Abstract
Background
Nephrotic syndrome is defined as urine total protein >3.5 g/d or total protein-creatinine ratio of >3.5 g/g, low serum albumin, high serum cholesterol, and peripheral edema. These threshold levels have not been rigorously evaluated in diabetic kidney disease or using urine albumin, the preferred measure of proteinuria in diabetes.
Study Design
Diagnostic test study
Setting and Participants
Adults with type 2 diabetes, hypertension and urine total protein >0.9 g/d enrolled in the Irbesartan in Diabetic Nephropathy Trial.
Index Test
Baseline measures of proteinuria (total protein, albumin, and protein-creatinine and albumin-creatinine ratios). Linear regression to relate measures.
Reference Test
Other signs and symptoms of nephrotic syndrome at baseline (serum albumin <3.5g/dl, serum total cholesterol >260mg/dl or use of a statin, edema or use of a loop diuretic); progression of chronic kidney disease during follow up (doubling of baseline serum creatinine or requirement for dialysis or kidney transplantation). Logistic regression to relate index and reference tests.
Results
Among 1608 participants, total urine protein of 3.5 g/d was equivalent to urine albumin of 2.2 (95% confidence interval 1.4–3.5) g/d. Of 1467 participants, 641 (44%) had urine total protein ≥3.5 g/d at baseline, 132 (9%) had other signs and symptoms of nephrotic syndrome at baseline and 385 (26%) had progression of kidney disease over a mean follow-up interval of 2.6 years. Areas under the receiver operating curves for measures of proteinuria were 0.80 to 0.83 for other signs and symptoms of nephrotic syndrome and 0.72 to 0.74 for kidney disease progression. The threshold levels for nephrotic-range proteinuria and albuminuria were close to the points of maximal accuracy for both outcomes.
Limitations
Study population limits generalizability; inability to adjust for several variables known to affect serum albumin; lack of spot urine samples
Conclusions
The historical definition for nephrotic-range proteinuria appears reasonable in diabetic kidney disease. The equivalent thresholds for nephrotic-range albuminuria and albumin-creatinine ratio are 2.2 g/d and 2.2 g/g, respectively.
Index words: nephrotic syndrome, urine protein, urine albumin, proteinuria, albuminuria, protein-creatinine ratio, albumin-creatinine ratio, diabetic kidney disease, edema, hypercholesterolemia, hypoalbuminemia, chronic kidney disease progression, CKD
Nephrotic syndrome is one of the principal presentations of kidney disease, reflecting the pathophysiologic effects of urinary losses of large quantities of protein. It is defined as urine total protein ≥3.5 g/d, low serum albumin, high serum cholesterol, and peripheral edema (1, 2). Nephrotic syndrome is seen only in glomerular diseases, thus its identification provides essential information about the cause of kidney disease, prognosis and treatment (3–6).
Proteinuria is the key finding differentiating edematous states due to kidney disease from other diseases (7–9). The threshold level of total urine protein of 3.5 g/d was proposed based on a case series of patients with predominantly primary glomerular disease (10), and later extended to a total protein–creatinine ratio of 3.5 g/g (11). Although these thresholds for the definition of nephrotic syndrome are widely accepted, they have not been rigorously tested to our knowledge.
Diabetic kidney disease has replaced primary glomerular diseases as the leading cause of proteinuria in the United States and Europe (12). However, there is evidence that diabetes may directly or indirectly affect the presentation of kidney disease with proteinuria(13) and urine albumin is now the preferred measure of urine protein in patients with diabetes (14, 15). For these reasons, we directly compared measures of proteinuria in adults with diabetic kidney disease and undertook a rigorous evaluation of specific threshold levels of these measures for the definition of nephrotic syndrome. Our goals were to define nephrotic-range albuminuria and to evaluate threshold levels of measures of proteinuria (total protein, albumin, and ratios of total protein-creatinine and albumin-creatinine) for the discrimination of outcomes of kidney disease (other signs and symptoms of nephrotic syndrome and subsequent kidney disease progression).
METHODS
Study Design and Population
We performed a diagnostic test evaluation using secondary analysis using data from the Irbesartan in Diabetic Nephropathy Trial (IDNT), a multicenter randomized controlled trial comparing the angiotensin receptor blocker irbesartan or the dihydropyridine calcium channel blocker amlodipine to placebo on the progression of diabetic kidney disease (16). The study included 1715 patients enrolled from the years 1996–1999 and used the following inclusion criteria: type 2 diabetes, hypertension (systolic or diastolic blood pressure >135 or >85 mm Hg, respectively or taking antihypertensive medications), baseline urine total protein ≥0.9 g/d, and baseline serum creatinine 1.0 to 3.0 mg/dl. We included patients with urine total protein values ≤30 g/day. Follow-up was until December 21, 2000 and mean time was 2.6 years. The investigational review board at Tufts Medical Center approved the study.
Definitions
The index tests were baseline measures of proteinuria (total protein, albumin, and ratios of protein-creatinine and albumin-creatinine), as reported from 24 hour collections. The reference tests were outcomes of kidney disease, including other signs and symptoms of nephrotic syndrome alone or in combination at baseline (for cross-sectional analysis) and progression of kidney disease during followup (for longitudinal analysis). In clinical practice, the nephrotic syndrome is considered as a binary characteristic, and therefore we dichotomized the individual signs and symptoms. Hypoalbuminemia was defined as serum albumin <3.5g/dl, hypercholesterolemia was defined as serum cholesterol >260mg/dl or use of a statin, edema was recorded on case report forms or use of a loop diuretic. Kidney disease progression during followup was defined as a doubling of baseline serum creatinine or requirement for dialysis or kidney transplantation.
