Abstract
Spinal cord injury (SCI) impaired sensory fiber transmission leads to chronic, debilitating neuropathic pain. Sensory afferents are responsive to neurotrophic factors, molecules that are known to promote survival and maintenance of neurons, and regulate sensory neuron transduction of peripheral stimuli. A subset of primary afferent fibers responds only to the glial cell-line derived neurotrophic factor (GDNF) family of ligands (GFLs) and are non-peptidergic. In peripheral nerve injury models, restoration of GDNF or artemin (another GFL) to pre-injury levels within the spinal cord attenuates neuropathic pain. One noninvasive approach to increase the levels of GFLs in the spinal cord is through exercise (Ex), and to date exercise training is the only ameliorative, nonpharmacological treatment for SCI-induced neuropathic pain. The purpose of this study was three fold: 1) to determine whether exercise affects the onset of SCI-induced neuropathic pain; 2) to examine the temporal profile of GDNF and artemin in the dorsal root ganglia and spinal cord dorsal horn regions associated with forepaw dermatomes after SCI and Ex; and 3) to characterize GFL-responsive sensory fiber plasticity after SCI and Ex. Adult, female, Sprague-Dawley rats received a moderate, unilateral spinal cord contusion at C5. A subset of rats was exercised (SCI+Ex) on automated running wheels for 20 minutes, 5d/week starting at 5 days post injury (dpi), continuing until 9 or 37 dpi. Hargreaves' and von Frey testing was performed preoperatively and weekly post SCI. Forty-two percent of rats in the unexercised group exhibited tactile allodynia of the forepaws while the other 58% retained normal sensation. The development of SCI-induced neuropathic pain correlated with a marked decrease in the levels of GDNF and artemin in the spinal cord and DRGs. Additionally, a dramatic increase in the density and the distribution throughout the dorsal horn of GFL-responsive afferents was observed in rats with SCI-induced allodynia. Importantly, in SCI rats that received Ex, the incidence of tactile allodynia decreased to 7% (1/17) and there was a maintenance of GDNF and artemin at normal levels, with a normal distribution of GFL-responsive fibers. These data suggest that GFLs and/or their downstream effectors may be important modulators of pain fiber plasticity, representing effective targets for anti-allodynic therapeutics. Furthermore, we highlight the potent beneficial effects of acute exercise after SCI.
Keywords: mechanical allodynia, thermal hyperalgesia, central pain, spinal cord injury, artemin, GDNF
INTRODUCTION
Damage to the cervical spinal cord that results in chronic debilitating neuropathic pain occurs in more than 60% of human spinal cord traumas (Siddall & Loeser, 2001; Widerstrom-Noga et al, 2008). Clinical hallmarks of central neuropathic pain are the development of allodynia, a condition where normally innocuous stimuli elicit a painful response, and hyperalgesia, a condition where noxious stimuli elicit an amplified pain response (Christensen et al, 1996). These types of neuropathic pain are further delineated based on the location of the pain relative to the SCI epicenter as above-level pain occurring in dermatomes rostral to the lesion site; as at-level pain occurring within 2 segments of the injury epicenter; or as below-level pain occurring in dermatomes caudal to the lesion site (Siddall & Loeser, 2001).
Following cervical spinal cord injury, deficits in sensation and the development of chronic neuropathic pain have been attributed to direct damage to the second order sensory neurons within the grey matter of the dorsal horn and/or direct interruption of their axons that ascend in the anterolateral and spinoreticular tracts. Additionally, peptidergic pain afferent fibers immunolabeled for calcitonin gene-regulated peptide (CGRP) or substance P (SP) sprout and exhibit robust arborization into the deep dorsal horn (laminas III–V) above, at and below the lesion epicenter in response to clinical (Calancie et al, 2005; Kakulas, 2004) and experimental SCI Hagg (Hagg, 2006; Krenz & Weaver, 1998; Murray & Goldberger, 1974; Ondarza et al, 2003; Weaver et al, 2002; Weaver et al, 2001; Zinck et al, 2007).
Another possible contributor to this change in pain afferent distribution and the concomitant development of neuropathic pain are the glial cell-line derived neurotrophic factor (GDNF) family of ligands (GFLs) within the spinal cord dorsal horn (Boucher & McMahon, 2001). In models of peripheral nerve injury, a decrease in GFLs such as GDNF and artemin correlates to the development of neuropathic pain, and restoration of these GFLs to normal levels is sufficient to attenuate dorsal horn remodeling and the development of neuropathic pain (Boucher et al, 2000; Gardell et al, 2003; Hao et al, 2003; Harvey et al, 2010; Pezet et al, 2006; Wang et al, 2003; Wang et al, 2008). Recent data by Harvey et al (2010) showed that restoration of artemin levels correlates with the appropriate laminar distribution of regenerating afferents after dorsal root crush injury. It is unlikely that artemin acts directly on peptidergic afferents, but rather on a separate and distinct class of pain afferents that are non-peptidergic and GFL-responsive (Malin et al, 2006; Orozco et al, 2001).
The levels of GDNF and its receptor are reduced in the spinal cord after SCI. One non-invasive, clinically useful approach to enhance the levels of neurotrophic factors in the spinal cord after injury is through exercise (Gomez-Pinilla et al, 2002; Côté et al 2011), and exercise is the only non-pharmacological treatment that has been shown to reduce SCI-induced neuropathic pain (Hutchinson et al, 2004). In the present study, we evaluated the effect of daily exercise therapy on the development of at-level neuropathic pain, the levels of GFLs in the dorsal horn as well as on the distribution of pain afferents within the dorsal horn after SCI. The results indicate a strong correlation between changes in GFL levels, reduction in primary afferent fiber sprouting and decreased incidence of allodynia in SCI rats that were exercised.
METHODS
Subjects and Surgeries
Eight-one adult, female Sprague-Dawley rats (225–250 g; Charles River Laboratories) were housed 2–3 per cage in a controlled environment (12 h light-dark cycles) with food and water ad libidum. All experimental procedures were approved by the Drexel University Institutional Animal Care and Use Committee.
We utilized a clinically-relevant and accepted model of SCI pain as described previously (Detloff et al 2012b). Briefly, rats were anesthetized with ketamine (60 mg/kg), xylazine (6 mg/kg) and acepromazine (6 mg/kg) and given antibiotics (ampicillin, s.c.,100 mg/kg, daily for 7d). After partial laminectomy at C5, the spinal column was stabilized in the Infinite Horizon Impact Device (Precision Systems and Instrumentation, Lexington, KY, Scheff et al, 2003). A custom impactor probe (1.6 mm in diameter) was lowered to within 2 mm of the mid portion of the right C5 spinal cord, and the spinal cord and surgical field was flooded with sterile saline. The spinal cord was then rapidly contused with a force of 200 kilodynes (no dwell time), resulting in tissue displacement of 1600–1800 μm. The incision was closed in layers and 5 cc of lactated Ringer's solution was administered subcutaneously to prevent dehydration.
