentrate and degrade multiple circulating exogenous39 and endogenous proteins amplify BBB breakdown in cases of pericyte deficiency.
BBB breakdown typically leads to an accumulation of various molecules in the brain. The build up of serum proteins such as immunoglobulins and albumin can cause brain oedema and suppression of capillary blood flow whereas high concentrations of thrombin lead to neurotoxicity and memory impairment40, and accelerate vascular damage and BBB disruption41. The accumulation of plasmin (derived from circulating plasminogen) can catalyze the degradation of neuronal laminin and, hence, promote neuronal injury42, and high fibrin levels accelerate neurovascular damage6. Finally, an increase in the number of RBCs causes deposition of haemoglobin-derived neurotoxic products including iron, which generates neurotoxic reactive oxygen species (ROS)8,43 (FIG. 3a). In addition to protein-mediated vasogenic oedema, local tissue ischaemia–hypoxia depletes ATP stores, causing (Na++K+) ATPase pumps and Na+-dependent ion channels to stop working and, consequently, the endothelium and astrocytes to swell (known as cytotoxic oedema)44. Upregulation of aquaporin 4 water channels in response to ischaemia facilitates the development of cytotoxic oedema in astrocytes45.
Hypoperfusion and hypoxia
CBF is regulated by local neuronal activity and metabolism, known as neurovascular coupling46. The pial and intracerebral arteries control the local increase in CBF that occurs during brain activation, which is termed ‘functional hyperaemia’. Neurovascular coupling requires intact pial circulation, and for VSMCs and pericytes to respond normally to vasoactive stimuli33,46,47. In addition to VSMC-mediated constriction and vasodilation of cerebral arteries, recent studies have shown that pericytes modulate brain capillary diameter through constriction of the vessel wall47, which obstructs capillary flow during ischaemia48. Astrocytes regulate the contractility of intracerebral arteries49,50.
Progressive CBF reductions have increasingly serious consequences for neurons (FIG. 3b). Briefly, mild hypoperfusion — termed oligaemia — affects protein synthesis, which is required for the synaptic plasticity mediating learning and memory46. Moderate to severe CBF reductions and hypoxia affect ATP synthesis, diminishing (Na++K+) ATPase activity and the ability of neurons to generate action potentials9. In addition, such reductions can lower or increase pH, and alter electrolyte balances and water gradients, leading to the development of oedema and white matter lesions, and the accumulation of glutamate and proteinaceous toxins (for example, amyloid-β and hyperphopshorylated tau) in the brain. A reduction of greater than 80% in CBF results in neuronal death2.
The effect of CBF reductions has been extensively studied at the molecular and cellular levels in relation to Alzheimer’s disease. Reduced CBF and/or CBF dysregulation occurs in elderly individuals at high risk of Alzheimer’s disease before cognitive decline, brain atrophy and amyloid-β accumulation10,46,51–54. In animal models, hypoperfusion can induce or amplify Alzheimer’s disease-like neuronal dysfunction and/or neuropathological changes. For example, bilateral carotid occlusion in rats causes memory impairment, neuronal dysfunction, synaptic changes and amyloid-β oligomerization55, leading to accumulation of neurotoxic amyloid-β oligomers56. In a mouse model of Alzheimer’s disease, oligaemia increases neuronal amyloid-β levels and neuronal tau phosphophorylation at an epitope that is associated with Alzheimer’s disease-type paired helical filaments57. In rodents, ischaemia leads to the accumulation of hyperphosphorylated tau in neurons and the formation of filaments that resemble those present in human neurodegenerative tauopathies and Alzheimer’s disease58. Mice expressing amyloid-β precursor protein (APP) and transforming growth factor β1 (TGFβ1) develop deficient neurovascular coupling, cholinergic denervation, enhanced cerebral and cerebrovascular amyloid-β deposition, and age-dependent cognitive decline59.
Recent studies have shown that ischaemia–hypoxia influences amyloidogenic APP processing through mechanisms that increase the activity of two key enzymes that are necessary for amyloid-β production; that is, β-secretase and γ-secretase60–63. Hypoxia-inducible factor 1α (HIF1α) mediates transcriptional increase in β-secretase expression61. Hypoxia also promotes phosphorylation of tau through the mitogen-activated protein kinase (MAPK; also known as extracellular signal-regulated kinase (ERK)) pathway64, downregulates neprilysin — an amyloid-β-degrading enzyme65 — and leads to alterations in the expression of vascular-specific genes, including a reduction in the expression of the homeobox protein MOX2 gene mesenchyme homeobox 2 (MEOX2) in brain endothelial cells5 and an increase in the expression of the myocardin gene (MYOCD) in VSMCs66. In patients with Alzheimer’s disease and in models of this disorder, these changes cause vessel regression, hypoperfusion and amyloid-β accumulation resulting from the loss of the key amyloid-β clearance lipoprotein receptor (see below). In addition, hypoxia facilitates alternative splicing of Eaat2 mRNA in Alzheimer’s disease transgenic mice before amyloid-β deposition67 and suppresses glutamate reuptake by astrocytes independently of amyloid formation68, resulting in glutamate-mediated neuronal injury that is independent of amyloid-β.
In response to hypoxia, mitochondria release ROS that mediate oxidative damage to the vascular endothelium and to the selective population of neurons that has high metabolic activity. Such damage has been suggested to occur before neuronal degeneration and amyloid-β deposition in Alzheimer’s disease69,70. Although the exact triggers of hypoxia-mediated neurodegeneration and the role of HIF1α in neurodegeneration versus preconditioning-mediated neuroprotection remain topics of debate, mitochondria-generated ROS seem to have a primary role in the regulation of the HIF1α-mediated transcriptional switch that can activate an array of responses, ranging from mechanisms that increase cell survival and adaptation to mechanisms inducing cell cycle arrest and death71. Whether inhibition of hypoxia- mediated pathogenic pathways will delay onset and/or control progression in neurodegenerative conditions such as Alzheimer’s disease remains to be determined.
When comparing the contributions of BBB break-down and hypoperfusion to neuronal injury, it is interesting to consider Meox2+/− mice. Such animals have normal pericyte coverage and an intact BBB but a substantial perfusion deficit5 that is comparable to that found in pericyte-deficient mice that develop BBB breakdown33. Notably, however, Meox2+/− mice show less pronounced neurodegenerative changes than pericyte- deficient mice, indicating that chronic hypoperfusion– hypoxia alone can cause neuronal injury, but not to the same extent as hypoperfusion–hypoxia combined with BBB breakdown.
Endothelial neurotoxic and inflammatory factors
Alterations in cerebrovascular metabolic functions can lead to the secretion of multiple neurotoxic and inflammatory factors72,73. For example, brain microvessels that have been isolated from individuals with Alzheimer’s disease (but not from neurologically normal age-matched and young individuals) and brain microvessels that have been treated with inflammatory proteins release neurotoxic factors that kill neurons74,75. These factors include thrombin, the levels of which increase with the onset of Alzheimer’s disease76. Thrombin can injure neurons directly40 and indirectly by activating microglia and astrocytes73. Compared with those from age-matched controls, brain microvessels from individuals with Alzheimer’s disease secrete increased levels of multiple inflammatory mediators, such as nitric oxide, cytokines (for example, tumour necrosis factor (TNF), TGFβ1, interleukin-1β (IL-1β) and IL-6), chemokines (for example, CC-chemokine ligand 2 (CCL2; also known as monocyte chemoattractant protein 1 (MCP1)) and IL-8), prostaglandins, MMPs and leukocyte adhesion molecules73. Endothelium-derived neurotoxic and inflammatory factors together provide a molecular link between vascular metabolic dysfunction, neuronal injury and inflammation in Alzheimer’s disease and, possibly, in other neurodegenerative disorders.
Neurovascular changes
This section examines evidence for neurovascular changes during normal ageing and for neurovascular and/or BBB dysfunction in various neurodegenerative diseases, as well as the possibility that vascular defects can precede neuronal changes.
Age-associated neurovascular changes
Normal ageing diminishes brain circulatory functions, including a detectable decay of CBF in the limbic and association cortices that has been suggested to underlie age-related cognitive changes77. Alterations in the cerebral microvasculature, but not changes in neural activity, have been shown to lead to age-dependent reductions in functional hyperaemia in the visual system in cats78 and in the sensorimotor cortex in pericyte-deficient mice33. Importantly, a recent longitudinal CBF study in neurologically normal individuals revealed that people bearing the apolipoprotein E (APOE) ε4 allele — the major genetic risk factor for late-onset Alzheimer’s disease79–81 — showed greater regional CBF decline in brain regions that are particularly vulnerable to pathological changes in Alzheimer’s disease than did people without this allele82.
