Abstract
Alzheimer’s disease (AD) is associated with increased brain levels of β-amyloid (Aβ) peptides, which readily self-aggregate into fibrils and oligomers that have particularly deleterious properties towards synapses of excitatory glutamatergic neurons. Here, we examined the neuroprotective effects of 1-methyl-1,2,3,4,-tetrahydroisoquinoline (1MeTIQ) against Aβ-induced loss of synaptic proteins in cultured primary hippocampal neurons. Exposure of mature primary hippocampal neurons to 10 μM synthetic Aβ1-40 over 72 h resulted in approximately 60% reduction in the surface expression of NR1 subunit of the NMDA receptor (NMDAR), PSD-95, and synaptophysin, without causing neuronal death. Concomitant treatment with 500 μM of 1MeTIQ, a low affinity NMDAR antagonist significantly ameliorated the loss of synaptic protein markers. The neuroprotective properties of 1MeTIQ were compared with those of MK-801, which at 0.5 μM concentration also prevented Aβ1-40-induced loss of synaptic proteins in primary neuronal cultures. Furthermore, we provide novel evidence demonstrating effectiveness of 1MeTIQ in reducing the level of reactive oxygen species (ROS) in primary neuronal culture system. As oxidative stress contributes importantly to neurodegeneration in AD, 1MeTIQ may provide a dual neuroproctective effect in AD both as a NMDARs antagonist and ROS formation inhibitor. 1MeTIQ occurs endogenously at low concentrations in the brain and its synthetic form readily penetrates the blood-brain-barrier after the systemic administration. Our results highlight a possibility of the application of 1MeTIQ as a neuroprotective agent in AD related neurodegeneration.
Keywords: 1MeTIQ, β-amyloid, NMDA receptor antagonist, neuronal culture, ROS production, synaptic protein
Introduction
Disturbance of amyloid β (Aβ) peptides homeostasis leading to their progressive accumulation in the brain is a culprit of the early AD pathology and precedes occurrence of neurofibrillary pathology and neuronal loss (Querfurth et al. 2010; Tanzi et al. 2004). Data from numerous in vitro and in vivo experiments suggest that Aβ oligomeric assemblies have a deleterious effect on the function of hippocampal synapses. Studies in primary cultures of cortical and hippocampal neurons have demonstrated that the application of Aβ oligomers promptly and dramatically reduces surface expression of the NR1 and NR2B subunits of N-methyl-D-aspartic acid receptors (NMDARs), which are essential for synaptic plasticity and memory formation, and post-synaptic density protein 95 (PSD-95), which is functionally and structurally associated with NMDARs (Dewachter et al. 2009; Snyder et al. 2005). Loss of surface NMDAR expression in neurons exposed to Aβ oligomers has also been associated with diminished NMDAR current and impairment of the cAMP response element-binding protein (CREB) activation (Dewachter et al. 2009; Snyder et al. 2005). The synaptotoxic effects of Aβ were confirmed in vivo using transgenic mouse models of AD (Dewachter et al. 2009; Snyder et al. 2005; Dong et al. 2008; Ray et al. 2011).
1-Methyl-1,2,3,4,-tetrahydroisoquinoline (1MeTIQ) is an endogenous alkaloid present in trace concentrations in the mammalian brain (for review see Abo et al. 2005; Antkiewicz-Michaluk et al. 2013; Vetulani et al. 2003). Our recent studies provide evidence that 1MeTIQ is a low affinity NMDAR antagonist. 1MeTIQ was found to inhibit binding of [3H]MK-801 (dizocilpine) to isolated neuronal membranes (Kuszczyk et al. 2010) and prevents glutamate-induced excitotoxicity and influx to neurons of calcium radio-labeled with the isotope 45Ca (Antkiewicz-Michaluk et al. 2006). What’s more, as was shown previously 1MeTIQ at concentrations of up to 500 μM does not decrease viability of neurons in primary culture, but exhibits neurotropic and neuroprotective properties (Antkiewicz-Michaluk et al. 2006; Kotake et al. 2005). In this study we investigated whether 1MeTIQ due to its NMDAR antagonistic properties can prevent Aβ evoked down-regulation of NMDAR associated proteins. The study was performed in 15 days in vitro (DIV) murine primary hippocampal neuronal culture system, which express functional NMDARs (Mattsson et al. 1991) and the synaptoprotective effects of 1MeTIQ were compared with those of an establish and highly specific NMDAR antagonist MK-801.
Irrespectively to its antagonistic effect on the NMDAR 1MeTIQ appears to poses anti-oxidative properties. Oxidative stress is a well establish factor, which contributes importantly to neurodegeneration in AD (for reviews see (Abeti and Duchen 2012; Kim et al., 2012; Quintanilla et al., 2012). Oligomeric assemblies of Aβ have been shown to precipitate formation of reactive oxygen species (ROS), which are deleterious to synapses (Ray et al. 2011). Another important source of ROS are microglia cells, which undergo activation in the course of formation of Aβ plaque deposits and in response to neurofibrillary degeneration (Sugama et al. 2009; von Bernhardi et al. 2010). Thus far anti-oxidative properties of 1MeTIQ has been directly demonstrated only in an abiotic system by showing inhibition of free radical production via the Fenton reaction (Antkiewicz-Michaluk et al. 2006), however observations that 1MeTIQ protect dopaminergic neurons from toxicity of toxins associated with oxidative stress e.g. MPTP or rotenone indicates that its anti-oxidative protection in vivo can be robust (for review see Antkiewicz-Michaluk et al. 2013). Seeking direct confirmation of anti-oxidative properties of 1MeTIQ in a biological experimental system we studied the effects of this compound on ROS production in H2O2-treated primary hippocampal neurons and found its significant effect on reduction ROS level. Collectively, our data indicate that 1MeTIQ can provide complex neuroprotective effect in AD pathology by simultaneous NMDAR inhibition and ROS reduction. Experimental treatments with a substance possessing both these properties might be more efficient than monotherapy and may provide new mechanistic information about the pathology of AD.