Prior to SCI, rats were randomly assigned to Exercise (n=27), SCI Alone (n=44), or Control (No SCI; n=10) groups. As we have previously shown, SCI Alone rats can be partitioned into 2 separate groups based on their paw withdrawal threshold in response to mechanical stimuli during von Frey testing (Detloff et al, 2012b). For a rat to be considered in the SCI Allodynia group, the animal must exhibit a 50% reduction in paw withdrawal threshold in both forepaws that is maintained from 14 dpi to the duration of the experiment. Additionally, each behavioral group was subdivided into short (9 day) or long (37 day) survival, and then separated at time of sacrifice for ELISA or immunohistochemical analysis (See table 1).
Table 1.
Number of rats at each time point used for behavior, ELISA or Immunocytochemistry (ICC).
| Days Post Spinal Cord Injury | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0 dpi | 9 dpi | 37 dpi | |||||||
| Group | n | Behavior | ELISA | ICC | Behavior | ELISA | ICC | Behavior | ELISA | ICC |
| Naïve | 10 | 10 | 5 | 5 | ||||||
|
| ||||||||||
| SCI No Allodynia | 24 | 10 | 5 | 5 | 14 | 5 | 8 | |||
|
| ||||||||||
| SCI Allodynia | 20 | 10 | 5 | 5 | 10 | 5 | 5 | |||
|
| ||||||||||
| SCI+Exercise | 27 | 10 | 5 | 5 | 17 | 5 | 12 | |||
Forced Exercise Paradigm
Rats in the SCI + Exercise group were acclimated to the forced exercise wheel walking system (Lafayette Instruments, Lafayette, IN). Rungs of the wheels were covered with vinyl material to create a continuous surface in order to minimize the potential of additional injury to SCI rats from limbs falling through the rungs due to a loss of grip and grasping function of the ipsilesional forepaw. Rats were placed in the automated wheels for 5 minutes prior to the start of exercise, with a subsequent running period of 20 minutes per day for 5 days per week. Rats began exercise 5 dpi with an initial speed of 5 m/ min with speed increasing at 1 minute intervals according to the forelimb capabilities of the rats or until a maximum speed of 14.0 m/min was achieved. The time spent at each speed, rather than the number of wheel revolutions was recorded. All rats reached the maximum speed by 21 dpi.
Behavioral Measures
Rats were acclimated to the individual testing environments for at least 7 days (20 min/day) prior to preoperative testing. Behavioral testing was conducted preoperatively to establish baseline responses and then weekly after SCI by blinded experimenters. Due to the unilateral nature of our injury model, we evaluated the ipsilesional (right) and contralesional (left) forepaws separately.
Tactile allodynia
The up-down method for von Frey hair monofilaments (VFH, Stoelting Co., Wood Dale, Il) was used to measure the degree of tactile sensory changes in the forepaws after SCI (Detloff et al, 2010; Detloff et al, 2012a; Detloff et al, 2012b). Post-injury assessment of nocifensive behavior was initiated only when there was evidence of weight support during locomotor testing for a given limb (Forelimb Locomotor Scale score ≥7; Sandrow et al, 2008). This ensured that the rat had suitable motor control to remove its paw from an unpleasant stimulus. Rats were enclosed in a metal chamber measuring 20 cm × 9 cm by 10 cm, with a wire mesh top and bottom (0.635 cm grid size) allowing access to the plantar surface of the forepaws. A total of ten VFH stimulus applications were collected for each paw for each day of testing, beginning with the 5.18 g VFH. Any supraspinally driven attention given to the tactile stimulus including vocalizing, licking or guarding of the stimulated paw was recorded to ensure that the rat had some supraspinal awareness of stimulus application. The paw withdrawal threshold was determined as the lowest force (g) that produced a forepaw withdrawal and supraspinal behaviors in at least 50% of the applications. Paw testing order was performed randomly to minimize an order effect.
As reported previously, spinal cord injured rats were labeled post hoc as allodynic after SCI if they exhibited pain-related behaviors (Detloff et al, 2012b). Specifically, the performance and selection criteria that led to assignation to the SCI Allodynia group were as follows: the rat must exhibit >50% reduction in the paw withdrawal threshold to von Frey stimulation by 7–14 days after SCI. This reduction in paw withdrawal threshold could not vacillate from week to week; it had to be stable and persist over time. Group means indicate that the SCI Allodynia rats had a significantly lower paw withdrawal threshold than both their baseline paw withdrawal threshold and that of SCI rats which maintained normal sensation (SCI No Allodynia; Figure 1, p<.05). Additionally, the rat must exhibit one or more signs of supraspinal awareness such as, vocalizing, licking, looking at, or guarding of the paw, or moving away from the stimulus after the application of the von Frey monofilament that elicits a paw withdrawal response. Importantly, SCI + Exercise rats were not able to be categorized in this manner as exercise therapy was initiated prior the ability to reliably assess tactile sensation after SCI. Data are presented as mean±SEM and include rats that were sacrificed at 9 or 35 dpi. Rats with short survival times were tested on the day of sacrifice to ensure the presence or absence of tactile forepaw allodynia.
Figure 1. At-level tactile allodynia is prevented by acute exercise.
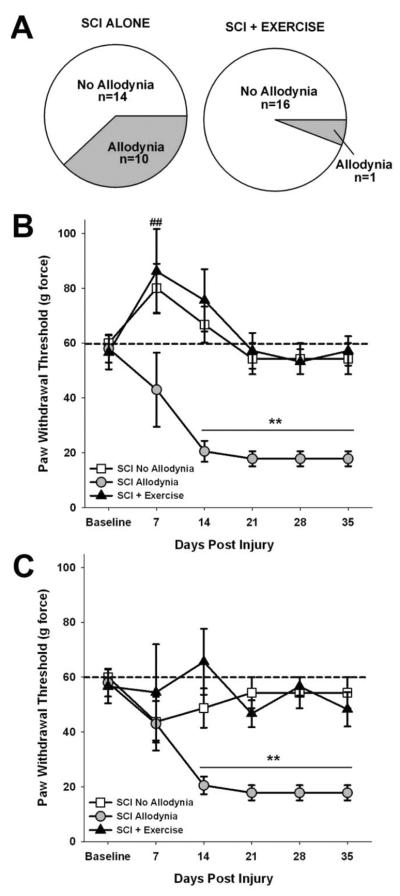
(A) Five weeks after SCI, at-level allodynia occurred in ~42% of the rats with SCI (10 out of 24 rats). Exercise intervention reduced the incidence of at-level allodynia to 6% (1 out of 17 rats; χ2=14.529, p=0.0001). The paw withdrawal threshold to tactile stimuli for the ipsilesional (B) and contralesional (C) forepaw were recorded over a period of 35 days. Before SCI, rats withdraw their forepaws from a stimulus force of 60 g (indicated by dashed line). Two behavioral cohorts emerge after SCI, those that maintain tactile paw withdrawal thresholds at or near 60g force (SCI No Allodynia) and those that exhibit a significant reduction in paw withdrawal thresholds that plateau near 30 g force (SCI Allodynia). These distinct responses to SCI were apparent by 14 dpi and persisted to at least 35 dpi (**p<.05 vs all other groups). Rats that were exercised (SCI + Exercise) starting at 5 dpi did not develop allodynia in either the ipsilesional or contralesional forepaw. There was significant hyposensitivity at 7 dpi in the contralesional forepaw of the SCI No Allodynia and SCI + Exercise groups that resolved by 14 dpi (##p<.05 vs. naïve).