Data analysis
We used linear regression to relate urine total protein to urine albumin in 24 hour samples. The measures were log transformed due to a skewed pattern on the residual diagnostic plot using non-transformed values. The resulting regression equation was used to calculate the albuminuia equivalent value of nephrotic-range proteinuria expressed both as excretion rate and protein-creatinine ratio in 24 hour urine samples.
We used logistic regression to relate the measures of urine protein and other signs and symptoms of the nephrotic syndrome at baseline. We used logistic regression as well as Cox proportional hazards analysis to relate measures of urine protein at baseline to kidney disease progression during follow-up. For both outcomes, we performed multivariate analyses, adjusting for age, sex, race, body mass index, duration of diabetes, insulin use, prior ACE (angiotensin converting enzyme) inhibitor use, systolic blood pressure, eGFR (estimated glomerular filtration rate), systolic blood pressure, and randomly assigned treatment group. The purpose of this step was to unequivocally establish the basic relationship between the measures of urine protein and outcomes by addressing potential confounding variables. Sensitivity analyses were performed using serum creatinine rather than eGFR. Model assumptions (e.g., proportional hazards) were checked with residual diagnostics.
We created receiver operating characteristic (ROC) curves to relate the measures of urine protein to outcomes using bivariate regression. The historical values for nephrotic syndrome (3.5 g/day urine total protein and 3.5 g/g protein-creatinine ratio) as well as the equivalent levels of urine albumin and albumin-creatinine ratio were compared with other potential cutoff levels. For the outcome of kidney disease progression, we compared the c-statistics derived from Cox models with the value derived from logistic regression to ensure concordance between the two methods (17).
Sensitivity analyses of logistic regression, Cox models, and ROC curves were preformed using different cutoff levels for hypercholesterolemia (200 mg/dl, 240 mg/dl, 300 mg/dl) and without the inclusion of statin use and considering edema without the inclusion of loop diuretic use. Sensitivity analyses were also preformed including the composite outcome of death or kidney disease progression.
RESULTS
Study Participants
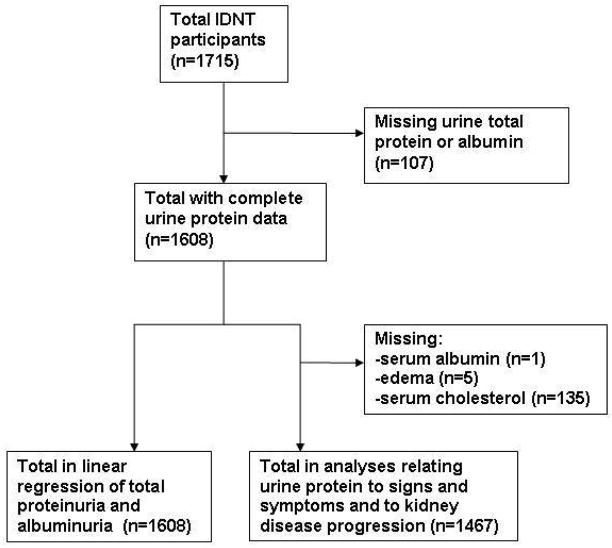
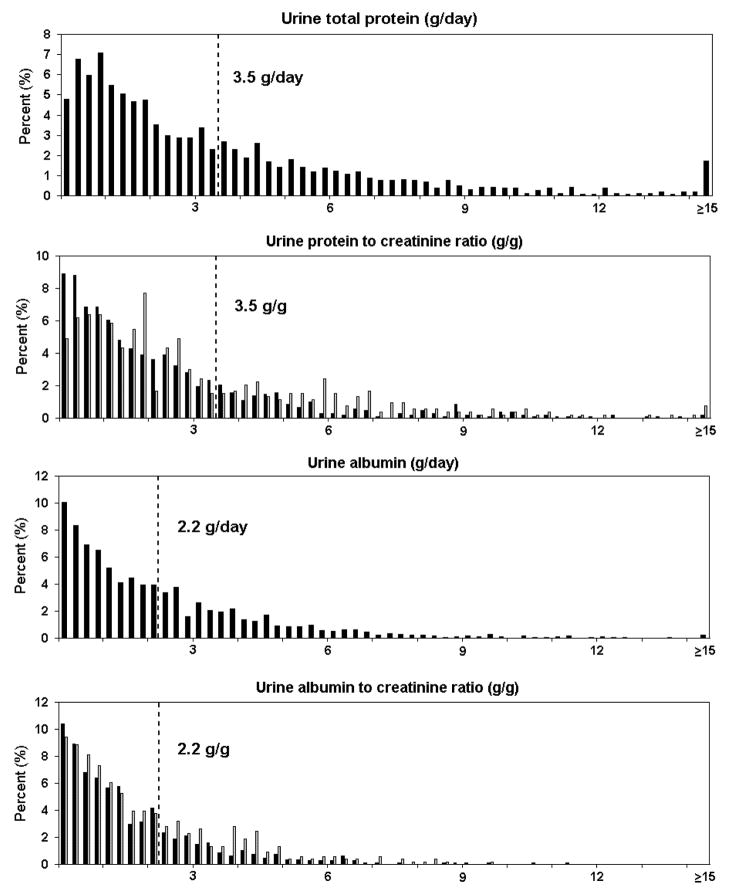
Of 1715 participants in IDNT, 1608 had complete data for measures of urine protein and 1467 patients for signs and symptoms of nephrotic syndrome (Figure 1). Characteristics of these samples were similar to the original study and to those with missing data (data not shown) (16). Distributions of urine total protein and albumin are shown in Figure 2. Table 1 show baseline characteristics and outcomes of the study sample according to baseline level of total urine protein. A total of 641 participants (44%) had urine total protein ≥3.5 g/d. Mean (SD) urinary creatinine excretion was 1.4 (0.5) g/d [1.6 (0.5) g/d in men and 1.1 (0.4) g/d in women]. Sex-specific distributions of ratios of total protein–creatinine and albumin-creatinine are shown in Figure 2.