Materials and Methods
Reagents
Aβ1-40 and fluorescein-tagged Aβ1-40 (FITC-Aβ1-40) were synthesized to order by the W.M. Keck Proteomic Facility of Yale University (New Haven, CT, USA). 1-Methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ) was synthesized in the Department of Medicinal Chemistry, Institute of Pharmacology, Polish Academy of Science, (Cracow, Poland), and its purity and homogeneity were verified by measurement of the melting point and chromatographic analysis, respectively. (+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK-801) was purchased from RBI (Natic, MA, USA). All other chemicals and antibodies were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Primary cultures of hippocampal neurons and their treatment with Aβ1-40
The treatment of animals and all experimental procedures involving them were approved by the Institutional Animal Care and Use Committee of New York University School of Medicine, and the 4th Local Ethical Committee in Warsaw. Primary cultures of hippocampal neurons were established from zero- or one-day old (P0-P1) pups of C57BL/6 mice according to Takahashi et al. (2004), as has been described in our previously published protocol (Kuszczyk et al. 2013). Neurons were seeded on removable poly-L–lysine-coated round coverslips placed at the bottom of tissue culture plate wells. Treatment of the hippocampal neurons with Aβ1-40 was commenced at 15 days in vitro (DIV). Aβ1-40 and FITC-Aβ1-40 were monomerized by treatment with 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) as previously described (Sadowski et al. 2004; Stine et al. 2003). Immediately prior the experiment they were reconstituted in neurobasal media, mixed in a 9:1 ratio, and added to the neurons (10 μM final concentration) without or with 1MeTIQ (500 μM final concentration) or MK-801 (0.5 μM final concentration). Experimental controls included DIV-matched untreated neurons and neurons grown in the presence of 1MeTIQ or MK-801 only. The neurons were maintained with the various experimental treatments for 72 h. At the conclusion of the experiment, the coverslips with attached neurons were washed three times in the phosphate buffered saline (PBS) at 37°C, immersed in ice-cold 80% methanol for 10 min to fix and then washed again three times in PBS.
MTT cytotoxicity assay
Monomeric Aβ1-40 was added to primary murine hippocampal neuronal cultures at the concentration 5, 10, 25 or 50 μM and the neurons were cultured in Aβ1-40 presence for 72 h. Then the viability of neurons was assessed using the (3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrasodium (MTT) metabolic assay kit (Ma et al. 1996) according to the manufacturer’s instructions (Roche Molecular Biochemicals, Indianapolis, IN).
Immunocytochemistry and synaptic protein expression analysis
The primary hippocampal neurons were immunostained with previously characterized commercially available primary monoclonal antibodies (mAbs) at the indicated dilutions: anti-NR1 subunit of NMDA receptor (1:100; Millipore Corporation, Billerica, MA, USA), anti-PSD-95 (1:500; Millipore Corporation) and anti-synaptophysin (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blocking of non-specific reactivity and detection of antigen-antibody complexes were performed using a Mouse on Mouse immunodetection kit (Vector Laboratories, Ltd., Burlingame, CA, USA) following the manufacturer’s instructions, except that VECTASTAIN ABC Reagent was replaced with Cy3 conjugated streptavidin (1:500; added for 30 min). Following extensive washing with PBS, the neuronal nuclei were counterstained with 4′,6-diamino-2-phenylindole (DAPI). Quantitative analysis of synaptic protein expression was performed following our published protocols (Kuszczyk et al. 2013). At least 20 neurons from each treatment per experiment from three independent experiments were randomly selected and photographed under a x100 oil immersion objective using a high-sensitivity monochrome DS-Qi1Mc camera attached to a Nikon 80i fluorescent microscope (Nikon Corp. Tokyo, Japan). Images were analyzed using NIH Image J software v1.42 (Bethesda, MD, USA). For each selected neuron, five rectangular test areas (20 × 4 μm) were randomly superimposed on the primary and secondary dendrites with the long axis of the test area oriented parallel to the long axis of each dendrite (Fig. 2 AI-IV). Synaptic densities within each test area were automatically thresholded and filtered according to the preset algorithm to discriminate non-specific staining (Kuszczyk et al. 2013). Synaptic protein densities were expressed relative to those in control DIV-matched primary hippocampal neurons that had not been treated with Aβ, 1MeTiQ or MK-801.
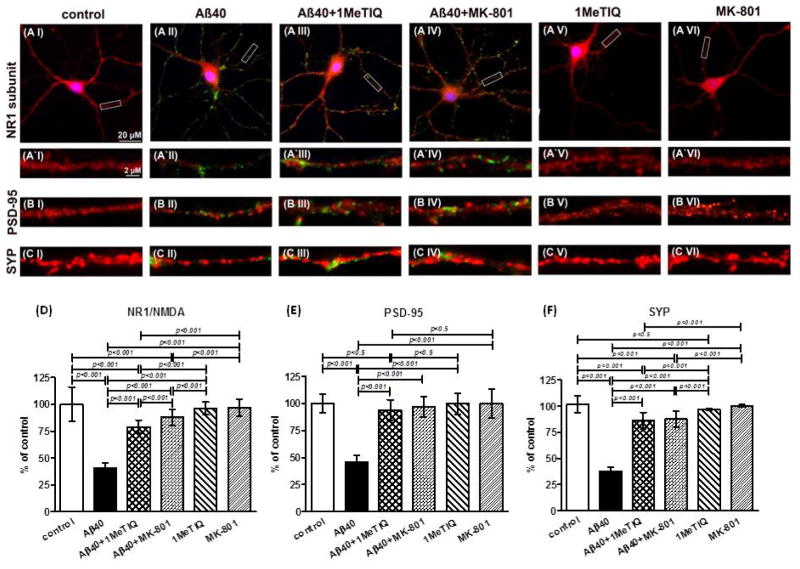
Figure 2.