Thermal hyperalgesia
Preoperatively and post-SCI once weight supported stepping occurred in the open field, we measured changes in thermal sensation using the Ugo Basile Plantar Heat test (Comerio VA, Italy) as first described by Hargraeves and colleagues (1988). Briefly, rats were enclosed in a clear Plexiglas box with a glass bottom. After a 10 min acclimation period, a noxious, infrared light beam was applied to the plantar surface of the forepaw, and paw withdrawal latency recorded in seconds. The infrared stimulus application automatically shut off at 30 sec. to avoid tissue damage. Five trials were collected randomly for each forepaw with at least a one minute delay between each trial. The trials for each paw were averaged to yield one score per paw. We recorded supraspinal behaviors that occurred in response to application of the thermal stimulus as described above for tactile allodynia.
Histology
Rats dedicated to histology were euthanized at 9 or 37 dpi (n=45, See Table 1 ICC Columns, Euthasol; 390 mg/kg sodium pentobarbital and 50 mg/kg phenytoin, ip.) and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. Eight millimeters of cervical spinal cord between C4 and C6 spanning the rostrocaudal extent of the lesion and a 4 mm block containing C7–8 cord was removed, post-fixed in paraformaldehyde at 4°C overnight and submersed in 30% sucrose for cryoprotection. Series of 25 micron thick transverse sections 250 μm apart through the extent of these tissue blocks were mounted on subbed slides.
Nissl-Myelin Staining of the lesion site
Sections from a single series were stained with cresyl violet (Sigma, St. Louis, MO) for Nissl substance and euriochrome cyanine (Sigma) for myelin. Sections were cover slipped with Permount mounting medium (Fisher Scientific, Pittsburgh, PA).
To determine the amount of spared tissue, the area of contralesional grey and white matter, spared grey and white matter on the ipsilesional side and the lesion cavity were measured separately in 10 non-serial sections (250 μm apart) spanning the rostrocaudal extent (C4–6) of the lesion using the Cavalieri estimator method (Stereo Investigator, MicroBrightfield, Burlington, VT) by an experimenter blinded to experimental groups. We then determined the proportion of the spared tissue area on the ipsilesional side of the spinal cord to the tissue area on the contralesional (uninjured) spinal cord (Côté et al, 2012; Detloff et al, 2012b).
C7–8 immunohiostchemistry
Non-peptidergic, GFL-responsive pain afferents were identified with Isolectin-B4 (IB-4) staining. Dried sections were incubated in 10% normal serum with 0.2% Triton-1×100 for 1 h and incubated for 48 h with primary antibody (1:2000; Sigma) in a humid chamber at room temperature. All sections were rinsed in buffer and then incubated with streptavidin conjugated Alexa 594 (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h in the dark at room temperature. Slides were rinsed in buffer and coverslipped with FluorSave Reagent (Calbiochem, Bedford, MA). Ten images of the ipsilesional and contralesional dorsal horn (250 microns apart) were taken for each rat at the same magnification, lens aperture and exposure time. Each image was then analyzed with a custom macro for ImageJ (NIH), where the proportional area of IB-4 immunoreactive tissue within the focal plane of the image was quantified. All sections in all rats were quantified using a constant optical density threshold.
Protein Analysis
Rats designated for protein analysis (n=35; see Table 1 ELISA columns) were euthanized at 9 or 37 dpi (Euthasol; 390 mg/kg sodium pentobarbital and 50 mg/kg phenytoin, ip.) and the C7–8 spinal cord and associated dorsal root ganglia (DRG) were rapidly dissected. C7 and C8 DRGs on the ipsilesional (right) or contralesional (left) side of the rat were pooled together for processing. The spinal cord segment was further prepared by removing the meninges and dissecting the ipsilesional and contralesional dorsal spinal cord into separate samples. All tissues were fast frozen in n-methyl butane cooled over dry ice and stored at −80°C. Tissue blocks were weighed (mg) and were placed in cold RIPA buffer, in the presence of protease and phosphotase inhibitors (Roche Diagnostics, Indianapolis, IN). Samples were homogenized, incubated on ice for 2h and then centrifuged at 14000 g for 45 min at 4°C. The supernatants were collected and GDNF or artemin protein was quantified by ELISA kits (Promega, Madison, WI; Shanghai BlueGene Biotech Co, LTD. Shanghai, China) and reported as pg/mg tissue.
Statistical Analysis
Two way repeated measure ANOVAs were performed for von Frey and Hargreaves' behavioral data (group × time) followed by Tukey's post-hoc comparisons using the harmonic mean to correct for unequal group sizes. A χ2 analysis was used to evaluate the incidence of allodynia in exercised and unexercised animals at 35 dpi. Epicenter sparing, ELISA and proportional area measurements for IB-4 immunoreactivity were analyzed via one-way ANOVA with Bonferroni's post-hoc analysis. Means and standard error of the mean (SEM) are reported throughout. Regression and Pearson's correlational analyses were completed to determine the relationship between allodynia and the proportional area of afferents in the dorsal horn.
RESULTS
Exercise therapy prevents the onset of neuropathic pain after SCI
To determine whether exercise was sufficient to prevent chronic neuropathic pain, we evaluated tactile and thermal sensation of the forepaws before and after SCI. In the lab, we define the development of tactile allodynia to be a >50% reduction of paw withdrawal threshold to von Frey monofilaments coupled with several incidence of supraspinal responses (licking, looking or guarding the paw) in response to stimulus application. Exercise significantly decreased the proportion of SCI rats which met these criteria (χ2=14.529, p=.0001). Five weeks after unilateral C5 contusion, 42% of unexercised SCI rats exhibited a >50% decrease in tactile sensory thresholds of both forepaws (n=10 out of 24, Figure 1A). Importantly, exercise therapy beginning at 5 dpi was able to reduce the incidence of SCI-induced at-level allodynia to just 6% (n= 1 out of 17), an 83% reduction in the number of rats developing chronic at-level allodynia (Figure 1A). That there are two significantly different cohorts of SCI rats with regards to their tactile sensation, for the remainder of the analysis we treated SCI rats with allodynia as one group, and SCI rats with normal sensation as another (SCI Allodynia and SCI No Allodynia, respectively). Since there was only 1 exercised rat (out of 17 total) who developed tactile allodynia, we did not divide the SCI Exercise group, but rather included the data from all the exercised SCI rats together.