A meta-analysis of BBB permeability in 1,953 individuals showed that neurologically healthy humans had an age-dependent increase in vascular permeability83. Moreover, patients with vascular or Alzheimer’s dis- ease-type dementia and leucoaraiosis — a small-vessel disease of the cerebral white matter — had an even greater age-dependent increase in vascular permeability83. Interestingly, an increase in BBB permeability in brain areas with normal white matter in patients with leukoaraiosis has been suggested to play a causal part in disease and the development of lacunar strokes84. Age-related changes in the permeability of the blood– CSF barrier and the choroid plexus have been reported in sheep85.
Vascular pathology
Patients with Alzheimer’s disease or other dementia-causing diseases frequently show focal changes in brain microcirculation. These changes include the appearance of string vessels (collapsed and acellular membrane tubes), a reduction in capillary density, a rise in endothelial pinocytosis, a decrease in mitochondrial content, accumulation of collagen and perlecans in the basement membrane, loss of tight junctions and/or adhe-rens junctions3–6,9,46,86, and BBB breakdown with leakage of blood-borne molecules4,6,7,9. The time course of these vascular alterations and how they relate to dementia and Alzheimer’s disease pathology remain unclear, as no protocol that allows the development of the diverse brain vascular pathology to be scored, and hence to be tracked with ageing, has so far been developed and widely validated87. Interestingly, a recent study involving 500 individuals who died between the ages of 69 and 103 years showed that small-vessel disease, infarcts and the presence of more than one vascular pathological change were associated with Alzheimer’s disease-type pathological lesions and dementia in people aged 75 years of age87. These associations were, however, less pronounced in individuals aged 95 years of age, mainly because of a marked ageing-related reduction in Alzheimer’s disease neuropathology relative to a moderate but insignificant ageing-related reduction in vascular pathology87.
Accumulation of amyloid-β and amyloid deposition in pial and intracerebral arteries results in CAA, which is present in over 80% of Alzheimer’s disease cases88. Inpatients who have Alzheimer’s disease with established CAA in small arteries and arterioles, the VSMC layer frequently shows atrophy, which causes a rupture of the vessel wall and intracerebral bleeding in about 30% of these patients89,90. These intracerebral bleedings contribute to, and aggravate, dementia. Patients with hereditary cerebral β-amyloidosis and CAA of the Dutch, Iowa, Arctic, Flemish, Italian or Piedmont L34V type have accelerated VSMC degeneration resulting in haemorrhagic strokes and dementia91. Duplication of the gene encoding APP causes early-onset Alzheimer’s disease dementia with CAA and intracerebral haemorrhage92.
Early studies of serum immunoglobulin leakage reported that patients with ALS had BSCB break- down and BBB breakdown in the motor cortex93. Microhaemorrhages and BSCB breakdown have been shown in the spinal cord of transgenic mice expressing mutant variants of human superoxide dismutase 1 (SOD1), which in mice cause an ALS-like disease8,94,95. In mice with ALS-like disease and in patients with ALS, BSCB breakdown has been shown to occur before motor neuron degeneration or brain atrophy8,11,95.
BBB breakdown in the substantia nigra and the striatum has been detected in murine models of Parkinson’s disease that are induced by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)96–98. However, the temporal relationship between BBB breakdown and neurodegeneration in Parkinson’s disease is currently unknown. Notably, the prevalence of CAA and vascular lesions increases in Parkinson’s dis- ease99,100. Vascular lesions in the striatum and lacunar infarcts can cause vascular parkinsonism syndrome101. A recent study reported BBB breakdown in a rat model of Huntington’s disease that is induced with the toxin 3-nitropropionic acid102.
Several studies have established disruption of BBB with a loss of tight junction proteins during neuroinflammatory conditions such as multiple sclerosis and its murine model, experimental allergic encephalitis. Such disruption facilitates leukocyte infiltration, leading to oliogodendrocyte death, axonal damage, demyelination and lesion development32.
Functional changes in the vasculature
In individuals with Alzheimer’s disease, GLUT1 expression at the BBB decreases103, suggesting a shortage in necessary metabolic substrates. Studies using 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) have identified reductions in glucose uptake in asymptomatic individuals with a high risk of dementia104,105. Several studies have suggested that reduced glucose uptake across the BBB, as seen by FDG PET, precedes brain atrophy104–108.
Amyloid-β constricts cerebral arteries109. In a mouse model of Alzheimer’s disease, impairment of endothelium-dependent regulation of neocortical microcirculation110,111 occurs before amyloid-β accumulation. Recent studies have shown that CD36, a scavenger receptor that binds amyloid-β, is essential for the vascular oxidative stress and diminished functional hyperaemia that occurs in response to amyloid-β exposure112. Neuroimaging studies in patients with Alzheimer’s disease have shown that neurovascular uncoupling occurs before neurode-generative changes10,51–53. Moreover, cognitively normal APOE ε4 carriers at risk of Alzheimer’s disease show impaired CBF responses to brain activation in the absence of neurodegenerative changes or amyloid-β accumulation54. Recently, patients with Alzheimer’s disease as well as mouse models of this disease with high cerebrovascular levels of serum response factor (SRF) and MYOCD, the two transcription factors that control VSMC differentiation, have been shown to develop a hypercontractile arterial phenotype resulting in brain hypoperfusion, diminished functional hyperaemia and CAA66,113. More work is needed to establish the exact role of SRF and MYOCD in the vascular dysfunction that results in the Alzheimer’s disease phenotype and CAA.
PET studies with 11C-verapamil, an ABCB1 substrate, have indicated that the function of ABCB1, which removes multiple drugs and toxins from the brain, decreases with ageing114 and is particularly compromised in the midbrain of patients with Parkinson’s disease, progressive supranuclear palsy or multiple system atrophy115. More work is needed to establish the exact roles of ABC BBB transporters in neurodegeneration and whether their failure precedes the loss of dopaminergic neurons that occurs in Parkinson’s disease.
In mice with ALS-like disease and in patients with ALS, hypoperfusion and/or dysregulated CBF have been shown to occur before motor neuron degeneration or brain atrophy8,116. Reduced regional CBF in basal ganglia and reduced blood volume have been reported in pre-symptomatic gene-tested individuals at risk for Huntington’s disease117. Patients with Huntington’s disease display a reduction in vasomotor activity in the cerebral anterior artery during motor activation118.
Vascular and neuronal common growth factors
Blood vessels and neurons share common growth factors and molecular pathways that regulate their development and maintenance119,120. Angioneurins are growth factors that exert both vasculotrophic and neurotrophic activities121. The best studied angioneurin is vascular endothelial growth factor (VEGF). VEGF regulates vessel formation, axonal growth and neuronal survival120. Ephrins, semaphorins, slits and netrins are axon guidance factors that also regulate the development of the vascular system121. During embryonic development of the neural tube, blood vessels and choroid plexus secrete IGF2 into the CSF, which regulates the proliferation of neuronal progenitor cells122. Genetic and pharmacological manipulations of angioneurin activity yielded various vascular and cerebral phenotypes121. Given the dual nature of angioneurin action, these studies have not been able to address whether neuronal dysfunction results from a primary insult to neurons and/or whether it is secondary to vascular dysfunction.
Increased levels of VEGF, a hypoxia-inducible angiogenic factor, were found in the walls of intraparenchymal vessels, perivascular deposits, astrocytes and intrathecal space of patients with Alzheimer’s disease, and were consistent with the chronic cerebral hypoperfusion and hypoxia that were observed in these individuals73. In addition to VEGF, brain microvessels in Alzheimer’s disease release several molecules that can influence angiogenesis, including IL-1β, IL-6, IL-8, TNF, TGFβ, MCP1, thrombin, angiopoietin 2, αVβ3 and αVβ5 integrins, and HIF1α73. However, evidence for increased vascularity in Alzheimer’s disease is lacking. On the contrary, several studies have reported that focal vascular regression and diminished microvascular density occur in Alzheimer’s disease4,5,73 and in Alzheimer’s disease transgenic mice123. The reason for this discrepancy is not clear. The anti-angiogenic activity of amyloid-β, which accumulates in the brains of individuals with Alzheimer’s disease and Alzheimer’s disease models, may contribute to hypo-vascularity123. Conversely, genome-wide transcriptional profiling of brain endothelial cells from patients with Alzheimer’s disease revealed that extremely low expression of vascular-restricted MEOX2 mediates aberrant angiogenic responses to VEGF and hypoxia, leading to capillary death5. This finding raises the interesting question of whether capillary degeneration in Alzheimer’s disease results from unsuccessful vascular repair and/or remodelling. Moreover, mice that lack one Meox2 allele have been shown to develop a primary cerebral endothelial hypoplasia with chronic brain hypoperfusion5, resulting in secondary neurodegenerative changes33.
Does vascular dysfunction cause neuronal dysfunction?