Expression of synaptic protein markers in murine primary hippocampal neurons. Examples of microphotographs demonstrating the immunostaining against the NR1 subunit of the NMDAR (A), post-synaptic density protein 95 (PSD-95) (B), and synaptophysin (SYP) (C) in 18 DIV primary mouse hippocampal neurons grown from 15 DIV under control conditions (I), in the presence of Aβ1-40/FITC-Aβ1-40 (10 μM) (II), Aβ1-40/FITC-Aβ1-40 + 1MeTIQ (III) and Aβ1-40/FITC-Aβ1-40 + MK-801 (IV), 1MeTIQ (500 μM) (V), MK-801 (0.5 μM) (IV). Rectangular areas covering sections of dendrites marked on the example images of neurons immunostained against NR1 subunit of the NMDAR in A I to A VI are shown enlarged beneath each picture as examples of the representative test fields selected for quantification of NR1 expression (A′I to A′ IV). For PSD-95 (B I–B IV) and synaptophysin (C I–C IV), only examples of the representative test fields selected for quantification of these synaptic proteins are shown. The red color represents immunostaining of specific synaptic proteins, green in II-IV is FITC-Aβ1-40, while blue in A is DAPI nuclear counterstaining. D–F: Quantitative analysis of synaptic protein expression. Values in the graphs show the mean expression level of synaptic proteins analyzed along primary and secondary dendrites relative to those in DIV-matched control neurons (±S.D.) in at least 60 neurons from three independent experiments. One-way ANOVA p<0.0001 for all three synaptic proteins. Post hoc Tukey-HSD test values are displayed on the graphs for pairs of columns with statistically significant differences.
Quantification of ROS production
The effect of 1MeTIQ on ROS formation was studied in 8 DIV primary hippocampal neuronal cultures established from P0-P1 pups of Wistar rats following the procedure used to establish neuronal cultures from murine pups. The neurons were incubated in medium containing 100 μM 6-carboxy-2′,7′-dichlorofluorescein diacetate (DCFH-DA, Molecular Probes, Invitrogen, Eugene, OR, USA) for 30 min at 37°C, according to adapted methodology originally described by Wang and Joseph (1999). After washing with DCFH-DA-free medium, the neurons were incubated with different concentrations of 1MeTIQ or 0.5 μM of MK-801 for 2 min at 37°C. After determining the basal DCF fluorescence, the neurons were challenged with 10 μM of H2O2 and fluorescence was recorded every 2 min for 30 min. DCF fluorescence was measured using a FLUOstar Omega microplate reader (BMG Labtech, Ortenberg, Germany) at 485 nm excitation and 538 nm emission wavelengths.
Statistical analysis
The results were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey-HSD post hoc test with GraphPad Prism v5.02 (GraphPad Software Inc., San Diego, CA, USA).
Results
Effect of Aβ1-40 on neuronal viability
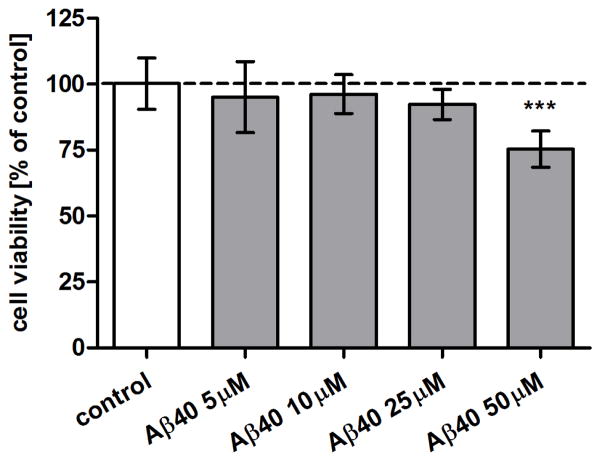
The intention of the present study was to examine effects of Aβ on the expression of synaptic proteins without causing general metabolic dysfunction or evoking neuronal death. To confirm that 10 μM concentration of Aβ1-40, which was used to evoke reduction of synaptic protein expression, do not produce significant cytotoxicity we first conducted the MTT metabolic assay on the primary murine neurons cultured in the presence of Aβ1-40 concentrations ranging from 5 to 50 μM for 72 h. In this experiment monomeric Aβ1-40 was added to the media once at the commencement of the experiment. As shown in Fig. 1, metabolic viability of primary murine hippocampal neurons remained unchanged up to 25 μM Aβ1-40 concentration. A significant cytotoxic effect was observed only at 50 μM Aβ, where cell metabolic viability was reduced by 24.1 % of the control neurons value (p < 0.001).
Figure 1.
Effect of Aβ1-40 peptide on viability of murine primary hippocampal neurons. Monomeric Aβ1-40 (5-50 μM) was added to 15 DIV neurons and their survival was assessed after 72-h incubation using MTT test. Cell viability values relative to the untreated control are given as the mean ± S.D. from three independent experiments, each with 4 replicates of each treatment. One-way ANOVA followed by post hoc Tukey-HSD test values identified a statistically significant difference from untreated cells; *** p<0.001.
Aβ-induced down-regulation of synaptic proteins in primary mice hippocampal neurons: effects of 1MeTIQ and MK-801
In the subsequent experiment we examined effects of growing primary murine hippocampal neurons in the presence of Aβ1-40 on the expression of the NMDAR NR1 subunit, PSD-95, and synaptophysin. The experiment was commenced when the neuronal cultures were 15 DIV and express functional NMDARs (Mattson et al. 1991). A mixture of momomeric Aβ1-40 and FITC-Aβ1-40 (9:1 ratio; 10 μM final Aβ concentration) was added to the growth medium once at the commencement of the experiment. The experiment was concluded after 72 h when the neurons were fixed and immunostained for the three studied synaptic proteins. Analysis of the neurons under FITC channels demonstrates visible aggregates of Aβ covering external surfaces of dendrites and perykaria (Fig 2 AII, A′II, BII, CII) which were absent in control neurons cultured in the absence of Aβ peptides (Fig 2 AI, A′I, BI, CI). Quantification of synaptic proteins expression revealed 59.4%, 54% and 62.1% reduction in the expression of NR1, PSD-95 and synaptophysin in neurons exposed to 10 μM Aβ1-40 compared to the control neurons, respectively (p < 0.001) (Fig. 2).