Figure 1.
Selection of the study population
Figure 2. Histograms of proteinuria in the study population.
Dotted line indicates the historical threshold value for definition of nephrotic-range proteinuria, and proposed threshold value for nephrotic range albuminuria. Urine total protein–creatinine and albumin-creatinine ratios are stratified by sex (men=black; women=grey).
Table 1.
Baseline characteristics and outcomes of study participants according to level of urine total protein
| Overall | <3.5 g/d | ≥3.5g/d | p value | |
|---|---|---|---|---|
|
|
|
|||
| n (%) or mean (std) | n (%) or mean (std) | n (%) or mean (std) | ||
| Total number | 1608 | 915 | 693 | |
| Age (years) | 59 (8) | 60 (7) | 58 (8) | <0.01 |
| Men | 1077 (67%) | 610 (67%) | 467 (67%) | 0.8 |
| White race | 1167 (73) | 672 (73%) | 495 (71%) | 0.4 |
| BMI (kg/m2) | 31 (6) | 30 (6) | 32 (6) | <0.01 |
| Insulin use | 924 (58%) | 498 (54%) | 426 (61%) | <0.01 |
| Duration of diabetes (years) | 15 (8) | 14 (8) | 15 (8) | 0.08 |
| Retinopathy | 1067 (69%) | 576 (66%) | 491 (74%) | <0.01 |
| History of cardiovascular disease | 725 (45%) | 383 (42%) | 342 (49%) | <0.01 |
| ACE inhibitor at baseline | 704 (44%) | 387 (42%) | 317 (46%) | 0.2 |
| SBP (mmHg) | 159 (20) | 157 (19) | 162 (20) | <0.01 |
| Serum creatinine (mg/dl) | 1.7 (0.6) | 1.6 (0.5) | 1.8 (0.6) | <0.01 |
| eGFR (ml/min/1.73m2) | 48 (18) | 51 (18) | 44 (16) | <0.01 |
| Hemoglobin (g/dl) | 13 (2) | 13 (2) | 13 (2) | <0.01 |
| Urine total protein (g/day)* | 2.9 (1.7–5.3) | 1.8 (1.2–2.5) | 5.8 (4.5–8.1) | <0.01 |
| Urine albumin (g/day)* | 1.9 (1.0–3.5) | 1.1 (0.7–1.6) | 3.9 (3.0–5.4) | <0.01 |
| Urine total protein–creatinine ratio (g/g)* | ||||
| men | 1.9 (1.1–3.5) | 1.2 (0.8–1.7) | 3.8 (2.8–5.5) | <0.01 |
| women | 2.7 (1.7–5.2) | 1.8 (1.2–2.5) | 5.5 (3.7–7.5) | <0.01 |
| Urine albumin-creatinine ratio (g/g)* | ||||
| men | 1.3 (0.7–2.4) | 0.7 (0.4–1.1) | 2.6 (1.9–3.7) | <0.01 |
| women | 1.8 (1.0–3.3) | 1.1 (0.7–1.6) | 3.4 (2.5–4.9) | <0.01 |
| Urine creatinine (g/day) | 1.4 (0.5) | 1.4 (0.5) | 1.5 (0.6) | <0.01 |
| men | 1.6 (0.5) | 1.5 (0.5) | 1.7 (0.6) | <0.01 |
| women | 1.1 (0.4) | 1.1 (0.4) | 1.2 (0.4) | <0.01 |
| Edema or use of loop diuretic+ | 729 (50%) | 321 (39%) | 408 (64%) | <0.01 |
| High serum cholesterol or use of statin+ | 663 (45%) | 303 (36%) | 360 (56%) | <0.01 |
| Low albumin+ | 345 (24%) | 75 (9%) | 270 (42%) | <0.01 |
| All three of the above+ | 132 (9%) | 21 (3%) | 111 (17%) | <0.01 |
| Doubling of serum creatinine (Scr)+ | 316 (22%) | 103 (13%) | 213 (33%) | <0.01 |
| End stage renal disease (ESRD)+ | 232 (16%) | 64 (8%) | 168 (26%) | <0.01 |
| Doubling of serum creatinine or ESRD+ | 385 (26%) | 126 (15%) | 259 (41%) | <0.01 |
| Death+ | 215 (15%) | 93 (11%) | 122 (19%) | <0.01 |
median (IQR)
total n = 1467 for edema or loop diuretic, high cholesterol or statin, low albumin, all three, or kidney disease outcomes, respectively
Conversion factors for units: estimated GFR in ml/min/1.73m2 to ml/s/1.73m2, x0.01667; serum creatinine in mg/dL to mol/L, x88.4; hemoglobin and albumin in g/dl to g/L, x10.