In summary, the evidence that is discussed above clearly indicates that vascular dysfunction is tightly linked to neuronal dysfunction. There are many examples to illustrate that primary vascular deficits lead to secondary neurodegeneration, including CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts), an hereditary small-vessel brain disease resulting in multiple small ischaemic lesions, neurodegeneration and dementia124; mutations in SLC2A1 that cause dysfunction of the BBB-specific GLUT1 transporter in humans resulting in seizures; cognitive impairment and microcephaly125; microcephaly and epileptiform discharges in mice with genetic deletion of a single Slc2a1 allele126; and neurodegeneration mediated by a single Meox2 homebox gene deletion restricted to the vascular system33. Patients with hereditary cerebral β-amyloidosis and CAA of the Dutch, Iowa, Arctic, Flemish, Italian or Piedmont L34V type provide another example showing that primary vascular dysfunction — which in this case is caused by deposition of vasculotropic amyloid-β mutants in the arterial vessel wall — leads to dementia and intracerebral bleeding. Moreover, as reviewed in the previous sections, recent evidence suggests that BBB dysfunction and/or breakdown, and CBF reductions and/or dysregulation may occur in sporadic Alzheimer’s disease and experimental models of this disease before cognitive decline, amyloid-β deposition and brain atrophy. In patients with ALS and in some experimental models of ALS, CBF dysregulation, BSCB breakdown and spinal cord hypoperfusion have been reported to occur before motor neuron cell death. Whether neurological changes follow or precede vascular dysfunction in Parkinson’s disease, Huntington’s disease and multiple sclerosis remains less clear. However, there is little doubt that vascular injury mediates, amplifies and/or lowers the threshold for neuronal dysfunction and loss in several neurological disorders.
Disease-specific considerations
This section examines how amyloid-β levels are kept low in the brain, a process in which the BBB has a central role, and how faulty BBB-mediated clearance mechanisms go awry in Alzheimer’s disease. On the basis of this evidence and the findings discussed elsewhere in the Review, a new hypothesis for the pathogenesis of Alzheimer’s disease that incorporates the vascular evidence is presented. ALS-specific disease mechanisms relating to the BBB are then examined.
Alzheimer’s disease
Amyloid-β clearance from the brain by the BBB is the best studied example of clearance of a proteinaceous toxin from the CNS. Multiple pathways regulate brain amyloid-β levels, including its production and clearance (FIG. 4). Recent studies127–129 have confirmed earlier findings in multiple rodent and non-human primate models demonstrating that peripheral amyloid-β is an important precursor of brain amyloid-β130–136. Moreover, peripheral amyloid-β sequestering agents such as soluble LRP1137, anti-amyloid-β antibodies138–140, gelsolin and the ganglioside GM1141, or systemic expression of neprilysin have been shown to reduce the amyloid burden in Alzheimer’s disease mice by eliminating contributions of the peripheral amyloid-β pool to the total brain pool of this peptide.
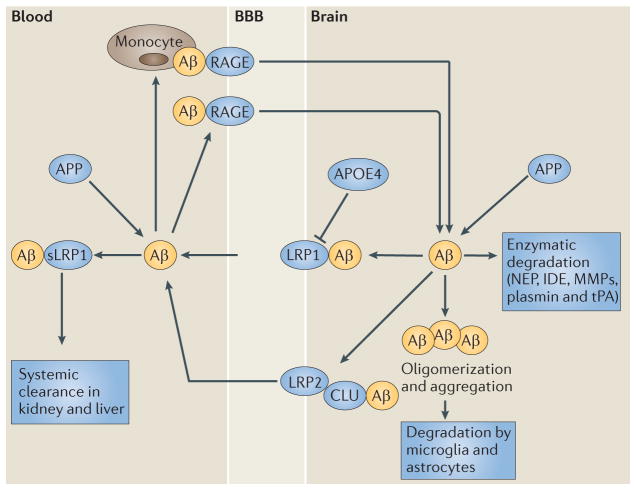
Figure 4. The role of blood–brain barrier transport in brain homeostasis of amyloid-β.
Amyloid-β (Aβ) is produced from the amyloid-β precursor protein (APP), both in the brain and in peripheral tissues. Clearance of amyloid-β from the brain normally maintains its low levels in the brain. This peptide is cleared across the blood– brain barrier (BBB) by the low-density lipoprotein receptor-related protein 1 (LRP1). LRP1 mediates rapid efflux of a free, unbound form of amyloid-β and of amyloid-β bound to apolipoprotein E2 (APOE2), APOE3 or α2-macroglobulin (not shown) from the brain’s interstitial fluid into the blood, and APOE4 inhibits such transport. LRP2 eliminates amyloid-β that is bound to clusterin (CLU; also known as apolipoprotein J (APOJ)) by transport across the BBB, and shows a preference for the 42-aminoacid form of this peptide. ATP-binding cassette subfamily A member 1 (ABCA1; also known as cholesterol efflux regulatory protein) mediates amyloid-β efflux from the brain endothelium to blood across the luminal side of the BBB (not shown). Cerebral endothelial cells, pericytes, vascular smooth muscle cells, astrocytes, microglia and neurons express different amyloid-β-degrading enzymes, including neprilysin (NEP), insulin-degrading enzyme (IDE), tissue plasminogen activator (tPA) and matrix metalloproteinases (MMPs), which contribute to amyloid-β clearance. In the circulation, amyloid-β is bound mainly to soluble LRP1 (sLRP1), which normally prevents its entry into the brain. Systemic clearance of amyloid-β is mediated by its removal by the liver and kidneys. The receptor for advanced glycation end products (RAGE) provides the key mechanism for influx of peripheral amyloid-β into the brain across the BBB either as a free, unbound plasma-derived peptide and/or by amyloid-β-laden monocytes. Faulty vascular clearance of amyloid-β from the brain and/or an increased re-entry of peripheral amyloid-β across the blood vessels into the brain can elevate amyloid-β levels in the brain parenchyma and around cerebral blood vessels. At pathophysiological concentrations, amyloid-β forms neurotoxic oligomers and also self-aggregates, which leads to the development of cerebral β-amyloidosis and cerebral amyloid angiopathy.
The receptor for advanced glycation end products (RAGE) mediates amyloid-β transport in brain and the propagation of its toxicity. RAGE expression in brain endothelium provides a mechanism for influx of amyloid-β144,145 and amyloid-β-laden monocytes146 across the BBB, as shown in Alzheimer’s disease models (FIG. 4). The amyloid-β-rich environment in Alzheimer’s disease and models of this disorder increases RAGE expression at the BBB and in neurons147,148, amplifying amyloid-β-mediated pathogenic responses. Blockade of amyloid-β–RAGE signaling in Alzheimer’s disease is a promising strategy to control self-propagation of amyloid-β-mediated injury.
Several studies in animal models of Alzheimer’s disease and, more recently, in patients with this disorder149 have shown that diminished amyloid-β clearance occurs in brain tissue in this disease. LRP1 plays an important part in the three-step serial clearance of this peptide from brain and the rest of the body150 (FIG. 4). In step one, LRP1 in brain endothelium binds brain-derived amyloid-β at the abluminal side of the BBB, initiating its clearance to blood, as shown in many animal models151–156 and BBB models in vitro151,157,158. The vasculotropic mutants of amyloid-β that have low binding affinity for LRP1 are poorly cleared from the brain or CSF151,159,160. APOE4, but not APOE3 or APOE2, blocks LRP1-mediated amyloid-β clearance from the brain and, hence, promotes its retention161, whereas clusterin (also known as apolipoprotein J (APOJ)) mediates amyloid-β clearance across the BBB via LRP2153. APOE and clusterin influence amyloid-β aggregation162,163. Reduced LRP1 levels in brain microvessels, perhaps in addition to altered levels of ABCB1, are associated with amyloid-β cerebrovascular and brain accumulation during ageing in rodents, non-human primates, humans, Alzheimer’s disease mice and patients with Alzheimer’s disease66,151,152,164–166. Moreover, recent work has shown that brain LRP1 is oxidized in Alzheimer’s disease167, and may contribute to amyloid-β retention in brain because the oxidized form cannot bind and/or trans- port amyloid-β137. LRP1 also mediates the removal of amyloid-β from the choroid plexus168.
In step two, circulating soluble LRP1 binds more than 70% of plasma amyloid-β in neurologically normal humans137. In patients with Alzheimer’s disease or mild cognitive impairment (MCI), and in Alzheimer’s disease mice, amyloid-β binding to soluble LRP1 is compromised due to oxidative changes137,169, resulting in elevated plasma levels of free amyloid-β isoforms comprising 40 or 42 amino acids (amyloid-β1–40 and amyloid-β1–42). These peptides can then re-enter the brain, as has been shown in a mouse model of Alzheimer’s disease137. Rapid systemic removal of amyloid-β by the liver is also mediated by LRP1 and comprises step three of the clearance process170.