Then we tested whether 1MeTIQ exerts protective effect on Aβ-induced loss of synaptic proteins. 500 μM 1MeTIQ was added to the neurons once at the commencement of the experiment together with Aβ. 1MeTIQ treatment reduced loss of NR1, PSD-95, and synaptophysin synaptic to 21%, 6%, and 14% of the control neurons values, respectively (p < 0.001 1MeTIQ+Aβ vs. Aβ and 1MeTIQ+Aβ vs. control; Fig. 2 D–F). For comparison we used MK-801 an established uncompetitive antagonist of NMDAR. Application of 0.5 μM MK-801 together with Aβ at the commencement of the experiment also prevented loss of NR1, PSD-95, and synaptophysin, which expression levels were reduced by 12%, 3%, and 12% of the control neurons values, respectively (p < 0.001 MK801+Aβ vs. Aβ Fig. 2 D–F). The magnitude of the neuroprotective effects of 1MeTIQ and MK801 were comparable for PSD-95, and synaptophysin, while for NR1 MK801 produced modestly better but statistically significant effect (p < 0.001 1MeTIQ+Aβ vs. MK801+Aβ). In a control experiment treatment of primary hippocampal neurons with 500 μM 1MeTIQ or 0.5 μM MK-801 in the absence of Aβ1-40 produced no significant changes in the expression level of NR1, PSD-95, and synaptophysin.
Effect of 1MeTIQ on ROS production induced by H2O2
The effect of 1MeTIQ on ROS generation in cell culture model system was investigated using 8 DIV primary rat hippocampal neurons. Accumulation of ROS as a marker for oxidative stress triggered by H2O2 was measured with the DCF fluorescent probe. Application of 10 μM H2O2 to neurons that had been pre- incubated with DCFH-DA resulted in an increase in DCF fluorescence, indicating ROS accumulation (Fig. 3). This effect was insensitive to MK-801, while 1MeTIQ (250 and 500 μM) significantly inhibited this effect in a concentration-dependent manner (51.7% and 82.3%, respectively) (p<0.05; Fig. 3), demonstrating the antiradical activity of 1MeTIQ in cultured neurons. The control experiments revealed that 0.5 μM MK-801 and 500 μM 1MeTIQ do not interfere with fluorescence of 100 μM DCFH-DA or DCF in the ionic medium, and have no significant effect on the spontaneous ROS production by cultured neurons (results not shown).
Figure 3.
Effects of 1MeTIQ and MK-801 on H2O2-induced ROS production in rat primary hippocampal neurons. Production of ROS in 8 DIV primary hippocampal neurons was stimulated by 10 μM H2O2 and quantified with the fluorescent probe DCF. Values on the graph show fluorescence intensity of control neurons and those treated with 1MeTIQ or MK-801 at various time points following challenge with H2O2, relative to the baseline fluorescence intensity. All values represent mean ± S.D. from three independent experiments, each with four replicates per treatment per time point. *p<0.05 vs. H2O2-treated cells at a given time point (Tukey-HSD).
Discussion
In this study we demonstrated that 1MeTIQ prevents Aβ evoked loss of synaptic proteins and reduces ROS level in primary neuronal culture model systems. To demonstrate protective effect of 1MeTIQ on synaptic protein expression we used previously published experimental model of Aβ evoked loss of synaptic proteins. In this model monomeric Aβ1-40 is added to primary hippocampal neurons for 72 h (Kuszczyk et al 2013) while the milieu of neuronal condition media promotes self-aggregation of Aβ into oligomeric assemblies (Stine et al 2003). Tracing Aβ1-40 with FITC-Aβ1-40 allows to direct visualize, aggregates of Aβ deposited on the external surface of dendrites and perykaria. While loss of synaptic proteins in this model is conspicuous, no significant reduction in metabolic activity or neuronal death is observed. In addition, our previous studies also showed no reduction in the steady-state level of cytoskeletal proteins (Kuszczyk et al 2013). Treatment of the primary hippocampal neurons with 500μM 1MeTiQ almost completely prevented loss of NR1 NMDAR subunit, PSD-95 and synaptophysin expression. Similar effect was observed when MK-801 an established uncompetitive antagonist of NMDAR was used. This effect illustrates previously described synaptoprotective effects of the antagonists of NMDARs in Aβ oligomer toxicity. Furthermore, our data revealed also inhibitory effect of 1MeTIQ on H2O2-induced ROS production in the immature hippocampal neuronal cultures. These results support the hypothesis that 1MeTIQ is the NMDAR antagonist with intrinsic antiradical activity. Because of these properties 1MeTIQ can be considered as a promising candidate for a model substance in studies on neuroprotection against Aβ toxicity.