Prior to SCI, the mean mechanical threshold to tactile stimuli was 58.3±5.1 g for either forepaw. By 14 dpi, SCI rats could be parceled into SCI Allodynia or SCI No Allodynia groups based upon change in the paw withdrawal threshold. In the SCI Allodynia group, moderate unilateral C5 SCI caused a significant reduction in the withdrawal threshold (17.8±2.8 g; Figure 1B) of the ipsilesional forepaw that was established by 14 dpi and persisted over the full testing period. Rats in the SCI No Allodynia group maintained normal paw withdrawal thresholds by the ipsilesional forepaw at all timepoints post SCI (Figure 1B). Rats that received exercise therapy starting at 5 dpi mirrored the SCI No Allodynia group, maintaining normal paw withdrawal thresholds after injury (Figure 1B). Assessment of paw withdrawal thresholds of the contralesional forepaw revealed a transient hyposensitivity in the first week after SCI that returned to normal levels at 14 dpi for the SCI No Allodynia and SCI+ Exercise groups (Figure 1C). The SCI Allodynia group exhibited robust hypersensitivity of the contralesional forepaw at 14 dpi that persisted throughout the duration of the study (Figure 1C, p<0.05). No rats in any group demonstrated overt evidence of spasticity, such as clonus or sustained anti-gravity posturing of either forepaw prior to or during sensory testing. Positive paw withdrawal responses to tactile stimuli in this group were often accompanied by hypervigilant behaviors including licking the stimulated paw as well as turning and looking at the stimulus, and occasionally moving away from the stimulus in animals with or without allodynia. No rats vocalized in response to von Frey monofilament application. These behaviors require supraspinal transmission of sensory information through the lesion to supraspinal centers suggesting that these animals are aware of the stimulus application and are responding in a manner consistent with it being unpleasant.
After unilateral C5 contusion, all rats developed significant thermal hyperalgesia in both the ipsilesional (Figure 2A) and contralesional (Figure 2B) forepaws (p<0.05 vs. preoperative levels) regardless of exercise treatment. Preoperatively, the mean withdrawal latency to a noxious heat stimulus was 10.7±0.28 s for either forepaw. After unilateral C5 contusion, both forepaws exhibited a progressive decline in paw withdrawal latency that plateaued by 21 dpi (p<0.05) and persisted for the duration of the study. There were no significant differences in paw withdrawal latency between groups at any time point for either the ipsilesional or contralesional forepaw regardless of either the presence or absence of tactile allodynia or the implementation of an exercise regimen.
Figure 2. Acute exercise does not prevent at-level thermal hyperalgesia.
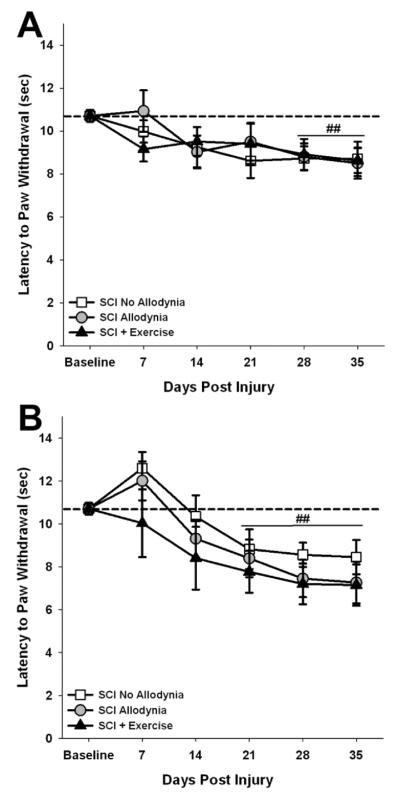
The latency to paw withdrawal to a noxious thermal stimulus for the ipsilesional (A) and contralesional (B) forepaw was recorded over a period of 35 days. Before SCI, rats withdraw their forepaws after 11 seconds (indicated by dashed line). After SCI, there was no difference in paw withdrawal latency between SCI No Allodynia, SCI Allodynia and SCI + Exercise groups at any time point for ipsilesional or contralesional forepaws, as all groups exhibited a significant decrease in paw withdrawal latency compared to baseline values (**p<.05 vs naïve control).
Exercise therapy does not improve tissue sparing
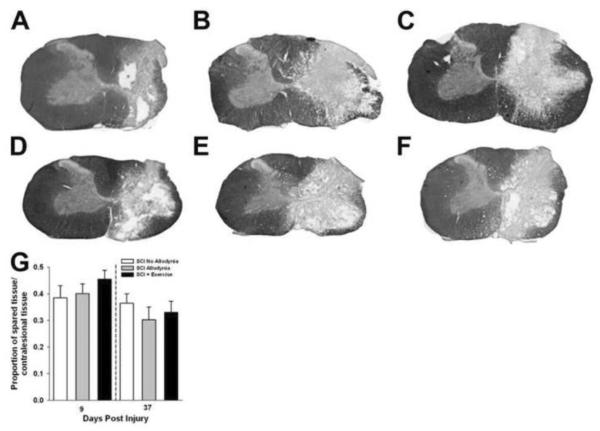
Contusive SCI produced a core lesion consisting of a cystic cavity surrounded by a rim of white matter that was confined to the injured side of the spinal cord, as previously described (Figure 3, Detloff et al, 2012b). For all groups, nearly all grey matter was eliminated and only a small outer rim of densly stained white matter remained, predominantly in the lateral and ventral funiculi of the ipsilesional spinal cord. There was no evidence of tissue damage in the contralesional spinal cord for any group at either 9 or 37 dpi (Figure 3A–F). Quantitatively, the proportion of spared tissue of the ipsilesional versus contralesional side of the spinal cord had no relationship to the presence of allodynic behavior (Figure 3G). In addition, exercise had no effect on tissue sparing (Figure 3G).
Figure 3. Anatomical assessments in SCI rats with and without tactile allodynia.
Representative transverse sections of the lesion epicenter stained for myelin sacrificed at 9 days post injury in groups SCI No Allodynia (A), SCI Allodynia (B), and SCI + Exercise (C). Sections from SCI rats sacrificed at 37 days post injury in groups SCI No Allodynia (D) SCI Allodynia (E) and SCI + Exercise (F) are presented. The 200 kdyne impact produced a moderate, unilateral lesion with near complete degeneration of grey matter and a spared rim of white matter on the right side of the spinal cord. Importantly, the grey and white matter of the contralesional spinal cord appears normal (scale bar=0.5mm). There were no significant differences in tissue sparing through the rostrocaudal extent of the lesion between any groups at either time point (G).