In brain, amyloid-β is enzymatically degraded by neprilysin171, insulin-degrading enzyme172, tissue plasminogen activator 173 and MMPs173,174 in various cell types, including endothelial cells, pericytes, astrocytes, neurons and microglia. Cellular clearance of this peptide by astrocytes and VSMCs is mediated by LRP1 and/or another lipoprotein receptor66,175. Clearance of amyloid-β aggregates by microglia has an important role in amyloid-β-directed immunotherapy176 and reduction of the amyloid load in brain177. Passive ISF–CSF bulk flow and subsequent clearance through the CSF might contribute to 10–15% of total amyloid-β removal152,153,178. In the injured human brain, increasing soluble amyloid-β concentrations in the ISF correlated with improvements in neurological status, suggesting that neuronal activity might regulate extracellular amyloid-β levels179.
The role of BBB dysfunction in amyloid-β accumulation, as discussed above, underlies the contribution of vascular dysfunction to Alzheimer’s disease (see FIG. 5 for a model of vascular damage in Alzheimer’s disease). The amyloid hypothesis for the pathogenesis of Alzheimer’s disease maintains that this peptide initiates a cascade of events leading to neuronal injury and loss and, eventually, dementia180,181. Here, I present an alternative hypothesis — the two-hit vascular hypothesis of Alzheimer’s disease — that incorporates the vascular contribution to this disease, as discussed in this Review (BOX 1). This hypothesis states that primary damage to brain microcirculation (hit one) initiates a non-amyloidogenic pathway of vascular-mediated neuronal dysfunction and injury, which is mediated by BBB dysfunction and is associated with leakage and secretion of multiple neurotoxic molecules and/or diminished brain capillary flow that causes multiple focal ischaemic or hypoxic micro-injuries. BBB dysfunction also leads to impairment of amyloid-β clearance, and oligaemia leads to increased amyloid-β generation. Both processes contribute to accumulation of amyloid-β species in the brain (hit two), where these peptides exert vasculotoxic and neurotoxic effects. According to the two-hit vascular hypothesis of Alzheimer’s disease, tau pathology develops secondary to vascular and/or amyloid-β injury.
Figure 5. A model of vascular damage in Alzheimer’s disease.
a, In the early stages of Alzheimer’s disease, small pial and intracerebral arteries develop a hypercontractile phenotype that underlies dysregulated cerebral blood flow (CBF). This phenotype is accompanied by diminished amyloid-β clearance by the vascular smooth muscle cells (VSMCs). In the later phases of Alzheimer’s disease, amyloid deposition in the walls of intracerebral arteries leads to cerebral amyloid angiopathy (CAA), pronounced reductions in CBF, atrophy of the VSMC layer and rupture of the vessels causing microbleeds. b, At the level of capillaries in the early stages of Alzheimer’s disease, blood–brain barrier (BBB) dysfunction leads to a faulty amyloid-β clearance and accumulation of neurotoxic amyloid-β oligomers in the interstitial fluid (ISF), microhaemorrhages and accumulation of toxic blood-derived molecules (that is, thrombin and fibrin), which affect synaptic and neuronal function. Hyperphosphorylated tau (p-tau) accumulates in neurons in response to hypoperfusion and/or rising amyloid-β levels. At this point, microglia begin to sense neuronal injury. In the later stages of the disease in brain capillaries, microvascular degeneration leads to increased deposition of basement membrane proteins and perivascular amyloid. The deposited proteins and amyloid obstruct capillary blood flow, resulting in failure of the efflux pumps, accumulation of metabolic waste products, changes in pH and electrolyte composition and, subsequently, synaptic and neuronal dysfunction. Neurofibrillary tangles (NFTs) accumulate in response to ischaemic injury and rising amyloid-β levels. Activation of microglia and astrocytes is associated with a pronounced inflammatory response. ROS, reactive oxygen species.
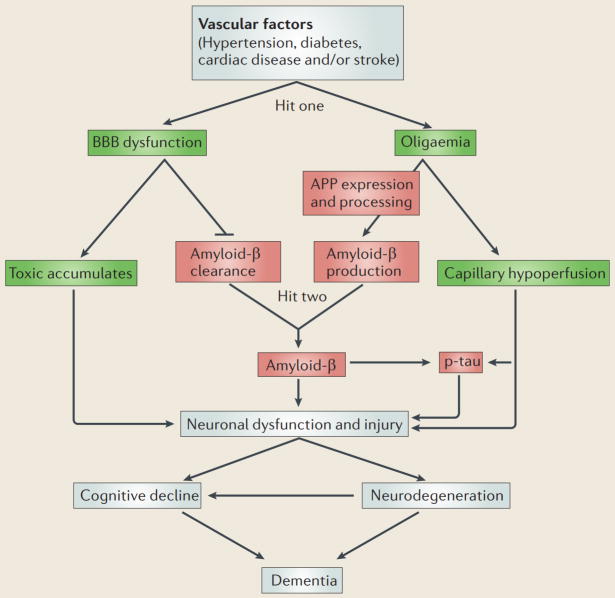
Box 1. The two-hit vascular hypothesis for Alzheimer’s disease.
Substantial overlap exists among risk factors for cerebrovascular disorder and Alzheimer’s disease9,88,205. For example, midlife diabetes10,206, hypertension207 and obesity208 are vascular risk factors that predispose individuals to Alzheimer’s disease and vascular dementia. It is now widely recognized that most cases of Alzheimer’s disease have mixed vascular pathology and small-vessel disease88,209. Moreover, brain hypoperfusion–hypoxia53, silent infarcts210, the presence of one or more infarctions211, stroke episodes and transient ischaemic or hypoxic attacks all increase the risk of Alzheimer’s disease. In this disorder, although the molecular and cellular events for each step in the disease process and for each risk factor are not absolutely clear, vascular factors might all converge on a common final disease pathway, involving brain microvascular dysfunction and/or degeneration, as well as amyloid-β and tau pathology. According to the two-hit vascular hypothesis of Alzheimer’s disease, vascular risk factors (hit one) lead to blood–brain barrier (BBB) dysfunction and a reduction in cerebral blood flow (oligaemia), initiating a cascade of events that precedes dementia. In the non-amyloid-β pathway (see the figure, shown in green boxes), toxic accumulates and capillary hypoperfusion induce early neuronal dysfunction. Vascular injury also reduces amyloid-β clearance at the BBB and increases production of this peptide from the amyloid-β precursor protein (APP), leading to amyloid-β accumulation (the amyloid-β pathway; see the figure, shown in red boxes). The increase in amyloid-β (hit two) amplifies neuronal dysfunction, accelerates neurodegeneration and dementia, and contributes to disease self-propagation. Amyloid-β and/or hypoperfusion can induce hyperphosphorylation of tau (p-tau), leading to neurofibrillary tangle formation.
Box 1.
The two-hit vascular hypothesis for Alzheimer’s disease
Amyotrophic lateral sclerosis
The cause of sporadic ALS, a fatal adult-onset motor neuron neurodegenerative disease, is not known182. In a relatively small number of patients with inherited SOD1 mutations, the disease is caused by toxic properties of mutant SOD1183. Mutations in the genes encoding ataxin 2 and TAR DNA-binding protein 43 (TDP43) that cause these proteins to aggregate have been associated with ALS182,184. Some studies have suggested that abnormal SOD1 species accumulate in sporadic ALS185. Interestingly, studies in ALS transgenic mice expressing a mutant version of human SOD1 in neurons, and in non-neuronal cells neighbouring these neurons, have shown that deletion of this gene from neurons does not influence disease progression186, whereas deletion of this gene from microglia186 or astrocytes187 substantially increases an animal’s lifespan. According to an emerging hypothesis of ALS that is based on studies in SOD1 mutant mice, the toxicity that is derived from non-neuronal neighbouring cells, particularly microglia and astrocytes, contributes to disease progression and motor neuron degeneration186–190, whereas BBB dysfunction might be critical for disease initiation8,43,94,95. More work is needed to determine whether this concept of disease initiation and progression may also apply to cases of sporadic ALS.
Human data support a role for angiogenic factors and vessels in the pathogenesis of ALS. For example, the presence of VEGF variations (which were identified in large meta-analysis studies) has been linked to ALS191. Angiogenin is another pro-angiogenic gene that is implicated in ALS because heterozygous missense mutations in angiogenin cause familial and sporadic ALS192. Moreover, mice with a mutation that eliminates hypoxia-responsive induction of the Vegf gene (Vegfδ/δ mice) develop lateonset motor neuron degeneration193. Spinal cord ischaemia worsens motor neuron degeneration and functional outcome in Vegfδ/δ mice, whereas the absence of hypoxic induction of VEGF in mice that develop motor neuron disease from expression of ALS-linked mutant SOD1G93A results in substantially reduced survival191.