Abbreviations: ACE, angiotensin-converting enzyme; BMI, body mass index; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate.
Definition of nephrotic-range albuminuria
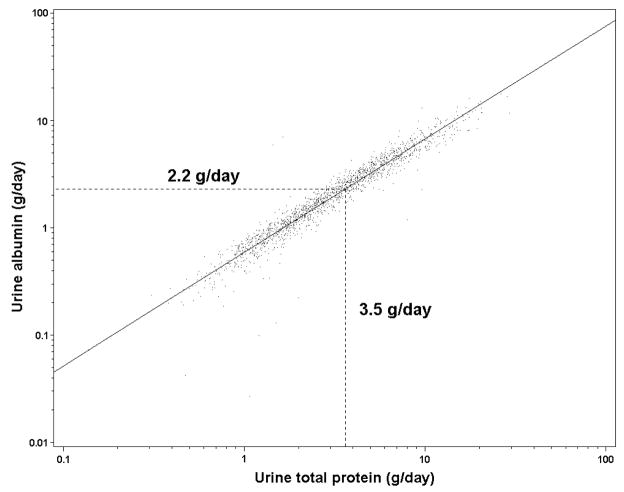
Figure 3 shows the relationship between log-transformed total urine protein and log-transformed urine albumin. The regression equation used to calculate urine albumin from urine total protein was: Albumin = 0.596 (95% CI [95% confidence interval] 0.58–0.60) × (Total Protein)1.054 (95% CI 1.04–1.06), r-squared value for the regression was 0.92). Therefore, a value of 3.5g/day for urine total protein was equivalent to 2.2 g/d for urine albumin and 3.5 g/g for urine total protein–creatinine ratio was equivalent to 2.2 g/g for urine albumin-creatinine ratio (95% CI for individual value 1.4–3.5 for both).
Figure 3. Relationship of log transformed urine albumin and log transformed total total protein.
Linear regression line (solid)Values for albumin are approximately 0.6 times the values for total protein; 2.2 g/d (95% confidence interval 1.4-3.5g/d) is the urine albumin equivalent of nephrotic-range total protein, 3.5 g/d (small dashed line).
Use of proteinuria cutoff levels to discriminate the signs and symptoms of nephrotic syndrome
Table 2 shows unadjusted and adjusted logistic regression analysis of measures of proteinuria with these outcomes. After multivariable adjustment, urine total protein (in log base 2 form: meaning OR (odds ratio) signifies risk for each doubling of urine protein measurement) was strongly associated with low serum albumin (OR and 95% confidence interval 3.33, 2.50–3.95), high serum cholesterol (1.52, 1.37–1.69), edema (1.47, 1.31–1.64), and a composite of all three (3.20, 2.53–4.06). Regressions using urine albumin and urine ratios of total protein–creatinine and albumin-creatinine as the independent variable gave similar results as regressions using urine total protein (Table 2). Regressions using creatinine rather than eGFR were not substantially different.
Table 2.
Relationship between log urine total protein and binary outcomes
| Unadjusted |
Adjusted* |
Unadjusted |
Adjusted* |
|
|---|---|---|---|---|
| Total protein |
Total protein-creatinine ratio |
|||
| Low serum albumin | 3.19 (2.74–3.71) | 3.33 (2.50–3.95) | 3.83 (3.25–4.50) | 3.65 (3.06–4.37) |
| High serum cholesterol | 1.48 (1.34–1.63) | 1.52 (1.37–1.69) | 1.48 (1.35–1.63) | 1.45 (1.31–1.61) |
| Edema | 1.66 (1.50–1.84) | 1.47 (1.31–1.64) | 1.64 (1.49–1.80) | 1.51 (1.36–1.69) |
| All three signs and symptoms | 3.15 (2.56–3.87) | 3.20 (2.53–4.06) | 3.44 (2.78–4.25) | 3.13 (2.48–3.95) |
| Kidney disease progression | 2.15 (1.90–2.43) | 1.83 (1.60–2.10) | 2.25 (1.99–2.54) | 1.83 (1.60–2.09) |
| Kidney disease progression or death | 2.04 (1.82–2.27) | 1.84 (1.62–2.07) | 2.22 (1.99–2.49) | 1.96 (1.73–2.21) |
|
Albumin |
Albumin-creatinine ratio |
|||
| Low serum albumin | 2.98 (2.58–3.44) | 3.19 (2.69–3.78) | 3.72 (3.16–4.38) | 3.52 (2.95–4.19) |
| High serum cholesterol | 1.45 (1.33–1.59) | 1.50 (1.36–1.66) | 1.47 (1.34–1.60) | 1.44 (1.31–1.59) |
| Edema | 1.59 (1.45–1.75) | 1.42 (1.28–1.58) | 1.59 (1.45–1.74) | 1.47 (1.33–1.62) |
| All three signs and symptoms | 3.01 (2.46–3.69) | 3.16 (2.49–4.00) | 3.49 (2.81–4.35) | 3.17 (2.51–4.02) |
| Kidney disease progression | 2.08 (1.85–2.34) | 1.83 (1.60–2.08) | 2.22 (1.97–2.50) | 1.84 (1.61–2.09) |
| Kidney disease progression or death | 1.91 (1.72–2.11) | 1.75 (1.57–1.96) | 2.10 (1.89–2.34) | 1.87 (1.66–2.09) |
Values are given as log base 2 odds ratio (95% confidence interval); OR signifies the risk for each doubling of urine protein measurement.