Exercise therapy prevents SCI-induced reduction of GFLs
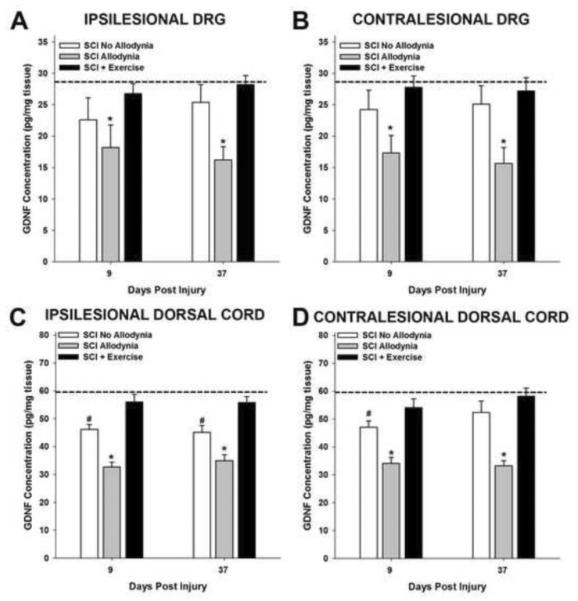
The level of GDNF and artemin were measured in the C7–8 segments of dorsal spinal cord and dorsal root ganglia which correspond to the dermatomes of the forepaw in naïve (n=5), SCI No Allodynia (n=5 at 9 and 37 dpi), SCI Allodynia (n=5 at 9 and 37 dpi) and SCI+Exercise (n=5 at 9 and 37 dpi) rats (Figure 4, 5). In naïve rats, the concentration of GDNF was 290 pg/ml in C7 and C8 DRG. The concentration of GDNF was significantly decreased in both the ipsilesional and contralesional DRGs of SCI rats with allodynia compared to all other groups at subacute and chronic timepoints after SCI (Figure 4A, B). Exercise intervention starting at 5 dpi out to 9 days or 37 days was sufficient to maintain normal concentrations of GDNF in the ipsilesional and contralesional cervical DRGs (Figure 4A, B).
Figure 4. Exercise prevents SCI-induced reduction of GDNF.
In naïve rats, the concentration of GDNF was 29 pg/mg tissue in the C7 and C8 dorsal root ganglia (dashed line in A and B). At 9 and 37 dpi, the concentration of GDNF was significantly reduced in the ipsilesional (A) and contralesional (B) DRGs of the SCI Allodynia group (*p<.05 vs all other groups). Exercise starting at 5 dpi maintained naïve concentrations of GDNF in the DRG. The concentration of GDNF in the naïve dorsal half of the spinal cord was 60 pg/mg tissue (dashed line in C and D). (C) The concentration of GDNF decreased in the ipsilesional dorsal spinal cord of the SCI Allodynia group compared to all other groups (*p<.05) at 9 and 37 dpi. (D) GDNF levels were significantly decreased in the contralesional dorsal cord of the SCI No Allodynia at 9 but not 37 dpi (#p<0.05 vs naïve). Exercise starting at 5 dpi maintained the levels of GDNF in the dorsal spinal cord at both time points.
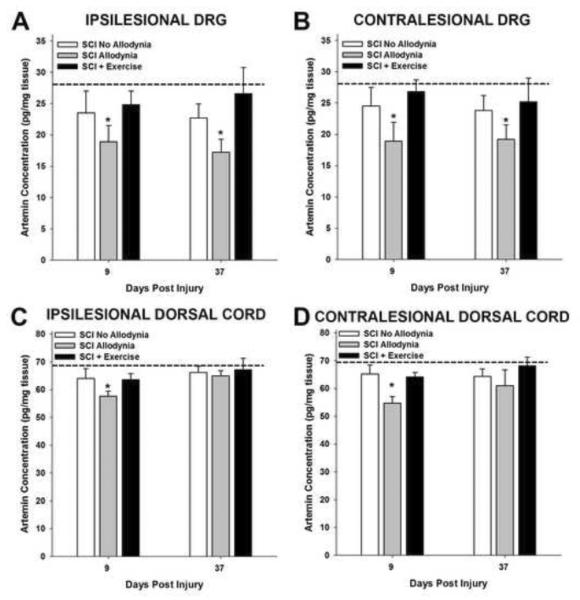
Figure 5. Exercise prevents SCI-induced reduction of artemin.
In naïve rats, the concentration of artemin was 29 pg/mg tissue in the C7 and C8 dorsal root ganglia (dashed line in A and B). At 9 and 37 dpi, the concentration of artemin was significantly reduced in the ipsilesional and contralesional DRGs of the SCI Allodynia group (*p<.05 vs all other groups). Exercise starting at 5 dpi maintained naïve concentrations of artemin in the DRG. The concentration of artemin in the normal dorsal half of the spinal cord was 69 pg/mg tissue (dashed line in C and D). The concentration of artemin decreased in both the ipsilesional (C) and contralesional (D) dorsal spinal cord of the SCI Allodynia group compared to all other groups (*p<.05) at 9 dpi. Exercise starting at 5 dpi maintained the levels of artemin in the dorsal spinal cord. At 37 dpi, there were no significant differences between groups.
The concentration of GDNF in the dorsal portion of the normal spinal cord was 600 pg/ml. Rats in the SCI Allodynia group exhibited a marked decrease in the concentration of GDNF at 9 and 37 dpi in the ipsilesional and contralesional dorsal spinal cord (Figure 4C, D) compared to all other groups (*p<.05). The concentration of GDNF was significantly decreased in the ipsilesional dorsal cord of rats in the SCI Allodynia group. However, in the contralesional dorsal cord of the SCI No Allodynia group, the decrease is only significant at 9 but not at 37 dpi (#p<0.05 vs naïve). Exercise starting at 5 dpi maintained the levels of GDNF in the dorsal spinal cord after 9 or 37 dpi.
Examination of the expression profile of artemin after SCI revealed significantly lower concentration in the ipsilesional and contralesional DRGs at 9 dpi and 37 dpi for rats with allodynia (*p<.05), while the concentration of artemin was similar to naïve levels of SCI rats that did not present with allodynia (Figure 5A,B). Exercise therapy beginning at 5 dpi was adequate to maintain normal concentrations of artemin in the DRGs (Figure 5A,B). Interestingly, in the dorsal spinal cord, the concentration of artemin was decreased in rats with allodynia at 9 but not 37 dpi (Figure 5C,D), while exercise starting at 5 dpi maintained the artemin concentration during the post injury periods (Figure 5C, D).