Therapeutic opportunities
Many investigators believe that primary neuronal dysfunction resulting from an intrinsic neuronal disorder is the key underlying event in human neurodegenerative diseases. Thus, most therapeutic efforts for neurodegenerative diseases have so far been directed at the development of so-called ‘single-target, single-action’ agents to target neuronal cells directly and reverse neuronal dysfunction and/or protect neurons from injurious insults. However, most preclinical and clinical studies have shown that such drugs are unable to cure or control human neurological disorders2,181,183,194,195. For example, although pathological overstimulation of glutaminergic NMDA receptors (NMDARs) has been shown to lead to neuronal injury and death in several disorders, includ- ing stroke, Alzheimer’s disease, ALS and Huntington’s disease16, NMDAR antagonists have failed to show a therapeutic benefit in the above-mentioned human neurological disorders.
Recently, my colleagues and I coined the term vascul-oneuronal-inflammatory triad195 to indicate that vascular damage, neuronal injury and/or neurodegeneration, and neuroinflammation comprise a common pathological triad that occurs in multiple neurological disorders. In line with this idea, it is conceivable that ‘multiple-target, multiple-action’ agents (that is, drugs that have more than one target and thus have more than one action) will have a better chance of controlling the complex disease mechanisms that mediate neurodegeneration than agents that have only one target (for example, neurons). According to the vasculo-neuronal-inflammatory triad model, in addition to neurons, brain endothelium, VSMCs, pericytes, astrocytes and activated microglia are all important therapeutic targets.
Here, I will briefly discuss a few therapeutic strategies based on the vasculo-neuronal-inflammatory triad model. VEGF and other angioneurins may have multiple targets, and thus multiple actions, in the CNS120. For example, preclinical studies have shown that treatment of SOD1G93A rats with intracerebroventricular VEGF196 or intramuscular administration of a VEGF-expressing lentiviral vector that is transported retrogradely to motor neurons in SOD1G93A mice197 reduced pathology and extended survival, probably by promoting angiogenesis and increasing the blood flow through the spinal cord as well as through direct neuronal protective effects of VEGF on motor neurons. On the basis of these and other studies, a phase I–II clinical trial has been initiated to evaluate the safety of intracerebroventricular infusion of VEGF in patients with ALS198. Treatment with angiogenin also slowed down disease progression in a mouse model of ALS199.
IGF1 delivery has been shown to promote amyloid-β vascular clearance and to improve learning and memory in a mouse model of Alzheimer’s disease200. Local intracerebral implantation of VEGF-secreting cells in a mouse model of Alzheimer’s disease has been shown to enhance vascular repair, reduce amyloid burden and improve learning and memory201. In contrast to VEGF, which can increase BBB permeability, TGFβ, hepatocyte growth factor and fibroblast growth factor 2 promote BBB integrity by upregulating the expression of endothelial junction proteins121 in a similar way to APC43. However, VEGF and most growth factors do not cross the BBB, so the development of delivery strategies such as Trojan horses is required for their systemic use25.
A recent experimental approach with APC provides an example of a neurovascular medicine that has been shown to favorably regulate multiple pathways in non- neuronal cells and neurons, resulting in vasculo-protection, stabilization of the BBB, neuroprotection and anti-inflammation in several acute and chronic models of the CNS disorders195 (BOX 2).
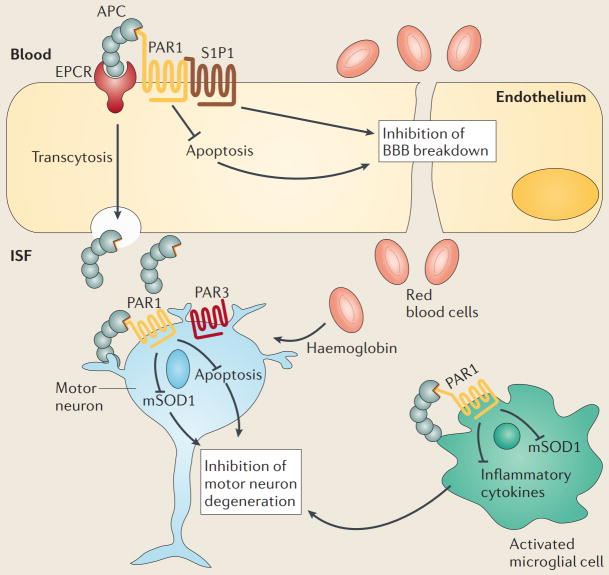
Box 2. A model of multiple-target, multiple-action neurovascular medicine.
Activated protein C (APC) is an endogenous plasma protease with antithrombotic, cytoprotective and anti-inflammatory activities in the CNS195. APC and recombinant analogues of this protease that have been engineered to have reduced or no anticoagulant activity have excellent therapeutic potential as disease-modifying therapies for neuropathologies that are driven by the vasculo-neuronal-inflammatory triad and, hence, involve vascular damage with blood–brain barrier (BBB) or blood–spinal cord barrier (BSCB) breakdown, neuronal injury and/or neurodegeneration, and neuroinflammation. Although much remains to be learned with respect to the biology of APC, APC-mediated signaling within the injured neurovascular unit (NVU) has beneficial effects in experimental models of amyotrophic lateral sclerosis (ALS)43, multiple sclerosis212, multiple models of stroke, spinal cord injury and brain trauma195. Here, drawing on data from a transgenic SOD1G93A mouse model of ALS, the multiple effects of APC on different cell types within the NVU in the spinal cord are shown43. APC protects brain endothelium from divergent inducers of apoptosis by first binding to the endothelial protein C receptor (EPCR). Activation of this receptor is required for activation of proteinase-activated receptor 1 (PAR1), which in turn induces cell protective signaling in the endothelium. PAR1 also cross-activates sphigosine 1 phosphate receptor 1 (S1P1), which enhances the integrity of endothelial barrier and inhibits BSCB breakdown195. APC’s action contributes to vascular repair of the NVU by ameliorating red blood cell extravasation, causing microhaemorrhages and inducing accumulation of neurotoxic reactive oxygen species in the spinal cord. EPCR also mediates transcytosis of APC across the BBB28 and BSCB43. Once in the spinal cord interstitial fluid (ISF), APC reaches motor neurons and exerts its direct neuronal protective activity by blocking the apoptotic pathways that are induced by divergent injurious stimuli, and by downregulating mutant superoxide dismutase 1 (mSOD1) expression. The effect of APC on neurons is mediated by PAR1 and PAR3 receptors. APC exerts its anti-inflammatory activity by activating PAR1 in microglia, which suppresses the activation of these cells and, therefore, inhibits the expression of inflammatory cytokines. PAR1 activation by APC also inhibits mSOD1 expression in microglia.
Box 2.
A model of multiple-target, multiple-action neurovascular medicine
The recognition of amyloid-β clearance pathways (FIG. 4), as discussed above, opens exciting new therapeutic opportunities for Alzheimer’s disease. Amyloid-β clearance pathways are promising therapeutic targets for the future development of neurovascular medicines because it has been shown both in animal models of Alzheimer’s disease1 and in patients with sporadic Alzheimer’s disease149 that faulty clearance from brain and across the BBB primarily determines amyloid-β retention in brain, causing the formation of neurotoxic amyloid-β oligomers56 and the promotion of brain and cerebrovascular amyloidosis3. The targeting of clearance mechanisms might also be beneficial in other diseases; for example, the clearance of extracellular mutant SOD1 in familial ALS, the prion protein in prion disorders and α-synuclein in Parkinson’s disease might all prove beneficial. However, the clearance mechanisms for these proteins in these diseases are not yet understood.
Conclusions and perspectives
Currently, no effective disease-modifying drugs are available to treat the major neurodegenerative disorders202–204. This fact leads to a question: are we close to solving the mystery of neurodegeneration? The probable answer is yes, because the field has recently begun to recognize that, first, damage to neuronal cells is not the sole contributor to disease initiation and progression, and that second, correcting disease pathways in vascular and glial cells may offer an array of new approaches to control neuronal degeneration that do not involve targeting neurons directly. These realizations constitute an important shift in paradigm that should bring us closer to a cure for neurodegenerative diseases. Here, I raise some issues concerning the existing models of neurodegeneration and the new neurovascular paradigm.
The discovery of genetic abnormalities and associations by linkage analysis or DNA sequencing has broadened our understanding of neurodegeneration. However, insufficient effort has been made to link genetic findings with disease biology. Another concern for neurodegenerative research is how we should interpret findings from animal models202. Genetically engineered models of human neurodegenerative disorders in Drosophila melanogaster and Caenorhabditis elegans have been useful for dissecting basic disease mechanisms and screening compounds. However, in addition to having much simpler nervous systems, insects and avascular species do not have cerebrovascular and immune systems that are comparable to humans and, therefore, are unlikely to replicate the complex disease pathology that is found in people.