for age, sex, race, body mass index, insulin use, duration of diabetes, baseline angiotensin-converting enzyme inhibitor, systolic blood pressure, estimated glomerular filtration rate, hemoglobin level, and randomization assignment
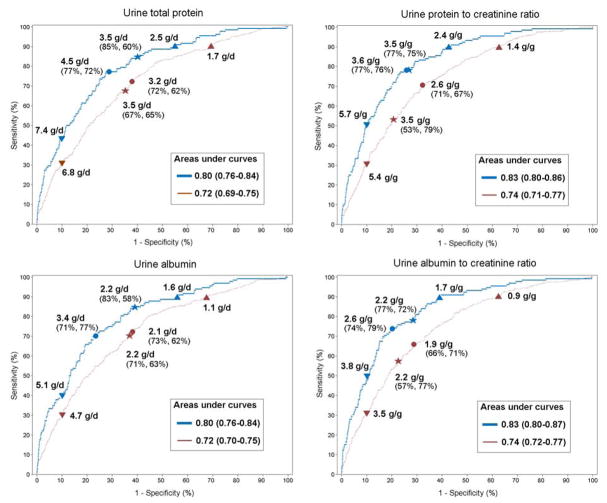
Figure 4 shows the ROC curves for discrimination of other signs and symptoms of nephrotic syndrome at baseline according to baseline level of measures of proteinuria. The area under the ROC was 0.80 (0.76–0.84) for urine total protein. The historical threshold level of 3.5 g/day had a sensitivity of 85% and specificity of 60%, which was close to the point of maximal accuracy (4.5 g/day, sensitivity of 77% and specificity of 72%). The area under the ROC was 0.83 (0.80–0.86) for urine total protein–creatinine ratio. The proposed definition of 3.5 g/g was almost identical to the point of maximal accuracy (3.6 g/g) and had a sensitivity and specificity 77% and 75%, respectively. The results were similar for albuminuria, yielding areas under the curve of 0.80 (0.76–0.84) for urine albumin and 0.83 (0.80–0.87) for albumin-creatinine ratio. The equivalent value for urine albumin of 2.2 g/d fell near the point of maximal accuracy (3.4 g/d) and the equivalent value for albumin-creatinine ratio of 2.2 g/g was close to the point of maximal accuracy (2.6 g/g) (Figure 4). Results of sensitivity analyses that varied the threshold level for high serum cholesterol and considered the outcomes without the effects of statins and loop diuretics were not substantially different.
Figure 4. Receiver operating curves relating measures of urine protein to outcomes.
All three other signs and symptoms of nephrotic syndrome (thick blue line) and kidney disease progression (thin red line) and sensitivity and specificity for selected values. Historical threshold value for nephrotic range urine total protein and equivalent value for nephrotic range albumin; point of maximum accuracy (circle); point of 90% sensitivity (triangle); point of 90% specificity (inverted triangle).
Use of proteinuria cutoff levels to discriminate kidney disease progression
During follow-up, a total of 385 participants (26%) had kidney disease progression [316 (22%) had doubling of serum creatinine and 232 (16%) began dialysis or underwent kidney transplantation] and 215 (15%) died. Table 2 shows unadjusted and adjusted logistic regression analysis of measures of proteinuria with these outcomes. After multivariable adjustment, urine total protein was strongly associated with kidney disease progression (OR and 95% confidence interval 1.83, 1.60–2.10). Using the composite outcome of progression of kidney disease or death, the association was similar (1.84, 1.62–2.07). Using Cox regression analysis, the results of survival analysis for kidney disease progression were similar, as was shown previously (18). Regressions using urine albumin and urine ratios of total protein–creatinine and albumin-creatinine as the independent variable gave similar results as regressions using urine total protein (Table 2). Regressions using creatinine rather than eGFR were not substantially different.
Figure 4 shows the ROC curve for discrimination of kidney disease progression during followup according to baseline level of proteinuria. The area under the ROC was 0.72 (0.69–0.75). The historical cutoff level 3.5 g/day had a sensitivity (67%) and specificity (65%), and was close to the point of maximal accuracy (3.2 g/day, sensitivity of 73% and specificity of 62%). The area under the ROC was 0.74 (0.71–0.77) for urine total protein–creatinine ratio. The proposed definition of 3.5 g/g was close to the point of maximal accuracy (2.6 g/g) and had a sensitivity and specificity 53% and 79%, respectively. The results were similar for albuminuria, yielding areas under the curve of 0.72 (0.70–0.75) for urine albumin and 0.74 (0.72–0.77) for albumin-creatinine ratio. The equivalent value for urine albumin of 2.2 g/d was almost identical to the point of maximal accuracy (2.1 g/d) and the equivalent value for albumin-creatinine ratio of 2.2 g/g was close to the point of maximal accuracy (1.9 g/g). Sensitivity analyses that included death in the composite outcome were not substantially different.