The reduction of the content of synaptic proteins in dendrites exposed to Aβ which was observed in our study is consistent with previously published data that oligomeric Aβ inhibits NMDARs-mediated signaling in neurons which may be ascribed to selective reduction of the expression of NMDARs subunits and other synaptic proteins in the neuronal membranes, without interference with the total level of these proteins. Ex vivo experiments have shown that the application of Aβ oligomers into organotypic hippocampal slices prepared from wild type rodents renders tetanic stimulus incapable of inducing long term potentiation (LTP) in the CA1 sector (Puzzo et al. 2005; Puzzo et al. 2008; Walsh et al. 2005). Furthermore, direct observations of impaired NMDAR mediated Ca2+ influx in the presence of Aβ oligomers ex vivo again support impairment of the NMDAR signaling (Dewachter et al. 2009; Shankar et al. 2007). Ex vivo studies utilizing surface biotinylation approach have shown that exposure of primary cortical and hippocampal neurons to Aβ oligomers results in marked reduction in the surface expression of NR1 and NR2B subunits of NMDARs while their total expression level in the neurons remained unchanged (Dewachter et al. 2009; Snyder et al. 2005). These observations suggest that Aβ oligomers evoke translocation of NMDARs from the membrane surface to the cytosol. Consistent with data from the in vitro experiments, reduction in the level of phosphorylated NR2B NMDAR subunit (which is the surface expressed form) but not in the total level of NR2B was found in the hippocampus of hAPP-J20 AD Tg mice (Palop et al. 2007). Likewise, analysis of NR2B and PSD-95 levels in the total brain homogenate of APPV717I AD Tg mice and its fraction containing postsynaptic density proteins, showed unchanged level of these two proteins in the total brain homogenate, but a marked reduction in the postsynaptic fraction when compared to wild type (WT) mice (Dewachter et al. 2009). These data from in vivo and in vitro models of early AD pathology consistently indicate that Aβ oligomers affect the surface expression of NMDAR and impair their signaling but they do not change the total expression of NMDAR subunits, as long as more profound neurodegenerative changes resulting in massive synaptic and neuronal loss do not occur.
In our study, using the in vitro model of Aβ-induced synaptotoxicity we demonstrated protection of the synaptic proteins by the established uncompetitive NMDA receptor antagonist 0.5 μM MK-801 (Fig. 2). The protection afforded by MK-801 against Aβ-induced synaptotoxicity is consistent with data showing that internalization of NMDARs evoked in glutamatergic synapses by Aβ may be triggered by NMDAR-mediated Ca2+ currents and dephosphorylation of NMDAR NR2B subunit on Tyr1472, and that NMDAR antagonists protect the dendritic spines and the expression of synaptic proteins synaptophysin and PSD-95 in hippocampal neurons challenged with Aβ (Dong et al. 2008, Lacor et al. 2007, Roselli et al. 2005, Shankar et al. 2007). Here we reveal that synaptoprotection concerns also NMDAR NR1 subunit, which is new, previously unpublished information. Other studies and our present data indicate that antagonists of different sites of NMDARs including blockers of the ionic channel MK-801 and memantine, competitive antagonist of glutamate binding site CPP and antagonist of poliamine site ifenprodil provide protection against Aβ-induced synaptotoxicity (Lacor et al. 2007; Dong et al. 2008; Shankar et al. 2007; Roselli et al. 2005). These data indicate that synaptoprotection provided by NMDAR antagonists in Aβ toxicity depends on the inhibition of these receptors regardless of their mechanisms of action.
Our results are the first demonstration that 500 μM 1MeTIQ can mimic the effect of 0.5 μM MK-801 in preventing the Aβ1-40-evoked reduction of surface levels of synaptophysin and PSD-95, and that this protection also extends to the NMDAR NR1 subunit. This finding is consistent with our previous demonstration of the anti-excitotoxic potential of 1MeTIQ, suggesting that this endogenous alkaloid antagonizes NMDA receptors (Antkiewicz-Michaluk et al. 2013). In particular, in an in vivo microdialysis experiment 1MeTIQ inhibited the release of excitatory amino acids from the frontal cortex induced by local infusion of kainate in freely moving rats (Antkiewicz-Michaluk et al. 2006). Also in vivo study demonstrated the anticonvulsant activity of 1MeTIQ which elevated the threshold for electroconvulsions in mice, and enhanced the protective action of carbamazepine and valproate against maximal electroshock-induced seizures in mice (Luszczki et al. 2006). What’s more, a neuroprotective effect of 1MeTIQ was shown in cultured rat cerebellar granule cells in a model of glutamate-evoked, NMDAR-mediated excitotoxicity (Antkiewicz-Michaluk et al. 2006; Kuszczyk et al. 2010). More specifically, 1MeTIQ in high μmolar concentrations which antagonized neuronal death also reduced 45Ca uptake induced by glutamate, and inhibited binding of radioactive [3H]MK-801 to isolated brain membranes (Antkiewicz-Michaluk et al. 2006). The exact mechanism of inhibiting NMDARs by 1MeTIQ is not clear, but there have been reports of inhibition of the ion channel (Gray et al. 1989; Ludwig et al. 2006) and the glycine binding site (Carling et al. 1992, Leeson et al. 1992) of NMDARs by some tetrahydroisoquinoline derivatives.
To verify in cultured neurons our previous finding that 1MeTIQ directly inhibits the generation of ROS in an abiotic system (Antkiewicz-Michaluk et al. 2006), and to eliminate the secondary excitotoxicity-related component of ROS production, which might be suppressed by the NMDA receptor antagonists (Nakamura and Lipton 2010, Wojda et al. 2008), we used immature 8 DIV rat hippocampal neuronal cultures that do not express functional NMDARs (Mattson et al. 1991). Utilizing that model we demonstrated that H2O2-induced ROS production was insensitive to MK-801 (Fig. 3), which confirms the lack of secondary involvement of NMDA receptor-mediated excitotoxicity in ROS production. In the same experimental system 1MeTIQ inhibited ROS production (Fig. 3). Recent study of Tetz et al. (2013) demonstrated that several factors and substances may interfere with DCF assay of ROS production, however our control experiments showed lack of effect of 250 and 500 μM 1MeTIQ on the fluorescence of DCF solution and on ROS production in the control neuronal cultures. Thus we conclude that inhibition of ROS production in neurons reflects the genuine antiradical activity of 1MeTIQ in cultured neurons. The mechanism of this phenomenon is not clear, however potential to inhibit the Fenton reaction seems to be a general property of various tetrahydroisoquinolines (Antkiewicz-Michaluk et al. 2006). In addition to these direct antiradical effects of 1MeTIQ one should remember that this compound may indirectly reduce ROS production using various mechanisms. Apart from the suppression of excitotoxicity-related oxidative stress caused by its antagonism of NMDARs, 1MeTIQ also interferes with other mechanisms of ROS production in the brain (Antkiewicz-Michaluk et al. 2013). It is known that apart from this direct antiradical activity 1MeTIQ may indirectly reduce ROS production using various mechanisms. Oxidative stress is a universal pathogenic mechanism involved in cell death, which may result among others from the mitochondrial dysfunction, an excessive dopamine catabolism by monoamine oxidase (MAO) and excitotoxicity (Federico et al. 2012; Forder and Tymianski 2009; Obata 2002). 1MeTIQ suppresses the inhibition by MPP+ of mitochondrial respiratory complex I (NADH-ubiquinone oxidoreductases) and this way prevents the ROS-mediated neurotoxic effect of MPP+ (Parrado et al. 2000). Moreover 1MeTIQ inhibits both MAO-A and MAO-B activities and the production of free radicals during dopamine degradation (Antkiewicz-Michaluk et al. 2001). 1MeTIQ which is a putative NMDAR antagonist (Antkiewicz-Michaluk et al. 2006; Kuszczyk et al. 2010) may indirectly inhibit excitotoxicity-evoked ROS production.