For most neurodegenerative disorders, early steps in the disease processes remain unclear, and biomarkers for these stages have yet to be identified. Thus, it is difficult to predict whether mammalian models expressing human genes and proteins that we know are implicated in the intermediate or later stages of disease pathophysiology, such as amyloid-β or tau in Alzheimer’s disease7,181, will help us to discover therapies for the early stages of disease and for disease prevention, because the exact role of these pathological accumulations during disease onset remains uncertain. According to the two-hit vascular hypothesis of Alzheimer’s disease, incorporating vascular factors that are associated with Alzheimer’s disease into current models of this disease may more faithfully replicate dementia events in humans. Alternatively, by focusing on the comorbidities and the initial cellular and molecular mechanisms underlying early neurovascular dysfunction that are associated with Alzheimer’s disease, new models of dementia and neurodegeneration may be developed that do not require the genetic manipulation of amyloid-β or tau expression.
The proposed neurovascular triad model of neurodegenerative diseases challenges the traditional neurocentric view of such disorders. At the same time, this model raises a set of new important issues that require further study. For example, the molecular basis of the neurovascular link with neurodegenerative disorders is poorly understood, in terms of the adhesion molecules that keep the physical association of various cell types together, the molecular crosstalk between different cell types (including endothelial cells, pericytes and astrocytes) and how these cellular interactions influence neuronal activity. Addressing these issues promises to create new opportunities not only to better understand the molecular basis of the neurovascular link with neurodegeneration but also to develop novel neurovascular-based medicines.
The construction of a human BBB molecular atlas will be an important step towards understanding the role of the BBB and neurovascular interactions in health and disease. Achievement of this goal will require identifying new BBB transporters by using genomic and proteomic tools, and by cloning some of the transporters that are already known. Better knowledge of transporters at the human BBB will help us to better understand their potential as therapeutic targets for disease.
Development of higher-resolution imaging methods to evaluate BBB integrity, key transporters’ functions and CBF responses in the microregions of interest (for example, in the entorhinal region of the hippocampus) will help us to understand how BBB dysfunction correlates with cognitive outcomes and neurodegenerative processes in MCI, Alzheimer’s disease and related disorders.
The question persists: are we missing important therapeutic targets by studying the nervous system in isolation from the influence of the vascular system? The probable answer is yes. However, the current exciting and novel research that is based on the neurovascular model has already begun to change the way that we think about neurodegeneration, and will continue to provide further insights in the future, leading to the development of new neurovascular therapies.
Acknowledgments
The author wishes to thank the US National Institutes of Health (grants R37 AG023084, R37 NS34467 and HL63290), the ALS Association (grant 1859) and the Zilkha family for research support. The author also wishes to thank A. Sagare for preparing the illustrations and for valuable help with the reference list.
Footnotes
Competing interests statement
The author declares competing financial interests: see web version for details.
References
- 1.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. Comprenesive review describing mechanisms of ischemic injury to the neurovascular unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, et al. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. Important study demonstrating that low expression of MEOX2 gene in brain endothelium leads to aberrant angiogenesis and vascular regression in Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 6.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. Important study showing the blood-brain barrier breakdown in models of Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipser BD, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Zhong Z, et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci. 2008;11:420–422. doi: 10.1038/nn2073. Important study demonstrating the blood-spinal cord barrier defects precede motor neuron degeneration in mice developing an ALS-like disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalaria RN. Vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr Rev. 2010;68 (Suppl 2):S74–87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knopman DS, Roberts R. Vascular risk factors: imaging and neuropathologic correlates. J Alzheimers Dis. 2010;20:699–709. doi: 10.3233/JAD-2010-091555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki K, et al. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. 2011;89:718–728. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- 12.Neuwelt EA, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40:S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J Biol Chem. 1999;274:31891–31895. doi: 10.1074/jbc.274.45.31891. [DOI] [PubMed] [Google Scholar]
- 16.Hardingham GE. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans. 2009;37:1147–1160. doi: 10.1042/BST0371147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elali A, Hermann DM. ATP-Binding Cassette Transporters and Their Roles in Protecting the Brain. Neuroscientist. 2011 doi: 10.1177/1073858410391270. [DOI] [PubMed] [Google Scholar]
- 18.Visser WE, Friesema EC, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlokovic BV, Begley DJ, Chain-Eliash DG. Blood-brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 20.Zlokovic BV, Lipovac MN, Begley DJ, Davson H, Rakic L. Transport of leucine-enkephalin across the blood-brain barrier in the perfused guinea pig brain. J Neurochem. 1987;49:310–315. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 21.Zlokovic BV, Mackic JB, Djuricic B, Davson H. Kinetic analysis of leucine-enkephalin cellular uptake at the luminal side of the blood-brain barrier of an in situ perfused guinea-pig brain. J Neurochem. 1989;53:1333–1340. doi: 10.1111/j.1471-4159.1989.tb08522.x. [DOI] [PubMed] [Google Scholar]
- 22.Zlokovic BV, et al. Kinetics of arginine-vasopressin uptake at the blood-brain barrier. Biochim Biophys Acta. 1990;1025:191–198. doi: 10.1016/0005-2736(90)90097-8. [DOI] [PubMed] [Google Scholar]
- 23.Zlokovic BV, Segal MB, Begley DJ, Davson H, Rakic L. Permeability of the blood-cerebrospinal fluid and blood-brain barriers to thyrotropin-releasing hormone. Brain Res. 1985;358:191–199. doi: 10.1016/0006-8993(85)90963-1. [DOI] [PubMed] [Google Scholar]
- 24.Dogrukol-Ak D, et al. Isolation of peptide transport system-6 from brain endothelial cells: therapeutic effects with antisense inhibition in Alzheimer and stroke models. J Cereb Blood Flow Metab. 2009;29:411–422. doi: 10.1038/jcbfm.2008.131. [DOI] [PubMed] [Google Scholar]
- 25.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Nishijima T, et al. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Banks WA. Blood-brain barrier as a regulatory interface. Forum Nutr. 2010;63:102–110. doi: 10.1159/000264398. [DOI] [PubMed] [Google Scholar]
- 28.Deane R, et al. Endothelial protein C receptor-assisted transport of activated protein C across the mouse blood-brain barrier. J Cereb Blood Flow Metab. 2009;29:25–33. doi: 10.1038/jcbfm.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iadecola C. Astrocytes take center stage in salt sensing. Neuron. 2007;54:3–5. doi: 10.1016/j.neuron.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, et al. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron. 2007;54:59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Henkel JS, Beers DR, Wen S, Bowser R, Appel SH. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology. 2009;72:1614–1616. doi: 10.1212/WNL.0b013e3181a41228. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. Important study demonstrating that loss of pericytes leads to blood-brain barrier breakdown and hypoperfusion resulting in secondary neurodegenerative changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 35.Cheng T, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 36.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. Important study describing that pericytes control the formation of blood-brain barrier during embryonic development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F, et al. Endothelial Smad4 maintains cerebrovascular integrity by activating N-cadherin through cooperation with Notch. Dev Cell. 2011;20:291–302. doi: 10.1016/j.devcel.2011.01.011. Important study demonstrating that N-cadherin mediates pericyte-endothelial attachment in the cerebral blood vessels preventing microhemorrhages. [DOI] [PubMed] [Google Scholar]
- 38.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. Important study demonstrating the role of pericytes in the maintenance of the blood-brain barrier in vivo during adulthood. [DOI] [PubMed] [Google Scholar]
- 39.Broadwell RD, Salcman M. Expanding the definition of the blood-brain barrier to protein. Proc Natl Acad Sci U S A. 1981;78:7820–7824. doi: 10.1073/pnas.78.12.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mhatre M, et al. Thrombin, a mediator of neurotoxicity and memory impairment. Neurobiol Aging. 2004;25:783–793. doi: 10.1016/j.neurobiolaging.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–2352. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- 42.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Z, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. Important study demonstrating that activated protein C prevents the blood-spinal cord barrier breakdown, suppresses microglia activation and protects motor neurons in ALS mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoshi A, Yamamoto T, Shimizu K, Sugiura Y, Ugawa Y. Chemical preconditioning-induced reactive astrocytosis contributes to the reduction of post-ischemic edema through aquaporin-4 downregulation. Exp Neurol. 2011;227:89–95. doi: 10.1016/j.expneurol.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 46.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 47.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. Important study demonstrating that pericytes control the diameter of brain capillaries in response to signals from neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yemisci M, et al. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 49.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takano T, Han X, Deane R, Zlokovic B, Nedergaard M. Two-photon imaging of astrocytic Ca2+ signaling and the microvasculature in experimental mice models of Alzheimer’s disease. Ann N Y Acad Sci. 2007;1097:40–50. doi: 10.1196/annals.1379.004. [DOI] [PubMed] [Google Scholar]