DISCUSSION
Proteinuria is central to the pathophysiology of nephrotic syndrome. Our study evaluated the relationship of measures of proteinuria and threshold levels for definition of nephrotic syndrome in diabetic kidney disease, the most common cause of proteinuria in the US and Europe. In this study, values for urine albumin and albumin-creatinine ratio of 2.2 g/d and 2.2 g/g, respectively, were equivalent to the accepted threshold levels for urine total protein and total protein-creatinine ratio of 3.5 g/d and 3.5 g/g, respectively. The threshold levels for urine total protein and the equivalent levels for urine albumin were close to the point of maximal accuracy for the discrimination of presence of other signs and symptoms of nephrotic syndrome. Thus our findings confirm these widely accepted threshold levels for application to diabetic kidney disease and define equivalent levels for urine albumin.
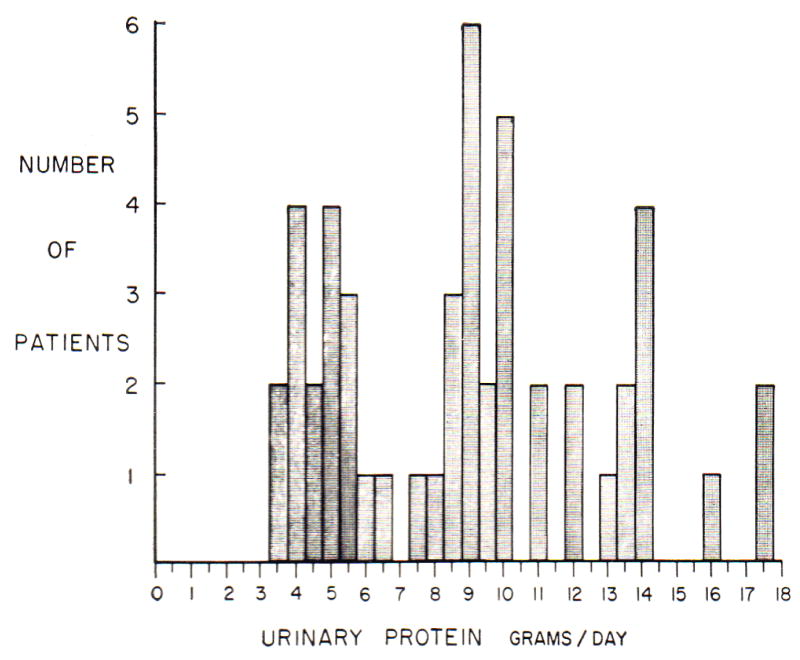
The recognition of signs and symptoms of nephrotic syndrome has evolved over many years. As early as the ancient Greek period, all edema was considered as one disease, termed “dropsy” (19). In 1827, Richard Bright identified a subset of patients with dropsy that also had proteinuria and kidney disease (“Bright’s Disease”) (8, 9). The association of proteinuria with other signs and symptoms of nephrotic syndrome was recognized in the early 20th century (7, 20–24). The threshold level for urine total protein of 3.5 g/d appears to have been proposed in the 1963 by Dr. George Schreiner who reported total urine protein in 186 patients with features of nephrotic syndrome (10). The lowest value was 3.5 g/d, observed in two patients (Figure 5). In that study, the mean age of the patients was 37 years, the majority had primary glomerular diseases, and the criteria for classification of patients as having nephrotic syndrome was not specified. Twenty years later, Ginsberg and colleagues proposed measuring urine total protein-creatinine ratio in random urine samples, and based on the assumption that urine creatinine excretion is approximately 1 g/d, suggested a ratio of 3.5 g/g as the threshold for nephrotic syndrome. Recently, urine albumin and albumin-creatinine ratio have been suggested to be more reliable measures of proteinuria than urine total protein– or protein-creatinine ratio (14, 15). Urine creatinine excretion was greater than 1.0 g/d in our study (Table 1), thus we observed values for ratios of total protein–creatinine and albumin-creatinine ratio that were lower than values for urine total protein and urine albumin expressed as g/d (Figure 2). Nonetheless, the accepted thresholds were close to the points of maximal accuracy for all measures.
Figure 5. Urine total protein excretion in 186 patients with nephrotic syndrome reported by Schreiner.
Reproduced with permission from Strauss and Welt.10
In our study, albumin accounted for approximately 60% of urine total protein, which is similar to the relative contribution of albumin to total serum protein in normal individuals (25). This is consistent with the understanding of pathophysiology of diabetic kidney disease. Detailed studies in humans with type 2 diabetes suggest the filtration barrier is altered both by an uniform widening of usual “pores” for filtration that allow filtration of small serum proteins, as well as emergence of large pores (“shunts”) that allow filtration of large serum proteins (26, 27). Patients with higher urine albumin exhibit a larger fraction of filtered macromolecules via the shunt pathway (28), thus the composition of urine protein should mirror that of plasma proteins in patients with nephrotic syndrome due to diabetic kidney disease.