Exercise therapy prevents SCI-induced redistribution of GFL-responsive pain afferents
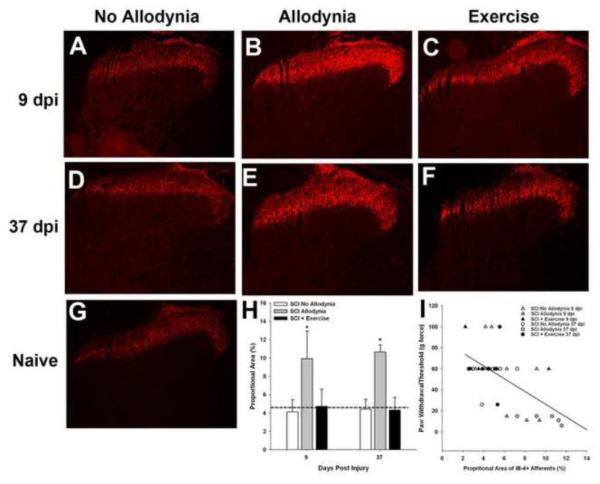
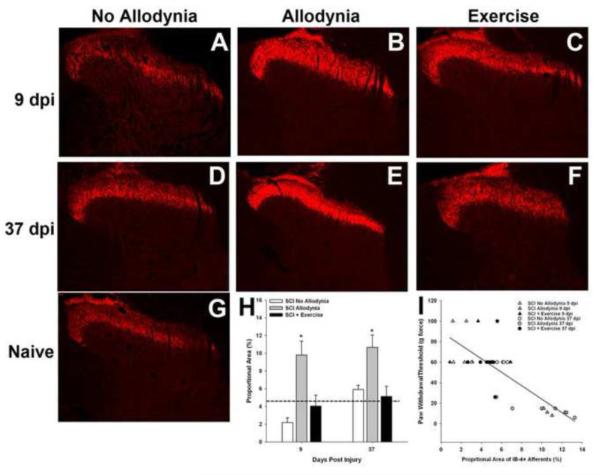
We evaluated the change in distribution of GFL-responsive c fibers in the C7–8 dorsal horn which corresponds to the dermatomes of the forepaw. In naïve dorsal horn, GFL-responsive afferents terminate in the inner layer of lamina II (Figure 6G). At 9 and 37 dpi, the SCI No Allodynia group exhibited a normal distribution of GFL-responsive c fibers in lamina IIi of the ipsilesional dorsal horn (Figure 6A, D). The SCI Allodynia group displayed robust changes in the distribution and density of the GFL-responsive c-fibers were evident in the ipsilesional dorsal horn (Figure 6B, E). The band of GFL-responsive c fibers for the SCI Allodynia group extended beyond the normal distribution pattern invading both lamina IIo and III. Additionally, the thickness and intensity of the band of GFL-responsive c fibers was uniformly distributed in the medial and lateral dorsal horn. Importantly, exercise therapy beginning at 5 dpi was sufficient to prevent the prominent changes in distribution and density of the GFL-responsive afferents (Figure 6C, F). The proportional area of the ipsilesional dorsal horn that was positively labeled for GFL-responsive c fibers (IB4+) was significantly increased by 9 and 37dpi in the SCI Allodynia group compared to naïve, SCI No Allodynia and SCI + Exercise groups (p<.05; Figure 6H). The degree of afferent density within the dorsal horn positively correlated with the severity of at-level allodynia after SCI. As the density and distribution pattern of GFL-responsive fibers increased, greater hypersensitivity of the ipsilesional forepaw developed (Figures 6I; r2=0.52; p<.05). Rats generally fell into one of two groups, those with normal distribution and density of GFL-responsive pain afferents, and those with a 2 fold increase in the GFL-responsive afferent distribution within the ipsilesional dorsal horn compared to naïve controls.
Figure 6. Exercise prevents SCI-induced redistribution of GFL-responsive afferents in the ipsilesional dorsal horn.
Representative section of the ipsilesional C7 dorsal horn of Naïve (G),SCI No Allodynia with 9 (A) or 37 d (D) survival, SCI Allodynia with 9 (B) or 37 d (E) survival and SCI + Exercise with 9 (C) or 37 d (F) survival. Proportional area of positively labeled tissue within the dorsal horn was determined across the C7 and C8 spinal cord. The average proportional areas for these regions of interest are represented in H. Significantly greater proportional area was labeled for Isolectin-B4, a marker of non-peptidergic c-fibers at both 9 and 37 dpi of the SCI Allodynia group (*p<.05 vs all other groups; dashed line indicates the proportional area of Isolectin-B4+ afferents in the dorsal horn of naïve rats). (I)The degree of afferent labeling predicted at-level allodynia of the ipsilesional forepaw (r2=0.52; p<.05).
Interestingly, the distribution pattern and density of GFL-responsive afferents in the contralesional dorsal horn was similar to changes in the distribution pattern in the ipsilesional dorsal horn. At 9 and 37 dpi, the SCI No Allodynia group displayed a thin band of isolectin-B4 positive afferents located in the inner layer of lamina II similar to naïve (Figure 7A, D, G). The distribution and density of the SCI Allodynia group was increased in the contralesional dorsal horn at 9 and 37 dpi (Figure 7B, E). The thick, contiguous band of GFL-responsive c fibers terminated in lamina IIi that extended into lamina IIo and III of the contralesional dorsal horn. The distribution pattern GFL-responsive afferents in the contralesional dorsal horn of the SCI + Exercise group was similar to naïve and SCI No Allodynia (Figure 7C, F). The proportional area of the contralesional dorsal horn that was positively labeled for GFL-responsive c fibers (IB4+) was significantly increased by 9 and 37dpi in the SCI Allodynia group compared to naïve, SCI No Allodynia and SCI + Exercise groups (p<.05; Figure 7H). The degree of afferent density within the dorsal horn positively correlated with the severity of at-level allodynia after SCI. As the density and distribution pattern of GFL-responsive fibers increased, greater hypersensitivity of the ipsilesional forepaw developed (Figures 7I; r2=0.64; p<.05). Again, rats fell into one of two groups, those with normal distribution and density of GFL-responsive pain afferents and those with a 2 fold increase in the GFL-responsive afferent distribution within the ipsilesional dorsal horn compared to naïve.
Figure 7. Exercise intervention prevents SCI-induced redistribution of GFL-responsive afferents in the contralesional dorsal horn.
Representative section of the contralesional C7 dorsal horn of Naïve (G),SCI No Allodynia with 9 (A) or 37 d (D) survival, SCI Allodynia with 9 (B) or 37 d (E) survival and SCI + Exercise with 9 (C) or 37 d (F) survival. Proportional area of positively labeled tissue within the dorsal horn was determined across the C7 and C8 spinal cord. The average proportional areas for these regions of interest are represented in H. Significantly greater proportional area was labeled for Isolectin-B4, a marker of non-peptidergic c-fibers at both 9 and 37 dpi of the SCI Allodynia group (*p<.05 vs all other groups; dashed line indicates the proportional area of Isolectin-B4+ afferents in the dorsal horn of naïve rats). (I) The degree of afferent labeling predicted at-level allodynia of the contralesional forepaw (r2=0.64; p<.05).
DISCUSSION
The current work demonstrates that early exercise therapy reduces the development of at-level allodynia to less than 10% of SCI rats compared to nearly 40% of non-Exercised rats. While SCI-induced allodynia was associated with a decrease in GDNF and artemin in the DRG and dorsal horn, exercise maintained these GFLs to near normal levels at time points corresponding to the early onset (9 dpi) and persistence (37 dpi) of this pain. Moreover, exercise prevented the increased density and redistribution of GFL-responsive pain afferents in the dorsal horn that correlated with the development of at-level allodynia.