The results of this study, in conformity with the literature (Dong et al. 2008; Shankar et al. 2007), demonstrate that use of an NMDAR antagonist is an efficient synaptoprotective strategy against Aβ toxicity. This indicates that NMDARs mediate synaptotoxic effects of Aβ, and that these receptors may be a major therapeutic target in treatment of AD. High affinity blockers of the NMDAR channel, like MK-801 or even ketamine, produce neuropsychotoxicity (psychosis, addiction) in humans resembling a paranoid type schizophrenia (Krystal et al. 2005). In rodents, phencyclidine and MK-801 evoke schizophrenia-like behavioral abnormalities sensitive to neuroleptics, e.g. hyperactivity (Arnt and Skarsfeldt 1998). To avoid these adverse effects, the low affinity NMDAR antagonist memantine has been used successfully for the treatment of dementia in AD patients (Parsons et al. 2013), while its derivative NitroMemantine, was recently shown to restore synaptic loss in AD transgenic mice and effect improvement in neurobehavioral assessment of hippocampal function (Talantova et al. 2013).
The demonstration that oxidative stress stimulates amyloidogenesis (Shen et al. 2013) and is involved in Aβ-induced reduction of synaptophysin expression in neurons (Ray et al. 2011) indicated that the application of free radical scavengers may be synaptoprotective. Moreover, a substance with combined antioxidant and NMDAR-inhibiting properties like 1MeTIQ might prove even more potent in synaptoprotection than NMDAR antagonists alone. The affinity of 1MeTIQ for NMDARs is 1000 times weaker than that of MK-801, and about 100 times weaker than that of memantine (Antkiewicz-Michaluk et al. 2006; Kuszczyk et al. 2010). In agreement with these data, we found that 0.5 μM MK-801 and 500 μM 1MeTIQ induced comparable protection of synaptic protein expression, with the former producing a slightly more potent effect. Possibly at lower concentrations, the relative benefits of 1MeTIQ would become more apparent. Further studies are needed to clarify this issue and to compare the synaptoprotection against Aβ toxicity, and inhibition of ROS production in Aβ-treated neurons, provided by 1MeTIQ and memantine.
1MeTIQ is an endogenous substance, which occurs in the brain at low physiological concentrations (Antkiewicz-Michaluk et al. 2013). However, 1MeTIQ is blood-brain barrier (BBB) permeable and, as demonstrated in previous studies, administration of synthetic 1MeTIQ to animals resulted in an increase in its brain concentration and produced a therapeutic response. In particular, it was shown that when administered to rats, 1MeTIQ completely antagonized hyperactivity and other effects evoked by MK-801 (Pietraszek et al. 2009). Since 1MeTIQ can be synthesized in vitro, its structure could be modified through a structure-activity relationship process to produce refined derivatives for therapeutic application aimed at protection against Aβ-induced synaptotoxicity.
In conclusion, this study has revealed that the synaptoprotection provided by NMDAR antagonists against Aβ1-40-induced toxicity in hippocampal neurons comprises maintenance of the surface level of the NMDAR NR1 subunit. The synaptoprotection afforded by 500 μM 1MeTIQ was comparable with the effects of 0.5 μM MK-801. We have also demonstrated the inhibitory effect of 1MeTIQ on H2O2-induced ROS production in primary neurons, which suggests that it has antiradical activity in biological systems. Our findings indicate that 1MeTIQ is a promising candidate for further study, particularly on how the antiradical abilities of this compound influence its synaptoprotective potential in Aβ toxicity, both in vitro and in animal models of Alzheimer’s disease.
Acknowledgments
We thank Dr Jan Boksa (Department of Medicinal Chemistry, Institute of Pharmacology PAS, Cracow) for synthesizing 1MeTIQ and Dr Elzbieta Zieminska for expert analyzing the effects of 1MeTIQ on DCFH-DA and DCF fluorescence in abiotic systems. This study was supported by NIH/NIA grants R01 AG031221 and K02 AG034176 to MJS, by the statutory grant from the Mossakowski Medical Research Centre, PAS, Warsaw, by the Polish MNiSW Scientific Network Fund no. 26/E-40/SN-0023/2007, and by Project De-Me-Ter Innovative Economy 2007-2013, Institute of Pharmacology, PAS, Cracow.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Magdalena A. Kuszczyk, Department of Neurology, New York University School of Medicine, New York, NY 10016, USA. Department of Neurochemistry, Mossakowski Medical Research Centre, Polish Academy of Sciences, Pawinskiego 5, 02-106 Warsaw, Poland
Martin J. Sadowski, Department of Neurology, New York University School of Medicine, New York, NY 10016, USA. Departments of Psychiatry, and Biochemistry and Molecular Pharmacology, New York University School of Medicine, New York, NY 10016, USA
Lucyna Antkiewicz-Michaluk, Department of Neurochemistry, Institute of Pharmacology, Polish Academy of Sciences, Smetna 12, 31-343 Cracow, Poland.