The pathophysiology of nephrotic syndrome has been extensively studied in primary glomerular diseases (29). The primary abnormality is loss of urine albumin leading to low serum albumin and low serum oncotic pressure, with consequent renal sodium retention and increased hepatic synthesis of apolipoproteins. However, independent of kidney disease and proteinuria, diabetes is associated with a variety of alterations which could also affect serum albumin and cholesterol levels (30–33). Patients with diabetes exhibit differences in interstitial osmotic gradients (30, 31) and glycosylation of albumin can alter its transport properties across certain tissues, which could potentially influence the development of peripheral edema (34). Furthermore, diabetes can cause elevated triglyceride levels, decreased HDL (high-density lipoprotein) levels, and abnormal LDL metabolism (32, 33). Nonetheless, in our study the relationships of urine total protein and albumin with other signs and symptoms of nephrotic syndrome were strong and robust to multivariable adjustment.
Threshold values (or “cut-off values”) to categorize continuous variables can aid in medical teaching and practice by simplifying clinical decision making. In our study, the relationships between level of measures of proteinuria and outcomes appeared continuous. However, the historical threshold values for urine total protein and equivalent values for urine albumin provided an adequate level for discrimination for the other signs and symptoms of nephrotic syndrome. Interestingly, these same threshold values also provided reasonable discrimination for short-term progression of kidney disease or the composite outcome of kidney disease progression and survival over a relatively short interval. Other studies report levels of proteinuria far below these thresholds are associated with increased long-term risk for kidney disease progression and for occurrence of cardiovascular disease events (35–37).
There are several limitations. First, IDNT included only patients with type 2 diabetes and urine total protein >0.9 g/d, which restricts the generalizability of the results. However, the pathophysiology of nephrotic syndrome makes it is unlikely that signs and symptoms would occur at lower levels of proteinuria, and therefore IDNT may represent an appropriate study population in type 2 diabetes for this purpose. Additional studies in patients with type 1 diabetes and nondiabetic kidney disease are necessary to extend our findings to these populations. Second, we did not have sensitive measures of important factors that affect serum albumin (for example, C-reactive protein as a marker of inflammation and prealbumin as a marker of malnutrition). However, the analysis did include hemoglobin, which often mirrors inflammation in CKD (chronic kidney disease), and BMI (body mass index), which could roughly estimate patients in the poorest categories of nutritional status, and adjustment for these factors did not change the results (38). Third, current practice guidelines recommend the use of untimed (“spot”) collections to assess the albumin-creatinine or protein-creatinine ratio; however, only 24-hour samples were available to us (14, 39). However, previous literature has established that the ratios in spot samples correlate reasonably well with 24 hour urine excretion rates (11, 40).
In conclusion, the historical definition for nephrotic range proteinuria of >3.5 g/d and the more recent proposal for nephrotic range protein-creatinine ratio of >3.5 g/g appear reasonable in diabetic kidney disease. Equivalent thresholds for the definition of nephrotic-range albuminuria and albumin-creatinine ratio are >2.2 g/d and >2.2 g/g, respectively.
Acknowledgments
This work was presented in abstract form at 40th Annual Meeting of the American Society of Nephrology, San Francisco, CA on November 3rd 2007. The authors thank Maia Leppo for providing technical assistance.
Support: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant UO1 DK053869. Dr Stoycheff was supported by institutional training grant T32 DK07777 from the NIDDK.
Footnotes
Financial Disclosure: Dr Stevens consulted for Abbot Vascular in 2008. The remaining authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Because an author of this manuscript is an editor for AJKD, the peer-review and decisionmaking processes were handled entirely by an Associate Editor (Robert Nelson, MD, PhD, National Institutes of Health) who served as Acting Editor-in-Chief. Details of the journal's procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
References
- 1.Brenner BM, Levine SA, Rector FC. Brenner & Rector’s the kidney. 7. Philadelphia, Pa: Saunders; 2004. MD Consult LLC. [Google Scholar]
- 2.Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338(17):1202–11. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 3.Rivera F, Lopez-Gomez JM, Perez-Garcia R. Clinicopathologic correlations of renal pathology in Spain. Kidney Int. 2004;66(3):898–904. doi: 10.1111/j.1523-1755.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 4.Hunt LP, Short CD, Mallick NP. Prognostic indicators in patients presenting with the nephrotic syndrome. Kidney Int. 1988;34(3):382–8. doi: 10.1038/ki.1988.192. [DOI] [PubMed] [Google Scholar]
- 5.Ordonez JD, Hiatt RA, Killebrew EJ, Fireman BH. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int. 1993;44(3):638–42. doi: 10.1038/ki.1993.292. [DOI] [PubMed] [Google Scholar]
- 6.The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98(4):561–4. doi: 10.1016/s0022-3476(81)80760-3. [DOI] [PubMed] [Google Scholar]
- 7.The Nephrotic syndrome. Lancet. 1958;2(7054):1000–2. [PubMed] [Google Scholar]