- 51.Smith CD, et al. Altered brain activation in cognitively intact individuals at high risk for Alzheimer’s disease. Neurology. 1999;53:1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- 52.Bookheimer SY, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruitenberg A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 54.Sheline YI, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, et al. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-beta oligomerization in rats. J Alzheimers Dis. 2010;21:813–822. doi: 10.3233/JAD-2010-100216. [DOI] [PubMed] [Google Scholar]
- 56.Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. Important study showing that amyloid-β oligomers inhibit neuronal activity in the hipocampus. [DOI] [PubMed] [Google Scholar]
- 57.Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta} Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gordon-Krajcer W, Kozniewska E, Lazarewicz JW, Ksiezak-Reding H. Differential changes in phosphorylation of tau at PHF-1 and 12E8 epitopes during brain ischemia and reperfusion in gerbils. Neurochem Res. 2007;32:729–737. doi: 10.1007/s11064-006-9199-3. [DOI] [PubMed] [Google Scholar]
- 59.Ongali B, et al. Transgenic mice overexpressing APP and transforming growth factor-beta1 feature cognitive and vascular hallmarks of Alzheimer’s disease. Am J Pathol. 2010;177:3071–3080. doi: 10.2353/ajpath.2010.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun X, et al. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 62.Guglielmotto M, et al. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 63.Li L, et al. Hypoxia increases Abeta generation by altering beta- and gamma-cleavage of APP. Neurobiol Aging. 2009;30:1091–1098. doi: 10.1016/j.neurobiolaging.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Fang H, Zhang LF, Meng FT, Du X, Zhou JN. Acute hypoxia promote the phosphorylation of tau via ERK pathway. Neurosci Lett. 2010;474:173–177. doi: 10.1016/j.neulet.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, et al. Hypoxia-induced down-regulation of neprilysin by histone modification in mouse primary cortical and hippocampal neurons. PLoS One. 2011;6:e19229. doi: 10.1371/journal.pone.0019229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bell RD, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. Important study demonstrating that hypoxia leads to a failure of LRP-1-mediated amyloid-β clearance from brain arteries via elevated levels of myocardin and serum response factor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munch C, et al. Chemical hypoxia facilitates alternative splicing of EAAT2 in presymptomatic APP23 transgenic mice. Neurochem Res. 2008;33:1005–1010. doi: 10.1007/s11064-007-9540-5. [DOI] [PubMed] [Google Scholar]
- 68.Boycott HE, Dallas M, Boyle JP, Pearson HA, Peers C. Hypoxia suppresses astrocyte glutamate transport independently of amyloid formation. Biochem Biophys Res Commun. 2007;364:100–104. doi: 10.1016/j.bbrc.2007.09.102. [DOI] [PubMed] [Google Scholar]
- 69.Carvalho C, et al. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J Bioenerg Biomembr. 2009;41:433–440. doi: 10.1007/s10863-009-9247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Checa JC, et al. Oxidative stress and altered mitochondrial function in neurodegenerative diseases: lessons from mouse models. CNS Neurol Disord Drug Targets. 2010;9:439–454. doi: 10.2174/187152710791556113. [DOI] [PubMed] [Google Scholar]
- 71.Correia SC, et al. Mitochondria: the missing link between preconditioning and neuroprotection. J Alzheimers Dis. 2010;20 (Suppl 2):S475–485. doi: 10.3233/JAD-2010-100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer’s disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moser KV, Stockl P, Humpel C. Cholinergic neurons degenerate when exposed to conditioned medium of primary rat brain capillary endothelial cells: counteraction by NGF, MK-801 and inflammation. Exp Gerontol. 2006;41:609–618. doi: 10.1016/j.exger.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Yin X, Wright J, Wall T, Grammas P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer’s disease. Am J Pathol. 2010;176:1600–1606. doi: 10.2353/ajpath.2010.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab. 1991;11:684–689. doi: 10.1038/jcbfm.1991.121. [DOI] [PubMed] [Google Scholar]
- 78.Li B, Freeman RD. Neurometabolic coupling in the lateral geniculate nucleus changes with extended age. J Neurophysiol. 2010;104:414–425. doi: 10.1152/jn.00270.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol. 2010;67:93–98. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- 85.Chen RL, et al. Age-related changes in choroid plexus and blood-cerebrospinal fluid barrier function in the sheep. Exp Gerontol. 2009;44:289–296. doi: 10.1016/j.exger.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 86.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 87.Savva GM, et al. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 88.Jellinger KA. Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegener Dis. 2010;7:112–115. doi: 10.1159/000285518. [DOI] [PubMed] [Google Scholar]
- 89.Cordonnier C. Brain microbleeds: more evidence, but still a clinical dilemma. Curr Opin Neurol. 2011;24:69–74. doi: 10.1097/WCO.0b013e328341f8c0. [DOI] [PubMed] [Google Scholar]
- 90.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy (CAA) in the elderly. Ann Neurol. 2011 doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fossati S, et al. Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-beta variants in cells composing the cerebral vessel walls. FASEB J. 2010;24:229–241. doi: 10.1096/fj.09-139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rovelet-Lecrux A, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 93.Engelhardt JI, Appel SH. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol. 1990;47:1210–1216. doi: 10.1001/archneur.1990.00530110068019. [DOI] [PubMed] [Google Scholar]
- 94.Garbuzova-Davis S, et al. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PLoS One. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garbuzova-Davis S, et al. Amyotrophic lateral sclerosis: a neurovascular disease. Brain Res. 2011;1398:113–125. doi: 10.1016/j.brainres.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 96.Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen X, Lan X, Roche I, Liu R, Geiger JD. Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem. 2008;107:1147–1157. doi: 10.1111/j.1471-4159.2008.05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chao YX, He BP, Tay SS. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J Neuroimmunol. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Elbaz A, Moisan F. Update in the epidemiology of Parkinson’s disease. Curr Opin Neurol. 2008;21:454–460. doi: 10.1097/WCO.0b013e3283050461. [DOI] [PubMed] [Google Scholar]
- 100.Bertrand E, et al. Amyloid angiopathy in idiopathic Parkinson’s disease. Immunohistochemical and ultrastructural study. Folia Neuropathol. 2008;46:255–270. [PubMed] [Google Scholar]
- 101.Benamer HT, Grosset DG. Vascular parkinsonism: a clinical review. Eur Neurol. 2009;61:11–15. doi: 10.1159/000165343. [DOI] [PubMed] [Google Scholar]
- 102.Duran-Vilaregut J, et al. Blood-brain barrier disruption in the striatum of rats treated with 3-nitropropionic acid. Neurotoxicology. 2009;30:136–143. doi: 10.1016/j.neuro.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer’s disease. Neurobiol Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 104.Hunt A, et al. Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res. 2007;155:147–154. doi: 10.1016/j.pscychresns.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 105.Herholz K. Cerebral glucose metabolism in preclinical and prodromal Alzheimer’s disease. Expert Rev Neurother. 2010;10:1667–1673. doi: 10.1586/ern.10.136. [DOI] [PubMed] [Google Scholar]
- 106.Mosconi L, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- 107.Samuraki M, et al. Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2007;34:1658–1669. doi: 10.1007/s00259-007-0454-x. [DOI] [PubMed] [Google Scholar]
- 108.Mosconi L, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. Important study demonstrating that amyloid-β constricts blood vessels. [DOI] [PubMed] [Google Scholar]
- 110.Iadecola C, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci. 1999;2:157–161. doi: 10.1038/5715. Important study showing dysregulation in the cerebral blood flow prior to amyloid-β deposition in Alzheimer’s mice. [DOI] [PubMed] [Google Scholar]
- 111.Niwa K, et al. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park L, et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci U S A. 2011;108:5063–5068. doi: 10.1073/pnas.1015413108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chow N, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. Important study demonstrating that elevated levels of myocardin and serum response factor lead to a hypercontractile phenotype of brain arteries in Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartels AL, et al. Blood-brain barrier P-glycoprotein function decreases in specific brain regions with aging: a possible role in progressive neurodegeneration. Neurobiol Aging. 2009;30:1818–1824. doi: 10.1016/j.neurobiolaging.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Bartels AL, et al. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm. 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rule RR, Schuff N, Miller RG, Weiner MW. Gray matter perfusion correlates with disease severity in ALS. Neurology. 2010;74:821–827. doi: 10.1212/WNL.0b013e3181d3e2dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harris GJ, et al. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington’s disease. Brain. 1999;122 ( Pt 9):1667–1678. doi: 10.1093/brain/122.9.1667. [DOI] [PubMed] [Google Scholar]
- 118.Deckel AW, Duffy JD. Vasomotor hyporeactivity in the anterior cerebral artery during motor activation in Huntington’s disease patients. Brain Res. 2000;872:258–261. doi: 10.1016/s0006-8993(00)02506-3. [DOI] [PubMed] [Google Scholar]
- 119.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 120.Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- 121.Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- 122.Lehtinen MK, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paris D, et al. Impaired angiogenesis in a transgenic mouse model of cerebral amyloidosis. Neurosci Lett. 2004;366:80–85. doi: 10.1016/j.neulet.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 124.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 125.Rotstein M, et al. Glut1 deficiency: inheritance pattern determined by haploinsufficiency. Ann Neurol. 2010;68:955–958. doi: 10.1002/ana.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang D, et al. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. doi: 10.1093/hmg/ddl032. [DOI] [PubMed] [Google Scholar]