Jerzy W. Lazarewicz, Email: jerzyl@cmdik.pan.pl, Department of Neurochemistry, Mossakowski Medical Research Centre, Polish Academy of Sciences, Pawinskiego 5, 02-106 Warsaw, Poland, tel. +48226086533; fax: +48226086623
References
- Abe K, Saitoh T, Horiguchi Y, Utsunomiya I, Taguchi K. Synthesis and neurotoxicity of tetrahydroisoquinoline derivatives for studying Parkinson’s disease. Biol Pharm Bull. 2005;28(8):1355–1362. doi: 10.1248/bpb.28.1355. [DOI] [PubMed] [Google Scholar]
- Abeti R, Duchen MR. Activation of PARP by oxidative stress induced by β-amyloid: implications for Alzheimer’s disease. Neurochem Res. 2012;37(11):2589–2596. doi: 10.1007/s11064-012-0895-x. [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Lazarewicz JW, Patsenka A, Kajta M, Zieminska E, Salinska E, Wasik A, Golembiowska K, Vetulani J. The mechanism of 1,2,3,4-tetrahydroisoquinolines neuroprotection: the importance of free radicals scavenging properties and inhibition of glutamate-induced excitotoxicity. J Neurochem. 2006;97(3):846–856. doi: 10.1111/j.1471-4159.2006.03756.x. [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Michaluk J, Mokrosz M, Romańska I, Lorenc-Koci E, Ohta S, Vetulani J. Different action on dopamine catabolic pathways of two endogenous 1,2,3,4-tetrahydroisoquinolines with similar antidopaminergic properties. J Neurochem. 2001;78(1):100–108. doi: 10.1046/j.1471-4159.2001.00391.x. [DOI] [PubMed] [Google Scholar]
- Antkiewicz-Michaluk L, Wąsik A, Michaluk J. 1-Methyl-1,2,3,4-tetrahydroisoquinoline, an endogenous amine with unexpected mechanism of action: new vistas of therapeutic application. Neurotox Res. 2013 doi: 10.1007/s12640-013-9402.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18(2):63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Carling RW, Leeson PD, Moseley AM, Baker R, Foster AC, Grimwood S, Kemp JA, Marshall GR. 2-Carboxytetrahydroquinolines. Conformational and stereochemical requirements for antagonism of the glycine site on the NMDA receptor. J Med Chem. 1992;35(11):1942–1953. doi: 10.1021/jm00089a003. [DOI] [PubMed] [Google Scholar]
- Dewachter I, Filipkowski RK, Priller C, Ris L, Neyton J, Croes S, Terwel D, Gysemans M, Devijver H, Borghgraef P, Godaux E, Kaczmarek L, Herms J, Van LF. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol Aging. 2009;30(2):241–256. doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dong H, Yuede CM, Coughlan C, Lewis B, Csernansky JG. Effects of memantine on neuronal structure and conditioned fear in the Tg2576 mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2008;33(13):3226–3236. doi: 10.1038/npp.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, Radi E. Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci. 2012;322(1–2):254–262. doi: 10.1016/j.jns.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Forder JP, Tymianski M. Postsynaptic mechanisms of excitotoxicity: Involvement of postsynaptic density proteins, radicals, and oxidant molecules. Neuroscience. 2009;158(1):293–300. doi: 10.1016/j.neuroscience.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Gray NM, Cheng BK, Mick SJ, Lair CM, Contreras PC. Phencyclidine-like effects of tetrahydroisoquinolines and related compounds. J Med Chem. 1989;32(6):1242–1248. doi: 10.1021/jm00126a016. [DOI] [PubMed] [Google Scholar]
- Kim HA, Miller AA, Drummond GR, Thrift AG, Arumugam TV, Phan TG, Srikanth VK, Sobey CG. Vascular cognitive impairment and Alzheimer’s disease: role of cerebral hypoperfusion and oxidative stress. Naunyn Schmiedebergs Arch Pharmacol. 2012;385(10):953–959. doi: 10.1007/s00210-012-0790-7. [DOI] [PubMed] [Google Scholar]
- Kotake Y, Taguchi R, Okuda K, Sekiya Y, Tasaki Y, Hirobe M, Ohta S. Neuroprotective effect of 1-methyl-1,2,3,4-tetrahydroisoquinoline on cultured rat mesencephalic neurons in the presence or absence of various neurotoxins. Brain Res. 2005;1033(2):143–150. doi: 10.1016/j.brainres.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179(1):303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Kuszczyk MA, Sanchez S, Pankiewicz J, Kim J, Duszczyk M, Guridi M, Asuni AA, Sullivan PM, Holtzman DM, Sadowski MJ. Blocking the Interaction between Apolipoprotein E and Aβ Reduces Intraneuronal Accumulation of Aβ and Inhibits Synaptic Degeneration. Am J Pathol. 2013;182(5):1750–1768. doi: 10.1016/j.ajpath.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszczyk M, Słomka M, Antkiewicz-Michaluk L, Salińska E, Łazarewicz JW. 1-Methyl-1,2,3,4-tetrahydroisoquinoline and established uncompetitive NMDA receptor antagonists induce tolerance to excitotoxicity. Pharmacol Rep. 2010;62(6):1041–1050. doi: 10.1016/s1734-1140(10)70366-2. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson PD, Carling RW, Moore KW, Moseley AM, Smith JD, Stevenson G, Chan T, Baker R, Foster AC, Grimwood S. 4-Amido-2-carboxytetrahydroquinolines. Structure-activity relationships for antagonism at the glycine site of the NMDA receptor. J Med Chem. 1992;35(11):1954–1968. doi: 10.1021/jm00089a004. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Hoesl CE, Höfner G, Wanner KT. Affinity of 1-aryl-1,2,3,4-tetrahydroisoquinoline derivatives to the ion channel binding site of the NMDA receptor complex. Eur J Med Chem. 2006;41(8):1003–1010. doi: 10.1016/j.ejmech.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Antkiewicz-Michaluk L, Czuczwar SJ. 1-Methyl-1,2,3,4-tetrahydroisoquinoline enhances the anticonvulsant action of carbamazepine and valproate in the mouse maximal electroshock seizure model. Neuropharmacology. 2006;50(2):133–144. doi: 10.1016/j.neuropharm.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Ma J, Brewer BH, Potter H, Brewer HB., Jr Alzheimer Aβ neurotoxicity: promotion by antichymotrypsin, apoE4; inhibition by Aβ-related peptides. Neurobiol Aging. 