Exercise is a non-invasive therapeutic approach with the potential for profound effects on the SCI population. It induces cellular and molecular changes within the spinal cord, promoting plasticity and functional recovery in animal models of SCI. The primary goal of exercise training in the laboratory and the clinic has been to promote locomotor recovery after SCI, yet the most notable effect so far is the prevention of SCI-induced at- and below-level neuropathic pain (Figure 1 and Hutchinson et al, 2004). Our lab routinely initiates exercise therapy (passive cycling, treadmill training or automated wheel running) at 5 dpi, and we have shown that the neurotrophic factors within the spinal cord and DRGs are increased (Keeler et al, 2011;Côté et al, 2011). In this experiment, we wanted to maximize the response of neurotrophic factors to have the best opportunity to affect the development of neuropathic pain after SCI. Unfortunately, this timing prohibited us from partitioning SCI+Exercise rats into an Allodynia and No Allodynia group because the assessment of tactile sensation so early after SCI is not reliable. This is often due to an inability of the rat to exhibit adequate weight bearing in the ipsilesional forepaw to withstand the force of the von Frey monofilaments (Detloff et al, 2009, Detloff et al, 2012a). However, if exercise were ineffective, we would expect that ~40% of exercised rats would exhibit allodynic behavior. In actuality, we saw that there was a dramatic decrease in the incidence of tactile allodynia in exercised rats (Figure 1). To prevent biased groups, we were careful to randomize rats into groups prior to SCI surgery. Additionally, we carefully examined the tissue sparing at the lesion epicenter for any clear outliers. Certainly, whether exercise is able to attenuate or ameliorate allodynic like hypersensitivity after it develops is a clinically-relevant question that is currently being examined in the laboratory.
That exercise was able to normalize paw withdrawal responses to tactile but not thermal stimuli is intriguing. In this experiment, bilateral hypersensitivity to a noxious thermal stimuli persisted even with exercise therapy (Figure 2). Exercise on automated running wheels, similar to locomotor treadmill training, provides rhythmic loading of the paws and stimulates both proprioceptive and cutaneous but not thermal afferents. Moreover, nociceptive afferents are distinct in their laminar distribution in the spinal cord as well as molecular phenotype and responsiveness to different sensory modalities. Non-peptidergic c afferents correspond to mechanosensitive nociceptors found in the epidermis (Cavanaugh et al, 2009). Peptidergic c fibers play an important role in heat sensitivity. After SCI, these peptidergic afferents sprout within the dorsal horn that corresponds sensitization and increased synaptic strength on second order projection neurons (Krenz & Weaver, 1998; Murray & Goldberger, 1974; Ondarza et al, 2003; Weaver et al, 2002; Weaver et al, 2001). Here, we report that the allodynia-related increase in non-peptidergic afferent density and distribution within the dorsal horn can be modulated by exercise. These data indicate that rhythmic, load-bearing exercise is a form of task-specific training for to improve tactile sensation by maintaining normal distribution of mechanosensitive nociceptive afferents. Whether exercise can modulate peptidergic c afferent distribution and sprouting associated with neuropathic pain is unknown.
The mechanisms by which daily exercise therapy protects the nonpeptidergic c afferents from aberrant plasticity are unclear. As exercise is a non-specific therapy, it is possible that it acts as an upstream modulator of both inflammatory mediators and neurotrophic factors—two distinct processes that are altered after SCI. While previous work from our lab and others has shown the importance of the innate immune response on the development of SCI-induced pain (Detloff et al, 2008; Hains & Waxman, 2006; Peng et al, 2006; Saab et al, 2008; Zhao et al, 2007a; Zhao et al, 2007b), the impact of neurotrophic factors, GFLs in particular, on the development of SCI-induced neuropathic pain has received little attention.
Neurotrophic factors promote survival, growth and maintenance of neurons and regulate synaptic strength to improve signal transduction. After SCI, local increase of neurotrophic factors in the damaged spinal cord via drug delivery systems, genetically modified cells or exercise promotes regeneration of damaged axons, plasticity of spinal cord circuitry and improves functional locomotor recovery (Dolbeare & Houle, 2003) (Boyce & Lemay, 2009; Côté et al, 2011; Petruska et al, 2010; Tom et al, 2013). Importantly, the effects of exercise are not limited to the region of the spinal cord most likely stimulated by the exercise intervention, but rather increases some neurotrophic factors throughout the spinal cord in segments both associated with and far-removed from the exercised limbs (Côté et al, 2011). That exercise represents a method to deliver neurotrophic factors to the spinal cord may represent a method to modulate plasticity of pain afferents and the development of at- or below-level neuropathic pain. Indeed, Hutchinson et al (2004) first showed that exercise therapy normalized levels of BDNF in the spinal cord and muscle that correlated with the amelioration of below-level allodynia through a BDNF-mediated mechanism. We have extended these findings and demonstrated that exercise maintains levels of GDNF and artemin within the DRG and dorsal horn while preventing the development of at-level allodynia.
The stereotypic topographical organization within the dorsal horn is disrupted by SCI. Damage to descending spinal cord tracts which modulate pain transmission in the dorsal horn leads to vacated synaptic sites on many dorsal horn neurons. The increase in IB4+, GFL-responsive afferents within the dorsal horn seen in this study coupled with the well-established data that peptidergic pain afferents exhibit robust sprouting after SCI (Ondarza et al, 2003) may represent an attempt to restore pre-injury innervation of second order neurons. Aberrant nociceptive afferent density coupled with recent data from Walters et al (2012) that showed an increase in spontaneous activity of nociceptive DRG neurons suggests that amplification of nocifensive or neuropathic signaling begins at the primary sensory neuron.
Afferent sprouting and regeneration can be induced by many neurotrophic factors including nerve growth factor, neurotrophin-3 and GDNF after a dorsal root crush and SCI; however only artemin has been shown to promote regeneration of damaged afferents to their original targets in the dorsal horn (Boucher & McMahon, 2001; Boucher et al, 2000; Harvey et al, 2010; Ramer et al, 2000). One interpretation of these data is that there is a specific threshold of GFLs necessary to maintain or restore a normal distribution of non-peptidergic GFL-responsive afferents. Unlike dorsal root crush models, our SCI model does not directly damage the afferent fibers terminating in the dorsal horn of C7 and C8 corresponding to the dermatomes of the forepaw. Yet, we have shown that SCI caused a decrease in GFLs that lead to an expansion of the distribution of GFL-responsive afferents in the dorsal horn. We show normalization of GFL levels after SCI via exercise maintained normal topographic distribution of IB4+ pain afferents within the dorsal horn. This indicates that GFLs may be a key factor in managing and/or preventing aberrant pain fiber plasticity and the associated induction of allodynia after SCI. That exercise in this instance prevents sprouting of nociceptive afferents is interesting, as several laboratories have shown that exercise and/or increased levels of neurotrophic factors (namely the neurotrophins NT-3 and BDNF) increases neural plasticity within the spinal cord grey matter to improve functional recovery (Boyce et al, 2012, Côté et al, 2011, Petruska et al, 2010). Exercise did not over express the levels of GFLs within the dorsal horn, but rather kept them within normal ranges, and the distribution pattern was similar to that of naïve. Whether over expression of GFLs in the dorsal horn would promote aberrant sprouting of afferents (nociceptive or otherwise) is unknown.