- 127.Eisele YS, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. Important study demonstrating that peripheral amyloid-β contributes to the development of cerebral β-amyloidosis in Alzheimer’s mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sutcliffe JG, Hedlund PB, Thomas EA, Bloom FE, Hilbush BS. Peripheral reduction of beta-amyloid is sufficient to reduce brain beta-amyloid: Implications for Alzheimer’s disease. J Neurosci Res. 2011;89:808–814. doi: 10.1002/jnr.22603. [DOI] [PubMed] [Google Scholar]
- 129.Sagare AP, Winkler EA, Bell RD, Deane R, Zlokovic BV. From the liver to the blood-brain barrier: An interconnected system regulating brain amyloid-beta levels. J Neurosci Res. 2011;89:967–968. doi: 10.1002/jnr.22670. [DOI] [PubMed] [Google Scholar]
- 130.Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 131.Mackic JB, et al. Circulating amyloid-beta peptide crosses the blood-brain barrier in aged monkeys and contributes to Alzheimer’s disease lesions. Vascul Pharmacol. 2002;38:303–313. doi: 10.1016/s1537-1891(02)00198-2. [DOI] [PubMed] [Google Scholar]
- 132.Mackic JB, et al. Cerebrovascular accumulation and increased blood-brain barrier permeability to circulating Alzheimer’s amyloid beta peptide in aged squirrel monkey with cerebral amyloid angiopathy. J Neurochem. 1998;70:210–215. doi: 10.1046/j.1471-4159.1998.70010210.x. [DOI] [PubMed] [Google Scholar]
- 133.Poduslo JF, Curran GL, Haggard JJ, Biere AL, Selkoe DJ. Permeability and residual plasma volume of human, Dutch variant, and rat amyloid beta-protein 1–40 at the blood-brain barrier. Neurobiol Dis. 1997;4:27–34. doi: 10.1006/nbdi.1997.0132. [DOI] [PubMed] [Google Scholar]
- 134.Ghilardi JR, et al. Intra-arterial infusion of [125I]A beta 1–40 labels amyloid deposits in the aged primate brain in vivo. Neuroreport. 1996;7:2607–2611. doi: 10.1097/00001756-199611040-00040. [DOI] [PubMed] [Google Scholar]
- 135.Zlokovic BV, et al. Blood-brain barrier transport of circulating Alzheimer’s amyloid beta. Biochem Biophys Res Commun. 1993;197:1034–1040. doi: 10.1006/bbrc.1993.2582. [DOI] [PubMed] [Google Scholar]
- 136.Martel CL, Mackic JB, McComb JG, Ghiso J, Zlokovic BV. Blood-brain barrier uptake of the 40 and 42 amino acid sequences of circulating Alzheimer’s amyloid beta in guinea pigs. Neurosci Lett. 1996;206:157–160. doi: 10.1016/s0304-3940(96)12462-9. [DOI] [PubMed] [Google Scholar]
- 137.Sagare A, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. Important study showing that soluble LRP1 binds amyloid-β in the cirulation preventing its reentry into the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. Important study showing that a circulating anti-amyloid-β antibody promotes efflux of this peptide from brain to blood. [DOI] [PubMed] [Google Scholar]
- 139.Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer’s disease-associated pathology in transgenic mice. Am J Pathol. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.DeMattos RB, et al. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem. 2002;81:229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 141.Matsuoka Y, et al. Novel therapeutic approach for the treatment of Alzheimer’s disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y, et al. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol Ther. 2009;17:1381–1386. doi: 10.1038/mt.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y, et al. Circulating neprilysin clears brain amyloid. Mol Cell Neurosci. 2010;45:101–107. doi: 10.1016/j.mcn.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Deane R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. Important study demonstrating that RAGE mediates influx of amyloid-β across the bood-brain barrier. [DOI] [PubMed] [Google Scholar]
- 145.Mackic JB, et al. Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1–40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giri R, et al. beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- 147.Yan SD, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 148.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–853. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. Important study demonstrating faulty amyloid-β clearance from the brain in patients affected by Alzheimer’s disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–1089. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 152.Shibata M, et al. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. Pionering study demonstrating that LRP-1 medaites amyloid-β clearance from the brain to blood across the blood-brain barrier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bell RD, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jaeger LB, et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shinohara M, et al. Reduction of brain beta-amyloid (Abeta) by fluvastatin, a hydroxymethylglutaryl-CoA reductase inhibitor, through increase in degradation of amyloid precursor protein C-terminal fragments (APP-CTFs) and Abeta clearance. J Biol Chem. 2010;285:22091–22102. doi: 10.1074/jbc.M110.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaeger LB, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamada K, et al. The low density lipoprotein receptor-related protein 1 mediates uptake of amyloid beta peptides in an in vitro model of the blood-brain barrier cells. J Biol Chem. 2008;283:34554–34562. doi: 10.1074/jbc.M801487200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nazer B, Hong S, Selkoe DJ. LRP promotes endocytosis and degradation, but not transcytosis, of the amyloid-beta peptide in a blood-brain barrier in vitro model. Neurobiol Dis. 2008;30:94–102. doi: 10.1016/j.nbd.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Monro OR, et al. Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging. 2002;23:405–412. doi: 10.1016/s0197-4580(01)00317-7. [DOI] [PubMed] [Google Scholar]
- 160.Davis J, et al. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 161.Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.DeMattos RB, et al. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 163.DeMattos RB, et al. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2002;99:10843–10848. doi: 10.1073/pnas.162228299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bading JR, et al. Brain clearance of Alzheimer’s amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002;10:359–368. doi: 10.1080/10611860290031831. [DOI] [PubMed] [Google Scholar]
- 165.Donahue JE, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 166.Cirrito JR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Owen JB, et al. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic Biol Med. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Behl M, et al. Lead-induced accumulation of beta-amyloid in the choroid plexus: role of low density lipoprotein receptor protein-1 and protein kinase C. Neurotoxicology. 2010;31:524–532. doi: 10.1016/j.neuro.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sagare AP, et al. Impaired Lipoprotein Receptor-Mediated Peripheral Binding of Plasma Amyloid-beta is an Early Biomarker for Mild Cognitive Impairment Preceding Alzheimer’s Disease. J Alzheimers Dis. 2011;24:25–34. doi: 10.3233/JAD-2010-101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tamaki C, et al. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res. 2006;23:1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 171.Iwata N, et al. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 172.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 173.Melchor JP, Pawlak R, Strickland S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yin KJ, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Koistinaho M, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 176.Bacskai BJ, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brody DL, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–1224. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 181.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 182.Lagier-Tourenne C, Cleveland DW. Neurodegeneration: An expansion in ALS genetics. Nature. 2010;466:1052–1053. doi: 10.1038/4661052a. [DOI] [PubMed] [Google Scholar]
- 183.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Elden AC, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gruzman A, et al. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Boillee S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. Important study demonstrating that toxicity of a ALS-linked SOD1 mutant to microglia determines the life span in mice with this disease. [DOI] [PubMed] [Google Scholar]
- 187.Yamanaka K, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Beers DR, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lambrechts D, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 192.Greenway MJ, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38:411–413. doi: 10.1038/ng1742. [DOI] [PubMed] [Google Scholar]
- 193.Oosthuyse B, et al. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28:131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 194.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010;9:702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 195.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Storkebaum E, et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8:85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 197.Azzouz M, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 198.A safety and tolerability study of intracerebroventricular administration of sNN0029 to patients with amyotrophic lateral sclerosis [online] 2011 http://clinicaltrials.gov/ct2/show/NCT00800501.
- 199.Kieran D, et al. Control of motoneuron survival by angiogenin. J Neurosci. 2008;28:14056–14061. doi: 10.1523/JNEUROSCI.3399-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer’s disease. J Neurosci. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Spuch C, et al. The effect of encapsulated VEGF-secreting cells on brain amyloid load and behavioral impairment in a mouse model of Alzheimer’s disease. Biomaterials. 2010;31:5608–5618. doi: 10.1016/j.biomaterials.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 202.Jucker M. The benefits and limitations of animal models for translational research in neurodegenerative diseases. Nat Med. 2010;16:1210–1214. doi: 10.1038/nm.2224. [DOI] [PubMed] [Google Scholar]
- 203.Lo EH. Degeneration and repair in central nervous system disease. Nat Med. 2010;16:1205–1209. doi: 10.1038/nm.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Van Broeckhoven C. The future of genetic research on neurodegeneration. Nat Med. 2010;16:1215–1217. doi: 10.1038/nm.2225. [DOI] [PubMed] [Google Scholar]
- 205.de la Torre JC. Vascular risk factor detection and control may prevent Alzheimer’s disease. Ageing Res Rev. 2010;9:218–225. doi: 10.1016/j.arr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 206.Luchsinger JA, et al. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 207.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Whitmer RA, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 209.Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 210.Vermeer SE, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 211.Snowdon DA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 212.Han MH, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]