1996;17(5):773–780. doi: 10.1016/0197-4580(96)00112-1. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Wang H, Michaelis EK. Developmental expression, compartmentalization, and possible role in excitotoxicity of a putative NMDA receptor protein in cultured hippocampal neurons. Brain Res. 1991;565(1):94–108. doi: 10.1016/0006-8993(91)91740-r. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lipton SA. Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies. Cell Calcium. 2010;47(2):190–197. doi: 10.1016/j.ceca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T. Role of hydroxyl radical formation in neurotoxicity as revealed by in vivo free radical trapping. Toxicol Lett. 2002;32(2):83–93. doi: 10.1016/s0378-4274(02)00076-0. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55(5):697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrado J, Absi E, Ayala A, Castano A, Cano J, Machado A. The endogenous amine 1-methyl-1,2,3,4- tetrahydroisoquinoline prevents the inhibition of complex I of the respiratory chain produced by MPP+ J Neurochem. 2000;75(1):65–71. doi: 10.1046/j.1471-4159.2000.0750065.x. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Dekundy A, Pulte I. Mementine and cholinesterase inhibitors: complementary mechanisms in the treatment of Alzheimer’s Disease. Neurotox Res. 2013 doi: 10.1007/s12640-013-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietraszek M, Michaluk J, Romańska I, Wąsik A, Gołembiowska K, Antkiewicz-Michaluk L. 1-Methyl-1,2,3,4-tetrahydroisoquinoline antagonizes a rise in brain dopamine metabolism, glutamate release in frontal cortex and locomotor hyperactivity produced by MK-801 but not the disruptions of prepulse inhibition, and impairment of working memory in rat. Neurotox Res. 2009;16(4):390–407. doi: 10.1007/s12640-009-9097-y. [DOI] [PubMed] [Google Scholar]
- Puzzo D, Privitera L, Leznik E, Fa M, Staniszewski A, Palmeri A, Arancio O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J Neurosci. 2008;28(53):14537–14545. doi: 10.1523/JNEUROSCI.2692-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo D, Vitolo O, Trinchese F, Jacob JP, Palmeri A, Arancio O. Amyloid-beta peptide inhibits activation of the nitric oxide/cGMP/cAMP-responsive element-binding protein pathway during hippocampal synaptic plasticity. J Neurosci. 2005;25(29):6887–6897. doi: 10.1523/JNEUROSCI.5291-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, Orellana JA, von Bernhardi R. Understanding risk factors for Alzheimer’s disease: interplay of neuroinflammation, connexin-based communication and oxidative stress. Arch Med Res. 2012;43(8):632–644. doi: 10.1016/j.arcmed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011;117(3):388–402. doi: 10.1111/j.1471-4159.2010.07145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F, Tirard M, Lu J, Hutzler P, Lamberti P, Livrea P, Morabito M, Almeida OF. Soluble beta-amyloid1-40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J Neurosci. 2005;25(48):11061–11070. doi: 10.1523/JNEUROSCI.3034-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li YS, Schmidt SD, Mathews PM, Fryer JD, Holtzman DM, Sigurdsson EM, Wisniewski T. A synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am J Pathol. 2004;165(3):937–948. doi: 10.1016/s0002-9440(10)63355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27(1):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YE, Wang Y, Yu GC, Liu C, Zhang ZY, Zhang LM. Effects of Edaravone on Amyloid-β Precursor Protein Processing in SY5Y-APP695 Cells. Neurotox Res. 2013;24(2):139–147. doi: 10.1007/s12640-012-9370-3. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Stine WB, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278(13):11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8(4):277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, Yu FM, Lin MT, Milner TA, Gouras GK. Oligomerization of Alzheimer’s beta-amyloid within processes and synapses of cultured neurons and brain. J Neurosci. 2004;24(14):3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto SI, Dziewczapolski G, Nakamura T, Cao G, Pratt AE, Kang YJ, Tu S, Molokanova E, McKercher SR, Hires SA, Sason H, Stouffer DG, Buczynski MW, Solomon JP, Michael S, Powers ET, Kelly JW, Roberts A, Tong G, Fang-Newmeyer T, Parker J, Holland EA, Zhang D, Nakanishi N, Chen HS, Wolosker H, Wang Y, Parsons LH, Ambasudhan R, Masliah E, Heinemann SF, Piña-Crespo JC, Lipton SA. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA. 2013;110(27):E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s A beta peptide: The many roads to perdition. Neuron. 2004;43(5):605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Tetz LM, Kamau PW, Cheng AA, Meeker JD, Loch-Caruso R. Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. J Pharmacol Toxicol Methods. 2013;67(2):56–60. doi: 10.1016/j.vascn.2013.01.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetulani J, Antkiewicz-Michaluk L, Nalepa I, Sansone M. A possible physiological role for cerebral tetrahydroisoquinolines. Neurotox Res. 2003;5(1–2):147–155. doi: 10.1007/BF03033379. [DOI] [PubMed] [Google Scholar]
- von Bernhardi R, Tichauer JE, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112(5):1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid beta-peptide (A beta) fibrillogenesis block oligomerization of natural A beta and thereby rescue long-term potentiation. J Neurosci. 2005;25(10):2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27(5–6):612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60(9):575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]