Alternatively, the increase in isolectin-B4 positive afferent fibers seen in rats with SCI-induced allodynia may represent a change in the phenotypic composition of normally cutaneous sensory afferents to a nociceptive phenotype. After nerve injury, cutaneous afferents decrease expression of growth factor receptor-(GFR)-α2 (the receptor for the GFL neurturin) and begin to express GFR-α3 (the receptor for artemin) and the capsaicin receptor, thereby switching from a cutaneous to a nociceptive phenotype (Wang et al, 2011). To accurately assess a compositional change was beyond the scope of the present experiments. Pre-injury anatomical tracing of afferent fibers would be required to examine whether these types of compositional changes occur after SCI. While this information would be interesting, our ability to reliably and accurately deliver exercise not to mention assess sensation (our primary outcome) would be jeopardized.
Several laboratories have suggested that the timing of neurotrophic support after dorsal root insult is critical to not only regenerative and growth promoting processes, but also to topographic specificity and the modulation of synaptic strength (Ramer et al, 2001, Harvey et al, 2012). The experiments presented in this manuscript initiated an exercise paradigm early after SCI, prior to the onset of at-level allodynia. Further studies are needed to test whether initiating exercise therapy once SCI-induced neuropathic pain is established will restore normal sensation and or restore normal afferent distribution within the dorsal horn.
In summary, the studies conducted here identify marked decrease in GDNF and artemin within the DRGs and dorsal horn as predictors of at-level allodynia after SCI. The increase in isolectinB-4 positive afferent may have a significant role in the development of SCI-induced allodynia. Importantly, we have shown that exercise training beginning 5 days after injury is sufficient to prevent the development of this debilitating neuropathic pain while normalizing the levels of GFLs, and maintaining a normal distribution of GFL-responsive pain fibers.
HIGHLIGHTS
-
>
Noninvasive exercise modulates neurotrophic factor expression and afferent plasticity.
-
>
We examine changes in GDNF and artemin and nociceptive afferents after SCI.
-
>
We show that exercise prevents the development of at-level neuropathic pain after SCI.
-
>
We show that exercise regulates GDNF, artemin and associated non-peptidergic afferents.
-
>
Data implicate GDNF and ARTN regulation via exercise as an anti-allodynic therapy.
Acknowledgements
We express our sincerest thanks to Kassi Miller for her assistance with animal care and behavioral procedures, and to Dr. Marion Murray for her valuable comments and review of this manuscript. This study was supported by Paralyzed Veterans of America Fritz Krauth Memorial Fellowship #2707 (MRD) and NIH Grant NS 026380 (JDH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boucher TJ, McMahon SB. Neurotrophic factors and neuropathic pain. Curr Opin Pharmacol. 2001;1:66–72. doi: 10.1016/s1471-4892(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–7. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Boyce VS, Lemay MA. Modularity of endpoint force patterns evoked using intraspinal microstimulation in treadmill trained and/or neurotrophin-treated chronic spinal cats. J Neurophysiol. 2009;101:1309–20. doi: 10.1152/jn.00034.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce VS, Park J, Gage FH, Mendell LM. Differential effects of brain-derived neurotrophic factor and neurotrophin-3 on hindlimb function in paraplegic rats. Eur J Neurosci. 2012;35(2):221–32. doi: 10.1111/j.1460-9568.2011.07950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calancie B, Molano MR, Broton JG. Epidemiology and demography of acute spinal cord injury in a large urban setting. J Spinal Cord Med. 2005;28:92–6. doi: 10.1080/10790268.2005.11753804. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci. 2009;106(22):9075–80. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté MP, Detloff MR, Wade RE, Jr, Lemay MA, Houle JD. Plasticity in ascending long propriospinal and descending supraspinal pathways in chronic cervical spinal cord injured rats. Front Physiol. 2012;3:330. doi: 10.3389/fphys.2012.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol. 2010;225:366–76. doi: 10.1016/j.expneurol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, Deibert RJ, Basso DM. Acute and chronic tactile sensory testing after spinal cord injury in rats. J Vis Exp. 2012a:e3247. doi: 10.3791/3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;212:337–47. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff MR, Wade RE, Jr, Houle JD. Chronic at- and below-level pain following unilateral cervical spinal cord contusion in rats. J Neurotrauma. 2012b [Google Scholar]
- Dolbeare D, Houle JD. Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF) J Neurotrauma. 2003;20:1251–61. doi: 10.1089/089771503770802916. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, et al. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–9. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–95. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Hagg T. Collateral sprouting as a target for improved function after spinal cord injury. J Neurotrauma. 2006;23:281–94. doi: 10.1089/neu.2006.23.281. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Mol Ther. 2003;8:367–75. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harvey P, Gong B, Rossomando AJ, Frank E. Topographically specific regeneration of sensory axons in the spinal cord. Proc Natl Acad Sci U S A. 2010;107:11585–90. doi: 10.1073/pnas.1003287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–14. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–63. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houlé JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;15(1438):8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Changes in the morphology of sympathetic preganglionic neurons parallel the development of autonomic dysreflexia after spinal cord injury in rats. Neurosci Lett. 1998;243:61–4. doi: 10.1016/s0304-3940(98)00101-3. [DOI] [PubMed] [Google Scholar]
- Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008;31:538–47. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–99. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Goldberger ME. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J Comp Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;184:373–80. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13:2177–82. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–51. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Kitay B, Boyce VS, Kaspar BK, Pearse DD, Gage FH, et al. Intramuscular AAV delivery of NT-3 alters synaptic transmission to motoneurons in adult rats. Eur J Neurosci. 2010;32:997–1005. doi: 10.1111/j.1460-9568.2010.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Krzyzanowska A, Wong LF, Grist J, Mazarakis ND, Georgievska B, et al. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol Ther. 2006;13:1101–9. doi: 10.1016/j.ymthe.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–6. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J Neurosci. 2001;21(8):2651–60. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab CY, Waxman SG, Hains BC. Alarm or curse? The pain of neuroinflammation. Brain Res Rev. 2008;58:226–35. doi: 10.1016/j.brainresrev.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210(2):489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–93. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan K, Gullapalli RP, Zhuo J, Mirvis SE. Diffusion tensor MR imaging in cervical spine trauma. AJNR Am J Neuroradiol. 2008;29:655–9. doi: 10.3174/ajnr.A0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, et al. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol. 2013;239:91–100. doi: 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–24. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, et al. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11:488–96. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Molliver DC, Jing X, Schwartz ES, Yang FC, Samad OA, et al. Phenotypic switching of nonpeptidergic cutaneous sensory neurons following peripheral nerve injury. PLoS One. 2011;6:e28908. doi: 10.1371/journal.pone.0028908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LC, Marsh DR, Gris D, Meakin SO, Dekaban GA. Central mechanisms for autonomic dysreflexia after spinal cord injury. Prog Brain Res. 2002;137:83–95. doi: 10.1016/s0079-6123(02)37009-2. [DOI] [PubMed] [Google Scholar]
- Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR. Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma. 2001;18:1107–19. doi: 10.1089/08977150152693782. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga E, Biering-Sorensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain basic data set. Spinal Cord. 2008;46:818–23. doi: 10.1038/sc.2008.64. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007a;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nociceptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine cysteine chemokine ligand 21. J Neurosci. 2007b;27:8893–902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol. 2007;204:777–90. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]