- 8.Jay V. Richard Bright--physician extraordinaire. Arch Pathol Lab Med. 2000;124(9):1262–3. doi: 10.5858/2000-124-1262-RBPE. [DOI] [PubMed] [Google Scholar]
- 9.Peitzman SJ. From dropsy to Bright’s disease to end-stage renal disease. Milbank Q. 1989;67 (Suppl 1):16–32. [PubMed] [Google Scholar]
- 10.Strauss MB, Welt LG. Diseases of the kidney. 2. Boston: Little Brown; 1963. [Google Scholar]
- 11.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309(25):1543–6. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 12.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50(2):169–80. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 13.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1. [PubMed] [Google Scholar]
- 14.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Miller WG, Bruns DE, Hortin GL, et al. Current Issues in Measurement and Reporting of Urinary Albumin. Clinical Chemistry. 2008 doi: 10.1373/clinchem.2008.106567. (in press) [DOI] [PubMed] [Google Scholar]
- 16.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 17.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61(1):92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 18.Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2):281–7. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Eknoyan G. A history of edema and its management. Kidney Int Suppl. 1997;59:S118–26. [PubMed] [Google Scholar]
- 20.Heidland A, Gerabek W, Sebekova K. Franz Volhard and Theodor Fahr: achievements and controversies in their research in renal disease and hypertension. J Hum Hypertens. 2001;15(1):5–16. doi: 10.1038/sj.jhh.1001130. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AA. A Contribution To The Study of The Chemistry of Blood Serum. J Exp Med. 1912;16(6):719–731. doi: 10.1084/jem.16.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein AA. Further Studies on the Chemistry of Blood Serum. J Exp Med. 1913;17(4):444–452. doi: 10.1084/jem.17.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein AA. Studies on the Chemistry of Serous Effusions. J Exp Med. 1914;20(4):334–345. doi: 10.1084/jem.20.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein AA. Concerning the causation of edema in chronic parenchymatous nephritis; method for its alleviation. Am J Med Sci. 1917;154(638):638–647. doi: 10.1016/0002-9343(52)90020-x. [DOI] [PubMed] [Google Scholar]
- 25.Becker GJ. Which albumin should we measure? Kidney Int Suppl. 2004;(92):S16–7. doi: 10.1111/j.1523-1755.2004.09204.x. [DOI] [PubMed] [Google Scholar]
- 26.Nelson RG, Bennett PH, Beck GJ, et al. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. Diabetic Renal Disease Study Group. N Engl J Med. 1996;335(22):1636–42. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 27.Myers BD, Nelson RG, Tan M, et al. Progression of overt nephropathy in non-insulin-dependent diabetes. Kidney Int. 1995;47(6):1781–9. doi: 10.1038/ki.1995.246. [DOI] [PubMed] [Google Scholar]
- 28.Lemley KV, Blouch K, Abdullah I, et al. Glomerular permselectivity at the onset of nephropathy in type 2 diabetes mellitus. J Am Soc Nephrol. 2000;11(11):2095–105. doi: 10.1681/ASN.V11112095. [DOI] [PubMed] [Google Scholar]
- 29.Appel GB, Blum CB, Chien S, Kunis CL, Appel AS. The hyperlipidemia of the nephrotic syndrome. Relation to plasma albumin concentration, oncotic pressure, and viscosity. N Engl J Med. 1985;312(24):1544–8. doi: 10.1056/NEJM198506133122404. [DOI] [PubMed] [Google Scholar]
- 30.Fauchald P, Norseth J, Jervell J. Transcapillary colloid osmotic gradient, plasma volume and interstitial fluid volume in long-term type 1 (insulin-dependent) diabetes. Diabetologia. 1985;28(5):269–73. doi: 10.1007/BF00271683. [DOI] [PubMed] [Google Scholar]
- 31.Hommel E, Mathiesen ER, Aukland K, Parving HH. Pathophysiological aspects of edema formation in diabetic nephropathy. Kidney Int. 1990;38(6):1187–92. doi: 10.1038/ki.1990.332. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien T, Nguyen TT, Zimmerman BR. Hyperlipidemia and diabetes mellitus. Mayo Clin Proc. 1998;73(10):969–76. doi: 10.4065/73.10.969. [DOI] [PubMed] [Google Scholar]
- 33.Kissebah AH, Alfarsi S, Evans DJ, Adams PW. Plasma low density lipoprotein transport kinetics in noninsulin-dependent diabetes mellitus. J Clin Invest. 1983;71(3):655–67. doi: 10.1172/JCI110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampietro T, Bertuglia S, Colantuoni A, Bionda A, Lenzi S, Donato L. Increased permeability of hamster microcirculation to glycosylated albumin. Lancet. 1987;2(8566):994–6. doi: 10.1016/s0140-6736(87)92559-1. [DOI] [PubMed] [Google Scholar]
- 35.Eknoyan G, Hostetter T, Bakris GL, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42(4):617–22. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 36.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–47. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 37.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 38.Chonchol M, Lippi G, Montagnana M, Muggeo M, Targher G. Association of inflammation with anaemia in patients with chronic kidney disease not requiring chronic dialysis. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn109. [DOI] [PubMed] [Google Scholar]
- 39.Bakker AJ. Detection of microalbuminuria. Receiver operating characteristic curve analysis favors albumin-creatinine ratio over albumin concentration. Diabetes Care. 1999;22(2):307–13. doi: 10.2337/diacare.22.2.307. [DOI] [PubMed] [Google Scholar]
- 40.Chitalia VC, Kothari J, Wells EJ, et al. Cost-benefit analysis and prediction of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin Nephrol. 2001;55(6):436–47. [PubMed] [Google Scholar]